Abstract
Horizontal transfer of genes encoding virulence factors has played a central role in the evolution of many pathogenic bacteria. The unexpected discovery that the genes encoding cholera toxin (ctxAB), the main cause of the profuse secretory diarrhea characteristic of cholera, are encoded on a novel filamentous phage named CTXΦ, has resulted in a renewed interest in the potential mechanisms of transfer of virulence genes among Vibrio cholerae. We describe here an alternative mechanism of cholera toxin gene transfer into nontoxigenic V. cholerae isolates, including strains that lack both the CTXΦ receptor, the toxin coregulated pilus (TCP), and attRS, the chromosomal attachment site for CTXΦ integration. A temperature-sensitive mutant of the V. cholerae generalized transducing bacteriophage CP-T1 (CP-T1ts) was used to transfer a genetically marked derivative of the CTX prophage into four nontoxigenic V. cholerae strains, including two V. cholerae vaccine strains. We demonstrate that CTXΦ transduced by CP-T1ts can replicate and integrate into these nontoxigenic V. cholerae strains with high efficiency. In fact, CP-T1ts transduces the CTX prophage preferentially when compared with other chromosomal markers. These results reveal a potential mechanism by which CTXΦ+ V. cholerae strains that lack the TCP receptor may have arisen. Finally, these findings indicate an additional pathway for reversion of live-attenuated V. cholerae vaccine strains.
Vibrio cholerae is unusual among enteric pathogens both for its tendency to cause explosive outbreaks and for its predilection for pandemic spread. V. cholerae is the prototypical noninvasive enteric pathogen which is spread by the ingestion of contaminated food and water. Of the more than 150 serogroups that exist, until recently only the O1 serogroup was associated with epidemic cholera. The O1 serogroup is divided into two distinct biotypes, designated classical and El Tor. The first six pandemics of cholera are believed to have been caused by the classical biotype and the ongoing seventh pandemic, which began in 1961, is caused by the El Tor biotype (6). Remarkably, in 1992 a new epidemic serogroup, O139 Bengal, emerged and temporarily replaced the predominant O1 serogroup (1, 9, 35).
Most of the major symptoms caused by V. cholerae infection result from the production of cholera toxin (CT) in the small intestine after colonization (39). Recently, CT was found to be encoded in the genome of an unusual lysogenic filamentous phage, named CTXΦ (45, 47). The receptor for CTXΦ is thought to be a type IV pilus, the toxin coregulated pilus (TCP), since V. cholerae cells that do not express TCP were found to be resistant to CTXΦ infection (40, 42, 45).
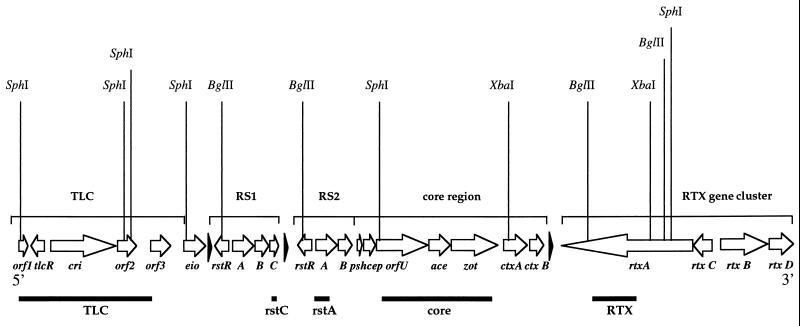
The CTXΦ genome consists of a 4.6-kb core region and a 2.4-kb repeated sequence (RS2) region. The 4.6-kb core region encodes the cholera toxin A and B subunits (ctxAB) and the psh, cep, orfU, ace, and zot genes which are required for phage morphogenesis and secretion. The 2.4-kb RS2 region encodes the rstR, rstA, and rstB genes required for repression, replication, and site-specific integration of CTXΦ (47) (Fig. 1).
FIG. 1.
Chromosomal arrangement of TLC, RS1, CTXΦ, and RTX in the V. cholerae chromosome. The open reading frames are shown as arrows. The black triangles represent attRS sequences. Vertical lines indicate restriction enzyme sites. Horizontal bars show the five DNA probes used in this study.
After infection of classical V. cholerae strains and El Tor strains lacking a CTXΦ integration site, CTXΦ can replicate as a plasmid. This plasmid form of CTXΦ was designated the phage replicative form (RF), since cells harboring the plasmid produce large amounts of viral particles. However, upon infection of most El Tor strains, CTXΦ integrates into the chromosome at a unique attachment site (attRS) to form a lysogen rather than maintaining the RF (33, 45, 47). Often, an RS1 sequence is found immediately adjacent to the CTX prophage on the V. cholerae chromosome (Fig. 1). RS1 is closely related to the RS2 region of the CTXΦ genome but contains an additional open reading frame of unknown function, rstC. The function and mechanism of transfer of RS1 is unknown. Upstream of the CTX prophage is an integrated 4.7-kb plasmid named pTLC (toxin-linked cryptic element) (36). Located 3′ of the CTX prophage is a large RTX toxin gene cluster (27) (Fig. 1).
The genes encoding the biosynthesis of TCP, the CTXΦ receptor, reside on a pathogenicity island known as the TCP island or vibrio pathogenicity island (VPI) (22, 24). Recently, Karaolis et al. have proposed that the VPI corresponds to the genome of another lysogenic filamentous bacteriophage, VPIΦ (23). Since TCP is required for CTXΦ infection of V. cholerae, it was proposed that there were two critical sequential steps in the evolution of pathogenic V. cholerae (14, 15, 30, 45, 46). First, strains had to acquire the TCP island (presumably via infection with VPIΦ) and, second, having acquired the CTXΦ receptor (as well as ToxT, a VPI-encoded transcription factor critical in expression of ctxAB), these TCP+ strains were then infected with and lysogenized by CTXΦ. Studies with the suckling mouse cholera model revealed that CTXΦ infection of nontoxigenic strains is particularly efficient within the intestine, an environment known to induce TCP expression, suggesting that the host environment may be the site where TCP+ mutant ctxAB strains acquire CTXΦ (25, 45). In fact, in a recent extensive strain survey, Faruque et al. (14) identified TCP+ CT+ strains and TCP+ CT− strains but found no TCP− CT+ strains, suggesting that CTXΦ was acquired subsequent to the acquisition of TCP. However, the two-step model of the sequential evolution of pathogenic V. cholerae is called into question by several V. cholerae O1 and non-O1 isolates that have been recently described that lack TCP but contain CTXΦ sequences (18, 38). These strains indicate that acquisition of CTXΦ and TCP may be independent of one another.
