Abstract
Flavonoids are naturally occurring compounds widely distributed in the Citrus genus. These natural compounds have many health benefits, mainly for metabolic and cardiovascular diseases. In fact, some these compounds are components of drug products with approved indications for peripheral vascular insufficiency and hemorrhoids. However, information on pharmacological effects of these compounds remains disperse and there is scarce comprehensive analysis of whole data and evidence. These kinds of evidence analyses could be necessary in drug design and the development of novel and innovate drug products in diabetes and hypertension. We aimed to systematically search for evidence on the efficacy of citroflavonoids in diabetes and hypertension in in vivo models. We searched four literature databases based on a PICO strategy. After database curation, twenty-nine articles were retrieved to analyze experimental data. There was high heterogeneity in both outcomes and methodology. Naringenin and hesperetin derivates were the most studied citroflavonoids in both experimental models. More investigation is still needed to determine its potential for drug design and development.
Keywords: biochemical parameters, blood markers, gene expression, hesperidin, naringenin, protein content
1. Introduction
Flavonoids comprise one of the significant natural compounds that have been interesting for researchers in the last two decades. They are a class of secondary metabolites widely spread in nature and found in several fruits and vegetables, mainly in Citrus genus [1]. Citroflavonoids (flavonoids extracted from Citrus species) are molecules characterized by multiple health benefits and biological activities, such as anticarcinogenic, antihyperglycemic, antihyperlipidemic, neuroprotective, and hepatoprotective activities reported in experimental and clinical frameworks. Hesperidin, nobiletin, naringin, diosmin, quercetin, rutin, hesperetin, naringenin and tangeretin are major citroflavonoids identified in nature [1]. It is common to find dietary flavonoids used as glycosylated derivates. The loss of glycosyl moiety is a critical step in the absorption and metabolism of citroflavonoids [2]. In fact, only aglycones and some glycosides can be absorbed. This evidence shows the importance of first-step metabolism on citroflavonoid bioactivity [3].
Some systematic reviews have exposed the impact of citroflavonoid consumption on cardiovascular disease and diabetes. [4,5,6]. An extensive systematic review found a reverse association between the consumption of flavonoids and a reduction in the risk of cardiovascular disease [6]. Until now, no published systematic review has assessed the effect of flavonoid-rich fruits on blood pressure [7]. On the other hand, although the association between the consumption of citrus fruits and the incidence of diabetes is not fully understood, some systematic reviews have shown that this behavior has a protective role [8].
Interestingly, some nutraceuticals have been developed for treating prediabetes as therapeutic alternatives. Eriomin®, a formula of citrus flavonoids composed of three single flavonoids, is a marketed end-product developed in Brazil. A double-blind, randomized, controlled trial showed evidence that this flavonoid mixture produces anti-inflammatory, antihyperglycemic, and antioxidant in those patients [9]. Furthermore, diosmin and hesperidin are well known components of the approved medicine Daflon®, a medication indicated for the treatment of peripheral vascular insufficiency and hemorrhoids [10]. An example such as this should be used as an encouraging factor to promote drug development of new and improved flavonoid-based end-products achievable for the population with cardiovascular risk factors.
Recently, systematic review and meta-analysis have become increasingly important in decision-making in the research field [11]. However, information on pharmacological effects of citroflavonoids remains disperse and there is scare comprehensive analysis of whole data and evidence. These kinds of evidence analyses could be necessary in drug design and the development of novel and innovate drug products in diabetes and hypertension. This work compares the efficacy outcomes reported for citroflavonoids evaluated in experimental diabetes and hypertension animal models. This investigation could be used as a reference for deciding what flavonoid is most promising for drug development and learning more about state-of-the-art flavonoid research.
