Abstract
Four-day-old BALB/c mice were infected by the oral administration of 50,000 Cryptosporidium parvum oocysts, and the resulting infection was scored histologically and by counting colonic oocysts. Infection occurred in the ileum and proximal colon (but not duodenum and jejunum), peaked on days 14 to 18, and was cleared between days 24 and 30. Nitric oxide (NO) appeared to play a protective role in this model as evidenced by the facts that plasma nitrite and nitrate levels increased during the period of peak parasitosis; immunohistochemically detected inducible nitric oxide synthase (iNOS) was increased in the ileum and colon enterocytes of infected animals; the NOS inhibitor l-N-iminoethyl lysine or N-nitro-l-arginine methyl ester (L-NAME) decreased the elevated plasma nitrite and nitrate levels while exacerbating the infection and increasing oocyst shedding; administration of a NO donor, S-nitroso-N-penicillamine, reduced oocyst and infection scores; and neonatal iNOS knockout mice exhibited a slightly longer infection than control animals. The oral administration of oocysts to L-NAME-treated BALB/c mice, but not control animals, between 24 and 40 days old resulted in the fecal excretion of oocysts 1 week later. Administration of the antioxidant ascorbic acid also exacerbated the C. parvum infection, suggesting a protective role for reactive nitrogen and/or reactive oxygen compounds, while administration of the superoxide scavenger superoxide dismutase exacerbated the infection. Taken together these data suggest that both reactive nitrogen and reactive oxygen species play protective roles in experimental cryptosporidiosis.
Cryptosporidiosis is a common self-limiting enteric disease caused by the protozoan parasite Cryptosporidium parvum. While immunocompetent individuals readily clear this parasite, immunodeficient and immunosuppressed individuals may experience a sustained infection with significant associated morbidity and mortality (5, 7, 23, 51). The infection is initiated when environmentally resistant oocysts are ingested. Contaminated water supplies constitute the most common source of such oocysts (23, 33). Excystation in the intestine yields four sporozoites that adhere to and invade adjacent epithelial cells, each establishing a unique parasitophorous vacuole just beneath the apical (luminal) membrane of the host cell. A proliferative asexual cycle ensues, giving rise to merozoites that further infect the mucosal epithelium, while a sexual cycle gives rise to oocysts that are mostly shed in the feces (17). Numerous mammalian species have been infected by this parasite (51). While primates, including humans, can be infected throughout their lifetimes, many immunocompetent animal species are resistant to C. parvum infection beyond the neonatal period (11, 38, 46). It is possible that the susceptibility of neonatal, but not older, animals to infection correlates with lower numbers of intraepithelial CD4+ and CD8+ lymphocytes in the former (39). The inability of AIDS patients with CD4+ counts of <50 cells/mm3 to clear a C. parvum infection (5) and observations that experimental murine cryptosporidiosis is exacerbated and prolonged by the depletion of CD4+ but not CD8+ T cells (52, 53) have led to an emphasis being placed on the role of CD4+ T-cell-dependent cellular immunity in this disease. Both Th1 and Th2 responses appear to be involved in protection and parasite clearance (2, 50). However, the fact that the administration of interleukin 12 (IL-12) protects the host against experimental cryptosporidiosis (54) and the many observations implicating gamma interferon (IFN-γ) in several aspects of the immune response to Cryptosporidium infection (8, 10, 18, 20, 26, 40, 49, 54) suggest that a Th1 inflammatory response predominates in cryptosporidiosis. Humoral immune responses seem to be less important in controlling infection, as infected AIDS patients can mount secretory and serum antibody responses without clearing the parasite (4), although persistent infections in patients with hypogammaglobulinemia have been reported (7).
The majority of experimental cryptosporidiosis studies have used rodent models. Three such models are a steriod-immunosuppressed-mouse or -rat model (40, 59); an immunodeficient-rodent model, most commonly the athymic nude mouse (22, 29, 34, 35) or SCID mouse (28, 34, 35); and a neonatal-mouse model (11, 21, 38, 54). Differences have been reported among these models in terms of the responses of the Cryptosporidium infection to chemotherapeutic agents (29) and to manipulation of putative protective cytokines or mediators such as nitric oxide (NO) (28, 30).
This study was undertaken to determine if reactive nitrogen and reactive oxygen compounds play protective roles in experimental cryptosporidiosis. The study was prompted by the conflicting results obtained in studies designed to test for a role for NO in this infection (28, 30) and the realization that not all of the protective effects of IFN-γ observed with this and other parasitic infections can be attributed to NO (14, 28, 44). This study demonstrates that NO and other reactive nitrogen compounds play a modest but significant role in limiting the severity and course of experimental cryptosporidiosis in one rodent model, the immunocompetent neonatal mouse. In addition, preliminary evidence is provided to suggest that reactive oxygen compounds are also protective in this cryptosporidiosis model.
MATERIALS AND METHODS
Animal models.
Pregnant BALB/c mice were obtained from Harlan Sprague- Dawley, Inc. (Indianapolis, Ind.), and housed in filter top cages. Litters were maintained in the range of 5 to 8 pups, and all animals were weaned at 21 days of age. At 4 days of age all animals in a litter were either administered 50,000 Cryptosporidium parvum oocysts in 50 μl of water or 50 μl of water by stomach tube. The C. parvum isolate was originally of bovine origin and has been maintained in nude mice for approximately 5 years (29). At appropriate times, pups were killed by cervical dislocation and weaned animals were killed with an intraperitoneal injection of pentobarbital (100 mg/kg of body weight). Segments of duodenum, jejunum, distal ileum, and proximal colon were placed in neutral formalin for subsequent histological evaluation, and the contents of the distal half of the colon and rectum were harvested and homogenized in 100 μl of neutral formalin for subsequent oocyst evaluation. In weaned animals the oocyst evaluation was performed with five fresh fecal pellets homogenized in 500 μl of neutral formalin. The choice of times at which animals were killed depended on the expected outcome of a given experiment. In experiments in which a drug was expected to reduce the parasite load, the animals were killed at or near the time of peak infection, while in experiments in which a drug was expected to exacerbate infection or delay parasite clearance, the animals were killed at a time when the infection was reduced or the intestine had just been cleared of parasites.
