Abstract
Toxoplasma gondii remains a serious cause of morbidity and mortality in individuals that are immunosuppressed, patients with AIDS in particular. The cellular immune response, especially by gamma interferon (IFN-γ)-producing CD8+ T cells, is an essential component of protective immunity against the parasite. In the present study the role of CD8+ T cells during the reactivation of Toxoplasma infection in an immunocompromised murine model was evaluated. Chronically infected mice were challenged with LP-BM5 virus, and the kinetics of CD8+ T-cell function was studied. At 10 weeks after viral infection, mice showed obvious signs of systemic illness and began to die. At this stage, CD8+ T cells were unresponsive to antigenic stimulation and unable to kill Toxoplasma-infected targets. IFN-γ production by the CD8+ T cells from dual-infected animals reached background levels, and a dramatic fall in the frequency of precursor cytotoxic T lymphocytes was observed. Histopathological analysis of the tissues demonstrated signs of disseminated toxoplasmosis as a result of reactivation of infection. However, treatment of the dual-infected animals with immune CD8+ T cells at 5 weeks post-LP-BM5 challenge prevented the reactivation of toxoplasmosis, and mice continued to live. Our study for the first time demonstrates a therapeutic role for CD8+ T cells against an opportunistic infection in an immunocompromised state.
Toxoplasmosis is an opportunistic infection that induces a strong cellular immune response in the normal host. Cell-mediated immunity is essential for host resistance (31) against this parasite. Infection in immunocompetent individuals results in asymptomatic chronic infection maintained by dormant tissue cysts. In AIDS and other immunocompromised situations, the infection is reactivated, resulting in severe morbidity and mortality in the infected host (11). Although Toxoplasma spp. can infect all cells and organ systems, the predominant manifestation of toxoplasma infection in patients with AIDS is encephalitis (4). Toxoplasmic encephalitis in AIDS patients results in multiple brain lesions, suggesting that reactivation of infection is multifocal within the brain (21).
Loss of CD4+ T cells from progressive human immunodeficiency virus (HIV) infection correlates with reactivation of T. gondii infection in these patients (5). However, the underlying mechanism that results in the activation of disease in these immunocompromised subjects is not clear. Studies with mice have shown that gamma interferon (IFN-γ), a cytokine produced by both CD4+ and CD8+ T cells, is critical for protection during both acute and chronic infection (33, 34). Our laboratory and others have shown that the CD8+ T-cell population is more important for long-term survival of infection (6, 17, 35).
Infection of C57BL/6 mice with the LP-BM5 mixture of helper and etiologic defective murine leukemia viruses (MuLV) leads to the development of murine AIDS (MAIDS), an inevitably fatal syndrome characterized by splenomegaly, lymphadenopathy, hypergammaglobunemia, and a progressive loss of B- and T-cell responses to antigens and mitogens (3, 26). Cytotoxic-T-lymphocyte (CTL) responses against alloantigens were reduced at 8 to 9 weeks after viral infection (25). The loss of T-cell responsiveness in vitro correlates with increased susceptibility to a variety of infections (8, 14). While MAIDS is not a perfect model of HIV infection, the patterns of immunodeficiency induced in both syndromes are strikingly similar. The similarities include early selective defects in CD4+ T-cell function and impaired CD8+ T-cell response late in the course of the disease.
Previous studies by Gazzinelli et al. have shown that CD8+ T cells are important for resistance to reactivation of toxoplasmosis in mice infected by LP-BM5 MuLV virus (10). Variation in the susceptibility of mice to LP-BM5 infection has been reported to be dependent in part on CD8+ T cells (22). For example, prior antibody depletion of CD8+ T cells in resistant A/J mice infected with LP-BM5 MuLV resulted in symptoms similar to those seen in the susceptible C57BL/6 strain. In the present study, we have analyzed the role of CD8+ T cells during the course of reactivation of T. gondii infection in mice infected with LP-BM5 MuLV. The reactivation of disease coincided with the loss of CD8+ function and could be prevented by adoptive immunotherapy with CD8+ T cells from toxoplasma-vaccinated mice.
MATERIALS AND METHODS
Mice.
Female C57BL/6 mice, 5 to 6 weeks old, obtained from Jackson Laboratory at Bar Harbor, Maine, were used for the study.
Infection with virus.
LP-BM5 MuLV (MAIDS) virus was prepared as previously described (19). G6 cells, originally provided to one of us by Janet Hartley and Herbert Morse as a cloned cell line from SC-1 cells infected with LP-BM5 virus mixture were cocultured with uninfected SC-1 cells. Mice were infected intraperitoneally with 100 μl of a virus stock that was quantitated by an XC plaque assay (29) to contain approximately 7 × 105 ecotropic PFU/ml.
Parasites and antigen preparation.
Chronic infection was established by 15 orally administered cysts of Me49 strain of T. gondii. Two weeks after feeding cysts, infection was confirmed by determining the toxoplasma serum antibody titer. Infected animals were challenged with LP-BM5 virus as described above.
Toxoplasma lysate antigen (TLA) was prepared from tachyzoites of the PLK strain of T. gondii. The parasites were cultured in human fibroblasts and released by forced extrusion through a 27-gauge needle. Parasites were isolated from host cell debris by separation by using a Percoll gradient. Purified parasites were essentially free of any fibroblast contamination. The parasites were pulse sonicated eight times (18,000 Hz) at 10-s intervals at 4°C. The sonicate was centrifuged at 10,000 × g for 15 min to remove nonsoluble antigen, and the protein concentration was determined by a commercial assay (Bio-Rad Laboratories, Cambridge, Mass.). The antigen was aliquoted and stored at −20°C until further use.
CD8+ T-cell purification and proliferation.
After the administration of general anesthesia, the spleens were removed and homogenized in a petri dish and contaminating erythrocytes were lysed in an erythrocyte lysis buffer (Sigma Chemical Co., St. Louis, Mo.). After two to three washes in Hanks balanced salt solution (Life Technologies Inc., Gaithersburg, Md.) with 3% fetal bovine serum (HyClone Laboratories, Logan, Utah), the CD8+ T cells were separated by microbeads (Miltenyl Biotec, Auburn, Calif.). The separation procedure was conducted as recommended by the manufacturer. The purity of separated cells was >95% as determined by fluorescence-activated cell sorter (FACS) analysis. The assay was performed by previously described standardized method (17).
