Abstract
The Pseudomonas aeruginosa quorum-sensing systems, las and rhl, control the production of numerous virulence factors. In this study, we have used the burned-mouse model to examine the contribution of quorum-sensing systems to the pathogenesis of P. aeruginosa infections in burn wounds. Different quorum-sensing mutants of P. aeruginosa PAO1 that were defective in the lasR, lasI, or rhlI gene or both the lasI and rhlI genes were utilized. The following parameters of the P. aeruginosa infection were examined: (i) lethality to the burned mouse, (ii) dissemination of the P. aeruginosa strain within the body of the infected mouse (by determining the numbers of CFU of P. aeruginosa within the liver and spleen), and (iii) spread of the P. aeruginosa strain within the burned skin (by determining the numbers of CFU of P. aeruginosa at the inoculation site and at a site about 15 mm from the inoculation site [distant site]). In comparison with that of PAO1, the in vivo virulence of lasI, lasR, and rhlI mutants was significantly reduced. However, the most significant reduction in in vivo virulence was seen with the lasI rhlI mutant. The numbers of CFU that were recovered from the livers, spleens, and skin of mice infected with different mutants were significantly lower than those of PAO1. At 8 and 16 h post burn infection, comparable numbers of CFU of PAO1 and lasI and rhlI mutants were obtained from both the inoculation and distant sites of the burned skin of infected mice. In contrast, CFU of the lasR mutant and the lasI rhlI double mutant were recovered only from the inoculation site of infected mice at 8 and 16 h post burn infection. The ability of a plasmid carrying either the lasI or rhlI gene or the lasI and rhlI genes to complement the defect of the lasI rhlI double mutant was also examined. The presence of any of these plasmids within the lasI rhlI double mutant significantly enhanced its in vivo virulence, as well as its ability to spread within the burned skin. These results suggest that the quorum-sensing systems play an important role in the horizontal spread of P. aeruginosa within burned skin and in the dissemination of P. aeruginosa within the bodies of burned-and-infected mice and contributed to the overall virulence of P. aeruginosa in this animal model.
Pseudomonas aeruginosa is an opportunistic gram-negative bacillus that rarely causes infections in healthy individuals but can cause serious infections in immunocompromised hosts (4). These immunocompromised hosts include cystic fibrosis patients (7), cancer patients (2), patients with human immunodeficiency virus infections (9), and patients with severe burn wounds (15). One of the most serious complications of burn injury is bacterial infection (such as P. aeruginosa infection) of the burn wound (15). The ability of P. aeruginosa to survive under different environmental conditions, combined with its inherent resistance to several antibiotics, allows it to colonize and proliferate within the burned tissues. This localized proliferation may lead to systemic sepsis, which is often associated with a high degree of mortality (21). The pathogenesis of P. aeruginosa infection is attributed to the production of both cell-associated and extracellular virulence factors. The cell-associated factors include the flagellum (19), the adhesion factors (e.g., pili and other possible adhesins [38]), and alginate (14, 20). The extracellular virulence factors include exotoxin A, exoenzyme S, elastases (LasA and LasB), alkaline protease, and phospholipase C (3, 14, 17, 22, 46). The virulence of P. aeruginosa (as well as the roles of specific factors in its virulence) has been examined by using different animal models that simulate the types of clinical infection caused by the organism (25, 41, 42, 47). However, the main problem inherent in the animal model is the difficulty in correlating the results obtained from the model with these clinical infections. Among the factors that contribute to this difficulty are the large dose of microorganisms required to produce an infection in the animal model and the severe traumatization of the animals. The nonlethally burned mouse model, which was developed by Stieritz and Holder (41), has been used successfully to examine the pathogenesis of P. aeruginosa infection of burn wounds. P. aeruginosa infection produced in the burned-mouse model resembles human wound sepsis to a great extent (41). By using the burned-mouse model, several previous studies have demonstrated the important roles of different virulence factors (such as the elastases, exotoxin A, and exoenzyme S) in the pathogenesis of P. aeruginosa infection of the burn wound (22, 27, 36).
In addition to the individual factors mentioned, the virulence of P. aeruginosa may also be affected by the newly described quorum-sensing systems which control the production of several virulence factors (44). The typical quorum-sensing system, which appears to function in response to cell density, is composed of a transcriptional activator protein and a small diffusible molecule (autoinducer) (11, 37). In P. aeruginosa, two complete quorum-sensing systems (las and rhl) have been described. The las quorum-sensing system is composed of the transcriptional activator LasR (which is encoded by lasR) and the diffusible extracellular signal N-(3-oxododecanoyl)homoserine lactone (12, 28). This signal (which is also called Pseudomonas autoinducer 1 [PAI1]) is synthesized by the P. aeruginosa autoinducer synthase LasI (which is encoded by the lasI gene) (28). At a certain cell density, P. aeruginosa produces sufficient levels of PAI1, which complexes with and activates LasR (26). Activated LasR then enhances the transcription of several virulence genes, including lasA, lasB, toxA, arpA, lasI, and rhlR (13, 26, 32, 43). Similar to the las system, the rhl system is composed of the transcriptional activator RhlR (which is encoded by rhlR) and the diffusible molecule N-butyryl-l-homoserine lactone (PAI2) (23, 24, 29). PAI2 is synthesized by the P. aeruginosa autoinducer synthase RhlI (which is encoded by the rhlI gene) (23, 24). RhlR is activated by binding to PAI2 (RhlR-PAI2) (29). Activated RhlR then enhances the transcription of the lasB, rpoS, rhlA, and rhlI genes (5, 18, 30).