2. Results
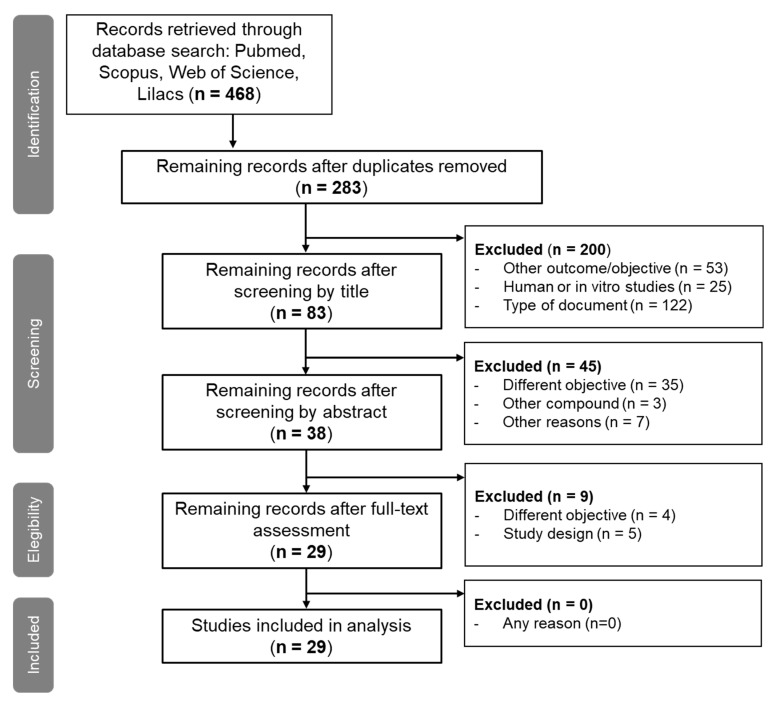
Overall, 468 articles were identified in primary searching (Pubmed = 110, Scopus = 187, Web of Science = 161, Lilacs = 10). After duplicate elimination, 283 unique articles remained for an abstract, title, and full-text revision. Only 29 articles (21 for diabetes and 8 for hypertension) remained for data analysis and discussion (Figure 1).
Figure 1.
PRISMA flowchart of the selection process of articles that fulfilled the criteria.
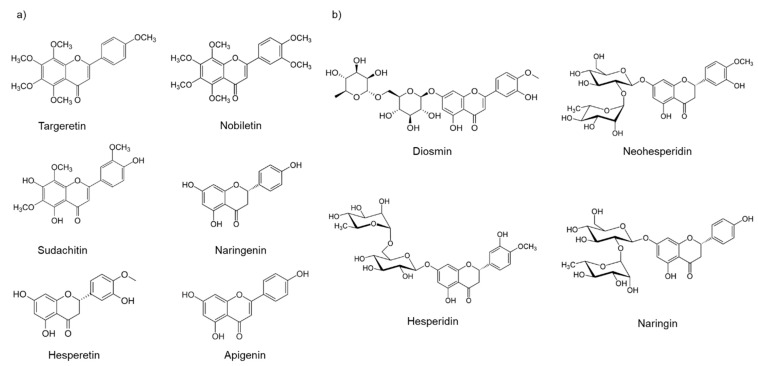
After full-text revision of selected articles, we found that the most studied flavonoids in diabetes and hypertension animal models were hesperidin (diabetes: 7, hypertension: 4), naringin (diabetes: 6, hypertension: 0), naringenin (diabetes: 5, hypertension: 1), diosmin (diabetes: 2, hypertension: 1), nobiletin (diabetes: 1, hypertension: 2), glycosyl-hesperidin (diabetes: 0, hypertension: 2), hesperitin (diabetes: 1, hypertension: 1), targeretin (diabetes: 1, hypertension: 0), sudachitin (diabetes: 1, hypertension: 0), apigenin (diabetes: 0, hypertension: 1), and neohesperidin (diabetes: 1, hypertension: 0). A list of these molecules is positioned below (Figure 2).
Figure 2.
Chemical structures of the citroflavonoids were found in selected articles of the systematic searching; (a) aglycones, (b) glycosides. Sketched structures were designed by ChemDraw Professional 20 software (PerkinElmer, Inc., Waltham, MA, USA).
Hesperidin, naringin, and naringenin (naringin aglycone) were the most studied citroflavonoids. Plasma biomarkers of carbohydrate and lipid metabolism, such as glucose and lipidic molecules (cholesterol, triglycerides, lipoproteins), liver function biomarkers (TGP, TGO, and alkaline phosphatase), were the most evaluated parameters for determining the bioactivity of citroflavonoids in diabetes. Moreover, body weight changes over time were assessed in almost all studies despite the low activity on this parameter. In addition, functional metabolic assays were also performed to establish changes in glucose uptake, insulin tolerance, and other metabolic pathways (Table 1).
Table 1.
