Abstract
Chlorpropham (CIPC) has been the dominant method of chemical sprout suppression for the last half-century. However, stricter regulations including outright bans on its use in several countries has prompted investigation into alternative products to replace it. Growing interest in organic foods has increased focus on the use of biopesticides, including essential oils (EOs), as potential sprout suppressants in stored potato. We evaluated the potential of ten EOs for sprout suppression in potato cultivar Ranger Russet at room temperature. Treatment with Cymbopogon citratus EO was found to be the most effective sprout suppressant, completely suppressing sprouting over the 90-day storage period. The EOs of Myrtus communis and Melaleuca quinquenervia significantly reduced sprout length relative to the control but did not have any effect on sprout number. These findings demonstrate the potential of select EOs as effective potato sprout suppressants that could replace CIPC use in this industry while also giving more power to organic potato producers and processors to control sprouting in their operations.
Keywords: potato storage, essential oils, sprout suppression, organic agriculture, room temperature
1. Introduction
Potato (Solanum tuberosum) is the fourth largest crop after maize, wheat, and rice, and is cultivated in over 100 countries [1]. The tubers are a staple in the diets of numerous cultures and are highly versatile in cooking; fresh potatoes can be prepared in many ways or processed into a variety of products [2]. Globally, over 370 million tons of potato were produced in 2019, and over 19 million tons were produced in the United States alone [3]. Sales of potatoes grown in the United States in 2020 totaled over $3.6 billion [4]. Potato is therefore of major economic importance in this region and on the global scale.
Potatoes must often be stored for several months before being consumed or used as seed for the establishment of the next crop [5]. Immediately following harvest, most potato cultivars are in a natural state of dormancy and will not sprout. The length of this innate dormancy is influenced by environmental, physiological, and hormonal factors, and is highly cultivar dependent [6,7]. However, even the longest periods of innate dormancy are often insufficient to meet the needs of overall markets [1]. Extended storage periods can promote increased water loss, occurrence of disease, and sprouting in tubers [8]. Control of sprouting during storage is crucial as sprouting leads to changes in tuber weight, texture, and nutritional value, and the formation of toxic alkaloids including solanine [6,9]. Sprouting thereby leads to economic losses as solanine accumulation renders the potatoes inedible and they become food waste [2].
Several strategies may be implemented to limit potato sprouting during storage. These include storage at low temperatures and the use of chemical sprout suppressants. Long-term storage (up to 9 months) at temperatures between 8–12 °C at 85–90% relative humidity is a common approach to preserve processing potato quality [5]. However, once the innate dormancy period of tubers has ended, these temperatures are not sufficient on their own to prevent sprouting and sprout elongation [5]. While storage at lower temperatures is possible, cold temperatures increase glucose concentrations in the tuber flesh which causes unacceptable darkening in processed potato products and the formation of acrylamide during frying, a compound that may pose health risks to humans [10,11]. The costs of installing, running, and maintaining cooling systems may also be prohibitive, particularly to small and medium facility owners [12]. Chemical sprout suppressants, on the other hand, may offer an effective and less expensive approach to achieving sprout suppression.
The potato industry has relied heavily on the use of chemical sprout suppressants such as chlorpropham (CIPC) since the mid-20th century [1]. CIPC is an inexpensive and highly effective sprout inhibitor that interferes with mitosis in developing bud tissue, requiring only a single application to achieve complete sprout suppression for up to 5 months [13,14]. However, growing concerns about potentially adverse health and environmental effects of CIPC and its metabolites has led to stricter limitations on allowable residues and an outright ban on CIPC in the European Union [15,16]. The total value of exported potatoes from the US in 2017 was around $3 billion [17]. With the ban of CIPC in the EU and other countries, US potato exports will suffer if American-grown potatoes cannot be sold to countries with zero-tolerance policies for CIPC residues. Meanwhile, a global market for organically produced foods and products has grown significantly in recent years; organic sales totaled $62 billion in the United States alone in 2021 [18]. Taken together, the new laws on CIPC use and growing interest in organic foods indicate high potential for expanded use of alternative chemical sprout suppressants such as essential oils (EOs) in potato sprout suppression. Doing so would not only reduce the economic impact that these bans have on the US potato industry but would also give organic potato growers and processors more control over sprouting in their operations.
Several EO-containing sprout suppressants are currently available including Biox-M, Biox-C, and Talent® [1]. Biox-M, containing 100% spearmint (Mentha spicata L.) EO, is the most common EO sprout suppressant used in the United States, accounting for 3% of treatments to stored potatoes in 2016 [16]. Biox-C contains 100% clove EO (Syzygium aromaticum L.), whereas Talent® contains caraway EO (Carum carvi L.) [1]. However, the efficacy of spearmint, clove, and caraway EOs on sprout suppression at room temperature may be inconsistent or inadequate and can vary with cultivar [12,16,19,20,21]. Furthermore, there may be other EOs with sprout suppressive qualities yet to be identified. This study was conducted to evaluate the effect of ten previously untested EOs on sprouting in one potato cultivar, Ranger Russet, with the objective of identifying those suitable for potato storage at room temperature.
2. Results and Discussion
2.1. Effects of Essential Oils (EOs) on Longest Sprout Length
After 90 days of storage, a statistically significant two-way interaction between treatment and time was observed on sprout length (Table 1). This suggests that the impact of treatment on sprout length depends on the amount of time that has passed. Furthermore, the main effects of both treatment and time were significant (Table 1).
Table 1.
ANOVA p-values (Pr[>F]) of the linear mixed model for sprout length and number in response to treatment and time and their interactions (*** = statistically significant).
| Variables | Sprout Length | Sprout Number |
|---|---|---|
| Treatment | <0.001 *** | <0.001 *** |
| Time | <0.001 *** | <0.001 *** |
| Treatment × Time | <0.001 *** | <0.001 *** |
The EOs of Myrtus communis, Melaleuca quinquenervia, and Cymbopogon citratus resulted in significant differences in sprout length relative to the control (Table 2). Treatment with C. citratus EO resulted in significant differences in sprout length from the control at all time points, whereas the effects of M. communis and M. quinquenervia EO treatments were significant between 45–90 days and 45–75 days of storage, respectively (Table 2).
Table 2.
Tukey’s test p-values describing the EOs’ effects on sprout length relative to the control at all time points (*, **, *** = statistically significant).
