Objective
Abdominal involvement of COVID-19 is a current issue. We aimed to evaluate hepatic and pancreatic density alterations on computed tomography (CT) and to analyze whether these alterations had a relationship with chest CT score and laboratory findings.
Methods
Patients with reverse transcription-polymerase chain reaction-confirmed COVID-19 from March 11, 2020, to February 6, 2021, were retrospectively analyzed. Patients were divided into nonprogressive and progressive groups according to their chest CT scores. Liver and pancreas density, and liver-to-spleen (L/S) ratio were calculated. Laboratory findings, medication, intensive care unit stay, and survival were noted.
Results
There were 51 patients in the nonprogressive group and 123 patients in the progressive group. The median (minimum to maximum) L/S value of the nonprogressive group was 1 (0.28–1.53) at admission and 1.06 (0.33–1.83) at follow-up (P < 0.001). In the progressive group, the median L/S value was 1.08 (0.35–1.51) at admission and 0.92 (0.33–1.75) at follow-up (P < 0.001). A significant difference was found between the 2 groups at admission and follow-up (P = 0.010 and P < 0.001, respectively). Pancreatic density measured at follow-up was significantly lower in the progressive group (P = 0.045). In the progressive group, aspartate aminotransferase, total bilirubin, creatinine, urea, C-reactive protein, D-dimer, and white blood cell values were higher; albumin and lymphocyte values were lower (P < 0.05).
Conclusions
Patients with COVID-19 with progressive CT scores may have a decrease in L/S values, and their pancreatic density is lower than nonprogressives. Aspartate aminotransferase, total bilirubin, creatinine, urea, C-reactive protein, D-dimer, and white blood cell values tend to be higher in patients with a high chest CT score.
Key Words: computed tomography, coronavirus, density, liver, pancreas
Severe acute respiratory syndrome coronavirus-2–induced coronavirus disease has caused a pandemic that has continued since the end of 2019 and caused significant health and economic problems. The clinical spectrum of this disease, named coronavirus disease 2019 (COVID-19), ranges from asymptomatic patients to severe viral pneumonia, acute respiratory distress syndrome and also death.1 Besides the respiratory system, COVID-19 affects other body parts, such as hepatobiliary, gastrointestinal, central nervous, cardiovascular, and urogenital systems.2–6
The reverse transcription-polymerase chain reaction (RT-PCR) test is the reference method for the diagnosis of the disease.7 However, the presence of false-negative results in clinical use necessitated the use of some auxiliary modalities in the diagnosis. The most prominent of these is chest computed tomography (CT), because it is widely available in emergency departments and provides fast results.8 In this pandemic, chest CT became an important diagnostic tool and was even used in the triage of patients.7,9–11 Moreover, the severity of COVID-19 pulmonary involvement was graded by various methods using chest CT.8,12–14
Multiorgan involvement is the most important factor affecting mortality and morbidity in COVID-19. Apart from the respiratory system, liver and kidney involvement also have an important place in the course of the disease.15–18 Various degrees of liver function test abnormalities were detected in 16% to 53% of patients with COVID-19.12,19,20 In a few studies, it has been reported that there is a decrease in liver density on CT, especially in severe cases.4,21 Diseases, such as acute hepatitis, cirrhosis, and hepatotoxicity, as well as hepatic steatosis, can alter the liver parenchyma density.22 Most chest CTs include upper abdominal organs, such as liver, spleen, and pancreas. Density alterations of the liver and pancreas appear useful to assess their involvement.
Although respiratory system findings have been defined comprehensively in the literature, abdominal involvement, especially liver and pancreas, is still a popular topic of research. Early detection of these involvements and knowing their results provide important advantages in both treatment and follow-up. In our study, we aimed to determine whether there was a relationship between progressive and nonprogressive chest CT scores, liver and pancreatic density alterations, and laboratory findings of patients with COVID-19.
MATERIALS AND METHODS
Study Design
This study was approved by the institutional review board (protocol number: 2021/08-33), and the requirement for informed consent was waived. We retrospectively evaluated patients with RT-PCR confirmed COVID-19 between March 11, 2020, and February 6, 2021. Adult patients (aged ≥18 years) who had both 2 unenhanced chest CTs and laboratory examinations at admission and follow-up were included. The exclusion criteria were as follows: patients (a) who received antiviral treatment before admission, (b) without CT images of the upper abdomen, (c) with motion or respiratory artifacts on CT images, (d) with receiving chemotherapy, (e) with underlying liver and pancreas diseases.
Laboratory findings on the days of the first and second CTs were recorded. We obtained patients' laboratory values, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, gamma-glutamyl transferase (GGT), total bilirubin (TB), amylase, lipase, albumin, urea, creatinine, C-reactive protein (CRP), ferritin, D-dimer, white blood cells (WBCs), neutrophils, monocytes, lymphocytes, platelets, and hemoglobin (Hb). Medication, history of intensive care unit (ICU) stays, and exitus of the patients were recorded.
