Abstract
In the last decade, an upsurge of human leishmaniasis has been reported in the Emilia-Romagna region, Northeast Italy. Epidemiologic data have raised doubts about the role of dogs as the main reservoirs for Leishmania infantum. In the present study, a total of 1077 wild animals were screened for L. infantum DNA in earlobe and spleen samples from 2019 to 2022. The lymph nodes were tested only in 23 animals already positive in the earlobe and/or spleen. A total of 71 (6.6%) animals resulted positive in at least one of the sampled tissues, including 3/18 (16.7%) wolves, 6/39 (15.4%) European hares, 38/309 (12.3%) roe deer, 1/11 (9.1%) red deer, 8/146 (4.9%) wild boars, 13/319 (4.1%) red foxes, 1/54 (1.9%) porcupine, and 1/59 (1.7%) European badger. Most of the infected animals (62/71) tested positive only in the earlobe tissue, only four animals (two roe deer and two wild boars) tested positive only in the spleen, and five animals (three roe deer and two red foxes) resulted positive for both tissues. L. infantum DNA was detected in the lymph nodes of 6/23 animals. L. infantum detection occurred in all seasons associated with low real-time PCR Ct values. Further research is needed in order to clarify the role of wildlife in the re-emerging focus of leishmaniasis in Northeast Italy.
Keywords: Leishmania infantum, reservoir, wildlife, artiodactyls, roe deer, European hare, red fox, wild boar
1. Introduction
Leishmaniasis caused by the protozoan Leishmania (L.) infantum Nicoll, 1908 is a neglected vector-borne zoonotic disease that is endemic in the Mediterranean basin [1,2]. L. infantum is the causative agent of zoonotic visceral (VL), cutaneous (CL) and mucosal (ML) leishmaniasis in humans [3,4]. It is also the cause of canine leishmaniasis (CanL) in dogs, which are considered the major domestic reservoir of infection for people [5]. In the Old World, transmission occurs via the bite of female sand flies of the genus Phlebotomus [1].
L. infantum is endemic in Italy, as it is throughout the entire Mediterranean region, with a median CanL seroprevalence of 17.7% [6]. The classical endemic areas are the rural and hilly peri-urban zones along the Tyrrhenian littoral, the southern peninsular regions, and the islands. Since the 1990s, the incidence of leishmaniasis in both dogs and humans has been on the rise in northern areas, previously regarded as non-endemic [7]. Among the northern Italian regions, the Emilia-Romagna region (RER) is characterized by a distinctive epidemiological situation: since 1934, CL has been frequently reported—with an incidence exceeding 2000 cases in the 1950s—before declining, most likely as a result of pesticide use in agriculture [8]. In contrast, until the early 1970s, there were only four recorded autochthonous cases of VL. In 1971–1972, a severe VL outbreak with 60 cases occurred in the foothill areas close to Bologna municipality, which is located in the central RER [9]. This outbreak was regarded as atypical, as only human cases were diagnosed, but a canine reservoir was not clearly evidenced [9]. Furthermore, follow-up surveillance of this VL focus failed to reveal human and canine cases over the next 15 years [10].
Since 2012, an upsurge in VL cases has been described in RER [11,12]. Similar to the VL outbreak in the 1970s, no rise in L. infantum infection among dogs was reported, which motivated research to better understand the regional L. infantum epidemiology. Molecular typing studies showed that L. infantum strains circulating in dogs belonged to a distinct population in comparison with strains circulating in VL cases and sand flies [13,14]. Moreover, the blood-meal analysis of Phlebotomus (Ph). perfiliewi Parrot, 1930—the strongly suspected vector of L. infantum in RER [15]—showed a biting preference for wild animals and humans [16]. These findings led to hypothesize a role for an unknown wildlife species as a reservoir for the parasite, also according to the increasing number of studies reporting infection of Leishmania spp. in wild animals [17].
The aim of the present study was to evaluate the role of different wild mammals as natural hosts of L. infantum in RER by testing them for L. infantum DNA over a 3-year period.
2. Materials and Methods
2.1. Study Area
RER is located in the northeastern part of the Italian peninsula. It covers an area of approximately 22,500 km2 and is inhabited by a population of 4.5 million people. The region is geographically divided into two homogeneous areas: the northern half of the region is entirely occupied by the Po Valley, while in the southern half, the flat area gives way to the hilly and then to the mountainous part of the Apennine chain. On its eastern side, it borders the Adriatic Sea. The climate is sub-continental in the inner part of the region and becomes Mediterranean near the coasts. The average temperature from 1991 to 2015 was 12.8 °C. The rainfall ranges from 650 to 1200 mm per year [18], depending on the altitude and the distance from the sea: the minimum value is recorded in the plains and increases toward the hills and the mountains. The rains are concentrated mainly in autumn and spring; the summer is often characterized by severe dryness.
RER is divided into 9 administrative provinces; this study included the warm-blooded fauna of the 6 provinces most affected by human leishmaniasis in the last decade: Reggio Emilia, Modena, Bologna, Ravenna, Forlì-Cesena, and Rimini [19]. This territory comprises the central-eastern part of the region, characterized by flat, hilly, and mountainous areas as well as the Adriatic coast.
2.2. Sampling
From February 2019 to March 2022, a total of 1077 wild mammal carcasses were collected as part of the wildlife health surveillance program set up in RER [20].
They consisted of: 508 artiodactyls, including 309 roe deer (Capreolus capreolus Linnaeus, 1758), 164 wild boars (Sus scrofa Linnaeus, 1758), 24 fallow deer (Dama dama Linnaeus, 1758), 11 red deer (Cervus elaphus Linnaeus, 1758); 401 carnivores, including 319 red foxes (Vulpes vulpes Linnaeus, 1758), 59 European badgers (Meles meles Linnaeus, 1758), 18 wolves (Canis lupus italicus Altobello, 1921), 2 stone martens (Martes foina Erxleben, 1777), 1 pine marten (Martes martes Linnaeus, 1758), 1 raccoon (Procyon lotor Linnaeus, 1758), 1 Western polecat (Mustela putorius Linnaeus, 1758); 67 rodents, including 54 porcupines (Hystrix cristata Linnaeus, 1758), 7 squirrels (Sciurus vulgaris Linnaeus, 1758), 5 dormices (Glis glis Linnaeus, 1766), 1 common dormouse (Muscardinus avellanarius Linnaeus, 1758); 62 hedgehogs (Erinaceus europaeus Linnaeus, 1758) belonging to the Eulipotyphla order; 39 lagomorphs European hares (Lepus europaeus Pallas, 1778).
The animals were legally hunted, found dead by local authorities, accidentally road-killed, or conferred by wildlife rescue and rehabilitation centers.
