Abstract
It is well known that, historically, plants have been an important resource of anticancer agents, providing several clinically approved drugs. Numerous preclinical studies have shown a strong anticancer potential of structurally different phytochemicals, including polyphenolic constituents of plants, flavonoids. In this review article, suppressing effects of equol in different carcinogenesis models are unraveled, highlighting the mechanisms involved in these anticancer activities. Among flavonoids, daidzein is a well-known isoflavone occurring in soybeans and soy products. In a certain part of population, this soy isoflavone is decomposed to equol under the action of gut microflora. Somewhat surprisingly, this degradation product has been shown to be more bioactive than its precursor daidzein, revealing a strong and multifaceted anticancer potential. In this way, it is important to bear in mind that the metabolic conversion of plant flavonoids might lead to products that are even more efficient than the parent compounds themselves, definitely deserving further studies.
Keywords: cancer, natural compounds, gut microbial synthesis, plant flavonoids, equol
1. Introduction
During the last decade, the role of gut microbiota in the metabolism of plant food-derived flavonoids has been highlighted [1]. While the structural characteristics and biological effects of many catabolites are still unknown, one of the best-described examples revealing the formation of more active agents within colonic degradation is the conversion of soy isoflavone, daidzein (4′,7-dihydroxyisoflavone). In fact, daidzein is metabolized to equol (4′,7-isoflavandiol) under the action of intestinal microflora; however, the knowledge about the exact bacterial species involved in this process is still limited [2]. Moreover, only about 30% of the Western population and 60% of the Asian population have been shown to be able to produce equol [3], whereas only the S-enantiomeric form of equol is generated within the colonic degradation of daidzein in the human gut [4].
A range of in vitro and in vivo studies have demonstrated numerous important bioactivities of equol in the model systems of several common diseases, including different types of malignancies. Recent studies have indeed shown strong anticancer effects of equol, inhibiting the development of both hormone-dependent malignancies such as breast and prostate tumors [5,6], as well as hormone-independent neoplasms such as gastric cancer [7], hepatocellular carcinoma [8] and non-small cell lung cancer [9]. Different molecular mechanisms behind these effects have been described, including the suppression of proliferation, migration and invasion of malignant cells besides promoting also their apoptotic death, through attacking diverse molecular targets and regulating a variety of cellular signal transduction pathways [6,7,10,11,12]. Several studies have also demonstrated the ability of equol to modulate the hormonal balance by binding to estrogen receptors (ERs), doing it with a higher affinity than its parent compound daidzein [2].
In this review article, the chemistry of equol and its biosynthesis from daidzein are described, discussing also the important biological activities of equol in different types of malignant tissues. Additionally, the synergistic effects of equol with clinically used standard anticancer drugs are under discussion to reveal the possibilities for a reduction in adverse side effects by lowering the doses of chemotherapeutics by co-administering with equol. Finally, the advances of nanotechnology in improving the delivery of equol directly to the malignant sites are presented, opening additional possibilities for decreasing the drug-related toxicities to normal healthy tissues. It is expected that all these efforts together might bring us a step closer to the development of more efficient cancer therapies in the future.
2. Biosynthesis, Absorption to Metabolism
Equol, a potent isoflavone, has steadily been gaining pharmacological importance, owing to the wide range of benefits that it offers. Bacterial daidzein conversion in the intestine yields S-equol, which has been shown to exhibit greater stability, absorbance and a lowered rate of clearance, as compared to its parent drug [2]. This enantiomeric form of the compound has been linked with promising activity on sex-hormone receptors, and the modulatory effects exerted have been related to the amelioration of a variety of metabolic disorders, such as cancer, cardiovascular disease and neurodegenerative conditions [13]. Of all the known isoflavones in nature and their metabolites (Table 1 and Table 2), equol has been observed to have the highest affinity for estrogen receptors [14].
Table 1.
