Abstract
Simple Summary
Conventional pesticides are synthetic chemicals that are toxic, have hazardous effects on living organisms and may be carcinogenic. Pests can be controlled in an alternative way using less hazardous and more ecofriendly methods, such as using bio-pesticides. Plants based pesticides are chemicals isolated from various plants which could be used to control pests in a non-toxic mechanism. Several plants have certain bioactive compounds which could be used as alternatives to hazardous synthetic pesticides for pest control. Some beetles could damage stored grains and thus causes huge economic losses. For example, Callosbruchus maculatus is a stored grain pest which causes more than 90% damage to sored grains in a few months. Here, in this work, the insecticidal potentials of six plants, including Melia azedarach, Nicotiana rustica, Azadirachta indica, Nicotiana tabacum and Thuja orientalis, were investigated against C. maculatus. Crude extracts of these plants at different concentration were checked against C. maculatus and mortality was observed. Maximum mortality was caused by N. tabacum and N. rustica (100%) followed by A. indica (82%), whereas minimum mortality was observed in T. orientalis (64%) at 2.5%. The results of this study revealed that the extracts of these plants could be used for the control of stored grain pests.
Abstract
Plant based insecticides are considered among the most economic and ecofriendly chemicals for the protection of plants and stored grains. The cowpea weevil (Callosbruchus maculatus) causes more than 90% damage to sored grains in three to six months. The current study investigates insecticidal potentials of five selected botanicals: Melia azedarach, Nicotiana rustica, Azadirachta indica, Nicotiana tabacum and Thuja orientalis. They are explored at six different concentrations (0.5, 1.0, 1.5, 2.0, 2.5 and 3.0%) against C. maculatus and compared to effects of distilled water which is used as a control. Toxicities of 3%(V/V) extracts of N. tabacum, N. rustica, A. indica and T. orientalis against C. maculatus were 100%, 86.11%, 80.56% and 72.22%, respectively. Maximum mortality was caused by N. tabacum and N. rustica (100%), followed by A. indica (82%), whereas minimum mortality was observed in T. orientalis (64%) at 2.5%. Several phytochemicals, alkaloids, saponins, diterphenes, phytosterol, flavonoids and phenols were identified in N. tabacum and N. rustica, while few were present in A. indica. Phytosterol was present in greatest abundance. Saponins were only detected in aqueous extracts of N. rustica and N. tabacum. Taken together, these results indicate the utility of N. tabacum, N. rustica and A. indica as potential botanicals to control pest beetle and cowpea weevil.
Keywords: entomotoxicity, stored product pests, plant extracts, phytochemicals, bruchid beetle, bio-pesticides
1. Introduction
The demand for food increases with an ever-growing population. Thus, it is necessary to protect crops and stored grains from pests. The multivoltine pest, cowpea weevil Callosobruchus maculatus Fab. (Coleoptera: Bruchidae), causes significant damage to stored pulses [1]. It has been reported that C. maculatus alone can cause as much as 90% damage during the three to six months of storage [2]. Due to their potencies to cause lethality in most life stages of a range of pests, synthetic pesticides are frequently used to protect both crop plants and stored grains [3]. Synthetic pesticides can adversely affect non-target organisms, including humans, accumulate in the environment, pollute soil and ground water. Some of the synthetic pesticides are also carcinogenic [4]. The overuse of synthetic pesticides for insect control poses risks to wildlife and even humans [5]. Toxic potency of synthetic pesticides and their potential effects have stirred interest from the public and regulatory agencies in alternative options for pest management [6].
Until the relatively recent development of synthetic pesticides and their widespread application since the 1940s, phytochemicals have long been successfully used to manage pests in crops [7]. Extracts of plants and other secondary metabolites of plants, microorganisms and enzymes are becoming increasingly popular as alternatives to synthetic pesticides [8]. Bio-insecticides can be very effective, selective and have little potential for developing resistance to target pests, as well as having minimal effects on non-target organisms [9].
Several plant extracts have been used to control various stored insect pests. Essential oils of some aromatic plants have been recognized to have cytotoxic, antioxidant, antifungal, insecticidal and antibacterial properties [10]. The aqueous extract of neem kernel has been used to protect crops from infestation with pests [11,12]. The species of the deciduous tree in the mahogany family, Meliaceae, commonly known as the chinaberry tree, pride of India, bead-tree, Cape lilac, syringa berry tree, Persian lilac, Indian lilac and white cedar (Melia azedarach) have insecticidal properties against several pest species [13]. The tobacco plants Nicotiana. tabacum and Nicotiana. rustica are also able to control several insect pests. Nicotine present in N. tabacum and N. rustica cause uncontrolled nerve firing and masking acetylcholine in insects, which results in death [13]. The present study was conducted to evaluate insecticidal potencies of extracts of five plants against C. maculatus. The toxic potencies of the five botanicals, M. azedarach, N. rustica, A. indica, N. tabacum and T. orientalis, against the cowpea weevil, C. maculatus, are investigated.
2. Materials and Methods
2.1. Collection of Insects
Cowpea weevil, Callosobruchus maculatus, were collected from various regions of the Swabi and Haripur districts of the Khyber Pakhtunkhwa province in Pakistan. C. maculatus were taken to the entomological laboratory and identified according to previous reports [14]. Adults of C. maculatus were chosen to initiate the culture at the entomological laboratory under laboratory conditions of 27.5 °C, 60 ± 5% RH and a 12L:12D photoperiod on the entire mung bean (Vigna radiate L.), which is known to be an ideal host in plastic jars (10 × 12 cm) enclosed in muslin fabric [15].
2.2. Plants Collection
Leaves and fruits of the specified selected plants were collected from various areas in the Swabi and Haripur districts of the Khyber Pakthunkhwa in Pakistan, according to previously described procedures [16] (Table 1).
Table 1.
