Idiopathic pulmonary fibrosis (IPF) is an incurable disease that affects approximately 3 million people worldwide [1]. Although two drugs (nintedanib and pirfenidone) have been approved for treatment of IPF, currently there is a lack of effective pharmacotherapy that could stop the progression or offer a cure for this devasting disease.
Programmed death-ligand 1 (PD-L1) is an immune checkpoint protein that serves as a mechanism of immune surveillance. It limits excessive immune response through binding with its receptor PD-1 on T cells and suppressing their activation [2]. Increasing evidence also unveils an important role of PD-L1 in cancer cell proliferation, metastasis, and drug resistance to targeted therapy [2,3]. Recently, a few papers published support targeting PD-L1/PD-1 axis for treatment of IPF [4-7]. The treatment with an anti-PD-L1 antibody attenuates/blocks the progression of pulmonary fibrosis in preclinical models of IPF. Beyond the immune cells, new evidence has also suggested a novel role of PD-L1 in fibroblasts [5,8], particularly through a process termed fibroblast to myofibroblast transition (FMT) or myofibroblast differentiation.
Lung fibroblasts play a critical role in the progression of pulmonary fibrosis. One critical feature lies in the differentiation of fibroblasts to myofibroblasts, which express structural protein α-SMA and excessive extracellular matrix, thus contributing to the structural remodeling of the lung. Myofibroblasts also entails positive feedback through secretion of profibrotic mediators, especially TGF-β1, the most potent inducer of profibrotic changes. Geng et al. identified a subgroup of invasive lung fibroblasts that possess high levels of PD-L1. Those fibroblasts with high PD-L1 expression can induce fibrotic changes in the mouse lungs upon tail vein injection [6]. The finding implicates PD-L1 in the development of pulmonary fibrosis in vivo. Kang et al. reported that TGF-β1 induces PD-L1 expression using established human and murine fibroblast cell lines and PD-L1 is implicated in FMT [5]. However, the underlying mechanisms remain elusive.
The recent publication by our group [8] provides further insight on how PD-L1 mediates FMT and how it is intervened with the TGF-β signaling. In this manuscript, the authors first demonstrated an enhanced expression of PD-L1 in the fibrotic lungs of IPF patients and mice models of pulmonary fibrosis. The finding provides a proof of concept of the implication of PD-L1 in pulmonary fibrosis. The authors further showed that TGF-β1 induces the expression of PD-L1 in several lines of primary lung fibroblasts from both normal and IPF donors. These data, together with a previous report using established cell lines [5], strengthen a role of PD-L1 induction by TGF-β1 in the FMT process. In addition, the expression of PD-L1 has also been identified in alveolar/bronchial epithelial cells [9] and alveolar macrophages [10] of IPF patients, implying various sources of PD-L1 in the pathogenesis of IPF.
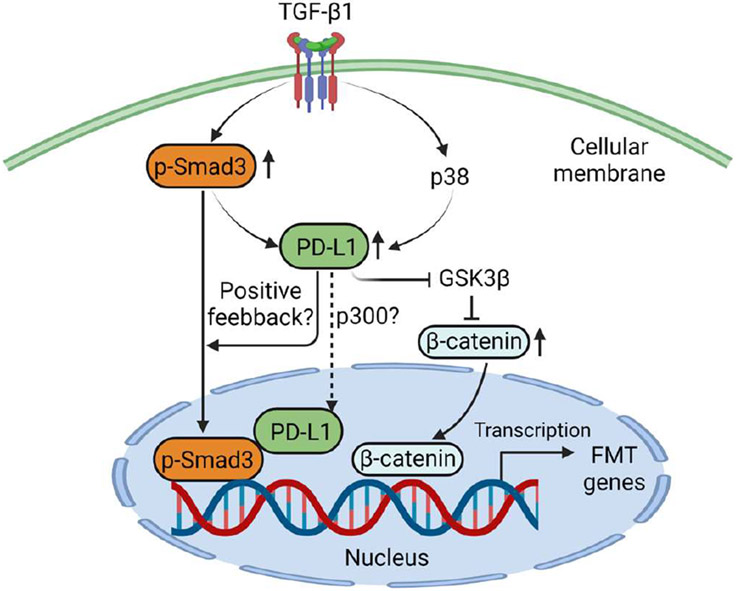
Both canonical and non-canonical pathways of TGF-β1 signaling contribute to the FMT process and pulmonary fibrosis development. The manuscript demonstrated that PD-L1 is involved in different pathways to promote FMT. The interaction between PD-L1 and Smad3 was confirmed through immunofluorescent co-staining of PD-L1 and phosphor-Smad3 and co-immunoprecipitation (co-IP) assay. The interaction increased with the treatment of TGF-β1, although a basal level of interaction was also found. It remains unclear, however, whether such increased interaction (co-IP) is due to elevated expression of PD-L1 or perhaps a result of increased binding affinity. Further study is also warranted to examine the transport of PD-L1 from cytoplasm to the nucleus where co-staining of PD-L1 and phosphor-Smad3 was noted. The recent identification of the p300 as the acetyltransferase of PD-L1 provides a mechanism for the nuclear transport of PD-L1 in cancer cells [11], which deserves further investigation in the setting of lung fibroblasts, especially in view of the interactions between TGF-β and p300 [12,13].