In the current study we investigate whether ctxAB is transmissible by means other than via CTXΦ. We found that CP-T1ts, a V. cholerae generalized transducing phage (32), transfers the entire CTXΦ genome at a high frequency. CP-T1ts-transduced CTXΦ can integrate into alternative attachment sites, and TCP is not required for CP-T1ts-mediated acquisition of CTXΦ. These findings cast doubt on the assumption that CTXΦ is the sole mediator of horizontal transfer of CT genes and that transfer occurs primarily within the host environment.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used in this study are shown in Table 1. Bacterial strains were stored at −70°C in Luria-Bertani (LB) broth containing 20% glycerol. Antibiotics were used at the following concentrations: kanamycin (Kn), 50 μg/ml; streptomycin (Sm), 200 μg/ml; and tetracycline (Tc), 1 μg/ml.
TABLE 1.
Characteristics of the El Tor V. cholerae strains used in this study
| Strain | Description | Source or reference |
|---|---|---|
| Donorsa | ||
| SM115 | E7946 ΔctxABN4::Kn, TLC+-attRS+-CTXΦ+-RTX+ | 19 |
| V66 | C6709 lacZ::res Tc res, TLC+-attRS+-CTXΦ+-RTX+ | 8 |
| SM115-Tc | SM115; lacZ::res Tc res | This study |
| Recipientsb | ||
| 468-83 | TCP− TLC− CTXΦ− RTX+; attRS mutant | 36 |
| 2740-80 | TCP+ TLC+attRS+ CTXΦ− RTX+ | 33 |
| Bah-2 | E7946 derivative, TCP+, ΔTLC ΔattRS ΔCTXΦ ΔRTX | 33 |
| Bah-3 | Bah-2 derivative, recA::htpG-ctxB | 46 |
CP-T1ts lysates were made on these strains.
Strains used as recipients for CP-T1 lysates.
CP-T1 transduction.
We performed experiments with a temperature-sensitive (ts) mutant of the V. cholerae transducing phage, CP-T1 (32), to determine whether ctxAB was transmissible by transduction to nontoxigenic V. cholerae O1 recipient strains. To detect transduction of ctxAB, a CP-T1ts phage was cultured and purified from strain SM115 (19), which contains Kn-marked ctxAB, to produce a high-titer lysate as follows. Serial dilutions of CP-T1ts were added to 20-μl aliquots of SM115 overnight cultures, mixed, and incubated for 10 min to allow CP-T1 infection of strain SM115. Infected cells were then added to 5.5 ml of top agar, mixed, and spread on LB agar plates. The LB plates were incubated overnight, and 5 ml of LB broth was added to those plates with confluent plaques. The LB broth and top agar were recovered in a large culture tube, mixed, and left at 4°C overnight to allow phage diffusion from the top agar. The top agar-phage mixture was centrifuged for 1 min at top speed in a bench-top centrifuge; the supernatant was then recovered and further centrifuged for 30 s to remove agar. The supernatant was then centrifuged for 2 h at 4°C at top speed in an Eppendorf microcentrifuge to concentrate the phage, followed by immediate aspiration of all but 60 μl of the supernatant, in which the phage was resuspended. A drop of chloroform was added to kill any remaining bacterial cells, and the high-titer lysate (1010 to 1011 PFU/ml) was stored at 4°C.
Transduction experiments were carried out with 20 μl of this high-titer lysate treated with 5,000 μJ of UV light in a Stratagene Stratalinker, which inactivates 90 to 99% of the phage. UV-treated phage were added to 100 μl of late-log-phase recipient cells, mixed briefly, and then incubated for 6 min to allow CP-T1 infection of recipient cells. To this mixture, 1 ml of LB medium was added, and it was then incubated at 39°C for 2 h with shaking. Next, cells were pelleted and resuspended in 100 μl of LB broth. Aliquots of 10 and 90 μl were plated on Kn-containing selection plates and incubated overnight at 39°C, which prohibits CP-T1ts replication.
To determine relative transfer efficiency of CTX-KnΦ by CP-T1, a second chromosomal marker was transduced from SM115 for comparison purposes. This was accomplished by initially infecting V. cholerae V66 (8), which contains a Tc-marked lacZ gene, with CP-T1. This lysate was then used to transduce SM115 to tetracycline resistance. The resulting strain, SM115-Tc, then contains a Tc-marked lacZ and a Kn-marked CTXΦ.
Molecular analyses.
Total genomic DNA from each bacterial isolate was extracted by using the G-nome DNA isolation kit from Bio 101 (Vista, Calif.). DNA was digested with several restriction enzymes, and the fragments were separated by electrophoresis in 0.6% agarose. The fragments were transferred to nylon membranes for hybridization as previously described (7). Primers for PCR amplification were designed from published DNA sequences (Table 2). PCR products were purified by using the Qiaquick PCR purification kit (Qiagen, Valencia, Calif.). A total of five DNA fragments were produced by PCR amplification (Table 2 and Fig. 1) for use as nonradioactive probes by labeling with fluorescein-conjugated nucleotides and, after hybridization, were detected by the Amersham ECL System (Arlington Heights, Ill.). The attRS probe was a PCR-amplified 328-bp fragment derived from strain 2740-80 (33) by using previously described primers (33). The rstA, rstC, core, and TLC probes were made by PCR amplification from strain SM115.
TABLE 2.
PCR primers used to amplify segments of CTXΦ and flanking regions from V. cholerae
| Primer | Primer sequence | Positiona | PCR product size (bp) | Probe | Reference |
|---|---|---|---|---|---|
| attB1 | 5′-CGCAGCAGACGAACTCTATGTC-3′ | NA | |||
| attB2 | 5′-GACTTTGGTGCACACAATTGACG-3′ | NA | 330 | attB | 33 |
| rstC1 | 5′-AACAGCTACGGGCTTATTC-3′ | −46 | |||
| rstC2 | 5′-TGAGTTGCGGATTTAGGC-3′ | 178 | 238 | rstC | 47 |
| rstA1 | 5′-ACTCGATACAAACGCTTCTC-3′ | 36 | |||
| rstA2 | 5′-AGAATCTGGAAGGTTGAGTG-3′ | 1045 | 1,009 | rstA | 47 |
| orfU1 | 5′-CGTCACACCAGTTACTTTTCG-3′ | 85 | 43 | ||
| zot2 | 5′-AACCCCGTTTCACTTCTAC-3′ | 1131 | 2,545 | core | 16 |
| tlc1 | 5′-ATGCGCCGGAGAAGTTCGAG-3′ | 1 | |||
| tlc2 | 5′-GACCGTCATTTCCTTTAA-3′ | 4078 | 4,000 | tlc | 36 |
| rtxA1 | 5′-CACTCATTCCGATAACCAC-3′ | 412 | |||
| rtxA2 | 5′-GCGATTCTCAAAGAGATGC-3′ | 1791 | 1,366 | rtxA | 27 |
Position is based on the start codon of gene. NA, not applicable.
