Abstract
The lack of safe and cost-effective treatments against leishmaniasis highlights the urgent need to develop improved leishmanicidal agents. Antimicrobial peptides (AMPs) are an emerging category of therapeutics exerting a wide range of biological activities such as anti-bacterial, anti-fungal, anti-parasitic and anti-tumoral. In the present study, the approach of repurposing AMPs as antileishmanial drugs was applied. The leishmanicidal activity of two synthetic anti-lipopolysaccharide peptides (SALPs), so-called 19-2.5 and 19-4LF was characterized in Leishmania major. In vitro, both peptides were highly active against intracellular Leishmania major in mouse macrophages without exerting toxicity in host cells. Then, q-PCR-based gene profiling, revealed that this activity was related to the downregulation of several genes involved in drug resistance (yip1), virulence (gp63) and parasite proliferation (Cyclin 1 and Cyclin 6). Importantly, the treatment of BALB/c mice with any of the two AMPs caused a significant reduction in L. major infective burden. This effect was associated with an increase in Th1 cytokine levels (IL-12p35, TNF-α, and iNOS) in the skin lesion and spleen of the L. major infected mice while the Th2-associated genes were downregulated (IL-4 and IL-6). Lastly, we investigated the effect of both peptides in the gene expression profile of the P2X7 purinergic receptor, which has been reported as a therapeutic target in several diseases. The results showed significant repression of P2X7R by both peptides in the skin lesion of L. major infected mice to an extent comparable to that of a common anti-leishmanial drug, Paromomycin. Our in vitro and in vivo studies suggest that the synthetic AMPs 19-2.5 and 19-4LF are promising candidates for leishmaniasis treatment and present P2X7R as a potential therapeutic target in cutaneous leishmaniasis (CL).
Keywords: leishmaniasis, drug repurposing, antimicrobial peptides (AMPs), peptide 19-2.5, peptide 19-4LF, drug resistance, proliferation, cytokines
1. Introduction
Leishmaniasis is a parasitic disease causing more than 13,700 deaths annually [1] and is classified by the World Health Organization (WHO) as one of the 20 neglected tropical diseases (NTDs) [2,3]. It is considered a vector-borne disease since Leishmania parasites (the causative agents of the disease) are transmitted to humans by the bite of phlebotomine sand flies [4]. According to some studies, humans are facilitating the worldwide dissemination of this disease due to increasing migration flows, deforestation and climate change [5,6]. Currently, this disease is endemic in 92 countries [7], and more than one billion people live in those endemic regions and are at risk of infection [7].
Leishmania parasites possess two distinct morphologies, the freely living promastigotes in the vector stage and the intracellular amastigotes in mammalian hosts. The promastigotes have an elongated shape and display an active flagellar motility. Once phagocytosed by the immune cells of vertebrate hosts, the parasite losses its flagellum and becomes spherical [8]. In patients infected with Leishmania there are three main clinical presentations of the disease: cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis (MCL) and visceral leishmaniasis (VL) [9]. The most common presentation, CL, is endemic in more than 70 countries around the world where it accounts for 700,000 to 1.2 million cases per year [9,10]. Although less common than CL, VL is the most severe presentation, ranking second after malaria in mortality rate among parasitic diseases [1,7].
There are few available drugs against leishmaniasis, with generic pentavalent antimonials being the first line of treatment [11]. However, these compounds are far from ideal due to their severe side effects and the increasing resistance developed by Leishmania [12]. Amphotericin B (Ampho B) and miltefosine are alternative treatments showing a higher efficacy against leishmaniasis, but they are a high-cost option compared to antimonials [3,13]. Finally, paromomycin (PM), a natural aminoglycoside antibiotic synthesized by Streptomyces riomosus, is more affordable and also has significant activity against Leishmania.
Importantly, treatment with all the available anti-Leishmania treatments is associated with numerous side effects, ranging from mild pain at the injection site to severe symptoms derived from hepatic and renal toxicity [3]. Due to these limitations and bearing in mind, the lack of an effective human vaccine against leishmaniasis, the search for new treatment strategies is of utmost importance.
Drug repurposing remains an insightful strategy within drug discovery and development processes performed to find new applications for medicines that are already used for other diseases. Therefore, drug repurposing has also emerged as an attractive strategy for neglected tropical disease drug discovery and development, including for antileishmanial treatment [14]. Currently, anti-microbial peptides (AMPs) are attractive molecules due to their high potential as therapeutic agents. AMPs are short molecules (<100 amino acids) produced by all types of living organisms including bacteria, fungi, plants, invertebrates, nonmammalian vertebrates, and mammals [15]. In many multicellular organisms and also in humans, AMPs are key components of the innate immune response where they exert a broad spectrum of biological activities, being not only capable of killing pathogens (fungi, parasites, bacteria and viruses) but also of modulating the host immune responses [16,17]. Furthermore, AMPs have, in general, low toxicity, and their target organisms are less likely to develop resistance against them than when treated with conventional drugs [18].
Several groups of AMPs, such as melittin, cecropin, cathelicidin, defensin, magainin, temporin, dermaseptin, eumenitin and histatin have been proven to have significant action against diverse Leishmania species, mainly acting through parasite membrane disruption. Besides, other modes of action were reported by some of these peptides including apoptosis, mitochondrial dysfunction, immune response modulation, and DNA damage [19]. There is active research focused on improving the pharmacological properties of AMPs by reducing their potential limitations such as toxicity and susceptibility to degradation by host proteases [17,20,21].
It is well known that AMPs can be fully synthetic or modified chemically. They can be replicas of the sequences available in nature, or improved versions thereof—shorter, hybrid, or amino acid substitutions [20]. In this context, a new class of synthetic anti-lipopolysaccharide peptides (SALPs) has been successfully designed and synthesized. SALPs derived from the lipopolysaccharide (LPS)-binding domain of the Limulus anti-LPS factor (LALF) and their primary sequence were extensively modified for optimal binding to the lipid A portion of LPS [22]. SALPs were shown to combine excellent selectivity for LPS, with high neutralizing activity in vitro. In addition, they were shown to efficiently protect against septic shock in murine and rabbit models of this pathology [22,23,24]. Interestingly, SALPs showed very low cytotoxicity under physiological conditions, thereby highlighting their therapeutic potential [22,23]. The antimicrobial peptides 19-2.5 and 19-4LF belonging to SALPs family are currently under preclinical tests. They have been essentially studied against bacterial infections showing remarkable activity against Gram-positive bacteria while they had modest activity against Gram-negative strains. In addition, these AMPs have an increased ability to neutralize toxins from both types of organisms, namely LPS and lipoproteins (LP) [25,26].
In the present study, the effects of both AMPs 19-2.5 and 19-4LF were studied for the first time against CL in vitro and in vivo. Our results supported the approach of repurposing both AMPs as antileishmanial drugs.
2. Materials and Methods
2.1. Compounds. Physicochemical Properties and Bioavailability
The peptides included in this study, 19–2.5, also termed Aspidasept®, (GCKKYRRFRWKFKGKFWFWG) and 19-4LF (GKKYRRFRWKFKGKLFLFG), were synthesized at the Borstel Research Institute with an amidated C terminus by the solid-phase peptide synthesis technique in an automatic peptide synthesizer (model 433A; Applied Biosystems, Waltham, MA, USA) on Fmoc-Rink amide resin, according to the 0.1-mmol FastMoc synthesis protocol of the manufacturer, including the removal of the N-terminal Fmoc group. The purity of both peptides was ≥95%, as determined by High-Performance Liquid Chromatography HPLC and mass spectrometry [22,27,28,29].
The properties for drug similarity were analyzed according to Lipinski’s rule of five (molecular mass of ≤500 Da, logP [logarithm of compound partition coefficient between n-octanol and water] of ≤5, H-bond donors [HBD] of ≤5, and H-bond acceptors [HBA] of ≤10) and using topological polar surface area (TPSA) values from the Molinspiration online property calculator tool kit, using the Molinspiration property calculation program (http://www.molinspiration.com/services/properties.html (accessed on 23 February 2021)). TPSA was used to calculate the percentage of absorption (% Abs) according to the equation % Abs = 109 − (0.345 × TPSA) [30]. PM, NipajinTM and ethylenediamine tetraacetic acid (EDTA) were obtained from Sigma-Aldrich (Rome, Italy). Stearic acid, cetyl alcohol, glycerol monoestearate, solid paraffin and white vaseline were purchased by Fagron (Barcelona, Spain). Liquid paraffin was obtained from Guinama (Valencia, Spain). All other reagents were of analytical grade.