List of plant species and plant parts tested against C. maculatus during 2021.
| Sr. No. | Common Name | Botanical Name | Family | Part Used |
|---|---|---|---|---|
| 1. | White Patta | Nicotiana rustica | Solanaceae | leaf |
| 2. | Virginia tobacco | Nicotiana tabacum | Solanaceae | leaf |
| 3. | Chinese arborvitae | Thuja orientalis | Cupressaceae | Fruit |
| 4. | Neem | Azadirachta indica | Meliaceae | Seed |
| 5. | Bakion | Melia azadarech | Meliaceae | Fruit |
2.3. Phytochemical Screening
Extracts of selected plants were screened for the presence of various bio-active compounds, such as alkaloids, phenols, phytosterol, terpenes and flavonoids. The detection of these compounds was carried out using standard tests, as reported in literature.
2.3.1. Maceration
Coarsely powdered plant material was extracted in a container of methanol and ethyl acetate and hexane) and agitated for a defined period.
2.3.2. Detection of Alkaloids, Phenols, Phytosterol, Terpenes and Flavonoids in Plant Extract
A few drops of iodine and two to three drops of potassium iodide were dissolved individually in diluted hydrochloric acid (1.5%). One milliliter of this reagent was added to plant extracts and stirred for five minutes. Dark reddish precipitates were observed in the samples, which indicates the presence of alkaloids in plants extracts [17,18]. For the screening of phenol, three to four drops of ferric chloride solution were added to plant aqueous extracts. The formation of a bluish black color indicates the presence of phenols in the extract [18]. Similarly, for the detection of phytosterol, extracts of the selected plants were cured with chloroform and then filtered. Few drops of concentrated sulfuric acid were added into the filtered extract and allowed to be undisturbed for a few minutes. The formation of a golden yellow color indicates the presence of phytosterol [19]. For the determination of diterpenes in the selected plants extracts, three to four drops of copper acetate solution were added into the aqueous extracts of the selected plants. The formation of emerald green color indicates the presence of diterpenes [20]. Flavonoids were detected in plants extracts by adding two to three drops of lead acetate solutions to the plant aqueous extracts. A bright yellow color appeared, which indicates the presence of flavonoids in the sample. The color disappeared when few drops of dilute acid was added [21].
2.4. Residual Toxicity
Residual toxicity of extracts was determined according to previously described methods (22). In this study, five plant extracts and six different concentrations (Table 2) were tested, following the CRD design with (6 × 5) factorial arrangement with four replicates. Sterilized test sample of mung bean (20 g) were treated with plant extracts (Table 2) separately, then air-dried for 30 min. Treated legumes were then put in plastic petri dishes (12 cm diam.). Five pairs of newly emerged adults were released in each petri dish and covered with muslin cloths to prevent the escapes of beetles from the testing arena. Mortality of C. maculatus in each petri dish was assessed after 24, 48, 72, 168 and 336 h of exposure. Dead beetles from each petri dish were removed and counted. Corrected mortality (23) for each treatment was calculated as per the following formula (Equation (1)).
| (1) |
Table 2.
Plant extract and their concentrations used against C. maculatus during 2021.
| Sr. No. | Common Name | Botanical Name | Concentration Used |
|---|---|---|---|
| 1. | White Patta | Nicotiana rustica | 0.5, 1, 1.5, 2, 2.5 and 3% |
| 2. | Virginia tobacco | Nicotiana tabacum | 0.5, 1, 1.5, 2, 2.5 and 3% |
| 3. | Chinese arborvitae | Thuja orientalis | 0.5, 1, 1.5, 2, 2.5 and 3% |
| 4. | Neem | Azadirachta indica | 0.5, 1, 1.5, 2, 2.5 and 3% |
| 5. | Bakion | Melia azadarech | 0.5, 1, 1.5, 2, 2.5 and 3% |
2.5. Topical Toxicity
Topical toxicity of the plant extracts (Table 2) was also investigated against C. maculatus adult beetles. The same experimental design and treatment in Section 2.4 were applied here. The concentrations of plant extracts (Table 2) were applied through pipet onto the thoracic segment of C. maculates, then wrapped in aluminum foil and stored in the refrigerator for five minutes before being treated. All treated C. maculatus adults were put in plastic petri plates with the help of a camel hairbrush. After exposure for 24, 48, 72, 168 and 336 h, the number of dead beetles was counted and the percentage mortality (%) for each concentration was calculated.
2.6. Statistical Analysis
While normality was checked using the Shapiro–Wilks test, the assumption of homogeneity of variance was evaluated using Levene’s test. The Tukey HSD analysis was applied at 5% probability of Type I error (α) to separate the means of the obtained data from recent research using Analysis of Variance (ANOVA). Statistical analyses were carried out using STATISTIX 8.1 (24). The mortality percentages were corrected using Abbott’s formula. Thereafter, the Log-Probit model analysis was applied to percentage mortality of the adult C. maculatus to determine the 50% and 90% lethal concentrations (LC50/LC90) (25). The Analysis of Variance and the Probit analyses were done using the Statistical Package for the Social Sciences (SPSS) version 20.
3. Results
Several phytochemicals, including alkaloids, saponins, di-terphenes, phyto-sterol, flavonoids and phenols, were identified in N. tabacum and N. rustica, while few were present in A. indica. Phytosterol was present in greatest abundance. Saponins were detected only in aqueous extracts of N. rustica and N. tabacum (Table 3).
Table 3.
Composition of phytochemicals in aqueous extracts of five selected plants species.
| Phytochemical Constituents of Five Plant Species | ||||||
|---|---|---|---|---|---|---|
| Plant Species | Alkaloids | Flavonoids | Saponins | Di-Terpenes | Phyto-Sterol | Phenols |
| T. orientalis | Low | low | low | low | Low | low |
| M. azedarach | low | low | low | low | moderate | moderate |
| N. rustica | low | low | not present | low | Low | low |
| A. indica | High | high | moderate | moderate | High | high |
| N. tabacum | moderate | moderate | moderate | high | moderate | high |
The insecticidal activity of plant aqueous extracts was tested against C. maculatus to six concentrations (0.5, 1.0, 1.5, 2.0, 2.5, and 3.0%). Data pertaining to insecticidal activity of the selected plant extracts both in residual (Figure 1 and Table 4) and direct (Figure 2 and Table 5 forms are represented.
