Abstract
The biocontrol fungus Trichoderma harzianum, from both marine and terrestrial environments, has attracted considerable attention. T. harzianum has a tremendous potential to produce a variety of bioactive secondary metabolites (SMs), which are an important source of new herbicides and antibiotics. This review prioritizes the SMs of T. harzianum from 1988 to June 2022, and their relevant biological activities. Marine-derived SMs, especially terpenoids, polyketides, and macrolides compounds, occupy a significant proportion of natural products from T. harzianum, deserving more of our attention.
Keywords: natural products, Trichoderma harzianum, marine sources, bioactivity, secondary metabolites
1. Introduction
The unique marine environment with high pressure, high salinity, and low temperature, breeds unique marine microorganisms [1,2]. Secondary metabolites obtained from marine-derived fungi have attracted considerable attention in recent years for potential use in the discovery of unique structures and diverse biological properties [3,4].
The biocontrol fungi Trichoderma spp. (sordariomycetes) are widely spread in the environment [5], such as in the ocean. With the deepening of marine science and technology exploration, more and more Trichoderma sp. strains have been discovered from marine sources. From marine and terrestrial environments, there are no fewer than 250 Trichoderma species discovered so far [6]. Trichoderma species are famous for producing plentiful secondary metabolites [7]. Among them, Trichoderma harzianum probably contributed the most secondary metabolites (SMs) originating from Trichoderma species [8,9]. The SMs from T. harzianum showed antifungal activity [10]. Additionally, cytotoxicity [11] and antimicrobial activity [12], and so on, have also been found in its SMs.
The SMs of T. harzianum have not been summarized in detail or systematically. Up to now, nearly 200 compounds of T. harzianum have been reported. The secondary metabolites of T. harzianum include terpenoids, polyketides, peptides, alkaloids, and lactones. Herein, this review reports the isolated compounds of T. harzianum and their bioactivities. Furthermore, details of the source organisms were analyzed for marine and terrestrial sources. A total number of 180 compounds are presented in this review with 58 cited references. These references cover the time period from 1988 to June 2022.
2. Structural and Biological Activity Studies
2.1. Terpenoids
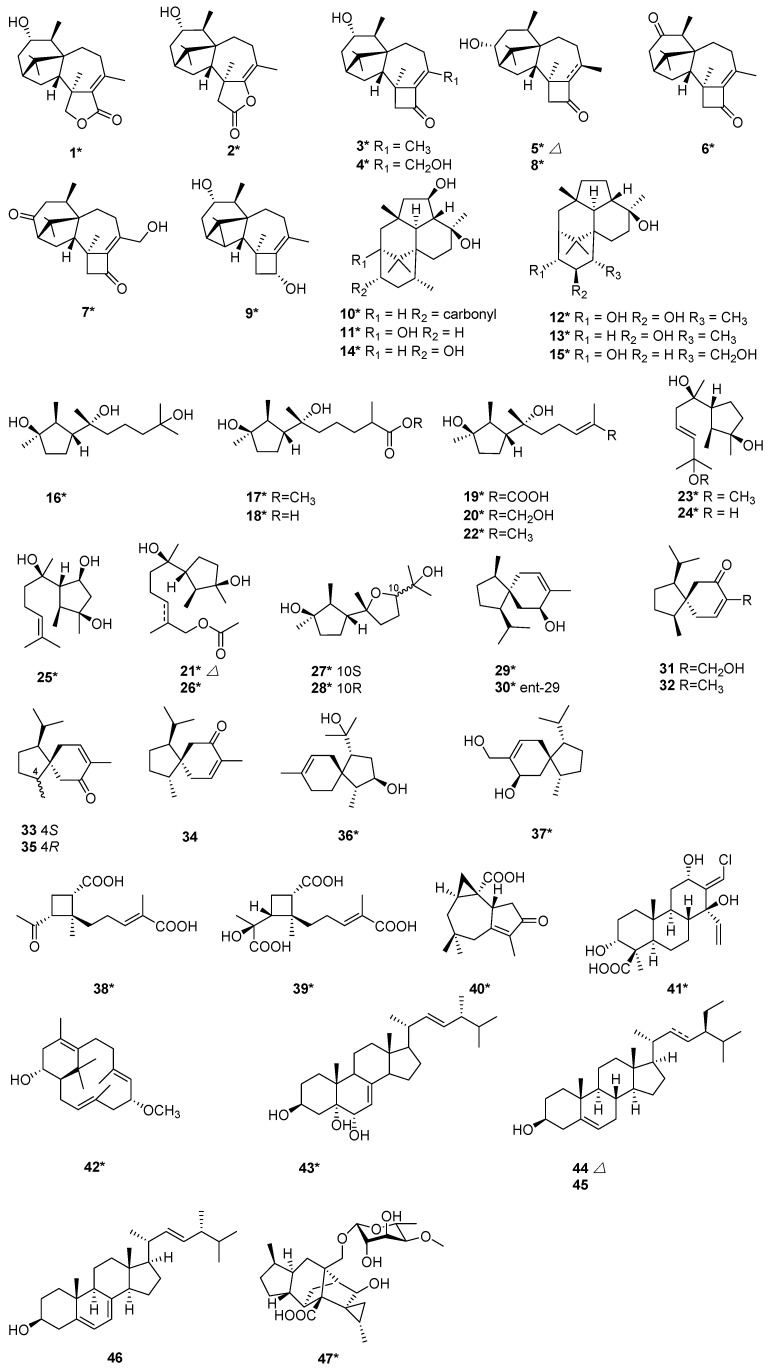
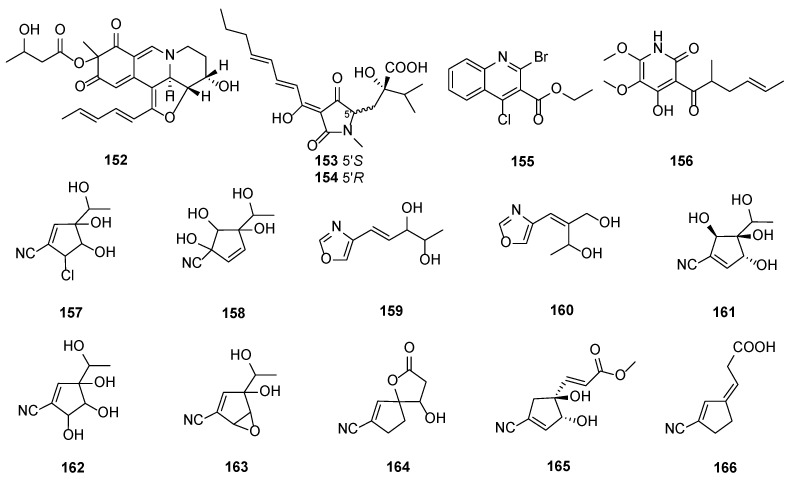
Seven new potent phytotoxic harziane diterpenes harzianelactones A and B (1 and 2), harzianones A–D (3–6) and harziane (9) were isolated from the soft coral-derived fungus T. harzianum XS-20090075 [13]. Compounds 1 and 2 belonged to a unique class of terpenes with a 6-5-7-5-fused carbocyclic core and a lactone ring. Harzianones A–D (3–6) consisted of a fused tetracyclic 6-5-7-4-fused tetra-cyclic skeleton. Chemical epigenetic manipulation was applied to activate silent genes of T. harzianum XS-20090075 by appending a histone deacetylase (HDAC) inhibitor. With this experimental technique, two new diterpenoids harzianone E (7) and harzianolic acid A (41), and one new sesquiterpenoid 3,7,11-trihydroxy-cycloneran (16) were isolated from the same strain T. harzianum XS-20090075. At the same time, 11 known sesquiterpenoids, methyl 3,7-dihydroxy-15-cycloneranate (17), catenioblinc (18), ascotrichic acid (19), cyclonerotriol (20), (10E)-12-acetoxy-10-cycloneren-3,7-diol (21), cyclonerodiol (22), cyclonerodiol oxide (27), epicyclonerodiol oxide (28), ent-trichoacorenol (29), trichoacorenol (30), and ophioceric acid (40) were isolated from T. harzianum XS-20090075 [14]. It was the first time for obtaining cleistanthane diterpenoid from T. harzianum XS-20090075. Trichodermanins C–H (10–15) were new diterpenes with a rare fused 6-5-6-6 ring system, and have been isolated from a fungus T. harzianum OUPS-111D-4 [15,16]. This strain was separated from a piece of sponge Halichondria okadai. Compounds 10–15 were evaluated for their cytotoxicity by using murine P388 leukemia, human HL-60 leukemia, and murine L1210 leukemia cell lines. Compound 10 with a fused 6-5-6-6 ring system exhibited potent cytotoxic activity [15], and compounds 12 and 13 exhibited modest activity [16]. Six new terpenes, including one harziane diterpene, 3R-hydroxy-9R,10R-dihydroharzianone (8), three cyclonerane sesquiterpenes, methyl 3,7-dihydroxy-15-cycloneranate (17), 11-methoxy-9-cycloneren-3,7-diol (23), 10-cycloneren-3,5,7-triol (25), and one acorane sesquiterpene, 8-acoren-3,11-diol (36), and one cyclonerane 11R-methoxy-5,9,13-proharzitrien-3-ol (42), together with four known sesquiterpenes, cyclonerodio (22), 9-cycloneren-3,7,11-triol (24), trichoacorenol (30) and trichoacorenol B (37) were isolated from T. harzianum X-5 [17]. The strain X-5 was an endophytic fungus isolated from the marine brown alga Laminaria japonica. The above six new compounds (8, 17, 23, 25, 36, and 42) were evaluated to inhibit four marine phytoplankton species and four marine-derived pathogenic bacteria [17]. Compounds 23 and 42 exhibited potent inhibition activity [17]. Harzianoic acid A (38) is a sesquiterpene, and harzianoic acid B (39) is a norsesquiterpene with a cyclobutane nucleus. They were isolated from a sponge-isolated fungus, T. harzianum LZDX-32-08 [18], and were found to have new natural scaffolds to exert anti-HCV activity for their capability to inhibit multi-targets, including those for virus replication and entry [18]. (10E)-12-Acetoxy-10-cycloneren-3,7-diol (21) and 12-acetoxycycloneran-3,7-diol (26) were two new cyclonerane sesquiterpenoids, which were isolated from the marine sediment-derived fungus T. harzianum P1-4 [9]. A new acorane-type sesquiterpene, 15-hydroxyacorenone (31), was isolated from T. harzianum [19], together with acorenone (32), acorenone-B (33), 4-epiacorenone (34), and 4-epiacorenone-B (35). Stigmasta-7,22-dien-3β,5α,6α-triol (43) was isolated from T. harzianum XS-20090075, cultivated by the Czapekʹs culture [20]. Compound 43 exhibited antifouling activity with an EC50 value of 39.2 μg/mL and Topo I inhibitory activity with an MIC value of 50.0 μM [20]. Two fungal strains of T. harzianum T-4 and T. harzianum T-5 were obtained from Palampur, Himachal Pradesh (India). Stigmasterol (44) and β-sitosterol (45) were isolated from T. harzianum T-4 [21]. Ergosterol (46) was isolated from T. harzianum T-5 [21]. Trichosordarin A (47), a unique norditerpene aglycone, was isolated from T. harzianum R5 [22]. Compound 47 was toxic to the marine zooplankton Artemia salina with an LC50 value of 233 µM [22] (Figure 1).