Study design of selected articles on diabetes animal models.
| No | Compound Name | Dosing | Follow Up Period | Animal Model | Study Outcomes | Reference | |
|---|---|---|---|---|---|---|---|
| Type of Animal | Age/Weight | ||||||
| 1 | Naringin | 100 mg/kg | 4 weeks | STZ-NA Wistar rat | 130–150 g | Blood biomarkers, OGTT, liver-specific enzyme activity, gene expression, and biomarkers content | [12] |
| Naringenin | |||||||
| 2 | Hesperidin | 10 mg/kg (1%) * | 4 weeks | Goto-Kakizaki rat | 3 weeks old | Blood biomarkers, liver-specific enzyme activity and gene expression | [13] |
| 3 | Hesperidin | 10 g/kg (1%) * | 4 weeks | STZ Wistar rat | 3 weeks old | Blood biomarkers, tissue-specific biomarkers content, and enzyme activity, serum flavonoid content, bone density | [14] |
| 4 | Naringenin | 30 mg/kg (3%) * | 4 weeks | Lep ob/ob mouse | 8–12 weeks old | Blood biomarkers, OGTT, ITT, tissue-specific biomarkers content, and gene expression, IHC analysis, anthropometric micro-CT imaging | [15] |
| 5 | Naringenin | 30 mg/kg (3%) * | 12 weeks | C57BL/6J Ldlr−/− mouse | 10–12 weeks old | Blood biomarkers, tissue-specific biomarkers content and gene expression, OGTT, ITT, IHC analysis, flow cytometry analysis for cell identification | [16] |
| 6 | Hesperetin | 40 mg/kg | 6 weeks | Wistar rat | 170–200 g | Blood biomarkers, tissue-specific biomarkers content, IHC analysis, oxidative stress assessment | [17] |
| 7 | Neohesperidin | 50 mg/kg | 6 weeks | KK-Ay mice | 8 weeks old | OGTT, ITT, blood biomarkers, liver-specific parameters, tissue-specific gene expression and protein quantification | [8] |
| 8 | Hesperidin | 0.2 g/kg * | 5 weeks | db/db mice | 5 weeks old | Blood biomarkers, liver-specific parameters, IHC analysis | [18] |
| Naringin | (23 g) | ||||||
| 9 | Hesperidin | 0.2 g/kg * | 5 weeks | db/db mice | 5 weeks old | Blood biomarkers, tissue-specific gene expression, protein quantification, and enzyme activity | [19] |
| Naringin | |||||||
| 10 | Hesperidin | 50 mg/kg | 4 weeks | HFD/STZ-Wistar rat | 180–200 g | OGTT, blood biomarkers, liver-specific enzyme activity, in situ intestinal glucose absorption, in vitro insulin secretion test | [20] |
| Naringin | |||||||
| 11 | Naringin | 100 mg/kg | 4 weeks | High fructose diet Sprague- Dawley rat | 180–200 g | Vascular reactivity, blood biomarkers, artery-specific protein quantification | [21] |
| 12 | Naringenin | 10, 30 mg/kg (1% or 3%) * | 4 weeks | C57BL/6J Ldlr−/− mouse | 12 weeks old | OGTT, ITT, blood biomarkers, tissue-specific parameters, gene expression, and enzyme activity, in situ intestinal lipid absorption, energy expenditure | [22] |
| 13 | Nobiletin | 10, 30 mg/kg (1% or 3%) * | 8–26 weeks | C57BL/6J Ldlr−/− mouse | 12 weeks old | Tissue- and cell-specific gene expression, blood biomarkers, hyperinsulinemic—euglycemic clamp and pyruvate tolerance test energy expenditure | [23] |
| 14 | Naringenin | 6, 12.5, 25 mg/kg | 6 weeks | HFD/STZ Wistar rat | 12–13 weeks old (75–100 g) | Blood biomarkers, lipid peroxidation determination, tissue-specific enzyme activity, gene expression, and protein quantification, IHC analysis | [24] |
| 15 | Naringin | 50, 100, 200 mg/kg * | 3 weeks | HFD/STZ Wistar rat | 150–200 g | Blood biomarkers, tissue-specific lipid determination, enzyme activity, and gene expression | [25] |
| 16 | Diosmin | 100 mg/kg | 6 weeks | STZ Wistar rat | 200–220 g | Blood biomarkers, tissue-specific enzyme activity, IHC analysis | [26] |
| 17 | Diosmin | 100 mg/kg | 6 weeks | STZ Wistar rat | 180–220 g | Blood biomarkers, tissue-specific lipid determination and enzyme activity | [27] |
| 18 | Targeretin | 100 mg/kg | 4 weeks | STZ Wistar rat | 180–200 g | OGTT, blood biomarkers, liver-specific enzyme activity and glycogen content, IHC analysis | [28] |
| 19 | Hesperidin | 25, 50, 100 mg/kg | 4 weeks | STZ Wistar rat | 200–220 g | OGTT, blood biomarkers, liver-specific enzyme activity and glycogen content, IHC analysis | [29] |
| 20 | Hesperidin | 20 ppm ** | 2 weeks, 3 days | STZ albino mouse † | ~30 g | Blood biomarkers, malformations rate, number of diabetic fetuses, whole body staining analysis | [30] |
| 21 | Sudachitin | 5 mg/kg | 12 weeks | HFD and db/db mouse | 4 weeks old | Blood biomarkers, anthropometric computed tomography analysis, OGTT, ITT, tissue-specific gene expression, energy expenditure | [31] |
Note: On some studies flavonoid preparations were supplemented by pellet-based diet (*) or drinking water (**). † This study was performed on pregnant diabetic mice. HFD: high-fat diet; IHC: immunohistochemistry staining technique; ITT: insulin tolerance test; OGTT: oral glucose tolerance test, STZ: streptozotocin-induced diabetic animal, STZ-NA: streptozotocin-nicotinamide-induced diabetic animal.