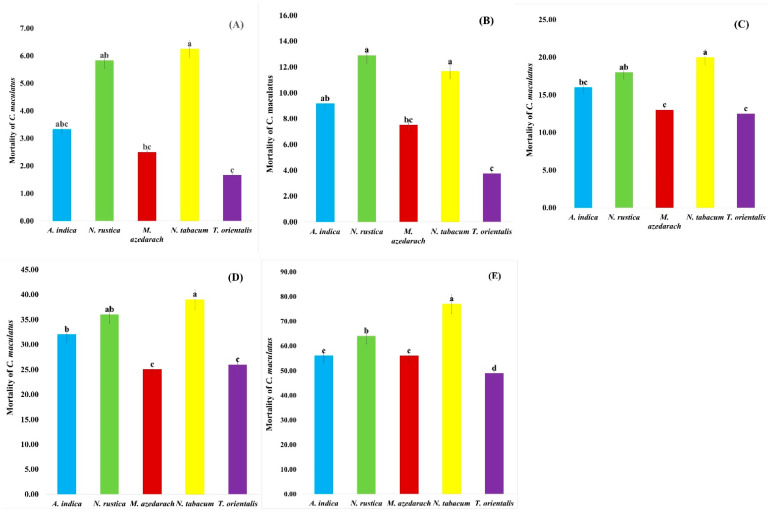
Figure 1.
Mean percent Mortality of C. maculatus treated with plant extracts after (A) 24 h, (B) 48 h, (C) 72 h, (D) 168 h and (E) 336 h exposure (Residual effect). (a, b, c, d put over the bars indicating that different letters are significantly different from each other at 0.5% level of significance).
Table 4.
Mortality of C. maculatus exposed to aqueous extracts for 24, 48, 72, 168 and 336 h.
| Time Hours | Plant Species | N* | LC50 95% LC | X2 | P | Slope + SE |
|---|---|---|---|---|---|---|
| 24 | A. indica | 240 | 38.44 (15.77–740.47) | 0.85 | 0.93 | 0.96 ± 0.26 |
| N. rustica | 240 | 37.19 (16.02–495.66) | 1.59 | 0.80 | 1.07 ± 0.28 | |
| M. azedarach | 240 | 29.73 (16.01–359.42) | 2.28 | 0.68 | 1.44 ± 0.37 | |
| N. tabacum | 240 | 24.73 (18.12–1308.62) | 0.69 | 0.95 | 1.05 ± 0.29 | |
| T. orientalis | 240 | 54.85 (18.42–1892.92) | 1.37 | 0.84 | 1.50 ± 0.53 | |
| 48 | A. indica | 240 | 32.24 (12.10–366.33) | 1.73 | 0.78 | 2.36 ± 0.75 |
| N. rustica | 240 | 30.27 (14.92–224.42) | 0.82 | 0.58 | 0.63 ± 0.37 | |
| M. azedarach | 240 | 24.20 (9.91–335.57) | 1.62 | 0.80 | 3.43 ± 1.40 | |
| N. tabacum | 240 | 20.75 (23.91–6190.66) | 0.99 | 0.91 | 1.22 ± 0.37 | |
| T. orientalis | 240 | 35.50 (13.60–7305.06) | 1.76 | 0.78 | 2.13 ± 0.79 | |
| 72 | A. indica | 240 | 28.36 (13.71–217.70) | 1.17 | 0.88 | 1.06 ± 0.26 |
| N. rustica | 240 | 24.39 (12.49–148.61) | 0.40 | 0.98 | 1.06 ± 0.26 | |
| M. azedarach | 240 | 29.57 (14.34–220.30) | 0.49 | 0.97 | 1.16 ± 0.28 | |
| N. tabacum | 240 | 17.53 (10.27–62.95) | 1.39 | 0.84 | 1.12 ± 0.24 | |
| T. orientalis | 240 | 29.01 (14.35–194.43) | 2.37 | 0.66 | 1.22 ± 0.29 | |
| 168 | A. indica | 240 | 6.32 (4.89–9.95) | 1.75 | 0.78 | 1.17 ± 0.21 |
| N. rustica | 240 | 5.44 (4.23–8.48) | 3.06 | 0.54 | 1.06 ± 0.20 | |
| M. azedarach | 240 | 9.15 (5.40–101.82) | 7.04 | 0.13 | 1.18 ± 0.22 | |
| N. tabacum | 240 | 4.75 (3.74–7.01) | 3.16 | 0.53 | 1.03 ± 0.20 | |
| T. orientalis | 240 | 8.39 (6.20–14.91) | 1.76 | 0.77 | 1.23 ± 0.22 | |
| 336 | A. indica | 240 | 1.86 (1.43–2.24) | 4.94 | 0.29 | 1.40 ± 0.20 |
| N. rustica | 240 | 1.34 (0.93–1.69) | 4.83 | 0.30 | 1.39 ± 0.20 | |
| M. azedarach | 240 | 1.72 (1.21–2.15) | 6.58 | 0.16 | 1.19 ± 0.20 | |
| N. tabacum | 240 | 0.92 (0.04–1.61) | 17.36 | 0.00 | 1.79 ± 0.23 | |
| T. orientalis | 240 | 2.55 (2.10–3.02) | 3.94 | 0.41 | 1.41 ± 0.20 |
N* = number of insects used; LC Lethal concentration are indicated with 95% confidence limit (CL,). LC50 of plant extract g/mL.
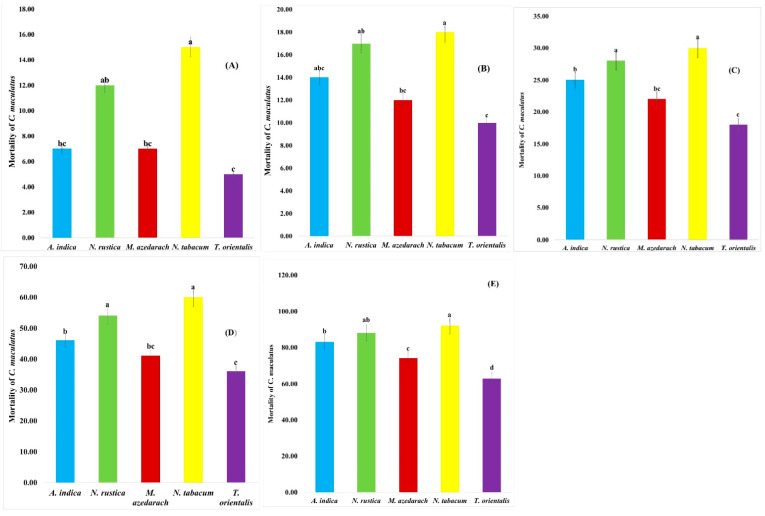
Figure 2.