Figure 1.
Chemical structures of terpenoids (1–47) from T. harzianum. * Means marine source compounds.
2.2. Polyketides
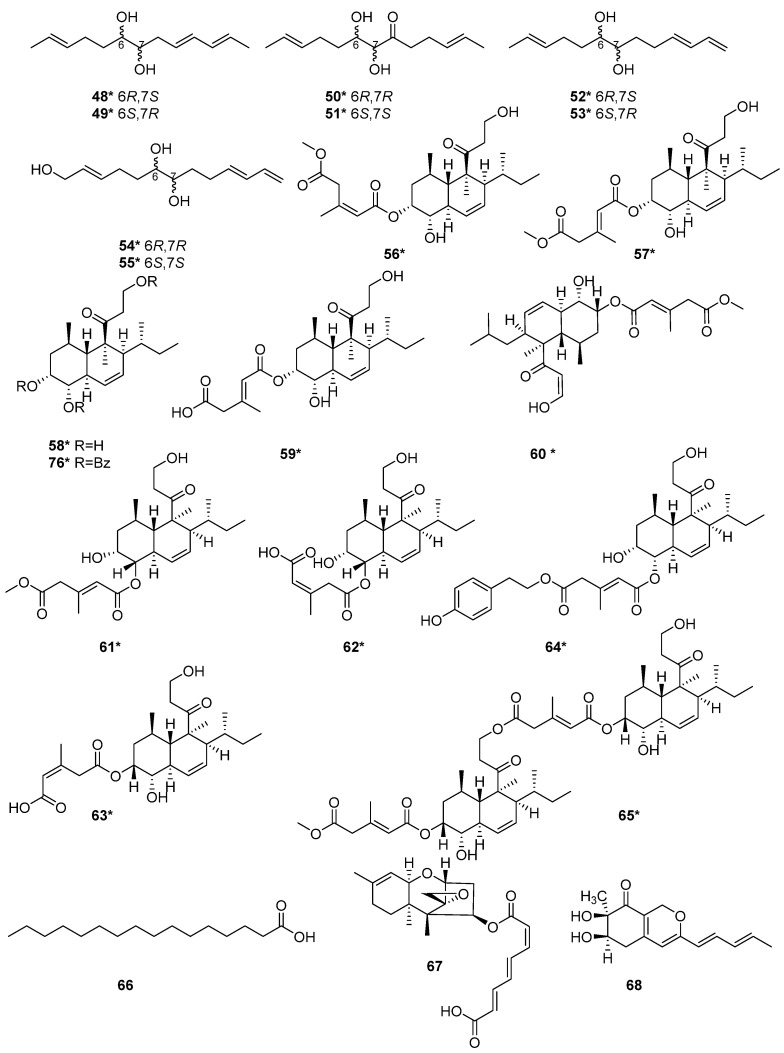
The fermentation of a sponge-associated fungus T. harzianum HMS-15-3 led to the isolation of four pairs of new C13 lipid enantiomers harzianumols A–H (48–55) [23]. Four polyketides, trichoharzin B (56), methyl-trichoharzin (57), trichoharzin (58), and eujavanicol A (59), were isolated from T. harzianum XS-20090075 [20], which was fermented in rice medium by one strain many compounds (OSMAC) strategy. New naphthalene compound 57, and known naphthalene compound 58 exhibited antifouling activity with the EC50 values of 29.8 and 35.6 μg/mL [20]. Six new tandyukisins, tandyukisins A–F (60–65), were isolated from T. harzianum OUPS-111D-4 [11,24,25], which were initially derived from the sponge Halichondria okadai. Among the tandyukisins A–F (60–65), compounds 60, 64 and 65 exhibited cytotoxicity against murine P388 leukemia, human HL-60 leukemia, and murine L1210 leukemia cell lines inferior to the control 5-fluorouracil [24]. Compounds 61–63 showed slightly selective growth inhibition against the central nervous system cancer SNB-75 cell line in the HCC panel [25]. Compounds 64 and 65 exhibited significant cytotoxicity against the cancer cell lines P388, HL-60, and L1210 [24]. The structure-activity relationship may be relevant to the terminals of the side chains. T. harzianum T-4 was obtained from Palampur, Himachal Pradesh in India, and a polyketide palmitic acid (66) was isolated from the T-4 [21]. Harzianum A (67), was a new trichothecene isolated from the soil-borne fungus T. harzianum in 1994 [26]. Harziphilone (68) was a new polyketide isolated from T. harzianum WC 47695 [27], which was isolated from sandy soil with plant debris collected in Fort Lauderdale. The REV/RRE binding assay and HIV assay revealed that compound 68 showed inhibitory activity against REV-protein binding to RRE RNA with IC50 values of 2.0 μM. In contrast, this compound did not show protection against HIV infection at concentration levels up to 200 μg/mL. The cytotoxicity assay on the murine tumor cell line M-109 showed that 68 exhibited cytotoxicity at 38 μM [27]. Seven polyketides, keto triol 3 (69), keto diol 7 (70), keto diol 6 (71), keto diol 8 (72), triacetate 9 (73), triol 10 (74) and acetal diol 2 (75) were isolated from T. harzianum [28]. One new trichoharzin (58), and two known compounds, tribenzoate (76) and triacetate (77), were isolated from T. harzianum Rifai in 1993 [29]. A new polyketide, T22azaphilone (78), was isolated from T. harzianum T22 [30]. A new compound, trichoharzianol (79), isolated from T. harzianum F031, exhibited antifungal activity against Colletotrichum gloeosporioides with a MIC of 128 μg/mL [31]. Three novel polyketides trichodenones A–C (80–82) were isolated from T. harzianum OUPS-N115 [32]. This strain was separated from the sponge Halichondria okadai. Trichodenones A–C (80–82) showed cytotoxicities against P388 cell line with the ED50 values of 0.21, 1.21, and 1.45 μg/mL, respectively. Homodimericin A (83) was isolated from T. harzianum WC13 [33,34]. In their model, compound 83 was the biologically inert aftermath of a fungal counter to a bacterial attack. The discovery of cryptenol (84) from T. harzianum WC13 [34] indicated that the interactions among microbes in a termite nest were not bipartite but a multipartite system.
The structure and activity relationships of anthraquinones (AQs) in T. harzianum have been studied. AQs represent an important class of SMs occurring in T. harzianum strains, which exhibited a variety of biological functions [12]. The alkylating functionalities in the AQs maximize the anticancer activity by binding tightly with DNA to disrupt the DNA function [35]. Moreover, anthraquinone derivatives were proposed to have an anticancer function by inhibiting protein kinase CK2 [36]. Pachybasin (85) and chrysophanol (86) were isolated from T. harzianum ETS 323 [37]. 1,7-Dihydroxy-3-hydroxymethyl-9,10-anthraquinone (87), 1,5-dihydroxy-3-hydroxymethyl-9,10-anthraquinone (88), emodin (89), and ω-hydroxypachybasin (90) were isolated from T. harzianum strain Th-R16 [38]. These compounds exhibited effective antifungal activity against Botrytis cinerea (Ascomycete) and Rhizoctonia solani (Basidiomycete). At a 500 μg/mL concentration, compound 88 showed comparatively higher activity against R. solani and B. cinerea than 89 [38]. Phomarin (91), (+)-2′S-isorhodoptilometrin (92), 1,6-dihydroxy-3-(hydroxymethyl)anthracene-9,10-dione (93), harzianumnone A (94) and harzianumnone B (95) were isolated from the soft coral-derived fungus T. harzianum XS-20090075 [12]. Compounds 94 and 95 were identified as a pair of epimers, the first example of hydroanthraquinones from T. harzianum XS-20090075. Compound 92 with Topo I inhibition activity, was further assessed for cytotoxic activity against human tumor cell lines. It exhibited cytotoxic activity against HepG2 cell line with an IC50 value of 2.10 µM, and showed cytotoxicity against Hela cell with an IC50 value of 8.59 µM [12] (Figure 2 and Figure 3).
Figure 2.
Chemical structures of polyketides (48–68 and 76) from T. harzianum. * Means marine source compounds.
Figure 3.
Chemical structures of polyketides (69–75 and 77–95) from T. harzianum. * Means marine source compounds.