CT Protocol
All CT scans were performed using a 128-slice CT scanner (Ingenuity Core 128; Philips Medical Systems, Best, The Netherlands). The axial images included body part extending craniocaudally from the thoracic inlet to the midsection of the kidneys. Computed tomography images were obtained using 100 kVp, 100 to 170 mA (with automatic tube current modulation), 2 × 2 mm collimation, 0.5-mm section thickness, and without the application of contrast material.
Image Assessment
The CT images were evaluated in consensus by 2 radiologists (G.K. and Y.G., with 8 and 12 years of experience, respectively). Lung parenchyma was evaluated for ground-glass opacities, consolidation, crazy paving pattern and pleural effusion. The CT scores were assigned for the first scan that was obtained at admission and follow-up CT scan of each patient to evaluate the extent of the lung involvement. Each lung lobe was scored from 0 to 5 as no involvement, less than 5% involvement, 5% to 25% involvement, 26% to 49% involvement, 50% to 75% involvement, and greater than 75% involvement, respectively. The total chest CT score was from 0 to 25.23–25 Patients were divided into 2 groups according to their chest CT scores: the nonprogressive group (patients with lower or same scores on the second CT) and the progressive group (patients with higher scores on the second CT).
The upper abdominal parts that were included on the chest CT scans were evaluated, and the liver-to-spleen ratio (L/S) was calculated. Liver density values were calculated by placing regions of interest (ROIs) greater than 100 mm2 in size in all segments of the liver, avoiding the large vessels and any focal lesions. Splenic density was obtained by placing 1 ROI larger than 100 mm2 in size (Fig. 1). The L/S ratio was calculated by averaging the Hounsfield unit (HU) measurements of all liver segments and dividing by the spleen HU value.22,26 The spleen is a suitable organ where liver density can be normalized.27
FIGURE 1.
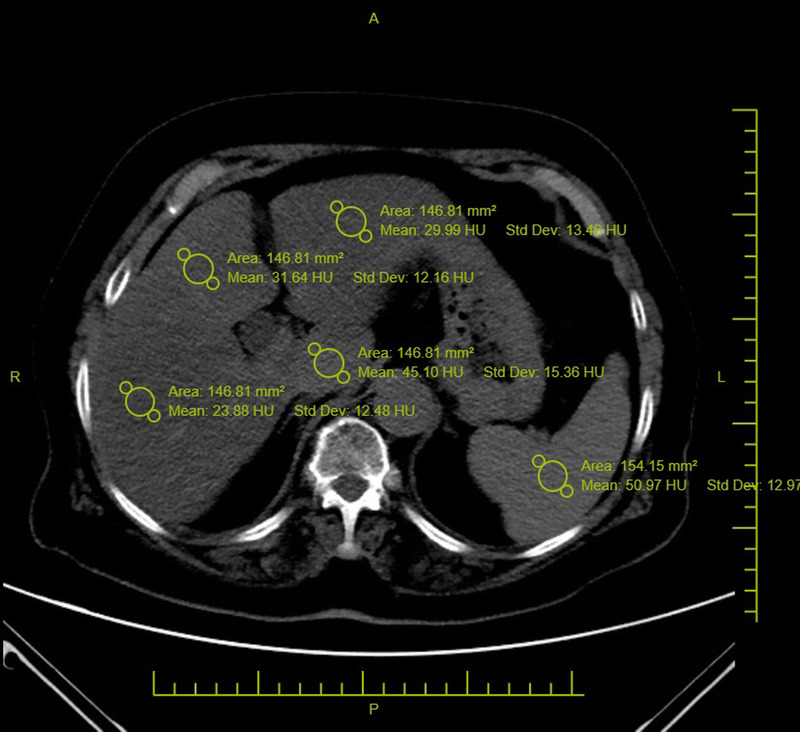
Hepatic density values were measured by placing ROIs greater than 100 mm2 in size on all liver segments. These values were then averaged (L). Splenic density values were obtained by placing an ROI greater than 100 mm2 in size (S). The liver-to-spleen ratio was calculated as L/S. Figure 1 can be viewed online in color at www.jcat.org.
Pancreas HU values were obtained by placing an average of 50 mm2 ROIs on the pancreatic head, corpus, and tail on noncontrast CT images of each patient. Pancreatic density was determined by taking the average value of the 3 ROIs. Care was taken not to include peripancreatic tissue in the ROIs, and large visible vascular structures were avoided wherever possible (Fig. 2).
FIGURE 2.