Mean percent Mortality of C. maculatus treated with plant extracts after (A) 24 h, (B) 48 h, (C) 72 h, (D) 168 h and (E) 336 h exposure (Topical effect). (a, b, c, d put over the bars indicating that different letters are significantly different from each other at 0.5% level of significance).
Table 5.
Toxicological effect (Direct) of plant extracts against C. maculatus after 24, 48, 72, 168 and 336 h’ exposure period.
| Time | Plant Species | N* | LC-50 95% LC | X2 | P | Slope + SE |
|---|---|---|---|---|---|---|
| 24 | A. indica | 240 | 44.55 (17.88–987.72) | 1.40 | 0.57 | 0.55 ± 0.16 |
| N. rustica | 240 | 36.33 (15.83–462.57) | 0.23 | 0.99 | 0.47 ± 0.12 | |
| M. azedarach | 240 | 36.33 ((15.83–462.57) | 0.23 | 0.99 | 0.47 ± 0.12 | |
| N. tabacum | 240 | 28.84 (13.47–278.34) | 0.05 | 1.00 | 0.42 ± 0.11 | |
| T. orientalis | 240 | 51.87(19.25–1985.44) | 2.92 | 0.84 | 0.54 ± 0.15 | |
| 48 | A. indica | 240 | 29.78 (13.83–287.89) | 0.89 | 0.99 | 0.40 ± 0.11 |
| N. rustica | 240 | 28.59 (12.92–362.31) | 0.04 | 1.00 | 0.38 ± 0.10 | |
| M. azedarach | 240 | 32.31 (14.06–474.14) | 0.32 | 0.98 | 0.39 ± 0.10 | |
| N. tabacum | 240 | 16.23 (9.38–65.90) | 0.31 | 0.98 | 0.42 ± 0.10 | |
| T. orientalis | 240 | 40.86 (16.18–921.48) | 0.825 | 0.92 | 0.39 ± 0.11 | |
| 72 | A. indica | 240 | 13.08 (6.60–742.10) | 1.43 | 0.83 | 0.92 ± 0.21 |
| N. rustica | 240 | 10.21 (5.71–176.58) | 2.43 | 0.65 | 0.94 ± 0.21 | |
| M. azedarach | 240 | 15.13 (7.23–1536.29) | 0.91 | 0.92 | 0.93 ± 0.22 | |
| N. tabacum | 240 | 7.73 (4.92–37.88) | 2.37 | 0.66 | 1.05 ± 0.21 | |
| T. orientalis | 240 | 23.97 (11.25–287.51) | 2.00 | 0.73 | 0.68 ± 0.23 | |
| 168 | A. indica | 240 | 2.73 (1.92–3.68) | 2.12 | 0.71 | 1.32 ± 0.32 |
| N. rustica | 240 | 1.94 (1.17–2.57) | 7.90 | 0.09 | 1.35 ± 0.32 | |
| M. azedarach | 240 | 3.24 2.03–5.76) | 4.33 | 0.36 | 0.94 ± 0.31 | |
| N. tabacum | 240 | 1.66 (1.06–2.16) | 11.09 | 0.02 | 1.64 ± 0.32 | |
| T. orientalis | 240 | 4.25 (2.93–9.40) | 3.84 | 0.42 | 1.00 ± 0.32 | |
| 336 | A. indica | 240 | 0.31 (0.00–0.082) | 7.88 | 0.09 | 1.29 ± 0.25 |
| N. rustica | 240 | 0.32 (0.00–0.89) | 12.85 | 0.01 | 1.55 ± 0.28 | |
| M. azedarach | 240 | 0.20 (0.00–0.54) | 1.46 | 0.83 | 0.79 ± 0.22 | |
| N. tabacum | 240 | 0.19 (0.001–0.56) | 8.82 | 0.06 | 1.43 ± 0.31 | |
| T. orientalis | 240 | 0.55 (20.01–9.40) | 3.89 | 0.42 | 0.61 ± 0.20 |
N*= number of insects used; LC Lethal concentration are indicated with 95% confidence limit (CL,). LC50 of plant extract gm/mL.
Among the plant species extracts, the highest residual mortalities of C. maculatus was observed with N. tabacum with 6.25%, followed by N. rustica with 5.83% and A. indica with 3.33%. Lowest mortalities were observed with T. orientalis, i.e., 1.66%, followed by M. azedarach, i.e., 2.50% (df = 4, F = 10.48, p = 0.000), after 24 h of exposure period (Figure 1A). The LC50 values towards C. maculatus among the plant extracts were 24.73 for N. tabacum, followed by 29.73 for M. azedarach. The extract of T. orientalis with an LC50 of 54.85 was the least efficacious of the extracts (Table 4).
The exposure time was further increased to 48 h to check the effect of these selected five plants extracts on the mortality of pests. Figure 1B shows the effects of the six different concentrations of five different plant species crudely extracted after the 48-h exposure period. Crude extracts showed significant mortality on C. maculatus. Maximum mortalities of 12.91% were recorded with N. rustica, followed by N. tabacum with 11.67% and A. indica with 9.16%, while minimum mortalities to C. maculatus were recorded with T. orientalis with 3.75%, followed by M. azedarach with 7.50% (df = 4, F = 06.46, p = 0.0001). The LC50 values, based on mortality of C. maculatus, were 20.75 for N. tabacum, followed by 24.20 for M. azedarach (Table 4). T. orientalis, which exhibited values for mortality of 35.50, were the least efficacious.