Postmortem examination and tissue sampling were conducted at the Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia-Romagna. The presence of pathological alterations in the organs was recorded. Spleen and earlobe samples were collected from all animals included in the study. Superficial lymph nodes of the head were sampled in a limited number of carcasses and tested only if the spleen and/or earlobe resulted positive.
All the sampled tissues were stored frozen (−20 °C) until molecular analysis.
2.3. Molecular Analysis
Genomic DNA was extracted from 25 mg sample of the spleen, lymph node, and the central hairless part of the earlobe. Tissue samples were homogenized and incubated overnight at 56 °C in 200 μL buffered lysis solution containing proteinase K (10 μg/mL). DNA was extracted using the NucleoSpin Tissue Kit (Macherey Nagel, Duren, Germany) according to the manufacturer’s instructions and eluted in 200 μL of elution buffer. Each sample was co-extracted and amplified with a commercial internal control DNA template (QuantiFast Pathogen PCR +IC Kit, Qiagen GmbH, Hilden, Germany). The detection of Leishmania DNA was carried out by a TaqMan MGB probe real-time PCR targeting a highly repetitive kinetoplast minicircle DNA sequence [21]. Each amplification was performed in a 25 μL of reaction mixture which contained 1x QuantiFast Pathogen Master Mix, 1x Internal Control Assay, 300 nM of each primers (leish-F: 5′-ACTTTTCTGGTCCTCCGGGTAG-3′, leish-R: 5′ -CCTATTTTACACCAACCCCCAGT-3′) 200 nM of probe (Leish Pb FAM-ATTTCTGCACCCATTTT-MGB), and 5 μL of sample DNA. The reactions were carried out on BioRad CFX96 thermal cycler (Bio-Rad Laboratories, Hercules, CA, USA) with the following temperature profile: 5 min at 95 °C, then 45 cycles of 15 s at 95 °C and 30 s at 60 °C. The PCR was considered positive when a threshold cycle (Ct) value lower than 40 was detected.
2.4. Data Analysis
Data were summarized with the proportion of positive samples, with exact confidence intervals [22]. Analysis was performed with R 4.0.2 [23]. The spatial distribution of samples was provided with QGis (version 3.14.15-Pi).
3. Results
A total of 2177 tissue samples were analyzed for the presence of L. infantum DNA: spleen and earlobe samples from each of the 1077 animals included in the study, and the lymph nodes of 23 animals found positive in the earlobe and/or spleen.
The overall species and tissue-specific PCR detection is summarized in Table 1. A total of 71 (6.6%) animals resulted positive in at least one of the sampled tissues, including 3/18 (16.7%) wolves, 6/39 (15.4%) European hares, 38/309 (12.3%) roe deer, 1/11 (9.1%) red deer, 8/164 (4.9%) wild boars, 13/319 (4.1%) red foxes, 1/54 (1.9%) porcupine, and 1/59 (1.7%) European badger.
Table 1.
L. infantum real-time PCR positive wildlife species in the Emilia-Romagna region in the period 2019-2022. Detection rates (including 95% confidence interval) for the target species and tissues are reported.
| Order | Species | Earlobe | Spleen | Total | |||
|---|---|---|---|---|---|---|---|
| Positive (Tested) | Prevalence (CI 95%) | Positive (Tested) | Prevalence (CI 95%) |
Positive (Tested) | Prevalence (CI 95%) |
||
| Artiodactyla | Roe deer | 36 (309) | 11.7 (8.3–15.8) | 5 (309) | 1.6 (0.5–3.7) | 38 (309) | 12.3 (8.9–16.5) |
| Red deer | 1 (11) | 9.1 (0.2–41.3) | 0 (11) | 0.0 (0.0–28.5) | 1 (11) | 9.1 (0.2–41.3) | |
| Wild boar | 6 (164) | 3.7 (1.4–7.8) | 2 (164) | 1.2 (0.2–4.3) | 8 (164) | 4.9 (2.1–9.4) | |
| Carnivora | Wolf | 3 (18) | 16.7 (3.6–41.4) | 0 (18) | 0.0 (0.0–18.5) | 3 (18) | 16.7 (3.6–41.4) |
| Red fox | 13 (319) | 4.1 (2.2–6.9) | 2 (319) | 0.6 (0.1–2.3) | 13 (319) | 4.1 (2.2–6.9) | |
| European badger | 1 (59) | 1.7 (0.0–9.1) | 0 (59) | 0.0 (0.0–6.1) | 1 (59) | 1.7 (0.0–9.1) | |
| Lagomorpha | European hare | 6 (39) | 15.4 (5.9–30.5) | 0 (39) | 0.0 (0.0–9.0) | 6 (39) | 15.4 (5.9–30.6) |
| Rodentia | Porcupine | 1 (54) | 1.9 (0.1–9.9) | 0 (54) | 0.0 (0.0–6.6) | 1 (54) | 1.9 (0.1–9.9) |
| Other species 1 | 0 (104) | - | 0 (104) | - | 0 (104) | - | |
| All Species | 67 (1077) | 6.2 (4.9–7.8) | 9 (1077) | 0.8 (0.4–1.6) | 71 (1077) | 6.6 (5.2–8.2) | |
1 species with no positive individuals, namely 62 hedgehogs, 24 fallow deer, 2 stone martens, 1 pine marten, 1 raccoon, 1 Western polecat, 7 squirrels, 5 dormices, 1 common dormouse.
In relation to the tissue location of the parasite, five animals (three roe deer and two foxes) tested positive in both the spleen and earlobe, four animals (two roe deer and two wild boars) tested positive only in the spleen, while the large majority of the positive animals (62/71) tested positive only in the earlobe. Earlobe samples showed the highest L. infantum DNA detection rate (6.2%; CI 95%: 4.9–7.8) compared to spleen samples (0.8%; CI 95%: 0.4–1.6). The superficial lymph nodes were tested on a subgroup of 23 animals already positive for the ear (19), spleen (1), or both (3). Among them, six animals tested positive, and one roe deer resulted positive in all the three organs (earlobe, spleen and lymph nodes) (Table 2).
Table 2.
Lymph node analysis on 23 animals already tested positive for L. infantum DNA in the earlobe and/or spleen.
| Order | Species | No. of Lymph Node Positive Animals (No. Tested) | |||
|---|---|---|---|---|---|
| Earlobe | Spleen | Both | Total | ||
| Artiodactyla | Roe deer | 2 (10) | 0 (1) | 1 (2) | 3 (13) |
| Red deer | 1 (1) | 1 (1) | |||
| Wild boar | 0 (1) | 0 (1) | |||
| Carnivora | Wolf | 1 (3) | 1 (3) | ||
| Red fox | 1 (3) | 0 (1) | 1 (4) | ||
| European badger | 0 (1) | 0 (1) | |||
| total | 5 (19) | 0 (1) | 1 (3) | 6 (23) | |
None of the positive animals showed macroscopic dermal and/or internal lesions suggestive of Leishmania infection, with the exception of one roe deer with a localized ulcerative lesion located on the positive earlobe (further investigations are underway).