Conversely, tail blood pressure measurement was the significant endpoint studied in hypertensive animal models. In addition, vascular reactivity to contraction or relaxation inducers in vessels extracted from these animals was the second most evaluated endpoint (Table 2). A comprehensive and detailed set of experimental data from each selected article is described in Tables S1 and S2.
Table 2.
Study design of selected articles on hypertension animal models.
| No | Compound Name | Dosing | Treatment Duration | Animal Model | Efficacy Outcome | Reference | |
|---|---|---|---|---|---|---|---|
| Type of Animal | Age/Weight | ||||||
| 1 | Hesperidin | 50 mg/kg | 4 weeks | SHR rat | 15 weeks | Blood pressure changes, vascular reactivity | [32] |
| 2 | Nobiletin | 20, 40 mg/kg * | 4 weeks | SHR (stroke prone) rat | 7 weeks | Blood pressure changes, cerebral vessels thrombogenesis test | [33] |
| 3 | Hesperidin | 30 mg/kg * | 25 weeks | SHR rat | 3 weeks | Blood pressure and heart rate changes | [34] |
| Glucosyl hesperidin | |||||||
| 4 | Apigenin | 1.44 mg/kg ** | 6 weeks | L-NAME Sprague–Dawley | 300–325 g | Blood pressure and heart rate changes, vascular reactivity, IHC analysis | [35] |
| Diosmin | 7.16 mg/kg ** | ||||||
| 5 | Nobiletin | 20, 40 mg/kg | 2 weeks | L-NAME Sprague–Dawley | 220–250 g | Conscious and unconscious blood pressure changes, vascular reactivity, artery-specific protein quantification, blood nitrate/nitrite quantification, IHC analysis | [36] |
| 6 | Hesperidin-naringenin mixture | 150 mg/kg | 4 weeks | SHR rat | 250–300 g | Blood pressure and heart rate changes, vascular reactivity | [37] |
| 7 | Hesperidin | 1 mg/kg * | 12 weeks | HFaD Apo-E KO mouse | 9 weeks old | Blood biomarkers, vascular reactivity, IHC analysis | [38] |
| Glucosyl hesperidin | 5 mg/kg * | ||||||
| 8 | Hesperidin | 20, 40 mg/kg | 4 weeks | One-clipped kidney Sprague-Dawley rat | 150–180 g | Conscious and unconscious blood pressure changes, blood biomarkers, vascular reactivity, artery-specific enzyme activity and protein content, blood nitrate/nitrite quantification | [39] |
Note: On some studies flavonoid preparations were supplemented by pellet-based diet (*) or drinking water (**). This study was performed on pregnant diabetic mice. HFD: high-fat diet; IHC: immunohistochemistry staining technique; ITT: insulin tolerance test; OGTT: oral glucose tolerance test, STZ: streptozotocin-induced diabetic animal, STZ-NA: streptozotocin-nicotinamide-induced diabetic animal.
3. Discussion
Citrus fruits are rich in flavonoid compounds, such as hesperidin, hesperetin, naringin, naringenin, diosmin, quercetin, rutin, nobiletin, tangeretin, and others. Mainly, citroflavonoids are present in many citrus fruits, such as bergamots, grapefruit, lemons, limes, mandarins, oranges, and pomelos [20]. Since the early 1950s, flavonoid research has been continuously growing due to the widespread health benefits found for these natural compounds and their applicability in managing non-communicable and infective diseases. Interestingly, from 1996 to the present, the investigation of these natural compounds has increased exponentially (Figure S1). However, evidence searching based on systematic reviews has arisen in the last two decades (Figure S2). For this reason, our systematic review focused on updating and acquiring more knowledge about the biological effects reported in experimental studies extensively.
Our systematic review showed that hesperidin or its aglycone was used in doses from 10 to 100 mg/kg, as naringin and its aglycone were administered. There was not a reasonable explanation as to why researchers selected this dose range. However, regarding other systematic reviews on flavonoid bioactivity, this dose range is employed for any experimental study that evaluates bioactivity in animal species [29].