Extracts of the five different tested plant species had significantly impacted the mortality of C. maculatus after the 72-h exposure period (Figure 1C). C. maculatus mortalities were higher at N. tabacum with 20.00%, followed by N. rustica with 18.00% and A. indica with 16.00%. Lower mortalities of 12.50% were seen with T. orientalis, followed by M. azedarach with 13.00% (df = 30, F = 07.11, p = 0.0001). The LC50 value for N. tabacum against C. maculatus was 17.53, followed by N. rustica with 24.39 and T. orientalis with 29.01 (Table 4).
Results represented in Figure 1D indicate significant mortality rates of the five different plant species crudely extracted for six different concentrations after 168 h of exposure to C. maculatus. The mortality rates of C. maculatus increase as the exposure time increases. C. maculatus mortalities were higher with N. tabacum with 39.00%, followed by N. rustica with 36.00% and A. indica with 32.00%. Lower mortalities of 25.00% were recorded with T. orientalis, followed by M. azedarach with 26.00% (df = 4, F = 20.30, p = 0.0000). From Table 4, it was clear that the LC50 of N. tabacum is 4.75, followed by N. rustica with 5.44 and A. indica with 6.32, whereas T. orientalis and M. azedarach have LC50 values of 8.39 and 9.15, respectively.
Finally, results presented in Figure 1E revealed significant mortality rates of five different plants species crude extracts of six different concentrations after fourteen days of exposure to C. maculatus. The highest mortalities of C. maculatus were noted with N. tabacum with 77.00%, followed by N. rustica with 56.00%. A. indica and M. azedarach exhibited the same mortalities of 56.00%, while the lowest mortalities of 49.00% were seen with T. orientalis (df = 4, F = 33.80, p = 0.0000). In case of LC50 values from Table 4, it was clear that N. tabacum revealed 0.92, followed by N. rustica with 1.34 and A. indica with 1.72, whereas T. orientalis and M. azedarach resulted in 2.55 and 1.86, respectively. The mean percent (±SE) residual mortality of C. maculatus after various time intervals is given in the supplementary file (Tables S1–S5) and the mean percent (±SE) topical mortality of C. maculatus after various time intervals is given in the supplementary file (Tables S6–S10).
Among the plant species extracts, the highest mortalities of C. maculatus were observed with N. tabacum of 15.00%, followed by N. rustica with 12.00%. The lowest mortality was observed with T. orientalis with 7.00%, followed by A. indica and M. azedarach, both with 7.00% (df = 4, F= 10.48, p = 0.00) after the 24-h exposure period (Figure 2A). In case of LC50, N. tabacum had 28.84, followed by N. rustica and M. azedarach with 36.33, were most effective. T. orientalis (51.87) was least effective against C. maculatus (Table 5).
The exposure time was further increased to 48 h to check the effect of these selected five plants extracts on the mortality of pests. Figure 2B shows the effects of six different concentration of five different plant species crudely extracted after the 48-h exposure period. Crude extracts showed significant mortality on C. maculatus. A maximum mortality of 18.00% was recorded with N. tabacum, followed by N. rustica with 17.00% and A. indica with 14.00%. A minimum mortality of 10.00% to C. maculatus was recorded with T. orientalis, followed by M. azedarach with 14.00% (df = 4, F = 06.46, p = 0.0001). From Table 5, it was clear that among the plant species N. tabacum (16.23), followed by N. rustica (28.59) and M. azedarach (32.31), gave promising results in killing 50% of the tested population of C. maculatus. T. orientalis’s (40.86) mortality showed the lowest effectiveness.
Extracts of five different tested plant species had significantly impacted the mortality of C. maculatus after the 72-h exposure period (Figure 2C). C. maculatus mortalities were higher at N. tabacum with 30.00%, followed by N. rustica with 28.00%, A. indica with 25.00% and M. azedarach with 22.00%. Lower mortalities of 18.00% were seen with T. orientalis (df = 30, F = 07.11, p = 0.0001). In case of LC50, N. tabacum (7.73), followed by N. rustica (10.21) and A. indica (13.08), were most effective, while T. orientalis (44.99) was found least effective against C. maculatus, as shown in Table 5.
Results represented in Figure 2D indicate significant mortality rates of five different plant species crudely extracted at six different concentrations after a 168-h exposure period on C. maculatus. The mortality rates of C. maculatus increases as the exposure time increases. C. maculatus mortalities were higher with N. tabacum by 60.00%, followed by N. rustica at 54.00%, A. indica at 46.00% and M. azedarach at 41.00%. A lower mortality of 36.00% was recorded with T. orientalis (df = 4, F = 20.30, p = 0.0000). In case of LC50, N. tabacum at 1.66, followed by N. rustica at 1.94 and A. indica at 2.73, were the most effective, while T. orientalis at 4.25 and M. azedarach at 3.24 were found the least effective against C. maculatus, as shown in Table 5.
Finally, results presented in Figure 2E revealed significant mortality rates of five different plants species crudely extracted at six different concentrations after fourteen days of exposure on C. maculatus. The highest mortalities of C. maculatus were noted with N. tabacum at 92.00%, followed by N. rustica at 88.00% and A. indica at 83.00%, while the lowest mortalities were seen at 63.00% with T. orientalis and 74.00% with M. azedarach (df = 4, F = 33.80, p = 0.0000). Among the plant extracts, N. tabacum at 0.19, followed by N. rustica at 0.20, were more promising in killing 50% of the tested population of C. maculatus. T. orientalis at 0.31 mortality showed the lowest effectiveness, as shown in Table 5.