By using the burned-mouse model, we have recently examined the role of lasR in the pathogenesis of P. aeruginosa infections of burn wounds (34). This was done by using a specific lasR deletion mutant of PAO1, PAO-R1 (34). In comparison with PAO1, PAO-R1 showed a significant reduction in in vivo virulence, systemic spread within the bodies of infected mice, and spread within burned skin (34). Based on these results, and since lasR is a major regulator of lasB and lasA, we suggested that the observed defects in the virulence of PAO-R1 were due to the loss of the elastolytic activity produced by LasA or LasB (34). However, further analysis (using specific PAO1 deletion mutants defective in lasA, lasB, or lasA and lasB) did not support this hypothesis. The decrease in virulence seen with PAO-R1 in the burned mouse was not detected in any of these specific mutants (35). Since LasR plays an important role in the function of the las and rhl quorum-sensing systems, we speculated that the defect in PAO-R1 is due to inactivation of the quorum-sensing systems. In this study, we have examined the contribution of quorum sensing to the pathogenesis of P. aeruginosa infections in burn wounds. This was done by using specific PAO1 mutants that are defective in the production of certain components of the quorum-sensing systems. Our results showed that the virulence of PAO1 mutants defective in either lasI, lasR, or rhlI is reduced. However, the most significant reduction was detected with the mutant that is defective in both lasI and rhlI.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli and P. aeruginosa strains were routinely grown in Luria-Bertani (LB) medium (1). Antibiotics (Sigma, St. Louis, Mo.) were used at the following concentrations: for E. coli, carbenicillin at 100 μg/ml and nalidixic acid at 20 μg/ml; for P. aeruginosa, carbenicillin at 300 μg/ml, rifampin at 80 μg/ml, streptomycin at 100 μg/ml, and tetracycline at 50 μg/ml. Mercuric chloride (Sigma) was used at 15 μg/ml in solid medium and at 7.5 μg/ml in LB medium.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotypea | Reference |
|---|---|---|
| Pseudomonas aeruginosa | ||
| PAO1 | Wild-type, prototrophic strain; clinical isolate | 16 |
| PAO-R1 | PAO1 ΔlasR Tcr | 12 |
| PDO100 | PAO1 ΔrhlI::Tn501 Hgr | 5 |
| JP1 | PAO1 ΔlasI Tcr | 30 |
| JP2 | PDO100 ΔlasI Hgr Tcr | 30 |
| Escherichia coli DH5α | supE44 thi-1 recA1 gyrA Nalr | 1 |
| Plasmids | ||
| pSW200 | pUC18 cloning vector carrying 1.8-kb PstI fragment that allows plasmid ColE1 to replicate stably in P. aeruginosa | 12 |
| pLASI-2 | Cbr; pSW200 carrying 989-bp fragment which contains lacP-lasI | 26 |
| pJPP42 | pLASI-2 carrying 1.5-kb fragment which contains lacP-rhlI | 30 |
| pJPP45 | pSW200 carrying 1.5-kb fragment which contains lacP-rhlI | 30 |
Abbreviations: Tcr, tetracycline resistance; Hgr, mercuric chloride resistance; Nalr, nalidixic acid resistance; Cbr, carbenicillin resistance.
Burned-mouse model.
The virulence of different P. aeruginosa mutants was examined by using the modified burned-mouse model of Stieritz and Holder (34, 41). In this modified model, the type of induced burn is a scald and not a flame burn. The experiments were conducted with adult female ND4 Swiss Webster mice weighing 20 to 24 g. The mice were anesthetized by intraperitoneal injection of 0.4 ml of Nembutal at 5 mg/ml (5% sodium pentobarbital; Abbott Laboratories, North Chicago, Ill.), and their backs were shaved. The mice were securely placed into a template with an opening (4.5 by 1.8 cm) exposing their shaved backs. About 15% of the total surface area of the mouse was exposed through the opening on the template. The thermal injury was induced by placing the exposed area of the shaved skin in 90°C water for 10 s. Such an injury is nonlethal but causes a third-degree (full-thickness) burn. Fluid replacement therapy consisting of a subcutaneous injection of 0.8 ml of 0.9% NaCl solution was administered immediately following the burn. Mice were challenged by the subcutaneous inoculation of 100 μl of the bacterial inoculum (see below) directly under the burn. Control mice were subcutaneously injected with 100 μl of sterile phosphate-buffered saline (PBS) directly under the burn. During recovery, the mice were kept under warming lights and observation. Mortality among infected mice was recorded at 48 h post burn infection. In some cases, infected mice were monitored for death until 4 to 5 days post burn infection. Animals were treated humanely and in accordance with the protocol approved by the Animal Care and Use Committee at Texas Tech University Health Sciences Center in Lubbock, Tex.
Preparation of the P. aeruginosa inoculum.
Aliquots (50 μl) of overnight cultures of PAO1 and different P. aeruginosa mutants were subcultured in fresh LB broth with antibiotics. The cultures were grown at 37°C for 4 h to an optical density at 540 nm of approximately 0.9 (some cultures were adjusted to an optical density at 540 nm of 0.9 by the addition of sterile PBS). A 100-μl aliquot of each culture was then pelleted, washed in PBS, and serially diluted (10-fold serial dilutions) in PBS. A 100-μl aliquot of the 10−5 dilution was injected into each animal. As we have previously determined (34), this dilution contains approximately 2 × 102 to 3 × 102 CFU of P. aeruginosa. We have also shown previously that this dose of PAO1 produces 94 to 100% lethality in ND4 Swiss Webster mice by 48 h post burn infection (34). The number of preinjection CFU of each strain was determined by plating serial dilutions of the inoculum on LB agar plates.
Quantitation of bacteria within the skin, livers, and spleens of infected mice at 24 h post burn infection.
At 24 h post burn infection, the mice were euthanized by intracardial injection of 0.2 ml of Sleepaway (sodium pentobarbital–7.8% isopropyl alcohol euthanasia solution; Fort Dodge Laboratories, Inc., Fort Dodge, Iowa). Skin sections of approximately 5 by 5 mm were obtained from the burned skin of both the control and challenged mice. Similarly, the heart, liver and spleen of each animal were obtained. Individual skin sections and organs were weighed, suspended in 2 ml of PBS, and homogenized (Wheaton overhead stirrer; Wheaton Instruments, Millville, N.J.). A 100-μl aliquot of each homogenate was plated on LB agar plates to determine the number of post burn infection CFU. The number of CFU from each organ was calculated per gram of tissue.
Spread of P. aeruginosa strains within the skin of infected mice.
Groups of mice (four or five mice per group) were burned and infected with different P. aeruginosa strains as described above. At a specific time period post burn infection, the mice were euthanized and specific skin sections (approximately 5 by 5 mm) were obtained from the burned skin of each animal. One section was obtained from the inoculation site of the burned skin. The other section was obtained from a site about 15 mm distant from the inoculation site (distant-site section). In addition to determining the spread of P. aeruginosa within the burned skin (horizontal spread), we tried to determine the spread of P. aeruginosa from the infected skin to the underlying tissue (vertical spread). Sections of the connective tissue underneath the skin at the inoculation site were obtained. The skin and connective tissue sections were suspended in PBS, homogenized, and plated on LB agar plates as described above.
Transfer of plasmids into P. aeruginosa mutant JP2.
Plasmid DNA was isolated from E. coli by the alkaline lysis procedure (1). The plasmids were then introduced into JP2 by electroporation as previously described (39).
In vitro and in vivo stability of plasmids in JP2.
In vitro stability was examined by the repeated subculturing (five or six times) of JP2 containing different plasmids in antibiotic-free LB broth. Individual colonies from the last subculture were inoculated onto LB agar plates and LB agar plates containing carbenicillin. The stability of different plasmids in JP2 was determined in vivo (within the infected mice) by inoculating the individual colonies from the spleens, livers, and skin of infected mice onto LB agar plates and LB agar plates containing carbenicillin.
Statistical analysis.