Several studies investigate the effect of flavonoid-rich fruit extract on blood pressure but with conflicting results. Moreover, herbal extracts are composed of multiple compound groups, mostly polyphenol-like structures that have been to prove also improve cardiovascular and metabolic status in patients [32].
Other findings showed that STZ-induced diabetic rat (Sprague Dawley or Wistar strain) and SHR models were the most used for flavonoid bioactivity assessment. Notably, although the Sprague Dawley model was developed from the Wistar strain, data show that the Wistar strain is prone to metabolic impairments [33]. For this reason, a Sprague Dawley model might be a more acceptable and similar model of diabetes than those on Wistar rats.
The analysis showed a variety of oral administration (by drinking water, enriched diet, or intragastric administration) and time framework (4–25 weeks). Furthermore, using different vehicles such as distilled water and carboxymethylcellulose might be a bias factor. For this reason, a meta-analysis was not possible. Additionally, animal species were possibly crucial for representing biological behavior and processes than chemical- or diet-induced models to surgery-based models with unreal conditions of essential hypertension (renal involved hypertension) [31]. The most used animal models for diabetes and hypertension were streptozotocin (STZ)-induced diabetic rat and spontaneously hypertensive rat (SHR) models.
Currently, some articles have systematically searched to analyze natural products’ proficiency in specific illnesses (e.g., triterpenes on wound healing). Recently, Chen et al. reviewed phenylated flavonoids and their biological effects; this investigation focused on the structural properties of the phenylated flavonoids and the implication for biological activity [17].
Finally, the precise mechanism by which flavonoids exert their antidiabetic and/or antihypertensive effects is unclear. However, evidence suggests that flavonoids might improve the oxidant/antioxidant status imbalance. For example, Jayaraman et al. reported that hesperetin might improve the antioxidant capacity of the liver tissue by restoring enzymatic activity and content [22]. On the other hand, it has been reported that citroflavonoids regulate inflammatory response and deposition of extracellular matrix [12]. More investigation on mechanistic evaluations must be encouraged.
4. Materials and Methods
4.1. Literature Sources
We performed a systematic search of the literature in four major databases: PubMed (Medline), Scopus, Web of Science, and Lilacs. PRISMA statement was followed to establish the evidence-based minimum set of items for reporting on systematic review (Figure 3).
Figure 3.
Graphic representation of the methodological process applied to perform this systematic review.
4.2. Eligibility Criteria
A PICO strategy was developed as described in Table S1. Briefly, the databases were searched using combinations of the following terms: citrus, flavonoid, citroflavonoid, diabetes, and hypertension. A filter was applied to retrieve only experimental animal-based studies up to 2019. Clinical trials, case reports, narrative reviews, editorial letters, and comments were excluded. Systematic reviews and meta-analyses were used only for comparative analysis and results in discussion. Duplicated articles and those without abstract or full-text documents were also excluded from this study. Language limits were not considered.
4.3. Studies Selection
The inclusion criteria used for selecting studies are described below. All articles containing the above terms in title, abstract or full text and those reporting experimental outcomes on diabetes and hypertension induced by mixed or single citroflavonoids were selected. Only studies where oral administration is employed were included. The selection was conducted by two independent reviewers for discrepancies.
4.4. Meta-Analysis
A meta-analysis of the retrieved data could not be carried out due to methodological heterogeneity such as dosing, mode of administration, animal species, and exposure time.
4.5. Risk of Bias Assessment
To assess the risk of biases in the selected studies, we applied a modified version of Cochrane’s RoB tool, the so-called Systematic Review Centre for Laboratory animal Experimentation (SYRCLE). This tool considers six biases: selection, performance, detection, attrition, reporting, and other biases [12,40].
5. Conclusions
Systematic searching and comprehensive literature analysis should be the first-line task in pharmacology research to perform an evidence-based decision and to know what compound or substance family needs to be investigated and what will be the way and strategy to be applied to produce more original and relevant data. Furthermore, systematic analysis of evidence could be a promising area to find well-known lead molecules able to be repositioned in the clinical management of hypertension and diabetes.
Acknowledgments
Sánchez Recillas thanks CONACyT for the postdoctoral scholarship (EPN 2018–2; Reference number: 298553). All authors are grateful to Tabatha Rios-Inurreta for technical and statistical assistance. Finally, authors are fully grateful with the R&D consulting agency Hypermedic MX for providing technical assistance in concept design, methodology and reporting.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27227933/s1. Figure S1: Growth in total publications on flavonoid research from the early 1950s to the present regarding human health benefits; Figure S2: The increased systematic search for previous reports on the efficacy and safety of citroflavonoids in any disease model or clinical trial has emerged in the last two decades. Table S1: Major outcomes reported in selected articles in diabetes animal models; Table S2: Major outcomes reported in selected articles in hypertension animal models.