Anticancer effects of daidzein and its metabolites including equol based on in vitro studies.
| Type of Cancer | Cell Lines | Effects | Mechanisms | Concentration | References |
|---|---|---|---|---|---|
| Osteosarcoma | 143B and U2OS | Induces apoptosis | ↓ proliferation and migration of 143B and U2OS osteosarcoma cells, ↑ %age of S phase cells, ↓ %age of G0/G1 phase cells, ↓ p-Src-ERK, ↓ p-Src, ↓ p-ERK, No change in expression levels of Src, JNK, p-JNK, ERK, p38 and p-p38 | Daidzein—0, 10, 20, 50, 100, 200 or 500 µM | [15] |
| MG-63 | Induces apoptosis | ↑ ROS, ↓ mitochondrial membrane potential, ↑ apoptosis rate, ↑ cell cycle arrest at the G2/M phase, ↓ Bcl-2, ↓ Bcl-x and ↓ Baid proteins, ↑ Bim protein | Daidzein—IC50 value of 59.7 µM | [16] | |
| Colon | HT-29 | Induces apoptosis | ↓ growth of cancer cells, significant increase in cells in the G0/G1 phase, ↓ Lipid droplets accumulation, ↓ Perilipin-1, ↓ ADRP and↓ Tip-47 family proteins, ↓ vimentin, ↑ PPAR, ↑ Fas, ↑ FABP, ↑ GPAT3, ↑MTTP, ↓ UCP2. ↓ PI3K, ↑ FOXO3a, ↑ caspase-8 | Genistein and Daidzein—0, 25, 50, 100, 200, and 400 μM | [17] |
| DLD1, HCT15, COLO205, LOVO, SW480 | Induces apoptosis | ↓ growth of HCT-15 cells with the expression of ERα and ERβ, ↓ growth of LOVO, and SW480 cells with the ERβ expression, ↑ ERα and ERβ in HCT-15. ↑ ERα and ERβ, ↑ Nrf2 | Equol—0, 0.5, 1, 5, 10 μM | [18] | |
| Breast | MCF-7 | Induces apoptosis | ↑ % age of apoptotic cells, ↑ Caspase 3/7 activity, ↑ Bax, ↓ Bcl2, ↑ ROS, ↓ ERα, ↑ ERβ | Daidzein—IC50—50 µM | [19] |
| MCF-7 and T47D | Induces apoptosis | ↑ cytotoxic effects towards cancer cells, ↓ NGB, ↑p- AKT, ↑ p38 phosphorylation, ↑ cleaved PARP-1 | Daidzein—1–10 µM and Equol 1 μM | [20] | |
| MCF7 and MCF7/ADR | Enhances the anticancer effect of topotecan (tpt) and reverses BCRP-mediated drug resistance |
↑ anti-proliferative effect with TPT on MCF7 and MCF7/ADR cells, ↑ inhibitory effect of TPT on Topo Ⅰ activity, ↑ inhibition of TPT on the catalytic activity of Topo Ⅰ, ↑ cells arresting at the G2/M phase, ↑ apoptosis rate, ↓ resistance of MCF7/ADR cells to TPT, ↓ ERα and BCRP, ↑ TPT accumulation intracellularly | Daidzein—0, 2.13, 6.25, 12.5, 25, 50, 100, 200 and 400 µM and Topotecan 0, 0.78, 1.56, 3.13, 6.25, 12.5, 25, 50 and 100 µM |
[21] | |
| MCF10DCIS.com | Induces apoptosis | ↓ TNF-α induced cell migration and invasion, no effect on IκBα expression and NF-κB p65 phosphorylation, ↓ Gli1, ↓ MMP-9 |
Daidzein—0, 5, 10, 30 and 50 µM and Equol—10 μM | [22] | |
| MCF-7 | Induces apoptosis | ↓ MCF-7 viability, ↑ %age of apoptotic cells, ↑ % age cells sub-G1 phase, ↓ % age cells in G0/G1, S and G2/M phase, ↑ p53, ↑ p21, ↑ PARP cleavage, ↑ α-fodrin proteolysis, ↑ pro-caspase-7 and pro-caspase-9 cleavage, ↓ Bcl-2, ↑ cytochrome-c release to the cytosol, ↓ Bcl-2: Bax ratio, ↑ tamoxifen’s anti-tumor activity | Equol—0, 25, 50 and 100 μM and 4-OHT 0, 0.01, 0.1, 1.0, 10.0 μM | [23] | |
| MCF-7 and MDA-MB-453 | Induces apoptosis | ↓ cell proliferation of cancer cells, ↑ cell cycle arrest in the G1 and G2/M phases, ↑ %age cells in sub-G0 phase, ↓ cyclin D, ↓ CDK2, ↓ CDK4, No Change in the expression of CDK6 and cyclin E, ↓ CDK 1, ↑ p21Cip1 and ↑ p57Kip2, No change in p27Kip1 | Daidzein—1–100 μM | [24] | |
