Abstract
Human brucellosis can be acquired from infected animal tissues by ingestion, inhalation, or contamination of the conjunctiva or traumatized skin by infected animal products. A vaccine to protect humans from occupational exposure or from zoonotic infection in areas where the disease is endemic would reduce an important cause of morbidity worldwide. Vaccines currently used in animals are unsuitable for human use. We tested a live, attenuated, purine-auxotrophic mutant strain of Brucella melitensis, WR201, for its ability to elicit cellular and humoral immune responses and to protect mice against intranasal challenge with B. melitensis 16M. Mice inoculated intraperitoneally with WR201 made serum antibody to lipopolysaccharide and non-O-polysaccharide antigens. Splenocytes from immunized animals released interleukin-2 (IL-2), gamma interferon, and IL-10 when cultured with Brucella antigens. Immunization led to protection from disseminated infection but had only a slight effect on clearance of the challenge inoculum from the lungs. These studies suggest that WR201 should be further investigated as a vaccine to prevent human brucellosis.
Human brucellosis, caused mostly by Brucella abortus, Brucella melitensis, and Brucella suis, can be acquired by ingestion, inhalation, or contamination of the conjunctiva or traumatized skin by infected animal products (4). Bacteria spread, presumably via lymphatics and blood (11), from the site of entry to the reticuloendothelial system. Although generalized symptoms of fever, sweats, and fatigue are nearly universal in patients with acute brucellosis, onset can be insidious, and many patients present with or develop localized foci of infection, especially in the bones and joints (36). Control of brucellosis in domestic food animals has markedly reduced the incidence of human brucellosis in the United States, but the disease represents an important cause of morbidity worldwide. A human vaccine would be valuable for individuals who may be occupationally exposed to brucellae and for persons who consume unpasteurized dairy products from brucella-endemic areas.
Crucial to the development of a human vaccine are attractive vaccine candidates and a suitable animal model. Live vaccines generate higher levels of protection against brucellosis in animals than do killed vaccines (19). Unfortunately, the genetic basis of attenuation of effective live vaccines for animals is unknown. Moreover, some of these vaccines (B. melitensis Rev1 and B. abortus 19) cause brucellosis in humans (28, 36); another, RB51, has unacceptable antibiotic resistance (26). On the other hand, an appropriately attenuated and genetically defined live vaccine may be effective against human brucellosis. A variant of strain 19 administered by subcutaneous injection or scarification to at least three million people in the former Soviet Union is credited with substantial reduction of human brucellosis in the 1950s (34). Our group previously described a novel, live, attenuated strain (WR201) derived from B. melitensis 16M by disruption of the purEK operon and replacement with a kanamycin resistance gene (8). WR201 requires purine supplementation for growth on minimal medium and fails to replicate in cultured human monocyte-derived macrophages (8). After intraperitoneal administration to mice, this strain colonizes the liver, lung, and spleen, persists in the spleen for at least 4 weeks, and is cleared from all three organs by 8 weeks (7). These characteristics suggest that, if sufficiently immunogenic, WR201 may be a useful vaccine candidate.
Since Verger (33) reported that mice were resistant to oral challenge with brucellae, workers have generally used intraperitoneal or intravenous routes for challenge infection (25) in vaccine studies. Vaccine efficacy is conveniently expressed as the reduction in the number of CFU per spleen in vaccinated compared to control animals at selected times after challenge (18). This approach has proven useful to demonstrate the antibacterial effects of live and killed vaccines, delineate cellular and humoral components of immunity, and support further development of vaccines destined for trials in large animals (25). On the other hand, most Brucella infections are initiated through mucosal routes (ingestion or inhalation). An animal model that uses a mucosal challenge route may provide advantages by allowing investigators to choose which vaccine candidates should be pursued for trials in nonhuman primates or humans. In the present report, we show that intraperitoneal administration of WR201 induces cellular and humoral immune responses. Moreover, this vaccine protects mice against systemic spread of bacteria following intranasal challenge with 16M and promotes clearance of bacteria from the lung.
MATERIALS AND METHODS
Bacteria and bacterial products.
B. melitensis 16M was obtained from Gerhardt Schurig (Virginia Polytechnic Institute, Blacksburg, Va.). Strain WR201, which lacks the entire purE gene and the first seven bases of purK, was derived from 16M as described (8). Strain WR51 was derived from 16M by replacement of rfbU, which codes for mannosyltransferase, with a chloramphenicol resistance cassette. The resulting strain has a rough phenotype, does not agglutinate with anti-brucella serum, and yields lipopolysaccharide (LPS) with a pattern after sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver staining consistent with absence of O-polysaccharide (OPS) side chains (18a). Bacteria were stored at −70°C. Before injection into animals, aliquots of 16M or WR201 stocks were grown overnight in shaker flasks in brucella broth at 37°C. Smooth LPS for target antigen in enzyme-linked immunosorbent assay (ELISA) was prepared from 16M by a minor modification of the method of Bundle et al. (5). Briefly, bacterial cells from 48-h shaker flask cultures were extracted with Tris-buffered (pH 7.2) 2% phenol. After centrifugation to remove bacteria and extensive dialysis against water to remove phenol, the supernatant was concentrated by ultrafiltration, and the crude LPS was pelleted by ultracentrifugation. Pellets were lyophilized and extracted twice with chloroform-methanol (2:1) then partitioned between chloroform and water. The water phase was lyophilized and digested with DNase, RNase, and proteinase K. Purified LPS was pelleted by ultracentrifugation, resuspended in water, and lyophilized. The 2-keto-3-deoxyoctonic acid contents of LPS samples were determined by the method of Karkhanis et al. (16), and the protein content was determined by using bicinchoninic acid reagent (27). The yield of purified LPS was 2 to 3 mg per liter of culture. As another target antigen in ELISA, a whole bacterial lysate (RFBL) was prepared from WR51. Bacterial cells from broth cultures were killed by treatment for 16 h with 0.5% phenol at 5°C, were pelleted by centrifugation, were washed once with water, and were resuspended in a solution containing 0.01 M Tris, 1% NaCl, and 2% phenol, pH 7.2. After being stirred for 3 days at 5°C, the suspension was washed again in water, was resuspended in 0.5% Sarkosyl in 0.01 M Tris-HCl buffer (pH 8.5), and was stirred for 60 min at room temperature, and the cell residue was pelleted by centrifugation. The supernatant fluid was concentrated threefold by ultrafiltration on a PM-10 membrane then extensively dialyzed against a solution containing 0.01 M Tris and 0.1% Sarkosyl, pH 7.5, at 5°C. The final product contained approximately 3.0 mg of protein/ml as estimated by bicinchoninic acid protein assay. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the protein extract showed multiple protein bands in the range of 5 to 200 kDa after Coomassie blue staining. Silver stain for LPS also showed the presence of rough LPS in this preparation. For some experiments, 16M from an overnight broth culture was washed twice in 0.9% saline and was heated at 65°C for 1 h to prepare heat-killed B. melitensis (HKBM).