The Wilcoxon signed rank test for significance (Statworks; Cricket Software, Inc., Malvern, Pa.) (40) was done to determine significant differences between the numbers of CFU obtained from the livers and spleens of groups of mice infected with PAO1 and the different quorum-sensing mutant strains. To determine the significance between groups in the mortality experiments, the one-way analysis of variance with the Tukey-Kramer multiple-comparisons test (40) was performed by using GraphPad InStat version 3.00 for Windows 95 (GraphPad Software, San Diego, Calif.).
RESULTS
The role of the quorum-sensing systems in the pathogenesis of P. aeruginosa infection of burn wounds was examined by using several P. aeruginosa mutants that are defective in certain aspects of the quorum-sensing systems (Table 1). These mutants were defective in the lasR (PAO-R1), lasI (JP1), rhlI (PDO100), or lasI and rhlI (JP2) genes (5, 12, 30). All mutants were generated from P. aeruginosa PAO1, which is a virulent strain originally isolated from a wound infection (41). Mutants PAO-R1 and JP1 were generated by the gene replacement technique using tetracycline resistance (Tcr) cassettes (12, 30). PAO1 mutant PDO100 was generated by transposon (Tn501) mutagenesis (5). JP2 was generated by both gene replacement (Tcr cassette) and transposon (Tn501) mutagenesis (5, 30). We have excluded the possibility that mutations in the quorum-sensing genes affect the general growth characteristic of PAO1. Strain PAO1 and the different quorum-sensing mutants were grown individually in antibiotic-free LB broth, and their growth was monitored throughout the growth cycle (about 20 to 22 h). No major differences between the growth rate of PAO1 and that of any of the quorum-sensing mutants were detected (data not shown). We have also excluded the possibility that the elastase-deficient phenotype of PAO-R1, JP1, JP2, and PDO100 was altered upon the growth of these mutants in antibiotic-free medium. The strains were extensively subcultured in LB broth and plated on LB agar plates for isolated colonies. Two hundred isolated colonies of each mutant were inoculated onto LB agar plates, selective antibiotic plates (tetracycline or mercuric chloride plates), and elastin plates. All isolated colonies were antibiotic resistant and elastase deficient (data not shown).
In vivo virulence of quorum-sensing mutants.
Groups of mice (four to seven per group) were burned and inoculated with approximately 2 × 102 CFU of each of the tested strains as described in Materials and Methods. Three separate experiments were conducted with each strain. The mortality of the mice was recorded at 48 h post burn infection. As shown in Table 2, the percent mortality of mice infected with PAO-R1 was significantly (P < 0.001) lower than that of those infected with PAO1. These results are similar to our previously reported results (34). In addition, the percent mortality among mice infected with PDO100 or JP1 was 46.7%, which is also significantly (P < 0.01) lower than the mortality of mice infected with PAO1 (Table 2). However, the most significant reduction in percent mortality was seen in mice infected with JP2 (only 6.7%; P < 0.001) (Table 2). The observed reduction in the percent mortality of mice infected with different quorum-sensing mutants correlates with the increase in the survival time of burned-and-infected mice. The percent mortalities in Table 2 were recorded at 48 h post burn infection. However, there was no increase in these percent mortalities, even at 3 or 4 days postinfection (data not shown). These results suggest that in the burn infection model, the in vivo virulence of P. aeruginosa is significantly reduced upon the loss of any one of three quorum-sensing system components (lasI, rhlI, or lasR). However, the reduction is most prominent when both quorum-sensing system components lasI and rhlI are defective (Table 2).
TABLE 2.
Mortality of burned mice infected with P. aeruginosa PAO1 and quorum-sensing mutant strains
| Strain | No. of mice dead/total (% mortality)a
|
Avg % mortalityb | ||
|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | ||
| PAO1 | 7/7 (100) | 5/6 (83.3) | 6/6 (100) | 94.3 |
| PAO-R1 | 2/5 (40) | 1/4 (25) | 1/5 (20) | 28.3 |
| PDO100 | 2/5 (40) | 3/5 (60) | 2/5 (40) | 46.7 |
| JP1 | 3/5 (60) | 2/5 (40) | 2/5 (40) | 46.7 |
| JP2/pSW200c | 0/5 (0) | 1/5 (20) | 0/5 (0) | 6.7 |
A total of 2 × 102 to 3 × 102 CFU of each strain was injected subcutaneously at the burn site immediately after burning. The mice were burned and then inoculated with each strain as described in Materials and Methods. The mortality among mice infected with each strain was determined at 48 h post burn infection as previously described (34). Three separate experiments were conducted with each strain.
The average percent mortality of three independent experiments is shown.
Systemic spread of quorum-sensing mutants within burned-and-infected mice.
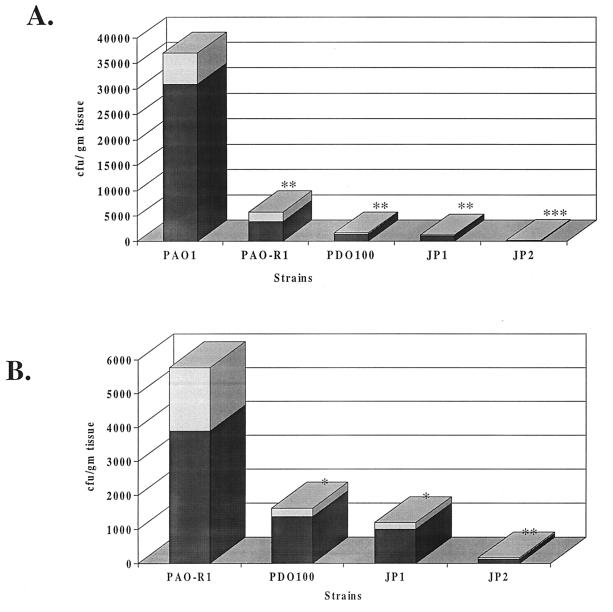
Quorum-sensing system mutations may affect the dissemination (the systemic spread) of P. aeruginosa within the bodies of burned mice. The systemic spread of PAO1 and the quorum-sensing mutants was examined by determining the numbers of CFU of these different strains within the livers and spleens of burned-and-infected mice. The livers and spleens of infected mice were harvested at 24 h post burn infection and homogenized. The homogenates were plated on LB agar plates to determine the number of CFU of each strain. As shown in Fig. 1A, at 24 h post burn infection, the numbers of microorganisms of the different quorum-sensing mutants (CFU per gram of tissue) that were recovered from the livers of infected mice were significantly (P < 0.01) lower than that of PAO1. Comparison of the quorum-sensing mutants with each other revealed that the numbers of microorganisms recovered from the livers of mice infected with JP2 were significantly (P < 0.01) lower than the number of microorganisms recovered from livers of mice infected with either JP1, PDO100, or PAO-R1 (Fig. 1B). Similar results were obtained from the spleens of infected mice (data not shown). Previous in vitro analysis has shown that the phenotypes of these mutants are stable (30). We have also confirmed the stability of these phenotypes in vivo. About 200 colonies of PAO-R1, PDO100, JP1, and JP2 that were obtained from the livers, spleens, and skin of infected mice were examined for antibiotic resistance and elastase production by using elastin plates. All tested colonies were carbenicillin resistant and elastase deficient (data not shown). These results suggest that a mutation in either one of the quorum-sensing systems interferes with the systemic spread of P. aeruginosa within the bodies of burned-and-infected mice.