The PCR Ct values ranged from 13.9 to 39.1; 15.9% of the samples had Ct values lower than 25, and 17.1% of the samples had Ct values greater than 35 (Figure 1). Overall, median Ct values were lower in the earlobe than in spleen and lymph node samples, with results of 29.0, 33.9, and 34.8, respectively. In particular, the median Ct values were less than 35 only in the earlobes of roe deer, red deer, red foxes, European hares, and porcupines. In addition, Ct values resulted less than 20 in four earlobe samples (two from roe deer and two from red foxes), always exceeding 30 in all spleen samples.
Figure 1.
Distribution of L. infantum real-time PCR cycle threshold (Ct) values for the target species and tissues.
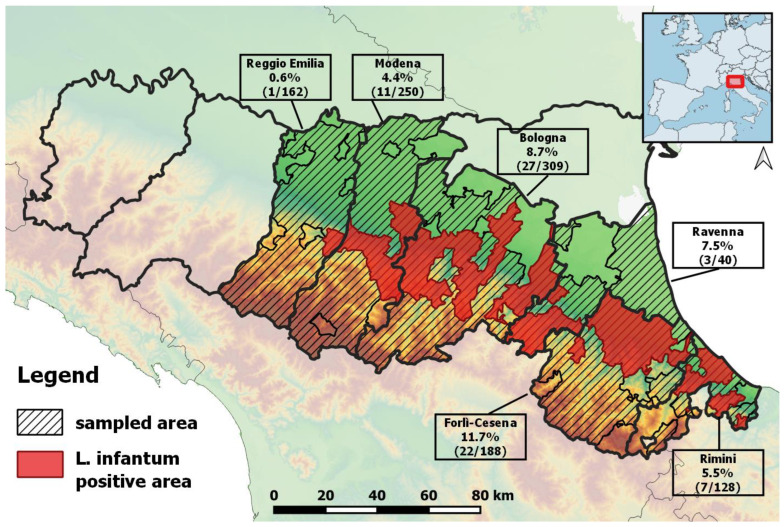
The spatial distribution of both the sampled and positive animals is shown in Figure 2. The highest detection rates were concentrated in the provinces of Forlì-Cesena (11.7%) and Bologna (8.7%) (Figure 2 and Table 3). At the province level, the highest number of positive species was also recorded in the Bologna and Forlì-Cesena provinces, with five and four species, respectively (Table S1).
Figure 2.
Sampled and L. infantum real-time PCR positive areas, Emilia-Romagna region, Italy.
Table 3.
Overall prevalence (including 95% confidence interval) of L. infantum DNA in wildlife in the provinces of the Emilia-Romagna region.
| Province | No. of Positive (Tested Animals) | Prevalence (%) | CI 95% |
|---|---|---|---|
| Bologna | 27 (309) | 8.7 | (5.8–12.5) |
| Forlì-Cesena | 22 (188) | 11.7 | (7.5–17.2) |
| Modena | 11 (250) | 4.4 | (2.2–7.7) |
| Ravenna | 3 (40) | 7.5 | (1.6–20.4) |
| Reggio Emilia | 1 (162) | 0.6 | (0.0–3.4) |
| Rimini | 7 (128) | 5.5 | (2.2–10.9) |
CI: confidence interval.
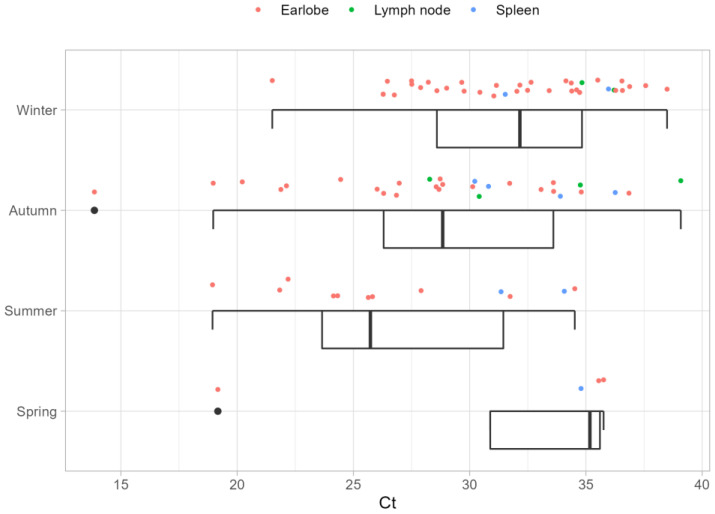
The yearly temporal distribution of Leishmania DNA detection showed that the animals tested positive in all seasons, and Ct values lower than 25 (from 13.9 to 21.6) were recorded throughout the whole year (Figure 3). Roe deer tested positive in all seasons (Table 4), with minimum associated Ct values ranging from 13.9 to 26.3. Overall, one roe deer, positive in both the earlobe and spleen, one badger, and one wild boar tested positive for L. infantum DNA during spring (Table 4).
Figure 3.
Distribution of L. infantum real-time PCR cycle threshold (Ct) values among the weather seasons.
Table 4.
Prevalence (including 95% confidence interval) of L. infantum real-rime PCR positive species along the weather seasons.
| Season | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Order | Species | Spring | Summer | Autumn | Winter | ||||||||
| N° Positive (n° Tested) |
Prevalence | 95% CI | N° Positive (n° Tested) |
Prevalence | 95% CI | N° Positive (n° Tested) |
Prevalence | 95% CI | N° Positive (n° Tested) |
Prevalence | 95% CI | ||
| Artiodactyla | Red deer | 1 (6) | 16.7 | (0.4–64.1) | 0 (5) | 0 | (0–52.2) | ||||||
| Roe deer | 1 (75) | 1.3 | (0–7.2) | 6 (53) | 11.3 | (4.3–23) | 13 (64) | 20.3 | (11.3–32.2) | 18 (117) | 15.4 | (9.4–23.2) | |
| Wild boar | 1 (10) | 10 | (0.3–44.5) | 0 (21) | 0 | (0–16.1) | 3 (77) | 3.9 | (0.8–11) | 4 (56) | 7.1 | (2–17.3) | |
| Carnivora | European badger | 1 (6) | 16.7 | (0.4–64.1) | 0 (10) | 0 | (0–30.8) | 0 (21) | 0 | (0–16.1) | 0 (22) | 0 | (0–15.4) |
| Red fox | 0 (4) | 0 | (0–60.2) | 5 (157) | 3.2 | (1–7.3) | 4 (64) | 6.3 | (1.7–15.2) | 4 (94) | 4.3 | (1.2–10.5) | |
| Wolf | 0 (1) | 0 | (0–97.5) | 0 (6) | 0 | (0–45.9) | 3 (11) | 27.3 | (6–61) | ||||
| Lagomorpha | European hare | 0 (7) | 0 | (0–41) | 0 (4) | 0 | (0–60.2) | 1 (16) | 6.3 | (0.2–30.2) | 5 (12) | 41.7 | (15.2–72.3) |
| Porcupine | 0 (8) | 0 | (0–36.9) | 0 (13) | 0 | (0–24.7) | 0 (17) | 0 | (0–19.5) | 1 (16) | 6.3 | (0.2–30.2) | |
| All species | 3 (113) | 2.7 | (0.6–7.6) | 11 (288) | 3.8 | (1.9–6.7) | 22 (329) | 6.7 | (4.2–10.0) | 35 (347) | 10.1 | (7.1–13.8) | |
CI: confidence interval.