Whether the binding between PD-L1 with Smad3 also stabilizes phosphor-Smad3 and thereby increasing its activity needs to be addressed in future studies. Another shortcoming of the study is the overlook of Smad2 in the FMT process. Whether PD-L1 also binds with Smad2 to form a tri-complex with Smad3 deserves further investigation. Nevertheless, this manuscript presents novel findings that help explain how PD-L1 is intervened with the Smad3 signaling. This finding might be of general interest in terms of the complex role of TGF-β in tumor progression and tissue fibrosis of other organs. Another mechanism reported in this manuscript involves the modulation of the GSK3β/β-catenin signaling by PD-L1. Previous studies have reported that Wnt/β-catenin signaling upregulates PD-L1 transcription in diverse cancer cells [14-16]. Interestingly, PD-L1 has also been found to activate β-catenin signaling in different cancer cell types [14,17], suggesting potential positive feedback, β-catenin is a known target of GSK3β for ubiquitin dependent degradation. It is also implicated in lung myofibroblast differentiation [18]. The link between PD-L1 and β-catenin signaling provides an additional layer of control over the FMT process in the context of IPF.
The identification of the upstream regulator of PD-L1 by TGF-β provides novel insight on the control of PD-L1 levels. Interestingly, Smad3 inhibition by a specific small molecule inhibitor SIS3 attenuates the induction of PD-L1 by TGF-β. It seems that there exists a positive loop between Smad3 and PD-L1, i.e., Smad3 upregulates PD-L1 level which in turn binds to and increase the activity of Smad3. However, more experiments are needed to test this hypothesis. On the other hand, p38 pathway as a critical non-canonical TGF-β signaling has also been implicated in the regulation of PD-L1. Inhibition of p38 also attenuates the induction of PD-L1 by TGF-β. Beyond IPF, asthma related subepithelial fibrosis also benefits from the inhibition of the p38 pathway [19]. Whether targeting PD-L1 will likewise benefit fibrosis of other organs remains to be explored.
The transcriptional regulation of PD-L1 was demonstrated through an array of assays in this manuscript. It is well known that multiple mechanisms control the level of PD-L1 including manipulating its protein stability [20], e.g., the identification of March8 as a novel E3 ligase for PD-L1 degradation [21].Whether TGF-β signaling also affects the stability of PD-L1 remains an interesting question and warrants further experimental testing. The authors put together these findings nicely into a diagram. TGF-β induces PD-L1 expression that is dependent on Smad3 and p38 signaling. Increased PD-L1 in turn binds to Smad3 to enhance its activation and transcriptional activity (via α-SMA luciferase reporter assay). In addition, PD-L1 also contributes to upregulated β-catenin levels induced by TGF-β, which is likely mediated through GSK3β pathway. Through those two mechanisms, PD-L1 is implicated in TGF-β induced FMT process and potentially the development of pulmonary fibrosis. A schematic presentation of the mechanisms by which PD-L1 mediates FMT is shown in Figure 1 (Created with BioRender.com).