FIG. 1.
Numbers of CFU of PAO1 and different quorum-sensing mutants obtained from the livers of burned-and-infected mice. Groups of mice were burned and then infected with each strain, and the numbers of CFU within the livers were determined as described in Materials and Methods. (A) Comparison of the numbers of CFU from the livers of PAO1-, PAO-R1-, PDO100-, JP1-, and JP2-infected mice. (B) Comparison of the numbers of CFU obtained from the livers of mice infected with quorum-sensing mutants (PAO-R1, PDO100, JP1, and JP2). Values represent the averages of three independent experiments ± the standard error of the mean (shaded areas). Symbols: ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
Horizontal spread of quorum-sensing mutants within burned skin.
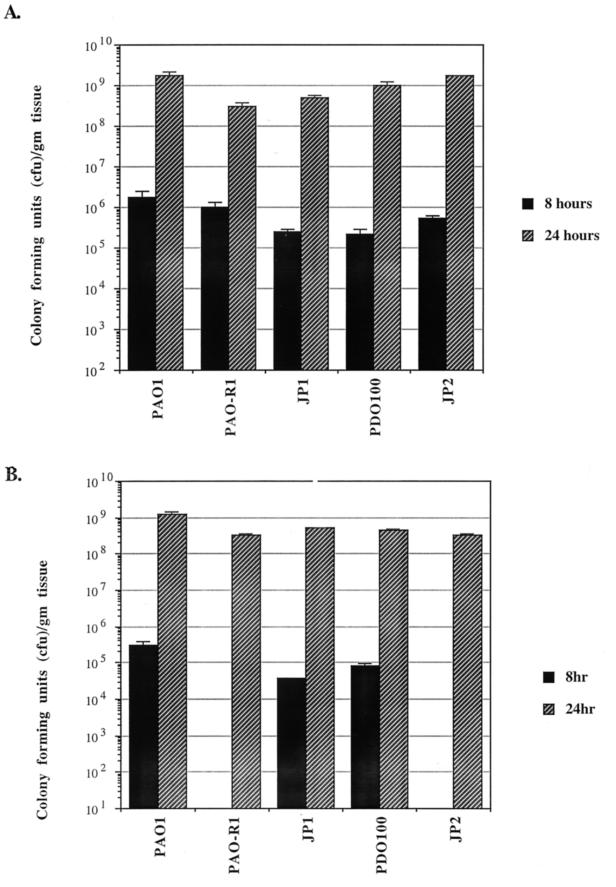
We have tried to determine if the defect in the in vivo virulence and the systemic spread of the different quorum-sensing mutants within the bodies of burned mice is due in part to the inability of these mutants to efficiently spread within burned skin. At 8 and 24 h post burn infection, mice were euthanized and two sections (about 5 by 5 mm each) of burned skin were removed from each animal. One section was obtained from the inoculation site, while the other was obtained at a site about 15 mm distant from the inoculation site (distant site). At 8 h postinfection, comparable numbers of microorganisms (CFU per gram of tissue) of PAO1, PDO100, and JP1 were obtained from both the inoculation and distant sites (Fig. 2A and B). In contrast, at 8 h postinfection, comparable numbers of microorganisms of PAO-R1 and JP2 were obtained from the inoculation site only and no microorganisms were obtained from the distant site (Fig. 2A and B). At 24 h postinfection, comparable numbers of all of the quorum-sensing mutant strains were obtained from both the inoculation and distant sites (Fig. 2A and B). These results suggest that PAO1 carrying a mutation(s) in either the lasR gene (PAO-R1) or the lasI and rhlI genes (JP2) is defective in horizontal spread within burned skin at early stages of infection (8 h postinfection).
FIG. 2.
Spreading of PAO1 and different quorum-sensing mutants within burned skin at 8 and 24 h post burn infection. A total of 2 × 102 to 3 × 102 CFU of each strain was injected subcutaneously at the burn site immediately after burning as described in Materials and Methods. At the specified time, the mice were euthanized, two separate skin sections were obtained, and the number of CFU in each section was determined. A section of the burned-and-infected skin (5 by 5 mm) was isolated at the site of inoculation and homogenized, and the number of CFU was determined. A similar section (5 by 5 mm) was obtained from the burned-and-infected skin at a distance of 15 mm from the inoculation site (distant site). (A) Number of CFU at the inoculation site. (B) Number of CFU at the distant site. Each value represents the average of three independent experiments ± the standard error of the mean.
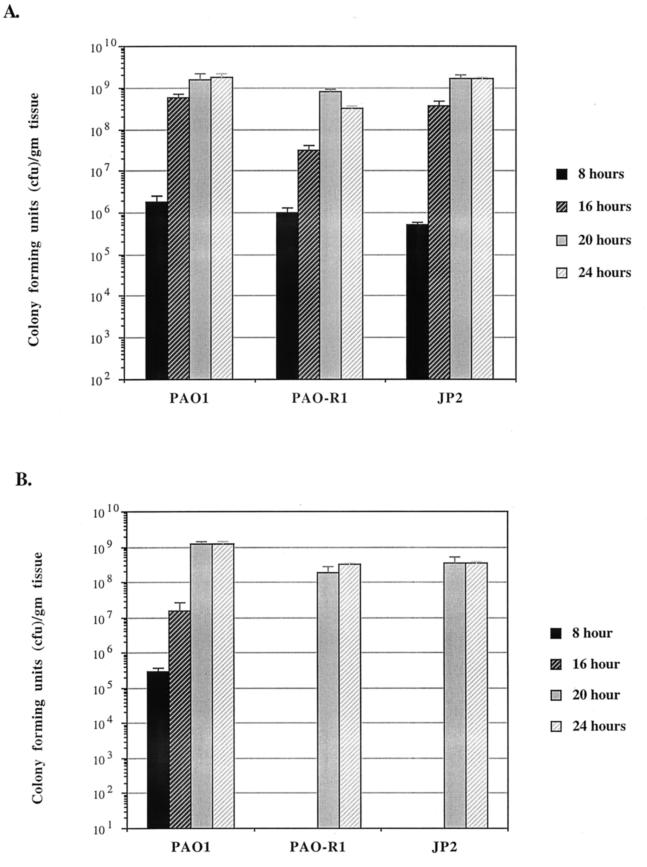
Although PAO-R1 and JP2 are defective in their spread within burned skin at 8 h postinfection, they spread efficiently by 24 h postinfection (Fig. 2A and B). Thus, we tried to determine the possible time (between 8 and 24 h) at which PAO-R1 and JP2 start to spread within burned skin. Groups of mice were infected post burn with either PAO1, PAO-R1, or JP2. Skin sections were obtained at 8, 16, 20, and 24 h postinfection, respectively. As shown in Fig. 3A and B, at 8, 16, 20, and 24 h postinfection, comparable numbers of CFU were obtained from the inoculation and distant sites of mice infected with PAO1. However, at 8 and 16 h postinfection, both PAO-R1 and JP2 were recovered from the inoculation site only (Fig. 3B). These results suggest that PAO-R1 and JP2 would not overcome the defect in spreading within the burned skin until after 20 h postinfection. None of the mutants appeared to be defective in spreading from burned skin to the tissues immediately underlying it (vertical spread). Besides the two skin sections described above, we have obtained sections of the connective tissues underneath the skin at the inoculation site only. The numbers of CFU of PAO1 and all tested mutants per gram of these connective tissue sections were comparable (data not shown).