Author Contributions
Conceptualization, R.O.-A., J.A.A.L. and J.C.S.-S.; Data curation, J.A.A.L., J.C.S.-S., A.S.-R. and P.V.-G.; Formal analysis, P.V.-G.; Investigation, R.O.-A. and E.H.-N.; Methodology, R.O.-A., J.A.A.L., J.C.S.-S., A.S.-R., P.V.-G. and E.H.-N.; Software, A.S.-R.; Writing—original draft, R.O.-A.; Writing—review and editing, J.A.A.L., J.C.S.-S. and E.H.-N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the project “Estudio preclínico de una forma farmacéutica sólida de citroflavonoides con propiedades hipoglucemiantes e hipotensores: Caracterización farmacocinética y farmacodinámica de un potencial fitofármaco” (CONACYT-Project ID number: PN-756).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barreca D., Trombetta D. Flavonoids as Promising Therapeutics of the Future: A Hub for Cells Survival or Death. Curr. Med. Chem. 2019;26:5092–5093. doi: 10.2174/092986732627191001091228. [DOI] [PubMed] [Google Scholar]
- 2.Walle T., Browning A.M., Steed L.L., Reed S.G., Walle U.K. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. J. Nutr. 2005;135:48–52. doi: 10.1093/jn/135.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollman P.C., Katan M.B. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed. Pharmacother. 1997;51:305–310. doi: 10.1016/S0753-3322(97)88045-6. [DOI] [PubMed] [Google Scholar]
- 4.Hooper L., Kroon P.A., Rimm E.B., Cohn J.S., Harvey I., Le Cornu K.A., Ryder J.J., Hall W.L., Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 5.Shi G.J., Li Y., Cao Q.H., Wu H.X., Tang X.Y., Gao X.H., Yu J.Q., Chen Z., Yang Y. In vitro and in vivo evidence that quercetin protects against diabetes and its complications: A systematic review of the literature. Biomed. Pharmacother. 2019;109:1085–1099. doi: 10.1016/j.biopha.2018.10.130. [DOI] [PubMed] [Google Scholar]
- 6.Wang X., Ouyang Y.Y., Liu J., Zhao G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014;111:1–11. doi: 10.1017/S000711451300278X. [DOI] [PubMed] [Google Scholar]
- 7.Ellwood L., Torun G., Bahar Z., Fernandez R. Effects of flavonoid-rich fruits on hypertension in adults: A systematic review. Libr. Syst. Rev. 2019;17:2075–2105. doi: 10.11124/JBISRIR-D-19-00050. [DOI] [PubMed] [Google Scholar]
- 8.Jia X., Zhong L., Song Y., Hu Y., Wang G., Sun S. Consumption of citrus and cruciferous vegetables with incident type 2 diabetes mellitus based on a meta-analysis of prospective study. Prim. Care Diabetes. 2016;10:272–280. doi: 10.1016/j.pcd.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Ribeiro C.B., Ramos F.M., Manthey J.A., Cesar T.B. Effectiveness of Eriomin® in managing hyperglycemia and reversal of prediabetes condition: A double-blind, randomized, controlled study. Phytother. Res. PTR. 2019;33:1921–1933. doi: 10.1002/ptr.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakkos S.K., Nicolaides A.N. Efficacy of micronized purified flavonoid fraction (Daflon®) on improving individual symptoms, signs and quality of life in patients with chronic venous disease: A systematic review and meta-analysis of randomized double-blind placebo-controlled trials. Int. Angiol. 2018;37:143–154. doi: 10.23736/S0392-9590.18.03975-5. [DOI] [PubMed] [Google Scholar]
- 11.Sánchez-Salgado J.C., Estrada-Soto S., García-Jiménez S., Montes S., Gómez-Zamudio J., Villalobos-Molina R. Analysis of Flavonoids Bioactivity for Cholestatic Liver Disease: Systematic Literature Search and Experimental Approaches. Biomolecules. 2019;9:102. doi: 10.3390/biom9030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulvihill E.E., Allister E.M., Sutherland B.G., Telford D.E., Sawyez C.G., Edwards J.Y., Markle J.M., Hegele R.A., Huff M.W. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes. 2009;58:2198–2210. doi: 10.2337/db09-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akiyama S., Katsumata S., Suzuki K., Nakaya Y., Ishimi Y., Uehara M. Hypoglycemic and hypolipidemic effects of hesperidin and cyclodextrin-clathrated hesperetin in Goto-Kakizaki rats with type 2 diabetes. Biosci. Biotechnol. Biochem. 2009;73:2779–2782. doi: 10.1271/bbb.90576. [DOI] [PubMed] [Google Scholar]