One experiment was designed to detect a role for NO in determining at what age BALB/c mice could no longer be infected with C. parvum oocysts. Animals were administered water orally or 10 mg of N-nitro-l-arginine methyl ester (L-NAME) per kg in water for 4 days prior to the administration of oocysts as described above. In a preliminary experiment using animals of various ages, it was found that a small number of oocysts, presumably originating from the oral innoculum, were shed in the feces for 2 to 3 days. None were detected by 5 days postinoculation. In those animals that were to go on to support an infection, fecal oocysts were detected on days 5 to 8 postinoculation. For this reason fecal oocyt shedding on day 7 postinoculation was used as the criterion for the establishment of an infection in older animals.
One experiment employed an inducible NO synthase (iNOS) knockout mouse to determine the effect of this defect on the severity and course of experimental cryptosporidiosis. The iNOS knockout breeding stock used was developed on a B6,129 background, and control animals were provided from the B6,129F2/J colony (Jackson Laboratory, Bar Harbor, Maine).
Oocyst and infection scores.
C. parvum oocysts in 10 μl of either colonic content or fecal homogenate were visualized by a commercial immunofluorescent assay (Meridian Diagnostics, Cincinnati, Ohio) and counted. The oocyst score was derived from this count (29, 30) as follows: 1 indicated 1 to 9 oocysts, 2 indicated 10 to 49 oocysts, 3 indicated 50 to 99 oocysts, and 4 indicated ≥100 oocysts.
Two separate hematoxylin- and eosin-stained, paraffin-embedded cross sections of each intestinal segment were assessed to generate the infection scores, which were as follows: 1 indicated a single parasite or a cluster of parasites, limited to one villus/crypt unit or one colonic crypt per section; 2 indicated two or three villi/crypt unit or two or three colonic crypts per section showing various numbers of parasites; 3 indicated parasites observed in many crypts with some villus or surface epithelium involvement; and 4 indicated extensive infection of the crypts and villi or surface epithelium, usually associated with epithelial damage and mucopenia.
Plasma NO2 and NO3.
No attempt was made to place the nursing mothers or weaned pups on a nitrate-restricted diet in these experiments. Prior to being killed, animals were heparinized by the intraperitoneal injection of 200 U of heparin. Immediately following cervical dislocation, blood was removed by intracardiac puncture and centrifuged, and the plasma was stored at −20°C prior to analysis. Plasma nitrate was reduced to nitrite and measured with endogenous nitrite as described by Lyall and colleagues (32). Briefly, by this method plasma samples were incubated for 2 h at 37°C in the presence of nitrate reductase, flavin adenine dinucleotide, and reduced nicotinamide adenine dinucleotide phosphate, buffered to pH 7.5. Excess reduced nicotinamide adenine dinucleotide phosphate was then oxidized by further incubation following the addition of lactate dehydrogenase and pyruvate. Greiss reagent was then added to the samples, and after incubation in the dark at room temperature the absorbance was measured at 548 nm and the values were compared against a nitrite standard curve. As the sample nitrite concentrations represented both nitrite and nitrate, values are expressed as concentrations of NO2 plus NO3.
iNOS immunohistochemistry.
Formalin-fixed, paraffin-embedded sections were deparaffinized, rehydrated, and incubated in 2% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for 1 h at room temperature. The anti-iNOS polyclonal antibody (Upstate Biotechnology, Lake Placid, N.Y.) was made up at a concentration of 3 μg/ml in 1% BSA in PBS, and sections were incubated with this first antibody overnight at 4°C. The secondary antibody used for the purposes of screening sections was a rabbit anti-mouse immunoglobulin G (PK-6101; Vector Laboratories, Inc., Burlingame, Calif.) visualized with Vectastain ABC reagent according to the directions of the manufacturer (Vector Laboratories, Inc.). Sections were then screened by light microscopy. For the purpose of illustration in this work, after tissue sections had been exposed to the first antibody overnight, they were washed three times for 5 min each time in PBS and then incubated in a 1:300-diluted biotinylated goat anti-rabbit immunoglobulin G (Jackson Immunoresearch, West Grove, Pa.) in 1% BSA in PBS at 37°C for 1 h, followed by three rinses in PBS and incubation in streptavidin-Oregon green 488 (Molecular Probes, Eugene, Oreg.) in PBS for 45 min at room temperature. Sections were then viewed by confocal microscopy with a laser excitation wavelength of 488 nm and an emission wavelength of 510 nm.
Drug administration.
The NOS inhibitors L-NAME and l-N-iminoethyl lysine (L-NIL) and the NO donor S-nitroso-N-penicillamine (SNAP) were dissolved in water and administered at a final volume of 100 μl by stomach tube in two divided doses for 4 days prior to killing of the animals. The antioxidant ascorbic acid was also dissolved in water and administered orally once a day at a dose of 5 mg/animal for 4 days prior to the killing. In these experiments the control animals were given the same volume of water by gastric tube. With the superoxide scavenger, 500 U of superoxide dismutase-polyethylene glycol (Sigma Chemical Co., St. Louis, Mo.) was suspended in 200 μl of corn oil and this suspension was injected subcutaneously daily for 4 days prior to the killing. Control animals for this experiment received corn oil alone.
Statistical analyses.
Data are means ± standard errors of the means of values from experiments with at least five animals. When data were evaluated in experiments involving more than a single drug dose, a one-way analysis of variance was performed to evaluate any treatment effect, followed by post hoc Tukey protected t tests to evaluate the differences between individual group mean values. Student t tests were used to evaluate the difference between mean values when there were only two groups in an experiment. Mean values were considered significantly different from one another when P was <0.05.