FIG. 3.
Spreading of PAO1, PAO-R1, and JP2 within burned skin at 8, 16, 20, and 24 h post burn infection. At the specified time, the mice were euthanized and the numbers of CFU within the inoculation site and the distant site of the burned skin were determined as described in the legend to Fig. 2. (A) Numbers of CFU of PAO1, PAO-R1, and JP2 at the inoculation site. (B) Numbers of CFU of PAO1, PAO-R1, and JP2 at the distant site. Each value represents the average of three independent experiments ± the standard error of the mean.
Complementation of the defect of JP2 with plasmids that carry the lasI and rhlI genes.
The above-described results showed that the loss of either the lasR gene or both the lasI and rhlI genes contributed to the significantly reduced in vivo virulence, systemic spread, and local spread of PAO1 within burned skin. Thus, to confirm this possibility, we conducted several complementation experiments. Plasmids that carry lasI, rhlI, or both lasI and rhlI (pLASI-2, pJPP45, and pJPP42, respectively) were introduced into JP2. The ability of these plasmids to complement the defect of JP2 in elastase and rhamnolipid production was previously confirmed (30). A JP2 strain carrying each of the three plasmids was compared with PAO1 for in vivo virulence, systemic spread, and local spread within the skin of burned mice. JP2 carrying cloning vector pSW200 was used as a negative control. Prior to these in vivo experiments, the segregation of the plasmids from JP2 was determined). As shown in Table 3, the presence of a plasmid carrying either lasI or rhlI in JP2 increased its in vivo virulence. In comparison with that of the control strain (JP2/pSW200), the percent mortality of JP2/pLASI-2 was increased by 11-fold while that of JP2/pJPP45 was increased by 7-fold (Table 3). However, the most significant enhancement (P < 0.001) occurred when both genes were complemented (JP2/pJPP42). The percent mortality of JP2/pJPP42 was 14-fold higher than that of JP2/pSW200 (Table 3). Similarly, the presence of lasI, rhlI, or both significantly enhanced the systemic spread of JP2 within the burned mice. The numbers of microorganisms of JP2/pLASI-2, JP2/pJPP45, and JP2/pJPP42 obtained from the livers and spleens of infected mice were significantly higher (P < 0.01) than those of JP2/pSW200 (data not shown). Finally, the defect of JP2 in horizontal spreading within burned skin was complemented by lasI, rhlI, or both (Table 4). In contrast to mice infected with JP2/pSW200 (where the microorganisms were obtained from the inoculation site only), comparable numbers of microorganisms were obtained from both the inoculation and distant sites of burned skin of mice infected with JP2/LASI-2, JP2/pJPP45, and JP2/pJPP42 (Table 4). Individual colonies of JP2/pLASI-2, JP2/pJPP45, and JP2/pJPP42 that were obtained from livers, spleens, and skin of infected mice were tested and produced elastolytic activity, which indicates that the plasmids did not segregate from JP2 in vivo (data not shown). These results suggest that the defect in the virulence of JP2 is due to the loss of the PAI1 and PAI2 (the synthesis of which are directed by lasI and rhlI, respectively). We have tried to determine if plasmids carrying the lasR gene would complement the defect of PAO-R1 (specifically, the defect in its spreading within the skin). However, despite several attempts (including the utilization of two different lasR plasmids), both plasmids segregated from PAO-R1 at a high rate under in vitro and in vivo conditions (data not shown).
TABLE 3.
Effects of plasmids that carry the lasI or rhlI gene or both the lasI and rhlI genes on the virulence of P. aeruginosa JP2
| Strain | No. of mice dead/total (% mortality)a
|
Avg % mortalityb | ||
|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | ||
| PAO1 | 7/7 (100) | 5/6 (83.3) | 6/6 (100) | 94.3 |
| JP2/pSW200c | 0/5 (0) | 1/5 (20) | 0/5 (0) | 6.7 |
| JP2/pLASI-2c | 4/5 (80) | 3/5 (60) | 4/5 (80) | 73.3 |
| JP2/pJPP45c | 2/5 (40) | 3/5 (60) | 2/5 (40) | 46.7 |
| JP2/pJPP42c | 4/5 (80) | 5/5 (100) | 5/5 (100) | 93.3 |
A total of 2 × 102 to 3 × 102 CFU of each strain was injected subcutaneously at the burn site immediately after burning. The mice were burned and then inoculated with each strain as described in Materials and Methods. The mortality among mice infected with each strain was determined at 48 h post burn infection as previously described (34). Three separate experiments were conducted with each strain.
The average percent mortality of three independent experiments is shown.
Plasmid pSW200 is a cloning vector (negative control), plasmid pLASI-2 carries a DNA fragment containing the intact lasI gene, plasmid pJPP45 carries a DNA fragment containing the intact rhlI gene, and plasmid pJPP42 carries a DNA fragment containing the intact lasI and rhlI genes.
TABLE 4.
Effects of the lasI or rhlI gene or both the lasI and rhlI genes on the spread of P. aeruginosa quorum-sensing mutant JP2 within burned skin at 8 h post burn infection
| Strain | Mean no. of CFUa (± SEM) recovered from:
|
|
|---|---|---|
| Inoculation siteb | Distant siteb | |
| PAO1 | 1.8 × 106 ± 7.0 × 105 | 3.0 × 105 ± 7.6 × 104 |
| PAO-R1 | 1.0 × 106 ± 2.8 × 105 | 0.0 |
| JP2/pSW200c | 5.3 × 105 ± 7.2 × 104 | 0.0 |
| JP2/pLASI-2c | 8.3 × 105 ± 2.8 × 104 | 4.6 × 104 ± 1.4 × 103 |
| JP2/pJPP45c | 3.4 × 105 ± 8.5 × 104 | 5.4 × 103 ± 2.4 × 103 |
| JP2/pJPP42c | 9.2 × 105 ± 8.2 × 104 | 3.2 × 105 ± 5.2 × 104 |
A total of 2 × 102 to 3 × 102 CFU of each strain was injected subcutaneously at the burn site immediately after burning. The mice were burned and then inoculated with each strain as described in Materials and Methods. At 8 h post burn infection, the mice were euthanized and two separate skin sections were obtained. The number of CFU in each section was determined as described in Materials and Methods.
