Abstract
Vaccination it is considered a vital strategy in order to mitigate monkeypox by protecting from severe disease and helping in reduction of hospitalisations. In this sense, this study aims to estimate the global prevalence of vaccination acceptance against monkeypox. We conducted a systematic review with a comprehensive search strategy for the following databases: PubMed, Scopus and Web of Science. A random-effect model meta-analysis was carried out using observational studies assessing the intention of vaccines against monkeypox from multiple continents. The quality assessment was developed using the Newcastle-Ottawa Scale adapted for cross-sectional studies. In addition, a subgroup analysis by study location and population and a sensitivity analysis was developed.Eleven cross-sectional studies were included. A total of 8045 participants were included. The pooled prevalence of monkeypox vaccination acceptance in all participants was 56.0% (95%CI: 42.0–70.0%). In the subgroup analysis of monkeypox vaccine acceptance according to continents, the prevalence of vaccine acceptance was 50.0% (95%CI: 24.0–76.0%) in Asian countries and 70.0% (95%CI: 55.0–84.0%) in European countries. The prevalence of vaccine acceptance was 43.0% (95%CI: 35.0–50.0%) in the general population, 63.0% (95%CI: 42.0–70.0%) in healthcare workers, and 84.0% (95%CI: 83.0–86.0%) in the LGBTI community. Despite the high prevalence of monkeypox vaccination acceptance in the LGBTI community found in our study, vaccination acceptance from healthcare workers and the general population are lower. Governments could use these results for planning, developing or promoting vaccination strategies and public health policies focused on these populations.
Keywords: monkeypox, vaccination acceptance, public health, systematic review
1. Introduction
Monkeypox is considered a viral re-emerging zoonotic disease. This threat was declared a Public Health Emergency of International Concern, after meetings of the Emergency Committee under the International Health Regulations, by the World Health Organization, on 23 July 2022 [1,2]. Up to 25 October 2022, there have been reported 75,885 confirmed cases, 74,994 in more than 101 countries not previously reporting monkeypox [3,4,5,6]. Although in most cases, the disease is self-limited [6,7], some patients may evolve due to risk factors to complicated forms and severe illness that may lead to fatal outcomes [3,8]. In 2022, a total of 34 deaths, 21 in countries not considered endemic for monkeypox, have been reported. Therefore, in addition to enhanced case definition, clinical, epidemiological, molecular and genomic surveillance, education, and prevention are critical for disease control [9,10]. That also includes the use of vaccines against monkeypox in specific populations (e.g., LGBTI community and health care workers) under certain circumstances and approaches (e.g., ring vaccination) [11,12].
Globally, efforts to develop effective and safe vaccines are ongoing, with the already use of licensed vaccines being used in countries such as the United States, Canada, and the United Kingdom, among others, for high-risk populations. However, as occurred with SARS-CoV-2/COVID-19, to reach the appropriate vaccination coverage, the willingness to receive the vaccine from people is an essential aspect that vaccination programs should consider [13,14,15].
Even though vaccines are essential for controlling communicable diseases such as monkeypox [16,17], there are multiple motivations for reluctance to vaccines, including fear to their negative adverse effects, misinformation and its impact, or distrust of medical personnel or the health system, among others [18,19]. Likewise, sociodemographic factors such as age, sex, and geographical residence zone would be connected differently with vaccine disposition subject on the context where them are investigated [20,21]. During the multi-country outbreak, monkeypox surveys have identified vaccine-hesitant subgroups [22,23,24,25].
Vaccine hesitancy is not a new public health issue and predates the COVID-19 pandemic but also other emerging and re-emerging disease outbreaks, including monkeypox [26,27]. Thus, the current systematic review aims to estimate the prevalence of the vaccination intention for monkeypox. This information could target interventions to promote vaccination, especially among risk groups.
2. Methods
2.1. Registration and Reporting
A short version of the protocol of this systematic review was uploaded to the International Prospective Register of Systematic Reviews (PROSPERO) [CRD42022363406]. The manuscript followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting the results [28].
2.2. Search Strategy and Databases
The search strategy was built using MeSH, Emtree, free terms and following the Peer Review of Electronic Search Strategies (PRESS) Checklist [29]. Afterwards, it was adapted for all the databases employed, and no date or language restrictions were applied. In addition, we developed a hand-search in preprints databases and the list of references of the included studies. Finally, the systematic search was performed on 20 September 2022, in the following databases: PubMed, Scopus, Embase, Ovid Medline, and Web of Science. The complete search strategy is attached as Supplemental Material (Table S1).
2.3. Study Selection and Data Extraction
At least two authors independently screened all study selection and critical appraisal phases. The eligibility criteria included (i) cross-sectional studies assessing the (ii) prevalence of vaccination intention against monkeypox. We excluded narrative reviews, scoping reviews, systematic reviews, and conference abstracts. Duplicates were removed with EndNote 20.1© (Clarivate, London, UK) and Rayyan QCRI (Rayyan Systems Inc.©, Cambridge, MA, USA) [30]. The remaining references were exported to Rayyan for screening by titles and abstracts. After identifying the potential references to be included, two authors (J.R.U.-B. and A.A.-C.) independently assessed the full text of each one. Any conflict or discrepancy in any phase of the study selection process was resolved by consensus. Data extraction was independently performed by two authors (E.A.H-B. and E.A.A.B.) using a standardised data extraction sheet designed in Google Sheets© (Google, Mountain View, CA, USA). The following information was extracted: author, publication date, country, type of population, sample size, survey modality, the prevalence of vaccination intention, and prevalence of vaccination hesitancy.
2.4. Risk of Bias and Publication Bias
Two authors performed the quality assessment process independently (J.R.U.-B. and E.A.H.-B.). We used the adapted version of the Newcastle-Ottawa Scale for cross-sectional studies (NOS-CS). A score ≥7 stars were considered a low risk of bias, while a score <7 stars was considered a high risk of bias. The publication bias assessment was not performed as it is not recommended in proportional meta-analysis for the following reasons: (i) conventional funnel plots and Egger’s test are inaccurate for these analyses, and (ii) there is no evidence that proportions adjust correctly to funnel plots or Egger’s tests [31,32].