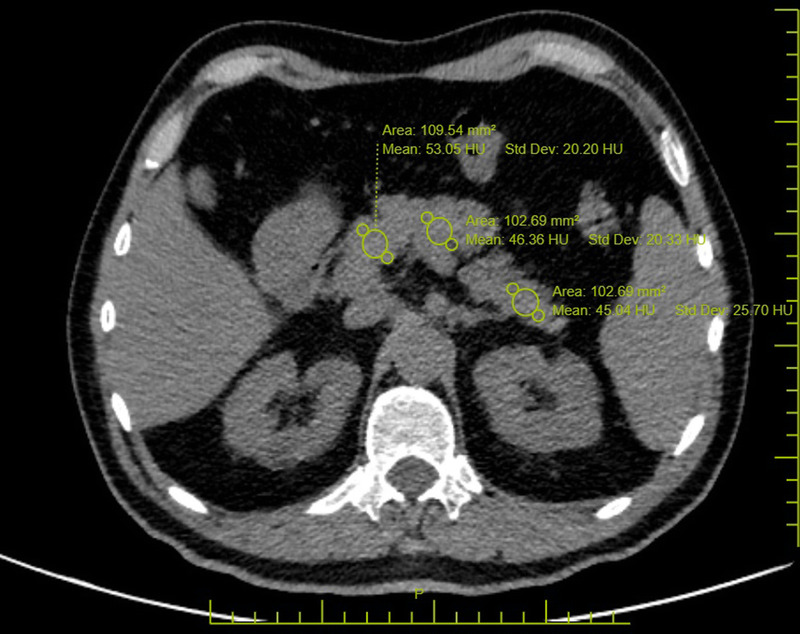
Pancreatic attenuation values were obtained by placing an average of 50 mm2 ROIs on the pancreatic head, corpus, and tail. These values were then averaged. Figure 2 can be viewed online in color at www.jcat.org.
Statistical Analysis
The IBM SPSS Statistics for Windows, Version 25.0 software was used in the statistical analysis of the study. The conformity of the quantitative data to normal distribution was evaluated using the Shapiro-Wilk test. Descriptive statistics of quantitative variables that did not fit normal distribution were given as median (minimum to maximum [min-max]), and descriptive statistics of categorical variables were given as number and percentage (n; %). The comparison of quantitative variables between 2 independent groups was performed using the Mann-Whitney U test, before/after comparisons of dependent groups were made using Wilcoxon's signed rank test, comparisons between 2 groups for categorical variables were made using Pearson χ2 test, and comparisons of categorical variables of dependent groups were made using McNemar χ2 test. Spearman correlation coefficient (r) was used to determine the relationship between 2 quantitative variables. The significance level was taken as 0.05.
RESULTS
Patient Characteristics
There were 2073 patients with positive RT-PCR tests in the study period. Of these patients, 174 who met the study criteria were included. There were 51 patients (28 men, 23 women; median age, 68 years; range, 28–92 years) in the nonprogressive group and 123 patients (78 men, 45 women; median age, 68 years; range, 34–91 years) in the progressive group. No significant differences in age and sex were found between the groups (Table 1).
TABLE 1.
Demographic, Comorbidity, Medication, Hospitalization, and Mortality Data of the Study Patients
| Nonprogressive (n = 51) | Progressive (n = 123) | P | |
|---|---|---|---|
| Age, median (min-max), y | 68 (28–92) | 68 (34–91) | 0.899 |
| Sex, n (%) | |||
| Male | 28 (54.9%) | 78 (63.4%) | 0.295 |
| Female | 23 (45.1%) | 45 (36.6%) | 0.310 |
| Comorbidities, n (%) | |||
| Hypertension | 26 (51%) | 47 (38.2%) | 0.132 |
| Diabetes mellitus | 14 (27.5%) | 42 (34.1%) | 0.477 |
| COPD/asthma | 11 (21.6%) | 25 (20.3%) | 0.840 |
| Coronary artery disease | 12 (23.5%) | 24 (20.3%) | 0.544 |
| Previous history of malignancy | 1 (2%) | 2 (1.6%) | 0.877 |
| Medication, n (%) | |||
| Favipiravir | 50 (98%) | 118 (95.9%) | 0.489 |
| Hydroxychloroquine | 11 (21.6%) | 37 (30.1%) | 0.253 |
| Methylprednisolone | 13 (25.5%) | 81 (65.9%) | <0.001 |
| Tocilizumab | 0 | 6 (4.9%) | |
| Antibiotics | 51 (100%) | 123 (100%) | |
| Low-molecular weight heparin | 51 (100%) | 123 (100%) | |
| ICU, n (%) | 2 (3.9%) | 123 (100%) | <0.001 |
| Dead, n (%) | 4 (7.8%) | 71 (57.7%) | <0.001 |
COPD indicates chronic obstructive pulmonary disease.
The median time between consecutive CT scans was 10 days (range, 2–51 days) in the nonprogressive group and 6 days (range, 1–39 days) in the progressive group (P = 0.013). All patients with high chest CT scores stayed in the ICU, whereas only 2 (3.9%) patients with low or stable chest CT scores required ICU treatment. Four (7.8%) patients died in the nonprogressive group, and 71 (57.7%) patients died in the progressive group. There was a significant difference in mortality rates between the 2 groups (P < 0.001).