Figure 1:
A diagram of the mechanisms by which PD-L1 mediates fibroblast to myofibroblast transition (FMT).
As mentioned above, PD-L1 expression has been reported in diverse cell types including alveolar macrophages [10], alveolar epithelial cells [9,22], and lung fibroblasts [4-6,8]. Therefore, the generation of fibroblast-specific mouse model is a prerequisite for testing the role of PD-L1 in lung fibroblasts towards pulmonary fibrosis development. Currently, the conditional model is not available and future work is needed to generate the fibroblast-specific PD-L1 knockout mouse model. A recent study [23] has identified a subpopulation of alveolar type II epithelial cells (AECII) that express PD-L1 and expand in the lung of IPF patients. Due to a progenitor role of these cells, a concern is raised that PD-L1 inhibition might injure the already compromised epithelial compartment in the IPF lung. Similarly, this speculation remains to be tested using conditional PD-L1 knockout mice model.
In summary, the immune checkpoint protein PD-L1 has emerged as a novel target for the treatment of IPF. Accumulating evidence supports the targeting of PD-L1 to block the development of pulmonary fibrosis. Experiments are needed, however, to test whether inhibition of PD-L1 could reverse the already existent fibrotic lesions in the lung of IPF patients, which is often the case in the clinic. It is likely that the role of PD-L1 in the progression of pulmonary fibrosis is complex, arising from diverse cell types and involving different mechanisms. The generation of cell-specific PD-L1 knockout animal models might hold the key to unveil the full view of PD-L1 in the development of IPF.
Acknowledgements
This work was supported by the University of Texas Health Science Center at Tyler Startup fund (to GQ and XG) and seed grant (to GQ); National Institutes of Health (R00HL141583 to XG); and the University of Texas Rising Star Award (to XG).
Footnotes
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- 1.Olson AL, Gifford AH, Inase N, Fernandez Perez ER, Suda T. The epidemiology of idiopathic pulmonary fibrosis and interstitial lung diseases at risk of a progressive-fibrosing phenotype. Eur Respir Rev. 2018;27(150). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patsoukis N,Wang Q, Strauss L, Boussiotis VA. Revisiting the PD-1 pathway. Sci Adv. 2020;6(38):eabd2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10:727–42. [PMC free article] [PubMed] [Google Scholar]
- 4.Cui L, Chen SY, Lerbs T, Lee JW, Domizi P, Gordon S, et al. Activation of JUN in fibroblasts promotes pro-fibrotic programme and modulates protective immunity. Nat Commun. 2020;11:2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang JH, Jung MY, Choudhury M, Leof EB. Transforming growth factor beta induces fibroblasts to express and release the immunomodulatory protein PD-L1 into extracellular vesicles. FASEB J. 2020;34:2213–26. [DOI] [PubMed] [Google Scholar]
- 6.Geng Y, Liu X, Liang J, Habiel DM, Kulur V, Coelho AL, et al. PD-L1 on invasive fibroblasts drives fibrosis in a humanized model of idiopathic pulmonary fibrosis. JCI Insight. 2019;4(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celada LJ, Kropski JA, Herazo-Maya JD, Luo W, Creecy A, Abad AT, et al. PD-1 up-regulation on CD4(+) T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-beta1 production. Sci Transl Med. 2018;10(460):eaar8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo X, Sunil C, Adeyanju o, Parker A, Huang S, Ikebe M, et al. PD-L1 mediates lung fibroblast to myofibroblast transition through Smad3 and beta-catenin signaling pathways. Sci Rep. 2022:12:3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kronborg-White S, Madsen LB, Bendstrup E, Poletti V. PD-L1 Expression