RESULTS
The severity and course of cryptosporidiosis in animals in this study were similar to what has been reported in the literature for neonatal BALB/c mice (38, 50, 56). Briefly, of the four sections of intestine studied, only the ileum and colon and never the duodenum and jejunum exhibited infection. The infection peaked between day (day of age) 14 and day 18 and cleared between day 24 and day 30. The infection in the colon was always less than in the ileum of the same animal, and the colonic infection cleared before the ileal infection (data not shown).
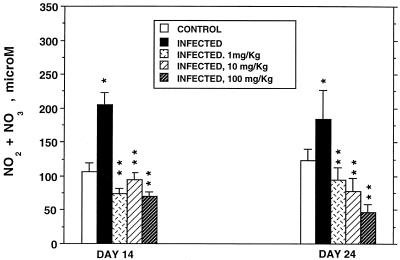
Two NOS inhibitors, L-NAME and L-NIL, were used to inhibit NO production and unmask any effect of endogenous NO on the severity and duration of the C. parvum infection. While the same results were obtained with both inhibitors, L-NIL is a more specific iNOS inhibitor and proved to be less toxic to these neonatal mice than was the more general NOS inhibitor L-NAME. Concentrations of NO2 plus NO3 in plasma were used as an indicator of host NO production. Figure 1 illustrates the effects of C. parvum infection and of L-NIL administration on concentrations of these NO metabolites in plasma on day 14 when the infection was at its peak and on day 24 when it had begun to clear. Infection significantly increased NO2 plus NO3 levels on both days compared to values for uninfected animals. All three doses of L-NIL significantly reduced plasma NO metabolite values in infected animals.
FIG. 1.
Concentrations of NO2 plus NO3 in the plasma of 14- and 24-day-old uninfected (control) and C. parvum-infected BALB/c mice and infected mice given 1, 10, or 100 mg of L-NIL per kg daily for 4 days prior to being killed. ∗, significantly different from corresponding mean value for uninfected mice; ∗∗, significantly different from corresponding mean value for infected mice not treated with L-NIL.
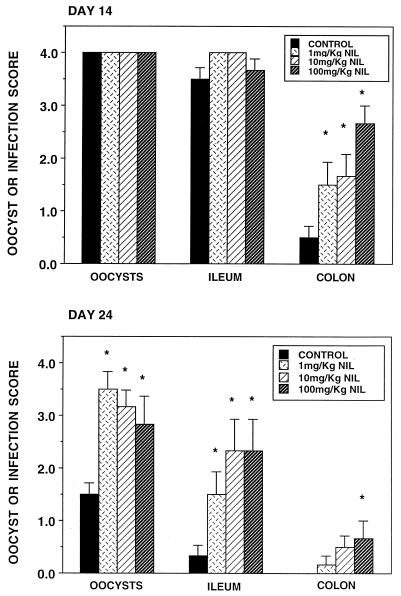
Figure 2 illustrates the oocyst, ileum, and colon infection scores for the same infected animals used to obtain the results shown in Fig. 1. On day 14 both the oocyst and ileum infection scores were at or near peak values, precluding the manifestation of any drug effect. The mean colonic infection score was less than 1.0 in untreated, infected animals, and L-NIL significantly exacerbated this score in a dose-related manner. Parasite clearance was well under way by day 24. The mean ileal infection score was below 0.5 and none of the untreated, infected colonic samples showed signs of infection. All three doses of the NOS inhibitor showed significant increases in oocyst and ileum infection scores, and the highest dose caused a significant increase in the colon infection score. In the ileum, the drug-induced increase in parasite burden occurred both in the crypts and on the villus surfaces. Similar results were obtained with the NOS inhibitor L-NAME (data not shown). The administration of the general NOS inhibitor L-NAME and of the more specific iNOS inhibitor L-NIL for 4 days prior to the killing of infected animals on days 14 and 24 reduced the infection-induced elevation of NO product levels in plasma and resulted in an exacerbation of the infection and a delay in parasite clearance from the large intestine. Parasites were not detected in either the duodenum or jejunum of any of these untreated or drug-treated, infected animals.
FIG. 2.
Oocyst, ileum, and colon infection scores of C. parvum-infected (control) 14- and 24-day-old BALB/c mice and infected animals given 1, 10, or 100 mg of L-NIL per kg daily. The same animals used to obtain the results shown in Fig. 1 were used for this experiment. ∗, significantly different from the value for control mice.