Antigen-specific proliferation of purified CD8+ T cells was determined by thymidine incorporation assay. CD8+ T cells were suspended in RPMI 1640 (Life Technologies) and cultured in 96-well flat-bottomed plates in a 200-μl volume at a concentration of 2 × 105 cells/well. The cells were stimulated with either 5 μg/ml of concanavalin A (ConA) or 15 μg of TLA per ml in the presence of 105 irradiated feeder cells. The feeder cells were obtained from syngeneic mice and were irradiated at 3,000 rads. After 72 h of incubation at 37°C in 5% CO2, [3H]thymidine (0.5 μCi/well; Amersham, Arlington Heights, Ill.) was added to the wells. Pulsed splenocytes were harvested on a glass filter by use of an automated multiple sample harvester and dried, and incorporation of radioactive thymidine was then determined by liquid scintillation according to a standard protocol (17).
IFN-γ assay.
The IFN-γ assay of purified CD8+ T cells from infected animals was performed at various time points after LP-BM5 MuLV infection. CD8+ T cells from the infected animals were isolated as described above and stimulated in vitro with TLA and irradiated feeder cells in a 24-well plate. After 72 h of incubation, the cultures were harvested. Supernatants were then collected, centrifuged, and stored at −70°C till further use. The supernatants were assayed for IFN-γ production by cytokine enzyme-linked immunosorbent assay (ELISA) (Genzyme, Cambridge, Mass.).
Bulk cytotoxic assay.
A CTL assay was performed according to a standard procedure in our laboratory (17). Briefly, mouse peritoneal macrophages were obtained by lavage 2 days after intraperitoneal inoculation with 1 ml of thioglycolate. The macrophages were washed three times in phosphate-buffered saline (PBS) and dispensed at a concentration of 3 × 104 cells/well in U-bottom tissue culture plates in medium. Macrophages were incubated overnight, and the next morning were labeled with 51Cr (0.5 μCi/well; New England Nuclear Research Products, Boston, Mass.) for 3 h at 37°C. After several washes in PBS (or until the supernatant contained <500 cpm), the macrophages were infected with freshly isolated cell culture-grown tachyzoites of the PLK strain at a concentration of 5 × 104 parasites/well and incubated overnight. The next morning, spontaneous lysis caused by overnight parasite infection was measured, and wells exhibiting >500 cpm in the supernatants were excluded form the experiment. Macrophages were washed three times in PBS and incubated with purified CD8+ T cells at various effector/target (E/T) ratios in a final volume of 200 μl of culture medium. CD8+ T cells from T. gondii or dual-infected animals were purified by use of magnetic beads as described above, and the purity was determined by FACS. After the addition of T cells, the microtiter plates were centrifuged at 200 × g for 3 min and incubated at 37°C for 3 h. Supernatant samples of 100 μl were removed and assayed for released counts per minute (cpm) by scintillation counting. The percent lysis was calculated as follows: (mean cpm of test sample − mean cpm of spontaneous release)/(mean cpm of maximal release − mean cpm of spontaneous release) × 100.
pCTL assay.
The cytolytic activity of the CD8+ T cells was quantitated by determining the precursor CTL (pCTL) frequency of the infected mice by using the limiting dilution assays. CD8+ T cells from the splenocytes of infected animals were isolated by magnetic separation with a resulting purity of >95%, as determined by FACS analysis. Purified cells were cultured, by limiting dilution, in 96-well round-bottom plates. Cells were grown in RPMI medium containing appropriate growth factors, including 15 U of recombinant interleukin-2 (R & D Chemicals, Minneapolis, Minn.) per ml, TLA (15 μg/ml), and 105 feeder cells per well. Dilution of cells was carried from 1,250, 2,500, 5,000, 10,000, to 20,000 cells/well. Wells containing only antigen and feeder cells, without effector cells, served as controls. After 1 week, the cells were harvested and incubated with 51Cr-labeled parasite-infected and uninfected macrophages. Macrophages were collected and labeled as described above. The pCTL frequency was calculated according to a standard formula (39).
Adoptive transfer of CD8+ T cells.
Mice were infected orally with 10 to 15 T. gondii cysts as described above. At 4 weeks after the infection the animals were splenectomized, and spleen cells from immunized and nonimmune controls were isolated and collected. CD8+ T cells were separated and purified. A total of 107 CD8+ T cells were adoptively transferred via intravenous route to Toxoplasma-infected animals at 5 to 8 weeks postchallenge with LP-BM5 virus. The survival of the animals treated with immune or nonimmune CD8+ T cells was monitored.
Histopathology.
Tissues (lung, spleen, liver, and brain) from the dual-infected animals transferred with immune CD8+ T cells were collected at 10 weeks after LP-BM5 infection. The tissues were immediately fixed in buffered 10% formalin, embedded, sectioned, and stained with hematoxylin and eosin for routine histological examination. Sections were examined and photographed on Kodak TMax film with an Olympus Van OX photomicroscope.
Statistical analysis.
Student's t test was used to determine the differences in the proliferation of CD8+ T cells (27).
RESULTS
CD8+ T cells from dual-infected animals show decreased antigen-specific proliferation.