2.5. Data Synthesis
The statistical analysis was performed using STATA 17.0 © (StataCorp LLC, College Station, TX, USA). We conducted a pooled analysis of the prevalence of vaccination intention and vaccination hesitancy against monkeypox. The 95% Confidence Intervals (CI) for the proportions reported in each study were calculated using the Clopper-Pearson Method. The Freeman-Tukey Double Arcsine Transformation was used as the variance stabiliser. We used a random-effects model (Dersimonian and Laird) for the quantitative analysis. We assessed the between-study heterogeneity using Cochran’s Q test and the I2 statistic. Values equal to or greater than 60% were defined as high heterogeneity for the I2 statistic, and p-values < 0.05 were considered indicators of heterogeneity in Cochran’s Q test. In addition, we carried out subgroup analysis by continents and population groups and a sensitivity analysis excluding those studies with a high risk of bias.
3. Results
3.1. Study Selection
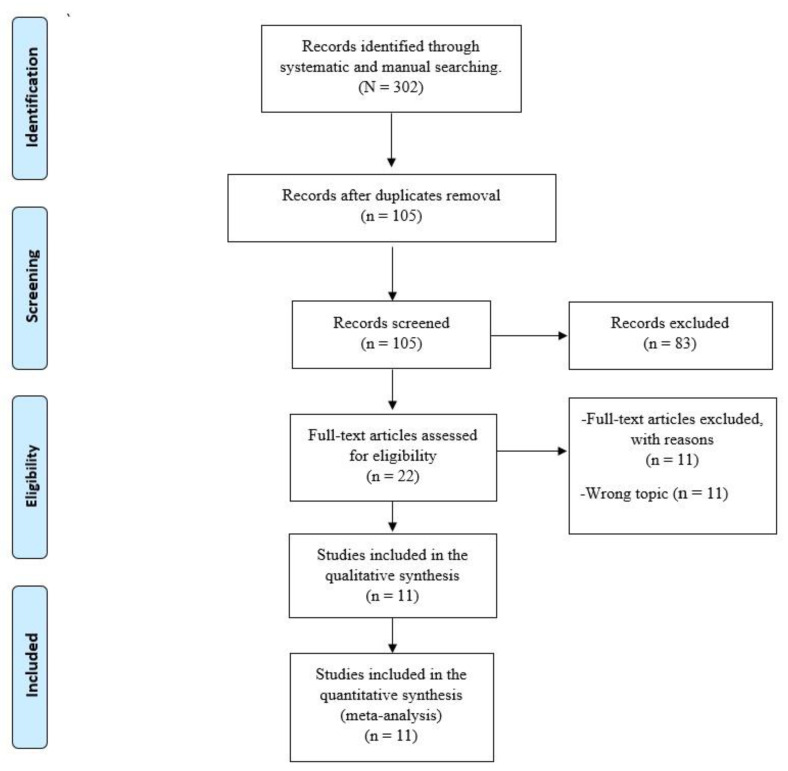
A total of 302 articles were identified through systematic and manual searches. Of these, 105 articles remained after eliminating 197 duplicate records. Finally, a careful assessment of the complete texts found 11 studies that fully complied with the eligibility criteria [33,34,35,36,37,38,39,40,41,42,43]. The detailed flow chart of the literature selection is shown in Figure 1.
Figure 1.
PRISMA Flow Diagram.
3.2. Study Characteristics
The characteristics of the included studies are summarized in Table 1. All the included studies were of cross-sectional design. A total of 8045 participants were included, of whom 4855 were men and 3300 were women. The studies were published between 2020 and 2022. Two studies were carried out in Indonesia, two in Saudi Arabia, one in Iraq, one in the United States, one in the Netherlands, one in the United Kingdom, one in Nigeria, one in Italy, and one in France and Belgium. The age of all participants ranged from 18 to 57 years. All the questionnaires were administered through an online survey and developed in different populations, such as the general population, healthcare workers, and the LGBTI community.
Table 1.
Characteristics of the included studies.
| Author | Year of Publication | Country | Target Population | Participants (Male/Female/Nonbinary) | Age (Mean ± SD or Age Ranges) | Survey Type | Data Collection Date | Response Recorded as Vaccine Acceptance | Prevalence of Vaccination Acceptance | Prevalence of Monkeypox Vaccination Hesitancy |
|---|---|---|---|---|---|---|---|---|---|---|
| Ahmed SK, et al. [33] | 2022 | Iraq | General population | 510 (277/233) | 18–57 | Online survey | 27 July 2022–30 July 2022 | Yes | 26% | 42% |
| Winter MS, et al. [34] | 2022 | United States | General population | 856 (419/437) | NR | Online survey | June 2022 | Yes | 46% | 25% |
| Wang H, et al. [35] | 2022 | Netherlands | LGBTI community | 394 (394/0) | NR | Online survey | July 2022 | High/very high vaccination intention (scale 4 and 5) | 70% | NR |
| Gagneux-Brunon A, et al. [36] | 2022 | France and Belgium | Healthcare workers | 397 (101/296) | 43.3(12) | Online survey | 15 June 2022–8 August 2022 | Intention to get vaccinated | 55.4% | NR |
| Salim NA, et al. [43] | 2022 | Indonesia | Healthcare workers | 75 (49/26) | 26–38 | Online survey | 2 August 2022–5 August 2022 | Yes | 36% | 22.70% |
| Ricco M, et al. [37] | 2022 | Italy | Healthcare workers | 446 (149) | 42.9 (10) | Online survey | 4 May 2022–31 May 2022 | Totally Agree/Agree | 66.36% | NR |
| Meo SA et al. [38] | 2022 | Saudi Arabia | General population | 1020 (466/554) | NR | Online survey | 15 May 2022–15 July 2022 | Yes | 43.7% | NR |
| Temsah MH, et al. [39] | 2022 | Saudi Arabia | General population | 1546 (650/896) | NR | Online survey | 27 May 2022–5 June 2022 | Yes | 50.6% | NR |
| Paparini S, et al. [40] | 2022 | United Kingdom | LGBTI community | 1932 (1750/88/94) | NR | Online survey | 15 June 2022–27 July 2022 | Yes | 86% | 8% |
| Harapan H, et al. [41] | 2020 | Indonesia | Healthcare workers | 407 (128/279) | NR | Online survey | 25 May 2019–25 July 2019 | Yes | 93.6% | NR |
| Al-Mustapha AI, et al. [42] | 2022 | Nigeria | General population | 822 (472/342/8) | NR | Online survey | 16 August 2022–29 August 2022 | NR | 46.37% | NR |
NR: Not Reported.