Antibody titer.
ELISAs were performed in 96-well flat-bottom polystyrene microtiter plates (Costar, Cambridge, Mass.) by the method of Engvall and Perlmann (10) with slight modification. Briefly, the wells were coated with 10 μg of brucella LPS or RFBL in phosphate-buffered saline (PBS) (0.01 M Na phosphate, 0.14 M NaCl, 0.02% NaN3, pH 7.4) by adding 100 μl of solution to each well and then incubating the plate for 3 h at 37°C. Excess binding sites were then blocked with 1% casein (Fisher Scientific, Columbia, Md.) in PBS at 37°C for 1 h. The wells were washed with PBS between steps to remove unbound material. The antigen-coated plates were incubated with serial twofold dilutions of primary antibodies for 16 h at room temperature (25°C). The plates were then incubated with phosphatase-labeled goat anti-mouse immunoglobulins (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) for 20 h at room temperature. Disodium p-nitrophenylphosphate (Sigma Chemical Corporation, St. Louis, Mo.) at a concentration of 1 mg/ml (in 1 M diethanolamine buffer containing 1 mM MgCl2, pH 9.8) was used as substrate. Absorbance was read at 410 nm (A410) on a plate reader (Dynatech, Alexandria, Va.). Antibody titers were calculated by using the dilution of serum that gave an A410 reading nearest to 0.5 (which falls within the linear part of the optical density [OD] dilution curve). The titer, expressed in OD units, was obtained by multiplying the reciprocal dilution of the serum by the actual A410 at that dilution.
Determination of splenocyte cytokine production.
Individual spleens from four naive control mice or animals immunized 9 weeks previously were ground lightly with the frosted ends of two glass slides. After lysis of erythrocytes by suspension in 8.3 g of NH4Cl per liter of 0.01 M Tris-HCl, pH 7.5 (red blood cell lysing buffer; Sigma), cell suspension was washed in RPMI 1640 medium and was adjusted to 2 × 106 cells/ml of medium containing 10% heat-inactivated (56°C for 30 min) fetal bovine serum, 5 × 10−5 M 2-mercaptoethanol, and 50 μg of gentamicin per ml. Two milliliters of cell suspension was cultured with 2 μg of concanavalin A (ConA) per ml, 108 HKBM cells, or 2 μg of RFBL in 16-mm-diameter wells in a tissue culture plate. Cells in control wells received medium only. After 24 to 72 h, cell suspensions were filtered through a 0.22-μm-pore-size filter to remove cell debris and to ensure sterility. Filtrates were analyzed by ELISA for interleukin-2 (IL-2), IL-10, and gamma interferon (IFN-γ) using monoclonal antibody pairs and protocols obtained from Pharmingen (San Diego, Calif.). Preliminary studies indicated that IL-2 and IFN-γ contents of filtrates peaked at 24 h, and IL-10 content peaked at 48 h. Filtrates from these time points were used in the present study.
Immunization and challenge of mice.
Groups of female BALB/cJ mice (Jackson Laboratories, Bar Harbor, Maine) were immunized by intraperitoneal administration of 105 WR201 cells. Nonimmunized, control mice received 0.9% NaCl intraperitoneally. Nine weeks later, after the immunizing inoculum had cleared from tissues, animals were anesthetized with 0.3 mg of xylazine and 1 mg of ketamine and were then inoculated intranasally with 104 CFU of 16M in 30 μl of 0.9% NaCl, administered dropwise into the external nares with a micropipette. In selected experiments, mice from immunized and nonimmunized groups were euthanized by CO2 inhalation prior to challenge in order to obtain sera to test antibody and spleen cells for cytokine production in response to LPS or RFBL. At various times after challenge, animals were euthanized, serum was collected, and spleen, lungs, and/or liver were removed. Organs were suspended in 1 ml of 0.9% NaCl and individually homogenized in tissue grinders. One-half milliliter of neat homogenates and 10 μl of serial 10-fold saline dilutions of homogenates were cultured on brucella agar. After incubation for 3 to 5 days at 37°C, the number of brucella colonies was enumerated and expressed as CFU per organ.
Statistical methods.