We assayed each of the transductants for the production of infectious CTX-KnΦ particles. To accomplish this, supernatants from mid-log-phase Knr transductants were filtered sterilized and then used to transduce agglutinated (TCP+) classical strain 0395 to Knr (31) according to an established protocol (45). To determine whether transductants derived from the transduction experiments contained plasmid DNA, Qiagen plasmid spin kits were used to isolate DNA from 1 ml of overnight cultures of Knr transductants. These plasmid DNA preparations were digested with SphI, separated on agarose gels, and stained with ethidium bromide to visualize the ∼7-kb plasmid CTXΦ DNA band.
RESULTS
CP-T1ts transduction of CTXΦ.
The V. cholerae phage CP-T1 has been shown to transduce a number of V. cholerae loci (21, 32). We used CP-T1ts, a temperature-sensitive mutant of CP-T1, to study whether ctxAB could be mobilized from an El Tor CTXΦ lysogen to a number of CTXΦ− O1 strains. To facilitate detection of ctxAB transduction, CP-T1 lysates were made on strain SM115 (19). This El Tor strain contains tandemly arranged CTX prophages that each contain an insertion of a Knr marker in place of most of ctxAB (ΔctxABN4::Kn). Thus, kanamycin could be used to select for CP-T1 transduction of ctxAB. In cases where Knr transductants were isolated, we investigated whether the transductants contained the entire CTXΦ DNA sequence as well as chromosomal DNA sequences that flank the CTX prophage.
We assayed four CTXΦ− RS1− El Tor V. cholerae strains for transduction to Knr. We found that lysates of SM115 could transduce Knr to all four recipient strains even though not all strains possessed both TCP and attRS. For example, strain 468-83, an El Tor strain that lacks both attRS and TCP, the CTXΦ receptor (36), could be transduced to Knr at 1.5 × 10−6 transductants per PFU, indicating efficient transduction of the ΔctxABN4::Kn marker by CP-T1ts. Similarly, there was a high frequency of Knr transductant colonies of strain 2740-80, an attRS+ U.S. Gulf Coast isolate (33), and of Bah-2 and Bah-3, two live-attenuated El Tor vaccine constructs, which contain the “attRS” deletion of the entire CTX prophage, RS1, and attRS from strain E7946 (33). Thus, CP-T1ts could efficiently transduce the ΔctxABN4::Kn marker into a variety of El Tor strains.
To examine whether CTXΦ genes other than ctxAB had been transferred, we tested whether infectious CTX-KnΦ virions were produced by the Knr transductants of 468-83, 2740-80, Bah-2, and Bah-3. Filtered supernatants of individual transductants were incubated with classical strain O395, a strain that is very proficient in uptake of CTXΦ in vitro (45), and the growth of Kn-resistant O395 was then assayed. Cell-free supernatants from 468-83Kn, 2740-80Kn, Bah-2Kn, and Bah-3Kn transductants were found to be capable of transducing O395 to Knr. The titer of Kn transducing particles in supernatants derived from these four strains did not differ by more than 1 order of magnitude (from 2 × 105 to 2 × 106 transducing particles/ml of supernatant). Therefore, the Knr transductants of 468-83, 2740-80, Bah-2, and Bah-3 all produce infectious CTX-KnΦ virions, indicating that the entire CTXΦ genome, not just the ΔctxABN4::Kn allele, was transduced by CP-T1ts. Thus, CP-T1ts enables the horizontal transfer of CTXΦ genes to V. cholerae strains that lack the CTXΦ receptor TCP as well as to TCP+ strains.
Determination of the structural state of the CP-T1ts-transduced CTX-KnΦ.
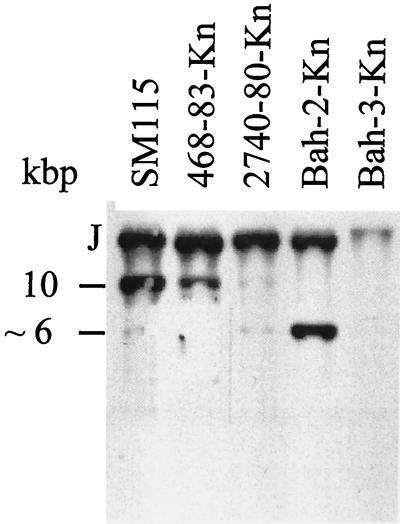
To determine the form in which CTX-KnΦ was maintained in the Knr transductants, we analyzed plasmid DNA and chromosomal DNA from a single Knr transductant of each of the recipient strains. First, plasmid DNA prepared from one of each of the Knr transductants, 468-84Kn, 2740-80Kn, Bah-2Kn, and Bah-3Kn, was examined for the presence of the 6.9-kb plasmid RF of CTX-KnΦ, pCTX-Kn. Plasmid DNA was isolated from strains 468-83Kn, 2740-80Kn, Bah-2Kn, Bah-3Kn, and O395 (pCTX-Kn) digested with SphI, which cuts once within pCTX-Kn, and subsequently probed with a core region probe (Fig. 1) in a Southern blot. All four of the transductants yielded hybridizing DNA which migrated at 6.9 kb, the size of the CTXΦ RF; thus, each of the transductants contained recoverable pCTX-Kn (Fig. 2).
FIG. 2.
Southern blot analysis of plasmid DNAs derived from transductants 468-83Kn, 2740-80Kn, Bah-2Kn, Bah-3Kn, and O395 (pCTX-Kn). Plasmid DNAs from the indicated strains were digested with SphI, which cuts once in CTXΦ, separated on an agarose gel, transferred to nitrocellulose, and then probed with a CTXΦ core region fragment.