| Plant EO | Duration of Storage | ||||
|---|---|---|---|---|---|
| 30 Days | 45 Days | 60 Days | 75 Days | 90 Days | |
| Myrtus communis | 0.3310 | <0.001 *** | <0.001 *** | 0.00246 ** | 0.0257 * |
| Melaleuca quinquenervia | 0.2200 | <0.001 *** | <0.001 *** | 0.0286 * | 0.513 |
| Myristica fragrans | 0.9680 | 0.098 | 0.387 | 0.920 | 0.984 |
| Commiphora erythraea | 0.9990 | 0.985 | 1.000 | 1.000 | 0.999 |
| Citrus aurantium | 1.0000 | 0.967 | 1.000 | 1.000 | 0.999 |
| Citrus sinensis | 0.9910 | 1.000 | 0.998 | 0.998 | 1.000 |
| Bursera graveolens | 1.0000 | 0.999 | 1.000 | 1.000 | 0.997 |
| Petroselinum sativum | 1.0000 | 0.590 | 0.300 | 0.313 | 0.221 |
| Pogostemon cablin | 1.0000 | 0.265 | 0.356 | 0.891 | 0.796 |
| Cymbopogon citratus | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** |
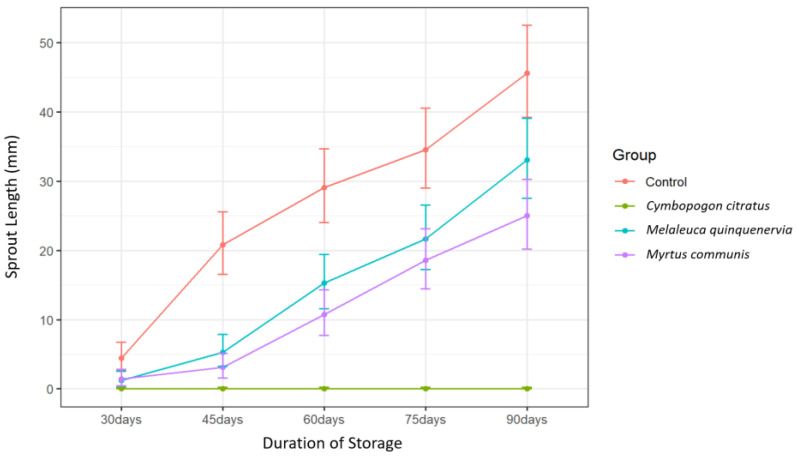
Complete suppression of sprouting was obtained over the entire 90-day storage period with C. citratus EO treatment (Figure 1). Sprout length due to C. citratus EO treatment differed significantly from that due to either M. communis or M. quinquenervia EO treatment from 45 days until the end of the storage period (p < 0.001, Tukey’s test) (Table 3). Sprout length due to M. communis EO treatment did not differ from that of M. quinquenervia EO treatment at any time point (p > 0.05, Tukey’s test) (Table 3). Repeated applications of EOs are often required to maintain adequate sprout suppression over longer storage periods [16,22,23]. Perhaps more effective sprout suppression could be achieved with M. communis and M. quinquenervia EOs if repeated applications were used.
Figure 1.
Sprout length (mm) over time of potatoes treated with distilled water (control), Cymbopogon citratus, Myrtus communis, and Melaleuca quinquenervia EOs. Error bars represent the 95% confidence level of the back-transformed means (emmeans method).
Table 3.
Longest sprout length (mm) of potato tubers treated with different essential oils at different time points.
| Duration of Storage | |||||
|---|---|---|---|---|---|
| 30 Days | 45 Days | 60 Days | 75 Days | 90 Days | |
| Control | 4.46 ± 1.07 a | 20.83 ± 2.32 a | 29.09 ± 2.74 a | 34.55 ± 2.99 a | 45.63 ± 3.43 a |
| Myrtus communis | 1.42 ± 0.60 ac | 3.11 ± 0.89 c | 10.77 ± 1.67 c | 18.55 ± 2.19 b | 24.99 ± 2.54 b |
| Melaleuca quinquenervia | 1.20 ± 0.55 ac | 5.29 ± 1.17 c | 15.28 ± 1.98 bc | 21.66 ± 2.36 bc | 33.07 ± 2.92 ab |
| Myristica fragrans | 2.75 ± 0.84 a | 13.11 ± 1.84 ab | 22.84 ± 2.43 ab | 29.32 ± 2.75 ab | 38.99 ± 3.17 a |
| Commiphora erythraea | 5.70 ± 1.21 a | 17.97 ± 2.15 ab | 29.15 ± 2.74 a | 32.87 ± 2.91 ac | 41.09 ± 3.26 a |
| Citrus aurantium | 3.70 ± 0.97 a | 17.63 ± 2.13 ab | 27.77 ± 2.68 a | 32.64 ± 2.90 ac | 41.00 ± 3.25 a |
| Citrus sinensis | 6.19 ± 1.26 ab | 21.32 ± 2.35 ab | 31.50 ± 2.85 a | 37.87 ± 3.13 a | 42.32 ± 3.31 a |
| Bursera graveolens | 4.92 ± 1.12 a | 18.79 ± 2.20 ab | 28.55 ± 2.71 a | 32.53 ± 2.90 ac | 40.19 ± 3.22 a |
| Petroselinum sativum | 4.17 ± 1.04 a | 15.63 ± 2.01 ab | 22.42 ± 2.41 ab | 25.53 ± 2.57 ab | 30.13 ± 2.79 a |
| Pogostemon cablin | 3.50 ± 0.95 a | 14.29 ± 1.92 ab | 22.70 ± 2.42 ab | 29.03 ± 2.74 ab | 35.58 ± 3.03 a |
| Cymbopogon citratus | 0 c | 0 d | 0 d | 0 d | 0 c |
Values are the back-transformed means ± SE (emmeans method). Different letters (a–d) within columns indicate statistically significant differences between treatments (Tukey’s test p < 0.05).
Myrtus communis is a medicinal plant used in the food, pharmaceutical, and cosmetic industries [24]. GC analysis of M. communis EO reveals α-pinene, eucalyptol, linalool, p-cymene, geranyl acetate, and α-terpineol as major constituents (Table 4). Geranyl acetate and α-pinene have previously been reported as an effective and somewhat effective sprout suppressant, respectively [1]. Furthermore, these monoterpenes have also been shown to inhibit germination and growth in other species, perhaps through their induction of oxidative stress [25,26,27]. It is possible that these compounds are responsible for this EO’s sprout suppressive properties. M. communis EO has been associated with decreased weight and fruit firmness losses in strawberries [28]. Furthermore, sunflower oil fortified with M. communis EO can enhance the physiochemical properties of potato chips and could significantly increase their shelf-life [29]. These reports and the findings of the current study suggest that M. communis EO could be an effective component in EO sprout suppressant formulations.
Table 4.
Myrtus communis EO constituents identified via GC–MS–FID analysis.