2.2. Animals
Six-week-old female BALB/c mice were purchased from Harlan Interfauna Ibérica S.A. (Barcelona, Spain). Animals were randomly housed in groups and kept in controlled environmental conditions (12:12 h light/dark cycle and 22 °C). All the procedures involving animals were approved by the Animal Care Ethics Commission of the University of Navarra [approval number: E5-16(068-15E1) 25 February 2016].
2.3. Cells and Culture Conditions
Leishmania major promastigotes (Lv39c5) were grown at 26 °C in Schneider’s Drosophila medium (Gibco) supplemented with heat-inactivated fetal bovine serum (FBS), and an antibiotic cocktail (50 U/mL penicillin, 50 mg/mL streptomycin). To maintain their infectivity, Leishmania cells were isolated from infected BALB/c mouse spleen and parasites were maintained in culture for not more than five passages.
Murine bone marrow-derived macrophages (BMDMs) were obtained as previously described [31].
2.4. Cytotoxicity Assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test (Sigma, St. Louis, MO, USA) was performed to determine the cytotoxicity of peptides on BMDMs. MTT solutions were prepared at 5 mg/mL in phosphate buffer solution (PBS), filtered and maintained at −20 °C until use. Briefly, macrophages were detached with a scraper and 5 × 104 cells were seeded per well in 96-well plates and allowed to adhere for 24 h at 37 °C in a 5% CO2 humidified atmosphere. The culture medium was replaced by fresh medium with increasing concentrations of peptides (from 0 to 4 µg/mL) and after 72 h of incubation, 100 µg/well of MTT was added and the plates were incubated for 4 h under the same conditions. Then, 80 µL of dimethyl sulfoxide (DMSO) was added to each well to dissolve formazan crystals. The optical density (OD) was measured in a Multiskan EX microplate photometer plate reader at 540 nm [32,33] and the half-maximal inhibitory concentration (IC50) was calculated. The IC50 represents the concentration required for 50% growth inhibition of treated cells with respect to untreated cells (controls) and was obtained by fitting a sigmoidal Emax model to dose-response curves. The results were expressed as means (±standard deviation, SD) from two independent experiments.
2.5. Activity against Intracellular Amastigotes
Macrophages were seeded in 8-well culture chamber slides (Lab-TekTM; BD Biosciences, East Rutherford, NJ, USA) at a density of 5 × 104 cells per well in RPMI medium and allowed to adhere overnight at 37 °C in 5% CO2. In order to perform the infection assay, metacyclic L. major promastigotes isolated by the peanut agglutinin (PNA) method [34] were used to infect macrophages at a macrophage/parasite ratio of 1/20. The plates were incubated for 24 h under the same conditions until promastigotes were phagocytized by macrophages. The extracellular parasites were removed by washing with medium, and plates were incubated with fresh medium containing 19-2.5 and 19-4LF peptides at 1 µg/mL [35]. Similarly, to analyze the effect of peptides in combination with leishmanicidal drugs (PM or Ampho B), infected macrophages were exposed to a mix containing 1 µg/mL of each peptide and 50 µM of PM or 0.025 µM of Ampho B; 72 h later, cells were washed with PBS, fixed with ice-cold methanol for 5 min and stained with Giemsa stain. To determine the parasite burden, the number of amastigotes per 200 infected macrophages was counted under a light microscope. The mean number of amastigotes per infected macrophage was determined by dividing the total number of amastigotes counted by the number of infected macrophages. Three independent experiments were performed with duplicates.
2.6. Gene Expression Changes upon Treatment
2.6.1. RNA Retrieval from L. major Infected BMDMs
RNA from L. major amastigotes was obtained as follows. BMDMs were plated with a density of 106 cells per well in 24-well plates. After 24 h of incubation, macrophages were infected with L. major PNA (-) metacyclic promastigotes parasites, at a ratio of 20:1 (parasites/macrophage). At 24 h post-infection, non-phagocytosed parasites were washed off with PBS and infected BMDMs were further treated with the peptides (1 µg/mL) or left untreated as a control. After 48 h of treatment, the adherent BMDMs containing L. major amastigotes were stored in TRI reagent (1 mL per well) at −80 °C until RNA extraction.
2.6.2. RNA Retrieval from L. major Infected Mice
Animals were infected by subcutaneous inoculation with 105 infective metacyclic promastigotes of L. major in the base of the tail. Eight weeks after the infection, lesions of measurable size had developed and animals were incorporated into the assay gradually once lesions reached an average area of 12 mm2 [31]. Three treatments were evaluated: PM (used as positive control), 19-2.5 and 19-4LF, and seven mice were used in each group, including the negative control corresponding to untreated mice. Treatments, topically administered twice daily for a period of 30 days, consisted of 50 mg of cream containing 15% of PM (1.4 g/kg/day) or a 20 µL drop of the peptide solution containing 400 µg/mL of the peptide (19-2.5 or 19-4LF) dissolved in pyrogen-free saline (PFS) (0.8 mg/kg/day) [36]. PM cream was prepared as previously described [31]. After the 30-day treatment regimen, animals were kept untreated for 3 days and then they were sacrificed. Fragments from skin lesions as well as from the spleen of infected mice were aseptically removed, immersed in 0.5 mL of RNAlater solution (Invitrogen, Waltham, MA, USA) to preserve them from RNA degradation, and conserved at 2–4 °C.
2.6.3. RNA Extraction and Gene Expression Analysis
Total RNA from L. major amastigotes was extracted following the TRI reagent manufacturer’s protocol (Sigma, St. Louis, MO, USA) and subsequently treated with DNase (Gibco-BRL). The RNA from mice tissue samples was also extracted following the TRI reagent manufacturer’s protocol (Sigma, St. Louis, MO, USA) and subsequently treated with DNase (Gibco-BRL). One microgram of total RNA from each sample of amastigotes and mice tissues was used for retrotranscription with M-MLV reverse transcriptase (Invitrogen), following the protocol of the manufacturer. Real-time PCR was performed with iQ SYBR Green supermix (Bio-Rad) in a CFX96 system from Bio-Rad, using specific primers for each gene. Leishmania and mouse primers used for qPCR are summarized in Table 1 and Table 2, respectively. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Table 1) [32] was used as a housekeeping gene to normalize L. major gene expression. For mouse genes, the β-actin reference gene was used to normalize the expression (Table 2) [37]. The amount of each transcript was expressed by the formula 2ct(actin or GAPDH)−ct(gene), with ct being the point (PCR cycle) at which the fluorescence rises appreciably above the background fluorescence [38].
Table 1.
Primer sequences used for the quantification of Leishmania genes.
| Gene | Sense Primer (5′→3′) | Antisense Primer (5′→3′) |
|---|---|---|
| Yip1 | AAGCTCCTTGGCAGCAAGAT | TGTGTCGGAAAAAGCGCAAG |
| ABCC6 | TGTCCTCTCAACACGCATCC | TCGCAGAGCTCTTCAGTTGG |
| gp63 | ACTGCCCGTTTGTTATCGAC | CCGGCGTACGACTTGACTAT |
| Cyclin 1 | CCCCAACACCGCTGACTAAT | TCCGACTGGCGGTCTATGTA |
| Cyclin 6 | AGTACCCTGCACGCCTACTA | TTGTTGTTGGCGCAGGAAAG |
| Lm18S | CCAAAGTGTGGAGATCGAAG | GGCCGGTAAAGGCCGAATAG |
| GAPDH | ACCACCATCCACTCCTACA | CGTGCTCGGGATGATGTTTA |
Table 2.
Primer sequences used for the quantification of mouse genes.
| Gene | Sense Primer (5′→3′) | Antisense Primer (5′→3′) |
|---|---|---|
| IL12p35 | CACGCTACCTCCTCTTTTTG | AGGCAACTCTCGTTCTTGTG |
| TNFα | CTTCCAGAACTCCAGGCGGT | GGTTTGCTACGACGTGGG |
| iNOS | TCCTACACCACACCAAACTG | AATCTCTGCCTATCCGTCTC |
| IL4 | GCTATTGATGGGTCTCAACC | TCTGTGGTGTTCTTGTTGC |
| IL6 | ACAAAGCCAGAGTCCTTCAG | TGGATGGTCTTGGTCCTTAG |
| TNFR1 | CGATAAAGCCACACCCACAA | ACCTTTGCCCACTTTTCACC |
| IL4Rα | TGACCTACAAGGAACCCAGGC | GAACAGGCAAAACAACGGGAT |
| IL6R | GGAGATCCTGGAGGGTGACA | CGTTGTGGCTGGACTTGCTT |
| CDKN1A | TTGTCGCTGTCTTGCACTCT | GGCACTTCAGGGTTTTCTC |
| β-actin | CGCGTCCACCCGCGAG | CCTGGTGCCTAGGGCG |
2.7. Parasite Burden Evaluation from Infected Mice
The quantification of L. major burden in different tissues from mice was performed by measuring mRNA levels of the 18S ribosomal gene from Leishmania spp. (Lm18S) by reverse transcription, followed by real-time PCR as previously described [38], using specific primers (see Table 1).