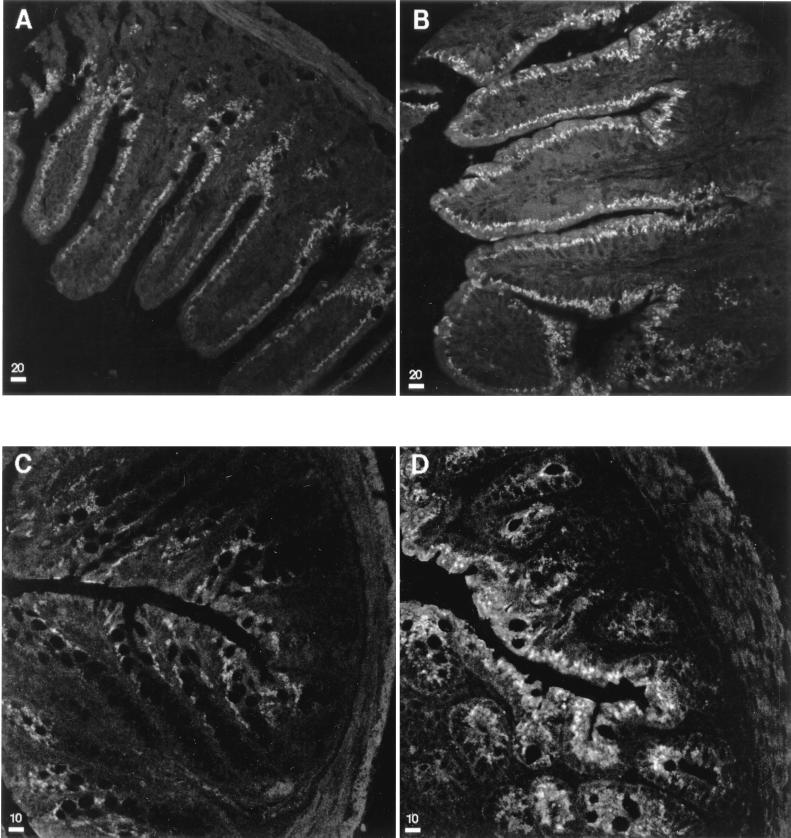
Inducible NOS was visualized immunohistochemically in paraffin-embedded sections. When these sections were screened, it was found that the iNOS activity was undetectable in duodenum and jejunum sections but that it was detectable in the ileal and colonic epithelia of the same animals. In ileum tissues from both uninfected and infected animals the enzyme was concentrated in the villus epithelial cells rather than crypt cells. This finding is similar to the observation that the more differentiated villus enterocytes possess the highest iNOS enzymatic activity in rat small intestine (48). Conversely, histological samples from infected animals showed that the parasite number was always greater in the crypts than in the villi. Sections from infected animals showed a consistently greater iNOS activity in ileal and colonic epithelia than in the same tissues from uninfected animals of the same age. Figure 3 shows representative confocal microscopic images of immunofluorescent staining for iNOS in uninfected and infected ileum and colon sections. This figure demonstrates that C. parvum infection is associated with an increase in epithelial cell iNOS.
FIG. 3.
Representative confocal microscopic immunohistochemical images of ileum (A and B) and colon (C and D) sections from 18-day-old uninfected (A and C) and infected (B and D) BALB/c mice illustrating the presence of iNOS in the mucosal epithelium. Bars represent 20 μm in the ileum sections and 10 μm in the colon sections.
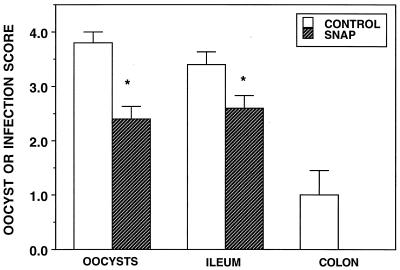
The NO donor SNAP was administered orally to infected animals for four days prior to killing of the animals on day 14. When compared with scores for untreated, infected animals, the oocyst and the ileum infection scores were significantly reduced for animals receiving the NO donor (Fig. 4). No SNAP-treated animal showed signs of colonic infection, but because of the variance in the colonic infection scores from untreated animals, this apparently protective effect was not statistically significant.
FIG. 4.
Oocyst, ileum, and colon infection scores of 14-day-old C. parvum-infected (control) BALB/c mice and infected mice treated for 4 days with 100 mg of SNAP per kg per day. ∗, significantly different from the value for control mice.
Uninfected animals of various ages were treated orally for 4 days with 10 mg of L-NAME per kg in two divided doses. On the fourth day of treatment they were infected with 50,000 C. parvum oocysts and fecal oocyst shedding was monitored. On the seventh day following oocyst administration, five fresh fecal pellets were collected and homogenized in 2 ml of neutral formalin. Five oocysts per 10 μl of fecal homogenate was chosen as a cutoff point that indicated the presence of a low-grade infection. Table 1 indicates the numbers of animals in untreated control groups and in L-NAME-treated groups that met this criterion. The age at which mice were susceptible to the establishment of a C. parvum infection was extended by L-NAME treatment. It was not usually possible to histologically confirm these low-grade infections. L-NAME was chosen for these experiments because, while it is a less specific iNOS inhibitor than L-NIL, it is expected to inhibit enterocyte and macrophage iNOS as well as constitutive NOS in enterocytes and other mucosal cells.
TABLE 1.
Age dependence of susceptibility of BALB/c mice to C. parvum infection
| Treatment | No. of animals meeting the criterion for infection/ no. of animals administered oocysts at the indicated age (days)
|
||||
|---|---|---|---|---|---|
| 4 | 24 | 30 | 40 | 60 | |
| None (control) | 10/10 | 0/6 | 0/6 | 0/5 | 0/6 |
| L-NAMEa | 7/8 | 2/6 | 5/5 | 0/6 | |
Mice in the L-NAME group were given 10 mg/kg/day for 4 days prior to the day of oocyst administration.
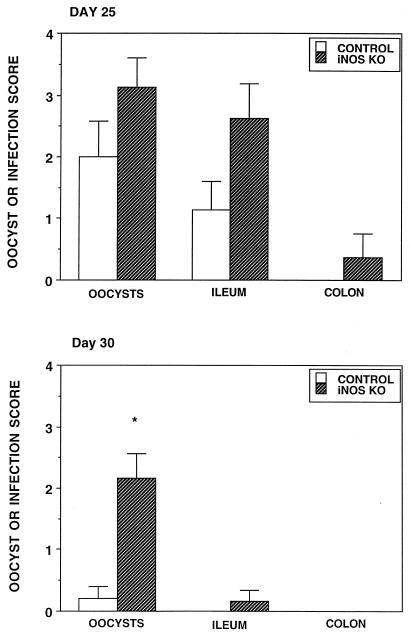
Figure 5 illustrates the oocyst, ileum, and colon infection scores of infected iNOS knockout animals and their controls on day 25 and day 30. While the mean scores were higher in the iNOS knockout mice than in control animals on day 25, these differences were not statistically significant. By day 30 the iNOS knockout animals shed significantly more oocysts than the control animals and while six of six iNOS animals still exhibited some sign of infection (shed oocysts or histologically detectable parasites), only one of six control animals remained infected, suggesting a delay in parasite clearance in animals lacking this NOS isoform.