The NOS was used for the quality assessment of the studies (see Supplementary Material Table S2). Two studies were at high risk of bias, and nine were at low risk.
3.3. Prevalence of Monkeypox Vaccination Acceptance
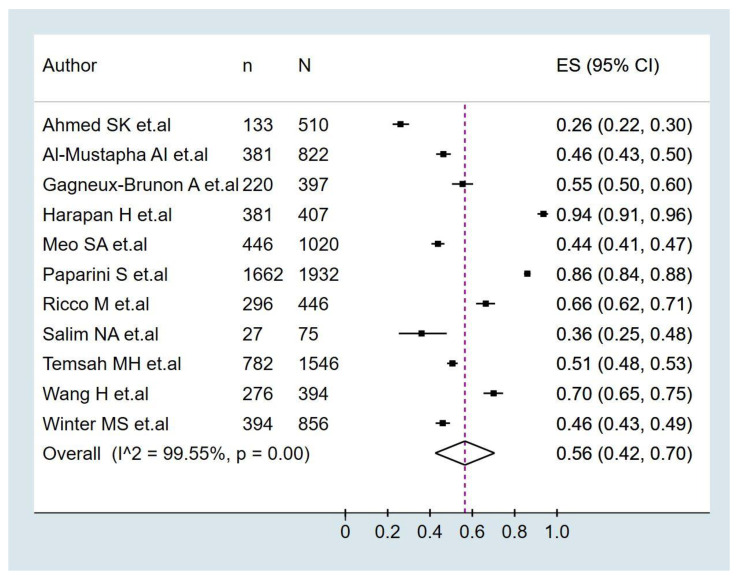
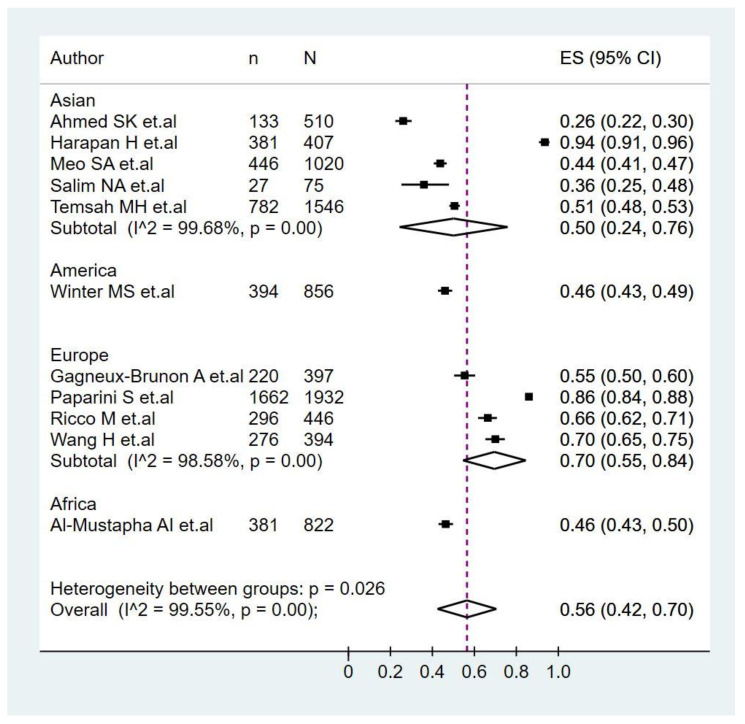
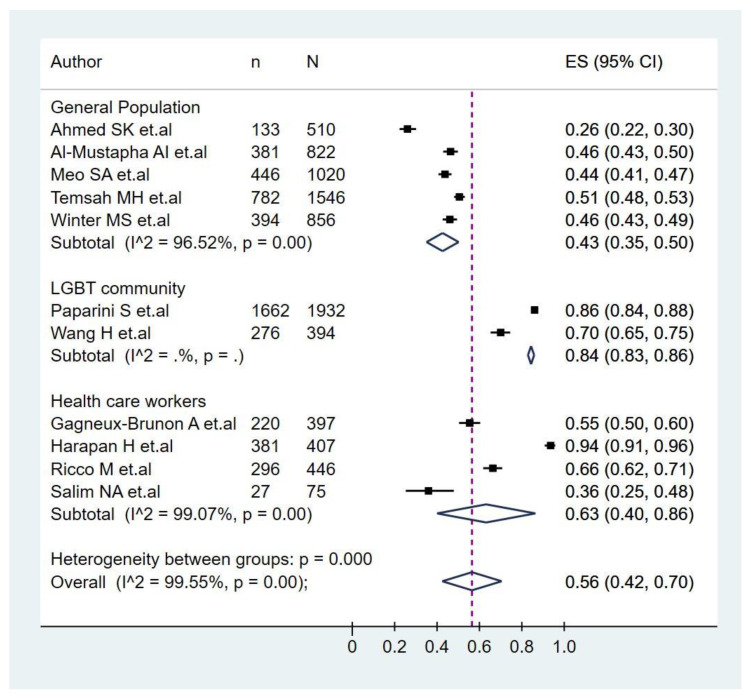
The pooled prevalence of monkeypox vaccination acceptance in all participants was 56.0% (95%CI: 42.0–70.0%), with significant heterogeneity among studies (I2 = 99.5%) (Figure 2). In the subgroup analysis of monkeypox vaccine acceptance according to continents (Figure 3), the prevalence of vaccine acceptance was 50.0% (95%CI: 24.0–76.0%) in Asian countries and 70.0% (95%CI: 55.0–84.0%) in European countries. Meanwhile, in the subgroup analysis of monkeypox vaccine acceptance according to the target population of the studies (Figure 4), the prevalence of vaccine acceptance was 43.0% (95%CI: 35.0–50.0%) in the general population, 63.0% (95%CI: 42.0–70.0%) in healthcare workers, and 84.0% (95%CI: 83.0–86.0%) in the LGBTI community. In the sensitivity analysis, after removing studies with a high risk of bias, the prevalence of vaccine acceptance was 60.0% (95%CI: 44.0–75.0%), with no decrease in heterogeneity (I2 = 99.61%).