2.8. Statistical Analyses
Statistical analyses were performed using PRISM version 5.0 (GraphPad: San Diego, CA, USA). The data are presented as means ± the SD. Comparisons between the two groups were made using the two-tailed unpaired t-test. The statistical significance was determined (***, p < 0.001; **, p < 0.01; *, p < 0.05).
3. Results
3.1. Both Peptides 19-2.5 and 19-4 LF Displayed Leishmanicidal Activities against L. major Intracellular Amastigotes
The cytotoxicity of 19-2.5 and 19-4 LF peptides on BMDMs was tested using MTT assay with increasing concentrations of the peptides. The corresponding macrophages viability was calculated compared to the untreated control. Both peptides displayed no toxicity (100% cell viability) at all the tested concentrations (from 0.5 to 4 µg/mL). According to the obtained cell viability values, in further experiments, we decided to use a concentration of 1 µg/mL for the two peptides, namely four times lower than the maximum (non-toxic) concentration tested.
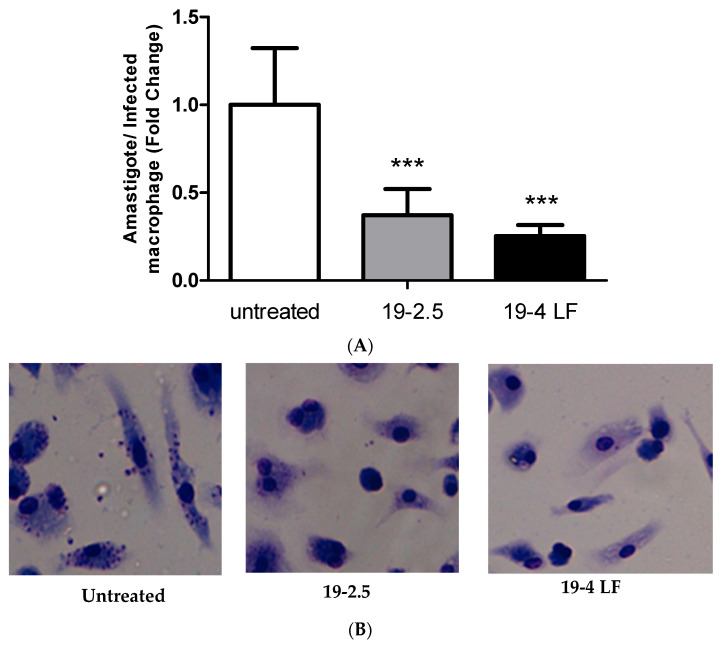
To investigate the leishmanicidal effect of 19-2.5 and 19-4 LF peptides, we studied their activity against the intracellular form of the parasite, the amastigote. For this purpose, macrophages were infected in vitro with metacyclic L. major promastigotes. After 24 h, infected macrophages were treated with the two peptides 19-2.5 and 19-4 LF at a final concentration of 1 µg/mL. The results showed that both peptides caused a significant reduction in the number of amastigotes per infected macrophage (p ≤ 0.001) compared to untreated cells (Figure 1).
Figure 1.
Leishmanicidal activity of 19-2.5 (1 µg/mL) and 19-4 LF (1 µg/mL) against intramacrophage amastigotes (A). Bars represent the mean ± SD values from three independent experiments. Significant reductions in the number of amastigotes per infected macrophage were detected when compared to untreated controls (***, p < 0.001). Representative images of untreated infected cells and infected macrophages treated with 1 µg/mL of 19-2.5 or 19-4LF peptides (B).
3.2. 19-2.5 and 19-4 LF Reduced the Expression Levels of Genes Related to Proliferation and Drug Resistance of L. major Amastigotes
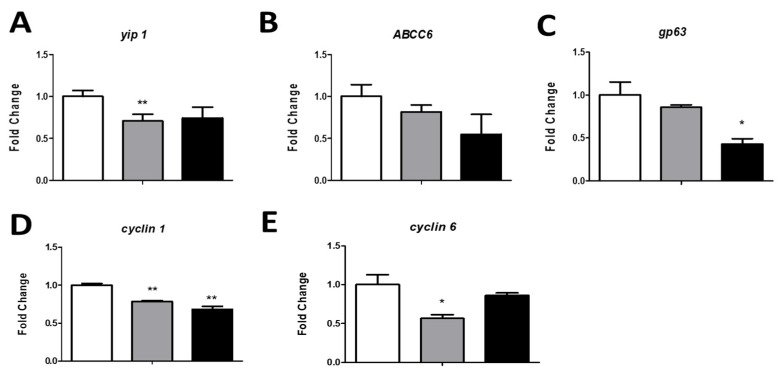
To identify the molecular mechanism involved in peptide activity, we studied the effect of 19-2.5 or 19-4LF on the expression levels of L. major genes related to drug resistance (yip1 and ABCC6) [39,40], parasite virulence (gp63) [41] and cell cycle proliferation (Cyclin 1 and Cyclin 6) [42]. To this end, the gene expression level was quantified by RT-PCR using mRNA purified from BMDM previously infected with L. major and treated with 19-2.5 or 19-4LF as a template. As shown in Figure 2, mRNA expression levels of all studied genes were downregulated in parasites exposed to 19-2.5 and 19-4LF peptides, compared to untreated parasites (control). Significant reductions (p < 0.01) were detected in (Yip1, Cyclin 1 and Cyclin 6) gene expression levels when treated with 19-2.5, while Cyclin 1 and gp63 mRNA levels were significantly decreased (p < 0.05) by 19-4LF (Figure 2).
Figure 2.
The effect of 19-2.5 and 19-4 LF on L. major amastigotes gene expression levels. The graphs show the expression levels of genes related to drug resistance, [yip 1 (A), ABCC6 (B)], virulence [gp63 (C)], and Leishmania proliferation [cyclin 1 (D), and cyclin 6 (E)] after 24 h of treatment with 19-2.5 (1 µg/mL) and 19-4 LF (1 µg/mL). GAPDH was used as a housekeeping gene to normalize L. major gene expression. The amount of each transcript was expressed by the formula 2ct(GAPDH)−ct(gene). Bars represent the mean ± SD from three independent experiments. Significant differences compared to control (untreated cells) were indicated (*, p < 0.05; **, p < 0.01).
3.3. Both Peptides Significantly Reduced the Parasite Burden In Vivo When Topically Administered
Lipinski descriptors for bioavailability estimation were calculated for 19-4 LF and 19-2.5 using the freely accessible program MIPC (Molinspiration Property Calculator) (http://www.molinspiration.com/services/properties.html (accessed on 23 February 2021)). These descriptors identify molecular properties important for drug pharmacokinetics in the human body including their potential oral absorption [32]. Our results predicted poor oral absorption for both peptides since they violated most of the parameters of Lipinski’s Rule of Five (Table 3). Preliminary tests were performed by administering them topically in further in-vivo studies.
Table 3.
Drug oral bioavailability parameters of 19-2.5 and 19-4 LF peptides.
| Peptide. | LogP (≤5) |
TPSA | MW (≤500) |
nON (≤10) |
nOHNH (≤5) |
Vol (Å3) |
Linpinski’s Violation (≤1) |
|---|---|---|---|---|---|---|---|
| 19-2.5 | −5.27 | 999.61 | 2712.28 | 59 | 48 | 2500.49 | 3 |
| 19-4LF | −5.28 | 938.93 | 2463.03 | 55 | 45 | 2316.94 | 3 |
LogP, logarithm of compound partition coefficient between n-octanol and water. TPSA, topological polar surface area. MW, molecular weight. nON, number of hydrogen bond acceptors, nOHNH, number of hydrogen bond donors.