| No. | Compound Name | Retention Time | Calculated KI | Actual KI | Identified | Area % |
|---|---|---|---|---|---|---|
| 1 | isobutyl isobutyrate | 5.414 | 909 | 911 | Kovat, NIST, Adams, [30,31,32] | 0.495 |
| 2 | α-thujene | 5.82 | 926 | 930 | Kovat, NIST, Adams, [30,31,32] | 0.354 |
| 3 | α-pinene | 6.089 | 936 | 939 | Kovat, NIST, Adams, Commercial Standard | 49.086 |
| 4 | β-pinene | 7.23 | 975 | 979 | Kovat, NIST, Adams, Commercial Standard | 0.4 |
| 5 | unknown | 7.954 | - | - | - | 0.47 |
| 6 | 3-carene | 8.27 | 1006 | 1011 | Kovat, NIST, Adams, Commercial Standard | 0.195 |
| 7 | unknown | 8.394 | - | - | - | 0.185 |
| 8 | p-cymene | 8.768 | 1023 | 1024 | Kovat, NIST, Adams, Commercial Standard | 2.123 |
| 9 | eucalyptol | 9.069 | 1032 | 1031 | Kovat, NIST, Adams, Commercial Standard | 33.119 |
| 10 | α-pinene oxide | 11.493 | 1099 | 1099 | Kovat, NIST, Adams, Commercial Standard | 0.664 |
| 11 | linalool | 11.584 | 1101 | 1096 | Kovat, NIST, Adams, Commercial Standard | 2.266 |
| 12 | unknown | 11.666 | - | - | - | 0.582 |
| 13 | unknown | 11.866 | - | - | - | 0.891 |
| 14 | α-campholenal | 12.575 | 1126 | 1126 | Kovat, NIST, Adams, [33] | 0.22 |
| 15 | trans-pinocarveol | 13.168 | 1141 | 1139 | Kovat, NIST, Adams, [31,33] | 0.48 |
| 16 | trans-verbenol | 13.431 | 1147 | 1144 | Kovat, NIST, Adams, Commercial Standard | 0.866 |
| 17 | terpinen-4-ol | 14.749 | 1177 | 1177 | Kovat, NIST, Adams, Commercial Standard | 0.193 |
| 18 | α-terpineol | 15.353 | 1189 | 1188 | Kovat, NIST, Adams, Commercial Standard | 1.215 |
| 19 | verbenone | 16.061 | 1205 | 1205 | Kovat, NIST, Adams, Commercial Standard | 0.42 |
| 20 | trans-carveol | 16.576 | 1219 | 1216 | Kovat, NIST, Adams, Commercial Standard | 0.289 |
| 21 | (R)-carvone | 17.523 | 1243 | 1243 | Kovat, NIST, Adams, Commercial Standard | 0.114 |
| 22 | linalyl acetate | 18.004 | 1255 | 1257 | Kovat, NIST, Adams, [31,32] | 0.581 |
| 23 | unknown | 20.026 | - | - | - | 1.248 |
| 24 | unknown | 20.714 | - | - | - | 0.552 |
| 25 | α-terpinyl acetate | 21.992 | 1348 | 1349 | Kovat, NIST, Adams, Commercial Standard | 0.294 |
| 26 | geranyl acetate | 23.416 | 1379 | 1381 | Kovat, NIST, Adams, Commercial Standard | 1.766 |
| 27 | methyl eugenol | 24.322 | 1398 | 1403 | Kovat, NIST, Adams, Commercial Standard | 0.321 |
| 28 | caryophyllene oxide | 31.426 | 1577 | 1583 | Kovat, NIST, Adams, Commercial Standard | 0.477 |
| 29 | unknown | 32.43 | - | - | - | 0.133 |
Melaleuca quinquenervia is a medicinal plant with applications in aromatherapy, cosmetics, and pharmaceuticals, although its use in the food industry has been suggested [34]. GC analysis reports eucalyptol, α-terpineol, α-pinene, viridiflorol, β-pinene, caryophyllene, viridiflorene, α-terpinyl acetate, and p-cymene as major constituents (Table 5). Of these compounds, only α-pinene has been reported as a somewhat effective sprout suppressant [1]. It is possible that the other major compounds, perhaps in combination with α-pinene, are responsible for the sprout suppressive capabilities of this EO. The findings of the current study encourage further investigation of both M. quinquenervia and M. communis EOs as sprout suppressants in other cultivars and using different application schemes, such as repeated or continuous application, to achieve more effective sprout control.
Table 5.
Melaleuca quinquenervia essential oil (EO) constituents identified via GC–MS–FID analysis.
| No. | Compound Name | Retention Time | Calculated KI | Actual KI | Identified | Area % |
|---|---|---|---|---|---|---|
| 1 | α-thujene | 5.81 | 926 | 930 | Kovat, NIST, Adams, [35] | 0.132 |
| 2 | α-pinene | 6.028 | 934 | 939 | Kovat, NIST, Adams, Commercial Standard | 7.081 |
| 3 | camphene | 6.42 | 948 | 954 | Kovat, NIST, Adams, Commercial Standard | 0.056 |
| 4 | benzaldehyde | 6.731 | 959 | 960 | Kovat, NIST, Adams, Commercial Standard | 0.154 |
| 5 | β-pinene | 7.232 | 975 | 979 | Kovat, NIST, Adams, Commercial Standard | 2.037 |
| 6 | myrcene | 7.597 | 986 | 990 | Kovat, NIST, Adams, Commercial Standard | 0.974 |
| 7 | unknown | 8.063 | - | - | - | 0.105 |
| 8 | α-terpinene | 8.482 | 1012 | 1017 | Kovat, NIST, Adams, Commercial Standard | 0.19 |
| 9 | p-cymene | 8.8 | 1024 | 1024 | Kovat, NIST, Adams, Commercial Standard | 1.31 |
| 10 | eucalyptol | 9.125 | 1034 | 1031 | Kovat, NIST, Adams, Commercial Standard | 64.754 |
| 11 | γ-terpinene | 9.976 | 1059 | 1059 | Kovat, NIST, Adams, Commercial Standard | 0.764 |
| 12 | terpinolene | 11.091 | 1088 | 1088 | Kovat, NIST, Adams, Commercial Standard | 0.494 |
| 13 | linalool | 11.561 | 1100 | 1096 | Kovat, NIST, Adams, Commercial Standard | 0.135 |
| 14 | δ-terpineol | 14.329 | 1167 | 1166 | Kovat, Adams, [36] | 0.177 |
| 15 | terpinen-4-ol | 14.753 | 1177 | 1177 | Kovat, NIST, Adams, Commercial Standard | 0.793 |
| 16 | α-terpineol | 15.409 | 1190 | 1188 | Kovat, NIST, Adams, Commercial Standard | 7.439 |
| 17 | α-terpinyl acetate | 21.998 | 1348 | 1349 | Kovat, NIST, Adams, Commercial Standard | 1.434 |
| 18 | α-gurjunene | 24.522 | 1402 | 1409 | Kovat, Adams, [35,36] | 0.165 |
| 19 | caryophyllene | 24.938 | 1414 | 1408 | Kovat, NIST, Adams, Commercial Standard | 1.787 |
| 20 | unknown | 25.796 | - | - | - | 0.292 |
| 21 | α-humulene | 26.317 | 1452 | 1454 | Kovat, NIST, Adams, Commercial Standard | 0.372 |
| 22 | alloaromadendrene | 26.611 | 1459 | 1460 | Kovat, NIST, Adams, [35,36] | 0.524 |
| 23 | viridiflorene | 28.012 | 1495 | 1496 | Kovat, Adams, Commercial Standard, [35] | 1.56 |
| 24 | γ-cadinene | 28.747 | 1514 | 1513 | Kovat, NIST, Adams, Commercial Standard | 0.178 |
| 25 | δ-cadinene | 29.1 | 1523 | 1523 | Kovat, NIST, Adams, Commercial Standard | 0.255 |
| 26 | trans-nerolidol | 30.706 | 1561 | 1563 | Kovat, NIST, Adams, Commercial Standard | 0.676 |
| 27 | viridiflorol | 31.866 | 1588 | 1592 | Kovat, NIST, Adams, Commercial Standard | 5.262 |
| 28 | ledol | 32.249 | 1596 | 1602 | Kovat, NIST, Adams, Commercial Standard | 0.901 |
Cymbopogon citratus is another medicinal plant commonly used in the pharmaceutical and cosmetic industries with potential applications in the food industry [37]. C. citratus EO is previously reported to be high in citral, a compound associated with effective sprout suppression in potato [1,37]. GC analysis of C. citratus EO used in this study confirms the presence of citral, an aldehyde mixture of neral and geranial. Major compounds of C. citratus EO include geranial, neral, geraniol, geranyl acetate, 6-methyl-5-heptene-2-one, camphene, linalool, 4-nonanone, γ-cadinene, caryophyllene, and limonene (Table 6). In addition to geranial and neral, geranyl acetate has also been reported as an effective sprout suppressant [1]. Given their high proportions within C. citratus EO, it is likely that these compounds are responsible for the complete suppression of sprouting observed in this study.