Laboratory and Medication
In the nonprogressive group, there was a downward trend of the values of AST, albumin, creatinine, CRP, and Hb; and there was an upward trend of the values of GGT, TB, WBC, neutrophil, monocyte, lymphocyte, and platelet (P < 0.05). In the progressive group, there was a downward trend of the values of albumin, lymphocyte, and Hb; and there was an upward trend of the values of AST, ALT, GGT, TB, urea, CRP, ferritin, D-dimer, WBC, neutrophil, and platelet (P < 0.05) (Table 2).
TABLE 2.
Comparison of Laboratory Data as Median (Min-Max) Within and Between Groups
| Nonprog. | Progressive | Intergroup (P) | Intragroup (P) | |||||
|---|---|---|---|---|---|---|---|---|
| Adm. | Fol. | Adm. | Fol. | Adm. | Fol. | Nonprog. | Prog. | |
| AST (U/L) | 39 (15–177) | 29 (16–578) | 36 (11–218) | 43 (16–5909) | 0.183 | 0.001 | 0.003 | 0.001 |
| ALT (U/L) | 23 (7–135) | 29 (8–430) | 24 (1–227) | 29 (3–2149) | 0.659 | 0.786 | 0.302 | 0.003 |
| ALP (U/L) | 71 (37–333) | 71 (25–227) | 74 (31–240) | 73 (31–327) | 0.498 | 0.409 | 0.918 | 0.301 |
| GGT (U/L) | 37 (9–240) | 41 (11–475) | 34 (7–184) | 49 (8–396) | 0.785 | 0.118 | 0.015 | 0.001 |
| TB (mg/dL) | 0.44 (0.09–0.98) | 0.49 (0.13–2.19) | 0.49 (0.10–36.0) | 0.59 (0.12–2.15) | 0.079 | 0.056 | 0.003 | 0.001 |
| Amylase (U/L) | 62 (24–453) | 64 (17–245) | 62 (25–577) | 62 (17–459) | 0.636 | 0.805 | 0.104 | 0.373 |
| Lipase (U/L) | 31 (4–952) | 30 (4–232) | 28 (2–296) | 28 (2–208) | 0.440 | 0.654 | 0.279 | 0.424 |
| Albumin (gr/L) | 36 (27–46) | 34 (25–47) | 36 (24–48) | 30 (22–41) | 0.763 | 0.001 | 0.001 | 0.001 |
| Urea (mg/dL) | 34.9 (15.4–126.8) | 31.2 (12.2–214.9) | 42.1 (17.2–115.9) | 49.4 (0.94–280.1) | 0.023 | 0.001 | 0.764 | 0.001 |
| Creatinine (mg/dL) | 0.86 (0.47–2.76) | 0.78 (0.38–2.64) | 1 (0.24–4.5) | 1 (0.14–8.37) | 0.009 | 0.002 | 0.001 | 0.241 |
| CRP (mg/L) | 69.1 (3.17–234.0) | 18.7 (1.82–148.0) | 50.5 (1.29–476.0) | 129 (1.48–961.0) | 0.9 | 0.001 | 0.001 | 0.001 |
| Ferritin (μg/L) | 203 (12–1106) | 220 (15–1120) | 166 (9–1550) | 410 (21–1600) | 0.15 | 0.001 | 0.383 | 0.001 |
| D-dimer (μg/L) | 977 (155–23,100) | 808 (78–20,900) | 783 (0.59–20,600) | 1290 (1.28–100,000) | 0.129 | 0.026 | 0.701 | 0.001 |
| WBC (×103/μL) | 6.1 (1.8–25.7) | 7.9 (1.4–17.0) | 6.7 (2.3–96.7) | 10.2 (4.0–27.2) | 0.069 | 0.003 | 0.001 | 0.001 |
| Neutrophil (×109/L) | 4.39 (0.94–24.22) | 5.41 (0.75–14.28) | 4.83 (1.18–84.14) | 8.6 (2.41–25.21) | 0.085 | 0.001 | 0.005 | 0.001 |
| Monocyte (×109/L) | 0.46 (0.12–1.09) | 0.60 (0.10–2.52) | 0.56 (0.15–1.17) | 0.56 (0.04–10.39) | 0.034 | 0.211 | 0.001 | 0.792 |
| Lymphocyte (×109/L) | 1.01 (0.33–3.60) | 1.41 (0.29–4.00) | 1.03 (0.29–7.19) | 0.86 (0.20–13.34) | 0.338 | 0.001 | 0.022 | 0.001 |
| Platelet (×109/L) | 181 (88–631) | 251 (99–671) | 186 (68–543) | 223 (52–519) | 0.5 | 0.065 | 0.001 | 0.001 |
| Hb (g/dL) | 13 (9.4–17.7) | 13 (9.2–17.0) | 13.8 (7.4–17.8) | 13.3 (6.8–17.3) | 0.144 | 0.899 | 0.037 | 0.001 |
ALP indicates alkaline phosphatase; Adm., Admission; Fol., follow-up; Nonprog., nonprogressive; Prog., progressive.