2.3. Peptides
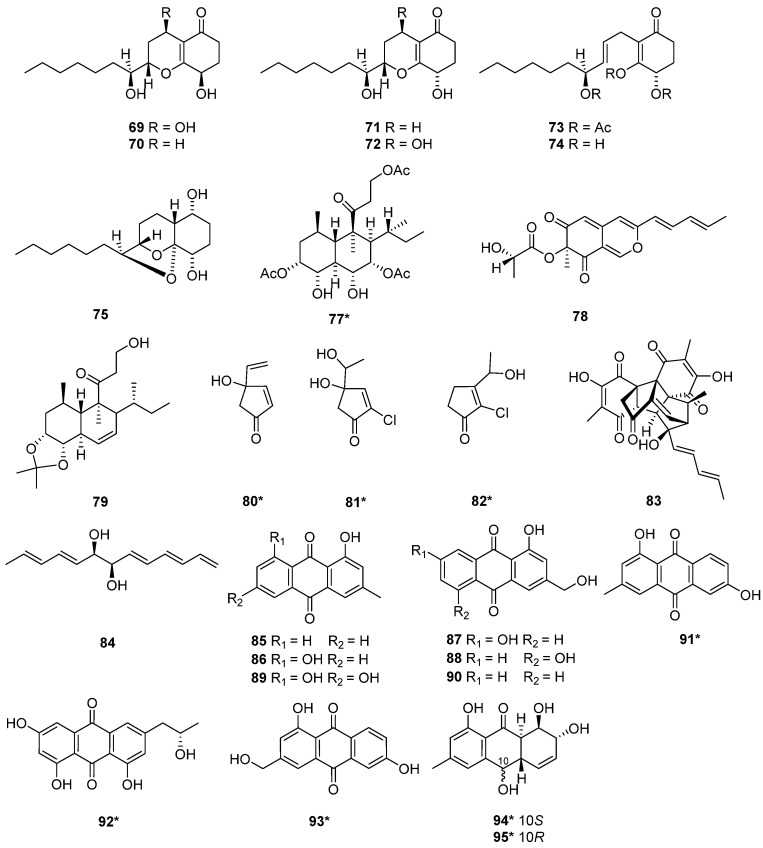
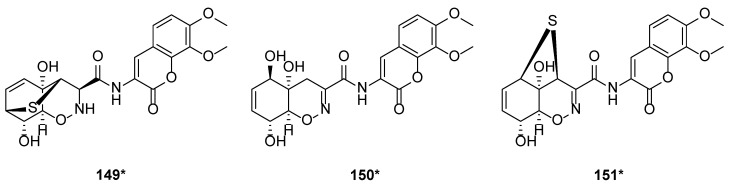
Peptaibols are linear antibiotic peptides consisting of 5 to 20 amino acids [39]. It could be biosynthesized by T. harzianum. Peptaibols were characterized by the structures of alpha-aminoisobutyric acid (Aib), and C-terminal hydroxylated amino acid. Two new series peptaibols, trichokindins (TKs) and trichorozins (TZs), were isolated from T. harzianum collected at Nara in Japan. TKs and TZs comprised 18 and 11 amino acid residues, respectively, while TKs were rich in isovaline (Iva). TK-VII (106) is the most hydrophobic of TKs with 18-residue peptides. Compound 106 induced Ca2+-dependent catecholamine secretion from bovine adrenal medullary chromaffin cells [40]. TKs (96–106), with a single peak on HPLC and typical IR absorptions at 3300, 1600, and 1530 cm−1, were confirmed as peptaibols by polarization transfer spectra [40]. With incubating 10 μM of TK-VII (106), 27% of the total catecholamines in bovine adrenal chromaffin cells were secreted in the presence of the Ca2+. In contrast, only 5% of the total catecholamines were secreted without Ca2+ [40]. Hydrophobicity is vital to the interaction between membranes and peptaibols [41]. HB I (107) was isolated from T. harzianum M-903603 [42]. Trichorzins HA (108–113) and MA (114–116) were isolated from T. harzianum M-903602 and T. harzianum M-922835, respectively. Compounds 108–116 are a series of 18-residue peptides [43]. Bioassays on the antifungal activity of trichorzins and harzianins on the phytopathogenic fungus Sclerotium cepivorum revealed that trichorzins were more potent (75% inhibition at 100 μg/mL) than harzianins (40% inhibition at 100 μg/mL) [44]. Research on the structured-activity relationships (SARs) revealed that the peptide chain length and superhydrophobicity played an essential part in the peptide/membrane interaction and the subsequent permeability by perturbing the ironic balance of the cell [44]. As new membrane-modifying peptides isolated from T. harzianum, trichorozins I–IV (117–120), belonged to peptaibols with 11 residues. It was reported that compounds 117–120 exhibited voltage-dependent ion channel-like activity in lipid bilayers [45]. Eleven peptides were isolated from T. harzianum M-903603, and named harzianins HC (121–131) [46]. The detailed study of such proline-rich 14-residue peptaibols revealed that harzianins HC increased the permeability of liposomes and improved voltage-dependent conductance [46]. An exogenous amino acid supply simplified the microheterogeneous peptide mixtures when Aib, Glu, or Arg was added to the fermentation media of T. harzianum M-902608. Harzianin PCU4 (132), trichorzin PAU4 (133), trichorzin PA II (134), trichorzin PA IV–VIII (135–139) and trichorzin PA IX (140) were isolated from this T. harzianum M-902608 [47]. When cultured in the Aib-enriched media, compounds 132 and 133 were isolated, while trichorzins PA was obtained from the standard culture media [47]. Trichorzianines A (TA) and B (TB) are peptaibols isolated from T. harzianum. TA IIIc (141) induced the growth inhibition and lysis of the amoeba Dictyostelium [48]. With the aid of positive ion FAB mass spectrometry, COSY and NOESY experiments, seven peptides of trichorzianines B isolated from T. harzianum were identified, and these peptides included trichorzianine TB IIa (142), trichorzianine TB IIIc (143), trichorzianine TB IVb (144), trichorzianine TB Vb (145), trichorzianine TB VIa (146), trichorzianine TB VIb (147) and trichorzianine TB VII (148) [49]. From a mangrove-derived fungus, T. harzianum D13, a novel heterocyclic dipeptide trichodermamide G (149), two known biogenetically related compounds, trichodermamide A (150) and aspergillazin A (151) were isolated. A unique sulfur bridge was observed in the structures of compounds 149 and 151 [50] (Table 1 and Figure 4).
Table 1.
The sequences of peptides (96–148) from T. harzianum.
| Compounds | Sequences of Peptides | |
|---|---|---|
| 96 | Trichokindin Ia | Ac Aib Ser Ala Aib Aib Gln Iva Leu Aib Ala Aib Aib Pro Leu Aib Aib Gln Ile OH |
| 97 | Trichokindin Ib | Ac Aib Ser Ala Aib Iva Gln Aib Leu Aib Ala Aib Aib Pro Leu Aib Aib Gln Ile OH |
| 98 | Trichokindin IIa | Ac Aib Ser Ala Aib Aib Gln Aib Leu Aib Ala Iva Aib Pro Leu Aib Aib Gln Ile OH |
| 99 | Trichokindin IIb | Ac Aib Ser Ala Aib Iva Gln Iva Leu Aib Ala Aib Aib Pro Leu Aib Aib Gln Leu OH |
| 100 | Trichokindin IIIa | Ac Aib Ser Ala Aib Aib Gln Iva Leu Aib Ala Iva Aib Pro Leu Aib Aib Gln Leu OH |
| 101 | Trichokindin IIIb | Ac Aib Ser Ala Aib Iva Gln Aib Leu Aib Ala Iva Aib Pro Leu Aib Aib Gln Leu OH |
| 102 | Trichokindin IV | Ac Aib Ser Ala Aib Iva Gln Iva Leu Aib Ala Aib Aib Pro Leu Aib Aib Gln Ile OH |
| 103 | Trichokindin Va | Ac Aib Ser Ala Aib Aib Gln Iva Leu Aib Ala Iva Aib Pro Leu Aib Aib Gln Ile OH |
| 104 | Trichokindin Vb | Ac Aib Ser Ala Aib Iva Gln Aib Leu Aib Ala Iva Aib Pro Leu Aib Aib Gln Ile OH |
| 105 | Trichokindin VI | Ac Aib Ser Ala Aib Iva Gln Iva Leu Aib Ala Iva Aib Pro Leu Aib Aib Gln Leu OH |
| 106 | Trichokindin VII | Ac Aib Ser Ala Aib Iva Gln Iva Leu Aib Ala Iva Aib Pro Leu Aib Aib Gln Ile OH |
| 107 | Harzianin HB I | Ac Aib Asn Leu Ile Aib Pro Iva Leu Aib Pro Leu OH |
| 108 | Trichorzin HA I | Ac Aib Gly Ala Aib Aib Gln Aib Val Aib Gly Leu Aib Pro Leu Aib Aib Gln Leu OH |
| 109 | Trichorzin HA II | Ac Aib Gly Ala Aib Aib Gln Aib Val Aib Gly Leu Aib Pro Leu Aib Iva Gln Leu OH |
| 110 | Trichorzin HA III | Ac Aib Gly Ala Aib Iva Gln Aib Val Aib Gly Leu Aib Pro Leu Aib Aib Gln Leu OH |
| 111 | Trichorzin HA V | Ac Aib Gly Ala Aib Iva Gln Aib Val Aib Gly Leu Aib Pro Leu Aib Iva Gln Leu OH |
| 112 | Trichorzin HA VI | Ac Aib Gly Ala Aib Iva Gln Iva Val Aib Gly Leu Aib Pro Leu Aib Iva Gln Leu OH |
| 113 | Trichorzin HA VII | Ac Aib Gly Ala Aib Iva Gln Val Val Aib Gly Leu Aib Pro Leu Aib Iva Gln Leu OH |
| 114 | Trichorzin MA I | Ac Aib Ser Ala Aib Aib Gln Aib Leu Aib Gly Leu Aib Pro Leu Aib Aib Gln Val OH |
| 1 15 | Trichorzin MA II | Ac Aib Ser Ala Aib Iva Gln Aib Leu Aib Gly Leu Aib Pro Leu Aib Aib Gln Val OH |
| 1 16 | Trichorzin MA III | Ac Aib Ser Ala Aib Iva Gln Iva Leu Aib Gly Leu Aib Pro Leu Aib Aib Gln Val OH |
| 1 17 | Trichorozin I | Ac Aib Asn Ile Leu Aib Pro Ile Leu Aib Pro Val OH |
| 1 18 | Trichorozin II | Ac Aib Gln Ile Leu Aib Pro Ile Leu Aib Pro Val OH |
| 1 19 | Trichorozin III | Ac Aib Asn Ile Leu Aib Pro Ile Leu Aib Pro Leu OH |
| 1 20 | Trichorozin IV | Ac Aib Gln Ile Leu Aib Pro Ile Leu Aib Pro Leu OH |
| 1 21 | Harzianin HC I | Ac Aib Asn Leu Aib Pro Ser Val Aib Pro Aib Leu Aib Pro Leu OH |