Table 6.
Cymbopogon citratus essential oil (EO) constituents identified via GC–MS–FID analysis.
| No. | Compound Name | Retention Time | Calculated KI | Actual KI | Identified | Area % |
|---|---|---|---|---|---|---|
| 1 | tricyclene | 5.723 | 922 | 921 | Kovat, NIST, Adams, Commercial Standard | 0.3055 |
| 2 | α-pinene | 6.015 | 933 | 932 | Kovat, NIST, Adams, Commercial Standard | 0.446 |
| 3 | camphene | 6.429 | 948 | 946 | Kovat, NIST, Adams, Commercial Standard | 2.3915 |
| 4 | 6-methyl-5-heptene-2-one | 7.435 | 981 | 981 | Kovat, NIST, Adams, Commercial Standard | 2.6885 |
| 5 | limonene | 8.899 | 1027 | 1024 | Kovat, NIST, Adams, Commercial Standard | 1.23 |
| 6 | trans-β-ocimene | 9.171 | 1035 | 1032 | Kovat, NIST, Adams, Commercial Standard | 0.171 |
| 7 | 4-nonanone | 10.436 | 1072 | - | NIST, Adams, Commercial Standard | 1.5955 |
| 8 | linalool | 11.552 | 1100 | 1095 | Kovat, NIST, Adams, Commercial Standard | 1.5965 |
| 9 | unknown | 11.677 | - | - | - | 0.218 |
| 10 | unknown | 13.084 | - | - | - | 0.231 |
| 11 | unknown | 13.516 | - | - | - | 0.3575 |
| 12 | citronellal | 13.646 | - | - | Kovat, NIST, Adams, Commercial Standard | 0.521 |
| 13 | unknown | 14.126 | - | - | - | 0.4845 |
| 14 | endo-borneol | 14.31 | 1168 | 1165 | Kovat, NIST, Adams, Commercial Standard | 0.213 |
| 15 | unknown | 14.88 | - | - | - | 0.9955 |
| 16 | α-terpineol | 15.323 | 1189 | 1186 | Kovat, NIST, Adams, Commercial Standard | 0.2205 |
| 17 | neral | 17.53 | 1243 | 1235 | Kovat, NIST, Adams, Commercial Standard | 30.295 |
| 18 | geraniol | 18.115 | 1258 | 1249 | Kovat, NIST, Adams, Commercial Standard | 6.0465 |
| 19 | geranial | 18.85 | 1275 | 1264 | Kovat, NIST, Adams, Commercial Standard | 41.4925 |
| 20 | geranyl acetate | 23.422 | 1379 | 1379 | Kovat, NIST, Adams, Commercial Standard | 4.3295 |
| 21 | caryophyllene | 24.935 | 1414 | 1408 | Kovat, NIST, Adams, Commercial Standard | 1.418 |
| 22 | α-humulene | 26.311 | 1451 | 1452 | Kovat, NIST, Adams, Commercial Standard | 0.3225 |
| 23 | γ-cadinene | 28.748 | 1513 | 1513 | Kovat, NIST, Adams | 1.4695 |
| 24 | δ-cadinene | 29.095 | 1522 | 1522 | Kovat, NIST, Adams, Commercial Standard | 0.423 |
| 25 | caryophyllene oxide | 31.402 | 1577 | 1582 | Kovat, NIST, Adams, Commercial Standard | 0.537 |
Owolabi et al. [38] demonstrated the potential of C. citratus EO as a sprout suppressant in Russet Burbank potatoes, corroborating the findings in the present study. Furthermore, Belay et al. [39] reported lower weight loss over a 14-week storage period in tubers treated with C. citratus EO, however, no difference in sprout length relative to a control was observed in either cultivar tested. EOs are known to show differences in sprout suppression depending on potato cultivar [16,23,39]. Therefore, it is possible that C. citratus EO is more effective in cultivars Ranger Russet, used in the present study, and Russet Burbank than it is in Gudene or Jalene [38,39]. C. citratus EO application has also been associated with reductions in potato tuber moth (Phthorimaea opperculella) infestation, suggesting that its use could provide additional benefits besides sprout suppression [40].
2.2. Effects of Essential Oils (EOs) on Number of Germinated Eyes
After 90 days of storage, a statistically significant two-way interaction between treatment and time was observed on sprout number (Table 1). This suggests that the impact of treatment on sprout number depends on the amount of time that has passed. Furthermore, the main effects of both treatment and time were significant (Table 1).
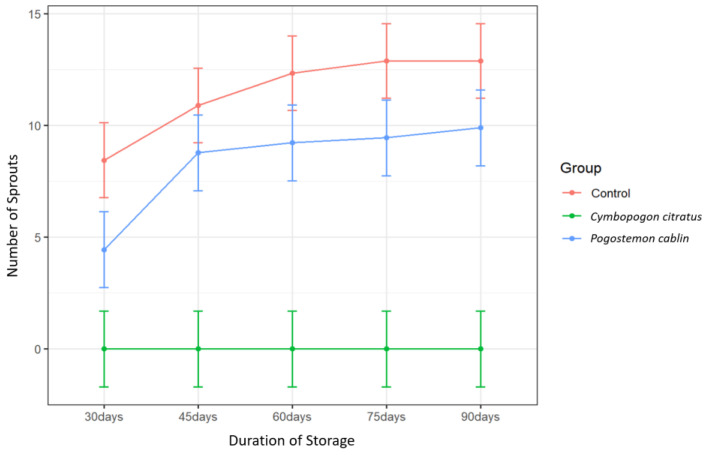
Only treatment with C. citratus EO resulted in a significant difference in sprout number relative to the control (Table 7). Though not significant, treatment with Pogostemon cablin EO resulted in slightly fewer sprouts relative to the control from 60–90 days (Figure 2). Complete suppression of sprouting was obtained over the entire 90-day storage period with C. citratus EO treatment, resulting in zero sprouting throughout the study (Figure 2). This contradicts findings by Belay et al. [39] reporting no effect of C. citratus EO on sprout numbers over the course of 16 weeks in cultivars Jalene and Gudene. However, like effects on sprout length, EO treatment effects on sprout number vary with cultivar [39]. The results of the present study suggest that C. citratus is a particularly effective sprout suppressant in Ranger Russet potatoes at room temperature.
Table 7.
Tukey’s test p-values describing the essential oils (EOs’) effects on sprout number relative to the control at all time points (*** = statistically significant).
| Plant EO | Duration of Storage | ||||
|---|---|---|---|---|---|
| 30 Days | 45 Days | 60 Days | 75 Days | 90 Days | |
| Myrtus communis | 0.9910 | 1.000 | 1.000 | 1.000 | 1.000 |
| Melaleuca quinquenervia | 0.2460 | 1.000 | 1.000 | 1.000 | 1.000 |
| Myristica fragrans | 0.9950 | 0.929 | 0.999 | 1.000 | 1.000 |
| Commiphora erythraea | 1.0000 | 0.992 | 0.999 | 1.000 | 0.999 |
| Citrus aurantium | 1.0000 | 1.000 | 1.000 | 0.997 | 0.999 |
| Citrus sinensis | 1.0000 | 1.000 | 0.995 | 1.000 | 0.998 |
| Bursera graveolens | 0.9220 | 0.992 | 1.000 | 1.000 | 0.999 |
| Petroselinum sativum | 0.9750 | 1.000 | 1.000 | 1.000 | 0.999 |
| Pogostemon cablin | 0.1870 | 0.903 | 0.146 | 0.053 | 0.085 |
| Cymbopogon citratus | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** |
Figure 2.