To investigate the therapeutic potential of 19-4 LF and 19-2.5 against Leishmania in vivo, we first infected mice subcutaneously and once the areas of the lesions were 12 mm2, we treated them topically with the drug candidates. Specifically, peptides were administered on the skin lesions of the animal twice a day for 30 days and parasite burden was determined by measuring the Lm18S mRNA expression levels in the skin lesion and the spleen. When compared to untreated samples (control), the parasite burden was lower in both, skin lesions and the spleen after the administration of the two peptides (Figure 3). Significant differences in parasite burden (**, p < 0.01) were detected in the skin lesion with 19-4LF treatment, compared to the control (Figure 3A).
Figure 3.
Effect of peptides 19-2.5 and 19-4 LF on parasite burden in the skin lesions (A) and the spleen (B) of L. major infected mice after treatment compared to controls (untreated mice). The parasite burden was evaluated by quantifying the Lm18S mRNA gene expression levels using qPCR method. Bars represent the mean ± the SD. Significant difference was indicated (**, p < 0.01 compared with the untreated control).
3.4. Both 19-2.5 and 19-4 LF Modulated the Expression of Host Cell Genes In Vivo
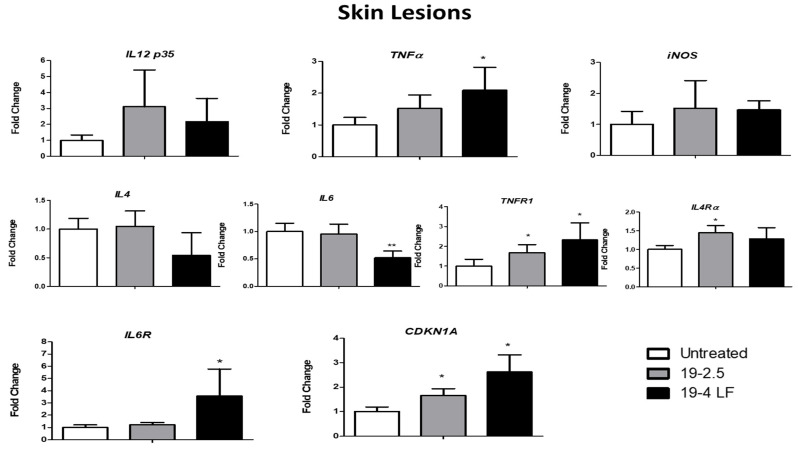
It has been demonstrated that pro-inflammatory and anti-inflammatory cytokines play a crucial role in immunity against CL inducing resistance/protection against the parasite (Th1 pro-inflammatory cytokines) and pathogen proliferation (Th2 cytokines) [43]. Therefore, four selected inflammatory cytokine genes (IL 12p35, TNFα, IL-4 and IL-6) and some of their corresponding receptors (TNFR1, Il4Rα and IL-6R) were analyzed by quantitative RT-PCR in the skin lesion (Figure 4) and the spleen (Figure 5) of infected BALB/c mice, treated with 19-2.5 and 19-4 LF (1 µg/mL) or left untreated. Two more genes were also studied, the inducible nitric oxide synthase (iNOS) whose expression is induced by cytokines [44] and the cell cycle repressor gene (CDKN1A) [45]. Gene expression analyses in the skin lesion of infected BALB/c mice, showed an induction of Th1 cytokine (IL12p35 and TNFα) and iNOS gene expression levels when treated with 19-2.5 and 19-4 LF peptides. A significant variation was detected for TNFα expression levels (*, p ≤ 0.05 compared to control) when the mice were treated with 19-4 LF (Figure 4). After analyzing the spleens of mice treated with peptides, significant inductions in IL12p35 and iNOS mRNA levels were detected with both treatments, as well as in TNFα gene with 19-2.5 (Figure 5). IL-4 and IL-6 (Th2 cytokines) gene expression levels were not altered either in skin lesions or in spleens after the treatment with 19-2.5. In fact, the IL-6 mRNA level augmented only in the spleen after treatment with 19-2.5 peptide (*, p < 0.05) (Figure 5). On the contrary, in skin lesions of 19-4LF treated mice, a decrease in expression levels of Th2 cytokines was detected. Such a reduction was very significant (**, p < 0.01) for IL-6 (Figure 4), while no significant change was observed in the spleen of the 19-4LF-treated mice for both IL-4 and IL-6. Regarding cytokine receptors, TNFR1, IL4Rα and IL6R expression levels tended to increase in both skin lesions and spleen tissues (Figure 4 and Figure 5). The treatment with 19-2.5 increased the levels of IL-4 gene receptors in both, skin lesions and the spleen while a significant induction of the TNF receptor was detected in the skin. On the other hand, 19-4LF peptide was able to significantly increase the expression levels of TNF and IL-6 receptors in both types of samples, skin lesions and spleens. Lastly, the expression analysis of the CDKN1A gene (a negative regulator of cell cycle progression genes) showed a significant increase (*, p < 0.05) in the skin lesions of mice treated with 19-2.5 and 19-4LF versus untreated mice) (Figure 4), while no significant changes were detected in the spleen tissues after applying the same treatments (Figure 5).
Figure 4.
Gene expression levels of Th1 cytokines (IL 12 p35 and TNFα), iNOS gene, Th2 cytokines (IL 4 and IL 6), cytokine receptors (TNFR1, IL4 Rα and IL6 R) and CDKN1A gene from the skin lesions of BALB/c mice treated with 19-2.5 and 19-4 LF peptides or untreated. β-actin was used as a reference gene to normalize mouse gene expression. The amount of each transcript was expressed by the formula 2ct(actin)−ct(gene). Bars represent the means (±SD) (*, p < 0.05; **, p < 0.01).
Figure 5.
Gene expression levels of Th1 cytokines (IL 12 p35 and TNFα), iNOS gene, Th2 cytokines (IL 4 and IL 6), cytokine receptors (TNFR1, IL4 Rα and IL6 R) and CDKN1A gene from the spleen of BALB/c mice treated with 19-2.5 and 19-4 LF peptides or untreated. β-actin was used as a housekeeping gene to normalize mouse gene expression. The amount of each transcript was expressed by the formula 2ct(actin)−ct(gene). Bars represent the means (±SD) (*, p < 0.05; **, p < 0.01).
3.5. P2X7 Receptor Gene Expression Was Highly Reduced by Both Peptides in Skin Lesions of BALB/c Mice
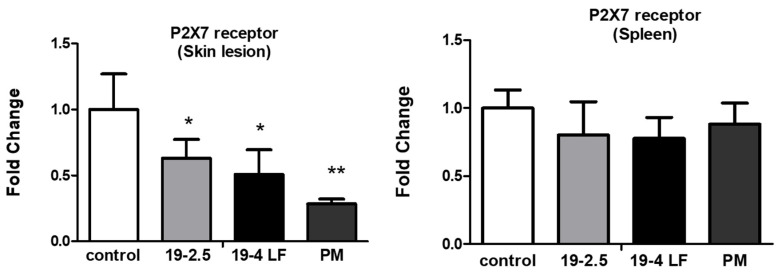
It was reported that the P2X7 receptor, expressed in immune cells, has a key role in the regulation of the inflammatory response leading to infection control [46]. To test whether the expression of the P2X7 receptor was affected by the treatment with the two peptides, mRNA expression levels were quantified in the skin lesions and the spleen of BALB/c mice infected with L. major and treated with 19-2.5, 19-4 LF (1 µg/mL) and PM (50 µM) or untreated (control). The P2X7 receptor was significantly downregulated in the skin lesions of the mice treated with 19-2.5 and 19-4LF (*, p < 0.05) and PM (**, p < 0.01), while no significant variation was detected in the spleen tissues with the same treatments (Figure 6).
Figure 6.
Effect of PM and the two peptides (19-2.5 and 19-4LF) on the expression levels of P2X7 receptor gene in the skin lesion and the spleen of treated BALB/c mice compared to untreated mice (controls). The bars represent the means ± SD. Significant differences were indicated (*, p < 0.05; **, p < 0.01 compared to controls).
3.6. The Combination of Both Peptides with the Leishmanicidal Drugs Paromomycin and Amphotericin B Greatly Enhanced In Vitro Their Activity against L. major Amastigotes
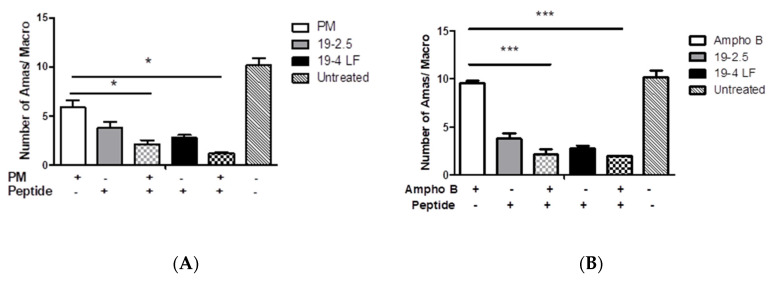
Lastly, the effect of both peptides in combination with the currently used anti-leishmanial drugs PM and Ampho B was studied. The results showed that the combination of 19-2.5 (1 µg/mL) or 19-4 LF (1 µg/mL) with PM (50 µM) dramatically reduced the number of amastigotes per macrophage from 6 (PM) to 2 or 1 (*, p < 0.05) when using 19-2.5 or 19-4LF, respectively (Figure 7).