We then tested whether the transduced CTX-KnΦ genome was also integrated into the chromosome of 468-83Kn, 2740-80Kn, Bah-2Kn, and Bah-3Kn, in addition to being present as a plasmid. For these studies, Southern hybridization analyses were carried out with chromosomal DNA prepared from each of the Knr transductants, as well as with DNA derived from the original strains. These DNA preparations were digested with restriction enzymes that cut once in the CTXΦ genome. Thus, strain 468-83Kn chromosomal DNA was digested with XbaI, which cuts within ctxA at the site where the Knr gene is inserted. Hybridization with a CTXΦ core region probe (Fig. 1 and Table 2) yielded a banding pattern identical to that of the donor strain SM115 (Fig. 3). Two hybridization bands were obtained: an ∼10-kb band corresponding to a CTX prophage and an adjacent copy of RS1 and a >10-kb band indicating the left junction fragment of the most 5′ copy of RS1 (Fig. 3). This finding suggested that at least two copies of CTXΦ and at least two copies of RS1 had been transferred by CP-T1ts. To confirm the presence of the copies of RS1, 468-83Kn DNA was digested with BglII, which cuts once within the RS sequence, and then probed with an RS1-specific probe, rstC (Fig. 4). This also resulted in a banding pattern similar to that for SM115 strain (Fig. 4). Two bands were visualized (Fig. 4), an ∼2.7-kb band that represents the two RS1 sequences 5′ of the two CTX prophages and an ∼4-kb 3′ junction fragment corresponding to an RS1 3′ of the CTX prophages (Fig. 4 and 5). Thus, strain 468-83Kn has two copies of RS1 arranged similar to those of strain SM115.
FIG. 3.
Southern blot analysis of the integrated copies of CTXΦ in strains SM115, 468-83Kn, 2740-80Kn, and Bah-2-Kn. Equal amounts of chromosomal DNAs from the indicated strains were digested with XbaI, electrophoresed, transferred to nitrocellulose, and probed with a CTXΦ core region fragment. J, left junction fragment of integrated CTXΦ. The ∼10- and ∼6-kb bands represent CTX prophages with or without an intervening RS1 sequence, respectively.
FIG. 4.
Southern blot analysis of the integrated copies of CTXΦ in strains SM115, 468-83Kn, 2740-80Kn, and Bah-2Kn. BglII-digested chromosomal DNA was separated on an agarose gel, transferred to nylon membranes, and hybridized with the RS1-specific probe rstC. The ∼4.0-kb bands represent the right chromosomal junction of an integrated RS1, and the ∼2.7-kb bands represent RS1 sequences 5′ of the CTX prophage.
FIG. 5.
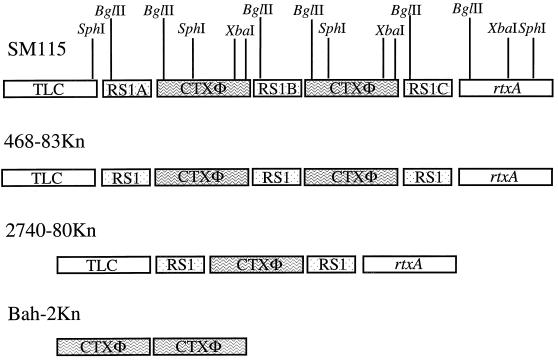
Chromosomal arrangement of CTXΦ, RS1, TLC, and rtxA in the original donor strain SM115 and in the transductants 468-83Kn, 2740-80Kn, and Bah-2Kn as determined by Southern blot and PCR analysis. Vertical lines indicate restriction enzyme sites; XbaI cuts twice in the Knr gene in SM115. The flanking regions of the integrated CTXΦ were determined by PCR analyses with core CTXΦ, TLC, and rtxA primers (Table 2). Open boxes indicate the TLC sequence and the rtxA sequence. Stippled boxes represent the RS1 sequence, and solid boxes represent the CTXΦ.
In the donor strain SM115, at the 5′ end of RS1A (Fig. 5), there are tandemly arranged copies of an integrated cryptic plasmid, the TLC element, that is found in all toxigenic V. cholerae (37). In the region 3′ of RS1C (Fig. 5) there is the recently described RTX toxin gene cluster (27). Strain 468-83 was found to lack TLC hybridizing sequences; however, DNA of strain 468-83Kn hybridized with a TLC probe, indicating that this region was cotransduced with CTXΦ by CP-T1ts (not shown), as was suggested by the equivalent sizes of the left junction fragments in Fig. 3. To further confirm the tight linkage between TLC and CTXΦ and to assess whether RTX genes and CTXΦ genes are linked in strain 468-83Kn, PCR analyses were performed. TLC, RTX, and CTXΦ primers were used to amplify the 5′ and 3′ flanking regions of the transduced RS1 and CTXΦ copies. PCR analysis confirmed the presence of a TLC element located 5′ of RS1A and an RTX element located 3′ of RS1C in strain 468-83Kn (data not shown) (Fig. 5). These results demonstrate that not only CTXΦ but also flanking regions from donor strain SM115 have been transduced by CP-T1ts into 468-83, suggesting that nontoxigenic strains may obtain relatively large chromosomal regions encoding a number of virulence genes in a single transfer event. Also, since strain 468-83 lacks the chromosomal attachment site for CTXΦ, it is likely that the large CP-T1ts-transduced fragment from donor strain SM115 integrated into the 468-83 chromosome by homologous or illegitimate recombination, rather than by CTXΦ-mediated site-specific recombination.
We performed a similar analysis of the Knr transductants of strain 2740-80, a nontoxigenic attRS+ El Tor isolate from the U.S. Gulf Coast (33). Southern hybridization analysis of DNA prepared from 2740-80Kn transductants digested with XbaI and probed with a core region probe revealed only a single hybridizing band of similar size to the 5′ junction fragments detected in SM115 and 468-83Kn, suggesting a single integration of CTXΦ (Fig. 3). Hybridization with the RS1-specific probe rstC revealed a banding pattern similar to that of 468-83Kn with two hybridization species. The ∼2.7-kb band corresponds to an integrated copy of RS1 located 5′ of the integrated CTXΦ (RS1A) and the ∼4 kb corresponds to an RS1 integrated located 3′ of the CTX prophage (RS1B) (Fig. 4 and 5). In strain 2740-80Kn, TLC and RTX sequences were found 5′ and 3′, respectively, of the integrated CTXΦ by both Southern blot and PCR analyses (Fig. 5 and data not shown). The reason why only a single RS1-CTXΦ-RS1 was transduced from SM115 into 2740-80 and the entire RS1-CTXΦ-RS1-CTXΦ-RS1 and flanking DNA was transduced into 468-83 is not clear. Since 2740-80 possesses attRS and 468-83 lacks attRS, this discrepancy may reflect the possibility that RS1-CTXΦ can integrate as a single genetic element at attRS, perhaps via an RS-encoded site-specific integration mechanism.