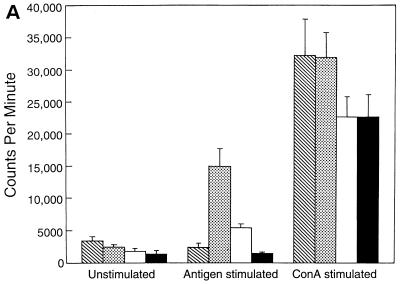
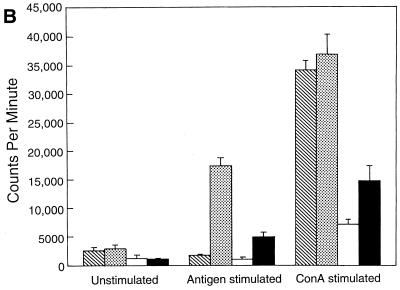
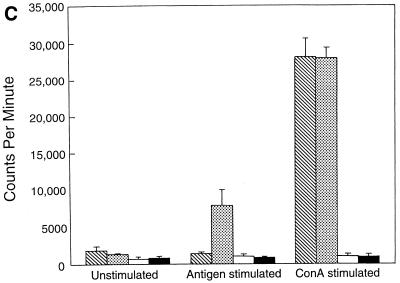
A proliferation assay was performed to study the kinetics of antigen-specific CD8+ T-cell response in the T. gondii-infected animals after LP-BM5 MuLV infection. As expected, CD8+ T cells from the mice infected with T. gondii alone showed a significant (P = 0.005) proliferation in response to stimulation with TLA compared to control unstimulated cultures (Fig. 1A). On the contrary, CD8+ T cells from Toxoplasma-infected mice, which were challenged 5 weeks earlier with LP-BM5 MuLV virus, exhibited significantly reduced antigen-specific proliferative response (P = 0.001). Although CD8+ T cells from the mice carrying dual infection or LP-BM5 MuLV virus alone proliferated in response to ConA, the level of stimulation was lower than for the uninfected animals or those infected with T. gondii alone. At 8 weeks after viral infection CD8+ T cells from the dual-infected mice continued to show significantly lower (P = 0.001) proliferation in response to antigenic stimulation (Fig. 1B). At this time point the downregulation of mitogenic response of CD8+ T cells from virally infected animals was further enhanced. After 10 weeks after viral infection, CD8+ T cells from dual-infected animals completely fail to respond to antigenic stimulation (Fig. 1C). The ConA response of the cells from both dual-infected animals and the mice treated with virus alone at this time is completely absent.
FIG. 1.
Proliferation of CD8+ T cells from T. gondii-infected mice after LP-BM5 challenge. C57BL/6 mice chronically infected with T. gondii were challenged with LP-BM5 virus as described in Materials and Methods. Pooled splenocytes (n = three per group) were collected from dual-infected animals at 5 (A), 8 (B), and 10 (C) weeks after LP-BM5 challenge. CD8+ T cells were purified by magnetic separation (>95% pure, as determined by FACS) and cultured in 96-well plates at the concentration of 2 × 105 cells/well. The cultures were stimulated with ConA or TLA in the presence of 105 irradiated feeder cells. After 72 h, proliferation was measured by determining thymidine incorporation. The data are representative of two separate experiments. Columns: ▧, UI; , Toxo; □ LP-BM5; ■, LP-BM5 plus Toxo.
Effect of LP-BM5 infection on the IFN-γ production by memory CD8+ T cells.
Cytokine production upon antigenic restimulation is an important characteristic of memory T cells (36). IFN-γ is known to be critical for protection against both acute and chronic toxoplasma infections (33). Immune CD8+ T cells have been reported to produce IFN-γ during T. gondii infection (6, 18, 35). The effect of LP-BM5 virus on the IFN-γ production of immune CD8+ T cell was evaluated. Purified CD8+ T cells from the T. gondii-infected mice challenged 5 weeks earlier with LP-BM5 virus produced almost threefold-less IFN-γ compared to the control animals infected with T. gondii alone (Table 1). The IFN-γ production of these cells was further compromised at 10 weeks after viral infection, and nearly background levels of cytokine were released. CD8+ T cells from nonviral controls continued to produce high cytokine titers in response to antigenic stimulation at this time point. IFN-γ levels could not be detected in the wells containing feeder cells alone (data not shown).
TABLE 1.
IFN-γ levels of CD8+ T cells from T. gondii-infected mice challenged with LP-BM5 virusa
| Group | IFN-γ
levels (pg/ml) at:
|
|||||
|---|---|---|---|---|---|---|
| 5 wks after LP-BM5
infection
|
10 wks after LP-BM5 infection
|
|||||
| Uninfected | T. gondii | T. gondii plus LP-BM5 | Uninfected | T. gondii | T. gondii plus LP-BM5 | |
| Unstimulated | ND | 20 ± 5.8 | 32 ± 6.4 | ND | 18.7 ± 1.6 | 9 ± 3.7 |
| Stimulated | 10 ± 0.5 | 2,587 ± 360 | 875 ± 160 | 7.8 ± 0.6 | 1,876 ± 175 | 30 ± 15.9 |
CD8+ T cells from T. gondii-infected animals carrying LP-BM5 virus were isolated at 5 or 10 weeks after viral challenge. A total of 106 purified CD8+ T cells (>95% pure) were cultured in 24-well plates in the presence of 15 μg of TLA per ml and 5 × 105 irradiated feeder cells. After 72 h of incubation, the cultures were collected, centrifuged, and stored at −70°C. The supernatants were assayed for IFN-γ by ELISA. ND, not detected.
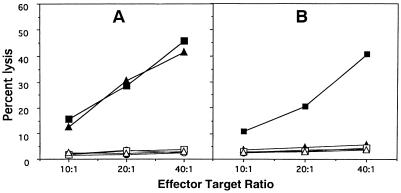
CD8+ T cells from dual-infected animals show reduced cytolytic response against T. gondii-infected targets.
One of the important characteristics of CD8+ T cells is their ability to exhibit cytolytic response against antigen-specific target (1). Induction of CD8+ CTLs against T. gondii infection has been demonstrated earlier (12, 16, 32). Secondary in vitro CTL response was assessed to determine the effect of LP-BM5 virus on CD8+ T-cell function. At 5 weeks postinfection no difference in the cytolytic response between the CD8+ T cells from dual-infected animals and the mice infected with T. gondii alone was observed. CD8+ T cells from both of these animals showed almost 40 to 45% lysis of infected targets at 40:1 E/T ratio (Fig. 2A). At 10 weeks after LP-BM5 MuLV infection, CD8+ T cells from dual-infected animals were unable to kill the toxoplasma-infected targets. The percent lysis at all E/T ratios were equal to the background levels (Fig. 2B). However, CD8+ T cells from mice infected with T. gondii alone continued to show 40% lysis of the infected targets at a 40:1 E/T ratio.