Figure 7.
Leishmanicidal effect of PM (50 µM) (A), Ampho B (0.025 µM) (B) and their combinations with peptides Pep19-2.5 (1 µg/mL) and Pep19-4 LF (1 µg/mL). Bars represent the means (±SD) from three independent experiments (*, p < 0.05; ***, p < 0.001).
Similarly, the anti-parasitic effect of Ampho B (0.025 µM) was also significantly induced when Ampho B was used in combination with the peptides. In fact, there was a very significant decrease in the number of amastigotes/macrophages from 9 (Ampho B) to 2 after such a combination (***, p < 0.001) (Figure 7).
4. Discussion
The attractive biological activities of AMPs are prompting active research in the therapeutic application of these agents to combat many infectious diseases, including leishmaniasis [20]. Herein, we evaluated the leishmanicidal activity of two synthetic AMPs, 19-2.5 and 19-4LF, against Leishmania major, the causative agent of CL. In vitro experiments demonstrated that both peptides displayed potent amastigote-killing activity in infected macrophages when added at low concentrations (1 µg/mL). Interestingly, we proved that 19-2.5 and 19-4LF caused no toxicity in host macrophages at 4 µg/mL (>4 times the effective concentration on amastigotes).
We also showed that the treatment of Leishmania-infected macrophages with 19-2.5 and 19-4LF lowered the expression levels of several Leishmania genes implicated in proliferation (Cyclin 1 and Cyclin 6), pathogen virulence (gp63) and treatment resistance (yip1) In line with our results, these genes were similarly downregulated by CPE2, a recently reported leishmanicidal compound [47]. Importantly, our results demonstrated that 19-2.5 and 19-4LF retained their anti-parasitic potential in vivo since they dramatically reduced the parasite burden in the skin lesion as well as in the spleen of infected mice. It is worth highlighting that both peptides exerted their therapeutic activity upon topical application on the skin lesions with no need for systemic administration. Previous studies have shown the application of both AMPs as skin drugs [29]. In fact, these peptides may become insensitive to the action of skin proteases after their adequate cream formulation [29]. This strategy will likely reduce toxicity concerns associated with the systemic route and it may facilitate their clinical use if one day these peptides reach the market.
Since the modulation of the immune response was one of the mechanisms of action reported for AMPs [19] we studied whether 19-2.5 and 19-4 LF peptides altered the cytokine expression profile in skin lesions and spleens of infected BALB/c mice. Although each peptide displayed its own pattern of cytokine modulatory activity, our results suggested that they caused an increase in Th1 cytokine mRNA levels (IL-12p35, TNF-a and iNOS) in both, the skin lesion and the spleen. Interestingly, the Th1 type immune response induction was reported to be of critical importance for the control of cutaneous leishmaniasis [48]. In contrast, in skin lesions from Leishmania-infected mice treated with the peptide 19-4LF, a decrease in IL-4 and IL-6 gene levels was detected, in agreement with the significant reduction in parasite burden in those samples. Such cytokines have been related to a non-protective Th2 response during CL in mice [48].
It has been demonstrated that Leishmania clearance was enhanced by the Th1-type response, in which IL-12 expression played a crucial role [49]. Consistent with this, an increase in IL-12, TNF and NO production by macrophages was shown to be characteristic of an efficient anti-parasitic response [50]. In addition, immunotherapy with IL-12 was reported to mediate the control of L. major infection in BALB/c mice and was associated with wound healing promotion, parasite burden reduction and IL-4 downregulation [43]. Recently, it was demonstrated that the overexpression of the protein kinase LmjF.22.0810 in L. major (cell line LmJ3OE) resulted in a phenotype with reduced virulence. Remarkably, this cell line was shown to repress the gene expression of Th2-associated cytokines (IL-4, IL-10 and arginase 1) leading to a Th1 immune response predominance (and an increase in IL12-p35 cytokine). This immune modulation likely explained the enhanced leishmanicidal ability that mice (infected with this cell line) exhibited compared to control (mice infected with L. major wild type) [38]. In accordance with previous studies, our findings suggested that the Th1 response induced by 19-4LF and 19-2.5, might be responsible for the parasite burden reduction in the skin lesion and in the spleen of L. major infected BALB/c mice. Previous studies performed to explore the effect of Leishmania infection on host macrophage gene expression showed a general suppression of the cytokine receptor genes (IL-4Ra, IL-6R and TNFR1) in conjunction with the parasite multiplication [51]. These data were consistent with our results showing significant upregulation of three receptors IL-4Ra, IL-6R and TNFR1 in both the skin lesion and the spleen of L. major BALB/c infected mice treated with both peptides 19-2.5 and 19-4LF. This overexpression could be related to the parasite burden reduction enhanced by 19-2.5 and 19-4LF peptides.
We also investigated the effect of both peptides on the purinergic receptor P2X7 gene expression. P2X7R had been associated with tissue damage in Mycobacterium tuberculosis infection [52], as well as in ulcerative colitis disease, where a reduction in tissue damage was detected in mice lacking P2X7 receptors [53]. Interestingly, in both the skin lesion and the spleen, our peptides 19-2.5 and 19-4LF were acting similarly to the PM. We observed that both AMPs downregulated P2X7R in the skin lesion of infected mice, while no significant change in the receptor gene expression was observed in the spleen [16,46,54]. However, P2X7R was reported to play a crucial role in L. amazonensis elimination by a leukotriene (LT) B4-dependent mechanism [55], and its absence was associated with a more severe lesion in mice infected with L. amazonensis [46]. P2X7 KO mice exhibited an increased Th1 inflammation during L. amazonensis infection, higher levels of INF-γ and a decrease in TGF-α [46]. Such a cytokine profile is related to L. major parasite clearance [56,57]. It is well known that the immune response against L. amazonensis is different from that against L. major and does not implicate the Th1/Th2 balance [58]. Our findings showed an activation of Th1-associated genes (IL-12, TNFa and iNOS) when the gene expression level of the P2X7 receptor was reduced in skin lesions of L. major infected mice. In addition, a significant increase in IL-6 gene expression levels was observed in the spleen in accordance with studies revealing that the P2X7 receptor promoted the secretion of the inflammatory cytokine IL-6 [59,60]. Our findings supported the P2X7 receptor as a therapeutic target for cutaneous leishmaniasis.
As previously described, cyclins are proteins involved in the cell cycle control which active the activity of kinases by forming a complex with those proteins (cyclin-CDKs) [61]. Inhibitors of cyclin-dependent kinases (CDKs) have been reported as suitable candidates for the treatment of leishmaniasis [62,63,64,65]. Recently discovered compounds with leishmanicidal activity have demonstrated the ability to inhibit the expression of such cyclins in Leishmania major [47]. On the other hand, terbinafine resistance locus protein (yip 1) codifying gene, has been detected to be overexpressed in drug-resistant Leishmania strains [42,66,67] Since the peptide 19-2.5 showed activity by inhibiting yip1 gene levels, it might be also considered as a potential candidate against resistant strains. Whereas the peptide 19-4LF might reduce the parasite burden of skin lesions by inhibiting the expression levels of the gene codifying GP63 (a metalloprotease and a virulence factor also involved in host cell signaling mechanisms) among other pathways [68,69].
Lastly, the combination of those peptides with two known drugs administered against CL showed a higher effect, increasing the leishmanicidal activity of such treatments alone. During in vitro assays, Ampho B and PM displayed higher activity against intracellular amastigotes when administered in combination with those peptides. It is known that the destruction of skin and severe tissue damage caused by CL can be produced by an exacerbated immune response [70]. Therefore, the control of both, immune response and parasite burden might be considered when looking for new therapeutic options. The combination of existing drugs and immunomodulatory compounds remains a promising strategy. For instance, the local administration of anti-TNF-α antibodies in combination with PM had shown a reduction in skin damage, although such a decrease did not correlate with parasite burden [31]. Similarly, the combination of PM and chloroquine during murine CL was able to decrease the lesion size but not the parasite load compared to data obtained from mice treated with the same doses of PM alone [31].