Bah-2 is a live-attenuated vaccine strain derived from El Tor strain E7946 (33). Bah-2 contains a deletion of the two CTX prophages, the three RS1 elements, the attRS attachment sites, and some flanking DNA from E7946 (Table 1). Perhaps because of this deletion CTX-KnΦ was integrated into the Knr transductants of Bah-2 in a manner that differed from that of the 468-83Kn and 2740-80Kn transductants. In Southern analyses of XbaI-digested DNA prepared from Bah-2Kn transductants, the core region probe detected two bands (Fig. 3), indicating that there are two copies of CTXΦ tandemly integrated into the Bah-2 chromosome. The >10-kb band represents the 5′ junction fragment of CTXΦ in the chromosome, and the ∼6-kb band corresponds to tandem integrated copies of CTXΦ lacking an intervening RS1 (Fig. 3). We believe that this ∼6-kb band represents tandemly arranged CTX prophages (and not a CTXΦ plasmid form) because the intensity of this band in this blot is at least equal to the intensity of the plasmid band detected in a Southern blot of plasmid DNA derived from more than 100 times the number of cells that the chromosomal DNA used in this blot was derived from. The RS1-specific probe rstC gave no hybridization bands indicating the absence of RS1 sequences in these transductants (Fig. 4). PCR analyses of the 5′ and 3′ flanking regions of the tandemly integrated CTX prophages failed to reveal TLC and RTX sequences linked to these CTX prophages (Fig. 5). These results indicate that transfer and integration of CTXΦ in this vaccine strain is not dependent on attRS, RS1, TLC, or RTX sequences and reveal the presence of an alternative CTXΦ attachment site.
To begin to address whether CTXΦ integration into the Bah-2 chromosome occurs at more than one chromosomal location, we performed Southern analyses on DNA isolated from five Bah-2Kn strains that had been transduced to kanamycin resistance by the CP-T1ts lysate made on SM115. We found two additional hybridization patterns with DNA digested with BglII and probed with an rstA probe (data not shown). Thus, in the absence of attRS, CP-T1ts-transduced CTX-KnΦ can integrate at multiple sites on the Bah-2 chromosome. Some Bah-2Kn transductants did not harbor integrated CTXΦ but only pCTX-Kn. The factors which determine whether or not CTXΦ integrates into the ΔattRS background are unknown. Analysis of Knr transductants of Bah-3, a mutant recA derivative of Bah-2 (46), demonstrated the requirement for homologous recombination in the integration of CP-T1ts-transduced CTXΦ DNA in a ΔattRS background. No evidence of chromosomal integration of CTXΦ within Bah-3Kn was found by Southern analysis (the weak band seen in Fig. 3 reflects background hybridization). Instead, in Bah-3Kn the CTXΦ was recovered only as a plasmid copy (Fig. 2). Thus, unlike CTXΦ integration into attRS, which can occur independent of recA (33), integration of CP-T1ts-transduced CTXΦ into a ΔattRS background is recA dependent.
Relative efficiency of CP-T1ts ctxAB transduction.
Our preliminary observations suggested that CP-T1 preferentially transduces ΔctxABN4::Kn, as indicated by the high number of Knr transductants recovered for each of the four V. cholerae recipient strains. To test the relative efficiency of ΔctxABN4::Kn CP-T1ts transduction, we first constructed an SM115 derivative, SM115-Tc, which contains lacZ::Tc. We then made a CP-T1ts lysate on SM115-Tc and compared the number of Knr 2740-80 transductants (indicative of ctxAB transduction) with the number of Tcr 2740-80 transductants (indicative of lacZ transduction). The Kn-marked ctxAB was transduced from SM115-Tc more than 200 times more frequently than the Tc-marked lacZ into the same recipient cells. There were 980 Knr 2740-80 transductants and only 4 Tcr 2740-80 transductants. Similarly, when this lysate was used to transduce Bah-2, we found 1,146 Knr Bah-2 transductants and only 5 Tcr Bah-2 transductants.
A potential explanation for the relative high efficiency of CP-T1ts transduction of CTXΦ is the presence of pCTX-Kn in SM115. To determine whether CP-T1ts can package pCTX-Kn DNA, we made use of the Bah-3Kn transductants that we found only contain the plasmid form of CTX-KnΦ. CP-T1ts was grown on this strain, and then this lysate was used to transduce 468-83, 2740-80, and Bah-2. We obtained Knr transduced cells for all three strains, indicating that CP-T1ts can package pCTX-Kn DNA efficiently.
Host range of CP-T1ts ΔctxABN4::Kn transduction.
To examine whether CP-T1 could transduce ctxAB to non-O1 V. cholerae strains, we attempted to transduce strains of 20 different non-O1 serogroups of V. cholerae by using the same CP-T1ts lysate made on SM115 that had been used to transduce the El Tor O1 strains described above. Interestingly, only one non-O1 serogroup was transduced to Kn resistance by CP-T1ts, serogroup O37. As previously reported (20), this finding suggests that the CP-T1ts receptor is the O1 serogroup antigen and that infection of non-O1 strains by this phage is very unusual.
DISCUSSION
Bacterial species exchange DNA by the parasexual means of conjugation, transduction, and transformation. These mechanisms of horizontal gene transfer play an important role in increasing the genetic variability of a bacterial species and also confer new phenotypes, such as virulence, to the recipient. Horizontal transfer of the genes encoding O139 lipopolysaccharide to an El Tor V. cholerae strain is thought to underlie the emergence of V. cholerae O139, a novel epidemic variant of V. cholerae (3–5, 12, 41, 44). This remarkable event stimulated renewed interest in the role of horizontal gene transfer in the emergence and evolution of pathogenic V. cholerae strains.
More recently, it has been demonstrated that CT is encoded by a lysogenic inovirus, CTXΦ (45). Interestingly, the receptor for CTXΦ, TCP (42), is itself encoded by a recently described novel lysogenic inovirus, VPIΦ (22–24). Hence, it has been presumed that only nontoxigenic V. cholerae strains that already contain TCP can acquire ctxAB via lysogenic conversion by CTXΦ infection. However, the exact molecular sequence of the acquisition of CTXΦ is unsettled, with reports in the literature of toxigenic strains of V. cholerae that lack the genes encoding TCP (18, 38). Some authors have suggested that such isolates arose by TCP-mediated CTXΦ infection, with subsequent loss of the TCP island (VPIΦ) (14, 15, 22). Alternatively, such strains may possess an as-yet-unidentified receptor and/or modes of CTXΦ acquisition that account for the presence of a CTXΦ prophage in these strains.
In our current study, we show that the generalized transducing phage CP-T1 can transfer the entire CTXΦ genome to a number of V. cholerae strains. We found that the CP-T1-transduced CTXΦ integrates into strain 468-83, a V. cholerae strain that lacks both the CTXΦ receptor TCP and the attRS attachment site. Therefore, it appears that TCP may not always be a limiting factor in the conversion of nontoxigenic strains to toxigenicity. Also, these results suggest that disruption of gene products required for TCP-mediated CTXΦ uptake in live-attenuated vaccine strains will not completely protect vaccine strains from reacquiring ctxAB.