| MCF-7/MDA MB-231 | Induces apoptosis | ↓ viability of MCF-7 and MDA MB-231 cell lines, no significant growth inhibition was observed in MCF-10A cells, ↑ no of rounded cells due to shrinkage and condensation of cytoplasm, ↑ apoptotic cells, ↑ tunnel +live cells, ↑ ROS, ↓ ∆ψm, ↓ Bcl-xL, ↑ BAX, ↑ Caspase 3/7/9, ↑ cleaved PARP, ↓ PI3K, ↓ p-Akt, ↓ p-mTOR, ↑ affectivity of Centchroman | Centchroman—1–30 µM and Daidzein 10–200 µM | [25] | |
| MCF-7 and MDA-MB-231 | Induces apoptosis | ↑ MRP2, ↓ MRP1, ↓ ABCC2 and ABCC1 mRNA | Daidzein—0.05, 0.5 and 5 µM, R-equol and S-equol—0.1, 1 and 10 µM | [26] | |
| MCF-7 | Enhances apoptosis-inducing activity of genistein | ↑ cytotoxicity of genistein, ↑ G2/M phase cells, ↓ G1/S blockade and G2/M progression ↑ sub-G0/G1 population ↑ apoptosis rate, ↑ Bax/Bcl-xL expression ratio, No change in activities of Akt and mTOR, ↑ c-PARP | Genistein—0–100 µM, Equol—0–100 µM | [27] | |
| MDA-MB-435 (ER) | Induces apoptosis | ↑ eIF4GI, ↑ c-Myc, ↑ Cyclin D ↑Bcl-XL ↑ p120 catenin | (R, S) Equol—25 μM | [28] | |
| MDA-MB-231 | Inhibit metastasis | ↓ invasive capacity, ↓ MMP-2, No Change on n the expression levels of MMP- 9, TIMP-1 or TIMP-2 | Daidzein, R—and S-Equol—0, 2.5, 10, 50 µM |
[29] | |
| MCF-7 | Induces apoptosis | ↑ ROS, ↓ Bcl-2, ↑ Bax, ↑ release of cytochrome C from the mitochondria into the cytosol, ↑ caspase-9, ↑ caspase-7 | Daidzein—25–100 µM | [30] | |
| MCF-7 | -- | ↑ antiproliferative effects, ↑ pS2 mRNA | Daidzein and (±)-equol 0.001 to 50 µM | [31] | |
| Lung | A594 and 95D | Induces apoptosis | ↓ proliferation and colony formation property of cancer cells ↓ IL-6, ↓IL-8, ↓ p65-NFκB expression and activation, ↓ level of p65-NFκB upregulation induced by C/EBPβ | Daidzein—0, 5, 10, and 25 μM | [32] |
| A549, HepG-2 | Induces apoptosis | ↑ ROS, ↓ mitochondrial membrane potential, ↑ apoptosis rate, ↑ cell cycle arrest at the G2/M phase, ↓ Bcl-2, ↓ Bcl-x and ↓ Baid proteins, ↑ Bim protein | Daidzein—IC50 value of 59.7 µM | [33] | |
| Gastric | MGC-803 | Induces apoptosis | ↓ viability of MGC-803 cells, ↑ G0/G1 cell cycle arrest, ↓ CDK2/4, ↓ Cyclin D1/Cyclin E1 ↑ P21WAF1, ↑ apoptosis frequency, ↑ cleaved PARP, ↑ caspase-3. ↑ P-Akt (Ser473 and Thr308) |
Equol—5, 10, 20, 40, or 80 μM | [34] |
| BGC-823 | Induces apoptosis | ↓ growth and proliferation of gastric carcinoma cells, ↓ mitochondrial transmembrane potential ↑ cleaved PARP, ↑ cleaved caspase-9, ↑ cleaved caspase-3, ↑ Bax, ↓ Bcl-2, ↓ Bcl-2/Bax |
Daidzein—0, 20, 40, and 80 µM | [35] | |
| Hepatocellular | SMMC-7721 and HepG2 | Induces apoptosis | ↓ proliferation, migration and invasion of cancer cells, ↓ concentrations of pyruvate, glutamate and glucose, ↓ activities of hexokinase, phosphofructokinase and pyruvate kinase, ↓ pyruvate kinase M2, ↑ levels of glycerophosphocholine, ethanolamine, taurine, fumarate, leucine, acetate, ↓ levels of pyruvate, glutamate, glutamine, adenosine monophosphate, creatine, glycine |
(−)—5-hydroxy Equol—0, 10, 20, 30, 40 and 50 µM | [36] |
| SMMC-7721 | Induces apoptosis | ↓ proliferation of SMMC-7721 cells, ↑ apoptosis frequency, ↑ S-phase cell cycle arrest, ↑ p21, ↓ cyclin A2; No change in expression of cyclin D1 and H2AX, ↑ caspase-9, ↑ caspase-3, ↑ c-PARP, ↑ Bax ↓ Bcl-2, ↑ caspase-8, ↑ caspase-12, ↑ Chop, ↑ Bip | (±)—Equol, R-(+)-Equol, and S-(–)-Equol—0, 5, 10, 20, 50, and 100 µM | [37] | |