in Patients with Idiopathic Pulmonary Fibrosis. J Clin Med. 2021;10(23):5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jovanovic D, Roksandic Milenkovic M, Kotur Stevuljevic J, Markovic J, Ceriman V, Kontic M, et al. Membrane PD-L1 expression and soluble PD-L1 plasma levels in idiopathic pulmonary fibrosis-a pilot study. J Thorac Dis. 2018;10:6660–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Y, Nihira NT, Bu X, Chu C, Zhang J, Kolodziejczyk A, et al. Acetylation-dependent regulation of PD-L1 nuclear translocation dictates the efficacy of anti-PD-1 immunotherapy. Nat Cell Biol. 2020;22:1064–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh AK, Bhattacharyya S, Lafyatis R, Farina G,Yu J, Thimmapaya B, et al. p300 is elevated in systemic sclerosis and its expression is positively regulated by TGF-beta.epigenetic feed-forward amplification of fibrosis. J Invest Dermatol. 2013;133:1302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishihara A, Hanai JI, Okamoto N, Yanagisawa J, Kato S, Miyazono K, et al. Role of p300, a transcriptional coactivator, in signalling of TGF-beta. Genes Cells. 1998;3:613–23. [DOI] [PubMed] [Google Scholar]
- 14.Fu L, Fan J, Maity S, McFadden G, Shi Y, Kong W. PD-L1 interacts with Frizzled 6 to activate beta-catenin and form a positive feedback loop to promote cancer stem cell expansion. Oncogene. 2022;41:1100–13. [DOI] [PubMed] [Google Scholar]
- 15.Du L, Lee JH, Jiang H, Wang C, Wang S, Zheng Z, et al. beta-Catenin induces transcriptional expression of PD-L1 to promote glioblastoma immune evasion. J Exp Med. 2020; 217(11):e20191115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang T, Li F, Cheng X, Wang J, Zhang W, Zhang B, et al. Wnt Inhibition Sensitizes PD-L1 Blockade Therapy by Overcoming Bone Marrow-Derived Myofibroblasts-Mediated Immune Resistance in Tumors. Front Immunol. 2021;12:619209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu W, Hua Y, Qiu H, Hao J, Zou K, Li Z, et al. PD-L1 promotes tumor growth and progression by activating WIP and beta-catenin signaling pathways and predicts poor prognosis in lung cancer. Cell Death Dis. 2020;11:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao H, Wang C, Chen X, Hou J, Xiang Z, Shen Y, et al. Inhibition of Wnt/beta-catenin signaling suppresses myofibroblast differentiation of lung resident mesenchymal stem cells and pulmonary fibrosis. Sci Rep. 2018;8:13644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paw M, Wnuk D, Nit K, Bobis-Wozowicz S, Szychowski R, Slusarczyk A, et al. SB203580-A Potent p38 MAPK Inhibitor Reduces the Profibrotic Bronchial Fibroblasts Transition Associated with Asthma. Int J Mol Sci. 2021; 22(23):12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucibello G, Mograbi B, Milano G, Hofman P, Brest P. PD-L1 regulation revisited.impact on immunotherapeutic strategies. Trends Mol Med. 2021;27:868–81. [DOI] [PubMed] [Google Scholar]
- 21.Qian G, Guo J, Vallega KA, Hu C, Chen Z, Deng Y, et al. Membrane-associated RING-CH 8 functions as a novel PD-L1 E3 ligase to mediate PD-L1 degradation induced by EGFR inhibitors. Mol Cancer Res. 2021;19(10):1622–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmadvand N, Khosravi F, Lingampally A, Wasnick R, Vazquez-Armendariz AI, Carraro G, et al. Identification of a novel subset of alveolar type 2 cells enriched in PD-L1 and expanded following pneumonectomy. Eur Respir J. 2021;58(5):2004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmadvand N, Carraro G, Jones MR, Shalashova I, Noori A, Wilhelm J, et al. Cell-Surface Programmed Death Ligand-1 Expression Identifies a Sub-Population of Distal Epithelial Cells Enriched in Idiopathic Pulmonary Fibrosis. Cells. 2022;11(10):1593. [DOI] [PMC free article] [PubMed] [Google Scholar]