FIG. 2.
LP-BM5 infection downregulates the cytolytic response of immune CD8+ T cells. Splenocytes from saline injected, toxoplasma infected and dual infected animals (n = three/gp) were isolated and pooled at 5 (A) and 10 (B) weeks after LP-BM5 challenge. The cells were cultured in vitro with TLA (toxoplasma lysate antigen; 15 μg/ml). After 5 days of incubation viable cells were separated on Ficoll and CD8+ T cells were isolated by magnetic separation. Purified CD8+ T cells were cultured with 51Cr-labeled macrophages infected with the PLK strains of T. gondii or uninfected targets at various E/T ratios. At 4 h after incubation the cytolytic activity was determined by measuring radioisotope release in the culture supernatant. The experiment was performed twice with similar findings each time. The data presented are representative of one experiment. Infected targets: ●, saline; ■, Toxo; ▴, Toxo plus LP-BM5. Uninfected targets: ○, saline; □, Toxo; ▵, Toxo plus LP-BM5.
LP-BM5 MuLV infection downregulates the pCTL frequency of anti-CD8+ T cells from T. gondii-infected mice.
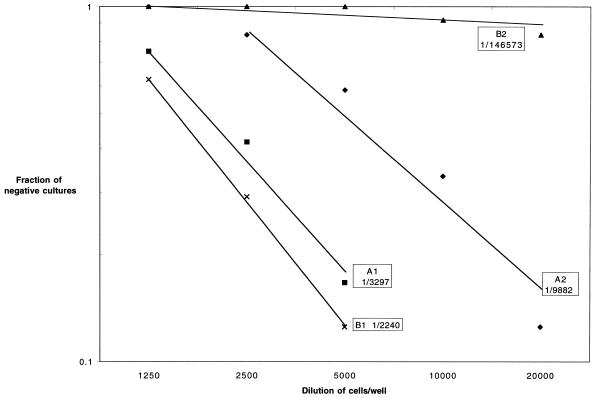
In order to better ascertain the effect of LP-BM5 MuLV infection on the CTL function of CD8+ T cells, pCTL frequency analyses were performed. At 7 weeks after T. gondii infection, the pCTL frequency of the infected animals was 1/3,297 cells (Fig. 3, A1) compared to 1/9,982 cells (Fig. 3, A2) in the group of animals challenged with LP-BM5 virus 5 weeks earlier. These values are within the range of variability (7), suggesting that early LP-BM5 MuLV infection does not significantly affect the cytotoxic function of CD8+ T cells. However, when the assay was performed 10 weeks after viral infection, the pCTL frequency of the dual-infected animals was severely diminished (1/146,537 cells) (Fig. 3, B2) compared to mice infected with T. gondii alone (1/2,240 cells) (Fig. 3, B1).
FIG. 3.
LP-BM5 infection reduces the pCTL frequency in the T. gondii-infected animals. CD8+ T cells from the pooled splenocytes (n = three/group) from dual-infected animals were separated at 5 (A) or 10 (B) weeks after LP-BM5 challenge. Purified CD8+ T cells (>95% pure) were cultured by limiting dilution in the presence of antigen and irradiated feeder cells. After 1 week in culture, the pCTL frequency of CD8+ T cells was determined. The experiment was performed twice. The data presented are representative of one experiment.
Adoptive transfer of immune CD8+ T cells prevents reactivation of toxoplasmosis in immunocompromised animals.
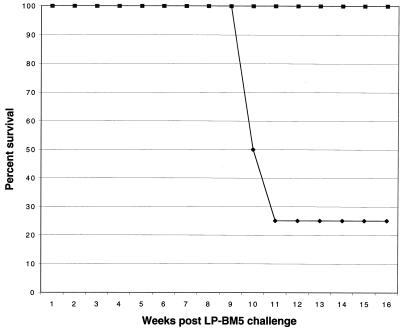
Treatment of naive animals with toxoplasma-immune CD8+ T cells results in almost complete protection against acute T. gondii infection (15). The role of CD8+ T cells during chronic toxoplasmosis, however, is not well studied. Experiments were performed to determine whether CD8+ T cells from toxoplasma-immunized animals can prevent the reactivation of infection in the LP-BM5 immunocompromised mice chronically infected with T. gondii. Mice carrying dual infection were treated with 107 purified immune CD8+ T cells at 5 to 8 weeks after LP-BM5 infection. None of the animals treated with immune CD8+ T cells 5 weeks after viral infection died for up to 16 weeks after viral challenge (Fig. 4). These mice did not develop any signs of sickness (ruffled fur, sluggishness, etc.). In comparison, 75% of the control animals treated with naive CD8+ T cells were dead by this time. Treatment of the dual-infected animals with immune CD4+ T cells had no effect on the mortality of these animals (data not shown). Immune CD8+ T cells failed to protect the dual-infected animals when the transfer was performed 8 weeks after LP-BM5 infection (data not shown). Like the mice treated with nonimmune CD8+ T cells, 70 to 80% of these animals succumbed to infection by 12 weeks after LP-BM5 challenge.
FIG. 4.
Adoptive transfer of immune CD8+ T cells prevents the reactivation of T. gondii infection in the LP-BM5-infected host. C57BL/6 mice infected with T. gondii were challenged with LP-BM5 virus as described above. Dual-infected animals were injected intravenously with 107 immune CD8+ T cells at 5 weeks after LP-BM5 infection. The animals were monitored daily for morbidity or mortality. There were 8 animals per group, and the experiment was performed twice with similar findings. The data presented are representative of one experiment. Symbols: ⧫, naive CD8; ■, immune CD8.