Number of sprouts per tuber over time of potatoes treated with distilled water (control), Cymbopogon citratus, and Pogostemon cablin EOs. Error bars represent the 95% confidence level of the means (emmeans method).
Essential oils (EOs) are generally believed to achieve sprout suppression by damaging the meristematic tissue of developing sprouts [22]. Indeed, 1,8-cineole application has been associated with complete necrosis of potato sprout tissue, whereas α-pinene and citral, an aldehyde mixture of geranial and neral, have been associated with necrosis of just the sprout tips [41]. However, sprouting was completely inhibited with C. citratus EO treatment, and no visible sprouts were observed throughout the 90-day storage period (Figure 3). SEM of tuber eyes from C. citratus treated potatoes reveals healthy, undeveloped bud tissue (Figure 4). Due to lack of discernable damage to the tuber bud tissue, it is therefore possible that C. citratus treatment may achieve sprout suppression through a mechanism that is different from the physical damage typically associated with EO sprout suppressants. For example, it is possible that C. citratus EO treatment may suppress sprouting by interfering with the plant hormone balance within the tubers or through another mechanism entirely. Several plant hormones including auxins, gibberellins, cytokinins, and abscisic acid are known to regulate dormancy release and sprouting in potato tubers [42]. Previous studies suggest that compounds such as citral may play a role in gibberellin and indole-3-acetic acid suppression in potato [43], whereas pinene isomers have been associated with fluctuations in abscisic acid concentrations [25]. Furthermore, 1,8-cineole and α-pinene have been shown to alter mitochondrial metabolism in corn, inhibiting root growth and interfering with germination [44] Similar mechanisms may be present in potato. Additionally, garlic EO application on potato tubers has been shown to alter the abundance of specific proteins associated with seed germination [45]. It is therefore possible that EOs achieve sprout suppression through a variety of mechanisms. Further studies performing proteomic analysis of EO-treated potatoes or investigating the effect of C. citratus EO and its pure components on the levels of various plant hormones in treated potatoes could help to determine the active ingredient(s) and provide important insights into their mode of action. This could hasten the identification of additional EOs with sprout suppressive capabilities and identify new target genes in tuber breeding programs.
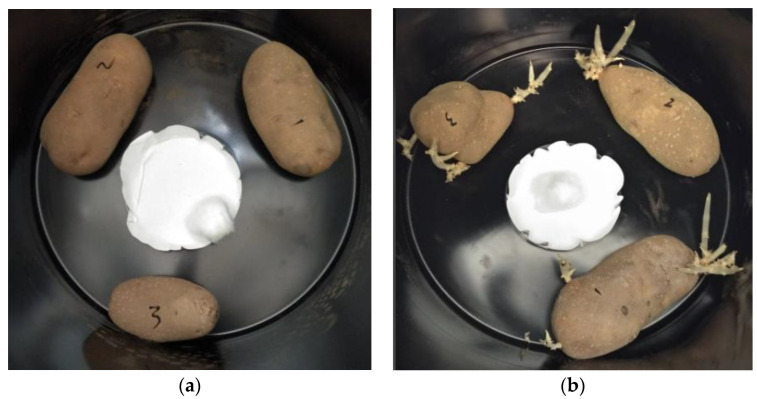
Figure 3.
Ranger Russet tubers treated with (a) Cymbopogon citratus essential oil (EO) and (b) distilled water at 90 days of storage.
Figure 4.
Scanning electron microscopy images of three potato eyes (a–c) from tubers treated with Cymbopogon citratus EO after 90 days of storage.
Recent studies suggest that various Cymbopogon spp. EOs may be promising potato sprout manipulators. Cymbopogon martini EO has been associated with sprout suppression at temperatures above 20 °C [12,39]. Similarly, Cymbopogon nardus EO can completely suppress sprouting for up to 30 days after dormancy break [46]. Conversely, Cymbopogon schoenanthus EO has been associated with sprout enhancement and increased yields [12]. The present study suggests that C. citratus EO is also an effective sprout suppressant at room temperature, corroborating previous findings by Owolabi et al. [38]. Greater focus and investigation into other species in this genus as potato sprout modulators in a wider range of cultivars is thus warranted.
The EO composition can vary widely depending on the plant parts used for extraction [35]. For this reason, it is possible that EOs not observed to be effective sprout suppressants in the present study could possess sprout suppressive properties if different plant parts are used for extraction. Similarly, different extractions of EOs shown to be effective in the present study from one or more plant parts may display variable effects on potato sprouting due to varying compositions. Indeed, studies comparing the effects of EOs extracted from the bark, leaves, or fruit of these species on potato sprouting could more fully illustrate their potential as sprout suppressants while expediting the identification of active ingredients and the best sources of EOs for use in this industry. Nevertheless, the EO of C. citratus, the most potent treatment, is commonly extracted from the whole aboveground plant parts in vegetative stage, that is stems and leaves.
Potatoes may be stored for many months before use, requiring multiple applications of sprout suppressants [13]. As only a single application of each EO was used, and typically, EO-based products are applied every 2 or 4 weeks, the storage period in the present study was set to 90 days. Future studies could investigate longer storage lengths or may test different concentrations of the most effective EOs. Importantly, even if an EO possesses significant sprout suppressive or inhibitory properties, it is possible that its application may alter the flavor, texture, or nutritional quality of treated potatoes. Therefore, additional studies are needed to investigate C. citratus and other EOs for their effects on other aspects of potato quality before commercial products may be developed.
Furthermore, larger studies comparing the effectiveness of C. citratus EO to conventional methods such as CIPC in semi-commercial and commercial settings are also needed. These types of studies are necessary to evaluate the efficacy of C. citratus EO in industrial settings and determine the feasibility of scaling up its use to a commercial scale.
3. Materials and Methods
3.1. Plant Material
Potato tubers of cultivar Ranger Russet were obtained from Oregon State University Hermiston Agricultural Research and Extension Center in Hermiston, OR, USA. Tubers were harvested in September 2021 and subsequently stored at 4 °C. All tubers were left untreated by any chemicals prior to the start of the experiment. Container studies were initiated in November 2021.
3.2. Experimental Materials
A total of 10 essential oils (EOs) including myrtle (Myrtus communis), niaouli (Melaleuca quinquenervia), nutmeg (Myristica fragrans), opopanax (Commiphora erythraea), bitter orange (Citrus aurantium), sweet orange (Citrus sinensis), palo santo (Bursera graveolens), parsley seed (Petroselinum sativum), patchouli (Pogostemon cablin), and lemongrass (Cymbopogon citratus) were used (Table 8). Cymbopogon citratus EO was purchased from Greenway Biotech, Inc. All other EOs were purchased from Mountain Rose Herbs (Eugene, OR, USA).
Table 8.