Integration of CTXΦ into the chromosome of isolates lacking attRS (e.g., 468-83 and Bah-2) indicates that there are alternative attachment sites or mechanisms of integration in these strains for CTXΦ. Examination of multiple independent CP-T1ts transductions of CTXΦ into strain Bah-2 also showed the presence of at least three alternative CTXΦ attachment sites in this strain and demonstrates that the attRS sequence is nonessential for CTXΦ integration. Among the four El Tor V. cholerae strains we used as recipients to study CP-T1 transduction of CTXΦ, three different patterns of CTXΦ integration into the chromosome were found. Strain 468-83 lacks homology to attRS, CTXΦ, RS1, and TLC sequences but contains the rtxA gene. A Knr transductant of this strain gave a hybridization pattern almost identical to the donor strain SM115, suggesting that CTXΦ and flanking DNA integrated into the 468-83 chromosome via homologous recombination involving the 5′ end of the RTX region. In contrast, a CP-T1ts transductant of strain 2740-80, which contains the attRS attachment site and both the TLC and the RTX regions, contains a single copy of CTXΦ flanked by RS1 sequences integrated into the chromosome. Integration in this strain probably occurred by CTXΦ-mediated site-specific recombination. The chromosomal arrangement of the 2740-80Kn transductant suggests that RS1-CTXΦ-RS1 may integrate as a single genetic element. Bah-2Kn transductants contained two CTX prophages in tandem, and no RS1, TLC, or RTX sequences were transduced. The mechanism by which this strain obtained two tandem copies of CTXΦ that lacked an RS1 sequence is not clear.
The morbidity and mortality of cholera, as well as the significant negative effects of this dreaded diarrheal disease on the economics of developing countries, make this disease a major public health problem and a target for the development of a safe and effective vaccine. Ideally, to prevent vaccine reversion to toxigenicity, live-attenuated V. cholerae vaccine strains should be resistant to CTXΦ infection. Mekalanos and coworkers have engineered El Tor-derived live vaccine strains which include a deletion of the CTXΦ attachment site, attRS, in pursuit of this goal (33). We report here that CP-T1ts CTX-KnΦ transductants of the vaccine strain Bah-2 lacking attRS still contained chromosomally integrated CTX-KnΦ, indicating that even this deletion of the CTXΦ attachment site does not entirely safeguard this type of vaccine construct against lysogenic conversion. However, CP-T1ts CTX-KnΦ transductants of Bah-3, a recA derivative of Bah-2, only contained the plasmid form of CTX-KnΦ, thereby demonstrating the necessity for recA in the chromosomal integration of CTXΦ into ΔattRS strains, as well as the value of this deletion in live vaccine constructs.
Since our examination of the host range of CP-T1 transduction of CTXΦ revealed that only 2 of 20 different V. cholerae serogroups, i.e., O1 and O37, were transduced, CP-T1 may not be important in the transfer of ctxAB to most non-O1 strains. Interestingly, the O37 strain has been shown to be closely related to epidemic O1 V. cholerae strains and was associated with a serious cholera outbreak in Sudan in 1968 (2, 5, 49). Although the CP-T1 host range appears to be limited to a subset of V. cholerae serogroups, it has been proposed that non-O1 serogroups may convert to the O1 serogroup and vice versa under certain environmental conditions, which may allow for the extension of the CP-T1 V. cholerae host range (10, 11). Furthermore, the importance of CP-T1 in particular and transducing phage in general cannot be overemphasized. Since the aquatic ecosystems that constitute the natural habitat of V. cholerae are known to contain enormous numbers and varieties of bacteriophages (13, 34, 48), it is reasonable to hypothesize that other V. cholerae generalized transducing phages exist, which transfer genes among V. cholerae populations independent of the serogroup. Our studies not only demonstrate an alternative evolutionary scenario for the emergence of toxigenic V. cholerae isolates but also suggest the potential, under the right selective pressures, for the horizontal transfer of CT genes to a broader range of bacterial isolates.
We found that ctxAB is preferentially transduced by CP-T1. Comparison of the frequency of ΔctxABN4::Kn transduction with the frequency of lacZ::Tc transduction from the same strain demonstrated that CP-T1 transduction of CTXΦ was markedly more efficient than the transduction of lacZ::Tc. The reason that CP-T1 preferentially transduces CTXΦ DNA is unknown, but several possibilities can be envisioned. Previously, others have identified potential CP-T1 pac sites (20); we therefore searched the DNA sequence of CTXΦ and its flanking regions for CP-T1 pac sites and identified a large number of candidates, which might explain the relatively high frequency of ΔctxABN4::Kn transfer compared with lacZ::Tc transfer. Alternatively, and not mutually exclusive with this possibility, the preferential transduction of CTXΦ DNA may be in part accounted for by the higher copy number of CTXΦ sequences in SM115, the donor strain used in these transductions. The presence of both the tandemly duplicated CTX-Kn prophage and the CTX-KnΦ RF in SM115 increase the copy number of CTX-KnΦ DNA in this strain. It is also possible that there is preferential packaging by CP-T1 of the CTX-KnΦ RF (which is present in SM115) compared to chromosomal sequences. In fact, we found that CP-T1 could efficiently transduce pCTX-Kn from Bah-3, a strain that lacks chromosomal CTXΦ sequences. Since CP-T1 was initially isolated from V. cholerae (21, 32), it may constitute an important mechanism of horizontal ctxAB DNA transfer in V. cholerae populations. Furthermore, the elevated frequency of CTXΦ transduction by CP-T1 may suggest coevolution of these bacteriophages.
The preferential transduction of CTXΦ by phage CP-T1 illustrates the potential importance of interactions between bacteriophages in the horizontal transfer of genes encoding virulence factors. The roles that interactions between bacteriophages play in the evolution of bacterial pathogens is becoming increasingly recognized. For example, Lindsay et al. recently described a phage-like element that carries the gene for toxic shock syndrome toxin 1 (TSST-1), which is excised and circularized by staphylococcal phages φ13 and 80α (28). Moreover, 80α transduces TSST-1 at high frequency and may be responsible for the spread of TSST-1 production among Staphylococcus aureus strains (28). Figueroa-Bossi and Bossi recently described how interactions between the Gifsy-1 and Gifsy-2 prophages play a role in Salmonella enterica serovar Typhimurium virulence (17). Furthermore, recent studies have demonstrated that R-type pyocin particles induced from Pseudomonas aeruginosa C contain closed circular single-stranded DNA that shows homology to filamentous phages (26). Finally, the demonstration that TCP, an essential V. cholerae colonization factor and the receptor for CTXΦ, is itself encoded by a filamentous phage (VPIΦ) indicates that a cell-surface form of a phage (TCP) can act as a receptor for another phage, a novel bacteriophage interaction. Whether bacteriophage interactions generally occur primarily within the human host, as seems to be the case for VPIΦ and CTXΦ (25, 29, 45), or within other environments remains to be investigated. In either case, the complexity of the apparent coevolution of diverse bacteriophages and their bacterial hosts in the evolution of bacterial pathogens is remarkable.