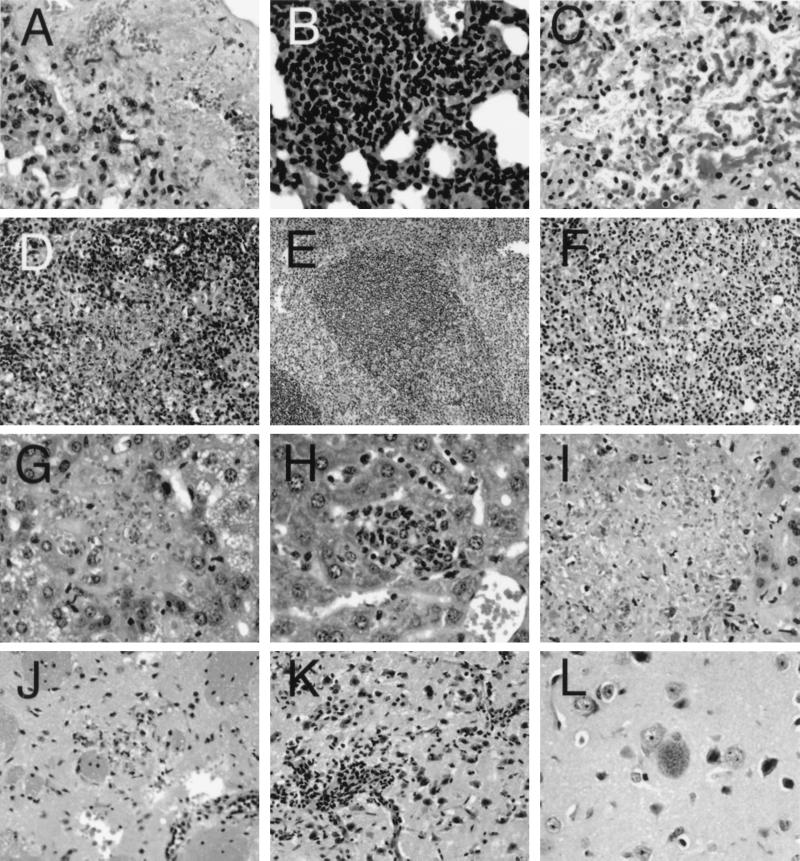
To confirm that the observed mortality in these animals was due to the reactivation of T. gondii infection, histopathological analysis of the tissues was performed at 10 weeks after LP-BM5 infection. This is the time point when complete loss of CD8+ T-cell function in the dual-infected animals was observed. Sections of various organs of the mice treated with naive CD8+ T cells revealed extensive T. gondii replication and necrosis. Areas of interstitial lymphocytic infiltration in the lung contained patches of necrosis in which abundant Toxoplasma tachyzoites could be identified (Fig. 5A). Within these areas of necrosis there were scattered lymphocytes, mononuclear cells, and polymorphonuclear granulocytes (PMN). The follicular architecture of the spleen was effaced, with loss of the normal organization replaced by a lymphocytic infiltrate in which numerous large phagocytic cells with engulfed pycnotic mononuclear cells were seen (Fig. 5D). Areas of necrosis and acute inflammatory infiltrate were seen, including many intracellular and extracellular T. gondii tachyzoites. Examination of LP-BM5 MuLV-infected mice at 8, 10, and 12 weeks after infection showed that the splenic effacement is seen in the absence of infection with T. gondii (data not shown). There was moderate fatty metamorphosis of the cytoplasm, with scattered small nodules of necrosis throughout the liver parenchyma (Fig. 5G). These nodules had few lymphocytes, scattered PMNs, and abundant T. gondii tachyzoites. There were scattered foci of necrosis in the white matter of the brain, with T. gondii tachyzoites evident (Fig. 5J), and the meninges and penetrating vessels had perivascular lymphocytic infiltrates.
FIG. 5.
Microphotographs of sections of lung (A to C), spleen (D to F), liver (G to I), and brain (J to L) of T. gondii-infected animals challenged with LP-BM5 virus. Magnifications: A to C, ×170; D, ×85; E, ×42.5; F, ×85; G to I, ×170; J and K, ×85; L, ×170. (A) Lung of LP-BM5 and toxoplasma-infected mouse with adoptively transferred naive CD8+ T cells at 5 weeks after LP-BM5 infection showing necrosis of the lung parenchyma with identifiable tachyzoites. (B) Lung of dual-infected mouse with adoptively transferred immune CD8+ T cells at 5 weeks after LP-BM5 challenge showing lymphocytic infiltration of the interstitial space (no tachyzoites can be identified). (C) Lung of dual-infected mouse with adoptively transferred immune CD8+ T cells at 8 weeks after LP-BM5 infection showing extensive necrosis of lung tissue with rare tachyzoites. (D) Spleen of dual-infected mouse with adoptively transferred naive CD8+ T cells showing effacement of follicular architecture and necrosis. (E) Spleen of dual-infected mouse with adoptively transferred immune CD8+ T cells showing preservation of follicular architecture. (F) Spleen of dual-infected mouse with adoptively transferred immune CD8+ cells at 8 weeks after LP-BM5 infection showing necrosis and effacement of follicular architecture. (G) Liver of dual-infected mouse with adoptively transferred naive CD8+ T cells showing extensive tachyzoite replication and necrosis. (H) Liver of dual-infected mouse with adoptively transferred immune CD8+ T cells at 5 weeks showing lymphocytic infiltrate within the parenchyma but no identifiable tachyzoites. (I) Liver of dual-infected mouse with adoptively transferred immune CD8+ cells at 8 weeks showing extensive necrosis and tachyzoite multiplication. (J) Brain of dual-infected mouse with adoptively transferred naïve CD8+ T cells at 5 weeks showing a microscopic focus of inflammation and necrosis and perivascular lymphocytic infiltrates. (K) Brain of dual-infected mouse with adoptively transferred immune CD8+ T cells at 5 weeks showing extensive perivascular lymphocytic infiltration but no necrosis. (L) Brain of dual-infected mouse with adoptively transferred immune CD8+ T cells at 5 weeks showing cyst without surrounding cellular reaction.