4. Discussion
4.1. Mortality of C. maculatus Exposed to Each of Five Plants
Crude extracts of A. indica, N. tabacum. N. rustica, M. azedarach and T. orientalis were mixed with 20 g mung bean at the rate of 0.5%, 1%. 1.5, 2%. 2.5% and 3%, respectively. At all the concentrations tested, crude extracts of N. tabacum, N. rustica, A. indica and M. azedarach showed more residual toxicity against C. maculatus. Our findings are similar to the findings of a past study [22] suggesting that crude extracts of N. tabacum had the highest residual toxicity against Tribolium castaneum, i.e., 89% at a 3% concentration. Botanical insecticides contain a range of bioactive compounds that have potential to cause adverse effects on organisms that either consume them or are exposed to them. In particular, plants have co-evolved with insects that eat them, so they have developed defense mechanisms, including production of compounds that disrupt normal physiology and behavior of insects, thus, affecting eating, mating, mortalities and oviposition [23]. Secondary metabolites, N. tabacum and N. rustica, including alkaliods, flavoniods, saponins and di-tarphene can repel or kill insects. Alkaloids, including nicotine, act as stomach poison in insects. Tobacco leaves contain physiologically active chemicals that paralyze insects by acting on their central nervous system. When they feed on plants containing these compounds insects ultimately die [24]. It has recently been observed that when relatively large amounts of A. indica was consumed in the diet of C. maculatus, significant mortalities were observed [25]. The major mechanism of action of azadirichtin has been reported to limit the release of neurosecretory material from the corpora cardiaca, resulting in a slower turnover rate, as well as altering the prothoracicotropic hormone (PTTH) via brain neurosecretory cells [26]. The results of this study are consistent with those reported previously suggesting that pulses sprayed with crude extracts of M. azedarach were well-preserved for up to six months without evidence of infestation [27]. Results of this study revealed insecticidal potency via topical application through consumption in the diet of C. maculatus without treating any mung bean seeds. Among the plants tested, N. tabacum, followed by N. rustica, A. indica and M. azedarach, exhibited significantly maximum accumulative mortality, whereas T. orientalis caused minimum mortality. The mechanism of entrance of the active components into the target location in insects can also be attributed to variations in potencies to cause mortality of the extracts. Biocidal ingredients of extracts are thought to enter the insect through the integument [28]. Specifically, toxins have been reported to gain access to the target areas through the lipophilic and hydrophilic cuticle, where they cause a variety of effects through multiple mechanisms. The hydrophilic-hydrophobic structure of the cuticle has been reported to influence penetration of pesticides which also controls their efficacy [29]. Chemical properties of active principals and polarity influence passage of bioactive compounds, like insecticides through the cuticle. The cuticle’s outermost lipophilic phase promotes nonpolar molecular mobility. As a result, only toxins in extracts with a favorable polarity and chemical composition were able to penetrate to an internal site of toxic action, which could initiate molecular responses that result in lethality. A dose-response relationship was observed between the concentration of a given crude plant extract and the corresponding percent mortality, which is consistent with the results reported previously [30].
4.2. Mortality of Selected Plants Extracts against C. maculatus
The harmful effects of the compounds in extracts of the studied plants observed in this study might have contributed to the mortality of C. maculatus, which is in close agreement with other similar works reported in literature [31]. Even though all of the plants exhibited promise as insecticides, their toxic potencies against C. maculatus differed, most likely due to differences in phytochemical composition. Currently N. tabacum exhibited the greatest mortality of C. maculatus at concentrations of 3% [32]. This also confirms the same results that N. tabacum exhibits high mortality of pulse beetle at 3% concentration. As suggested previously, secondary metabolites contained in these plants might be responsible incapacity of adult C. maculatus [33].
4.3. Phytochemical Analysis Five Plants Extracts
Nicotiana tabacum contains relatively great concentrations of alkaloids, phenolic compounds, flavonoid, tannins, saponins, terpenoids, various proteins and carbohydrates. This result is consistent with previous reports of constituents in extracts of this plant. Nicotine, the active ingredient of N. tabacum, has been documented to have contact, stomach, and respiratory poisoning effects [33]. Saponins, alkaloids, flavonoids, tannins and cyanogenic glucosides are all found in crude extracts of A. indica. The natural phytochemicals from plants have a potential of being eco-friendly and replace synthetic pesticides for insect pests [34].
Acknowledgments
We thank to Researchers Supporting Project number (RSP-2021/99), King Saud University, Riyadh, Saudi Arabia. This research was supported in part by a Discovery Grant from the Natural Science and Engineering Research Council of Canada (Project # 326415-07). J.P. Giesy was supported by the Canada Research Chair program, and a Distinguished Visiting Professorship in the Department of Environmental Sciences, Baylor University in Waco, TX, USA.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects13111047/s1, Table S1: Mean percent (±SE) residual mortality of C. maculatus after 24 h exposure period treated with six different concentrations of plant crude-extracts of five plant species under laboratory conditions during 2018–2019. Table S2: Mean percent (±SE) residual mortality of C. maculatus after 48 h exposure period treated with six different concentrations of plant crude-extracts of five plant species under laboratory conditions during 2018–2019. Table S3: Mean percent (±SE) residual mortality of C. maculatus after 72 h exposure period treated with six different concentrations of plant crude-extracts of five plant species under laboratory conditions during 2018–2019. Table S4: Mean percent (±SE) residual mortality of C. maculatus after 168 h exposure period treated with six different concentrations of plant crude-extracts of five plant species under laboratory conditions during 2018–2019. Table S5: Mean percent (±SE) residual mortality of C. maculatus after 336 h exposure period treated with six different concentrations of plant crude-extracts of five botanicals under laboratory conditions during 2018–2019. Table S6: Mean percent (±SE) topical mortality of C. maculatus after 24 h exposure period treated with six different concentrations of plant crude-extracts of five plant species under laboratory conditions during 2018–2019. Table S7: Mean percent (±SE) topical mortality of C. maculatus after 48 h exposure period treated with six different concentrations of plant crude-extracts of five plant species under laboratory conditions during 2018–2019. Table S8: Mean percent (±SE) topical mortality of C. maculatus after 72 h exposure period treated with six different concentrations of plant crude-extracts of five plant species under laboratory conditions during 2018–2019. Table S9: Mean percent (±SE) topical mortality of C. maculatus after 168 h exposure period treated with six different concentrations of plant crude-extracts of five plant species under laboratory conditions during 2018–2019. Table S10: Mean percent (±SE) topical mortality of C. maculatus after 336 h exposure period treated with six different concentrations of plant crude-extracts of five plant species under laboratory conditions during 2018–2019.