A section (5 by 5 mm) of burned-and-infected skin at the site of inoculation was isolated and homogenized, and the number of CFU was determined (the inoculation site). A similar section (5 by 5 mm) was obtained from the burned-and-infected skin at a distance of 15 mm from the inoculation site (distant site).
Plasmid pSW200 is a cloning vector (negative control), plasmid pLASI-2 carries a DNA fragment containing the intact lasI gene, plasmid pJPP45 carries a DNA fragment containing the intact rhlI gene, and plasmid pJPP42 carries a DNA fragment containing the intact lasI and rhlI genes.
DISCUSSION
In the present study, we have examined the role of quorum sensing in the pathogenesis of P. aeruginosa infection of burn wounds. Our results suggest that both quorum-sensing systems (las and rhl) contribute to the virulence of P. aeruginosa. In comparison with P. aeruginosa parent strain PAO1, lasI (JP1), and rhlI (PDO100) mutants showed a significant reduction in virulence (Table 2). In addition, both mutants were significantly less efficient than PAO1 in spreading within the bodies of burned-and-infected mice (Fig. 1A). Our results also suggest that both quorum-sensing systems are required for the optimum virulence of P. aeruginosa in the burn infection model. Although the in vivo virulence of JP1 and PDO100 is reduced, the most significant reduction in in vivo virulence was detected in JP2, in which both quorum-sensing systems are inactivated (Table 2). This defect of JP2 was partially complemented by plasmids that carry either lasI or rhlI (Tables 3 and 4) but completely complemented by a plasmid that carries both lasI and rhlI (Tables 3 and 4). Our results provide additional evidence supporting the hypothesis that the P. aeruginosa quorum-sensing systems function in a hierarchy and that the lasR gene plays an essential role in this hierarchical function (31). As we have previously shown (34), and confirmed in this study, the in vivo virulence of the lasR mutant (PAO-R1) is significantly lower than that of PAO1 (Table 2). In comparison with JP1 and PDO100, the in vivo virulence of PAO-R1 is lower (Table 2). In addition, similar to JP2, PAO-R1 was defective in spreading within burned skin by 8 and 16 h post burn infection (Fig. 3). Unfortunately, we were not able to provide a definite confirmation of these results by complementation experiments. In the absence of antibiotic selective pressure, plasmids carrying the lasR gene segregated from PAO-R1 at a high rate (data not shown).
Among the different mutants that carry deletions in quorum-sensing genes, the PAO-R1 mutant that carries a deletion in the lasR gene was analyzed by several previous studies (33, 34, 42). In these studies, the effect of a lasR mutation on the virulence of P. aeruginosa was examined by different animal models. Using the burned mouse model, we have previously shown that the in vivo virulence of PAO-RI and its ability to disseminate within the bodies of burned-and-infected mice were significantly lower that those of PAO1 (34). In addition, PAO-R1 was not able to spread within burned skin at 8 h post burn infection (34). In contrast to the results of the burned-mouse studies, experiments with the murine model of corneal keratitis indicated that lasR is not necessary for the establishment or maintenance of corneal infections (33). There was no significant difference between the 50% infective doses of PAO1 and PAO-R1 (33). In addition, the numbers of microorganisms that were recovered from the corneas that were infected with either PAO1 or PAO-R1 were similar (33). In the neonatal-mouse model of P. aeruginosa pneumonia, PAO-R1 was shown to be significantly less virulent than PAO1 (42). In comparison with PAO1, PAO-R1 produced no mortality in infected mice (42). In both the burned-mouse model and the neonatal-mouse model of P. aeruginosa pneumonia, PAO-R1 was able to establish an infection at the inoculation site (42; Fig. 2). At 24 h postinfection, PAO-R1 had successfully colonized the lung tissue (42). However, it did not replicate efficiently within the lung tissue, produced very little damage, and did not disseminate as well as PAO1 (42). In the burned-mouse model, PAO-R1 failed to spread within burned skin at 8 and 16 h but not at 20 or 24 h post burn infection (Fig. 3). Thus, as previously suggested (42), the contribution of the lasR gene to the pathogenesis of P. aeruginosa may depend on the type of infected tissue. The defect in the virulence of PAO-R1 (in the burned-mouse model at least) is likely to be due to the loss of the elastases (LasB and LasA), as well as other virulence factors that are controlled by the quorum-sensing systems. We have recently observed that the reduction in the in vivo virulence of PAO1 mutants defective in lasB, lasA, or lasA and lasB is not as significant as that of PAO-R1 (35). Other than PAO-R1, none of the lasB or lasA mutants was examined by the neonatal-mouse model of P. aeruginosa pneumonia.
It is clear from the present results that mutations in the quorum-sensing systems interfered with the ability of P. aeruginosa to cause general and local damage in the burn wound infection (Fig. 1 and 3; Table 2). One possible explanation for these findings is that the spread of P. aeruginosa within burned skin requires a low level of either elastase, rhamnolipid, or other factors that are controlled by either quorum-sensing system. These factors include pyocyanin, the stationary-phase sigma factor RpoS, and the P. aeruginosa secretion apparatus XCP, which are controlled by the rhl system (6, 18, 31), and exotoxin A and alkaline protease, which are controlled by the las system (13). Based on our recent analysis of elastase-deficient mutants, elastase is not likely to be the required factor. In comparison with PAO1, a lasB lasA double mutant spread just as efficiently within burned skin at 8 and 16 h post burn infection (35). The role of rhamnolipid (and possibly other factors) is supported by previous analysis of JP1, PDO100, and JP2 (30). In comparison with PAO1, JP1 and PDO100 produced reduced levels of rhamnolipid whereas JP2 produced none (30). In addition, and similar to the present complementation in the spread of JP2 within burned skin, rhamnolipid production by JP2 was partially complemented by either a lasI or a rhlI plasmid and completely complemented by a plasmid that carries both genes (30). Thus, it is possible that the production of a sufficient amount of an rhl-controlled factor by JP1 and JP2/pJPP42/(rhlI+) facilitates their horizontal spread within the skin. The spread of PDO100 and JP2/pLASI-2/(lasI+) may be explained by the ability of lasI to provide some activation for the rhl system, bypassing the need for rhlI. Pearson et al. (30) have previously suggested that besides its involvement in the synthesis of the PAI1, LasI may synthesize small amounts of other autoinducers (such as VAI-1 [8]) that may activate the rhl system, thus partially complementing the defect of JP2. Similarly, rhlI may activate the las system and enhance the production of exotoxin A or alkaline protease, which might be needed for the spread of JP2 within burned skin. The failure of PAO-R1 to efficiently spread within burned skin is probably due to the lack of activation of the las or rhl system (18, 31, 32). Currently, we are conducting experiments to determine the spread of specific toxA, apr, rhlA, and rpoS mutants within burned skin.