Figure 2.
Prevalence of monkeypox vaccination intention in all participants [33,34,35,36,37,38,39,40,41,42,43].
Figure 3.
Subgroup analysis of monkeypox vaccine acceptance according to continents [33,34,35,36,37,38,39,40,41,42,43].
Figure 4.
Analysis of monkeypox vaccine acceptance subgroups according to the target population of the studies [33,34,35,36,37,38,39,40,41,42,43].
3.4. Prevalence of Monkeypox Vaccination Hesitancy
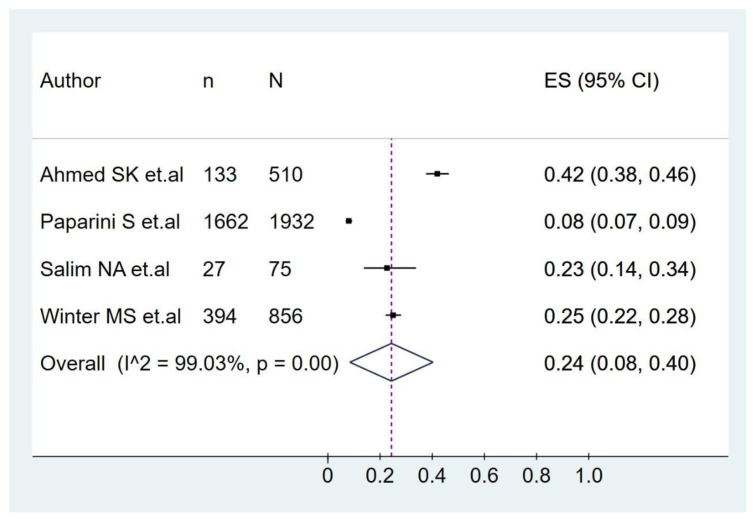
The pooled prevalence of monkeypox vaccination hesitancy in all participants was 24.0% (95%CI: 8.0–40.0%), with significant heterogeneity among studies (I2 = 99.03%) (Figure 5).
Figure 5.
Prevalence of monkeypox vaccination hesitancy [33,34,40,43].
4. Discussion
Better vaccination coverages are in general important for most diseases where vaccines are currently available. Then, it is not only important to develop efficacious and safe vaccines, but also to warrant the appropriate logistical issues, fair distribution and the populations acceptance in order to have the necessary demand of them [44]. Then vaccine hesitance and acceptance is a key determinant of vaccination coverage that should be assessed and consequently addressed with evidence, education and promotion as part of disease prevention campaigns, included now for monkeypox.
Our systematic review targeted to estimate the prevalence of monkeypox vaccination acceptance globally. Present findings indicated a moderate prevalence of monkeypox vaccine acceptance (56%), which was, as expected, higher in Europe (70%) and lower in Asia (50%) due to the incidence and probably associated perception of risk and impact. Nations such as Spain, the United Kingdom, France, and Germany are located in the top ten of incidence. Indeed, in May 2022, the outbreak started in the United Kingdom, and multiple European countries were rapidly affected. Still, multiples countries in Asia have not reported cases of monkeypox, and among those with confirmed patients, numbers would be low (e.g., India with 17; Thailand, 11; Saudi Arabia, 8; Japan, 7; Qatar, 5; China, 1) [24,45]. Although among the included studies in the present systematic reviews, there were studies from Asia, Europe, the USA, and Africa, but not Latin America. Before 2022, only one study addressed the acceptance of monkeypox vaccines. That is consistent with the past lack of studies on monkeypox and that monkeypox has been neglected [46,47,48,49].
A number of local, racial, religious, cultural and a number of other aspects may influence people’s perception on their acceptance of vaccination, as well as misinformation, as was clearly observed in the COVID-19 pandemic [50,51,52,53,54,55,56,57].
Also, as expected, the acceptance of monkeypox vaccines was low in the general population (43%), even may perceive this disease as only occurring in the LGBTI community, with its associated stigma [58]. However, at the same time, it was higher among the LGBTI population (84%), which has been predominantly affected by monkeypox [59,60,61]. LGBTI communities should be prioritized for education and promotion on monkeypox, its transmission and preventive practices, while their stigma should be avoided [62,63].
Curiously, although the general belief is that the acceptance should be 100% among healthcare workers, especially physicians, this systematic review found only 64%. This was even lower than studies for COVID-19 vaccine acceptance among physicians in different parts of the world, e.g., Colombia (90.7%) [64], and comparable with results from India (63.34%) [65].
The overall acceptance of monkeypox vaccines (56%) was lower than in some systematic reviews for COVID-19. For example, a systematic review, including 19 studies from Latin America, found that accepting COVID-19 vaccines was 78% [13].
A recent study on acceptance for the monkeypox vaccines among 32,902 male owners of smartphones, registered at online gay-dating apps in Europe found that the acceptance was 82% and higher in north (84.8–90.4%) and western (83.1–87.7%) countries, compared to south-east (60.9–70.2%) and eastern (59.9–71.1%) European countries [66].
All these findings underscore the relevance of education and the promotion of vaccines. The efficacy and safety of available vaccines against monkeypox should be divulgated among the general population, but especially among healthcare workers and LGBTI communities, which are risk groups. As the attitudes towards different vaccines as well as the trust degree and misinformation are probable to change through different periods according to outcomes and opinions, they require to be constantly assessed, considering their evolving nature. Challenges and attitudes towards vaccines will evolve and require close monitoring to improve compliance and distribution [19]. As far as the outbreak evolves, effectiveness studies are urgently required to improve the understanding of the real impact of the monkeypox vaccines. In the meantime, a recent study in the Netherlands found that among those non-primed vaccinated individuals, with a 2-shot immunization series with the modified vaccinia virus Ankara-Bavarian Nordic (MVA-BN, also known as Jynneos, Imvanex or Imvamune), this estimulated partially low titers of MPXV-protective neutralizing antibodies. Also, that the MVA-based influenza vaccine dose-sparing leads to low levels of MPXV-neutralizing antibodies, whereas a third dose with the same vaccine significantly enhance the antibody immune response. As the importance of MPXV-neutralizing antibodies as a possible protection correlate for disease and transmission is yet to be defined accurately, based on that preliminary data, authors concluded that follow-up studies at cohorts of vaccinated subjects would be important to evaluate vaccine protection in populations at risk [67].