Author Contributions
Conceptualization, R.A. and A.A.; Methodology, R.A.; Software, I.A.K.; Validation, B.F., A.U. and R.A.; Formal Analysis, R.A.A.; Investigation, A.M.A.; Resources, M.E.-S.; Data Curation, A.F.; Writing—Original Draft Preparation, R.A; Writing—Review & Editing, J.P.G.; Visualization, M.A.M.A.-S.; Supervision, A.A.; Project Administration, I.A.K.; Funding Acquisition, R.A.A.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data pertinent to this work are presented in the paper. Any requests should be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by King Saud University, Riyadh, Saudi Arabia, RSP-2021/99.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bibi R., Tariq R.M., Abdelgaleil S.A., Rasheed M. Insecticidal Potential of Botanicals from Red Seaweeds against Stored Grain Pests, Rice Weevil (Sitophilus oryzae L.) and Cowpea Weevil (Callosobruchus maculatus Fab.) Pak. J. Zool. 2022;54:1657–1664. doi: 10.17582/journal.pjz/20190819070846. [DOI] [Google Scholar]
- 2.Agour A., Mssillou I., Mechchate H., Es-Safi I., Allali A., Barnossi A.E., Al Kamaly O., Alshawwa S.Z., El Moussaoui A., Bari A. Brocchia cinerea (Delile) Vis. Essential Oil Antimicrobial Activity and Crop Protection against Cowpea Weevil Callosobruchus maculatus (Fab.) Plants. 2022;11:583. doi: 10.3390/plants11050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kedia A., Prakash B., Mishra P.K., Singh P., Dubey N.K. Botanicals as eco friendly biorational alternatives of synthetic pesticides against Callosobruchus spp. (Coleoptera: Bruchidae)—A review. J. Food Sci. Technol. 2015;52:1239–1257. doi: 10.1007/s13197-013-1167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahanta K., Rajkhowa D., Kumar M., Verma H. Impact of Herbicides Used in Agriculture: Benefits and Hazards. Biot. Res. Today. 2021;3:999–1001. [Google Scholar]
- 5.Rani L., Thapa K., Kanojia N., Sharma N., Singh S., Grewal A.S., Srivastav A.L., Kaushal J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021;283:124657. doi: 10.1016/j.jclepro.2020.124657. [DOI] [Google Scholar]
- 6.Xie F., Rizvi S.A.H., Zeng X. Fumigant toxicity and biochemical properties of (α+ β) thujone and 1, 8-cineole derived from Seriphidium brevifolium volatile oil against the red imported fire ant Solenopsis invicta (Hymenoptera: Formicidae) Rev. Bras. Farmacogn. 2020;29:720–727. doi: 10.1016/j.bjp.2019.04.013. [DOI] [Google Scholar]
- 7.Basile S., Badalamenti N., Riccobono O., Guarino S., Ilardi V., Bruno M., Peri E. Chemical composition and evaluation of insecticidal activity of Calendula incana subsp. maritima and Laserpitium siler subsp. siculum essential oils against stored products pests. Molecules. 2022;27:588. doi: 10.3390/molecules27030588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batiha G.E.-S., Hussein D.E., Algammal A.M., George T.T., Jeandet P., Al-Snafi A.E., Tiwari A., Pagnossa J.P., Lima C.M., Thorat N.D. Application of natural antimicrobials in food preservation: Recent views. Food Control. 2021;126:108066. doi: 10.1016/j.foodcont.2021.108066. [DOI] [Google Scholar]
- 9.Tozlu E., Cakir A., Kordali S., Tozlu G., Ozer H., Akcin T.A. Chemical compositions and insecticidal effects of essential oils isolated from Achillea gypsicola, Satureja hortensis, Origanum acutidens and Hypericum scabrum against broadbean weevil (Bruchus dentipes) Sci. Hortic. 2011;130:9–17. doi: 10.1016/j.scienta.2011.06.019. [DOI] [Google Scholar]
- 10.Zimmermann R.C., de Carvalho Aragao C.E., de Araújo P.J.P., Benatto A., Chaaban A., Martins C.E.N., do Amaral W., Cipriano R.R., Zawadneak M.A. Insecticide activity and toxicity of essential oils against two stored-product insects. Crop Prot. 2021;144:105575. doi: 10.1016/j.cropro.2021.105575. [DOI] [Google Scholar]
- 11.Ghoneim K., Hamadah K., Selim S., Waheeb H. Biopesticidal potential of Nerolidol, a sesquiterpene compound, and its drastic impact on growth and metamorphosis of the cotton leafworm Spodoptera littoralis (Lepidoptera: Noctuidae) Sch. Acad. J. Biosci. 2021;2:36–57. [Google Scholar]
- 12.Shah W.A., Qadir M. Chemical composition, antioxidant and antibacterial activity of Thuja orientalis essential oil. World J. Pharm. Sci. 2014;2:1–136. [Google Scholar]
- 13.Androutsopoulou C., Christopoulou S.D., Hahalis P., Kotsalou C., Lamari F.N., Vantarakis A. Evaluation of essential oils and extracts of rose geranium and rose petals as natural preservatives in terms of toxicity, antimicrobial, and antiviral activity. Pathogens. 2021;10:494. doi: 10.3390/pathogens10040494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bidar F., Razmjou J., Golizadeh A., Fathi S.A.A., Ebadollahi A., Naseri B. Effect of different legume seeds on life table parameters of Cowpea weevil, Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae) J. Stored Prod. Res. 2021;90:101755. doi: 10.1016/j.jspr.2020.101755. [DOI] [Google Scholar]