| 1 22 | Harzianin HC III | Ac Aib Asn Leu Aib Pro Ser Val Aib Pro Iva Leu Aib Pro Leu OH |
| 1 23 | Harzianin HC VI | Ac Aib Asn Leu Aib Pro Ala Val Aib Pro Aib Leu Aib Pro Leu OH |
| 1 24 | Harzianin HC VIII | Ac Aib Asn Leu Aib Pro Ala Val Aib Pro Iva Leu Aib Pro Leu OH |
| 1 25 | Harzianin HC IX | Ac Aib Asn Leu Aib Pro Ala Ile Aib Pro Iva Leu Aib Pro Leu OH |
| 1 26 | Harzianin HC X | Ac Aib Gln Leu Aib Pro Ala Val Aib Pro Iva Leu Aib Pro Leu OH |
| 1 27 | Harzianin HC XI | Ac Aib Asn Leu Aib Pro Ser Ile Aib Pro Aib Leu Aib Pro Leu OH |
| 1 28 | Harzianin HC XII | Ac Aib Asn Leu Aib Pro Ser Ile Aib Pro Iva Leu Aib Pro Leu OH |
| 1 29 | Harzianin HC XIII | Ac Aib Gln Leu Aib Pro Ser Ile Aib Pro Iva Leu Aib Pro Leu OH |
| 1 30 | Harzianin HC XIV | Ac Aib Asn Leu Aib Pro Ala Ile Aib Pro Aib Leu Aib Pro Leu OH |
| 1 31 | Harzianin HC XV | Ac Aib Gln Leu Aib Pro Ala Ile Aib Pro Iva Leu Aib Pro Leu OH |
| 1 32 | Harzianin PCU4 | Ac Aib Asn Leu Aib Pro Ser Ile Aib Pro Aib Leu Aib Pro Val OH |
| 1 33 | Trichorzin PAU4 | Ac Aib Ser Ala Aib Aib Gln Aib Val Aib Gly Leu Aib Pro Leu Aib Aib Gln Trp OH |
| 1 34 | Trichorzin PA II | Ac Aib Ser Ala Aib Iva Gln Aib Val Aib Gly Leu Aib Pro Leu Aib Aib Gln Trp OH |
| 1 35 | Trichorzin PA IV | Ac Aib Ser Ala Aib Iva Gln Iva Val Aib Gly Leu Aib Pro Leu Aib Aib Gln Trp OH |
| 1 36 | Trichorzin PA V | Ac Aib Ser Ala Iva Iva Gln Aib Val Aib Gly Leu Aib Pro Leu Aib Aib Gln Trp OH |
| 1 37 | Trichorzin PA VI | Ac Aib Ser Ala Aib Iva Gln Aib Val Aib Gly Leu Aib Pro Leu Aib Aib Gln Phe OH |
| 1 38 | Trichorzin PA VII | Ac Aib Ser Ala Iva Iva Gln Aib Val Aib Gly Leu Aib Pro Leu Aib Aib Gln Trp OH |
| 1 39 | Trichorzin PA VIII | Ac Aib Ser Ala Aib Iva Gln Iva Val Aib Gly Leu Aib Pro Leu Aib Aib Gln Phe OH |
| 1 40 | Trichorzin PA IX | Ac Aib Ser Ala Iva Iva Gln Aib Val Aib Gly Leu Aib Pro Leu Aib Aib Gln Phe OH |
| 1 41 | Trichorzianine TA IIIc | Ac Aib Ala Ala Aib Aib Gln Aib Aib Aib Ser Leu Aib Pro Val Aib Ile Gln Gln Trp OH |
| 1 42 | Trichorzianine TB IIa | Ac Aib Ala Ala Aib Aib Gln Aib Aib Aib Ser Leu Aib Pro Leu Aib Ile Gln Glu Trp OH |
| 1 43 | Trichorzianine TB IIIc | Ac Aib Ala Ala Aib Aib Gln Aib Aib Aib Ser Leu Aib Pro Val Aib Ile Gln Glu Trp OH |
| 1 44 | Trichorzianine TB IVb | Ac Aib Ala Ala Aib Iva Gln Aib Aib Aib Ser Leu Aib Pro Val Aib Ile Gln Glu Trp OH |
| 1 45 | Trichorzianine TB Vb | Ac Aib Ala Ala Aib Aib Gln Aib Aib Aib Ser Leu Aib Pro Leu Aib Ile Gln Glu Phe OH |
| 1 46 | Trichorzianine TB VIa | Ac Aib Ala Ala Aib Iva Gln Aib Aib Aib Ser Leu Aib Pro Leu Aib Ile Gln Glu Phe OH |
| 1 47 | Trichorzianine TB VIb | Ac Aib Ala Ala Aib Aib Gln Aib Aib Aib Ser Leu Aib Pro Val Aib Ile Gln Glu Phe OH |
| 1 48 | Trichorzianine TB VII | Ac Aib Ala Ala Aib Iva Gln Aib Aib Aib Ser Leu Aib Pro Val Aib Ile Gln Glu Phe OH |
Figure 4.
Chemical structures of peptides (149–151) from T. harzianum. * Means marine source compounds.
2.4. Alkaloids
Fleephilone (152), a new HIV REV/RRE binding inhibitor, was produced by T. harzianum WC 47695 [27] isolated from sandy soil with plant debris collected in Fort Lauderdale, FL, USA. Compound 152 showed inhibitory activity against REV-protein binding to RRE RNA with an IC50 value of 7.6 μM, and exhibited no protection against HIV infection at concentrations up to 200 μg/mL. Harzianic acid (153) was isolated from T. harzianum SY-307, which exhibited antimicrobial activity against Pasteurella piscicida sp. 6395 [51]. Isoharzianic acid (154), a new stereoisomer of compound 153, was isolated from the T. harzianum strain M10, together with Harzianic acid (HA) [52]. HA was able to promote plant growth and strongly bind iron [52]. An OSMAC approach using multiple culture conditions or co-cultures has been applied to access the chemical diversity of T. harzianum XS-20090075 [20]. A new halogenate quinoline natural product, ethyl 2-bromo-4-chloroquinoline-3-carboxylate (155), was isolated from T. harzianum XS-20090075 [20]. Harzianopyridone (156) was isolated from the T. harzianum T-5. This strain was obtained from Palampur, Himachal Pradesh, India [21]. Compound 156 inhibited more than 90% growth of Rhizoctonia solani, Sclerotium rolfsii, and Fusarium oxysporum (EC50 35.9–50.2 μg/mL), but was less active than Bavistin [21]. A new oxazole metabolite, MR93A (159) was isolated from T. harzianum KCTC 0114BP [53], while eight metabolites MR566A (157), MR566B (158), MR93B (160), MR304A (161), 1-(1,4,5-trihydroxy-3-isocyanocyclopenten-2-enyl)-ethanol (162), 2-hydroxy-4-isocyano-α -methyl-6-oxabicyclo[3.1.0]-hex-3-ene-2-methanol (163), 4-hydroxy-8-isocyano-1- oxaspiro[4.4]cyclonon-8-en-2-one (164), methyl-3-(1,5-dihydroxy-3-isocyanocyclopent- 3-enyl)prop-2-enoate (165) and 3-(3′-isocyanocyclopent-2′-eny1idene)propionic acid (166) were isolated from T. harzianum [54]. MR566A (157) strongly inhibited mushroom tyrosinase with an IC50 value of 1.72 µM compared with kojic acid with an IC50 value of 3.08 µM [55]. Compound 166 exhibited inhibitory activity against mushroom tyrosinase with an IC50 value of 0.0014 µM, which was more active than the kojic acid [55] (Figure 5).
Figure 5.
Chemical structures of alkaloids (152–166) from T. harzianum.
2.5. Lactones
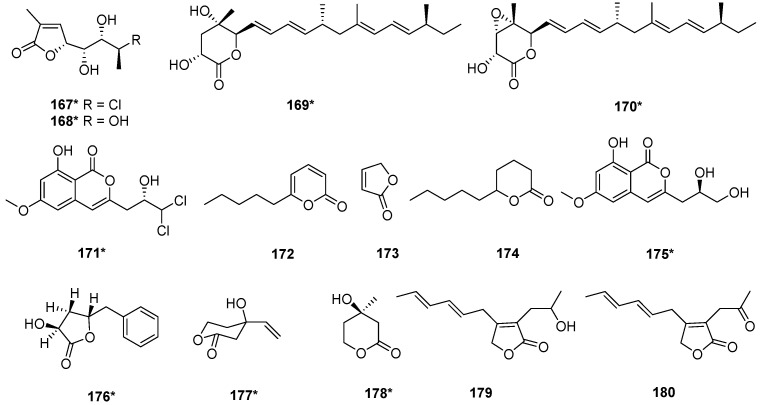
Two lactones, nafuredins C (169) and A (170), were isolated from the mangrove-derived fungus T. harzianum D13, and the new compound 169 exhibited antifungal activity against Magnaporthe oryzae, with an MIC value of 8.63 µM [50]. From T. harzianum XS-20090075, four known compounds, xylogibloactones A and B (167, and 168), nafuredin A (170), and dichlorodiaportin (171) [20,56,57] were isolated. Compound 170 exhibited antifouling activity with the EC50 value of 21.4 μg/mL [20]. 6-Pentyl-2H-pyran-2-one (172) and 2(5H)-furanone (173) were isolated from T. harzianum T-4 [21], while δ-decanolactone (174) was isolated from T. harzianum T-5 [21]. Compound 172, a volatile organic compound from T. harzianum [58], had the ability to inhibit primary root growth and induce lateral root formation. Peniisocoumarin H (175) was isolated from the mangrove-derived fungus T. harzianum D13 [50]. Two new lactones, harzialactones A (176) and B (177), together with a known compound R-mevalonolactone (178), were isolated from T. harzianum OUPS-N115 [32]. T. harzianum OUPS-N115 was separated from the sponge Halichondria okadai, and the cytotoxicity of compounds 176–178 against the P388 cell line was tested. The results showed no significant cytotoxicity [32]. Two lactones harzianolide (179) and T39butenolide (180) were isolated from T. harzianum T39 [30] (Figure 6).