| SK-HEP-1 | Induces apoptosis | ↓ cell proliferation of cancer cells, ↑ Prdx-3, ↑ Bak, ↓ Bcl-2, ↓ Bcl-xL, ↑ release of mitochondrial cytochrome c to cytosol, ↑ APAF-1, ↑ caspase 9, ↑ caspase 3 | Daidzein—0, 200, 400 and 600 µM | [38] | |
| Pancreatic | MiaPaCa-2 and PANC-1 | Induces apoptosis | ↓ growth and proliferation of pancreatic cancer cells, inhibitory effects on both ER positive and negative pancreatic cancer cells | Daidzein—0.1, 1, 10, 25, 50, 75 and 100 µmol/L |
[39] |
| Colorectal | SW620 | Anti-proliferative effects | ↓ p-ERK/ERK, ↓ p-AKT/AKT | Chrysin IC50 values 70 µM and Daidzein IC50 values 23.5 µM | [40] |
| HCT-15 | Induces apoptosis | Racemic equol ↓ proliferation of HCT-15 cells, whereas(S) equol had no effect on the proliferation of HCT-15 cells. Racemic equol ↓ ERβ and ↓ Nrf2, while (R) equol ↓ Nrf2 | Racemic equol and equol enantiomers—0, 0. 5, 1, 5 and 10 μM | [41] | |
| Bladder | RT112, RT4 and SW780 | Induces apoptosis | ↓ cell viability, Impaired colony formation, ↑ G1/S cell cycle arrest, ↑ apoptosis frequency, ↓ FGFR3 signaling pathway, ↓ p-FGFR3, ↓ p-Akt, p-ERK | Daidzein—0, 0.5, 1, 2.5, 5, 7.5, 10, 50 and 100 μM | [42] |
| Prostate | DU145, LNCaP and PC3 | Induces apoptosis | ↑ cytotoxic activity, ↑ ERβ binding activity, ↑ ERβ gene expression, ↓ cMYC, ↓ Cyclin D1 genes, ↑ caspase 3 and 9, No change in uterotropic and anti-androgenic activities | Novel daidzein molecules—1, 5, 10, 50, 100, 200, 300, 400, 500 µM |
[43] |
| LNCaP, DU145 and PC3 | Induces apoptosis | ↑ cell cycle arrest in the G2/M phase↓ Cyclin B1 ↓ CDK1, ↑ p21 and p27, ↑ apoptosis rate, ↑ FasL ↑ Bim. ↑ FOXO3a, ↓ p-FOXO3a, ↑ nuclear stability of FOXO3a, ↓ MDM2 | S-Equol—0, 0.5, 1, 5, 10 μM | [44] | |
| DU145 | Induces apoptosis | ↓ cell migration and invasion, ↓ MMP-, ↓ u-PA, ↓ secreted MMP-2 and MMP-9, ↑ SOD, ↑ Nrf2, ↑ PTEN | (±) Equol 5, 10, 50 µM, Daidzein and Genistein—0.5, 1 and 5 µM |
[45] | |
| PC3, DU145 cells | Induces apoptosis | ↓ MMP-2, ↓ MMP-9, ↑ ERγ, No change in Erβ | Equol—0, 0.5, 1, 5, 10 μM | [46] | |
| Choriocarcinoma | JAR and JEG-3 | Induces apoptosis | ↓ cell viability, ↑ early and late apoptotic cells, ↑ apoptosis frequency, ↑ caspase-9, ↑ caspase-3, ↑ c-PARP, ↓ Bcl-2/Bax | Daidzein—0, 25, 50 or 100 µM | [29] |
| Cervix | BEL-7402, HeLa, | Induces apoptosis | ↑ ROS, ↓ mitochondrial membrane potential, ↑ apoptosis rate, ↑ cell cycle arrest at the G2/M phase, ↓ Bcl-2, ↓ Bcl-x and ↓ Baid proteins, ↑ Bim protein | Daidzein—IC50 value of 59.7 µM | [47] |
| Ovarian | caov-3, OVAcAR-3, SKOV3 and A2780 |
Induces apoptosis | ↑ antiproliferative effects on SKVO3 cells, SKOV3 cancer cells became rounder, shrunken and detached from the substratum, ↑ apoptotic cells, ↑ release of cytochrome c into the cytoplasm, ↑ cytosolic levels of cyt c, ↑ Bax, ↑ cleaved caspase-3 and -9, ↑ cleaved PARP, ↑ G2 phase cells leading to G2/M cell cycle phase arrest, ↓ pcdc25c (Ser216), ↓ cdc25c, ↓ pcdc2 (Tyr15), ↓ cdc2, ↓ cyclin B1, ↑ p21, ↓ migratory capability of cancer cells, ↓ MMP-9, ↓ MMP-2, ↓ p-MEK, ↓ p-ERK |
Daidzein—0, 10, 20 and 40 µM |
[48] |
Table 2.