Data reported from lung tissue harvested soon after infection, in which the majority of organs were infected, were expressed as mean log CFU ± standard deviations (SDs) for each group, and the significance of differences between groups was analyzed by Student's t test. For this purpose, culture-negative organs were assigned a value of 1 CFU. At later time points, when numerous culture-negative spleens were obtained from immunized animals, log CFU data from spleens were presented graphically and analyzed descriptively. At these time points, the proportion of infected spleens in immunized versus nonimmunized groups was analyzed using Fisher's exact test. Correlation between anti-LPS immunoglobulin G (IgG) and anti-RFBL IgG was determined by using the regression module from Excel 98 (Microsoft Corporation, Seattle, Wash.).
RESULTS
Humoral and cellular immune responses.
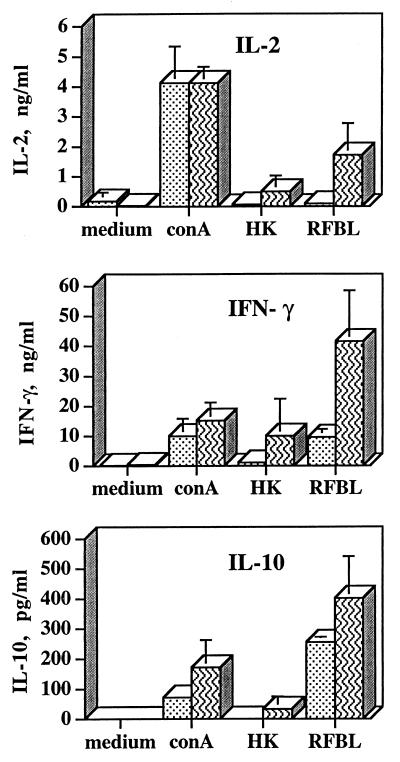
Immunization with WR201 led to antigen-specific T-cell responses (Fig. 1). Spleen cells obtained 9 weeks after inoculation of mice with WR201 produced IL-2, IL-10, and IFN-γ in response to RFBL. These responses were significantly greater (P < 0.02, P < 0.04, and P < 0.01, respectively) than those of spleen cells from nonimmunized, noninfected control mice. HKBM induced similar trends in cytokine production, but the difference between immune and nonimmune cells was significant (P < 0.02) only for induction of IL-2. ConA-induced production of all three cytokines was similar in immune and nonimmune cells.
FIG. 1.
Production of cytokines by splenocytes from noninfected mice immunized with WR201 9 weeks previously (striped bars) or from noninfected, nonimmunized mice (stippled bars). Splenocytes were cultured for 24 h (IL-2 and IFN-γ) or 48 h (IL-10) with medium, 2 μg of ConA, heat-killed 16M (HK), or 2 μg of RFBL per ml. Cytokine levels (mean ± SD) in culture supernatant fluids from cells of individual mice (n = 4) were determined by ELISA. One of three separate experiments with similar results is depicted.
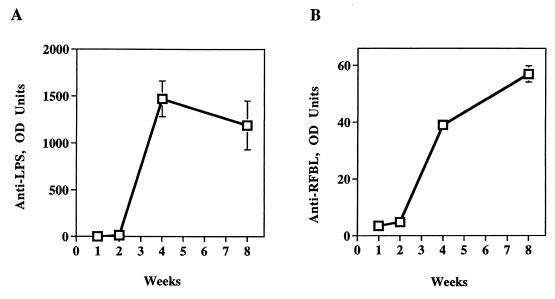
Mice immunized with WR201 also made anti-Brucella serum antibody. Sera obtained from immunized animals from 1 to 8 weeks after intraperitoneal administration of WR201 showed a rise in both anti-LPS and anti-protein IgG by week 4 (Fig. 2). These responses were sustained at week 8 (Fig. 2), and, in other experiments, these responses were sustained in samples taken just prior to challenge at week 9 (data not shown).
FIG. 2.
Production of antibody during the course of WR201 infection. Mice were inoculated with 105 CFU of WR201, and blood was collected at the indicated time points for determination of anti-LPS (A) or anti-RFBL (B) antibody by ELISA. Sera from five mice were pooled for each time point. Error bars denote SD. OD units represent the dilution of serum required to give an A410 of 0.5 (approximately the half-maximal value of the OD-serum dilution curve). Nonimmunized mice made no antibody at any time (not shown).
Protection against intraperitoneal challenge.
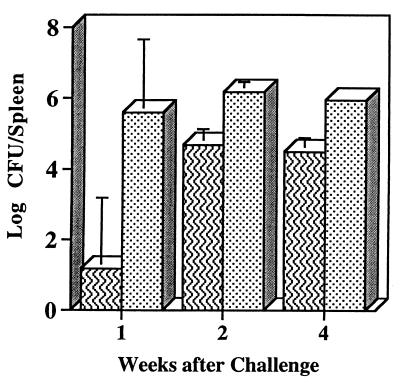
To determine whether these immune responses were associated with protective efficacy, we challenged WR201-immunized mice with 16M using two different routes of inoculation and evaluation timetables. First, in a preliminary experiment, two groups of eight mice that had been intraperitoneally inoculated with either WR201 or saline 9 weeks previously were inoculated intraperitoneally with 16M. The numbers of bacterial CFU in the spleens of groups of two or three mice were determined at 1, 2, and 4 weeks after inoculation. At each time point, immunized mice had significantly (P < 0.05) fewer splenic brucellae than nonimmunized animals (Fig. 3). The reduction in the number of CFU per spleen in immunized animals ranged from 4.4 log units at week 1 to 1.4 log units at weeks 2 and 4. This experiment indicated significant antibacterial activity following immunization.
FIG. 3.
Colonization of spleen after intraperitoneal inoculation of 16M. Immunized mice (striped bars) received WR201. Nonimmunized animals (stippled bars) received saline intraperitoneally 9 weeks before intraperitoneal challenge with 105 16M. Spleens were harvested at the indicated time points, and number of CFU was determined in disrupted tissue by serial dilution and plating.