Globally, nations, particularly those more affected by the monkeypox outbreak, must prioritise implementing strategies to deal with misinformation and vaccination hesitancy [26]. Current findings are key for the implementation of vaccination policies. Providing evidence-based data in means and forms understandable to everybody is key; regarding this, public health authorities and professionals should increase their use of social media, as these are value resources yet to be a more significant tool in disease prevention. Therefore, national and regional authorities should enhance their efforts in providing massively intense educational interventions, including higher presence in social and news media that are also key in providing trustworthy information to the population that needs to be vaccinated promptly and reduce the risk of monkeypox, especially in risk groups.
As occurred with COVID-19 [13], identifying specific groups with a lower rates of vaccination intention for monkeypox would help health authorities and governments to explore and design more efficient public health approaches for vaccination. Our study results constitute an input towards the implemented measures for global vaccination against monkeypox. They could be used as a guide for developing new public health policies focused on population subgroups with a low prevalence of monkeypox vaccination acceptance. Additionally, these results could be used as a reference in future outbreaks to stratify groups with low vaccine acceptance and build specific strategies for them. Further research is needed to study factors associated with low monkeypox vaccination acceptance among these groups.
Our systematic review has limitations, the main one being the high heterogeneity in the meta-analyses performed, which would not explained using the subgroup and sensitivity analyses. The high heterogeneity probably has to do with the differences in the populations surveyed as well as with the measurement instruments used. Likewise, we found few studies in America and Africa, so it is necessary to carry out more research in these continents.
5. Conclusions
Despite the high rate of monkeypox vaccination acceptance in the LGBTI community found in our study, the prevalence of vaccination from healthcare workers and the general population is lower. Governments could use these results for designing, developing or promoting vaccination approaches and public health policies focused on these vulnerable and at-risk populations. Immunoprevention remains an essential public health intervention to prevent disease and probably transmission, even in monkeypox.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens11111248/s1, Table S1: Search strategies; Table S2: Quality assessment of included studies; Figure S1. Sensitivity analysis of monkeypox vaccine acceptance according to the risk of bias.
Author Contributions
Conceptualisation, V.A.B.-Z.; methodology, J.R.U.-B., A.A.-k.-C., E.A.H.-B. and E.A.A.-B.; writing—original draft preparation, V.A.B.-Z., D.K.B.-A. and A.J.R.-M.; writing—review and editing, visualisation, supervision, project administration by all authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Available on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research has been funded by Universidad Peruana de Ciencias Aplicadas through Grant A-023-2021.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.León-Figueroa D.A., Bonilla-Aldana D.K., Pachar M., Romaní L., Saldaña-Cumpa H.M., Anchay-Zuloeta C., Diaz-Torres M., Franco-Paredes C., Suárez J.A., Ramirez J.D., et al. The never ending global emergence of viral zoonoses after COVID-19? The rising concern of monkeypox in Europe, North America and beyond. Travel Med. Infect. Dis. 2022;49:102362. doi: 10.1016/j.tmaid.2022.102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sah R., Reda A., Lashin B.I., Mohanty A., Abdelaal A., Rodriguez-Morales A.J. Public health emergencies of international concernin the 21st century. Ann. Med. Surg. 2022;81:104417. doi: 10.1016/j.amsu.2022.104417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benites-Zapata V.A., Ulloque-Badaracco J.R., Alarcon-Braga E.A., Hernandez-Bustamante E.A., Mosquera-Rojas M.D., Bonilla-Aldana D.K., Rodriguez-Morales A.J. Clinical features, hospitalisation and deaths associated with monkeypox: A systematic review and meta-analysis. Ann. Clin. Microbiol. Antimicrob. 2022;21:36. doi: 10.1186/s12941-022-00527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farahat R.A., Abdelaal A., Shah J., Ghozy S., Sah R., Bonilla-Aldana D.K., Rodriguez-Morales A.J., McHugh T.D., Leblebicioglu H. Monkeypox outbreaks during COVID-19 pandemic: Are we looking at an independent phenomenon or an overlapping pandemic? Ann. Clin. Microbiol. Antimicrob. 2022;21:26. doi: 10.1186/s12941-022-00518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Morales A.J., Lopardo G., Verbanaz S., Orduna T., Lloveras S., Azeñas-Burgoa J.M., Escalera-Antezana J.P., Alvarado-Arnez L.E., Barbosa A.N., Diaz-Quijano F., et al. Latin America: Situation and preparedness facing the multi-country human monkeypox outbreak. Lancet Reg. Health Am. 2022;13:100318. doi: 10.1016/j.lana.2022.100318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortiz-Martínez Y., Rodríguez-Morales A.J., Franco-Paredes C., Chastain D.B., Gharamti A.A., Vargas Barahona L., Henao-Martínez A.F. Monkeypox—A description of the clinical progression of skin lesions: A case report from Colorado, USA. Ther. Adv. Infect Dis. 2022;9:20499361221117726. doi: 10.1177/20499361221117726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farahat R.A., Sah R., El-Sakka A.A., Benmelouka A.Y., Kundu M., Labieb F., Shaheen R.S., Abdelaal A., Abdelazeem B., Bonilla-Aldana D.K., et al. Human monkeypox disease (MPX) Infez. Med. 2022;30:372–391. doi: 10.53854/liim-3003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sah R., Mohanty A., Abdelaal A., Reda A., Rodriguez-Morales A.J., Henao-Martinez A.F. First Monkeypox deaths outside Africa: No room for complacency. Adv. Infect Dis. 2022;9:20499361221124027. doi: 10.1177/20499361221124027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortiz-Martínez Y., Sarmiento J., Bonilla-Aldana D.K., Rodríguez-Morales A.J. Monkeypox goes viral: Measuring the misinformation outbreak on Twitter. J. Infect. Dev. Ctries. 2022;16:1218–1220. doi: 10.3855/jidc.16907. [DOI] [PubMed] [Google Scholar]