4. Discussion
L. infantum is a zoonotic parasite steadily endemic at a low prevalence (around 2%) in the canine population in RER [19,24]. In contrast, an increasing number of VL, CL, and ML cases have been reported in the region in the last decade [3,11,12,25]. Molecular typing of strains from canine and human hosts and from sand flies have raised doubts about the dogs’ role as main reservoirs to human infection in this region [13,14]. Moreover, studies on the blood-meals of Ph. perfiliewi—the most abundant sand fly species in the study area—showed a feeding preference for wild mammals (especially roe deer and European hares) and humans, while no trace of canine or rodents’ blood was observed [16]. In addition, high infection rates in L. infantum naturally infected sand flies were found, suggesting the existence of a dog-independent sylvatic cycle [15,16].
These findings led to the investigation of the potential role of wild mammals as natural reservoirs of L. infantum in the region. Different species of mammals have been found positive for L. infantum DNA in the study area, with an overall prevalence of 6.6% (CI 95%: 5.2–8.2), ranging among positives from 1.7% to 16.7% according to species. The overall highest positivity rates in wildlife were observed in the foothill areas of the Forlì-Cesena and Bologna provinces (Figure 1), which are the areas more affected by human leishmaniasis [19] and where a high density of sand flies has been historically reported [26,27]. Even though the results could be skewed by the non-homogeneous sample across provinces, the largest number of infected species was also detected in these provinces, perhaps as a result of higher infection pressure. The circulation of the Leishmania parasite in these provinces could be related to the abundance of sand flies [27]. Ph. perfiliewi is characterized by a patchy distribution, but it reaches higher densities in rural and semi-natural areas of the hills (hedges on the border of cultivated fields, woods, pastures) where wild animals are numerous and simple to access as blood sources.
Among the wild carnivores sampled in the present study, the highest Leishmania DNA detection rate was observed in wolves (16.7%; CI 95%: 3.6–41.4). This value was lower than those reported from other European countries, including other northern Italian regions, where prevalence rates of up to 50% were observed [28,29,30]. In addition, the prevalence found in foxes (4.1%; CI 95%: 2.2–6.9), ranging from 3.6% to 7.2% according to the sampling areas (Table S1), was lower than that reported previously in northwestern (12.3%), central (52.2%), and southern Italy (28.6%–40%) [30,31,32,33], and in Spain (14.1%) [34,35]. Similarly, a low L. infantum detection rate was found in European badgers (1.7%), when compared with the high values observed in northern Spain (26%) and northwestern Italy (53.3%) [30,36].
Among rodents, porcupines were the unique species found positive for L. infantum DNA with a detection rate of 1.9% (CI 95%: 0.1–9.9). To the best of our knowledge, there is only a single paper reporting porcupines of the genus Hystrix as a possible wild reservoir species for visceral leishmaniasis [37]. The small sample size of other species prevented a real assessment of the current prevalence in rodents in the study area. Recently, anthropophilic rodents have been found to be infected at a prevalence of 11% in plain areas of RER [38], which are historically less involved in human leishmaniasis than the foothill areas. However, the increase in seasonal abundance and the distribution of sand flies due to climate change, together with the recent detection of Leishmania DNA from lowland sand flies [39], indicate that the role of rodents in the epidemiology of leishmaniasis in RER needs to be monitored and further investigated.
Lagomorphs such as European hares were found infected in the study area with a detection rate of 15.4% (CI 95%: 5.9–30.6). Even though this value was lower than those reported from other Mediterranean countries, such as Spain (43.6%) and northern Greece (23.5%) [40,41], all the positive subjects (6/13, 46%) were sampled in the Bologna province (Table S1), which is the most ancient and re-emerging focus of VL in RER [11]. In addition to black rats (Rattus rattus) in Italy [42], infectiousness to sand flies has been demonstrated in the Iberian hare (Lepus granatensis), which was suggested to be the main sylvatic reservoir in the human leishmaniasis outbreak that occurred in Fuenlabrada, Spain [40,43].
Although rodents, carnivores, and lagomorphs are the orders more widely explored in leishmaniasis foci, being the most suitable wild reservoirs for the parasite [44,45], over the last decade, the list of wild animals infected with L. infantum has been enlarged with bats, primates, and different species of hedgehog [17,46,47,48]. L. infantum has been recently detected or isolated from domestic species belonging to the families Equidae and Bovidae, respectively [49,50]. However, wild artiodactyls have only been rarely investigated.
The present study provided convincing evidence for L. infantum DNA detection in wild boar (Sus scrofa) and in other two artiodactyls belonging to the family Cervidae, namely roe deer (Capreolus capreolus) and red deer (Cervus elaphus).
Moraes-Silva and colleagues [51], by combining serologic and molecular methods as well as experimental infection, concluded that domestic swine (Sus scrofa) is resistant to infection by L. infantum, excluding the possible involvement of this species in the epidemiology of visceral leishmaniasis. However, the same authors commented on a previous study [52] showing the presence of numerous amastigotes of an uncharacterized Leishmania species, probably L. braziliensis, in one pig. In the present study, wild boars showed a median Ct value of 35.9, with the lowest value above 30, probably indicating a natural resistance to the disease and a transient infection. Nevertheless, the detection of an infected animal in the spring indicates that additional research is required to clarify the role of wild boar.
Data generated in the present study suggest the need for an in-depth analysis of the role of cervids. The detection rate of Leishmania DNA in roe deer over the entire study area was 12.3% (95% CI: 8.9–16.5). Considering only the areas most affected by human leishmaniasis, it was found to be 9.4% in the province of Bologna, while the highest infection rate was detected in the province of Forlì-Cesena with 31.8% of infected roe deer. In addition, roe deer has been shown to be the preferred host species by Ph. perfiliewi in selected endemic sites in the RER region, according to blood-meal identification. In the same study, a Ph. perfiliewi specimen was found engorged with a double meal, i.e., roe deer and man, and it was infected by a high load of L. infantum as well [16].