- 14.Akiyama S., Katsumata S., Suzuki K., Ishimi Y., Wu J., Uehara M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J. Clin. Biochem. Nutr. 2010;46:87–92. doi: 10.3164/jcbn.09-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burke A.C., Sutherland B.G., Telford D.E., Morrow M.R., Sawyez C.G., Edwards J.Y., Huff M.W. Naringenin enhances the regression of atherosclerosis induced by a chow diet in Ldlr(-/-) mice. Atherosclerosis. 2019;286:60–70. doi: 10.1016/j.atherosclerosis.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Assini J.M., Mulvihill E.E., Burke A.C., Sutherland B.G., Telford D.E., Chhoker S.S., Sawyez C.G., Drangova M., Adams A.C., Kharitonenkov A., et al. Naringenin prevents obesity, hepatic steatosis, and glucose intolerance in male mice independent of fibroblast growth factor 21. Endocrinology. 2015;156:2087–2102. doi: 10.1210/en.2014-2003. [DOI] [PubMed] [Google Scholar]
- 17.Chen X., Mukwaya E., Wong M.S., Zhang Y. A systematic review on biological activities of prenylated flavonoids. Pharm. Biol. 2014;52:655–660. doi: 10.3109/13880209.2013.853809. [DOI] [PubMed] [Google Scholar]
- 18.Jung U.J., Lee M.K., Jeong K.S., Choi M.S. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J. Nutr. 2004;134:2499–2503. doi: 10.1093/jn/134.10.2499. [DOI] [PubMed] [Google Scholar]
- 19.Jung U.J., Lee M.K., Park Y.B., Kang M.A., Choi M.S. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int. J. Biochem. Cell Biol. 2006;38:1134–1145. doi: 10.1016/j.biocel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Mahmoud A.M., Hernández Bautista R.J., Sandhu M.A., Hussein O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxid. Med. Cell Longev. 2019;2019:5484138. doi: 10.1155/2019/5484138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahmoud A.M., Ahmed O.M., Ashour M.B., Abdel-Moneim A. In vivo and in vitro antidiabetic effects of citrus flavonoids; a study on the mechanism of action. Int. J. Diabetes Dev. Ctries. 2015;35:250–263. doi: 10.1007/s13410-014-0268-x. [DOI] [Google Scholar]
- 22.Jayaraman R., Subramani S., Sheik Abdullah S.H., Udaiyar M. Antihyperglycemic effect of hesperetin, a citrus flavonoid, extenuates hyperglycemia and exploring the potential role in antioxidant and antihyperlipidemic in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2018;97:98–106. doi: 10.1016/j.biopha.2017.10.102. [DOI] [PubMed] [Google Scholar]
- 23.Mulvihill E.E., Assini J.M., Lee J.K., Allister E.M., Sutherland B.G., Koppes J.B., Sawyez C.G., Edwards J.Y., Telford D.E., Charbonneau A., et al. Nobiletin attenuates VLDL overproduction, dyslipidemia, and atherosclerosis in mice with diet-induced insulin resistance. Diabetes. 2011;60:1446–1457. doi: 10.2337/db10-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Priscilla D.H., Jayakumar M., Thirumurugan K. Flavanone naringenin: An effective antihyperglycemic and antihyperlipidemic nutraceutical agent on high fat diet fed streptozotocin induced type 2 diabetic rats. J. Funct. Foods. 2015;14:363–373. doi: 10.1016/j.jff.2015.02.005. [DOI] [Google Scholar]
- 25.Malakul W., Pengnet S., Kumchoom C., Tunsophon S. Naringin ameliorates endothelial dysfunction in fructose-fed rats. Exp. Ther. Med. 2018;15:3140–3146. doi: 10.3892/etm.2018.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srinivasan S., Pari L. Ameliorative effect of diosmin, a citrus flavonoid against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Chem.-Biol. Interact. 2012;195:43–51. doi: 10.1016/j.cbi.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan S., Pari L. Antihyperlipidemic effect of diosmin: A citrus flavonoid on lipid metabolism in experimental diabetic rats. J. Funct. Foods. 2013;5:484–492. doi: 10.1016/j.jff.2012.12.004. [DOI] [Google Scholar]