In the present study, the synthetic AMPs were able to diminish the number of intracellular macrophages when administered alone as well as in combination with drugs currently used in the clinic. They also increased the protective Th1-type immune response. Moreover, the peptide 19-4LF decreased the expression of genes related to Th2-type immune response cytokines in skin lesions, yielding a reduction in the parasite load. More studies are needed to completely elucidate the mechanism of action of such AMPs during Leishmania infection. Likely, they act through different targets including both the parasite elimination and the host immune response modulation.
Acknowledgments
PN thanks the COST Actions CA18217 (ENOVAT), CA18218, CA21105 and CA21111 for their support. Klaus Brandenburg greatly acknowledges a kind gift for “Brandenburg Antiinfektiva GmbH” by Satoshi Fukuoka (Kumamoto, Japan). The authors acknowledge Susana Murillo and María Orbe for their assistance.
Author Contributions
Conceptualization, K.B., G.M.-d.-T. and P.N.; methodology, R.E.-D., C.F.-R., J.P.-G., E.M., E.L. and P.N.; validation, R.E.-D., C.F.-R., J.P.-G., E.M., E.L., S.E. and F.A.-S.; formal analysis, R.E.-D., C.F.-R., E.L. and P.N.; investigation, R.E.-D., C.F.-R., J.P.-G., E.M., E.L. and P.N.; resources, P.N.; data curation, R.E.-D., C.F.-R., E.L. and P.N.; writing—original draft preparation, R.E.-D. and P.N.; writing—review and editing, R.E.-D., C.F.-R., J.P.-G., E.M., E.L., S.E., F.A.-S., K.B., G.M.-d.-T. and P.N.; visualization, R.E.-D. and P.N.; supervision, P.N.; project administration, P.N.; funding acquisition, P.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All the procedures with animals were approved by the Animal Care Ethics Commission of the University of Navarra (CEEA) and by the institutional ethics committees [approval number: E5-16(068-15E1) 25 February 2016].
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Funding Statement
This research was funded by the Spanish Ministry of Science and Innovation (PID2020-112713RB-C21), Fundación La Caixa (LCF/PR/PR13/51080005), Fundación Roviralta and Ubesol.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Naghavi M., Abajobir A.A., Abbafati C., Abbas K.M., Abd-Allah F., Abera S.F., Aboyans V., Adetokunboh O., Afshin A., Agrawal A., et al. GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. Erratum in Lancet 2017, 390, e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartley M.-A., Kohl K., Ronet C., Fasel N. The Therapeutic Potential of Immune Cross-Talk in Leishmaniasis. Clin. Microbiol. Infect. 2013;19:119–130. doi: 10.1111/1469-0691.12095. [DOI] [PubMed] [Google Scholar]
- 3.WHO Expert Committee on the Control of the Leishmaniases and World Health Organization Control of the leishmaniases; Proceedings of the WHO Expert Commitee on the Control of Leishmaniases; Geneva, Switzerland. 22–26 March 2010. [Google Scholar]
- 4.Karunaweera N.D., Ferreira M.U. Leishmaniasis: Current Challenges and Prospects for Elimination with Special Focus on the South Asian Region. Parasitology. 2018;145:425–429. doi: 10.1017/S0031182018000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kevric I., Cappel M.A., Keeling J.H. New World and Old World Leishmania Infections: A Practical Review. Derm. Clin. 2015;33:579–593. doi: 10.1016/j.det.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Morales A.J., Silvestre J., Cazorla-Perfetti D.J. Imported Leishmaniasis in Australia. J. Travel Med. 2009;16:144–145. doi: 10.1111/j.1708-8305.2008.00296_1.x. [DOI] [PubMed] [Google Scholar]
- 7.WHO Leishmaniasis. [(accessed on 27 October 2021)]. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1.
- 8.Sunter J., Gull K. Shape, Form, Function and Leishmania Pathogenicity: From Textbook Descriptions to Biological Understanding. Open Biol. 2021;7:170165. doi: 10.1098/rsob.170165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reithinger R., Dujardin J.-C., Louzir H., Pirmez C., Alexander B., Brooker S. Cutaneous Leishmaniasis. Lancet Infect. Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Leishmaniasis Epidemiology & Risk Factors. [(accessed on 26 October 2021)]; Available online: https://www.cdc.gov/parasites/leishmaniasis/epi.html.
- 11.Blum J., Lockwood D.N.J., Visser L., Harms G., Bailey M.S., Caumes E., Clerinx J., van Thiel P.P.A.M., Morizot G., Hatz C., et al. Local or Systemic Treatment for New World Cutaneous Leishmaniasis? Re-Evaluating the Evidence for the Risk of Mucosal Leishmaniasis. Int. Health. 2012;4:153–163. doi: 10.1016/j.inhe.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira L.F., Schubach A.O., Martins M.M., Passos S.L., Oliveira R.V., Marzochi M.C., Andrade C.A. Systematic Review of the Adverse Effects of Cutaneous Leishmaniasis Treatment in the New World. Acta Trop. 2011;118:87–96. doi: 10.1016/j.actatropica.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Solomon M., Pavlotsky F., Leshem E., Ephros M., Trau H., Schwartz E. Liposomal Amphotericin B Treatment of Cutaneous Leishmaniasis Due to Leishmania Tropica. J. Eur. Acad. Dermatol. Venereol. 2011;25:973–977. doi: 10.1111/j.1468-3083.2010.03908.x. [DOI] [PubMed] [Google Scholar]
- 14.Andrade-Neto V.V., Cunha-Junior E.F., Faioes V.D.S., Martins T.P., Lopes Silva R., Leon L.L., Torres-Santos E.C. Leishmaniasis Treatment: Update of Possibilities for Drug Repurposing. Front. Biosci. 2018;23:967–996. doi: 10.2741/4629. [DOI] [PubMed] [Google Scholar]
- 15.Pasupuleti M., Schmidtchen A., Malmsten M. Antimicrobial Peptides: Key Components of the Innate Immune System. Crit. Rev. Biotechnol. 2012;32:143–171. doi: 10.3109/07388551.2011.594423. [DOI] [PubMed] [Google Scholar]
- 16.Hilchie A.L., Wuerth K., Hancock R.E.W. Immune Modulation by Multifaceted Cationic Host Defense (Antimicrobial) Peptides. Nat. Chem. Biol. 2013;9:761–768. doi: 10.1038/nchembio.1393. [DOI] [PubMed] [Google Scholar]
- 17.Kumar P., Kizhakkedathu J.N., Straus S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules. 2018;8:4. doi: 10.3390/biom8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robles-Loaiza A.A., Pinos-Tamayo E.A., Mendes B., Teixeira C., Alves C., Gomes P., Almeida J.R. Peptides to Tackle Leishmaniasis: Current Status and Future Directions. Int. J. Mol. Sci. 2021;22:4400. doi: 10.3390/ijms22094400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Dirany R., Shahrour H., Dirany Z., Abdel-Sater F., Gonzalez-Gaitano G., Brandenburg K., Martinez de Tejada G., Nguewa P.A. Activity of Anti-Microbial Peptides (AMPs) against Leishmania and Other Parasites: An Overview. Biomolecules. 2021;11:984. doi: 10.3390/biom11070984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu W., Zhu X., Tan T., Li W., Shan A. Design of Embedded-Hybrid Antimicrobial Peptides with Enhanced Cell Selectivity and Anti-Biofilm Activity. PLoS ONE. 2014;9:e98935. doi: 10.1371/journal.pone.0098935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de la Fuente-Núñez C., Cardoso M.H., de Souza Cândido E., Franco O.L., Hancock R.E.W. Synthetic Antibiofilm Peptides. Biochim. Biophys. Acta. 2016;1858:1061–1069. doi: 10.1016/j.bbamem.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas G., Iosu R.-O., Ina K., Yani K., Jörg H., Rainer B., Mathias H., Tobias S., Manfred R., Susana S.-G., et al. New Antiseptic Peptides To Protect against Endotoxin-Mediated Shock. Antimicrob. Agents Chemother. 