Absence of pathological alterations has been a common finding among wild animals infected by Leishmania spp., but in a study carried out in Spain, almost half the animals tested exhibited at least one lesion compatible with leishmaniasis in their earlobe [53]. In the present study, the earlobe tissue of a single roe deer showed an ulcerated area with an inflammatory histologic pattern typical of cutaneous leishmaniasis (data not shown). Further investigation is ongoing at the time of writing this paper.
In this study, three different tissues were targeted to detect the parasite in wildlife. Earlobe samples showed a higher level of Leishmania DNA detection than the spleen samples. Results of lymph node examination suggested a low tendency for L. infantum visceralization in wildlife from RER. Other authors have also compared different tissues, with results varying between studies as well as between animal species. Abbate and colleagues [33] showed that in most foxes and wild rabbits, L. infantum DNA was found mainly in the spleen. In other lagomorph surveys, L. infantum was more frequently detected in the skin than in other tissues [29,54].
The mere finding of L. infantum DNA in a given animal species does not necessarily imply that this species is involved in Leishmania epidemiology, acting as a reservoir. Xenodiagnoses should be carried out to demonstrate host infectiousness to sand flies. However, it is likely that the success of host to sand fly transmission depends on the extent of skin parasite load, which has been suggested as the best marker to identify potential reservoir animal species [36,55,56]. Although the molecular analysis performed did not allow for exact quantification, the real-time PCR threshold cycle (Ct) of positivity can serve as an estimate of parasite load [15]. A low-to-intermediate number of parasites (median Ct between 25 and 35) was found in the earlobes of red deer, wolves, European hares, porcupines, roe deer, and red foxes. In the latter two species, we also found high parasite loads (Ct values < 20) in several earlobe samples. Amastigotes in skin are directly accessible to sand flies, which are known to prefer safe, hairless parts of hosts, such as the ear pinnae, and feed copiously on them [57,58].
The results of the present study showed detection of Leishmania DNA also in winter and spring, seasons free of adult sand flies [27]. Roe deer tested positive in all seasons, but the limited number of sampled animals does not allow for a definitive assessment to be drawn on all other species. Furthermore, the occurrence of Ct values below 25 in earlobe samples independently of the season suggests that some species could not clear the L. infantum infection, thus fitting the most important of the five criteria to consider a species as a reservoir of Leishmania parasites, i.e., the long course of infection [59,60]. In light of this, roe deer need to be further investigated, as they are among the species with low Ct values in spring, thus representing a possible host for L. infantum overwintering.
The present study confirmed previous findings on the existence of a sylvatic cycle for L. infantum in the study area. We cannot exclude that differences observed in wild hosts and parasite’s tissue tropism in RER could be linked to: (i) the peculiar parasitic population circulating in humans and sand flies in the region [14], being phylogenetically distant from the L. infantum MON-1 strains commonly affecting dogs and humans [61,62,63,64]; (ii) the predominant sand fly species, i.e., Ph. perfiliewi, in comparison with other Mediterranean endemic areas, where Ph. pernicious is the main vector of L. infantum. Finally, the exhibited multi-host infection pattern and preferential tropism for skin tissues would allow the parasite to widen its reservoir spectrum, allowing for greater exploitation of the trophic (host) activity of the vector, determined by vector host preference and host abundance. The involvement of wild animals in the parasite’s epidemiology will be determined by ongoing molecular research on the L. infantum DNA detected in wildlife in RER.
5. Conclusions
A deep knowledge of the transmission dynamics of Leishmania parasites is essential in order to apply control measures or monitoring programs. This study provides evidence that L. infantum is widespread in wildlife in RER and gives preliminary data on tissue tropism and geographic and seasonal pattern of the parasite. Additionally, the study expands the number of mammalian hosts for L. infantum in Italy. Further investigation is needed in order to clarify whether wildlife species should be considered maintenance hosts, sources of infection, or sentinels for L. infantum circulation in the re-emerging focus of leishmaniasis in Northeast Italy.
Acknowledgments
The authors are grateful to all the local hunters, forest guards, and staff from the Wildlife Rescue and Rehabilitation Centers for their help in collecting samples. We would also like to express our gratitude to Elio Licata and Stefano Palminteri of the Veterinary Public Health Services for the implementation and coordination of the wildlife surveillance program in the Emilia-Romagna region.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens11111308/s1, Table S1: Prevalence (including 95% confidence interval) of L. infantum real-rime PCR positive species among the provinces of the study area.
Author Contributions
Conceptualization, G.R., R.T. and M.C.; methodology, R.T. and A.B.; validation, G.R., M.C. and R.T.; formal analysis, G.G., A.R. (Arianna Rossi) and A.S.; investigation, R.T., A.B. and G.R.; resources, G.P., M.F., L.F., M.C.F., C.M. and A.R. (Alessandro Reggiani); data curation, R.T. and G.G.; writing—original draft preparation, R.T. and G.R.; writing—review and editing, A.S., M.C., E.C., C.M. and M.T.; visualization, G.G. and A.R. (Arianna Rossi); supervision, G.R. and R.T.; project administration, G.R.; funding acquisition, G.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study such that it did not involve the killing of animals. The samples did not originate from experimental trial but took advantage from diagnostic activity with Regional Surveillance Plans for wildlife diseases (DGR, 13 November 2017, no. 1703). Therefore, since the sampling was not specifically programmed as an experimental study, but originating from diagnostic activity, we believe that it does not fall in the provisions of the National Law (i.e., DLSG 4/3 2014, n. 26. Application at national level of the EU Directive 2010/63/UE), and no ethical approval or permit for animal experimentation was required.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data generated or analyzed during this study are included in the published article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by the Ministry of Health, Italy (grant E54I19002870001—IZSLER PRC2019016).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moriconi M., Rugna G., Calzolari M., Bellini R., Albieri A., Angelini P., Cagarelli R., Landini M.P., Charrel R.N., Varani S. Phlebotomine sand fly-borne pathogens in the Mediterranean Basin: Human leishmaniasis and phlebovirus infections. PLoS Negl. Trop. Dis. 2017;11:e0005660. doi: 10.1371/journal.pntd.0005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berriatua E., Maia C., Conceicao C., Ozbel Y., Toz S., Baneth G., Perez-Cutillas P., Ortuno M., Munoz C., Jumakanova Z., et al. Leishmaniases in the European Union and Neighboring Countries. Emerg. Infect. Dis. 2021;27:1723. doi: 10.3201/eid2706.210239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaspari V., Zaghi I., Macri G., Patrizi A., Salfi N., Locatelli F., Carra E., Re M.C., Varani S. Autochthonous Cases of Mucosal Leishmaniasis in Northeastern Italy: Clinical Management and Novel Treatment Approaches. Microorganisms. 2020;8:588. doi: 10.3390/microorganisms8040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gradoni L., López-Vélez R., Mokni M. Manual on Case Management and Surveillance of the Leishmaniases in the WHO European Region. 2017. [(accessed on 25 October 2022)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/344118/9789289052511-eng.pdf?sequence=1&isAllowed=y.