Effect of immunization on lung infection after intranasal challenge.
Since most human and ruminant infections occur primarily via mucosal routes, including the respiratory and gastrointestinal tracts, we used a recently developed model of intranasal infection with 16M (32a) to test protection against systemic infection. In this model, 16M administered intranasally at 103 or 105 CFU/mouse infects 100% of mouse lungs and spreads to the spleen in 1 to 2 weeks. Administration of 103 CFU of 16M leads to the infection of 50% of spleens; administration of 104 CFU leads to the infection of approximately 90% of spleens. By 4 weeks postinfection, the proportion of animals that remain infected in the lung declines, but the proportion of animals infected in the spleen remains constant from 4 through 12 weeks. For the present study, we examined the effect of immunization with WR201 on early pulmonary and late splenic infection after intranasal challenge with 104 CFU of 16M. This inoculum, 10 times the dose that leads to spleen infection in 50% of mice, consistently led to splenic infection in at least 80% of nonimmunized animals by 2 weeks. Five experiments, denoted A to E, variously focused on early or later time points; some included both. At early time points (≤4 weeks) after infection, there were consistent tendencies toward reduced CFU and lower percentages of infection in lungs from mice immunized with WR201 compared to mice that had received saline intraperitoneally (Table 1). At only 3 of 10 separate data points, however, were differences in lung CFU between immunized and nonimmunized mice statistically significant.
TABLE 1.
Lung infection in mice immunized with WR201 and challenged 9 weeks later intranasally with 16Ma
| Experiment | No. of days after infection | No. infected/no. challenged after immunization withb:
|
Log CFU/lung (mean ± SD) after immunization with:
|
P valuec | ||
|---|---|---|---|---|---|---|
| Saline | WR201 | Saline | WR201 | |||
| A | 1 | 15/15 | 15/15 | 3.0 ± 0.20 | 2.1 ± 0.28 | <0.001 |
| 3 | 15/15 | 15/15 | 2.2 ± 0.73 | 1.9 ± 0.37 | 0.28d | |
| 7 | 14/15 | 12/14 | 1.9 ± 0.81 | 1.8 ± 1.03 | 0.68d | |
| B | 1 | 5/5 | 5/5 | 2.7 ± 0.64 | 2.6 ± 0.26 | 0.55d |
| 3 | 5/5 | 4/5 | 2.6 ± 0.49 | 1.6 ± 1.02 | 0.1d | |
| 14 | 4/5 | 3/4 | 2.6 ± 1.52 | 1.9 ± 1.28 | 0.43d | |
| C | 1 | 5/5 | 5/5 | 4.5 ± 0.17 | 2.8 ± 0.29 | <0.001 |
| 3 | 4/4 | 5/5 | 3.1 ± 1.15 | 2.7 ± 0.95 | 0.57d | |
| 7 | 5/5 | 4/5 | 3.1 ± 0.92 | 1.7 ± 1.11 | 0.062d | |
| 14 | 5/5 | 2/5 | 3.45 ± 1.40 | 0.8 ± 1.10 | 0.01 | |
| D | 28 | 6/10 | 1/10 | 3.6 ± 1.09 | 4.13 | |
Mice were immunized with WR201 or sham immunized with saline and challenged with 104 CFU of 16M intranasally. Animals were euthanized at various times after infection for determination of number of lung bacterial CFU.
Limit of detection = 2 CFU.
Student's t test.
Not significant.
Effect of immunization on disseminated infection after intranasal challenge.
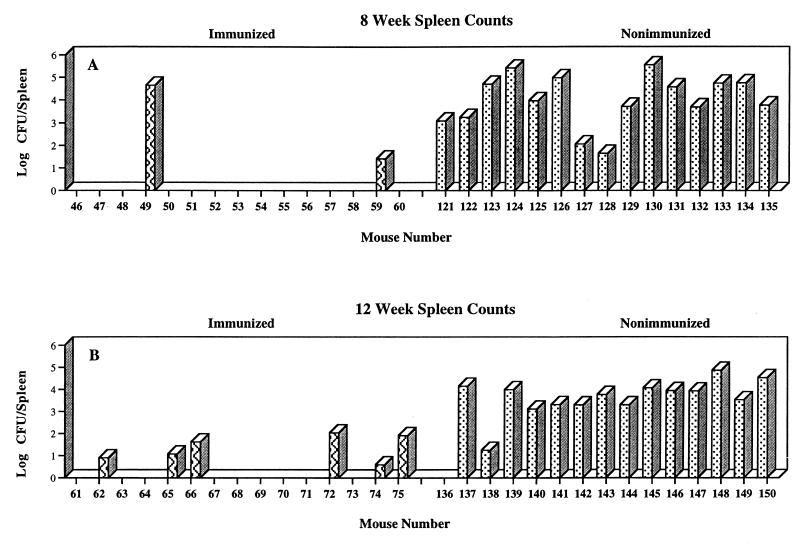
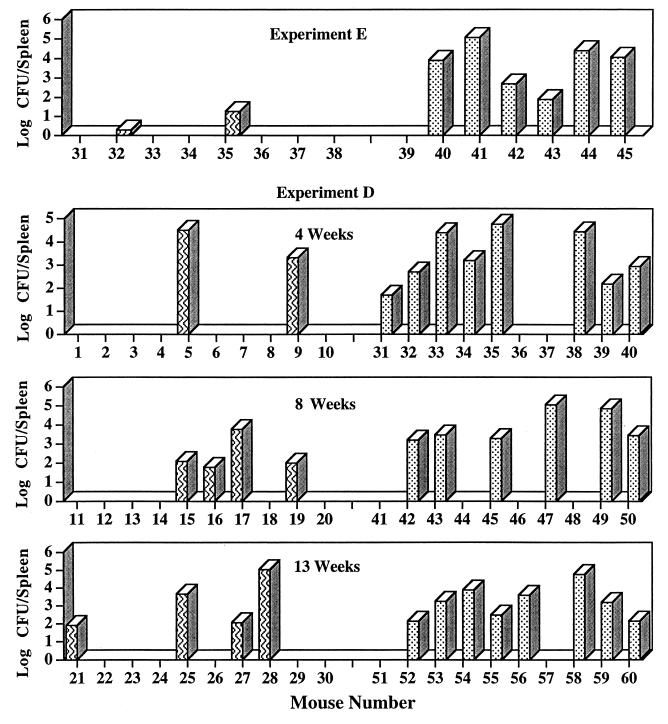