FIG. 5.
Oocyst, ileum, and colon infection scores of 25- and 30-day-old C. parvum-infected (control) B6,129 mice and iNOS knockout mice. ∗, significantly different from the value for control mice.
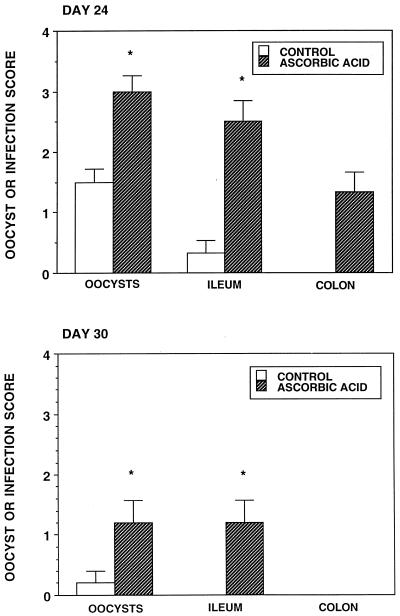
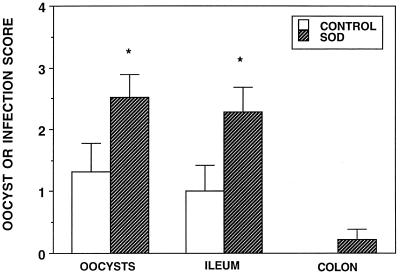
Ascorbic acid was administered orally to infected neonatal BALB/c mice daily for 4 days prior to their killing on days 24 and 30. On day 24 all scores were significantly increased by the administration of this antioxidant (Fig. 6). By day 30 parasite clearance had largely been accomplished in infected control animals and the ascorbic acid only significantly increased the oocysts and ileum infection scores. When the superoxide scavenger superoxide dismutase-polyethylene glycol was administered subcutaneously for 4 days prior to the killing of infected BALB/c animals on day 24, the oocyst and ileum infection scores were significantly greater than those for vehicle control animals (Fig. 7).
FIG. 6.
Oocyst, ileum, and colon infection scores of 24- and 30-day-old C. parvum-infected (control) BALB/c mice and infected animals treated for 4 days with 5 mg of ascorbic acid per animal per day. ∗, significantly different from the value for control mice.
FIG. 7.
Oocyst, ileum, and colon infection scores of 24-day-old C. parvum-infected (control) BALB/c mice and infected animals treated for 4 days with superoxide dismutase (SOD). ∗, significantly different from the value for control mice.
DISCUSSION
NO has been implicated in the killing of many infectious agents, including viruses (9), bacteria (16, 19, 45), fungi (3), and a wide variety of parasites (1, 25, 41, 55). Evidence that NO protects against C. parvum is less clear. Kuhls and colleagues (28) suggested that NO has no role in cryptosporidiosis after they observed that the NOS inhibitor aminoguanidine had no effect on the severity and course of the infection in either adult BALB/c or SCID mice. Similarly, those authors were unable to show an effect of administered IFN-γ. Those models differ significantly from the neonatal BALB/c model in that the SCID mouse develops a very severe infection, not restricted to the ileum and colon, that is expected to mask any but the most dramatic ameliorating effect of NO. Kuhls and colleagues were unable to detect infection in adult BALB/c mice killed 4 weeks after oocyst administration, even when the mice were administered aminoguanidine. In the present study we observed an extension of the period when an animal could be infected when L-NAME was administered. However, even with this agent, the observed infection was minimal and was expected to be cleared within 4 weeks. Putrescine has been shown to protect neonatal C57BL/6 mice against C. parvum infection, but this effect was apparently not NO mediated (56). On the other hand, we previously found that a arginine-rich diet was protective in C. parvum-infected nude mice, an effect that was prevented by the NOS inhibitor L-NAME, and that NO donors inhibited oocyst excystation and reduced sporozoite viability in vitro (30).
The present study employed the neonatal murine cryptosporidiosis model and suggests that NO or other reactive nitrogen compounds play roles in limiting the severity of infection, contributing to parasite clearance from the intestine and limiting the age window during which it is possible to establish a low-grade infection in this model. NO does not appear to be a factor in determining which section of the neonatal mouse intestine can be infected, as neither iNOS knockout animals nor animals administered NOS inhibitors exhibited duodenal or jejunal infection. In contrast, IFN-γ knockout mice (20) and SCID mice (35) exhibited C. parvum infection throughout the gastrointestinal tract. Differences in the small intestinal T-cell subtypes may be one explanation for the regional differences seen in susceptibility to C. parvum infection in neonatal BALB/c mice (6).
NO is known to play many roles in the gastrointestinal tract, acting as a neurotransmitter, controlling gastrointestinal motility, modulating blood flow, and contributing to the integrity of the mucosal barrier, as well as providing protection against invading microorganisms (27, 42). Excessive production of NO has, however, been implicated in host cell damage and inflammatory bowel disease (47).
The simultaneous accumulation of reactive nitrogen and reactive oxygen compounds increases the repertoire of highly toxic compounds available for killing microorganisms (16). While activated macrophages have been considered a major source of reactive compounds in the gastrointestinal tract (27, 42), the induction of NOS in intestinal epithelial cells by proinflammatory cytokines (13) suggests that the epithelium may also take a direct part in its own defense. This may be particularly true for C. parvum, as reactive nitrogen and reactive oxygen species derived from lamina propria macrophages may not be able to diffuse unaltered into the parasite's niche at the luminal surfaces of the enterocytes.