2010;54:3817–3824. doi: 10.1128/AAC.00534-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaconis Y., Kowalski I., Howe J., Brauser A., Richter W., Razquin-Olazarán I., Iñigo-Pestaña M., Garidel P., Rössle M., Martinez de Tejada G., et al. Biophysical Mechanisms of Endotoxin Neutralization by Cationic Amphiphilic Peptides. Biophys. J. 2011;100:2652–2661. doi: 10.1016/j.bpj.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez de Tejada G., Heinbockel L., Ferrer-Espada R., Heine H., Alexander C., Bárcena-Varela S., Goldmann T., Correa W., Wiesmüller K.-H., Gisch N., et al. Lipoproteins/Peptides Are Sepsis-Inducing Toxins from Bacteria That Can Be Neutralized by Synthetic Anti-Endotoxin Peptides. Sci. Rep. 2015;5:14292. doi: 10.1038/srep14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinbockel L., Sánchez-Gómez S., Martinez de Tejada G., Dömming S., Brandenburg J., Kaconis Y., Hornef M., Dupont A., Marwitz S., Goldmann T., et al. Preclinical Investigations Reveal the Broad-Spectrum Neutralizing Activity of Peptide Pep19-2.5 on Bacterial Pathogenicity Factors. Antimicrob. Agents Chemother. 2013;57:1480–1487. doi: 10.1128/AAC.02066-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuerholz T., Doemming S., Hornef M., Martin L., Simon T.P., Heinbockel L., Brandenburg K., Marx G. The Anti-Inflammatory Effect of the Synthetic Antimicrobial Peptide 19-2.5 in a Murine Sepsis Model: A Prospective Randomized Study. Crit. Care. 2013;17:R3. doi: 10.1186/cc11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinbockel L., Marwitz S., Barcena Varela S., Ferrer-Espada R., Reiling N., Goldmann T., Gutsmann T., Mier W., Schürholz T., Drömann D., et al. Therapeutical Administration of Peptide Pep19-2.5 and Ibuprofen Reduces Inflammation and Prevents Lethal Sepsis. PLoS ONE. 2015;10:e0133291. doi: 10.1371/journal.pone.0133291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jannadi H., Correa W., Zhang Z., Brandenburg K., Oueslati R., Rouabhia M. Antimicrobial Peptides Pep19–2.5 and Pep19-4LF Inhibit Streptococcus Mutans Growth and Biofilm Formation. Microb. Pathog. 2019;133:103546. doi: 10.1016/j.micpath.2019.103546. [DOI] [PubMed] [Google Scholar]
- 29.Kuhlmann N., Heinbockel L., Correa W., Gutsmann T., Goldmann T., Englisch U., Brandenburg K. Peptide Drug Stability: The Anti-Inflammatory Drugs Pep19-2.5 and Pep19-4LF in Cream Formulation. Eur. J. Pharm. Sci. 2018;115:240–247. doi: 10.1016/j.ejps.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 30.Ahsan M.J., Samy J.G., Khalilullah H., Nomani S., Saraswat P., Gaur R., Singh A. Molecular Properties Prediction and Synthesis of Novel 1,3,4-Oxadiazole Analogues as Potent Antimicrobial and Antitubercular Agents. Bioorg. Med. Chem. Lett. 2011;21:7246–7250. doi: 10.1016/j.bmcl.2011.10.057. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz J., Moreno E., Calvo A., Blanco L., Fernández-Rubio C., Sanmartín C., Nguewa P., Irache J.M., Larrea E., Espuelas S. Combination of Paromomycin plus Human Anti-TNF-α Antibodies to Control the Local Inflammatory Response in BALB/ Mice with Cutaneous Leishmaniasis Lesions. J. Dermatol Sci. 2018;92:78–88. doi: 10.1016/j.jdermsci.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Fernández-Rubio C., Campbell D., Vacas A., Ibañez E., Moreno E., Espuelas S., Calvo A., Palop J.A., Plano D., Sanmartin C., et al. Leishmanicidal Activities of Novel Methylseleno-Imidocarbamates. Antimicrob. Agents Chemother. 2015;59:5705–5713. doi: 10.1128/AAC.00997-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernández-Rubio C., Larrea E., Guerrero J.P., Herrero E.S., Gamboa I., Berrio C., Plano D., Amin S., Sharma A.K., Nguewa P.A. Leishmanicidal Activity of Isoselenocyanate Derivatives. Antimicrob. Agents Chemother. 2019;63:e00904–e00918. doi: 10.1128/AAC.00904-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sacks D.L., Perkins P.V. Identification of an Infective Stage of Leishmania Promastigotes. Science. 1984;223:1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- 35.Puig-Rigall J., Fernández-Rubio C., González-Benito J., Houston J.E., Radulescu A., Nguewa P., González-Gaitano G. Structural Characterization by Scattering and Spectroscopic Methods and Biological Evaluation of Polymeric Micelles of Poloxamines and TPGS as Nanocarriers for Miltefosine Delivery. Int. J. Pharm. 2020;578:119057. doi: 10.1016/j.ijpharm.2020.119057. [DOI] [PubMed] [Google Scholar]
- 36.Pfalzgraff A., Bárcena-Varela S., Heinbockel L., Gutsmann T., Brandenburg K., Martinez-de-Tejada G., Weindl G. Antimicrobial Endotoxin-Neutralizing Peptides Promote Keratinocyte Migration via P2X7 Receptor Activation and Accelerate Wound Healing in Vivo. Br. J. Pharm. 2018;175:3581–3593. doi: 10.1111/bph.14425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreno E., Schwartz J., Larrea E., Conde I., Font M., Sanmartín C., Irache J.M., Espuelas S. Assessment of β-Lapachone Loaded in Lecithin-Chitosan Nanoparticles for the Topical Treatment of Cutaneous Leishmaniasis in L. Major Infected BALB/c Mice. Nanomedicine. 2015;11:2003–2012. doi: 10.1016/j.nano.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Vacas A., Fernández-Rubio C., Larrea E., Peña-Guerrero J., Nguewa P.A. LmjF.22.0810 from Leishmania Major Modulates the Th2-Type Immune Response and Is Involved in Leishmaniasis Outcome. Biomedicines. 2020;8:452. doi: 10.3390/biomedicines8110452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marchini J.F.M., Cruz A.K., Beverley S.M., Tosi L.R.O. The H Region HTBF Gene Mediates Terbinafine Resistance in Leishmania Major. Mol. Biochem. Parasitol. 2003;131:77–81. doi: 10.1016/S0166-6851(03)00174-9. [DOI] [PubMed] [Google Scholar]
- 40.Leprohon P., Légaré D., Girard I., Papadopoulou B., Ouellette M. Modulation of Leishmania ABC Protein Gene Expression through Life Stages and among Drug-Resistant Parasites. Eukaryot Cell. 2006;5:1713–1725. doi: 10.1128/EC.00152-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Etges R., Bouvier J., Bordier C. The Major Surface Protein of Leishmania Promastigotes Is a Protease. J. Biol. Chem. 1986;261:9098–9101. doi: 10.1016/S0021-9258(18)67621-5. [DOI] [PubMed] [Google Scholar]
- 42.Patino L.H., Muskus C., Ramírez J.D. Transcriptional Responses of Leishmania (Leishmania) Amazonensis in the Presence of Trivalent Sodium Stibogluconate. Parasit Vectors. 2019;12:348. doi: 10.1186/s13071-019-3603-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maspi N., Abdoli A., Ghaffarifar F. Pro- and Anti-Inflammatory Cytokines in Cutaneous Leishmaniasis: A Review. Pathog. Glob. Health. 2016;110:247–260. doi: 10.1080/20477724.2016.1232042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kleinert H., Forstermann U. Inducible Nitric Oxide Synthase. In: Enna S.J., Bylund B.B., editors. xPharm: The Comprehensive Pharmacology Reference. Elsevier; New York, NY, USA: 2007. pp. 1–12. [Google Scholar]