Eleven (21.6%) patients in the nonprogressive group and 37 (30.1%) patients in the progressive group had hydroxychloroquine treatment. Fifty (98%) patients in the nonprogressive group received favipiravir treatment, and 118 (95.9%) patients in the progressive group were treated with favipiravir. Thirteen (25.5%) patients in the nonprogressive group and 81 (65.9%) patients in the progressive group received methylprednisolone. A significant difference in methylprednisolone treatment was found between the 2 groups (P < 0.001) (Table 1).
CT Findings
Although there was no significant difference in the prevalence of consolidation, crazy paving, and pleural effusion between the groups on CT at admission, there was a significant difference on CT at follow-up (P < 0.001, P = 0.003, and P = 0.009, respectively) (Table 3). Ground-glass opacities were observed in all patients in both groups at admission and follow-up. At the follow-up, there was an increase in consolidation, crazy paving, and pleural effusion in the progressive group (P < 0.001). In the nonprogressive group, the median (min-max) value of the admission chest CT score was 11 (3–2), and the median (min-max) value of the follow-up chest CT score was 10 (1–17) (P < 0.001). In the progressive group, the median (min-max) value of the admission chest CT score was 6 (1–18), and the median (min-max) value of the follow-up chest CT score was 15 (7–25) (P < 0.001) (Table 4).
TABLE 3.
Comparison of Lung Parenchyma Findings
| Consolidation | Crazy Paving | Pleural Effusion | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NP. | P. | P | NP. | P. | P | NP. | P. | P | |
| Adm. | n = 17, 33.3% | n = 43, 35% | 0.83 | n = 3, 5.9% | n = 7, 5.7% | 0.96 | n = 2, 3.9% | n = 6, 4.9% | 0.784 |
| Fol. | n = 11, 21.6% | n = 86, 69.9% | <0.001 | n = 2, 3.9% | n = 28, 22.8% | 0.003 | n = 2, 3.9% | n = 24, 19.5% | 0.009 |
| P | 0.146 | <0.001 | 0.999 | <0.001 | 0.999 | <0.001 | |||
Adm. indicates Admission; Fol., follow-up; NP., nonprogressive; P., progressive.
TABLE 4.
Intragroup and Intergroup Comparison of Chest CT Score, L/S, Liver and Pancreas Density Values (Median, Min-Max)
| Nonprogressive | Progressive | P | |
|---|---|---|---|
| CT score 1 | 11 (3–22) | 6 (1–18) | <0.001 |
| CT score 2 | 10 (1–17) | 15 (7–25) | <0.001 |
| P | <0.001 | <0.001 | |
| Liver HU 1 | 51.1 (13.1–73.8) | 52.6 (17.6–76.6) | 0.458 |
| Liver HU 2 | 56.8 (17.2–84.1) | 50.3 (16.3–81.8) | <0.001 |
| P | <0.001 | 0.052 | |
| L/S 1 | 1 (0.28–1.53) | 1.06 (0.33–1.83) | 0.010 |
| L/S 2 | 1.08 (0.35–1.51) | 0.92 (0.33–1.75) | <0.001 |
| P | <0.001 | <0.001 | |
| Pancreas HU 1 | 41 (16–72) | 38 (−97–63) | 0.144 |
| Pancreas HU 2 | 43.6 (11–76) | 39 (−65–69) | 0.045 |
| P | 0.151 | 0.06 |
A significant difference was observed in the L/S value on the CT scans taken at admission (L/S1) and follow-up (L/S2) between the groups (P = 0.010 and P < 0.001, respectively). The L/S2 value of the nonprogressive group was significantly higher; however, the L/S2 value of the progressive group was significantly lower than their L/S1 values (P < 0.001 for both) (Table 4).
Pancreatic density was measured lower in the progressive group compared with the nonprogressive group at admission, but it was not statistically significant (P = 0.144). Pancreatic density measured at follow-up was significantly lower in the progressive group (P = 0.045) (Table 4).
DISCUSSION
In this study, we analyzed if there was a correlation between liver and pancreas density, progression of chest CT scores, and laboratory findings of patients with COVID-19. The main findings enabled us to obtain some results in this disease, which has not been fully clarified. In the progressive group consisting of patients with an increased chest CT score, both liver HU, L/S, and pancreas HU values were significantly lower on follow-up CT. Among the laboratory findings, AST, TB, albumin, creatinine, CRP, D-dimer, WBC, and lymphocyte values did not differ significantly between the 2 groups at admission. However, AST, TB, creatinine, CRP, D-dimer, and WBC were found to be higher, and albumin and lymphocytes were found to be lower in the progressive group at the follow-up. Urea was higher in the progressive group both at admission and at follow-up.