Immunization with WR201 had a much more obvious effect on the dissemination of brucellae from lungs to spleen. In every experiment, at all time points, the proportion of infected spleens was lower in immunized than in nonimmunized animals (Table 2). Although the number of CFU per infected spleen was often less in the immunized group, substantial overlap in CFU per infected spleen between immunized and nonimmunized animals also frequently occurred. Figures 4 and 5 show the number of CFU per spleen from individual mice from the three experiments (A, D, and E) in which spleens were harvested at least 4 weeks after challenge. In experiment E and at the 12-week time point in experiment A, the number of CFU in infected spleens was smaller in the immunized animals. On the other hand, in experiment D and at the 8-week time point in experiment A, considerable overlap in number of CFU per infected spleen between immunized and nonimmunized animals occurred, even though at each point the proportion of infected spleens was lower in the immunized group. When data from all five experiments were pooled, they demonstrated significant protection from disseminated infection at all time points after the first week (Table 3). Protective efficacy {100 × [(1-number of infected immunized animals)/(1-number of infected nonimmunized animals)]} ranged from 50 to 70%.
TABLE 2.
Spleen colonization in mice immunized with WR201 and challenged 9 weeks later intranasally with 16Ma
| Experiment | No. of weeks after infection | No. infected/no. challenged after immunization with:
|
P valueb | |
|---|---|---|---|---|
| Saline | WR201 | |||
| C | 1 | 2/5 | 1/5 | 0.99c |
| 2 | 5/5 | 2/5 | 0.17c | |
| B | 2 | 4/5 | 1/4 | 0.21c |
| E | 4 | 6/7 | 2/8 | 0.041 |
| D | 4 | 8/10 | 2/10 | 0.023 |
| 8 | 6/10 | 4/10 | 0.66c | |
| 13 | 8/10 | 4/10 | 0.17c | |
| A | 8 | 15/15 | 2/15 | <0.001 |
| 12 | 14/15 | 7/15 | 0.014 | |
Mice were immunized with WR201 or sham immunized with saline and challenged with 104 CFU of 16M intranasally. Animals were euthanized at various times after infection for determination of number of splenic bacterial CFU. Limit of detection = 2 CFU.
Fisher's exact test.
Not significant.
FIG. 4.
Effect of immunization on spleen colonization after intranasal challenge with 16M (experiment A). Mice were immunized intraperitoneally with WR201 (striped bars) or sham immunized with saline (stippled bars). Nine weeks later, all animals were challenged with 104 16M. Spleens were harvested at the indicated time points, and number of CFU was determined in disrupted tissue by serial dilution and plating. Each bar indicates CFU from an individual mouse. Limit of detection was 2 CFU/spleen.
FIG. 5.
Effect of immunization on spleen colonization after intranasal challenge with 16M (experiments D and E). Mice were immunized intraperitoneally with WR201 (striped bars) or sham immunized with saline (stippled bars). Nine weeks later, all animals were challenged with 104 16M. Spleens were harvested at the indicated time points, and number of CFU was determined in disrupted tissue by serial dilution and plating. Each bar indicates CFU from an individual mouse. Limit of detection was 2 CFU/spleen.
TABLE 3.
Summary of all experiments of spleen colonization in mice immunized with WR201 and challenged 9 weeks later intranasally with 16M
| No. of weeks after infection | No. infected/no. challenged (%) after immunization witha:
|
P valueb | |
|---|---|---|---|
| Saline | WR201 | ||
| 1 | 2/5 (40) | 1/5 (20) | 0.99c |
| 2 | 9/10 (90) | 3/9 (30) | 0.020 |
| 4 | 14/17 (80) | 4/18 (20) | 0.006 |
| 8 | 21/25 (80) | 6/25 (20) | <0.001 |
| 12 or 13 | 22/25 (90) | 11/25 (40) | 0.002 |
Mice were immunized with WR201 or sham immunized with saline and challenged with 104 CFU of 16M intranasally. Animals were euthanized at various times after infection for determination of number of splenic bacterial CFU. Limit of detection = 2 CFU.
Fisher's exact test. Data are pooled from experiments A through E.
Not significant.
Serum antibody to LPS and RFBL after challenge.