Figure 6.
Chemical structures of lactones (167–180) from T. harzianum. * Means marine source compounds.
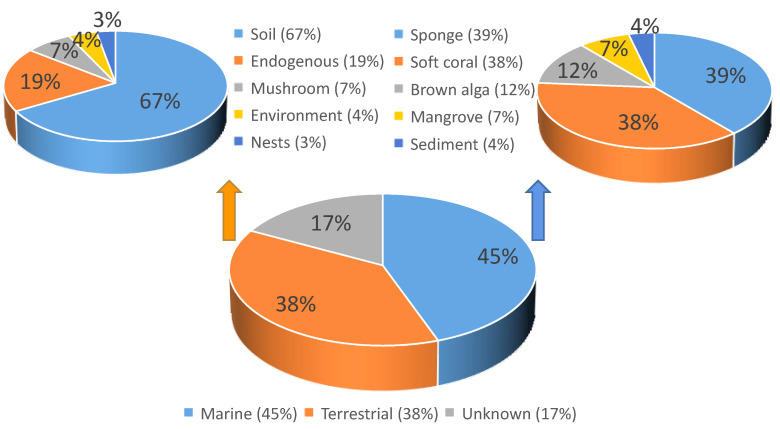
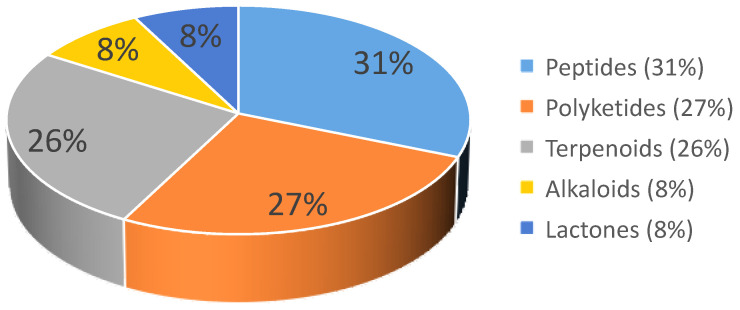
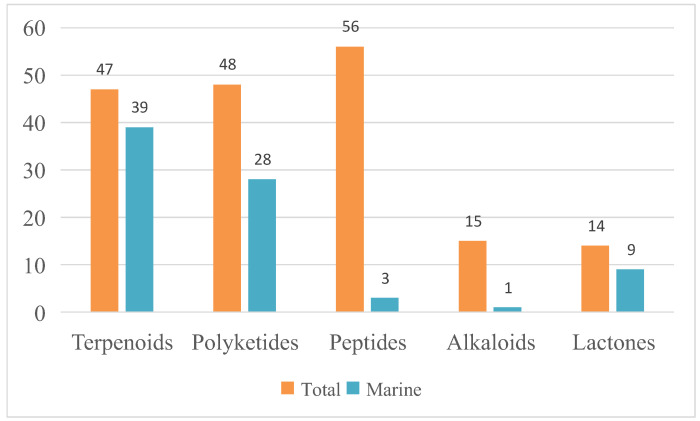
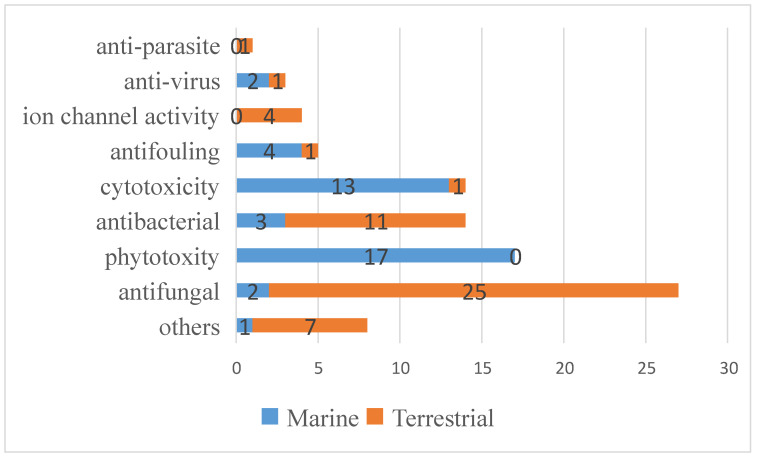
All compounds from T. harzianum with their biological activities and habitats were summaried in Table 2. As an analysis, the percentage of marine sources and terrestrial sources from the SMs distribution were exhibited, including the specific source ratio (Figure 7). The structure type proportion and the bioactivity distribution of the SMs isolated from T. harzianum were also shown (Figure 8, Figure 9 and Figure 10).
Table 2.
The bioactivities and habitats of SMs (1–180) from T. harzianum.
| Compounds | Bioactivities | Habitats | Refs |
|---|---|---|---|
| Harzianelactone A (1) * | Phytotoxicity | Soft coral | [13] |
| Harzianelactone B (2) * | phytotoxicity | Soft coral | [13] |
| Harzianone A (3) * | phytotoxicity | Soft coral | [13] |
| Harzianone B (4) * | phytotoxicity | Soft coral | [13] |
| Harzianone C (5) * | phytotoxicity | Soft coral | [13] |
| Harzianone D (6) * | phytotoxicity | Soft coral | [13] |
| Harzianone E (7) * | Antibacterial | Soft coral | [14] |
| 3R-Hydroxy-9R,10R-dihydroharzianone (8) * | phytotoxicity | Brown alga | [17] |
| Harziane (9) * | phytotoxicity | Soft coral | [13] |
| Trichodermanin C (10) * | Cytotoxicity | Sponge | [15,16] |
| Trichodermanin D (11) * | — | Sponge | [15,16] |
| Trichodermanin E (12) * | Cytotoxicity | Sponge | [15,16] |
| Trichodermanin F (13) * | Cytotoxicity | Sponge | [15,16] |
| Trichodermanin G (14) * | — | Sponge | [15,16] |
| Trichodermanin H (15) * | — | Sponge | [15,16] |
| 3,7,11-Trihydroxy-cycloneran (16) * | — | Soft coral | [14] |
| Methyl 3,7-dihydroxy-15-cycloneranate (17) * | Antibacterial | Soft coral | [14] |
| phytotoxicity | Brown alga | [17] | |
| Catenioblinc (18) * | — | Soft coral | [14] |
| Ascotrichic acid (19) * | — | Soft coral | [14] |
| Cyclonerotriol (20) * | — | Soft coral | [14] |
| (10E)-12-Acetoxy-10-cycloneren-3,7-diol (21) * | — | Sediment | [9] |
| — | Soft coral | [14] | |
| Cyclonerodiol (22) * | — | Soft coral | [14] |
| phytotoxicity | Brown alga | [17] | |
| 11-Methoxy-9-cycloneren-3,7-diol (23) * | phytotoxicity | Brown alga | [17] |
| 9-Cycloneren-3,7,11-triol (24) * | phytotoxicity | Brown alga | [17] |
| 10-Cycloneren-3,5,7-triol (25) * | phytotoxicity | Brown alga | [17] |
| 12-Acetoxycycloneran-3,7-diol (26) * | — | Sediment | [9] |
| Cyclonerodiol oxide (27) * | — | Soft coral | [14] |
| Epicyclonerodiol oxide (28) * | — | Soft coral | [14] |
| ent-Trichoacorenol (29) * | — | Soft coral | [14] |
| Trichoacorenol (30) * | — | Soft coral | [14] |
| phytotoxicity | Brown alga | [17] | |
| 15-Hydroxyacorenone (31) | — | Mushroom | [19] |
| Acorenone (32) | — | Mushroom | [19] |
| Acorenone-B (33) | — | Mushroom | [19] |
| 4-Epiacorenone (34) | — | Mushroom | [19] |
| 4-Epiacorenone-B (35) | — | Mushroom | [19] |
| 8-Acoren-3,11-diol (36) * | phytotoxicity | Brown alga | [17] |
| Trichoacorenol B (37) * | phytotoxicity | Brown alga | [17] |
| Harzianoic acid A (38) * | Antivirus | Sponge | [18] |
| Harzianoic acid B (39) * | Antivirus | Sponge | [18] |
| Ophioceric acid (40) * | — | Soft coral | [14] |
| Harzianolic acid A (41) * | — | Soft coral | [14] |
| 11R-Methoxy-5,9,13- proharzitrien-3-ol (42) * | phytotoxicity | Brown alga | [17] |
| Stigmasta-7,22-dien-3β,5α,6α-triol (43) * | Antifouling and DNA top I inhibitory activity |
Soft coral | [20] |
| Stigmasterol (44) | — | Soil | [21] |
| β-Sitosterol (45) | — | Soil | [21] |
| Ergosterol (46) | — | Soil | [21] |
| Trichosordarin A (47) * | Toxic to zooplankton | Sediment | [22] |
| Harzianumol A (48) * | — | Sponge | [23] |
| Harzianumol B (49) * | — | Sponge | [23] |
| Harzianumol C (50) * | — | Sponge | [23] |
| Harzianumol D (51) * | — | Sponge | [23] |
| Harzianumol E (52) * | — | Sponge | [23] |
| Harzianumol F (53) * | — | Sponge | [23] |
| Harzianumol G (54) * | — | Sponge | [23] |
| Harzianumol H (55) * | — | Sponge | [23] |
| Trichoharzin B (56) * | — | Soft coral | [20] |
| Methyl-trichoharzin (57) * | Antifouling | Soft coral | [20] |
| Trichoharzin (58) * | Antifouling | Soft coral | [20] |
| — | Sponge | [29] | |
| Eujavanicol A (59) * | — | Soft coral | [20] |
| Tandyukisin A (60) * | Cytotoxicity | Sponge | [11] |
| Tandyukisin B (61) * | Cytotoxicity | Sponge | [25] |
| Tandyukisin C (62) * | Cytotoxicity | Sponge | [25] |
| Tandyukisin D (63) * | Cytotoxicity | Sponge | [25] |
| Tandyukisin E (64) * | Cytotoxicity | Sponge | [24] |
| Tandyukisin F (65) * | Cytotoxicity | Sponge | [24] |
| Palmitic acid (66) | — | Soil | [21] |
| Harzianum A (67) | Antifungal | Soil | [26] |
| Harziphilone (68) | Cytotoxicity | Soil | [27] |
| Keto triol 3 (69) | Antifungal | Wheat roots | [28] |
| Keto diol 7 (70) | Antifungal | Wheat roots | [28] |
| Keto diol 6 (71) | Antifungal | Wheat roots | [28] |
| Keto diol 8 (72) | Antifungal | Wheat roots | [28] |
| Triacetate 9 (73) | Antifungal | Wheat roots | [28] |
| Triol 10 (74) | Antifungal | Wheat roots | [28] |
| Acetal diol 2 (75) | Antifungal | Wheat roots | [28] |
| Tribenzoate (76) * | — | Sponge | [29] |
| Triacetate (77) * | — | Sponge | [29] |
| T22azaphilone (78) | — | Commercial products | [30] |
| Trichoharzianol (79) | Antifungal | Soil | [31] |
| Trichodenone A (80) * | Cytotoxicity | Sponge | [32] |
| Trichodenone B (81) * | Cytotoxicity | Sponge | [32] |
| Trichodenone C (82) * | Cytotoxicity | Sponge | [32] |
| Homodimericin A (83) | — | Florida termite nest | [33,34] |
| Cryptenol (84) | — | Florida termite nest | [34] |
| Pachybasin (85) | — | Laboratory environment | [37] |
| Chrysophanol (86) | — | Laboratory environment | [37] |
| 1,7-Dihydroxy-3-hydroxymethyl-9,10-anthraquinone (87) | Antifungal | Plant roots | [38] |
| 1,5-Dihydroxy-3-hydroxymethyl-9,10- anthraquinone (88) | Antifungal | Plant roots | [38] |
| Emodin (89) | Antifungal | Plant roots | [38] |
| ω-Hydroxypachybasin (90) | Antifungal | Plant roots | [38] |
| Phomarin (91) * | — | Soft coral | [12] |
| (+)-2′S-Isorhodoptilometrin (92) * | Cytotoxicity | Soft coral | [12] |
| 1,6-Dihydroxy-3-(hydroxymethyl)anthracene-9,10-dione (93) * | — | Soft coral | [12] |
| Harzianumnone A (94) * | — | Soft coral | [12] |
| Harzianumnone B (95) * | — | Soft coral | [12] |
| Trichokindin_Ia (96) | — | Soil | [40] |
| Trichokindin_Ib (97) | — | Soil | [40] |
| Trichokindin_IIa (98) | — | Soil | [40] |