- 5.Solano-Gallego L., Koutinas A., Miro G., Cardoso L., Pennisi M.G., Ferrer L., Bourdeau P., Oliva G., Baneth G. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet. Parasitol. 2009;165:1–18. doi: 10.1016/j.vetpar.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 6.Maia C., Cardoso L. Spread of Leishmania infantum in Europe with dog travelling. Vet. Parasitol. 2015;213:2–11. doi: 10.1016/j.vetpar.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Maroli M., Rossi L., Baldelli R., Capelli G., Ferroglio E., Genchi C., Gramiccia M., Mortarino M., Pietrobelli M., Gradoni L. The northward spread of leishmaniasis in Italy: Evidence from retrospective and ongoing studies on the canine reservoir and phlebotomine vectors. Trop. Med. Int. Health. 2008;13:256–264. doi: 10.1111/j.1365-3156.2007.01998.x. [DOI] [PubMed] [Google Scholar]
- 8.Pampiglione S. La Leishmaniosi Viscerale in Emilia-Romagna. Ann. Sanità Pubblica. 1974;35:1021–1028. [Google Scholar]
- 9.Pampiglione S., La Placa M., Schlick G. Studies on mediterranean Leishmaniasis. I. An outbreak of visceral leishmaniasis in Northern Italy. Trans R. Soc. Trop. Med. Hyg. 1974;68:349–359. doi: 10.1016/0035-9203(74)90148-5. [DOI] [PubMed] [Google Scholar]
- 10.Baldelli R., Di Francesco A. Leishmaniosi in Italia: Risultati di indagini sierologiche condotte su cani di diversa provenienza geografica. Atti Della Soc. Delle Sci. Vet. 1992;46:1395–1399. [Google Scholar]
- 11.Varani S., Cagarelli R., Melchionda F., Attard L., Salvadori C., Finarelli A.C., Gentilomi G.A., Tigani R., Rangoni R., Todeschini R., et al. Ongoing outbreak of visceral leishmaniasis in Bologna Province, Italy, November 2012 to May 2013. Eurosurveillance. 2013;18:20530. doi: 10.2807/1560-7917.ES2013.18.29.20530. [DOI] [PubMed] [Google Scholar]
- 12.Franceschini E., Puzzolante C., Menozzi M., Rossi L., Bedini A., Orlando G., Gennari W., Meacci M., Rugna G., Carra E., et al. Clinical and Microbiological Characteristics of Visceral Leishmaniasis Outbreak in a Northern Italian Nonendemic Area: A Retrospective Observational Study. Biomed. Res. Int. 2016;2016:6481028. doi: 10.1155/2016/6481028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rugna G., Carra E., Corpus F., Calzolari M., Salvatore D., Bellini R., Di Francesco A., Franceschini E., Bruno A., Poglayen G., et al. Distinct Leishmania infantum Strains Circulate in Humans and Dogs in the Emilia-Romagna Region, Northeastern Italy. Vector Borne Zoonotic Dis. 2017;17:409–415. doi: 10.1089/vbz.2016.2052. [DOI] [PubMed] [Google Scholar]
- 14.Rugna G., Carra E., Bergamini F., Calzolari M., Salvatore D., Corpus F., Gennari W., Baldelli R., Fabbi M., Natalini S., et al. Multilocus microsatellite typing (MLMT) reveals host-related population structure in Leishmania infantum from northeastern Italy. PLoS Negl. Trop. Dis. 2018;12:e0006595. doi: 10.1371/journal.pntd.0006595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calzolari M., Carra E., Rugna G., Bonilauri P., Bergamini F., Bellini R., Varani S., Dottori M. Isolation and Molecular Typing of Leishmania infantum from Phlebotomus perfiliewi in a Re-Emerging Focus of Leishmaniasis, Northeastern Italy. Microorganisms. 2019;7:644. doi: 10.3390/microorganisms7120644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calzolari M., Romeo G., Bergamini F., Dottori M., Rugna G., Carra E. Host preference and Leishmania infantum natural infection of the sand fly Phlebotomus perfiliewi in northern Italy. Acta Trop. 2022;226:106246. doi: 10.1016/j.actatropica.2021.106246. [DOI] [PubMed] [Google Scholar]
- 17.Cardoso L., Schallig H., Persichetti M.F., Pennisi M.G. New Epidemiological Aspects of Animal Leishmaniosis in Europe: The Role of Vertebrate Hosts Other Than Dogs. Pathogens. 2021;10:307. doi: 10.3390/pathogens10030307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antolini G., Pavan V., Tomozeiu R., Marletto V. Atlante Climatico Dell’Emilia-Romagna 1961–2015. 2017th ed. Arpae Emilia-Romagna; Bologna, Italy: 2017. [Google Scholar]
- 19.Regione Emilia-Romagna. Piano Regionale di Sorveglianza e Controllo Della Leishmaniosi Canina. [(accessed on 25 October 2022)]. Available online: https://www.anagrafecaninarer.it/acrer/Default.aspx?tabid=160#.
- 20.Regione Emilia Romagna Sorveglianza e Monitoraggio della Fauna Selvatica. [(accessed on 25 October 2022)]. Available online: https://www.alimenti-salute.it/documentazione-regionale/90355.
- 21.Galletti E., Bonilauri P., Bardasi L., Fontana M.C., Ramini M., Renzi M., Dosa G., Merialdi G. Development of a minor groove binding probe based real-time PCR for the diagnosis and quantification of Leishmania infantum in dog specimens. Res. Vet. Sci. 2011;91:243–245. doi: 10.1016/j.rvsc.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Clopper C.J., Pearson E.S. The Use of Confidence or Fiducial Limits Illustrated in the Case of the Binomial. Biometrika. 1934;26:404–413. doi: 10.1093/biomet/26.4.404. [DOI] [Google Scholar]
- 23.R Core Team R: A language and Environment for Statistical Computing. 2022. [(accessed on 25 October 2022)]. Available online: https://www.R-project.org.