- 10.Ortiz-Martínez Y., Galvis-Cataño L.M., Arias-Rodríguez D., Romero-Dager C., Bonilla-Aldana D.K., Rodriguez-Morales A.J. YouTube and 2022 Monkeypox outbreak: Opportunities for awareness and infection control. J. Hosp. Infect. 2022 doi: 10.1016/j.jhin.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sah R., Abdelaal A., Asija A., Basnyat S., Sedhai Y.R., Ghimire S., Sah S., Bonilla-Aldana D.K., Rodriguez-Morales A.J. Monkeypox virus containment: The application of ring vaccination and possible challenges. J. Travel Med. 2022;29:taac085. doi: 10.1093/jtm/taac085. [DOI] [PubMed] [Google Scholar]
- 12.Abdelaal A., Reda A., Lashin B.I., Katamesh B.E., Brakat A.M., Al-Manaseer B.M., Kaur S., Asija A., Patel N.K., Basnyat S., et al. Preventing the Next Pandemic: Is Live Vaccine Efficacious against Monkeypox, or Is There a Need for Killed Virus and mRNA Vaccines? Vaccines. 2022;10:1419. doi: 10.3390/vaccines10091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alarcón-Braga E.A., Hernandez-Bustamante E.A., Salazar-Valdivia F.E., Valdez-Cornejo V.A., Mosquera-Rojas M.D., Ulloque-Badaracco J.R., Rondon-Saldaña J.C., Zafra-Tanaka J.H. Acceptance towards COVID-19 vaccination in Latin America and the Caribbean: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2022;49:102369. doi: 10.1016/j.tmaid.2022.102369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benites-Zapata V.A., Herrera-Añazco P., Benites-Meza J.K., Bonilla-Aguilar K., Urrunaga-Pastor D., Bendezu-Quispe G., Uyen-Cateriano A., Rodriguez-Morales A.J., Hernandez A.V. Prevalence of parents’ non-intention to vaccinate their children and adolescents against COVID-19: A comparative analysis in Colombia and Peru. Vaccine X. 2022;12:100198. doi: 10.1016/j.jvacx.2022.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urrunaga-Pastor D., Bendezu-Quispe G., Herrera-Añazco P., Uyen-Cateriano A., Toro-Huamanchumo C.J., Rodriguez-Morales A.J., Hernandez A.V., Benites-Zapata V.A. Cross-sectional analysis of COVID-19 vaccine intention, perceptions and hesitancy across Latin America and the Caribbean. Travel Med. Infect. Dis. 2021;41:102059. doi: 10.1016/j.tmaid.2021.102059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mezones-Holguin E., Al-kassab-Córdova A., Maguiña J.L., Rodriguez-Morales A.J. Vaccination coverage and preventable diseases in Peru: Reflections on the first diphtheria case in two decades during the midst of COVID-19 pandemic. Travel Med. Infect. Dis. 2021;40:101956. doi: 10.1016/j.tmaid.2020.101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russo A.T., Berhanu A., Bigger C.B., Prigge J., Silvera P.M., Grosenbach D.W., Hruby D. Co-administration of tecovirimat and ACAM2000™ in non-human primates: Effect of tecovirimat treatment on ACAM2000 immunogenicity and efficacy versus lethal monkeypox virus challenge. Vaccine. 2020;38:644–654. doi: 10.1016/j.vaccine.2019.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bendezu-Quispe G., Benites-Meza J.K., Urrunaga-Pastor D., Herrera-Añazco P., Uyen-Cateriano A., Rodriguez-Morales A.J., Toro-Huamanchumo C.J., Hernandez A.V., Benites-Zapata V.A. Mass Media Use to Learn About COVID-19 and the Non-intention to Be Vaccinated Against COVID-19 in Latin America and Caribbean Countries. Front. Med. 2022;9:877764. doi: 10.3389/fmed.2022.877764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Morales A.J., Franco O.H. Public trust, misinformation and COVID-19 vaccination willingness in Latin America and the Caribbean: Today’s key challenges. Lancet Reg. Health Am. 2021;3:100073. doi: 10.1016/j.lana.2021.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mangla S., Zohra Makkia F.T., Pathak A.K., Robinson R., Sultana N., Koonisetty K.S., Karamehic-Muratovic A., Nguyen U.D.T., Rodriguez-Morales A.J., Sanchez-Duque J.A., et al. COVID-19 Vaccine Hesitancy and Emerging Variants: Evidence from Six Countries. Behav. Sci. 2021;11:148. doi: 10.3390/bs11110148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortiz-Martínez Y., López-López M., Ruiz-González C.E., Turbay-Caballero V., Sacoto D.H., Caldera-Caballero M., Bravo H., Sarmiento J., Rodriguez-Morales A.J. Willingness to receive COVID-19 vaccination in people living with HIV/AIDS from Latin America. Int. J. STD AIDS. 2022;33:652–659. doi: 10.1177/09564624221091752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harapan H., Wagner A.L., Yufika A., Setiawan A.M., Anwar S., Wahyuni S., Asrizal F.W., Sufri M.R., Putra R.P., Wijayanti N.P., et al. Acceptance and willingness to pay for a hypothetical vaccine against monkeypox viral infection among frontline physicians: A cross-sectional study in Indonesia. Vaccine. 2020;38:6800–6806. doi: 10.1016/j.vaccine.2020.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ajman F., Alenezi S., Alhasan K., Saddik B., Alhaboob A., Altawil E.S., Alshahrani F., Alrabiaah A., Alaraj A., Alkriadees K., et al. Healthcare Workers’ Worries and Monkeypox Vaccine Advocacy during the First Month of the WHO Monkeypox Alert: Cross-Sectional Survey in Saudi Arabia. Vaccines. 2022;10:1408. doi: 10.3390/vaccines10091408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alshahrani N.Z., Alzahrani F., Alarifi A.M., Algethami M.R., Alhumam M.N., Ayied H.A.M., Awan A.Z., Almutairi A.F., Bamakhrama S.A., Almushari B.S., et al. Assessment of Knowledge of Monkeypox Viral Infection among the General Population in Saudi Arabia. Pathogens. 2022;11:904. doi: 10.3390/pathogens11080904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ansari Z., Ramzan H., Shakeel R. Is there a need of monkeypox vaccine amidst the hesitancy of COVID-19 immunization in Pakistan? Ann. Med. Surg. 2022;81:104391. doi: 10.1016/j.amsu.2022.104391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith M.J., Marshall G.S. Navigating parental vaccine hesitancy. Pediatr. Ann. 2010;39:476–482. doi: 10.3928/00904481-20100726-05. [DOI] [PubMed] [Google Scholar]