In this study, ascorbic acid was effective at exacerbating and prolonging the infection. This result was presumably due to the antioxidant effect of this vitamin and may have been the result of a reduction in either or both critical reactive nitrogen and critical reactive oxygen species (43). One reaction product of NO and superoxide, peroxynitrite, has been implicated in the killing of microorganisms (60). To protect themselves from this reactive compound, some bacteria have been reported to utilize superoxide dimutase to scavenge superoxide, resulting in the production of the less toxic NO and H2O2 (16). Similarly, the administration of exogenous superoxide dismutase in the present experiments may have exacerbated the infection by reducing the production of peroxynitrite, or alternatively, it may have functioned to reduce a critical superoxide pool. The administration of a similar superoxide dismutase preparation to mice reportedly protected the animals against reactive-oxygen-induced lung damage (12). Compared to many intracellular parasites (37), Cryptosporidium has a poor capacity to scavenge reactive oxygen species (15), making it potentially more susceptible to killing by such compounds.
Cytokines, notably IL-1β, IL-2, tumor necrosis factor alpha, and IFN-γ, have been shown to increase iNOS expression in cultured epithelial cells (13). While tumor necrosis factor alpha does not seem to play a significant role in experimental cryptosporidiosis in most species studied, IFN-γ is protective (8, 34, 50, 58). Many of the effects of IFN-γ on both host and microorganisms have been shown to be NO mediated (24, 31, 44), but increasingly it is recognized that some of the protective effects of this cytokine do not require NO (14, 28, 44). The relatively modest Cryptosporidium infection seen here in iNOS knockout mice contrasts with the severe and often fatal infections seen in IFN-γ knockout mice (20, 36) and adds to the argument that some of the protective effect of this cytokine in cryptosporidiosis is NO independent.
NOS inhibitors caused a more severe exacerbation in the infection than was seen in the iNOS knockout animals. This result may reflect the presence of redundant protective mechanisms in the latter case (57) rather than imply that NO was derived from other NOS isoforms in iNOS knockout animals.
This study indicates that reactive nitrogen and reactive oxygen species play modest but significant protective roles in experimental cryptosporidiosis in the neonatal mouse. The administration of a NO donor reduced infection, while the reduced production of reactive nitrogen species and, to a lesser extent, the scavenging of reactive oxygen species exacerbated the infection in the ileum and proximal colon, slightly delayed parasite clearance, and extended the age at which this mouse strain could be infected with C. parvum.
ACKNOWLEDGMENT
This work was supported in part by Public Health Service grant RR03034.
REFERENCES
- 1.Adams L B, Hibbs J B, Jr, Taintor R R, Krahenbuhl J L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from l-arginine. J Immunol. 1990;144:2725–2729. [PubMed] [Google Scholar]
- 2.Aguirre S A, Perryman L E, Davis W C, McGuire T C. IL-4 protects adult C57BL/6 mice from prolonged Cryptosporidium parvum infection; analysis of CD4+ alpha beta+IFN-γ+ and CD4+alpha beta+IL-4+ lymphocytes in gut-associated lymphoid tissue during resolution of infection. J Immunol. 1998;161:1891–1900. [PubMed] [Google Scholar]
- 3.Alspaugh J A, Granger D L. Inhibition of Cryptococcus neoformans replication by nitrogen oxides supports the role of these molecules as effectors of macrophage-mediated cytostasis. Infect Immun. 1991;59:2291–2296. doi: 10.1128/iai.59.7.2291-2296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benhamou Y, Kapel N, Hoang C, Matta H, Meillet D, Magne D, Raphael M, Gentilini M, Opolon P, Gobert J G. Inefficacy of intestinal secretory immune response to Cryptosporidium in acquired immunodeficiency syndrome. Gastroenterology. 1995;108:627–635. doi: 10.1016/0016-5085(95)90433-6. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard C, Jackson A M, Shanson D C, Francis N, Gazzard B G. Cryptosporidiosis in HIV-seropositive patients. Q J Med. 1992;85:813–823. [PubMed] [Google Scholar]
- 6.Boher Y, Perez-Schael I, Caceres-Dittmar G, Urbina G, Gonzalez R, Kraal G, Tapia F J. Enumeration of selected leukocytes in the small intestine of BALB/c mice infected with Cryptosporidium parvum. Am J Trop Med Hyg. 1994;50:145–151. [PubMed] [Google Scholar]
- 7.Campbell P N, Current W L. Demonstration of serum antibodies to Cryptosporidium sp. in normal and immunodeficient humans with confirmed infections. J Clin Microbiol. 1983;18:165–169. doi: 10.1128/jcm.18.1.165-169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Harp J A, Harmsen A G. Requirements for CD4+ cells and gamma interferon in resolution of established Cryptosporidium parvum infection in mice. Infect Immun. 1993;61:3928–3932. doi: 10.1128/iai.61.9.3928-3932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croen K D. Evidence for antiviral effect of nitric oxide. J Clin Investig. 1993;91:2446–2452. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Culshaw R J, Bancroft G J, McDonald V. Gut intraepithelial lymphocytes induce immunity against Cryptosporidium infection through a mechanism involving gamma interferon production. Infect Immun. 1997;65:3074–3079. doi: 10.1128/iai.65.8.3074-3079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Current E L, Reese N C. A comparison of endogenous development of three isolates of Cryptosporidium in suckling mice. J Protozool. 1986;33:98–108. doi: 10.1111/j.1550-7408.1986.tb05567.x. [DOI] [PubMed] [Google Scholar]