Sections from mice at 5 weeks after transfer of immune CD8+ T cells showed intense lymphocytic and mononuclear cell infiltrates in the lung with scattered plasma cells and PMNs, but there was no evidence of necrosis or T. gondii replication (Fig. 5B). Follicular architecture was preserved in the spleen, with scattered large phagocytes similar to those seen in the control mice receiving naive CD8+ T cells (Fig. 5E). Replicating T. gondii organisms were not evident. Hepatocytes had finely granular cytoplasm, and there were scattered small nodules of lymphocytic inflammatory cells throughout the parenchyma of the liver (Fig. 5H). The brain contained rare foci of necrosis and inflammation, with T. gondii tachyzoites and evidence of cyst formation (Fig. 5K and L). The meninges and penetrating vessels showed evidence of lymphocytic infiltration. On the contrary, mice treated with immune CD8+ T cells 8 weeks after LP-BM5 challenge showed extensive necrosis and T. gondii replication in the lung, spleen, and liver (Fig. 5C, F, and I), as well as in mesenteric fat and brain (data not shown), results similar to those seen in animals treated with naive CD8+ T cells.
DISCUSSION
Toxoplasmosis is a major problem in immunocompromised individuals, in particular in individuals with AIDS. Earlier studies by Lang et al. have shown that among the nonviral opportunistic infections, encephalitis due to T. gondii was the most frequent cause of mortality in the HIV-infected population (20). In a majority of these cases toxoplasmic encephalitis was due to recrudescence of chronic infection as a result of loss of cellular immune responses. We used a mouse model for retrovirus-induced immunodeficiency to study the reactivation of chronic T. gondii infection. Our findings suggest that reactivation of toxoplasmosis in the immunocompromised mice coincides with the loss of CD8+ T-cell function. The reactivation of the disease could be prevented if these animals are treated with immune CD8+ T cells from immunocompetent mice. These studies clearly demonstrate that immune CD8+ T cells are critical for the prevention of reactivation in mice chronically infected with T. gondii. A protective effect of adoptively transferred immune CD8+ T cells has been previously reported (15). However, the present findings for the first time demonstrate the role of these cells in preventing the reactivation of toxoplasma infection in an immunocompromised host.
One of the major complications of HIV infection is the progressive loss of CD4+ T cells (5). Both CD4+ and CD8+ T cells are known to play a role in resistance to T. gondii infection (9). However, immunity during chronic infection has been reported to be largely dependent on CD8+ T cells, with IFN-γ playing a central role in the protection (6, 35). Like HIV infection in humans, mice with MAIDS have been reported to exhibit a loss of CD4+ T-cell function with the passage of time (25, 28). This ultimately results in defective CD8+ T-cell response in these animals. Thus, the mechanism of reactivation of T. gondii infection in the dual-infected animals is due to lack of CD4+ T-cell help to the effector CD8+ T-cell population. Previous studies have shown that resistance to lethal reactivation of toxoplasmosis in animals with MAIDS is dependent on CD8+ T cells and IFN-γ (10). These findings are strengthened by the reports that reactivation of toxoplasmosis during HIV infection occurs in individuals with full-blown AIDS, when CD8+ T-cell immunity is compromised (30).
In the present study, CD8+ T cells start to show a loss of toxoplasma-specific proliferation starting at 5 weeks after MAIDS infection. By 10 weeks after viral challenge, the immune CD8+ T cells completely failed to respond to antigenic or mitogenic stimulation. The cells were unable to lyse infected targets, and IFN-γ production in response to antigenic stimulation was barely detectable. Animals at this time point look very sick and started to die as a result of the reactivation of toxoplasma infection. However, if the dual-infected animals were treated with immune CD8+ T cells at 5 weeks after infection, the reactivation of toxoplasmosis was prevented and there was no dissemination of infection. These animals showed no sign of sickness and continued to live for up to at least 16 weeks after viral challenge. Histopathological analysis of the tissues of these animals at 10 weeks after onset of MAIDS showed no evidence of reactivation of chronic toxoplasmosis. If the transfer was delayed to 8 weeks after LP-BM5 MuLV infection, the reactivation of toxoplasmosis could not be prevented. Evidence of disseminated infection similar to the control animals treated with naive CD8+ T cells was revealed upon histopathological examination of the tissues. The intracellular multiplication of T. gondii and necrosis of tissues was superimposed on the lymphoid infiltrates, a finding typical of LP-BM5 MuLV infection (38). Infection with LP-BM5 MuLV alone produces an appearance in the spleen similar to that seen in dual-infected mice, without the evidence of increased phagocytosis.
The inability of immune CD8+ T cells to protect the immunocompromised animals at 8 weeks after onset of MAIDS may be due to the fact that the toxoplasma infection by this time has completely reactivated and the parasites have disseminated to various tissues. Immune CD8+ T cells are thus ineffective in controlling overwhelming toxoplasma infection. This view is supported by the observations of Gazzinelli et al., who reported significantly decreased cyst numbers in the brains of dual-infected animals beginning 8 weeks after viral infection (10).
Although endogenous CD8+ T cells from the dual-infected animals are not severely compromised at 5 weeks after viral infection, these cells are unable to prevent the reactivation of toxoplasma infection in the host. This finding can be attributed to the reduced ability of these cells to proliferate and produce IFN-γ in response to T. gondii stimulation. In contrast, immune CD8+ T cells from the mice infected with T. gondii alone exhibit significantly higher proliferation and IFN-γ production when stimulated with toxoplasma antigen. When transferred to the immunocompromised host the immune CD8+ T cells prevent the reactivation of infection in immunocompromised animals, which results in their survival. The possibility that, like the endogenous CD8+ T-cell population, the donor CD8+ T cells ultimately may undergo immunosuppression cannot be ruled out. However, based on our current observations, these cells definitely play a protective role in the initial stages of reactivation of T. gondii infection.