It has been suggested that most patients with COVID-19 admitted to the hospital have liver blood test abnormalities, but the presence of clinically significant liver damage is not common.3,4,15 It was stated that aminotransferase and TB values were higher in patients with COVID-19 with a poor clinical course compared with other patients.16,28 However, despite these situations, the cause is uncertain. Although drug-related liver damage may occur, in a study, mild liver test abnormalities were found at the time of admission, even when significant medication was not administered.3,4,28–30 It has been suggested that elevated aminotransferase levels may also be the result of COVID-19–associated myositis.3 Liver damage may be due to COVID-19-related hepatitis, but this view is mostly not accepted.4 Postmortem liver biopsy examination of a patients with COVID-19 showed microvesicular steatosis.31 It may suggest that the virus directly causes hepatosteatosis or that secondary steatosis develops due to liver damage. Another route for liver damage is the development of hepatotoxicity with cytokines, immune mediators, and coagulation cascade.3,4 The presence of angiotensin-converting enzyme-2 receptors in the biliary system, which allows the virus to enter the cell, can cause direct liver damage of the virus.32 Regardless, only ICU stay also causes cholestasis and may cause an increase in liver function tests.33 The significant increase in ICU stay in progressive patients in our study may have contributed to this liver function test abnormality. Although Bhayana et al33 could not reach statistically significant results in their study, they found that patients with COVID-19 with ICU stay had a higher prevalence of fatty liver than those without. It has also been reported that hepatic steatosis may develop during the course of COVID-19. Although it is difficult to determine the underlying mechanism such as steatosis or hepatitis, our study showed alterations in hepatic density during the course of COVID-19.
There are some studies examining the relationship between COVID-19 severity and liver density and damage, and detecting a decrease in density and an increase in liver damage in severe/progressive disease.6,21,34–38 Guler et al21 found no significant difference in mean L/S values at admission to hospital, but the L/S value was significantly lower in the progressive group at follow-up. In group comparisons, there was a significant decrease in L/S values on the follow-up CT in the progressive group. Lei et al34 found liver hypodensity and pericholecystic fat stranding as the most common abnormal findings on the upper abdominal CT of patients with COVID-19 and also found liver hypodensity more frequently in critical patients. This supports the higher incidence of liver damage in the progressive patients in our study. Parlak et al35 detected a significantly lower liver density in patients with ICU stay, and the prevalence of severe disease was higher in patients with hepatic steatosis. In some other studies, nonalcoholic fatty liver disease is shown as an independent risk factor for COVID-19 severity.35,36,39–41 Uchida et al37 detected more liver injury in patients with severe COVID-19. In their studies, L/S and liver density were significantly lower in the severe group at admission, and a significant increase in liver density and L/S was found on CT performed in the remission phase compared with admission. From this result, it can be concluded that the decrease in liver density in patients with COVID-19 is temporary. However, more comprehensive studies and liver density follow-ups of the progressive group after discharge are required. If we evaluate these studies together with our results, it can be concluded that the decrease in liver density may be useful both in predicting the course of the disease and in showing liver damage in the course of the disease.
There were some studies on pancreatic damage of COVID-19, and it was thought that it could cause acute pancreatitis.42–44 Grusova et al45 demonstrated peripancreatic stranding or fluid collection was higher in patients with COVID-19. It is accepted that pancreatic damage is similar to liver damage mechanisms, but there is no definite finding.18,46,47 Pancreatic damage can be seen in up to 1–17% of patients with COVID-19 and is more common in severe infections.48 A decrease in parenchymal density is seen as a CT finding in pancreatic damage. This decrease may occur in acute pancreatitis as well as in processes such as steatosis. Most studies on COVID-19 in the literature are about amylase and lipase levels, but few have evaluated radiologic findings.45,47 Bozdag et al47 showed that pancreatic density was significantly lower in PCR-positive cases compared with negative cases on CTs taken with the suspicion of COVID-19. In our study, we focused only on pancreatic parenchymal density measurements without evaluating peripancreatic tissue changes, and we found that there was a significant decrease in density in the progressive group at follow-up. This finding radiologically proves the involvement of pancreatic parenchyma, especially in patients with a severe course. Also, elevated amylase levels were found to be significantly associated with the severity of COVID-19.18 However, in our study, we found no significant difference in amylase and lipase between the groups.