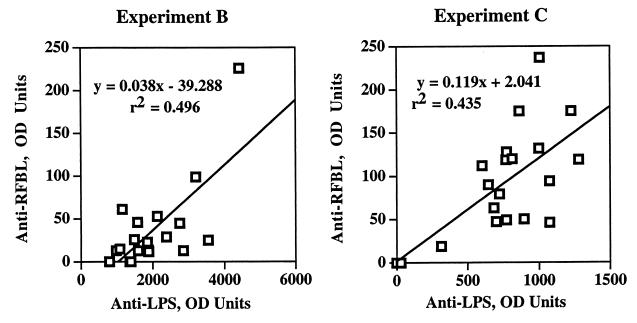
Serum anti-LPS and anti-rough cell lysate antibody did not change during the 2 weeks following intranasal challenge (data not shown). Mice previously immunized with WR201 maintained their prechallenge levels, and nonimmunized mice did not mount a detectable antibody response to either antigen in the first 2 weeks. In two separate experiments, we compared anti-LPS IgG and anti-RFBL IgG in sera collected from individual WR201-immunized mice from 1 to 14 days postchallenge with 16M (Fig. 6). In both experiments, anti-LPS levels significantly (P < 0.001 and P < 0.002) correlated with anti-RFBL levels. Antibody levels did not correlate with numbers of CFU per lung from these animals, although a trend toward increased anti-LPS or anti-RFBL antibodies in animals with fewer lung CFU was observed in one experiment (data not shown).
FIG. 6.
Comparison of anti-LPS and anti-RFBL antibodies in the serum of mice immunized with WR201. Animals were immunized intraperitoneally 9 weeks previously then challenged intranasally with 16M. Sera were collected from mice 1 to 14 days after challenge and were analyzed by ELISA for antibody to LPS or lysate of rough B. melitensis. OD units represent the dilution of serum required to give an A410 of 0.5 (approximately the half-maximal value of the OD-serum dilution curve). The regression line formula and correlation coefficient were determined by the least-squares method.
DISCUSSION
These studies demonstrate that mice immunized with WR201, a purine-auxotrophic mutant of B. melitensis, resist challenge with a virulent strain. This resistance was observed whether animals were challenged mucosally through the nose or by a traditional nonmucosal, intraperitoneal route. Immunization with WR201 protected against intraperitoneal challenge (Fig. 3), reducing splenic CFU by 4.4 to 1.4 log units over the 4-week time period studied. The degree of reduction in CFU induced by immunization with WR201 was greatest at early time points. This observation is consistent with a previous study in mice immunized with B. abortus 19 and challenged at least 6 weeks later with B. abortus 2308 (18). In contrast, a recent study using CD1 mice immunized with B. abortus 19 or B. melitensis Rev1 30 days before challenge with B. abortus 544 or B. melitensis H38 demonstrated persistent vaccine efficacy when numbers of spleen CFU were determined 2 or 8 weeks after challenge (32). WR201 may be less virulent and less persistent than Rev1 and hence may induce a weaker immune response than Rev1. This possibility is suggested by the comparison of survival curves from our previous study (7) to those from the work of Tibor et al. (32) and could be addressed by direct comparative studies.
The predominant mechanism by which WR201 induces immunity is unknown. Studies by a number of investigators (1, 3, 14, 18, 23, 24, 35, 38) have shown that the adoptive transfer of immune CD4, CD8, or mixed T cells and the passive transfer of the anti-OPS antibody from immunized mice to naive animals all mediate an antibacterial effect in animals challenged with strains that express OPS. Our demonstration of WR201-induced antibacterial immunity against intraperitoneal challenge is consistent with our finding that immunization with this live, attenuated, strain induces both humoral (anti-OPS and antiprotein antibody) and cellular (production of IL-2 and IFN-γ) immune responses. The studies reported here complement and extend those of Olsen et al., who showed that lymph node cells from goats infected with WR201 proliferate in response to protein fractions derived from 2308 (21). Antigen-specific lymphoproliferation and production of IL-2 and IFN-γ both reflect responses of sensitized T cells that should augment defense against Brucella. On the other hand, elicitation by bacterial lysate of IL-10 production in cells from immunized as well as nonimmunized mice may counterbalance this effect. A number of studies have demonstrated antagonistic roles of these two cytokines in murine brucellosis. Administration of IFN-γ, which enhances macrophage brucellacidal activity in vitro (13), ameliorates infection in mice (29). Conversely, treatment with anti-IFN-γ worsens infection (37), and IFN-γ-knockout mice die when challenged with Brucella (2). IL-10, which inhibits macrophage brucellacidal activity and brucella-induced secretion of IFN-γ by cultured splenocytes, also enhances Brucella survival in vivo (12). The administration of live (20) or dead (31) brucellae to mice leads to the production of both IFN-γ and IL-10 at an early time point, before the onset of specific immunity. We have not determined which cell type produced IL-10 in our studies; B cells, T cells, and mononuclear phagocytes all have that capability (17). The enhancement of IL-10 production in RFBL-stimulated cells from immunized animals could reflect counterregulation driven by increased IFN-γ by specifically sensitized lymphocytes. Alternatively, it may reflect antigenic stimulation of specifically sensitized Th2-type cells to make IL-10. The ability of RFBL to induce IL-10 production by splenocytes from nonimmunized animals, however, suggests that a portion of the IL-10 response reflects nonspecific stimulation by brucella components. It is likely that this induction of IL-10 production plays a role in the survival of brucellae during natural infection and may also reduce the immunogenicity of live, attenuated vaccines by inhibiting robust development of a Th1-type response. A vaccine that selectively induced cells to make IFN-γ or failed to induce production of IL-10 might be more protective than our current candidate.