- 45.Feriotto G., Tagliati F., Giriolo R., Casciano F., Tabolacci C., Beninati S., Khan M.T.H., Mischiati C. Caffeic Acid Enhances the Anti-Leukemic Effect of Imatinib on Chronic Myeloid Leukemia Cells and Triggers Apoptosis in Cells Sensitive and Resistant to Imatinib. Int. J. Mol. Sci. 2021;22:1644. doi: 10.3390/ijms22041644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Figliuolo V.R., Chaves S.P., Savio L.E.B., Thorstenberg M.L.P., Machado Salles É., Takiya C.M., D’Império-Lima M.R., de Matos Guedes H.L., Rossi-Bergmann B., Coutinho-Silva R. The Role of the P2X7 Receptor in Murine Cutaneous Leishmaniasis: Aspects of Inflammation and Parasite Control. Purinergic Signal. 2017;13:143–152. doi: 10.1007/s11302-016-9544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peña-Guerrero J., Fernández-Rubio C., Burguete-Mikeo A., El-Dirany R., García-Sosa A.T., Nguewa P. Discovery and Validation of Lmj_04_BRCT Domain, a Novel Therapeutic Target: Identification of Candidate Drugs for Leishmaniasis. Int. J. Mol. Sci. 2021;22:10493. doi: 10.3390/ijms221910493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scott P., Novais F.O. Cutaneous Leishmaniasis: Immune Responses in Protection and Pathogenesis. Nat. Rev. Immunol. 2016;16:581–592. doi: 10.1038/nri.2016.72. [DOI] [PubMed] [Google Scholar]
- 49.Menzies F.M., MacPhail D., Henriquez F.L. The Role of Chemokines and Their Receptors during Protist Parasite Infections. Parasitology. 2016;143:1890–1901. doi: 10.1017/S0031182016001694. [DOI] [PubMed] [Google Scholar]
- 50.Majumder N., Ganguly S., Ghosh A.K., Kundu S., Banerjee A., Saha S. Chlorogenic Acid Acts upon Leishmania Donovani Arresting Cell Cycle and Modulating Cytokines and Nitric Oxide in Vitro. Parasite Immunol. 2020;42:e12719. doi: 10.1111/pim.12719. [DOI] [PubMed] [Google Scholar]
- 51.Buates S., Matlashewski G. General Suppression of Macrophage Gene Expression During Leishmania Donovani Infection. J. Immunol. 2001;166:3416–3422. doi: 10.4049/jimmunol.166.5.3416. [DOI] [PubMed] [Google Scholar]
- 52.Amaral E.P., Ribeiro S.C.M., Lanes V.R., Almeida F.M., de Andrade M.R.M., Bomfim C.C.B., Salles E.M., Bortoluci K.R., Coutinho-Silva R., Hirata M.H., et al. Pulmonary Infection with Hypervirulent Mycobacteria Reveals a Crucial Role for the P2X7 Receptor in Aggressive Forms of Tuberculosis. PLoS Pathog. 2014;10:e1004188. doi: 10.1371/journal.ppat.1004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neves A.R., Castelo-Branco M.T.L., Figliuolo V.R., Bernardazzi C., Buongusto F., Yoshimoto A., Nanini H.F., Coutinho C.M.L.M., Carneiro A.J.V., Coutinho-Silva R., et al. Overexpression of ATP-Activated P2X7 Receptors in the Intestinal Mucosa Is Implicated in the Pathogenesis of Crohn’s Disease. Inflamm. Bowel Dis. 2014;20:444–457. doi: 10.1097/01.MIB.0000441201.10454.06. [DOI] [PubMed] [Google Scholar]
- 54.Santos A.A., Rodrigues-Junior V., Zanin R.F., Borges T.J., Bonorino C., Coutinho-Silva R., Takyia C.M., Santos D.S., Campos M.M., Morrone F.B. Implication of Purinergic P2X7 Receptor in M. Tuberculosis Infection and Host Interaction Mechanisms: A Mouse Model Study. Immunobiology. 2013;218:1104–1112. doi: 10.1016/j.imbio.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Chaves M.M., Sinflorio D.A., Thorstenberg M.L., Martins M.D.A., Moreira-Souza A.C.A., Rangel T.P., Silva C.L.M., Bellio M., Canetti C., Coutinho-Silva R. Non-Canonical NLRP3 Inflammasome Activation and IL-1β Signaling Are Necessary to L. Amazonensis Control Mediated by P2X7 Receptor and Leukotriene B4. PLoS Pathog. 2019;15:e1007887. doi: 10.1371/journal.ppat.1007887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kima P.E., Soong L. Interferon Gamma in Leishmaniasis. Front. Immunol. 2013;4:156. doi: 10.3389/fimmu.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olivier M., Gregory D.J., Forget G. Subversion Mechanisms by Which Leishmania Parasites Can Escape the Host Immune Response: A Signaling Point of View. Clin. Microbiol. Rev. 2005;18:293–305. doi: 10.1128/CMR.18.2.293-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ji J., Sun J., Qi H., Soong L. Analysis of T Helper Cell Responses during Infection with Leishmania Amazonensis. The American journal of tropical medicine and hygiene. Am. J. Trop. Med. Hyg. 2002;66:338–345. doi: 10.4269/ajtmh.2002.66.338. [DOI] [PubMed] [Google Scholar]
- 59.Magni L., Bouazzi R., Heredero Olmedilla H., Petersen P.S.S., Tozzi M., Novak I. The P2X7 Receptor Stimulates IL-6 Release from Pancreatic Stellate Cells and Tocilizumab Prevents Activation of STAT3 in Pancreatic Cancer Cells. Cells. 2021;10:1928. doi: 10.3390/cells10081928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solle M., Labasi J., Perregaux D.G., Stam E., Petrushova N., Koller B.H., Griffiths R.J., Gabel C.A. Altered Cytokine Production in Mice Lacking P2X7Receptors. J. Biol. Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 61.Banerjee S., Sen A., Das P., Saha P. Leishmania Donovani Cyclin 1 (LdCyc1) Forms a Complex with Cell Cycle Kinase Subunit CRK3 (LdCRK3) and Is Possibly Involved in S-Phase-Related Activities. FEMS Microbiol. Lett. 2006;256:75–82. doi: 10.1111/j.1574-6968.2006.00105.x. [DOI] [PubMed] [Google Scholar]
- 62.Grant K.M., Dunion M.H., Yardley V., Skaltsounis A.-L., Marko D., Eisenbrand G., Croft S.L., Meijer L., Mottram J.C. Inhibitors of Leishmania Mexicana CRK3 Cyclin-Dependent Kinase: Chemical Library Screen and Antileishmanial Activity. Antimicrob. Agents Chemother. 2004;48:3033–3042. doi: 10.1128/AAC.48.8.3033-3042.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cleghorn L.A.T., Woodland A., Collie I.T., Torrie L.S., Norcross N., Luksch T., Mpamhanga C., Walker R.G., Mottram J.C., Brenk R., et al. Identification of Inhibitors of the Leishmania Cdc2-Related Protein Kinase CRK3. ChemMedChem. 2011;6:2214–2224. doi: 10.1002/cmdc.201100344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walker R.G., Thomson G., Malone K., Nowicki M.W., Brown E., Blake D.G., Turner N.J., Walkinshaw M.D., Grant K.M., Mottram J.C. High Throughput Screens Yield Small Molecule Inhibitors of Leishmania CRK3:CYC6 Cyclin-Dependent Kinase. PLoS Negl. Trop. Dis. 2011;5:e1033. doi: 10.1371/journal.pntd.0001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wyllie S., Thomas M., Patterson S., Crouch S., de Rycker M., Lowe R., Gresham S., Urbaniak M.D., Otto T.D., Stojanovski L., et al. Cyclin-Dependent Kinase 12 Is a Drug Target for Visceral Leishmaniasis. Nature. 2018;560:192–197. doi: 10.1038/s41586-018-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Franssen S.U., Durrant C., Stark O., Moser B., Downing T., Imamura H., Dujardin J.-C., Sanders M.J., Mauricio I., Miles M.A., et al. Global Genome Diversity of the Leishmania Donovani Complex. Elife. 2020;9:e51243. doi: 10.7554/eLife.51243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Franssen S.U., Takele Y., Adem E., Sanders M.J., Müller I., Kropf P., Cotton J.A. Diversity and Within-Host Evolution of Leishmania Donovani from Visceral Leishmaniasis Patients with and without HIV Coinfection in Northern Ethiopia. mBio. 2021;12:e0097121. doi: 10.1128/mBio.00971-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Isnard A., Shio M.T., Olivier M. Impact of Leishmania Metalloprotease GP63 on Macrophage Signaling. Front Cell Infect Microbiol. 2012;2:72. doi: 10.3389/fcimb.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chan A., Ayala J.-M., Alvarez F., Piccirillo C., Dong G., Langlais D., Olivier M. The Role of Leishmania GP63 in the Modulation of Innate Inflammatory Response to Leishmania Major Infection. PLoS ONE. 2022;16:e0262158. doi: 10.1371/journal.pone.0262158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Costa-Da-silva A.C., de Oliveira Nascimento D., Ferreira J.R.M., Guimarães-Pinto K., Freire-De-lima L., Morrot A., Decote-Ricardo D., Filardy A.A., Freire-De-lima C.G. Immune Responses in Leishmaniases: An Overview. Trop. Med. Infect Dis. 2022;7:54. doi: 10.3390/tropicalmed7040054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.