- 24.Santi A., Renzi M., Baldelli R., Calzolari M., Caminiti A., Dell’Anna S., Galletti G., Lombardini A., Paternoster G., Tamba M. A surveillance program on canine leishmaniasis in the public kennels of Emilia-Romagna Region, Northern Italy. Vector Borne Zoonotic Dis. 2014;14:206–211. doi: 10.1089/vbz.2013.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cesinaro A.M., Nosseir S., Mataca E., Mengoli M.C., Cavatorta C., Gennari W. An outbreak of cutaneous leishmaniasis in Modena province (Northern Italy): Report of 35 cases. Pathologica. 2017;109:363–367. [PubMed] [Google Scholar]
- 26.Corradetti A. Phlebotomus and leishmaniasis in North-Central Italy (Apennine Region) Sci. Rep. Ist. Sup. Sanità. 1962;2:103–109. [Google Scholar]
- 27.Calzolari M., Romeo G., Callegari E., Bonilauri P., Chiapponi C., Carra E., Rugna G., Taddei R., Lelli D., Dottori M. Co-Circulation of Phleboviruses and Leishmania Parasites in Sand Flies from a Single Site in Italy Monitored between 2017 and 2020. Viruses. 2021;13:1660. doi: 10.3390/v13081660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oleaga A., Zanet S., Espi A., Pegoraro de Macedo M.R., Gortazar C., Ferroglio E. Leishmania in wolves in northern Spain: A spreading zoonosis evidenced by wildlife sanitary surveillance. Vet. Parasitol. 2018;255:26–31. doi: 10.1016/j.vetpar.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Risueno J., Ortuno M., Perez-Cutillas P., Goyena E., Maia C., Cortes S., Campino L., Bernal L.J., Munoz C., Arcenillas I., et al. Epidemiological and genetic studies suggest a common Leishmania infantum transmission cycle in wildlife, dogs and humans associated to vector abundance in Southeast Spain. Vet. Parasitol. 2018;259:61–67. doi: 10.1016/j.vetpar.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Battisti E., Zanet S., Khalili S., Trisciuoglio A., Hertel B., Ferroglio E. Molecular Survey on Vector-Borne Pathogens in Alpine Wild Carnivorans. Front. Vet. Sci. 2020;7:1. doi: 10.3389/fvets.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dipineto L., Manna L., Baiano A., Gala M., Fioretti A., Gravino A.E., Menna L.F. Presence of Leishmania infantum in red foxes (Vulpes vulpes) in southern Italy. J. Wildl. Dis. 2007;43:518–520. doi: 10.7589/0090-3558-43.3.518. [DOI] [PubMed] [Google Scholar]
- 32.Ranieri V., Poli A., Ariti G., Nardoni S., Bertuccelli M. Fanucchi Manciant Detection of Leishmania infantum DNA in tissues of free-ranging red foxes (Vulpes vulpes) in Central Italy. Eur. J. Wildl. Res. 2010;56:689–692. [Google Scholar]
- 33.Abbate J.M., Arfuso F., Napoli E., Gaglio G., Giannetto S., Latrofa M.S., Otranto D., Brianti E. Leishmania infantum in wild animals in endemic areas of southern Italy. Comp. Immunol. Microbiol. Infect. Dis. 2019;67:101374. doi: 10.1016/j.cimid.2019.101374. [DOI] [PubMed] [Google Scholar]
- 34.Criado-Fornelio A., Gutierrez-Garcia L., Rodriguez-Caabeiro F., Reus-Garcia E., Roldan-Soriano M.A., Diaz-Sanchez M.A. A parasitological survey of wild red foxes (Vulpes vulpes) from the province of Guadalajara, Spain. Vet. Parasitol. 2000;92:245–251. doi: 10.1016/S0304-4017(00)00329-0. [DOI] [PubMed] [Google Scholar]
- 35.Sobrino R., Ferroglio E., Oleaga A., Romano A., Millan J., Revilla M., Arnal M.C., Trisciuoglio A., Gortazar C. Characterization of widespread canine leishmaniasis among wild carnivores from Spain. Vet. Parasitol. 2008;155:198–203. doi: 10.1016/j.vetpar.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Del Rio L., Chitimia L., Cubas A., Victoriano I., de la Rua P., Gerrikagoitia X., Barral M., Munoz-Garcia C.I., Goyena E., Garcia-Martinez D., et al. Evidence for widespread Leishmania infantum infection among wild carnivores in L. infantum periendemic northern Spain. Prev. Vet. Med. 2014;113:430–435. doi: 10.1016/j.prevetmed.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Petrisceva P.A. The natural focality of leishmaniasis in the USSR. Bull. World Health Organ. 1971;44:567–576. [PMC free article] [PubMed] [Google Scholar]
- 38.Magri A., Galuppi R., Fioravanti M., Caffara M. Survey on the presence of Leishmania sp. in peridomestic rodents from the Emilia-Romagna Region (North-Eastern Italy) Vet. Res. Commun. 2022;2022:1–6. doi: 10.1007/s11259-022-09925-4. [DOI] [PubMed] [Google Scholar]
- 39.Calzolari M., Romeo G., Munari M., Bonilauri P., Taddei R., Sampieri M., Bariselli S., Rugna G., Dottori M. Sand Flies and Pathogens in the Lowlands of Emilia-Romagna (Northern Italy) Viruses. 2022;14:2209. doi: 10.3390/v14102209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruiz-Fons F., Ferroglio E., Gortazar C. Leishmania infantum in free-ranging hares, Spain, 2004–2010. Eurosurveillance. 2013;18:20541. doi: 10.2807/1560-7917.ES2013.18.30.20541. [DOI] [PubMed] [Google Scholar]
- 41.Tsokana C.N., Sokos C., Giannakopoulos A., Mamuris Z., Birtsas P., Papaspyropoulos K., Valiakos G., Spyrou V., Lefkaditis M., Chatzopoulos D.C., et al. First evidence of Leishmania infection in European brown hare (Lepus europaeus) in Greece: GIS analysis and phylogenetic position within the Leishmania spp. Parasitol. Res. 2016;115:313–321. doi: 10.1007/s00436-015-4749-8. [DOI] [PubMed] [Google Scholar]
- 42.Gradoni L., Pozio E., Gramiccia M., Maroli M., Bettini S. Leishmaniasis in Tuscany (Italy): VII. Studies on the role of the black rat, Rattus rattus, in the epidemiology of visceral leishmaniasis. Trans R. Soc. Trop. Med. Hyg. 1983;77:427–431. doi: 10.1016/0035-9203(83)90102-5. [DOI] [PubMed] [Google Scholar]
- 43.Molina R., Jimenez M.I., Cruz I., Iriso A., Martin-Martin I., Sevillano O., Melero S., Bernal J. The hare (Lepus granatensis) as potential sylvatic reservoir of Leishmania infantum in Spain. Vet. Parasitol. 2012;190:268–271. doi: 10.1016/j.vetpar.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Millan J., Ferroglio E., Solano-Gallego L. Role of wildlife in the epidemiology of Leishmania infantum infection in Europe. Parasitol. Res. 2014;113:2005–2014. doi: 10.1007/s00436-014-3929-2. [DOI] [PubMed] [Google Scholar]