- 28.Sundaram R., Shanthi P., Sachdanandam P. Effect of tangeretin, a polymethoxylated flavone on glucose metabolism in streptozotocin-induced diabetic rats. Phytomedicine. 2014;21:793–799. doi: 10.1016/j.phymed.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Sundaram R., Nandhakumar E., Haseena Banu H. Hesperidin, a citrus flavonoid ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Toxicol. Mech. Methods. 2019;29:644–653. doi: 10.1080/15376516.2019.1646370. [DOI] [PubMed] [Google Scholar]
- 30.Toumi M., Merzoug S., Boutefnouchet A., Tahraoui A., Ouali K., Guellati M. Hesperidin, a natural citrus flavanone, alleviates hyperglycaemic state and attenuates embryopathies in pregnant diabetic mice. J. Med. Plant Res. 2009;3:862–869. [Google Scholar]
- 31.Tsutsumi R., Yoshida T., Nii Y., Okahisa N., Iwata S., Tsukayama M., Hashimoto R., Taniguchi Y., Sakaue H., Hosaka T., et al. Sudachitin, a polymethoxylated flavone, improves glucose and lipid metabolism by increasing mitochondrial biogenesis in skeletal muscle. Nutr. Metab. 2014;11:32. doi: 10.1186/1743-7075-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dobiaš L., Petrová M., Vojtko R., Kristová V. Long-term Treatment with Hesperidin Improves Endothelium-dependent Vasodilation in Femoral Artery of Spontaneously Hypertensive Rats: The Involvement of NO-synthase and K(v) Channels. Phytother. Res. 2016;30:1665–1671. doi: 10.1002/ptr.5670. [DOI] [PubMed] [Google Scholar]
- 33.Ikemura M., Sasaki Y., Giddings J., Yamamoto J. Protective Effects of Nobiletin on Hypertension and Cerebral Thrombosis in Stroke-Prone Spontaneously Hypertensive Rats (SHRSP) Food Nutr. Sci. 2012;3:1539–1546. doi: 10.4236/fns.2012.311201. [DOI] [Google Scholar]
- 34.Ohtsuki K., Abe A., Mitsuzuwi H., Kondo M., Uemura K., Iwasaki Y., Kondo Y. Effects of long-term administration of hesperidin and glucosyl hesperidin to spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 2002;48:420–422. doi: 10.3177/jnsv.48.420. [DOI] [PubMed] [Google Scholar]
- 35.Paredes M.D., Romecín P., Atucha N.M., O’Valle F., Castillo J., Ortiz M.C., García-Estañ J. Beneficial Effects of Different Flavonoids on Vascular and Renal Function in L-NAME Hypertensive Rats. Nutrients. 2018;10:484. doi: 10.3390/nu10040484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Potue P., Wunpathe C., Maneesai P., Kukongviriyapan U., Prachaney P., Pakdeechote P. Nobiletin alleviates vascular alterations through modulation of Nrf-2/HO-1 and MMP pathways in l-NAME induced hypertensive rats. Food Funct. 2019;10:1880–1892. doi: 10.1039/C8FO02408A. [DOI] [PubMed] [Google Scholar]
- 37.Sánchez-Recillas A., Gonzalez N., Barrera V., Ibarra-Barajas M., Estrada-Soto S., Ortiz-Andrade R. Vasorelaxant and Antihypertensive Activities of Citroflavonoids (Hesperidin/Naringenin Mixture): Potential Prophylactic of Cardiovascular Endothelial Dysfunction. Pharmacogn. Mag. 2019;15:S84–S91. [Google Scholar]
- 38.Sugasawa N., Katagi A., Kurobe H., Nakayama T., Nishio C., Takumi H., Higashiguchi F., Aihara K.I., Shimabukuro M., Sata M., et al. Inhibition of Atherosclerotic Plaque Development by Oral Administration of α-Glucosyl Hesperidin and Water-Dispersible Hesperetin in Apolipoprotein E Knockout Mice. J. Am. Coll. Nutr. 2019;38:15–22. doi: 10.1080/07315724.2018.1468831. [DOI] [PubMed] [Google Scholar]
- 39.Wunpathe C., Potue P., Maneesai P., Bunbupha S., Prachaney P., Kukongviriyapan U., Kukongviriyapan V., Pakdeechote P. Hesperidin Suppresses Renin-Angiotensin System Mediated NOX2 Over-Expression and Sympathoexcitation in 2K-1C Hypertensive Rats. Am. J. Chin. Med. 2018;46:751–767. doi: 10.1142/S0192415X18500398. [DOI] [PubMed] [Google Scholar]
- 40.Hooijmans C.R., Rovers M.M., de Vries R.B., Leenaars M., Ritskes-Hoitinga M., Langendam M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.