Anticancer effects of daidzein and its metabolites including equol based on in vivo studies.
| Type of Cancer | Animal Models | Effects | Mechanisms | Dosage | Duration | References |
|---|---|---|---|---|---|---|
| Osteosarcoma | BALB/c nude mice xenografted with 143B (1 × 107) cells | Inhibited Tumor Growth | ↓ volume and weight of the tumors, ↑ number of necrotic cells, no systemic toxicity | Daidzein—20 mg/kg | 16 days | [15] |
| Breast | Athymic nude mice xenografted with MCF7 cells (5 × 106 cells) | Inhibited Tumor Growth | No obvious damage found in visceral organs of MCF7 xenograft nude mice, ↓ tumor volume, ↑ tumor inhibition rate of the combination group, ↑ Bax, ↑ p53 and ↑ p21 in the combination group than in the TPT monotherapy group, ↓ Bcl2 |
Topotecan—3 mg/ kg and Daidzein—5 mg/kg. |
15 days | [21] |
| MCF-7 cells implanted in ovariectomized athymic mice |
Inhibited Tumor Growth | No significant difference was observed in uterine weight, No significant induction of pS2 mRNA (an estrogen responsive marker) in tumors |
Daidzein—125, 250, 500 and 1000 p.p.m and (±)-Equol—250, 500 and 1000 p.p.m |
7 days | [31] | |
| Lung | Balb/c nude mice xenografted with A549 cells |
Inhibited Tumor Growth | ↓ Ki-67 and ↓ p65-NF-κB, ↓ tumor volume | Daidzein—5 mg/kg. | 21 days | [32] |
| Bladder | Nude mice xenografted with RT112 cells (1 × 106 cells) | Inhibited Tumor Growth | ↓ tumor volume and weight and size, ↓ tumor volume toxicity of daidzein to normal cells, | Daidzein—10 mg/kg and 20 mg/kg | 27 days | [42] |
| Colorectal | Albino rats subcutaneous injected with DMH (40 mg/kg) | Inhibited Tumor Growth | ↓ NO, ↓ MDA, ↑ GSH, ↓ CYP2E1 colon content, ↓ CXCL1, ↓ AREG level, ↓ colon content of MMP-9↓ DMH+DSS induced histopathological changes at both doses | Chrysin—125 and 250 mg/kg and Daidzein—5 and 10 mg/kg |
56 days | [40] |
| Prostate | C57B1/6 male mice xenografted with PC3 cell (5 × 104) | Inhibited Tumor Growth | ↑ doxorubicin anti-tumor activity, ↑ number of necrotic cells, shows hyperplastic acini lined by simple columnar epithelium and basal cells, ↑ recovery from prostate cancer | The novel metabolites 1 and 2–30 mg/ kg and Doxorubicin—6 mg/kg |
21 days | [43] |
| BALB/c nude mice xenografted with PC3 cells | Inhibited Tumor Growth | ↓ volume and weight of the tumors, ↑ no. number of necrotic cells, ↓ p-FOXO3a and ↑ nuclear stability of FOXO3a | Daidzein | -- | [44] | |
| Ovary | Nude mice xenografted with SKVO3 cells (5 × 106 cells). | Inhibited Tumor Growth | ↓ Ki-67 ↑ cleaved caspase-3 | Daidzein—10, 20 and 40 µg/kg |
27 days | [48] |
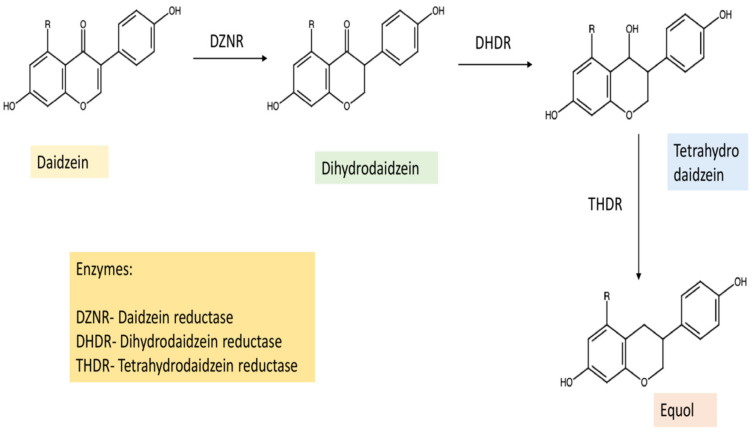
An increased focus on research has led to the discovery of a number of bacterial strains capable of equol biosynthesis, primarily concentrated in the intestinal microbiota. Most of these microorganisms are anaerobic in nature, belonging to the family Eggerthellaceae. Dihydrodaidzein and tetrahydrodaidzein are produced as intermediates in the process of synthesis, and the reactions are mediated by reductase enzymes, primarily occurring in oxygen-susceptible bacteria [49]. These include daidzein reductase, dihydrodaidzein reductase, tetrahydrodaidzein reductase and dihydrodaidzein reductase (linked with an increase in equol biosynthesis). In addition to this, related organisms belonging to the class Coriobacteria have been linked with equol production as well, and four key enzymes have been identified, as aforementioned [50]. The biosynthetic pathway may be summarized as shown in Figure 1.