- 45.Alcover M.M., Riera M.C., Fisa R. Leishmaniosis in Rodents Caused by Leishmania infantum: A Review of Studies in the Mediterranean Area. Front. Vet. Sci. 2021;8:702687. doi: 10.3389/fvets.2021.702687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munoz-Madrid R., Belinchon-Lorenzo S., Iniesta V., Fernandez-Cotrina J., Parejo J.C., Serrano F.J., Monroy I., Baz V., Gomez-Luque A., Gomez-Nieto L.C. First detection of Leishmania infantum kinetoplast DNA in hair of wild mammals: Application of qPCR method to determine potential parasite reservoirs. Acta Trop. 2013;128:706–709. doi: 10.1016/j.actatropica.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Miro G., Troyano A., Montoya A., Farinas F., Fermin M.L., Flores L., Rojo C., Checa R., Galvez R., Marino V., et al. First report of Leishmania infantum infection in the endangered orangutan (Pongo pygmaeus pygmaeus) in Madrid, Spain. Parasit. Vectors. 2018;11:185. doi: 10.1186/s13071-018-2772-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azami-Conesa I., Gomez-Munoz M.T., Martinez-Diaz R.A. A Systematic Review (1990–2021) of Wild Animals Infected with Zoonotic Leishmania. Microorganisms. 2021;9:1101. doi: 10.3390/microorganisms9051101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mhadhbi M., Sassi A. Infection of the equine population by Leishmania parasites. Equine Vet. J. 2020;52:28–33. doi: 10.1111/evj.13178. [DOI] [PubMed] [Google Scholar]
- 50.Paixao-Marques M.D.S., Alves-Martin M.F., Guiraldi L.M., Dos Santos W.J., de Lemos F.A., Sanchez G.P., Richini-Pereira V.B., Lucheis S.B. First isolation of Leishmania infantum by blood culture in bovines from endemic area for canine visceral leishmaniasis. Parasitology. 2019;146:911–913. doi: 10.1017/S0031182019000088. [DOI] [PubMed] [Google Scholar]
- 51.Moraes-Silva E., Antunes F.R., Rodrigues M.S., da Silva Juliao F., Dias-Lima A.G., Lemos-de-Sousa V., de Alcantara A.C., Reis E.A., Nakatani M., Badaro R., et al. Domestic swine in a visceral leishmaniasis endemic area produce antibodies against multiple Leishmania infantum antigens but apparently resist to L. infantum infection. Acta Trop. 2006;98:176–182. doi: 10.1016/j.actatropica.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Brazil R.P., Desterro M.D., Nascimento S.B., Macau R.P. Natural infection of a pig (Sus scrofa) by Leishmania in a recent focus of cutaneous leishmaniasis on the Island of Sao Luis, Maranhao. Mem. Inst. Oswaldo Cruz. 1987;82:145. doi: 10.1590/S0074-02761987000100025. [DOI] [PubMed] [Google Scholar]
- 53.Martin-Sanchez J., Torres-Medina N., Morillas-Marquez F., Corpas-Lopez V., Diaz-Saez V. Role of wild rabbits as reservoirs of leishmaniasis in a non-epidemic Mediterranean hot spot in Spain. Acta Trop. 2021;222:106036. doi: 10.1016/j.actatropica.2021.106036. [DOI] [PubMed] [Google Scholar]
- 54.Ortega-Garcia M.V., Salguero F.J., Rodriguez-Bertos A., Moreno I., Garcia N., Garcia-Seco T., Luz Torre G., Dominguez L., Dominguez M. A pathological study of Leishmania infantum natural infection in European rabbits (Oryctolagus cuniculus) and Iberian hares (Lepus granatensis) Transbound. Emerg. Dis. 2019;66:2474–2481. doi: 10.1111/tbed.13305. [DOI] [PubMed] [Google Scholar]
- 55.Aslan H., Oliveira F., Meneses C., Castrovinci P., Gomes R., Teixeira C., Derenge C.A., Orandle M., Gradoni L., Oliva G., et al. New Insights into the Transmissibility of Leishmania infantum From Dogs to Sand Flies: Experimental Vector-Transmission Reveals Persistent Parasite Depots at Bite Sites. J. Infect. Dis. 2016;213:1752–1761. doi: 10.1093/infdis/jiw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arumugam S., Scorza B.M., Petersen C. Visceral Leishmaniasis and the Skin: Dermal Parasite Transmission to Sand Flies. Pathogens. 2022;11:610. doi: 10.3390/pathogens11060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Svobodova M., Votypka J. Experimental transmission of Leishmania tropica to hamsters and mice by the bite of Phlebotomus sergenti. Microbes Infect. 2003;5:471–474. doi: 10.1016/S1286-4579(03)00066-2. [DOI] [PubMed] [Google Scholar]
- 58.Ready P.D. Biology of phlebotomine sand flies as vectors of disease agents. Annu. Rev. Entomol. 2013;58:227–250. doi: 10.1146/annurev-ento-120811-153557. [DOI] [PubMed] [Google Scholar]
- 59.World Health Organization Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, Switzerland, 22–26 March 2010; Proceedings of the WHO Technical Report Series; Geneva, Switzerland. 22–26 March 2010; Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 60.Chaves L.F., Hernandez M., Dobson A.P., Pascual M. Sources and sinks: Revisiting the criteria for identifying reservoirs for American cutaneous leishmaniasis. Trends Parasitol. 2007;23:311–316. doi: 10.1016/j.pt.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Chicharro C., Llanes-Acevedo I.P., Garcia E., Nieto J., Moreno J., Cruz I. Molecular typing of Leishmania infantum isolates from a leishmaniasis outbreak in Madrid, Spain, 2009 to 2012. Eurosurveillance. 2013;18:20545. doi: 10.2807/1560-7917.ES2013.18.30.20545. [DOI] [PubMed] [Google Scholar]
- 62.Cortes S., Mauricio I.L., Kuhls K., Nunes M., Lopes C., Marcos M., Cardoso L., Schonian G., Campino L. Genetic diversity evaluation on Portuguese Leishmania infantum strains by multilocus microsatellite typing. Infect. Genet. Evol. 2014;26:20–31. doi: 10.1016/j.meegid.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 63.Pomares C., Marty P., Banuls A.L., Lemichez E., Pratlong F., Faucher B., Jeddi F., Moore S., Michel G., Aluru S., et al. Genetic Diversity and Population Structure of Leishmania infantum from Southeastern France: Evaluation Using Multi-Locus Microsatellite Typing. PLoS Negl. Trop. Dis. 2016;10:e0004303. doi: 10.1371/journal.pntd.0004303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.El Hamouchi A., El Kacem S., Ejghal R., Lemrani M. Genetic polymorphism in Leishmania infantum isolates from human and animals determined by nagt PCR-RFLP. Infect Dis. Poverty. 2018;7:54. doi: 10.1186/s40249-018-0439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated or analyzed during this study are included in the published article.