The intranasal challenge model we have focused on in this report raises interesting issues about the compartmentalization of the immune response, since it permits examination of the frequency and intensity of infection at a portal of entry as well as at a distant site. There are at least four aspects of defense that we can evaluate. The first phase, colonization of the lung immediately after challenge, was not consistently affected by vaccination. The next phase, clearance of bacteria from the lung, was probably enhanced by immunization, although the magnitude of this effect was small and only reached statistical significance in a minority of experiments. The mechanism of this effect is unknown, but it could involve antibody-mediated antibrucella processes such as complement-dependent bacterial killing (6), antibody-dependent cellular cytotoxicity, or enhanced phagocytosis with killing by activated macrophages (9, 15). Cytotoxic T cells (20) or increased macrophage microbicidal capability induced by Th1 cytokine release from sensitized T cells (13) could also mediate enhanced clearance. The third phase, prevention of the spread of bacteria from lung to spleen, could be influenced by the same factors that enhance clearance from the lungs. Serum antibody might play an important role in this process. In studies of localization of live B. abortus 544 to popliteal lymph nodes after injection of organisms into footpads, prior administration of immune serum prevented dissemination to the spleen (22, 24). Similarly, Sulitzeanu (30) demonstrated that antibodies direct the localization of intraperitoneally administered B. abortus 2308 to mesenteric lymph nodes and limits dissemination to liver and spleen. Our studies do not exclude an effect of cell-mediated host defenses on either clearance or prevention of dissemination of infection. A fourth phase of antibrucella activity, reduction of numbers of CFU and elimination of those bacteria that arrived in the spleen, was not consistently observed in this study. The number of CFU per infected spleen of individual animals within groups of immunized mice often overlapped the number of CFU per infected spleen in animals from the nonimmunized groups. Whether the mechanisms leading to recovery from infection are different from those that limit dissemination is unknown. Of note, IFN-γ-knockout mice fail to control bacterial replication after intraperitoneal inoculation of B. abortus and eventually die from infection (2). This observation suggests that cell-mediated mechanisms play a major role in the elimination of brucellae from reticuloendothelial organs. The failure of immunization with WR201 to enhance elimination from the spleen suggests that the Th1-type response we documented by measurement of IFN-γ was not sufficiently robust to mediate bacterial clearance from reticuloendothelial organs, although it may have been sufficient to increase the rate of clearance from lungs. As discussed above, a vaccine strategy that minimizes the induction of IL-10 or promotes the production of IFN-γ might enhance recovery from disseminated infection if bacteria overcome the barrier effects of immunization and spread to the spleen and other reticuloendothelial organs. We are examining oral immunization and combinations of live, attenuated vaccines with LPS-based immunization to address this possibility.
ACKNOWLEDGMENTS
We thank Joseph Thompson, Kristine Sasala, and Lynnette Young for excellent technical assistance.
REFERENCES
- 1.Araya L N, Elzer P H, Rowe G E, Enright F M, Winter A J. Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J Immunol. 1989;143:3330–3337. [PubMed] [Google Scholar]
- 2.Baldwin, C. Personal communication.
- 3.Bascoul S, Cannat A, Huguet M F, Serre A. Studies on the immune protection to murine experimental brucellosis conferred by Brucella fractions. I. Positive role of immune serum. Immunology. 1978;35:213–221. [PMC free article] [PubMed] [Google Scholar]
- 4.Buchanan T M, Hendricks S L, Patton C M, Feldman R A. Brucellosis in the United States, 1960–1972: an abattoir-associated disease. Part III. Epidemiology and evidence for acquired immunity. Medicine (Baltimore) 1974;53:427–439. [PubMed] [Google Scholar]
- 5.Bundle D R, Cherwonogrodzky J W, Perry M B. Structural elucidation of the Brucella melitensis M antigen by high-resolution NMR at 500 MHz. Biochemistry. 1987;26:8717–8726. doi: 10.1021/bi00400a034. [DOI] [PubMed] [Google Scholar]
- 6.Corbeil L B, Blau K, Inzana T J, Nielsen K H, Jacobson R H, Corbeil R R, Winter A J. Killing of Brucella abortus by bovine serum. Infect Immun. 1988;56:3251–3261. doi: 10.1128/iai.56.12.3251-3261.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford R M, Van De Verg L, Yuan L, Hadfield T L, Warren R L, Drazek E S, Houng H H, Hammack C, Sasala K, Polsinelli T, Thompson J, Hoover D L. Deletion of purE attenuates Brucella melitensis infection in mice. Infect Immun. 1996;64:2188–2192. doi: 10.1128/iai.64.6.2188-2192.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drazek E S, Houng H S, Crawford R M, Hadfield T L, Hoover D L, Warren R L. Deletion of purE attenuates Brucella melitensis 16M for growth in human monocyte-derived macrophages. Infect Immun. 1995;63:3297–3301. doi: 10.1128/iai.63.9.3297-3301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elzer P H, Jacobson R H, Jones S M, Nielsen K H, Douglas J T, Winter A J. Antibody-mediated protection against Brucella abortus in BALB/c mice at successive periods after infection: variation between virulent strain 2308 and attenuated vaccine strain 19. Immunology. 1994;82:651–658. [PMC free article] [PubMed] [Google Scholar]
- 10.Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971;8:871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- 11.Enright F M. The pathogenesis and pathobiology of Brucella infection in domestic animals. In: Nielsen K, Duncan J R, editors. Animal brucellosis. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 301–320. [Google Scholar]
- 12.Fernandes D M, Baldwin C L. Interleukin-10 downregulates protective immunity to Brucella abortus. Infect Immun. 1995;63:1130–1133. doi: 10.1128/iai.63.3.1130-1133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang X, Baldwin C L. Effects of cytokines on intracellular growth of Brucella abortus. Infect Immun. 1993;61:124–134. doi: 10.1128/iai.61.1.124-134.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jimenez de Bagues M P, Elzer P H, Jones S M, Blasco J M, Enright F M, Schurig G G, Winter A J. Vaccination with Brucella abortus rough mutant RB51 protects BALB/c mice against virulent strains of Brucella abortus, Brucella melitensis, and Brucella ovis. Infect Immun. 1994;62:4990–4996. doi: 10.1128/iai.62.11.4990-4996.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones S M, Winter A J. Survival of virulent and attenuated strains of Brucella abortus in normal and gamma interferon-activated murine peritoneal macrophages. Infect Immun. 1992;60:3011–3014. doi: 10.1128/iai.60.7.3011-3014.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karkhanis Y D, Zeltner J Y, Jackson J J, Carlo D J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978;85:595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- 17.Lalani I, Bhol K, Ahmed A R. Interleukin-10: biology, role in inflammation and autoimmunity. Ann Allergy Asthma Immunol. 1997;79:469–483. doi: 10.1016/S1081-1206(10)63052-9. [DOI] [PubMed] [Google Scholar]
- 18.Montaraz J A, Winter A J. Comparison of living and nonliving vaccines for Brucella abortus in BALB/c mice. Infect Immun. 1986;53:245–251. doi: 10.1128/iai.53.2.245-251.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Nickolich, M. P., et al. Unpublished data.