| Trichokindin_IIb (99) | — | Soil | [40] |
| Trichokindin_IIIa (100) | — | Soil | [40] |
| Trichokindin_IIIb (101) | — | Soil | [40] |
| Trichokindin_IV (102) | — | Soil | [40] |
| Trichokindin_Va (103) | — | Soil | [40] |
| Trichokindin_Vb (104) | — | Soil | [40] |
| Trichokindin_VI (105) | — | Soil | [40] |
| Trichokindin_VII (106) | Induced catecholamine secretion | Soil | [40] |
| Harzianin_HB_I (107) | Membrane-modifying activity | Soil | [42] |
| Trichorzin_HA_I (108) | Antifungal | Soil | [43,44] |
| Trichorzin_HA_II (109) | Antifungal | Soil | [43,44] |
| Trichorzin_HA_III (110) | Antifungal | Soil | [43,44] |
| Trichorzin_HA_V (111) | Antifungal | Soil | [43,44] |
| Trichorzin_HA_VI (112) | Antifungal | Soil | [43,44] |
| Trichorzin_HA_VII (113) | Antifungal | Soil | [43,44] |
| Trichorzin_MA_I (114) | Antifungal | Soil | [43,44] |
| Trichorzin_MA_II (115) | Antifungal | Soil | [43,44] |
| Trichorzin_MA_III (116) | Antifungal | Soil | [43,44] |
| Trichorozin_I (117) | ion channel activity | Soil | [45] |
| Trichorozin_II (118) | ion channel activity | Soil | [45] |
| Trichorozin_III (119) | ion channel activity | Soil | [45] |
| Trichorozin_IV (120) | ion channel activity | Soil | [45] |
| Harzianin_HC_I (121) | Antibacterial | — | [46] |
| Harzianin_HC_III (122) | Antibacterial | — | [46] |
| Harzianin_HC_VI (123) | Antibacterial | — | [46] |
| Harzianin_HC_VIII (124) | Antibacterial | — | [46] |
| Harzianin_HC_IX (125) | Antibacterial | — | [46] |
| Harzianin_HC_X (126) | Antibacterial | — | [46] |
| Harzianin_HC_XI (127) | Antibacterial | — | [46] |
| Harzianin_HC_XII (128) | Antibacterial | — | [46] |
| Harzianin_HC_XIII (129) | Antibacterial | — | [46] |
| Harzianin_HC_XIV (130) | Antibacterial | — | [46] |
| Harzianin_HC_XV (131) | Antibacterial | — | [46] |
| Harzianin_PCU4 (132) | — | — | [47] |
| Trichorzin_PAU4 (133) | — | — | [47] |
| Trichorzin_PA_II (134) | — | — | [47] |
| Trichorzin_PA_IV (135) | — | — | [47] |
| Trichorzin_PA_V (136) | — | — | [47] |
| Trichorzin_PA_VI (137) | — | — | [47] |
| Trichorzin_PA_VII (138) | — | — | [47] |
| Trichorzin_PA_VIII (139) | — | — | [47] |
| Trichorzin_PA_IX (140) | — | — | [47] |
| Trichorzianine_TA_IIIc (141) | Anti-parasite | — | [48] |
| Trichorzianine_TB_IIa (142) | — | — | [49] |
| Trichorzianine_TB_IIIc (143) | — | — | [49] |
| Trichorzianine_TB_IVb (144) | — | — | [49] |
| Trichorzianine_TB_Vb (145) | — | — | [49] |
| Trichorzianine_TB_VIa (146) | — | — | [49] |
| Trichorzianine_TB_VIb (147) | — | — | [49] |
| Trichorzianine_TB_VII (148) | — | — | [49] |
| Trichodermamide G (149) * | — | Mangrove | [50] |
| Trichodermamide A (150) * | — | Mangrove | [50] |
| Aspergillazin A (151) * | — | Mangrove | [50] |
| Fleephilone (152) | Antivirus | Soil | [27] |
| Harzianic acid (153) * | Antibacterial | Water sample | [51] |
| Isoharzianic acid (154) | Plant growth promotion | Hardwood bark | [52] |
| Ethyl 2-bromo-4-chloroquinoline-3-carboxylate (155) | — | Soft coral | [20] |
| Harzianopyridone (156) | Antifungal | Soil | [21] |
| MR566A(157) | Melanin synthesis inhibition | Soil | [54,55] |
| MR566B (158) | Melanin synthesis inhibition | Soil | [54] |
| MR93A (159) | — | leaf | [53] |
| MR93B (160) | — | Soil | [54] |
| MR304A (161) | — | Soil | [54] |
| 1-(1,4,5-Trihydroxy-3-isocyanocyclopenten-2-enyl)-ethanol (162) | — | Soil | [54] |
| 2-Hydroxy-4-isocyano-α-methyl-6-oxabicyclo[3.1.0]-hex-3-ene-2-Methanol (163) | — | Soil | [54] |
| 4-Hydroxy-8-isocyano-1-oxaspiro[4.4]cyclonon-8-en-2-one (164) | — | Soil | [54] |
| Methyl-3-(1,5-dihydroxy-3-isocyanocyclopent-3-enyl)prop-2-enoate (165) | — | Soil | [54] |
| 3 -(3′-Isocyanocyclopent -2′-eny1idene)propionic acid (166) | Melanin synthesis inhibition | Soil | [54,55] |
| Xylogibloactone A (167) * | — | Soft coral | [20] |
| Xylogibloactone B (168) * | — | Soft coral | [20] |
| Nafuredin C (169) * | Antifungal | Mangrove | [50] |
| Nafuredin A (170) * | — | Mangrove | [50] |
| Antifouling | Soft coral | [20] | |
| Dichlorodiaportin (171) * | — | Soft coral | [20] |
| 6-Pentyl-2H-pyran-2-one (172) | Antifungal | Soil | [21,58] |
| 2(5H)-Furanone (173) | — | Soil | [21] |
| δ-Decanolactone (174) | — | Soil | [21] |
| Peniisocoumarin H (175) * | — | Mangrove | [50] |
| Harzialactone A (176) * | — | Sponge | [32] |
| Harzialactone B (177) * | — | Sponge | [32] |
| R-Mevalonolactone (178) * | — | Sponge | [32] |
| Harzianolide (179) | — | Commercial products | [30] |
| T39butenolide (180) | Antifungal | Commercial products | [30] |
* Means marine source fungal strains.
Figure 7.
The SMs of T. harzianum from marine and terrestrial sources, and its distribution.
Figure 8.
Proportion of SMs obtained from T. harzianum.
Figure 9.
Total numbers and marine source numbers of SMs with each chemical structure type.
Figure 10.
The bioactivities of SMs from T. harzianum and the territorial distribution.
3. Conclusions
This review covers papers on metabolites isolated from T. harzianum. From the SMs’ distribution point of view, marine sources account for 45%, while terrestrial sources were 38%. From marine sources, 31 compounds were from sponges-derived T. harzianum strains, 30 compounds were isolated from soft corals-derived T. harzianum strains, 10 compounds were from brown alga-derived T. harzianum strains, 6 compounds were from mangrove samples-derived T. harzianum strains, and 3 compounds were from marine sediment samples. T. harzianum strains and their secondary metabolites were mainly derived from sponges (39%) and soft corals (38%). From the terrestrial sources, 46 compounds were purified from soil samples-derived T. harzianum strains, 13 compounds were from endogenous and 5 compounds were purified from mushroom-derived fungal strains. Compounds derived from terrestrial soil samples account for 67%. For the structure type proportion of the SMs isolated from T. harzianum, the peptides, polyketides, and terpenoids account for 31%, 27%, and 26%, respectively, followed by alkaloids (8%) and lactones (8%). Marine-derived terpenoids and polyketides have 39 and 28 natural products among the 47 and 48 total compounds, respectively. Notably, 91 of the 180 SMs exhibited bioactivities. Antifungal activity was exhibited by 27 natural products, and 17 compounds possessed phytotoxicity activity, while antibacterial and cytotoxicity activity SMs number were all 14. In the research on phytotoxicity and cytotoxic active products, almost all the active natural products were from marine-derived T. harzianum strains. Moreover, 120 of the 180 compounds were new.