- 12.Denis M. Antioxidant therapy partially blocks immune-induced lung fibrosis. Inflammation. 1995;19:207–219. doi: 10.1007/BF01534462. [DOI] [PubMed] [Google Scholar]
- 13.Dignass A U, Podolsky D K, Rachmilewitz D. NOx generation by cultured small intestinal epithelial cells. Dig Dis Sci. 1995;40:1859–1865. doi: 10.1007/BF02208647. [DOI] [PubMed] [Google Scholar]
- 14.Dimier I H, Bout D T. Interferon-gamma activated enterocytes inhibit Toxoplasma gondii replication; a role for intracellular iron. Immunology. 1998;94:488–495. doi: 10.1046/j.1365-2567.1998.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Entrala E, Mascaro C, Barrett J. Anti-oxidant enzymes in Cryptosporidium parvum oocysts. Parasitology. 1997;114:13–17. doi: 10.1017/s0031182096008037. [DOI] [PubMed] [Google Scholar]
- 16.Fang F C. Perspective series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Investig. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fayer R, Speer C A, Dubey J P. The general biology of Cryptosporidium. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. New York, N.Y: CRC Press; 1997. pp. 1–41. [Google Scholar]
- 18.Gomez Morales M A, Ausiello C M, Guarino A, Urbani F, Spagnuolo M I, Pignata C, Pozio E. Severe, protracted intestinal cryptosporidiosis associated with interferon-gamma deficiency; pediatric case report. Clin Infect Dis. 1996;22:848–856. doi: 10.1093/clinids/22.5.848. [DOI] [PubMed] [Google Scholar]
- 19.Granger D I, Hibbs J B, Perfect J R, Durack D T. Specific amino acid (l-arginine) requirement for the microbiostatic effect of murine macrophages. J Clin Investig. 1987;81:1129–1136. doi: 10.1172/JCI113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffiths J K, Theodos C, Paris M, Tzipori S. The gamma interferon gene knockout mouse: a highly sensitive model for evaluation of therapeutic agents against Cryptosporidium parvum. J Clin Microbiol. 1998;36:2503–2508. doi: 10.1128/jcm.36.9.2503-2508.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harp J A, Sacco R E. Development of cellular immune functions in neonatal to weanling mice: relationship to Cryptosporidium parvum infection. J Parasitol. 1996;82:245–249. [PubMed] [Google Scholar]
- 22.Heine J, Moon H W, Woodmansee D B. Persistent Cryptosporidium infection in congenitally athymic (nude) mice. Immunity. 1984;43:856–859. doi: 10.1128/iai.43.3.856-859.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoxie N J, Davis J P, Vergeront J M, Nashold R D, Blair K A. Cryptosporidiosis-associated mortality following a massive waterborne outbreak in Milwaukee, Wisconsin. Am J Publ Health. 1997;87:2032–2035. doi: 10.2105/ajph.87.12.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hruby Z, Beck K F. Cytotoxic effect of autocrine and macrophage-derived nitric oxide on cultured rat mesangial cells. Clin Exp Immunol. 1997;107:76–82. doi: 10.1046/j.1365-2249.1997.d01-906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James S L, Glaven J. Macrophage cytotoxicity against schistosomula or Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989;143:4208–4212. [PubMed] [Google Scholar]
- 26.Kapel N, Benhamou Y, Buraud M, Magne D, Opolon P, Gobert J G. Kinetics of mucosal ileal gamma-interferon response during cryptosporidiosis in immunocompetent neonatal mice. Parasitol Res. 1996;82:664–667. doi: 10.1007/s004360050182. [DOI] [PubMed] [Google Scholar]
- 27.Konturek S K, Konturek P C. Role of nitric oxide in the digestive system. Digestion. 1995;56:1–13. doi: 10.1159/000201214. [DOI] [PubMed] [Google Scholar]
- 28.Kuhls T L, Mosier D A, Abrams V L, Crawford D L, Greenfield R A. Inability of interferon-γ and aminoguanidine to alter Cryptosporidium parvum infection in mice with severe combined immunodeficiency. J Parasitol. 1994;80:480–485. [PubMed] [Google Scholar]
- 29.Leitch G J, He Q. Putative anticryptosporidial agents tested with an immunodeficient mouse model. Antimicrob Agents Chemother. 1994;38:865–867. doi: 10.1128/aac.38.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leitch G J, He Q. Arginine-derived nitric oxide reduces oocyst shedding in nude mice infected with Cryptosporidium parvum. Infect Immun. 1994;62:5173–5176. doi: 10.1128/iai.62.11.5173-5176.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liew F Y, Wei X Q, Proudfoot L. Cytokines and nitric oxide as effector molecules against parasitic infection. Philos Trans R Soc Lond Ser B. 1997;352:1311–1315. doi: 10.1098/rstb.1997.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyall F, Young A, Greer I A. Nitric oxide concentrations are increased in the fetoplacental circulation in preeclampsia. Am J Obstet Gynecol. 1995;173:714–718. doi: 10.1016/0002-9378(95)90328-3. [DOI] [PubMed] [Google Scholar]
- 33.MacKenzie W R, Schell W L, Blair K A, Addiss D G, Peterson D E, Hoxie N J, Kazmierczak J J, Davis J P. Massive outbreak of waterborne Cryptosporidium infection in Milwaukee, Wisconsin; recurrence of illness and risk of secondary transmission. Clin Infect Dis. 1995;21:57–62. doi: 10.1093/clinids/21.1.57. [DOI] [PubMed] [Google Scholar]
- 34.McDonald V, Deer R, Uni S, Iseki M, Bancroft G J. Immune responses to Cryptosporidium muris and Cryptosporidium parvum in adult immunocompetent or immunocompromised (nude and SCID) mice. Infect Immun. 1992;60:3325–3331. doi: 10.1128/iai.60.8.3325-3331.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mead J R, Arrowood M J, Sidwell R W, Healey M C. Chronic Cryptosporidium parvum infections in congenitally immunodeficient SCID and nude mice. J Infect Dis. 1991;163:1297–1304. doi: 10.1093/infdis/163.6.1297. [DOI] [PubMed] [Google Scholar]