ACKNOWLEDGMENTS
We thank A. Camilli and D. Hava for kindly providing us with the CP-T1ts bacteriophage and for useful suggestions. We thank our colleagues, Andrew Heilpern, Katie Moyer, Brigid Davis, and Bianca Hochhut, for critically reading the manuscript. We are also grateful to Anne Kane and the New England Medical Center GRASP Center (grant P30DK-34928) for providing us with culture media.
This work was supported by National Institutes of Health grant AI-42347 to M.K.W. E.F.B. was supported by training grant T32 AI-07329. M.K.W. is a PEW Scholar of Biomedical Research.
REFERENCES
- 1.Albert M J, Siddique A K, Islam M S, Faruque A S, Ansaruzzaman M, Faruque S M, Sack R B. Large outbreak of clinical cholera due to Vibrio cholerae non-O1 in Bangladesh. Lancet. 1993;341:704. doi: 10.1016/0140-6736(93)90481-u. [DOI] [PubMed] [Google Scholar]
- 2.Beltran P, Delgado G, Navarro A, Trujillo F, Selander R K, Cravioto A. Genetic diversity and population structure of Vibrio cholerae. J Clin Microbiol. 1999;371:581–590. doi: 10.1128/jcm.37.3.581-590.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berche P, Poyart C, Abachin E, Lelievre H, Vandepitte J, Dodin A, Fournier J M. The novel epidemic strain O139 is closely related to the pandemic strain O1 of Vibrio cholerae. J Infect Dis. 1994;170:701–704. doi: 10.1093/infdis/170.3.701. [DOI] [PubMed] [Google Scholar]
- 4.Bik E M, Bunschoten A E, Gouw R D, Mooi F R. Genesis of the novel epidemic Vibrio cholerae O139 strain: evidence for horizontal transfer of genes involved in polysaccharide synthesis. EMBO J. 1995;14:209–216. doi: 10.1002/j.1460-2075.1995.tb06993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bik E M, Gouw R D, Mooi F R. DNA fingerprinting of Vibrio cholerae strains with a novel insertion sequence element: a tool to identify epidemic strains. J Clin Microbiol. 1996;34:1453–1461. doi: 10.1128/jcm.34.6.1453-1461.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blake P A. Historical perspective on pandemic cholera. In: Wachsmuth I K, Blake P A, Olsvik Ø, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C: ASM Press; 1994. pp. 293–295. [Google Scholar]
- 7.Boyd E F, Hartl D L. Chromosomal regions specific to pathogenic isolates of Escherichia coli have a phylogenetically clustered distribution. J Bacteriol. 1998;180:1159–1165. doi: 10.1128/jb.180.5.1159-1165.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camilli A, Beattie D T, Mekalanos J J. Use of genetic recombination as a reporter of gene expression. Proc Natl Acad Sci USA. 1994;91:2634–2638. doi: 10.1073/pnas.91.7.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cholera Working Group. Large epidemic of cholera-like disease in Bangladesh caused by Vibrio cholerae non-O139 Bengal. Lancet. 1993;342:387–390. [PubMed] [Google Scholar]
- 10.Colwell R R. Global climate and infectious disease: the cholera paradigm. Science. 1996;274:2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- 11.Colwell R R, Huq A, Chowdhury M A, Brayton P R, Xu B. Serogroup conversion of Vibrio cholerae. Can J Microbiol. 1995;41:946–950. doi: 10.1139/m95-131. [DOI] [PubMed] [Google Scholar]
- 12.Comstock L E, Maneval D, Jr, Panigrahi P, Joseph A, Levine M M, Kaper J B, Morris J G, Jr, Johnson J A. The capsule and O antigen in Vibrio cholerae O139 Bengal are associated with a genetic region not present in Vibrio cholerae O1. Infect Immun. 1995;63:317–323. doi: 10.1128/iai.63.1.317-323.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DePaola A, Motes M L, Chan A M, Suttle C A. Phages infecting Vibrio vulnificus are abundant and diverse in oysters (Crassostrea virginica) collected from the Gulf of Mexico. Appl Environ Microbiol. 1998;64:346–351. doi: 10.1128/aem.64.1.346-351.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faruque S M, Albert M J, Mekalanos J J. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev. 1998;62:1–14. doi: 10.1128/mmbr.62.4.1301-1314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faruque S M, Asadulghani, Saha M N, Alim A R, Albert M J, Islam K M, Mekalanos J J. Analysis of clinical and environmental strains of nontoxigenic Vibrio cholerae for susceptibility to CTXΦ: molecular basis for origination of new strains with epidemic potential. Infect Immun. 1998;66:5819–5825. doi: 10.1128/iai.66.12.5819-5825.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fasano A, Baudry B, Pumplin D W, Wasserman S S, Tall B D, Ketley J M, Kaper J B. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci USA. 1991;88:5242–5246. doi: 10.1073/pnas.88.12.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Figueroa-Bossi N, Bossi L. Inducible prophages contribute to Salmonella virulence in mice. Mol Microbiol. 1999;33:167–176. doi: 10.1046/j.1365-2958.1999.01461.x. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh C, Nandy R K, Dasgupta S K, Nair G B, Hall R H, Ghose A C. A search for cholera toxin (CT), toxin coregulated pilus (TCP), the regulatory element ToxR and other virulence factors in non-O1/non-O139 Vibrio cholerae. Microb Pathog. 1997;22:199–208. doi: 10.1006/mpat.1996.0105. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg I, Mekalanos J J. Cloning of the Vibrio cholerae recA gene and construction of a Vibrio cholerae recA mutant. J Bacteriol. 1986;165:715–722. doi: 10.1128/jb.165.3.715-722.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guidolin A, Manning P A. Molecular analysis of the packaging signal in bacteriophage CP-T1 of Vibrio cholerae. Mol Gen Genet. 1988;212:514–521. doi: 10.1007/BF00330858. [DOI] [PubMed] [Google Scholar]
- 21.Guidolin A, Morelli G, Kamke M, Manning P A. Vibrio cholerae bacteriophage CP-T1: characterization of bacteriophage DNA and restriction analysis. J Virol. 1984;51:163–169. doi: 10.1128/jvi.51.1.163-169.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karaolis D K, Johnson J A, Bailey C C, Boedeker E C, Kaper J B, Reeves P R. A Vibrio cholerae pathogenicity island associated with epidemic and pandemic strains. Proc Natl Acad Sci USA. 1998;95:3134–3139. doi: 10.1073/pnas.95.6.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karaolis D K, Somara S, Maneval D R, Jr, Johnson J A, Kaper J B. A bacteriophage encoding a pathogenicity island, a type-IV pilus and a phage receptor in cholera bacteria. Nature. 1999;399:375–379. doi: 10.1038/20715. [DOI] [PubMed] [Google Scholar]