In summary, organic compounds are abundant in the SMs of T. harzianum, they may be used as a fungicide, antibacterial, antineoplastic, and weedicide, both in clinical and agricultural applications. The marine sources molecules (marked * in this paper) with their unique molecular and diverse activities, could be the basis for the development of new drug-forming lead compounds.
Author Contributions
Conceptualization, X.P. and R.G.; writing—original draft preparation, R.G.; review and editing, X.P., R.G., G.L. and Z.Z. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China [grant numbers 41706077 and 81903494], and the China Postdoctoral Science Foundation [grant number 2019M652309].
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carroll A.R., Copp B.R., Davis R.A., Keyzers R.A., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2021;38:362–413. doi: 10.1039/D0NP00089B. [DOI] [PubMed] [Google Scholar]
- 2.Sutak R., Camadro J.M., Lesuisse E. Iron uptake mechanisms in marine phytoplankton. Front. Microbiol. 2020;11:566691. doi: 10.3389/fmicb.2020.566691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pang X., Lin X., Yang J., Zhou X., Yang B., Wang J., Liu Y. Spiro-phthalides and isocoumarins isolated from the marinesponge-derived fungus Setosphaeria sp. SCSIO41009. J. Nat. Prod. 2018;81:1860–1868. doi: 10.1021/acs.jnatprod.8b00345. [DOI] [PubMed] [Google Scholar]
- 4.Carroll A.R., Copp B.R., Davis R.A., Keyzers R.A., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2022;39:1122–1171. doi: 10.1039/D1NP00076D. [DOI] [PubMed] [Google Scholar]
- 5.Barra L., Dickschat J.S. Harzianone biosynthesis by the biocontrol fungus Trichoderma. ChemBioChem. 2017;18:2358–2365. doi: 10.1002/cbic.201700462. [DOI] [PubMed] [Google Scholar]
- 6.Bissett J., Gams W., Jaklitsch W., Samuels G.J. Accepted Trichoderma names in the year 2015. IMA Fungus. 2015;6:263–295. doi: 10.5598/imafungus.2015.06.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan R.A.A., Najeeb S., Hussain S., Xie B., Li Y. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms. 2020;8:817. doi: 10.3390/microorganisms8060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han M., Qin D., Ye T., Yan X., Wang J., Duan X., Dong J. An endophytic fungus from Trichoderma harzianum SWUKD3.1610 that produces nigranoic acid and its analogues. Nat. Prod. Res. 2019;33:2079–2087. doi: 10.1080/14786419.2018.1486311. [DOI] [PubMed] [Google Scholar]
- 9.Fang S.T., Wang Y.J., Ma X.Y., Yin X.L., Ji N.Y. Two new sesquiterpenoids from the marine-sediment-derived fungus Trichoderma harzianum P1–4. Nat. Prod. Res. 2019;33:3127–3133. doi: 10.1080/14786419.2018.1522314. [DOI] [PubMed] [Google Scholar]
- 10.Vinale F., Nigro M., Sivasithamparam K., Flematti G., Ghisalberti E.L., Ruocco M., Varlese R., Marra R., Lanzuise S., Eid A., et al. Harzianic acid: A novel siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013;347:123–129. doi: 10.1111/1574-6968.12231. [DOI] [PubMed] [Google Scholar]
- 11.Yamada T., Mizutani Y., Umebayashi Y., Inno N., Kawashima M., Kikuchi T., Tanaka R. Tandyukisin, a novel ketoaldehyde decalin derivative, produced by a marine sponge-derived Trichoderma harzianum. Tetrahedron Lett. 2014;55:662–664. doi: 10.1016/j.tetlet.2013.11.107. [DOI] [Google Scholar]
- 12.Shi T., Hou X.-M., Li Z.-Y., Cao F., Zhang Y.-H., Yu J.-Y., Zhao D.-L., Shao C.-L., Wang C.-Y. Harzianumnones A and B: Two hydroxyanthraquinones from the coral-derived fungus Trichoderma harzianum. RSC Adv. 2018;8:27596–27601. doi: 10.1039/C8RA04865G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao D.L., Yang L.J., Shi T., Wang C.Y., Shao C.L., Wang C.Y. Potent phytotoxic harziane diterpenes from a soft coral-derived strain of the fungus Trichoderma harzianum XS-20090075. Sci. Rep. 2019;9:13345. doi: 10.1038/s41598-019-49778-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi T., Shao C.L., Liu Y., Zhao D.L., Cao F., Fu X.M., Yu J.Y., Wu J.S., Zhang Z.K., Wang C.Y. Terpenoids from the coral-derived fungus Trichoderma harzianum (XS-20090075) induced by chemical epigenetic manipulation. Front. Microbiol. 2020;11:572. doi: 10.3389/fmicb.2020.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamada T., Suzue M., Arai T., Kikuchi T., Tanaka R. Trichodermanins C-E, new diterpenes with a fused 6-5-6-6 ring system produced by a marine sponge-derived fungus. Mar. Drugs. 2017;15:169. doi: 10.3390/md15060169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada T., Fujii A., Kikuchi T. New diterpenes with a fused 6-5-6-6 ring system isolated from the marine sponge-derived fungus Trichoderma harzianum. Mar. Drugs. 2019;17:480. doi: 10.3390/md17080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song Y.P., Fang S.T., Miao F.P., Yin X.L., Ji N.Y. Diterpenes and sesquiterpenes from the marine algicolous Fungus Trichoderma harzianum X-5. J. Nat. Prod. 2018;81:2553–2559. doi: 10.1021/acs.jnatprod.8b00714. [DOI] [PubMed] [Google Scholar]
- 18.Li B., Li L., Peng Z., Liu D., Si L., Wang J., Yuan B., Huang J., Proksch P., Lin W. Harzianoic acids A and B, new natural scaffolds with inhibitory effects against hepatitis C virus. Bioorg. Med. Chem. 2019;27:560–567. doi: 10.1016/j.bmc.2018.12.038. [DOI] [PubMed] [Google Scholar]
- 19.Tezuka Y., Tasaki M., Huang Q., Hatanaka Y., Kikuchi T. 15-Hydroxyacorenone: New acorane-type sesquiterpene from the culture broth of the mycoparasitic fungus Trichoderma harzianum. Liebigs Ann. Recl. 1997;12:2579–2580. doi: 10.1002/jlac.199719971224. [DOI] [Google Scholar]
- 20.Yu J.Y., Shi T., Zhou Y., Xu Y., Zhao D.L., Wang C.Y. Naphthalene derivatives and halogenate quinoline from the coral-derived fungus Trichoderma harzianum (XS-20090075) through OSMAC approach. J. Asian. Nat. Prod. Res. 2021;23:250–257. doi: 10.1080/10286020.2020.1729752. [DOI] [PubMed] [Google Scholar]
- 21.Ahluwalia V., Kumar J., Rana V.S., Sati O.P., Walia S. Comparative evaluation of two Trichoderma harzianum strains for major secondary metabolite production and antifungal activity. Nat. Prod. Res. 2015;29:914–920. doi: 10.1080/14786419.2014.958739. [DOI] [PubMed] [Google Scholar]
- 22.Liang X.R., Ma X.Y., Ji N.Y. Trichosordarin A, a norditerpene glycoside from the marine-derived fungus Trichoderma harzianum R5. Nat. Prod. Res. 2020;34:2037–2042. doi: 10.1080/14786419.2019.1574782. [DOI] [PubMed] [Google Scholar]
- 23.Li B., Huang Q.X., Gao D., Liu D., Ji Y.B., Liu H.G., Lin W.H. New C13 lipids from the marine-derived fungus Trichoderma harzianum. J. Asian. Nat. Prod. Res. 2015;17:468–474. doi: 10.1080/10286020.2015.1042371. [DOI] [PubMed] [Google Scholar]
- 24.Suzue M., Kikuchi T., Tanaka R., Yamada T. Tandyukisins E and F, novel cytotoxic decalin derivatives isolated from a marine sponge-derived fungus. Tetrahedron Lett. 2016;57:5070–5073. doi: 10.1016/j.tetlet.2016.10.004. [DOI] [Google Scholar]
- 25.Yamada T., Umebayashi Y., Kawashima M., Sugiura Y., Kikuchi T., Tanaka R. Determination of the chemical structures of tandyukisins B-D, isolated from a marine sponge-derived fungus. Mar. Drugs. 2015;13:3231–3240. doi: 10.3390/md13053231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corley D.G., Miller-Wideman M., Durley R.C. Isolation and structure of harzianum A: A new trichothecene from Trichoderma harzianum. J. Nat. Prod. 1994;57:422–425. doi: 10.1021/np50105a019. [DOI] [PubMed] [Google Scholar]
- 27.Qian-Cutrone J., Huang S., Chang L.P., Pirnik D.M., Klohr S.E., Dalterio R.A., Hugill R., Lowe S. Harziphilone and fleephilone, two new HIV REV/RRE binding inhibitors produced by Trichoderma harzianum. J. Antibiot. 1996;49:990–997. doi: 10.7164/antibiotics.49.990. [DOI] [PubMed] [Google Scholar]
- 28.Ghisalberti E.L., Rowland C.Y. Antifungal metabolites from Trichoderma harzianum. J. Nat. Prod. 1993;56:1799–1804. doi: 10.1021/np50100a020. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi M., Uehara H., Matsunami K., Aoki S., Kitagawa I. Trichoharzin, a new polyketide produced by the imperfect fungus Trichoderma harzianum separated from the marine sponge Micale cecilia. Tetrahedron Lett. 1993;34:7925–7928. doi: 10.1016/S0040-4039(00)61513-7. [DOI] [Google Scholar]