Another possible explanation for our results is that, besides their function in the activation of the quorum-sensing systems, the P. aeruginosa autoinducers themselves may function as virulence factors and facilitate the spread of JP2 within burned skin. A recent study has suggested that PAI1 may play a direct role in P. aeruginosa virulence by modulating the host inflammatory response (10). However, further experiments, including the inoculation of either PAI1 or PAI2 (alone or in conjunction with JP2) in the burned-mouse model, is necessary to determine if the autoinducers function as virulence factors.
The observed defect in the spreading of JP2 and PAO-R1 within burned skin is temporary (observed only until 20 h post burn infection). By 24 h post burn infection, JP2 and PAO-R1 spread efficiently (Fig. 2 and 3). The reason for their spreading at 24 h post burn infection is not known. It is unlikely that the observed spreading is due to the selection for suppressor mutations within PAO-R1 or JP2. Van Delden et al. (45) have recently shown that starvation of PAO-R1 (by using casein as the sole carbon and nitrogen source in the growth medium) resulted in the selection of rare suppressor mutations. At late stationary phase, the elastolytic activity produced by these suppressor mutants was about 30% of that of PAO1 (45). However, when the same approach was used with JP2, no suppressor mutants were obtained (45). The conditions within burned skin are less likely to induce the selection for PAO-R1 suppressor mutants (burned tissues may provide sufficient nutrient for PAO-R1 that can be used as carbon and nitrogen sources). In addition, we have excluded the possibility that PAO-R1 and JP2 that were recovered from the distant site at 24 h post burn infection represent possible suppressor mutants. Several colonies of PAO-R1 and JP2 that were obtained from the distant site were screened on elastin plates, and all were elastase deficient, even after 72 h of incubation (data not shown).
The defect in the spreading of P. aeruginosa within burned skin may contribute (directly or indirectly) to the observed general defect. The simplest explanation for this contribution is that a reduction in the horizontal spreading of P. aeruginosa within burned skin may cause a concomitant reduction in the numbers of the microorganisms that spread vertically (through the connective and lymphoid tissues underneath the burned skin). This may lead to a reduction in the number of microorganisms that are disseminated within the bodies of burned mice and an eventual reduction in in vivo virulence. Such a scenario may be plausible with JP2 but not with PAO-R1. In comparison with the other two quorum-sensing mutants (JP1 and PDO100), the systemic spreading, as well as the in vivo virulence, of JP2 is significantly reduced (Table 2 and Fig. 1). However, the systemic spreading of PAO-R1 is more efficient than that of JP1 and PDO100 (Fig. 1B). In addition, the in vivo virulence of PAO-R1 is not as significantly low as that of JP2 (while the percent mortality of infected mice is 28.3% with PAO-R1, it is 6.7% with JP2) (Table 2). The reasons for the differences between PAO-R1 and JP2 are not known. Analysis of additional quorum-sensing mutants may clarify these differences.
ACKNOWLEDGMENTS
This work was supported by grant 010674-037 from the Texas Higher Education coordinating Board to A. N. Hamood and Public Health Service grant AI 33713 to B. H. Iglewski. Kendra Rumbaugh is supported by grant P200A80102 from the U.S. Department of Education (GAANN Fellowship Program).
REFERENCES
- 1.Ausubel F, Brent R, Kingston R, Moor D, Seidman J, Smith J, Stauhle K. Current protocols in molecular biology. New York, N.Y: Wiley Intersciences; 1988. [Google Scholar]
- 2.Bergen G A, Shelhamer J H. Pulmonary infiltrates in the cancer patient. New approaches to an old problem. Infect Dis Clin N Am. 1996;10:297–325. doi: 10.1016/s0891-5520(05)70300-7. [DOI] [PubMed] [Google Scholar]
- 3.Berka R M, Gray G L, Vasil M L. Studies of phospholipase C (heat-labile hemolysin) in Pseudomonas aeruginosa. Infect Immun. 1981;34:1071–1074. doi: 10.1128/iai.34.3.1071-1074.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodey G P, Bolivar R, Fainstein V, Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983;5:279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- 5.Brint J M, Ohman D E. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J Bacteriol. 1995;177:7155–7163. doi: 10.1128/jb.177.24.7155-7163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapon-Herve V, Akrim M, Latifi A, Williams P, Lazdunski A, Bally M. Regulation of the xcp secretion pathway by multiple quorum-sensing modulons in Pseudomonas aeruginosa. Mol Microbiol. 1997;24:1169–1178. doi: 10.1046/j.1365-2958.1997.4271794.x. [DOI] [PubMed] [Google Scholar]
- 7.Doring G. Pseudomonas aeruginosa infection in cystic fibrosis patients. In: Campa M, Bendinelli M, Friedman H, editors. Pseudomonas aeruginosa as an opportunistic pathogen. New York, N.Y: Plenum Press; 1993. pp. 245–273. [Google Scholar]
- 8.Eberhard A, Burlingame A L, Eberhard C, Kenyon G L, Nealson K H, Oppenheimer J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981;20:2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- 9.Fichtenbaum C J, Woeltje K F, Powderly W G. Serious Pseudomonas aeruginosa infections in patients infected with human immunodeficiency virus: a case-control study. Clin Infect Dis. 1994;19:417–422. doi: 10.1093/clinids/19.3.417. [DOI] [PubMed] [Google Scholar]
- 10.Finch R G, Pritchard D I, Bycroft B W, Williams P, Gordon S, Stewart A B. Quorum sensing: a novel target for anti-infective therapy. J Antimicrob Chemother. 1998;42:569–571. doi: 10.1093/jac/42.5.569. [DOI] [PubMed] [Google Scholar]
- 11.Fuqua W C, Winans S C, Greenberg E P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gambello M J, Iglewski B H. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol. 1991;173:3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gambello M J, Kaye S, Iglewski B H. LasR of Pseudomonas aeruginosa is a transcriptional activator of the alkaline protease gene (apr) and an enhancer of exotoxin A expression. Infect Immun. 1993;61:1180–1184. doi: 10.1128/iai.61.4.1180-1184.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilligan P H. Microbiology of airway disease in patients with cystic fibrosis. Clin Microbiol Rev. 1991;4:35–51. doi: 10.1128/cmr.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holder I A. Pseudomonas aeruginosa burn infections: pathogenesis and treatment. In: Campa M, Bendinelli M, Friedman H, editors. Pseudomonas aeruginosa as an opportunistic pathogen. New York, N.Y: Plenum Press; 1993. pp. 275–295. [Google Scholar]