- 36.Mead J R, You X. Susceptibility differences to Cryptosporidium parvum infection in two strains of gamma interferon knockout mice. J Parasitol. 1998;84:1045–1048. [PubMed] [Google Scholar]
- 37.Mehlotra R K. Antioxidant defense mechanisms in parasitic protozoa. Crit Rev Microbiol. 1996;22:295–314. doi: 10.3109/10408419609105484. [DOI] [PubMed] [Google Scholar]
- 38.Novak S M, Sterling C R. Susceptibility dynamics in neonatal BALB/c mice infected with Cryptosporidium parvum. J Protozool. 1991;38:102S–104S. [PubMed] [Google Scholar]
- 39.Pasquali P, Fayer R, Almeria S, Trout J, Polidori G A, Gasbarre L C. Lymphocyte dynamic patterns in cattle during a primary infection with Cryptosporidium parvum. J Parasitol. 1997;83:247–250. [PubMed] [Google Scholar]
- 40.Rehg J E. Effect of interferon-gamma in experimental Cryptosporidium parvum infection. J Infect Dis. 1996;174:229–232. doi: 10.1093/infdis/174.1.229. [DOI] [PubMed] [Google Scholar]
- 41.Rockett K A, Awburn M M, Cowden W B, Clark I A. Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect Immun. 1991;59:3280–3283. doi: 10.1128/iai.59.9.3280-3283.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salzman A L. Nitric oxide in the gut. New Horiz. 1995;3:352–364. [PubMed] [Google Scholar]
- 43.Sandoval M, Zhang X J, Liu X, Mannick E E, Clark D A, Miller M J. Peroxynitrite-induced apoptosis in T84 and RAW 264.7 cells: attenuation by l-ascorbic acid. Free Radic Biol Med. 1997;22:489–495. doi: 10.1016/s0891-5849(96)00374-7. [DOI] [PubMed] [Google Scholar]
- 44.Scharton-Kersten T M, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shank J L, Silliker J H, Harper R H. The effect of nitric oxide on bacteria. Appl Microbiol. 1962;10:185–189. doi: 10.1128/am.10.3.185-189.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sherwood D, Angus K W, Snodgrass D R, Tzipori S. Experimental cryptosporidiosis in laboratory mice. Infect Immun. 1982;38:471–475. doi: 10.1128/iai.38.2.471-475.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singer I I, Kawka D W, Scott S, Weidner J R, Mumford R A, Riehl T E, Stenson W F. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology. 1996;111:871–885. doi: 10.1016/s0016-5085(96)70055-0. [DOI] [PubMed] [Google Scholar]
- 48.Tepperman B L, Brown J F, Whittle B J. Nitric oxide synthase induction and intestinal epithelial cell viability in rats. Am J Physiol. 1993;265:G214–G218. doi: 10.1152/ajpgi.1993.265.2.G214. [DOI] [PubMed] [Google Scholar]
- 49.Theodos C M, Sullivan K L, Griffiths J K, Tzipori S. Profiles of healing and nonhealing Cryptosporidium parvum infection in C57BL/5 mice with functional B and T lymphocytes: the extent of gamma interferon modulation determines the outcome of infection. Infect Immun. 1997;65:4761–4769. doi: 10.1128/iai.65.11.4761-4769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tilley M, McDonald V, Bancroft G J. Resolution of cryptosporidial infection in mice correlates with parasite-specific lymphocyte proliferation associated with both Th1 and Th2 cytokine secretion. Parasite Immunol. 1995;17:459–464. doi: 10.1111/j.1365-3024.1995.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 51.Tzipori S. Cryptosporidiosis in perspective. Adv Parasitol. 1988;27:63–129. doi: 10.1016/S0065-308X(08)60353-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ungar B L P, Burris J A, Quinn C A, Finkelman F D. New mouse model for chronic Cryptosporidium infection in immunodeficient hosts. Infect Immun. 1990;58:961–969. doi: 10.1128/iai.58.4.961-969.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ungar B L P, Kao T C, Burris J A, Finkelman F D. Cryptosporidium infection in an adult mouse model. Independent roles for IFN-γ and CD4+ T lymphocytes in protective immunity. J Immunol. 1991;147:1014–1022. [PubMed] [Google Scholar]
- 54.Urban J F, Jr, Fayer R, Chen S J, Gause W C, Gately M K, Finkelman F D. IL-12 protects immunocompetent and immunodeficient neonatal mice against infection with Cryptosporidium parvum. J Immunol. 1996;156:263–268. [PubMed] [Google Scholar]
- 55.Vincendeau P, Daulouede S. Macrophage cytostatic effect on Trypanosoma musculi involves an l-arginine dependent mechanism. J Immunol. 1991;146:4338–4343. [PubMed] [Google Scholar]
- 56.Waters W R, Reinhardt T A, Harp J A. Oral administration of putrescine inhibits Cryptosporidium parvum infection of neonatal C57BL-6 mice and is independent of nitric oxide synthesis. J Parasitol. 1997;83:746–750. [PubMed] [Google Scholar]
- 57.Wei X, Charles I G, Smith A, Ure J, Feng G, Huang F, Xu S, Muller W, Moncada S, Liew F Y. Altered immune response in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 58.Wyatt C R, Brackett E J, Perryman L E, Rice-Ficht A C, Brown W C, O'Rourke K I. Activation of intraepithelial T lymphocytes in calves infected with Cryptosporidium parvum. Infect Immun. 1997;65:185–190. doi: 10.1128/iai.65.1.185-190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang S, Healey M C. The immunosuppressive effects of dexamethasone administration in drinking water to C57BL/6N mice infected with Cryptosporidium parvum. J Parasitol. 1993;79:626–630. [PubMed] [Google Scholar]
- 60.Zhu L, Gunn C, Beckman J S. Bactericidal activity of peroxynitrite. Arch Biochem Biophys. 1992;298:452–457. doi: 10.1016/0003-9861(92)90434-x. [DOI] [PubMed] [Google Scholar]