- 24.Kovach M E, Shaffer M D, Peterson K M. A putative integrase gene defines the distal end of a large cluster of ToxR-regulated colonization genes in Vibrio cholerae. Microbiology. 1996;142:2165–2174. doi: 10.1099/13500872-142-8-2165. [DOI] [PubMed] [Google Scholar]
- 25.Lazar S, Waldor M K. ToxR-independent expression of cholera toxin from the replicative form of CTXΦ. Infect Immun. 1998;66:394–397. doi: 10.1128/iai.66.1.394-397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee F K, Dudas K C, Hanson J A, Nelson M B, LoVerde P T, Apicella M A. The R-type pyocin of Pseudomonas aeruginosa C is a bacteriophage tail-like particle that contains single-stranded DNA. Infect Immun. 1999;67:717–725. doi: 10.1128/iai.67.2.717-725.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin W, Fullner K J, Clayton R, Sexton J A, Rogers M B, Calia K E, Calderwood S B, Fraser C, Mekalanos J J. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc Natl Acad Sci USA. 1999;96:1071–1076. doi: 10.1073/pnas.96.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindsay J A, Ruzin A, Ross H F, Kurepina N, Novick R P. The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus. Mol Microbiol. 1998;29:527–543. doi: 10.1046/j.1365-2958.1998.00947.x. [DOI] [PubMed] [Google Scholar]
- 29.Mekalanos J J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983;35:253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- 30.Mekalanos J J, Rubin E J, Waldor M K. Cholera: molecular basis for emergence and pathogenesis. FEMS Immunol Med Microbiol. 1997;18:241–248. doi: 10.1111/j.1574-695X.1997.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 31.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogg J E, Timme T L, Alemohammad M M. General transduction in Vibrio cholerae. Infect Immun. 1981;31:737–741. doi: 10.1128/iai.31.2.737-741.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearson G D, Woods A, Chiang S L, Mekalanos J J. CTX genetic element encodes a site-specific recombination system and an intestinal colonization factor. Proc Natl Acad Sci USA. 1993;90:3750–3754. doi: 10.1073/pnas.90.8.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Proctor L M. Advances in the study of marine viruses. Microsc Res Tech. 1997;37:136–161. doi: 10.1002/(SICI)1097-0029(19970415)37:2<136::AID-JEMT3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 35.Ramamurthy T, Garg S, Sharma R, Bhattacharya S K, Nair G B, Shimada T, Takeda T, Karasawa T, Kurazano H, Pal A, et al. Emergence of novel strain of Vibrio cholerae with epidemic potential in southern and eastern India. Lancet. 1993;34:703–704. doi: 10.1016/0140-6736(93)90480-5. [DOI] [PubMed] [Google Scholar]
- 36.Rubin E J, Lin W, Mekalanos J J, Waldor M K. Replication and integration of a Vibrio cholerae cryptic plasmid linked to the CTX prophage. Mol Microbiol. 1998;28:1247–1254. doi: 10.1046/j.1365-2958.1998.00889.x. [DOI] [PubMed] [Google Scholar]
- 37.Rubin E J, Waldor M K, Mekalanos J J. Mobile genetic elements and the evolution of new epidemic strains of Vibrio cholerae. In: M. K R, editor. Emerging infections. San Diego, Calif: Academic Press; 1998. pp. 147–161. [Google Scholar]
- 38.Said B, Smith H R, Scotland S M, Rowe B. Detection and differentiation of the gene for toxin co-regulated pili (tcpA) in Vibrio cholerae non-O1 using the polymerase chain reaction. FEMS Microbiol Lett. 1995;125:205–209. doi: 10.1111/j.1574-6968.1995.tb07359.x. [DOI] [PubMed] [Google Scholar]
- 39.Sears C L, Kaper J L. Enteric bacterial toxins: mechanism of action and linkage to intestinal secretion. Microbiol Rev. 1996;60:167–215. doi: 10.1128/mr.60.1.167-215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skorupski K, Taylor R K. Control of the ToxR virulence regulon in Vibrio cholerae by environmental stimuli. Mol Microbiol. 1997;25:1003–1009. doi: 10.1046/j.1365-2958.1997.5481909.x. [DOI] [PubMed] [Google Scholar]
- 41.Stroeher U H, Parasivam G, Dredge B K, Manning P A. Novel Vibrio cholerae O139 genes involved in lipopolysaccharide biosynthesis. J Bacteriol. 1997;179:2740–2747. doi: 10.1128/jb.179.8.2740-2747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor R K, Miller V L, Furlong D B, Mekalanos J J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci USA. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trucksis M, Galen J E, Michalski J, Fasano A, Kaper J B. Accessory cholera enterotoxin (Ace), the third toxin of a Vibrio cholerae virulence cassette. Proc Natl Acad Sci USA. 1993;90:5267–5271. doi: 10.1073/pnas.90.11.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waldor M K, Mekalanos J J. Emergence of a new cholera pandemic: molecular analysis of virulence determinants in Vibrio cholerae O139 and development of a live vaccine prototype. J Infect Dis. 1994;170:278–283. doi: 10.1093/infdis/170.2.278. [DOI] [PubMed] [Google Scholar]
- 45.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 46.Waldor M K, Mekalanos J J. Progress toward live-attenuated cholera vaccines. In: kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines. New York, N.Y: Academic Press, Inc.; 1996. pp. 229–240. [Google Scholar]
- 47.Waldor M K, Rubin E J, Pearson G D, Kimsey H, Mekalanos J J. Regulation, replication, and integration functions of the Vibrio cholerae CTXΦ are encoded by region RS2. Mol Microbiol. 1997;24:917–926. doi: 10.1046/j.1365-2958.1997.3911758.x. [DOI] [PubMed] [Google Scholar]
- 48.Wichels A, Biel S S, Gelderblom H R, Brinkhoff T, Muyzer G, Schutt C. Bacteriophage diversity in the North Sea. Appl Environ Microbiol. 1998;64:4128–4133. doi: 10.1128/aem.64.11.4128-4133.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zinnaka Y, Carpenter C C. An enterotoxin produced by noncholera vibrios. Johns Hopkins Med J. 1972;131:403–411. [PubMed] [Google Scholar]