- 16.Holloway B W, Krishnapillai V, Morgan A F. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979;43:73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Homma J Y. Roles of exoenzymes and exotoxin in the pathogenicity of Pseudomonas aeruginosa and the development of a new vaccine. Jpn J Exp Med. 1980;50:149–165. [PubMed] [Google Scholar]
- 18.Latifi A, Foglino M, Tanaka K, Williams P, Lazdunski A. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhlR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol Microbiol. 1996;21:1137–1146. doi: 10.1046/j.1365-2958.1996.00063.x. [DOI] [PubMed] [Google Scholar]
- 19.Mahenthiralingam E, Campbell M E, Speert D P. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect Immun. 1994;62:596–605. doi: 10.1128/iai.62.2.596-605.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.May T B, Chakrabarty A M. Pseudomonas aeruginosa: genes and enzymes of alginate synthesis. Trends Microbiol. 1994;2:151–157. doi: 10.1016/0966-842x(94)90664-5. [DOI] [PubMed] [Google Scholar]
- 21.McManus A T, Mason A D, Jr, McManus W F, Pruitt B A., Jr Twenty-five year review of Pseudomonas aeruginosa bacteremia in a burn center. Eur J Clin Microbiol. 1985;4:219–223. doi: 10.1007/BF02013601. [DOI] [PubMed] [Google Scholar]
- 22.Nicas T I, Iglewski B H. The contribution of exoproducts to virulence of Pseudomonas aeruginosa. Can J Microbiol. 1985;31:387–392. doi: 10.1139/m85-074. [DOI] [PubMed] [Google Scholar]
- 23.Ochsner U A, Koch A K, Fiechter A, Reiser J. Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J Bacteriol. 1994;176:2044–2054. doi: 10.1128/jb.176.7.2044-2054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochsner U A, Reiser J. Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:6424–6428. doi: 10.1073/pnas.92.14.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohman D E, Burns R P, Iglewski B H. Corneal infections in mice with toxin A and elastase mutants of Pseudomonas aeruginosa. J Infect Dis. 1980;142:547–555. doi: 10.1093/infdis/142.4.547. [DOI] [PubMed] [Google Scholar]
- 26.Passador L, Cook J M, Gambello M J, Rust L, Iglewski B H. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science. 1993;260:1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- 27.Pavlovskis O R, Wretlind B. Assessment of protease (elastase) as a Pseudomonas aeruginosa virulence factor in experimental mouse burn infection. Infect Immun. 1979;24:181–187. doi: 10.1128/iai.24.1.181-187.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearson J P, Gray K M, Passador L, Tucker K D, Eberhard A, Iglewski B H, Greenberg E P. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci USA. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson J P, Passador L, Iglewski B H, Greenberg E P. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearson J P, Pesci E C, Iglewski B H. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pesci E C, Iglewski B H. The chain of command in Pseudomonas quorum sensing. Trends Microbiol. 1997;5:132–134. doi: 10.1016/S0966-842X(97)01008-1. . (Discussion, 5:134–135.) [DOI] [PubMed] [Google Scholar]
- 32.Pesci E C, Pearson J P, Seed P C, Iglewski B H. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 1997;179:3127–3132. doi: 10.1128/jb.179.10.3127-3132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preston M J, Seed P C, Toder D S, Iglewski B H, Ohman D E, Gustin J K, Goldberg J B, Pier G B. Contribution of proteases and LasR to the virulence of Pseudomonas aeruginosa during corneal infections. Infect Immun. 1997;65:3086–3090. doi: 10.1128/iai.65.8.3086-3090.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rumbaugh K P, Griswold J A, Hamood A N. Contribution of the regulatory gene lasR to the pathogenesis of Pseudomonas aeruginosa infection of burned mice. J Burn Care Rehabil. 1999;20:42–49. doi: 10.1097/00004630-199901001-00008. [DOI] [PubMed] [Google Scholar]
- 35.Rumbaugh, K. P., J. A. Griswold, and A. N. Hamood. Unpublished data.
- 36.Saelinger C B, Snell K, Holder I A. Experimental studies on the pathogenesis of infections due to Pseudomonas aeruginosa: direct evidence for toxin production during Pseudomonas infection of burned skin tissues. J Infect Dis. 1977;136:555–561. doi: 10.1093/infdis/136.4.555. [DOI] [PubMed] [Google Scholar]
- 37.Salmond G P, Bycroft B W, Stewart G S, Williams P. The bacterial ‘enigma’: cracking the code of cell-cell communication. Mol Microbiol. 1995;16:615–624. doi: 10.1111/j.1365-2958.1995.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 38.Simpson D A, Ramphal R, Lory S. Genetic analysis of Pseudomonas aeruginosa adherence: distinct genetic loci control attachment to epithelial cells and mucins. Infect Immun. 1992;60:3771–3779. doi: 10.1128/iai.60.9.3771-3779.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith A W, Iglewski B H. Transformation of Pseudomonas aeruginosa by electroporation. Nucleic Acids Res. 1989;17:10509. doi: 10.1093/nar/17.24.10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steel R G, Torrie J H. Principles and procedures of statistics: a biometrical approach, second edition. New York, N.Y: McGraw-Hill, Inc.; 1980. [Google Scholar]
- 41.Stieritz D D, Holder I A. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. J Infect Dis. 1975;131:688–691. doi: 10.1093/infdis/131.6.688. [DOI] [PubMed] [Google Scholar]
- 42.Tang H B, DiMango E, Bryan R, Gambello M, Iglewski B H, Goldberg J B, Prince A. Contribution of specific Pseudomonas aeruginosa virulence factors to pathogenesis of pneumonia in a neonatal mouse model of infection. Infect Immun. 1996;64:37–43. doi: 10.1128/iai.64.1.37-43.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toder D S, Gambello M J, Iglewski B H. Pseudomonas aeruginosa LasA: a second elastase under the transcriptional control of lasR. Mol Microbiol. 1991;5:2003–2010. doi: 10.1111/j.1365-2958.1991.tb00822.x. [DOI] [PubMed] [Google Scholar]
- 44.Van Delden C, Iglewski B H. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis. 1998;4:551–560. doi: 10.3201/eid0404.980405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Delden C, Pesci E C, Pearson J P, Iglewski B H. Starvation selection restores elastase and rhamnolipid production in a Pseudomonas aeruginosa quorum-sensing mutant. Infect Immun. 1998;66:4499–4502. doi: 10.1128/iai.66.9.4499-4502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woods D E, Iglewski B H. Toxins of Pseudomonas aeruginosa: new perspectives. Rev Infect Dis. 1983;5(Suppl. 4):S715–S722. doi: 10.1093/clinids/5.supplement_4.s715. [DOI] [PubMed] [Google Scholar]
- 47.Woods D E, Sokol P A, Bryan L E, Storey D G, Mattingly S J, Vogel H J, Ceri H. In vivo regulation of virulence in Pseudomonas aeruginosa associated with genetic rearrangement. J Infect Dis. 1991;163:143–149. doi: 10.1093/infdis/163.1.143. [DOI] [PubMed] [Google Scholar]