- 27.Larson H.J., Jarrett C., Eckersberger E., Smith D.M., Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: A systematic review of published literature, 2007–2012. Vaccine. 2014;32:2150–2159. doi: 10.1016/j.vaccine.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 28.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGowan J., Sampson M., Salzwedel D.M., Cogo E., Foerster V., Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barker T.H., Migliavaca C.B., Stein C., Colpani V., Falavigna M., Aromataris E., Munn Z. Conducting proportional meta-analysis in different types of systematic reviews: A guide for synthesisers of evidence. BMC Med. Res. Methodol. 2021;21:189. doi: 10.1186/s12874-021-01381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunter J.P., Saratzis A., Sutton A.J., Boucher R.H., Sayers R.D., Bown M.J. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J. Clin. Epidemiol. 2014;67:897–903. doi: 10.1016/j.jclinepi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed S.K., Abdulqadirb S.O., Omar R.M., Hussein S.H., M-Amin H.I., Chandran D., Sharma A.K., Dhama K., Ahmed Z.K., Essa R.A., et al. Study of knowledge, attitude and anxiety in Kurdistan-region of Iraqi population during the monkeypox outbreak in 2022: An online cross-sectional study. Res. Sq. 2022 doi: 10.21203/rs.3.rs-1961934/v2. [DOI] [Google Scholar]
- 34.Malik A.A., Winters M.S., Omer S.B. Attitudes of the US general public towards Monkeypox. medRxiv. 2022 doi: 10.1101/2022.06.20.22276527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H., D’Abreu De Paulo K.J.I., Gültzow T., Zimmermann H.M.L., Jonas K.J. Monkeypox self-diagnosis abilities, determinants of vaccination and self-isolation intention after diagnosis among MSM, the Netherlands, July 2022. Eurosurveillance. 2022;27:2200603. doi: 10.2807/1560-7917.ES.2022.27.33.2200603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gagneux-Brunon A., Dauby N., Launay O., Botelho-Nevers E. Intentions to get vaccinated against Monkeypox in Healthcare workers in France and Belgium correlates with attitudes toward COVID-19 vaccination. medRxiv. 2022 doi: 10.1101/2022.08.25.22279205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riccò M., Ferraro P., Camisa V., Satta E., Zaniboni A., Ranzieri S., Baldassarre A., Zaffina S., Marchesi F. When a Neglected Tropical Disease Goes Global: Knowledge, Attitudes and Practices of Italian Physicians towards Monkeypox, Preliminary Results. Trop. Med. Int. Health. 2022;7:135. doi: 10.3390/tropicalmed7070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meo S.A., Al-Khlaiwi T., Aljofan Z.F., Alanazi A.I., Meo A.S. Public Perceptions of the Emerging Human Monkeypox Disease and Vaccination in Riyadh, Saudi Arabia: A Cross-Sectional Study. Vaccines. 2022;10:1534. doi: 10.3390/vaccines10091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Temsah M.H., Aljamaan F., Alenezi S., Alhasan K., Saddik B., Al-Barag A., Alhaboob A., Bahabri N., Alshahrani F., Alrabiaah A., et al. Monkeypox caused less worry than COVID-19 among the general population during the first month of the WHO Monkeypox alert: Experience from Saudi Arabia. Travel Med. Infect. Dis. 2022;49:102426. doi: 10.1016/j.tmaid.2022.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paparini S., Whitacre R., Smuk M., Thornhill J., Mwendera C., Strachan S., Nutland W., Orkin C. Public understanding, awareness, and response to monkeypox virus outbreak: A cross-sectional survey of the most affected communities in the United Kingdom during the 2022 public health emergency. medRxiv. 2022 doi: 10.1101/2022.08.25.22279207. [DOI] [PubMed] [Google Scholar]
- 41.Harapan H., Setiawan A.M., Yufika A., Anwar S., Wahyuni S., Asrizal F.W., Sufri M.R., Putra R.P., Wijayanti N.P., Salwiyadi S., et al. Physicians’ willingness to be vaccinated with a smallpox vaccine to prevent monkeypox viral infection: A cross-sectional study in Indonesia. Clin. Epidemiol. Glob. Health. 2020;8:1259–1263. doi: 10.1016/j.cegh.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Mustapha A.I., Sikiru N.A., Kolawole B., Oyewo M., Ahmed H., Odukoya A., Ogundijo O.A., Asiegbu E.C., Nanven M.B., Lawal-Atolagbe T., et al. A Cross-sectional Survey of Public Knowledge of the Monkeypox Disease in Nigeria. Res. Sq. 2022 doi: 10.21203/rs.3.rs-2031058/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salim N.A., Septadina I.S., Permata M., Hudari H. Knowledge, attitude, and perception of anticipating 2022 global human monkeypox infection among internal medicine residents at Palembang Indonesia: An online survey. J. Ilmu Kedokt Kesehat J. Med. Health Sci. 2022;9:253–262. doi: 10.32539/JKK.V9I3.18799. [DOI] [Google Scholar]
- 44.Gates B. How to Prevent the Next Pandemic. Knopf; Seatle, WA, USA: 2022. [Google Scholar]
- 45.Sah R., Mohanty A., Reda A., Lashin B.I., Abdelaal A., Rath R.S., Rodriguez-Morales A.J. Monkeypox: A potential pandemic at door of Asia. Ann. Med. Surg. 2022;81:104509. doi: 10.1016/j.amsu.2022.104509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodríguez-Morales A.J., Ortiz-Martínez Y., Bonilla-Aldana D.K. What has been researched about monkeypox? a bibliometric analysis of an old zoonotic virus causing global concern. New Microbes New Infect. 2022;47:100993. doi: 10.1016/j.nmni.2022.100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haider N., Guitian J., Simons D., Asogun D., Ansumana R., Honeyborne I., Velavan T.P., Ntoumi F., Valdoleiros S.R., Petersen E., et al. Increased outbreaks of monkeypox highlight gaps in actual disease burden in Sub-Saharan Africa and in animal reservoirs. Int. J. Infect. Dis. IJID. 2022;122:107–111. doi: 10.1016/j.ijid.2022.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Musa A., Chou J., LaBere B. The resurgence of a neglected orthopoxvirus: Immunologic and clinical aspects of monkeypox virus infections over the past six decades. Clin. Immunol. 2022;243:109108. doi: 10.1016/j.clim.2022.109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization Multi-Country Monkeypox Outbreak: Situation Update. [(accessed on 14 October 2022)]. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON390.