AST, ALT, TB, and albumin are important markers for the evaluation of liver injury.38 There are studies evaluating the relationship of these and other laboratory data with COVID-19 and disease severity. High levels of neutrophil, D-dimer, ALT, AST, TB, GGT, CRP, and low levels of lymphocytes, albumin, and platelets were associated with ICU stay, severe disease, and high chest CT scores.6,21,25,34,35,37,38 The decreased albumin level may be secondary to liver dysfunction or damage. Of course, many other causes of hypoalbuminemia and increased AST values can be mentioned. These findings suggest that the higher the chest CT score, the greater the risk of liver injury. Patients with COVID-19 with abnormal liver function tests have higher values of inflammatory markers such as CRP and procalcitonin.20 This may be associated with liver function test abnormality with cell destruction due to increased inflammation in the liver, but studies that include histopathologic evaluations are needed.
In previous studies, it has been reported that antibiotics, antivirals, steroids, favipiravir, lopinavir/ritonavir, hydroxychloroquine, remdesivir, tocilizumab, and other drugs used for the treatment of patients with COVID-19 may result in abnormal liver function tests.5,6,20,21,49 In our study, patients had a history of methylprednisolone, favipiravir, hydroxychloroquine, tocilizumab, several antibiotics, and low-molecular weight heparin medication. Among these, only methylprednisolone use was statistically significantly higher in the progressive group. The significant difference in the use of methylprednisolone may also have been effective in the reduction of liver HU and L/S values in the progressive group. However, the time interval between the 2 CT scans of patients in the progressive group was significantly less than in the nonprogressive group. It is unlikely that methylprednisolone alone would cause the liver density alteration as there are factors contributing to the hypodensity Nevertheless, for a definitive result on this subject, prospective controlled studies are needed.
Our study has several limitations. The first and most important of these is its retrospective design and incomplete documentation of patient history. Despite the exclusion of patients with liver and pancreatic diseases, there might have been underlying diseases whose history we did not know. Another limitation of our study is the inability to evaluate changes in the liver and pancreas on contrast-enhanced CT. The time interval between 2 consecutive CTs, as well as the inhomogeneity of drug types and doses in each patient and between groups, may have affected the results. As mentioned before, liver density can alter for many reasons. However, prospective controlled studies are needed to examine them independently from each other. Therefore, in our retrospective-based study, we mainly investigated the relationship between liver density alterations and chest CT score progression. Another limitation is the lack of histopathologic evaluations in evaluating liver and pancreatic damage and parenchymal changes.
In conclusion, a decrease in L/S may be observed in patients with COVID-19 with progressive chest CT scores. In addition, AST, TB, creatinine, urea, CRP, D-dimer, and WBC values tend to be higher in patients with high chest CT scores. In our study, we also found the pancreatic density of patients with COVID-19 with progressive chest CT scores was significantly lower than in nonprogressives. Decreased density in the liver and pancreas may be associated with the progression of the disease. It will be useful to carefully evaluate these data in patients with COVID-19, especially in patients with a progressive course.
Footnotes
ORCID ID: 0000-0001-9280-3254
ORCID ID: 0000-0002-8800-7973
ORCID ID: 0000-0002-4073-9665
ORCID ID: 0000-0002-6752-6838
ORCID ID: 0000-0001-9049-5446
The authors declare no conflict of interest.
Contributor Information
Yeliz Gul, Email: yeliz_gul78@hotmail.com.
Gulhan Kilicarslan, Email: dr.gulhankilicarslan@gmail.com.
Mehtap Balaban, Email: mehtapbalaban40@hotmail.com.
Evrim Gul, Email: egul@firat.edu.tr.
REFERENCES
- 1.Chen N, Zhou M, Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rismanbaf A, Zarei S. Liver and kidney injuries in COVID-19 and their effects on drug therapy; a letter to editor. Arch Acad Emerg Med. 2020;8:e17. [PMC free article] [PubMed] [Google Scholar]
- 3.Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu L Liu J Lu M, et al. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo H Zhang Z Zhang Y, et al. Analysis of liver injury factors in 332 patients with COVID-19 in Shanghai, China. Aging (Albany NY). 2020;12:18844–18852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ai T Yang Z Hou H, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32–E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwee TC, Kwee RM. Chest CT in COVID-19: what the radiologist needs to know. Radiographics. 2020;40:1848–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang Y Zhang H Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;296:E115–E117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie X Zhong Z Zhao W, et al. Chest CT for typical coronavirus disease 2019 (COVID-19) pneumonia: relationship to negative RT-PCR testing. Radiology. 2020;296:E41–E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am J Roentgenol. 2020;214:1280–1286. [DOI] [PubMed] [Google Scholar]