Studies with LP-BM5 infection have shown that the infected animals show early loss of CD4+ T-cell effector functions, including help for the CD8+ T-cell response (26). The CD4+ help has been reported to be critical for certain infectious disease and tumor models, where protective immunity is dependent on CD8+ T cells (23, 24, 37), as is the case with T. gondii infection, where CD8+ T-cell immunity is required for resistance against the parasite (2, 15, 33). CD8+ T cells and IFN-γ keep the infection under check by restricting it to a chronic or dormant state. However, a lack of adequate CD4+ T-cell help, probably as a result of LP-BM5 infection, is followed by a poor CD8+ T-cell response against the parasite. This results in the absence of sufficient host immune surveillance required for preventing the reactivation of infection. Treatment with CD8+ T cells from an immunocompetent host can make up for this loss, and the infection can be controlled.
Adoptive immunotherapy with activated CD8+ T cells has been suggested as a therapy for HIV infection (13, 40). In one of these studies the transfer of activated CD8+ T cells to AIDS patients resulted in stable CD4+ and CD8+ T-cell counts, lower levels of antigenemia, and minimal toxicity over the period of study (40). The treated individuals improved clinically and remained stable. The ability of adoptively transferred CD8+ T cells to protect naive mice against acute toxoplasma infection has been demonstrated (15). The role of immune CD8+ T cells in the reactivation model of T. gondii infection has not been thoroughly studied. Our studies for the first time demonstrate that immunotherapy with immune CD8+ T cells can prevent reactivation of toxoplasmosis in a retrovirus-induced immunocompromised state.
ACKNOWLEDGMENTS
We thank Julie Weiss for her help with statistical analysis.
This work was supported by National Institute of Health grant AI33325.
REFERENCES
- 1.Bhardwaj N, Bender A, Gonzalez N, Bui L K, Garrett M C, Steinman R M. Stimulation of human anti-viral CD8+cytolytic T lymphocytes by dendritic cells. Adv Exp Med Biol. 1995;378:375–379. doi: 10.1007/978-1-4615-1971-3_84. [DOI] [PubMed] [Google Scholar]
- 2.Brown C R, McLeod R. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondiiinfection. J Immunol. 1990;145:3438–3441. [PubMed] [Google Scholar]
- 3.Cerny A, Hugin A W, Holmes K L, Morse H C., III CD4+T cells in murine acquired immunodeficiency syndrome: evidence for an intrinsic defect in the proliferative response to soluble antigen. Eur J Immunol. 1990;20:1577–1581. doi: 10.1002/eji.1830200725. [DOI] [PubMed] [Google Scholar]
- 4.Cingolani A, De Luca A, Ammassari A, Murri R, Linzalone A, Grillo R, Antinori A. PCR detection of Toxoplasma gondiiDNA in CSF for the differential diagnosis of AIDS-related focal brain lesions. J Med Microbiol. 1996;45:472–476. doi: 10.1099/00222615-45-6-472. [DOI] [PubMed] [Google Scholar]
- 5.Dannemann B, McCutchan J A, Israelski D, Antoniskis D, Leport C, Luft B, Nussbaum J, Clumeck N, Morlat P, Chiu J, et al. Treatment of toxoplasmic encephalitis in patients with AIDS: a randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. The California Collaborative Treatment Group. Ann Intern Med. 1992;116:33–43. doi: 10.7326/0003-4819-116-1-33. [DOI] [PubMed] [Google Scholar]
- 6.Denkers E Y, Sher A, Gazzinelli R T. CD8+ T-cell interactions with Toxoplasma gondii: implications for processing of antigen for class-I-restricted recognition. Res Immunol. 1993;144:51–57. doi: 10.1016/s0923-2494(05)80099-9. [DOI] [PubMed] [Google Scholar]
- 7.Doherty P C, Topham D J, Tripp R A. Establishment and persistence of virus-specific CD4+ and CD8+T cell memory. Immunol Rev. 1996;150:23–44. doi: 10.1111/j.1600-065x.1996.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 8.Doherty T M, Morse III H C, Coffman R L. Modulation of specific T cell responses by concurrent infection with Leishmania majorand LP-BM5 murine leukemia viruses. Int Immunol. 1995;7:131–138. doi: 10.1093/intimm/7.1.131. [DOI] [PubMed] [Google Scholar]
- 9.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- 10.Gazzinelli R T, Hartley J W, Fredrickson T N, Chattopadhyay S K, Sher A, Morse H C., III Opportunistic infections and retrovirus-induced immunodeficiency: studies of acute and chronic infections with Toxoplasma gondiiin mice infected with LP-BM5 murine leukemia viruses. Infect Immun. 1992;60:4394–4401. doi: 10.1128/iai.60.10.4394-4401.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant I H, Gold J W, Rosenblum M, Niedzwiecki D, Armstrong D. Toxoplasma gondiiserology in HIV-infected patients: the development of central nervous system toxoplasmosis in AIDS. AIDS. 1990;4:519–521. [PubMed] [Google Scholar]
- 12.Hakim F T, Gazzinelli R T, Denkers E, Hieny S, Shearer G M, Sher A. CD8+ T cells from mice vaccinated against Toxoplasma gondiiare cytotoxic for parasite-infected or antigen-pulsed host cells. J Immunol. 1991;147:2310–2316. [PubMed] [Google Scholar]
- 13.Ho M, Armstrong J, McMahon D, Pazin G, Huang X L, Rinaldo C, Whiteside T, Tripoli C, Levine G, Moody D, et al. A phase 1 study of adoptive transfer of autologous CD8+T lymphocytes in patients with acquired immunodeficiency syndrome (AIDS)-related complex or AIDS. Blood. 1993;81:2093–2101. [PubMed] [Google Scholar]
- 14.Hugin A W, Cerny A, Morse H C., III Mice with an acquired immunodeficiency (MAIDS) develop a persistent infection after injection with Listeria monocytogenes. Cell Immunol. 1994;155:246–252. doi: 10.1006/cimm.1994.1117. [DOI] [PubMed] [Google Scholar]