- 19.Nicoletti P. Vaccination. In: Nielsen K, Duncan J R, editors. Animal brucellosis. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 283–300. [Google Scholar]
- 20.Oliveira S C, Splitter G A. CD8+ type 1 CD44hi CD45 RBlo T lymphocytes control intracellular Brucella abortus infection as demonstrated in major histocompatibility complex class I- and class II-deficient mice. Eur J Immunol. 1995;25:2551–2557. doi: 10.1002/eji.1830250922. [DOI] [PubMed] [Google Scholar]
- 21.Olsen S C, Cheville N F, Stevens M G, Houng H H, Drazek E S, Hadfield T L, Warren R L, Hoover D L. Lymphocyte proliferative responses of goats vaccinated with Brucella melitensis 16M or a delta purE201 strain. Infect Immun. 1997;65:2987–2991. doi: 10.1128/iai.65.7.2987-2991.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pardon P, Marly J. Resistance of normal or immunized guinea pigs against a subcutaneous challenge of Brucella abortus. Ann Rech Vet. 1978;9:419–425. [PubMed] [Google Scholar]
- 23.Phillips M, Deyoe B L, Canning P C. Protection of mice against Brucella abortus infection by inoculation with monoclonal antibodies recognizing Brucella O-antigen. Am J Vet Res. 1989;50:2158–2161. [PubMed] [Google Scholar]
- 24.Plommet M, Plommet A M. Immune serum-mediated effects on brucellosis evolution in mice. Infect Immun. 1983;41:97–105. doi: 10.1128/iai.41.1.97-105.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plommet M, Serre A, Fensterbank R. Vaccines, vaccination in brucellosis. Ann Inst Pasteur Microbiol. 1987;138:117–121. doi: 10.1016/0769-2609(87)90089-5. [DOI] [PubMed] [Google Scholar]
- 26.Schurig G G, Roop R M, Bagchi T, Boyle S, Buhrman D, Sriranganathan N. Biological properties of RB51: a stable rough strain of Brucella abortus. Vet Microbiol. 1991;28:171–188. doi: 10.1016/0378-1135(91)90091-s. [DOI] [PubMed] [Google Scholar]
- 27.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. . (Erratum, 163:279.) [DOI] [PubMed] [Google Scholar]
- 28.Spink W W, Hall J W I, Finstad J, Mallet E. Immunization with viable Brucella organisms. Bull W H O. 1962;26:409–419. [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens M G, Pugh G J, Tabatabai L B. Effects of gamma interferon and indomethacin in preventing Brucella abortus infections in mice. Infect Immun. 1992;60:4407–4409. doi: 10.1128/iai.60.10.4407-4409.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sulitzeanu D. The fate of killed, radioiodinated Brucella abortus injected into mice. J Immunol. 1959;82:304–312. [PubMed] [Google Scholar]
- 31.Svetic A, Jian Y C, Lu P, Finkelman F D, Gause W C. Brucella abortus induces a novel cytokine gene expression pattern characterized by elevated IL-10 and IFN-gamma in CD4+ T cells. Int Immunol. 1993;5:877–883. doi: 10.1093/intimm/5.8.877. [DOI] [PubMed] [Google Scholar]
- 32.Tibor A, Jacques I, Guilloteau L, Verger J M, Grayon M, Wansard V, Letesson J J. Effect of P39 gene deletion in live Brucella vaccine strains on residual virulence and protective activity in mice. Infect Immun. 1998;66:5561–5564. doi: 10.1128/iai.66.11.5561-5564.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Van De Verg, L. L., et al. Unpublished data.
- 33.Verger J M. Comparaison des doses infectieuses 50 p. 100 (DI50) de Brucella melitensis inoculee par les voies conjonctivale, intragastrique et intraperitoneale a la souris. Ann Rech Vet. 1971;2:185–196. [Google Scholar]
- 34.Vershilova P A. The use of live vaccine for vaccination of human beings against brucellosis in the USSR. Bull W H O. 1961;24:85–89. [PMC free article] [PubMed] [Google Scholar]
- 35.Winter A J, Duncan J R, Santisteban C G, Douglas J T, Adams L G. Capacity of passively administered antibody to prevent establishment of Brucella abortus infection in mice. Infect Immun. 1989;57:3438–3444. doi: 10.1128/iai.57.11.3438-3444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young E J. Brucellosis: current epidemiology, diagnosis, and management. Curr Clin Top Infect Dis. 1995;15:115–128. [PubMed] [Google Scholar]
- 37.Zhan Y, Cheers C. Endogenous gamma interferon mediates resistance to Brucella abortus infection. Infect Immun. 1993;61:4899–4901. doi: 10.1128/iai.61.11.4899-4901.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhan Y, Kelso A, Cheers C. Differential activation of Brucella-reactive CD4+ T cells by Brucella infection or immunization with antigenic extracts. Infect Immun. 1995;63:969–975. doi: 10.1128/iai.63.3.969-975.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]