- 15.Hajam Y.A., Kumar R. Management of stored grain pest with special reference to Callosobruchus maculatus, a major pest of cowpea: A review. Heliyon. 2022;8:e08703. doi: 10.1016/j.heliyon.2021.e08703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashbrook A.R., Mikaelyan A., Schal C. Comparative Efficacy of a Fungal Entomopathogen with a Broad Host Range against Two Human-Associated Pests. Insects. 2022;13:774. doi: 10.3390/insects13090774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gharbi K., Tay J.-W. Fumigant Toxicity of Essential Oils against Frankliniella occidentalis and F. insularis (Thysanoptera: Thripidae) as Affected by Polymer Release and Adjuvants. Insects. 2022;13:493. doi: 10.3390/insects13060493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanal S. Qualitative and Quantitative Phytochemical Screening of Azadirachta indica Juss. Plant Parts. Int. J. Appl. Sci. Biotechnol. 2021;9:122–127. doi: 10.3126/ijasbt.v9i2.38050. [DOI] [Google Scholar]
- 19.Cruz J.P., Ribeiro F., de Oliveira Vasconcelos V. Molluscicidal activity of extracts of plants from the Cerrado against Biomphalaria glabrata (Say, 1818) Res. Soc. Dev. 2022;11:e20611830656. doi: 10.33448/rsd-v11i8.30656. [DOI] [Google Scholar]
- 20.Maliński M.P., Kikowska M.A., Soluch A., Kowalczyk M., Stochmal A., Thiem B. Phytochemical screening, phenolic compounds and antioxidant activity of biomass from Lychnis flos-cuculi L. In Vitro cultures and intact plants. Plants. 2021;10:206. doi: 10.3390/plants10020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Semwal P., Jai Singh R., Kumar A., Malik J. Pharmacognostical Exploration of Saccharum officinarum. Sch. Int. J. Tradit. Complement. Med. 2021;4:53–60. doi: 10.36348/sijtcm.2021.v04i04.003. [DOI] [Google Scholar]
- 22.Tavares W.R., Barreto M.d.C., Seca A.M. Aqueous and ethanolic plant extracts as bio-insecticides—Establishing a bridge between raw scientific data and practical reality. Plants. 2021;10:920. doi: 10.3390/plants10050920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adesina J.M. Bioactive constituents and fumigant toxicity of Datura metel extracts as grain protectant and progeny emergence inhibition of Callosobruchus maculatus (Coleoptera: Bruchidae) J. Plant Dis. Prot. 2022;129:819–829. doi: 10.1007/s41348-022-00615-6. [DOI] [Google Scholar]
- 24.Nigussie D., Davey G., Tufa T.B., Brewster M., Legesse B.A., Fekadu A., Makonnen E. Antibacterial and antifungal activities of Ethiopian medicinal plants: A systematic review. Front. Pharmacol. 2021;12:633921. doi: 10.3389/fphar.2021.633921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ifeanyieze F., Ameh H., Ejiofor T., Ikehi M., Onu F. Seed powder extract of physic nut (Jatropha curcas) as a biopesticide for weevils (Callosobruchus maculatus) in stored cowpea (Vigna unguiculata) Afr. J. Sci. Technol. Innov. Dev. 2021;13:1–6. doi: 10.1080/20421338.2021.1988408. [DOI] [Google Scholar]
- 26.Stejskal V., Vendl T., Aulicky R., Athanassiou C. Synthetic and natural insecticides: Gas, liquid, gel and solid formulations for stored-product and food-industry pest control. Insects. 2021;12:590. doi: 10.3390/insects12070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar A., Ray A. Biological and infestation studies on Araecerus fasciculatus DeGeer a new pest of Melia azadirach L. in India. Int. J. Trop. Insect Sci. 2022;42:1245–1254. doi: 10.1007/s42690-021-00643-z. [DOI] [Google Scholar]
- 28.Willis Chan D.S., Raine N.E. Population decline in a ground-nesting solitary squash bee (Eucera pruinosa) following exposure to a neonicotinoid insecticide treated crop (Cucurbita pepo) Sci. Rep. 2021;11:4241. doi: 10.1038/s41598-021-83341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alotaibi S.S., Darwish H., Alzahrani A.K., Alharthi S., Alghamdi A.S., Al-Barty A.M., Helal M., Maghrabi A., Baazeem A., Alamari H.A. Environment-Friendly Control Potential of Two Citrus Essential Oils against Aphis punicae and Aphis illinoisensis (Hemiptera: Aphididae) Agronomy. 2022;12:2040. doi: 10.3390/agronomy12092040. [DOI] [Google Scholar]
- 30.Singh K.D., Mobolade A.J., Bharali R., Sahoo D., Rajashekar Y. Main plant volatiles as stored grain pest management approach: A review. J. Agric. Food Res. 2021;4:100127. doi: 10.1016/j.jafr.2021.100127. [DOI] [Google Scholar]
- 31.Muhammad M.-u.-R., Khalid A., Muhammad N., Alizai A., Sajjad H. Entomocidal studies of some plant materials against pulse beetle, Callosobruchus chinensis (Bruchidae: Coleoptera) on stored chickpea (Cicer arietinum) Pak. Entomol. 2018;40:71–75. [Google Scholar]
- 32.Alamgir A. Therapeutic Use of Medicinal Plants and Their Extracts: Volume 1. Springer; Berlin/Heidelberg, Germany: 2017. Medicinal, non-medicinal, biopesticides, color-and dye-yielding plants; secondary metabolites and drug principles; significance of medicinal plants; use of medicinal plants in the systems of traditional and complementary and alternative medicines (CAMs) pp. 61–104. [Google Scholar]
- 33.Malaikozhundan B., Vaseeharan B., Vijayakumar S., Thangaraj M.P. Bacillus thuringiensis coated zinc oxide nanoparticle and its biopesticidal effects on the pulse beetle, Callosobruchus maculatus. J. Photochem. Photobiol. B Biol. 2017;174:306–314. doi: 10.1016/j.jphotobiol.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 34.Ashraf A., Qureshi N.A., Shaheen N., Iqbal A., Fatima H., Afzal M., Alhewairini S.S., Qureshi M.Z. Termiticidal and protozocidal potentials of eight tropical plant extracts evaluated against Odontotermes obesus Rambur (Blattodea; Termitidae) and Heterotermes indicola Wasmann (Blattodea; Rhinotermitidae) Pol. J. Environ. Stud. 2020;29:3493–3507. doi: 10.15244/pjoes/116105. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data pertinent to this work are presented in the paper. Any requests should be directed to the corresponding author.