The plant parts, their origin, and the method of extraction used to produce the essential oils (EOs) used in the present study. Information was obtained from supplier websites.
| Plant EO | Plant Parts | Country of Origin | Method of Extraction |
|---|---|---|---|
| Myrtus communis | Leaves and Twigs | Tunisia | Steam Distillation |
| Melaleuca quinquenervia | Leaves and Twigs | Madagascar | Steam Distillation |
| Myristica fragrans | Seeds | Sri Lanka | Steam Distillation |
| Commiphora erythraea | Resin | Somalia | Steam Distillation |
| Citrus aurantium | Peels | Egypt | Steam Distillation |
| Citrus sinensis | Peels | USA | Cold Pressed |
| Bursera graveolens | Wood | Ecuador | Steam Distillation |
| Petroselinum sativum | Seeds | France | Steam Distillation |
| Pogostemon cablin | Leaves | Sri Lanka | Steam Distillation |
| Cymbopogon citratus | Leaves and Stems | Sri Lanka | Steam Distillation |
3.3. Experimental Design
One (1) mL of EO was pipetted on to a cotton ball sitting in a glass Petri dish lined with filter paper in the center of a new, previously unused black 20 L plastic container. Three randomly selected tubers were placed in each container and the containers were then sealed with aluminum foil for fumigation with the EO vapor. The Petri dish with EO had no direct contact with the tubers. A loose-fitting lid was placed on the containers, which were then stacked and left undisturbed aside from scheduled intervals for data collection as described in 2.4. Observations. There were 3 replications per EO treatment and the control with 1 mL distilled water, for a total of 3 mL of each EO and distilled water used in the experiment. The experiment was conducted at room temperature and lasted 90 days.
3.4. Observations
The effects of the treatments were evaluated by recording data on sprout length and number of sprouts starting at 30 days and continuing every 15 days thereafter until a 90-day storage period was reached.
At all data collection time points, the longest sprout on each tuber in each replication was recorded in millimeters and reported as sprout length. The total number of germinated (≥1 mm) eyes was recorded for all tubers in each replication and reported as sprout number. Averages of observations for each replication were calculated for later analysis.
3.5. Statistical Analysis
R software, Version 3.6.3, was used for the statistical analysis [47]. A linear mixed model was used to analyze both sprout length and sprout number. Due to wide variability of the data and to fulfill the ANOVA assumptions, a square root transformation was used on the sprout length data to achieve homogeneity of variance and normality of residuals. A post hoc Tukey’s HSD test was used as a multiple comparison test to identify differences in sprout length and number due to the different treatments across all time points. For the sprout length data, estimated marginal means and confidence intervals were back-transformed for reporting and graphics. To perform the aforementioned analysis, data summary, and graphics, we used various R packages (“ggpubr” [48], “tidyverse”, “rstatix”, “nlme”, “emmeans”, and “ggplot2” [48,49,50,51,52]).
3.6. Gas Chromatography Mass Spectrometry Flame Ionization Detection (GC–MS–FID) Essential Oil Analysis
Gas chromatography (GC)–mass spectroscopy (MS)–flame ionization detection (FID) analysis of C. citratus, M. quinquenervia and M. communis EOs was performed at the Natural Products Center of the USDA-ARS, Natural Products Utilization Research Unit in University, MS, USA. Using a micropipette, 50 μL of oil (weight measured on a tared balance) from each sample was transferred into a 10 mL volumetric flask. Samples were brought to volume with CHCl3. A 1 mL aliquot of each diluted oil sample was placed by glass pipet into a GC vial for analysis.
Oil samples were analyzed by GC–MS–FID on an Agilent (Santa Clara, CA, USA) 7890A GC system coupled to an Agilent 5975C inert XL MSD. Chemical standards and oils were analyzed using a DB-5 column (30 m × 0.25 mm fused silica capillary column, film thickness of 0.25 µm) operated using an injector temp of 240 °C, column temperature of 60 to 240 °C at 3 °C/min and held at 240 °C for 5 min, helium as the carrier gas, an injection volume of 1 µL (split ratio 25:1), and an MS mass range from 50 to 550. FID temperature was 300 °C. Post-column splitting was performed so that 50% of outlet flow proceeded to FID and 50% to mass spectrometry (MS) detection.
Compounds were identified by Kovats Index analyses, direct comparison of MS and retention time to authentic standards, and comparison of mass spectra with those reported in the Adams and NIST mass spectra databases, unless otherwise noted. Commercial standards were obtained from Sigma-Aldrich (St. Louis, MO, USA) for direct comparison.
Compounds were quantified by performing area percentage calculations based on the total combined FID area. For example, the area for each reported peak was divided by total integrated area from the FID chromatogram from all reported peaks and multiplied by 100 to arrive at a percentage. The percentage of a peak is a percentage relative to all other constituents integrated in the FID chromatogram.
3.7. Scanning Electron Microscopy
Three eyes were randomly selected and removed from whole tubers treated with C. citratus EO and placed in micro-centrifuge tubes containing deionized water. The eyes were ultra-sonicated for 5 min to remove remaining soil. The wash water was then removed and chemical fixative (2.5% glutaraldehyde, 1% paraformaldehyde in 0.1 M sodium cacodylate buffer) was added. The potato eyes remained in the fixative for 60 h at 40 °F. Samples were rinsed and serial dehydrated with ethanol and critical point dried for scanning electron microscopy (SEM). Dried samples were attached to a SEM stub mount with carbon tape and sputter coated with gold-palladium. Images were acquired using a Quanta 600 FEG SEM at Oregon State University.
4. Conclusions
Growing interest in organic foods and stricter regulations on the use of CIPC make EO sprout suppressants uniquely poised for expanded use in potato sprout suppression. While several EO-containing sprout suppressants are currently available, they exhibit variable efficacy depending on storage conditions, potato cultivars, and application schemes. The wide variability in plant secondary metabolites and in the chemical profiles of EOs suggests that many additional, effective EO sprout suppressants have yet to be discovered. The present study identified C. citratus EO as a highly effective sprout suppressant that completely suppresses sprouting in Ranger Russet potatoes for up to 90 days at room temperature storage. Results also suggest that the efficacy of M. communis and M. quinquenervia EOs could be enhanced if they are applied repeatedly during storage. This study clearly demonstrated the ability of select EOs to control sprouting in stored potato, offering an organic alternative to present practices dependent on the use of CIPC. Application schemes of these EOs in commercial settings will need to be investigated and optimized and their modes of action can be explored. Doing so will realize the benefits of their use in the potato industry and could allow for identification of other promising sprout suppressants via composition alone.
Acknowledgments
We thank Teresa Sawyer for assistance in acquiring SEM images. Authors also acknowledge the help from Sagar Sathuvalli’s potato breeding program and the OSU Hermiston Agricultural Research and Extension Center crew for growing and storing the potato for us. Authors also thank Solomon Yilma from the OSU potato breeding program for his support and advise.