- 50.Guagliardo S.A.J., Monroe B., Moundjoa C., Athanase A., Okpu G., Burgado J., Townsend M.B., Satheshkumar P.S., Epperson S., Doty J.B., et al. Asymptomatic Orthopoxvirus Circulation in Humans in the Wake of a Monkeypox Outbreak among Chimpanzees in Cameroon. Am. J. Trop. Med. Hyg. 2020;102:206–212. doi: 10.4269/ajtmh.19-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Albuquerque T.R., Macedo L.F.R., de Oliveira E.G., Neto M.L.R., de Menezes I.R.A. Vaccination for COVID-19 in children: Denialism or misinformation? J. Pediatr. Nurs. 2022;64:141–142. doi: 10.1016/j.pedn.2022.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Saint Laurent C., Murphy G., Hegarty K., Greene C.M. Measuring the effects of misinformation exposure and beliefs on behavioural intentions: A COVID-19 vaccination study. Cogn. Res. Princ. Implic. 2022;7:87. doi: 10.1186/s41235-022-00437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ganie A.U.R., Mukhter I. Misinformation induced anxieties and fear affecting vaccination programs: Challenge for COVID-19 vaccination program. J. Fam. Med. Prim. Care. 2022;11:405–406. doi: 10.4103/jfmpc.jfmpc_1520_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalayou M.H., Awol S.M. Myth and Misinformation on COVID-19 Vaccine: The Possible Impact on Vaccination Refusal Among People of Northeast Ethiopia: A Community-Based Research. Risk Manag. Health Policy. 2022;15:1859–1868. doi: 10.2147/RMHP.S366730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim K., Lee C.J., Ihm J., Kim Y. A comprehensive examination of association between belief in vaccine misinformation and vaccination intention in the COVID-19 context. J. Health Commun. 2022:1–15. doi: 10.1080/10810730.2022.2130479. [DOI] [PubMed] [Google Scholar]
- 56.Li H.O., Pastukhova E., Brandts-Longtin O., Tan M.G., Kirchhof M.G. YouTube as a source of misinformation on COVID-19 vaccination: A systematic analysis. BMJ Glob. Health. 2022;7 doi: 10.1136/bmjgh-2021-008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pierri F., Perry B.L., DeVerna M.R., Yang K.C., Flammini A., Menczer F., Bryden J. Online misinformation is linked to early COVID-19 vaccination hesitancy and refusal. Sci. Rep. 2022;12:5966. doi: 10.1038/s41598-022-10070-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sah R., Mohanty A., Reda A., Padhi B.K., Rodriguez-Morales A.J. Stigma during monkeypox outbreak. Front. Public Health. 2022;10:1023519. doi: 10.3389/fpubh.2022.1023519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Narain K., Mkhize N. Monkeypox in South Africa: The need for responsible messaging to avoid stigmatising the LGBTI community. South Afr. Med. J. Suid-Afrik. Tydskr. Geneeskd. 2022;112:741. doi: 10.7196/SAMJ.2022.v112i9.16723. [DOI] [PubMed] [Google Scholar]
- 60.Gandrakota N., Lee H., Nwosu O., Kulshreshtha A. Monkeypox coinfection with Neurosyphilis in a transgender with HIV in Atlanta, USA. Travel Med. Infect. Dis. 2022;50:102454. doi: 10.1016/j.tmaid.2022.102454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hubach R.D., Owens C. Findings on the Monkeypox Exposure Mitigation Strategies Employed by Men Who Have Sex with Men and Transgender Women in the United States. Arch. Sex. Behav. 2022:1–6. doi: 10.1007/s10508-022-02423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reda A., Abdelaal A., Brakat A.M., Lashin B.I., Abouelkheir M., Abdelazeem B., Rodriguez-Morales A.J., Sah R. Monkeypox Viral Detection In Semen Specimens of Confirmed Cases: A Systematic Review and Meta-Analysis. J. Med. Virol. 2022 doi: 10.1002/jmv.28250. [DOI] [PubMed] [Google Scholar]
- 63.Ortiz-Martínez Y., Saul Z., Hutchinson K.A., Miljkovic G., Rodríguez-Morales A.J. Not Just Differential Diagnoses… Importance of Sexually Transmitted Infections as Coinfections with Monkeypox Amidst the Outbreak. Int. J. STD AIDS. 2022:9564624221127746. doi: 10.1177/09564624221127746. [DOI] [PubMed] [Google Scholar]
- 64.Alvarado-Socarras J.L., Vesga-Varela A.L., Quintero-Lesmes D.C., Fama-Pereira M.M., Serrano-Diaz N.C., Vasco M., Carballo-Zarate V., Zambrano L.I., Paniz-Mondolfi A., Rodriguez-Morales A.J. Perception of COVID-19 Vaccination Amongst Physicians in Colombia. Vaccines. 2021;9:287. doi: 10.3390/vaccines9030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ubale M.R., Bagle T.R., Gaikwad S., Baviskar P.A., Nanoty V.A. Attitude and perception of COVID-19 vaccines in healthcare workers. J. Fam. Med. Prim. Care. 2022;11:2448–2455. doi: 10.4103/jfmpc.jfmpc_1377_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reyes-Urueña J., D’Ambrosio A., Croci R., Bluemel B., Cenciarelli O., Pharris A., Dukers-Muijrers N., Nutland W., Niaupari S., Badran J., et al. High monkeypox vaccine acceptance among male users of smartphone-based online gay-dating apps in Europe, 30 July to 12 August 2022. Euro Surveill. 2022;27:2200757. doi: 10.2807/1560-7917.ES.2022.27.42.2200757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zaeck L.M., Lamers M.M., Verstrepen B.E., Bestebroer T.M., van Royen M.E., Götz H., Shamier M.C., van Leeuwen L.P.M., Schmitz K.S., Alblas K., et al. Low levels of monkeypox virus neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat. Med. 2022 doi: 10.1038/s41591-022-02090-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available on reasonable request.