- 15.Khan I A, Ely K H, Kasper L H. Antigen-specific CD8+ T cell clone protects against acute Toxoplasma gondiiinfection in mice. J Immunol. 1994;152:1856–1860. [PubMed] [Google Scholar]
- 16.Khan I A, Ely K H, Kasper L H. A purified parasite antigen (p30) mediates CD8+ T cell immunity against fatal Toxoplasma gondiiinfection in mice. J Immunol. 1991;147:3501–3506. [PubMed] [Google Scholar]
- 17.Khan I A, Kasper L H. IL-15 augments CD8+ T cell-mediated immunity against Toxoplasma gondiiinfection in mice. J Immunol. 1996;157:2103–2108. [PubMed] [Google Scholar]
- 18.Khan I A, Smith K A, Kasper L H. Induction of antigen-specific human cytotoxic T cells by Toxoplasma gondii. J Clin Investig. 1990;85:1879–1886. doi: 10.1172/JCI114649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klinken S P, Fredrickson T N, Hartley J W, Yetter R A, Morse H C., III Evolution of B cell lineage lymphomas in mice with a retrovirus-induced immunodeficiency syndrome, MAIDS. J Immunol. 1988;140:1123–1131. [PubMed] [Google Scholar]
- 20.Lang W, Miklossy J, Deruaz J P, Pizzolato G P, Probst A, Schaffner T, Gessaga E, Kleihues P. Neuropathology of the acquired immune deficiency syndrome (AIDS): a report of 135 consecutive autopsy cases from Switzerland. Acta Neuropathol. 1989;77:379–390. doi: 10.1007/BF00687372. [DOI] [PubMed] [Google Scholar]
- 21.Luft B J, Remington J S. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 22.Makino M, Chattopadhyay S K, Hartley J W, Morse H C., III Analysis of role of CD8+T cells in resistance to murine AIDS in A/J mice. J Immunol. 1992;149:1702–1706. [PubMed] [Google Scholar]
- 23.Matloubian M, Concepcion R J, Ahmed R. CD4+ T cells are required to sustain CD8+cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mercadal C M, Martin S, Rouse B T. Apparent requirement for CD4+T cells in primary anti-herpes simplex virus cytotoxic T-lymphocyte induction can be overcome by optimal antigen presentation. Viral Immunol. 1991;4:177–186. doi: 10.1089/vim.1991.4.177. [DOI] [PubMed] [Google Scholar]
- 25.Morse H, III C, Yetter R A, Via C S, Hardy R R, Cerny A, Hayakawa K, Hugin A W, Miller M W, Holmes K L, Shearer G M. Functional and phenotypic alterations in T cell subsets during the course of MAIDS, a murine retrovirus-induced immunodeficiency syndrome. J Immunol. 1989;143:844–850. [PubMed] [Google Scholar]
- 26.Mosier D E, Yetter R A, Morse H C., III Retroviral induction of acute lymphoproliferative disease and profound immunosuppression in adult C57BL/6 mice. J Exp Med. 1985;161:766–784. doi: 10.1084/jem.161.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neter J, Wasserman W, Kuter M H. Applied linear statistical models. 2nd ed. Homewood, Ill: Irwin; 1985. [Google Scholar]
- 28.Rosenberg A S, Maniero T G, Morse H C., III In vivo immunologic deficits in mice with murine acquired immunodeficiency syndrome and the effect of LP-BM5 infection on rejection of skin from infected mice. Transplant Proc. 1991;23:167–169. [PubMed] [Google Scholar]
- 29.Rowe W P, Pugh W E, Hartley J W. Plaque assay techniques for murine leukemia viruses. Virology. 1970;42:1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- 30.Shearer G M, Bernstein D C, Tung K S, Via C S, Redfield R, Salahuddin S Z, Gallo R C. A model for the selective loss of major histocompatibility complex self-restricted T cell immune responses during the development of acquired immune deficiency syndrome (AIDS) J Immunol. 1986;137:2514–2521. [PubMed] [Google Scholar]
- 31.Sher A, Denkers E Y, Gazzinelli R T. Induction and regulation of host cell-mediated immunity by Toxoplasma gondii. CIBA Found Symp. 1995;195:95–104. doi: 10.1002/9780470514849.ch7. [DOI] [PubMed] [Google Scholar]
- 32.Subauste C S, Koniaris A H, Remington J S. Murine CD8+ cytotoxic T lymphocytes lyse Toxoplasma gondii-infected cells. J Immunol. 1991;147:3955–3959. [PubMed] [Google Scholar]
- 33.Suzuki Y, Conley F K, Remington J S. Treatment of toxoplasmic encephalitis in mice with recombinant gamma interferon. Infect Immun. 1990;58:3050–3055. doi: 10.1128/iai.58.9.3050-3055.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki Y, Orellana M A, Schreiber R D, Remington J S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki Y, Remington J S. The effect of anti-IFN-gamma antibody on the protective effect of Lyt-2+immune T cells against toxoplasmosis in mice. J Immunol. 1990;144:1954–1956. [PubMed] [Google Scholar]
- 36.Swain S L, Croft M, Dubey C, Haynes L, Rogers P, Zhang X, Bradley L M. From naive to memory T cells. Immunol Rev. 1996;150:143–167. doi: 10.1111/j.1600-065x.1996.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 37.Tamada K, Harada M, Abe K, Li T, Tada H, Onoe Y, Nomoto K. Antitumor vaccination effect of dendritic cells can be augmented by locally utilizing Th1-type cytokines from OK432-reactive CD4+T cells. Cancer Immunol Immunother. 1998;46:128–136. doi: 10.1007/s002620050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang Y, Hugin A W, Giese N A, Gabriele L, Chattopadhyay S K, Fredrickson T N, Kagi D, Hartley J W, Morse H C., III Control of immunodeficiency and lymphoproliferation in mouse AIDS: studies of mice deficient in CD8+T cells or perforin. J Virol. 1997;71:1808–1813. doi: 10.1128/jvi.71.3.1808-1813.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981;126:1614–1619. [PubMed] [Google Scholar]
- 40.Whiteside T L, Elder E M, Moody D, Armstrong J, Ho M, Rinaldo C, Huang X, Torpey D, Gupta P, McMahon D, et al. Generation and characterization of ex vivo propagated autologous CD8+cells used for adoptive immunotherapy of patients infected with human immunodeficiency virus. Blood. 1993;81:2085–2092. [PubMed] [Google Scholar]