Author Contributions
J.L.T.: Formal analysis, Investigation, Writing—original draft, Writing—review and editing, Visualization. C.L.C.: GC–MS–FID analysis, Writing—reviewing and editing. V.D.Z.: Conceptualization, Methodology, Resources, Writing—review and editing, Supervision, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This work was partially supported by the Oregon Potato Commission, the Oregon Department of Agriculture—Specialty Crops BGP ODA6024GR, and by USDA-NIFA 2021-51106-35584 grants awarded to Valtcho Zheljazkov (Jeliazkov).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boivin M., Bourdeau N., Barnabé S., Desgagné-Penix I. Sprout Suppressive Molecules Effective on Potato (Solanum tuberosum) Tubers during Storage: A Review. Am. J. Potato Res. 2020;97:451–463. doi: 10.1007/s12230-020-09794-0. [DOI] [Google Scholar]
- 2.Plant Production and Protection Division . International Year of the Potato 2008—New Light on a Hidden Treasure: An End-of-Year Review. FAO; Rome, Italy: 2009. [Google Scholar]
- 3.FAO . FAO Statistical Yearbook—World Food and Agriculture. FAO; Rome, Italy: 2021. World Food and Agriculture—Statistical Yearbook 2021. [Google Scholar]
- 4.USDA NASS Press Release. [(accessed on 18 October 2022)]; Available online: https://www.nass.usda.gov/Statistics_by_State/Alaska/Publications/Current_News_Release/PT09_1.pdf.
- 5.Paul V., Ezekiel R., Pandey R. Sprout Suppression on Potato: Need to Look beyond CIPC for More Effective and Safer Alternatives. J. Food Sci. Technol. 2016;53:1–18. doi: 10.1007/s13197-015-1980-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonnewald S., Sonnewald U. Regulation of Potato Tuber Sprouting. Planta. 2014;239:27–38. doi: 10.1007/s00425-013-1968-z. [DOI] [PubMed] [Google Scholar]
- 7.Visse-Mansiaux M., Soyeurt H., Herrera J.M., Torche J.-M., Vanderschuren H., Dupuis B. Prediction of Potato Sprouting during Storage. Field Crops Res. 2022;278:108396. doi: 10.1016/j.fcr.2021.108396. [DOI] [Google Scholar]
- 8.Magdalena G., Dariusz M. Losses during Storage of Potato Varieties in Relation to Weather Conditions during the Vegetation Period and Temperatures during Long-Term Storage. Am. J. Potato Res. 2018;95:130–138. doi: 10.1007/s12230-017-9617-x. [DOI] [Google Scholar]
- 9.Sorce C., Lorenzi R., Parisi B., Ranalli P. Physiological Mechanisms Involved In Potato (Solanum tuberosum) Tuber Dormancy And The Control Of Sprouting By Chemical Suppressants. Acta Hortic. 2005;684:177–186. doi: 10.17660/ActaHortic.2005.684.24. [DOI] [Google Scholar]
- 10.Paul V., Ezekiel R., Pandey R. Acrylamide in Processed Potato Products: Progress Made and Present Status. Acta Physiol. Plant. 2016;38:276. doi: 10.1007/s11738-016-2290-8. [DOI] [Google Scholar]
- 11.Wiltshire J.J.J., Cobb A.H. A Review of the Physiology of Potato Tuber Dormancy. Ann. Appl. Biol. 1996;129:553–569. doi: 10.1111/j.1744-7348.1996.tb05776.x. [DOI] [Google Scholar]
- 12.Shukla S., Pandey S.S., Chandra M., Pandey A., Bharti N., Barnawal D., Chanotiya C.S., Tandon S., Darokar M.P., Kalra A. Application of Essential Oils as a Natural and Alternate Method for Inhibiting and Inducing the Sprouting of Potato Tubers. Food Chem. 2019;284:171–179. doi: 10.1016/j.foodchem.2019.01.079. [DOI] [PubMed] [Google Scholar]
- 13.Kleinkopf G.E., Oberg N.A., Olsen N.L. Sprout Inhibition in Storage: Current Status, New Chemistries and Natural Compounds. Am. J. Potato Res. 2003;80:317. doi: 10.1007/BF02854316. [DOI] [Google Scholar]
- 14.Vaughn K.C., Lehnen L.P. Mitotic Disrupter Herbicides. Weed Sci. 1991;39:450–457. doi: 10.1017/S0043174500073215. [DOI] [Google Scholar]
- 15.Arena M., Auteri D., Barmaz S., Bellisai G., Brancato A., Brocca D., Bura L., Byers H., Chiusolo A., European Food Safety Authority (EFSA) et al. Peer Review of the Pesticide Risk Assessment of the Active Substance Chlorpropham. EFSA J. 2017;15:e04903. doi: 10.2903/j.efsa.2017.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briddon A., Stroud G.P. Efficacy of Sprout Suppressants Used Alone, or in Combination, to Control Sprouting of Stored Potato. Agriculture and Horticulture Development Board; Sutton Bridge Crop Storage Research; Spalding, UK: 2019. [Google Scholar]
- 17.National Potato Council Potato Statistical Yearbook. [(accessed on 29 April 2022)]. Available online: https://www.flipsnack.com/nationalpotatocouncil/2018-npc-statistical-yearbook.html.
- 18.Organic Trade Association U.S. Organic Sales Soar to New High of Nearly $62 Billion in 2020|OTA. [(accessed on 5 April 2022)]. Available online: https://ota.com/news/press-releases/21755.
- 19.Gómez-Castillo D., Cruz E., Iguaz A., Arroqui C., Vírseda P. Effects of Essential Oils on Sprout Suppression and Quality of Potato Cultivars. Postharvest Biol. Technol. 2013;82:15–21. doi: 10.1016/j.postharvbio.2013.02.017. [DOI] [Google Scholar]
- 20.Şanlı A., Karadogan T. Carvone Containing Essential Oils as Sprout Suppressants in Potato (Solanum tuberosum L.) Tubers at Different Storage Temperatures. Potato Res. 2019;62:345–360. doi: 10.1007/s11540-019-9415-6. [DOI] [Google Scholar]
- 21.Teper-Bamnolker P., Dudai N., Fischer R., Belausov E., Zemach H., Shoseyov O., Eshel D. Mint Essential Oil Can Induce or Inhibit Potato Sprouting by Differential Alteration of Apical Meristem. Planta. 2010;232:179–186. doi: 10.1007/s00425-010-1154-5. [DOI] [PubMed] [Google Scholar]
- 22.Frazier M.J., Olsen N., Kleinkopf G. Organic and Alternative Methods for Potato Sprout Control in Storage. University of Idaho Extension; Moscow, ID, USA: 2004. [Google Scholar]
- 23.Saunders S., Harper G. Integrating Alternative Sprout Suppressants for the Fresh Market. Sutton Bridge Crop Storage Research; Spalding, UK: 2019. [Google Scholar]
- 24.Aleksic V., Knezevic P. Antimicrobial and Antioxidative Activity of Extracts and Essential Oils of Myrtus communis L. Microbiol. Res. 2014;169:240–254. doi: 10.1016/j.micres.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Areco V.A., Figueroa S., Cosa M.T., Dambolena J.S., Zygadlo J.A., Zunino M.P. Effect of Pinene Isomers on Germination and Growth of Maize. Biochem. Syst. Ecol. 2014;55:27–33. doi: 10.1016/j.bse.2014.02.013. [DOI] [Google Scholar]