- 30.Vinale F., Marra R., Scala F., Ghisalberti E.L., Lorito M., Sivasithamparam K. Major secondary metabolites produced by two commercial Trichoderma strains active against different phytopathogens. Lett. Appl. Microbiol. 2006;43:143–148. doi: 10.1111/j.1472-765X.2006.01939.x. [DOI] [PubMed] [Google Scholar]
- 31.Jeerapong C., Phupong W., Bangrak P., Intana W., Tuchinda P. Trichoharzianol, a new antifungal from Trichoderma harzianum F031. J. Agric. Food Chem. 2015;63:3704–3708. doi: 10.1021/acs.jafc.5b01258. [DOI] [PubMed] [Google Scholar]
- 32.Amagata T., Usami Y., Minoura K., Ito T., Numata A. Cytotoxic substances produced by a fungal strain from a sponge: Physico-chemical properties and structures. J. Antibiot. 1998;51:33–40. doi: 10.7164/antibiotics.51.33. [DOI] [PubMed] [Google Scholar]
- 33.Mevers E., Saurí J., Liu Y., Moser A., Ramadhar T.R., Varlan M., Williamson R.T., Martin G.E., Clardy J. Homodimericin A: A complex hexacyclic fungal metabolite. J. Am. Chem. Soc. 2016;138:12324–12327. doi: 10.1021/jacs.6b07588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mevers E., Chouvenc T., Su N.-Y., Clardy J. Chemical interaction among termite-associated microbes. Chem. Eng. J. 2017;43:1078–1085. doi: 10.1007/s10886-017-0900-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koyama M., Kelly T.R., Watanabe K.A. Novel type of potential anticancer agents derived from chrysophanol and emodin. Some structure-activity relationship studies. J. Med. Chem. 1988;31:283–284. doi: 10.1021/jm00397a002. [DOI] [PubMed] [Google Scholar]
- 36.De Moliner E., Moro S., Sarno S., Zagotto G., Zanotti G., Pinna L.A., Battistutta R. Inhibition of protein kinase CK2 by anthraquinone-related compounds. J. Biol. Chem. 2003;278:1831–1836. doi: 10.1074/jbc.M209367200. [DOI] [PubMed] [Google Scholar]
- 37.Liu S.-Y., Lo C.-T., Chen C., Liu M.-Y., Chen J.H., Peng K.C. Efficient isolation of anthraquinone-derivatives from Trichoderma harzianum ETS 323. J. Biochem. Biophys. Methods. 2007;70:391–395. doi: 10.1016/j.jbbm.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Liu S.-Y., Lo C.-T., Shibu M.A., Leu Y.-L., Jen B.-Y., Peng K.-C. Study on the anthraquinones separated from the cultivation of Trichoderma harzianum strain Th-R16 and their biological activity. J. Agric. Food Chem. 2009;57:7288–7292. doi: 10.1021/jf901405c. [DOI] [PubMed] [Google Scholar]
- 39.Hou X., Sun R., Feng Y., Zhang R., Zhu T., Che Q., Zhang G., Li D. Peptaibols: Diversity, bioactivity, and biosynthesis. Eng. Microbiol. 2022;2:100026. doi: 10.1016/j.engmic.2022.100026. [DOI] [Google Scholar]
- 40.Iida A., Sanekata M., Fujita T., Tanaka H., Enoki A., Fuse G., Kanai M., Rudewicz P.J., Tachikawa E. Fungal metabolites. XVI. Structures of new peptaibols, trichokindins I-VII, from the fungus Trichoderma harzianum. Chem. Pharma. Bull. 1994;42:1070–1075. doi: 10.1248/cpb.42.1070. [DOI] [PubMed] [Google Scholar]
- 41.Tsantrizos Y.S., Pischos S., Sauriol F., Widden P. Peptaibol metabolites of Tolypocladium geodes. Can. J. Chem. 1996;74:165–172. doi: 10.1139/v96-020. [DOI] [Google Scholar]
- 42.Augeven-Bour I., Rebuffat S., Auvin C., Goulard C., Prigent Y., Bodo B. Harzianin HB I, an 11-residue peptaibol from Trichoderma harzianum: Isolation, sequence, solution synthesis and membrane activity. J. Chem. Soc. Perkin Trans. 1997;1:1587–1594. doi: 10.1039/a605629f. [DOI] [Google Scholar]
- 43.Hlimi S., Rebuffat S., Goulard C., Duchamp S., Bodo B. Trichorzins HA and MA, antibiotic peptides from Trichoderma harzianum II. Sequence determination. J. Antibiot. 1995;48:1254–1261. doi: 10.7164/antibiotics.48.1254. [DOI] [PubMed] [Google Scholar]
- 44.Goulard C., Hlimi S., Rebuffat S., Bodo B. Trichorzins HA and MA, antibiotic peptides from Trichoderma harzianum I. Fermentation, isolation and biological properties. J. Antibiot. 1995;48:1248–1253. doi: 10.7164/antibiotics.48.1248. [DOI] [PubMed] [Google Scholar]
- 45.Iida A., Sanekata M., WADA S.-I., Fujita T., Tanaka H., Enoki A., Fuse G., Kanai M., Asami K. Fungal metabolities. XVIII. New membrane-modifying peptides, trichorozins I-IV, from the fungus trichoderma harzianum. Chem. Pharm. Bull. 1995;43:392–397. doi: 10.1248/cpb.43.392. [DOI] [PubMed] [Google Scholar]
- 46.Rebuffat S., Goulard C., Bodo B. Antibiotic peptides from Trichoderma harzianum: Harzianins HC, proline-rich 14-residue peptaibols. J. Chem. Soc. Perkin Trans. 1995;1:1849–1855. doi: 10.1039/p19950001849. [DOI] [Google Scholar]
- 47.Leclerc G., Rebuffat S., Goulard C., Bodo B. Directed biosynthesis of peptaibol antibiotics in two Trichoderma strains I. Fermentation and isolation. J. Antibiot. 1998;51:170–177. doi: 10.7164/antibiotics.51.170. [DOI] [PubMed] [Google Scholar]
- 48.Hajji M.E., Rebuffat S., Doan T.L., Klein G., Satre M., Bodo B. Interaction of trichorzianines A and B with model membranes and with the amoeba Dictyostelium. Biochim. Biophys. Acta. 1989;978:97–104. doi: 10.1016/0005-2736(89)90504-X. [DOI] [PubMed] [Google Scholar]
- 49.Rebuffat S., Hajji M.E., Hennig P., Davoust D., Bodo B. Isolation, sequence, and conformation of seven trichorzianines B from Trichoderma harzianum. Int. J. Pept. Protein Res. 1989;34:200–210. doi: 10.1111/j.1399-3011.1989.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 50.Zhao D.-L., Zhang X.-F., Huang R.-H., Wang D., Wang X.-Q., Li Y.-Q., Zheng C.-J., Zhang P., Zhang C.-S. Antifungal nafuredin and epithiodiketopiperazine derivatives from the mangrove-derived fungus Trichoderma harzianum D13. Front. Microbiol. 2020;11:1495. doi: 10.3389/fmicb.2020.01495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sawa R., Mori Y., Iinuma H., Naganawa H., Hamada M., Yoshida S., Furutani H., Kajimura Y., Fuwa T., Takeuchi T. Harzianic acid, a new antimicrobial antibiotic from a fungus. J. Antibiot. 1994;47:731–732. doi: 10.7164/antibiotics.47.731. [DOI] [PubMed] [Google Scholar]
- 52.Vinale F., Manganiello G., Nigro M., Mazzei P., Piccolo A., Pascale A., Ruocco M., Marra R., Lombardi N., Lanzuise S., et al. A novel fungal metabolite with beneficial properties for agricultural applications. Molecules. 2014;19:9760–9772. doi: 10.3390/molecules19079760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee C., Chung M., Lee H., Kho Y. Koshino, H. MR-93A, a new oxazole from Trichoderma harzianum KCTC 0114BP. J. Nat. Prod. 1995;58:1605–1607. doi: 10.1021/np50124a022. [DOI] [Google Scholar]
- 54.Lee C.H., Koshino H., Chung M.C., Lee H.J., Hong J.K., Yoon J.S., Kho Y.H. MR566A and MR566B, new melanin synthesis inhibitors produced by Trichoderma harzianum II. Physico-chemical properties and structural elucidation. J. Antibiot. 1997;50:474–478. doi: 10.7164/antibiotics.50.474. [DOI] [PubMed] [Google Scholar]
- 55.Lee C.H., Chung M.C., Lee H.J., Bae K.S., Kho Y.H. MR566A and MR566B, new melanin synthesis inhibitors produced by Trichoderma harzianum I. Taxonomy, fermentation, isolation and biological Activities. J. Antibiot. 1997;50:469–473. doi: 10.7164/antibiotics.50.469. [DOI] [PubMed] [Google Scholar]
- 56.Takano D., Nagamitsu T., Ui H., Shiomi K., Yamaguchi Y., Masuma R., Kuwajima I., Ōmura S. Absolute configuration of nafuredin, a new specific NADH-fumarate reductase inhibitor. Tetrahedron Lett. 2001;42:3017–3020. doi: 10.1016/S0040-4039(01)00355-0. [DOI] [PubMed] [Google Scholar]
- 57.Larsen T.O., Breinholt J. Dichlorodiaportin, diaportinol, and diaportinic acid: Three novel isocoumarins from Penicillium nalgiovense. J. Nat. Prod. 1999;62:1182–1184. doi: 10.1021/np990066b. [DOI] [PubMed] [Google Scholar]
- 58.Garnica-Vergara A., Barrera-Ortiz S., Muñoz-Parra E., Raya-González J., Méndez-Bravo A., Macías-Rodríguez L., Ruiz-Herrera L.F., López-Bucio J. The volatile 6-pentyl-2H-pyran-2-one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ETHYLENE INSENSITIVE 2 functioning. New Phytol. 2016;209:1496–1512. doi: 10.1111/nph.13725. [DOI] [PubMed] [Google Scholar]