- 12.Shi H Han X Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y Dong C Hu Y, et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020;296:E55–E64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prokop M van Everdingen W van Rees Vellinga T, et al. CO-RADS: a categorical CT assessment scheme for patients suspected of having COVID-19-definition and evaluation. Radiology. 2020;296:E97–E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C Wang Y Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan WJ Ni ZY Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D Hu B Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bacaksiz F Ebik B Ekin N, et al. Pancreatic damage in COVID-19: why? How? Int J Clin Pract. 2021;75:e14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu XW Wu XX Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan Z Chen L Li J, et al. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guler E Unal NG Cinkooglu A, et al. Correlation of liver-to-spleen ratio, lung CT scores, clinical, and laboratory findings of COVID-19 patients with two consecutive CT scans. Abdom Radiol (NY). 2021;46:1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma X Holalkere NS Kambadakone RA, et al. Imaging-based quantification of hepatic fat: methods and clinical applications. Radiographics. 2009;29:1253–1277. [DOI] [PubMed] [Google Scholar]
- 23.Chang YC Yu CJ Chang SC, et al. Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT. Radiology. 2005;236:1067–1075. [DOI] [PubMed] [Google Scholar]
- 24.Pan F Ye T Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology. 2020;295:715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francone M Iafrate F Masci GM, et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020;30:6808–6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kodama Y Ng CS Wu TT, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188:1307–1312. [DOI] [PubMed] [Google Scholar]
- 27.Johnston RJ Stamm ER Lewin JM, et al. Diagnosis of fatty infiltration of the liver on contrast enhanced CT: limitations of liver-minus-spleen attenuation difference measurements. Abdom Imaging. 1998;23:409–415. [DOI] [PubMed] [Google Scholar]
- 28.Boeckmans J Rodrigues RM Demuyser T, et al. COVID-19 and drug-induced liver injury: a problem of plenty or a petty point? Arch Toxicol. 2020;94:1367–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falcao MB Pamplona de Goes Cavalcanti L Filgueiras Filho NM, et al. Case report: hepatotoxicity associated with the use of hydroxychloroquine in a patient with COVID-19. Am J Trop Med Hyg. 2020;102:1214–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai Q Yang M Liu D, et al. Experimental treatment with Favipiravir for COVID-19: an open-label control study. Engineering (Beijing). 2020;6:1192–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Z Shi L Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chai X Hu L Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020. doi: 10.1101/2020.02.03.931766. [DOI] [Google Scholar]
- 33.Bhayana R Som A Li MD, et al. Abdominal imaging findings in COVID-19: preliminary observations. Radiology. 2020;297:E207–E215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lei P Zhang L Han P, et al. Liver injury in patients with COVID-19: clinical profiles, CT findings, the correlation of the severity with liver injury. Hepatol Int. 2020;14:733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parlak S Çivgin E Beşler MS, et al. The effect of hepatic steatosis on COVID-19 severity: chest computed tomography findings. Saudi J Gastroenterol. 2021;27:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palomar-Lever A Barraza G Galicia-Alba J, et al. Hepatic steatosis as an independent risk factor for severe disease in patients with COVID-19: a computed tomography study. JGH Open. 2020;4:1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uchida Y Uemura H Yamaba S, et al. Significance of liver dysfunction associated with decreased hepatic CT attenuation values in Japanese patients with severe COVID-19. J Gastroenterol. 2020;55:1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao X Lei Z Gao F, et al. The impact of coronavirus disease 2019 (COVID-19) on liver injury in China: a systematic review and meta-analysis. Medicine (Baltimore). 2021;100:e24369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji D Qin E Xu J, et al. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. 2020;73:451–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai Q Huang D Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742–1752. [DOI] [PubMed] [Google Scholar]
- 41.Garrido I, Liberal R, Macedo G. Review article: COVID-19 and liver disease—what we know on 1st May 2020. Aliment Pharmacol Ther. 2020;52:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barlass U Wiliams B Dhana K, et al. Marked elevation of lipase in COVID-19 disease: a cohort study. Clin Transl Gastroenterol. 2020;11:e00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McNabb-Baltar J Jin DX Grover AS, et al. Lipase elevation in patients with COVID-19. Am J Gastroenterol. 2020;115:1286–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goyal H Sachdeva S Perisetti A, et al. Hyperlipasemia and potential pancreatic injury patterns in COVID-19: a marker of severity or innocent bystander? Gastroenterology. 2021;160:946–948.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grusova G Bruha R Bircakova B, et al. Pancreatic injury in patients with SARS-Cov-2 (COVID-19) infection: a retrospective analysis of CT findings. Gastroenterol Res Pract. 2021;2021:5390337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geravandi S Mahmoudi-Aznaveh A Azizi Z, et al. SARS-CoV-2 and pancreas: a potential pathological interaction? Trends Endocrinol Metab. 2021;32:842–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bozdag A Eroglu Y Sagmak Tartar A, et al. Pancreatic damage and radiological changes in patients with COVID-19. Cureus. 2021;13:e14992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang F Wang H Fan J, et al. Pancreatic injury patterns in patients with coronavirus disease 19 pneumonia. Gastroenterology. 2020;159:367–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hundt MA Deng Y Ciarleglio MM, et al. Abnormal liver tests in COVID-19: a retrospective observational cohort study of 1,827 patients in a major U.S. hospital network. Hepatology. 2020;72:1169–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]