- 26.Zunino M.P., Zygadlo J.A. Effect of Monoterpenes on Lipid Oxidation in Maize. Planta. 2004;219:303–309. doi: 10.1007/s00425-004-1216-7. [DOI] [PubMed] [Google Scholar]
- 27.Luiza Ishii-Iwamoto E., Marusa Pergo Coelho E., Reis B., Sebastiao Moscheta I., Moacir Bonato C. Effects of Monoterpenes on Physiological Processes During Seed Germination and Seedling Growth. Curr. Bioact. Compd. 2012;8:50–64. doi: 10.2174/157340712799828223. [DOI] [Google Scholar]
- 28.Ulukanli Z., Oz A.T. The Effect of Oleum myrtle on the Fruit Quality of Strawberries during MAP Storage. J. Food Sci. Technol. 2015;52:2860–2868. doi: 10.1007/s13197-014-1325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boroujeni L.S., Hojjatoleslamy M. Using Thymus carmanicus and Myrtus communis Essential Oils to Enhance the Physicochemical Properties of Potato Chips. Food Sci. Nutr. 2018;6:1006–1014. doi: 10.1002/fsn3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mimica-Dukić N., Bugarin D., Grbović S., Mitić-Culafić D., Vuković-Gacić B., Orcić D., Jovin E., Couladis M. Essential Oil of Myrtus communis L. as a Potential Antioxidant and Antimutagenic Agents. Molecules. 2010;15:2759–2770. doi: 10.3390/molecules15042759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bekhechi C., Watheq Malti C.E., Boussaïd M., Achouri I., Belilet K., Gibernau M., Casanova J., Tomi F. Composition and Chemical Variability of Myrtus communis Leaf Oil From Northwestern Algeria. Nat. Prod. Commun. 2019;14:1934578X19850030. doi: 10.1177/1934578X19850030. [DOI] [Google Scholar]
- 32.Mulas M., Melis R.A.M. Essential Oil Composition of Myrtle (Myrtus communis) Leaves. J. Herbs Spices Med. Plants. 2011;17:21–34. doi: 10.1080/10496475.2011.556986. [DOI] [Google Scholar]
- 33.Khan M., Al-Mansour M.A., Mousa A.A., Alkhathlan H.Z. Compositional Characteristics of the Essential Oil of Myrtus communis Grown in the Central Part of Saudi Arabia. J. Essent. Oil Res. 2014;26:13–18. doi: 10.1080/10412905.2013.820671. [DOI] [Google Scholar]
- 34.Siddique S., Mazhar S., Parveen Z. Chemical Characterization, Antioxidant and Antimicrobial Activities of Essential Oil from Melaleuca quinquenervia Leaves. IJEB. 2018;56:686–693. [Google Scholar]
- 35.Chaverri C., Cicció J.F. Chemical Composition of Essential Oils of the Tree Melaleuca quinquenervia (Myrtaceae) Cultivated in Costa Rica. UNED Res. J. 2021;13:10. doi: 10.22458/urj.v13i1.3327. [DOI] [Google Scholar]
- 36.Ireland B.F., Hibbert D., Goldsack R.J., Doran J.C., Brophy J. Chemical Variation in the Leaf Essential Oil of Melaleuca quinquenervia (Cav.) S.T. Blake. Biochem. Syst. Ecol. 2002;30:457–470. doi: 10.1016/S0305-1978(01)00112-0. [DOI] [Google Scholar]
- 37.Majewska E., Kozłowska M., Gruczyńska-Sękowska E., Kowalska D., Tarnowska K. Lemongrass (Cymbopogon citratus) Essential Oil: Extraction, Composition, Bioactivity and Uses for Food Preservation—A Review. Pol. J. Food Nutr. Sci. 2019;69:327–341. doi: 10.31883/pjfns/113152. [DOI] [Google Scholar]
- 38.Owolabi M., Olowu R., Lajide L., Oladimeji M., Padilla E., Flores-Fernández J. Inhibition of Potato Tuber Sprouting during Storage by the Controlled Release of Essential Oil Using a Wick Application Method. Ind. Crops Prod. 2013;45:83–87. doi: 10.1016/j.indcrop.2012.11.043. [DOI] [Google Scholar]
- 39.Belay D.W., Asfaw Z., Lulekal E., Kassa B., Kifele H. Effects of Essential Oils on Potato Tuber Sprouting at Room Temperature Storage in Ethiopia. Heliyon. 2022;8:e09090. doi: 10.1016/j.heliyon.2022.e09090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharaby A., Rahman H.A., Abdel-Aziz S., Moawad S.S. Ecologia Balkanica. Ecol. Balk. 2014;6:1–10. [Google Scholar]
- 41.Vaughn S.F., Spencer G.F. Volatile Monoterpenes Inhibit Potato Tuber Sprouting. Am. Potato J. 1991;68:821–831. doi: 10.1007/BF02853856. [DOI] [Google Scholar]
- 42.Eshel D. Advances in Plant Dormancy. Springer International Publishing; Cham, Switzerland: 2015. Bridging dormancy release and apical dominance in potato tuber; pp. 187–196. [Google Scholar]
- 43.Huang H., Ettoumi F., Li L., Xu Y., Luo Z. Emulsification-Based Interfacial Synthesis of Citral-Loaded Hollow MIL-88A for the Inhibition of Potato Tuber Sprouting. Food Chem. 2022;393:133360. doi: 10.1016/j.foodchem.2022.133360. [DOI] [PubMed] [Google Scholar]
- 44.Abrahim D., Braguini W.L., Kelmer-Bracht A.M., Ishii-Iwamoto E.L. Effects of Four Monoterpenes on Germination, Primary Root Growth, and Mitochondrial Respiration of Maize. J. Chem. Ecol. 2000;26:611–624. doi: 10.1023/A:1005467903297. [DOI] [Google Scholar]
- 45.Li L., Chen J., Li Z., Li H., Yang S., Ren B., Lu Y., Zheng S., Yu L., Wang X., et al. Proteomic Analysis of Garlic Essential Oil-Treated Potato Reveals That StHSP26.5 as a Vital Gene Involving in Tuber Sprouting. Postharvest Biol. Technol. 2022;183:111725. doi: 10.1016/j.postharvbio.2021.111725. [DOI] [Google Scholar]
- 46.Jia B., Xu L., Guan W., Lin Q., Brennan C., Yan R., Zhao H. Effect of Citronella Essential Oil Fumigation on Sprout Suppression and Quality of Potato Tubers during Storage. Food Chem. 2019;284:254–258. doi: 10.1016/j.foodchem.2019.01.119. [DOI] [PubMed] [Google Scholar]
- 47.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2021. [(accessed on 18 October 2022)]. Available online: https://www.R-project.org/ [Google Scholar]
- 48.Kassambara A. Ggpubr: ‘ggplot2’ Based Publication Ready Plots. 2020. [(accessed on 18 October 2022)]. Available online: https://CRAN.R-project.org/package=ggpubr.
- 49.Kassambara A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 2021. [(accessed on 18 October 2022)]. Available online: https://CRAN.R-project.org/package=rstatix.
- 50.Lenth R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. 2022. [(accessed on 18 October 2022)]. Available online: https://CRAN.R-project.org/package=emmeans.
- 51.Pinheiro J., Bates D., DebRoy S., Sarkar D., R Core Team Nlme: Linear and Nonlinear Mixed Effects Models. 2022. [(accessed on 18 October 2022)]. Available online: https://CRAN.R-project.org/package=nlme.
- 52.Wickham H., Averick M., Bryan J., Chang W., McGowan L.D., François R., Grolemund G., Hayes A., Henry L., Hester J., et al. Welcome to the Tidyverse. J. Open Source Softw. 2019;4:1686. doi: 10.21105/joss.01686. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.