Figure 1.
Diagrammatic representation of equol biosynthesis with the enzymes involved.
A study conducted by [51] evaluated the effect of the administration of HXMB408 bacteria solution on daidzein metabolism, and the subsequent equol production, using a rat model. The results from the experiment indicated an increase in equol production in the test group as compared to the control group (both administered with daidzein), signifying a degradative role of bacteria on isoflavones. An improved microbiome environment, which is microbially diverse, has been linked with improved metabolite production [51].
An interesting trial conducted by [52] aimed to establish a relationship between the equol production in children and its link with maternal equol production, and it was observed that a child’s equol production status (producer/non-producer) bears a direct link with the mother’s status, as opposed to other factors such as dietary intake or excreted levels of the metabolite [52].
In a study undertaken by [53], the metabolism of daidzein was explored using an in silico and physiologic based model. The fecal samples used to mimic the gut microbiome as well as the rat model employed indicated the potent estrogenic nature of S-equol; however, lower plasma concentrations than the parent isoflavone were observed. This may vary on the basis of soy supplementation as well as isoflavone content in different types of diet, indicating the relation between dietary supplementation and equol production [53]. In a separate comparative study between equol producers and non-producers, it was observed that an increase in daidzein intake was linked with an increased level of Assaccharobacter celatus and Slackia isoflavoniconvertens, which are gut microbes associated with equol production [54], further supplementing the influence of dietary intake.
As we continue to discover the intestinal microbes responsible for equol production, it is important to note that inter-individual differences account for variation in the composition of the intestinal microbiota [55]. Owing to this, only about one-third of the world’s population is capable of equol biosynthesis, by the metabolism of daidzein [56] Advancements in biotechnological research would help in designing technological strategies to enhance the output and provide a sustainable source for equol to keep pace with its growing therapeutic benefits in biological systems.
3. Structure-Activity Relationship of Equol
In addition to being distinguished on the basis of a 3-phenylchromone structure, isoflavones are further classified as aglycones and glycosides—daidzein belonging to the former category. The affinity of isoflavones to estrogen receptors may be attributed to their structural similarity to estrogen [57]. A structure activity relationship (SAR) study conducted by Cho et al. [58] revealed that an increased saturation of the C-ring in the structure of isoflavones enhanced their ERβ selectivity, enhancing transactivation and exerting a neuroprotective effect. Among the metabolites of daidzein, equol and dehydroequol were observed to exert the most potent neuroprotective effect, and it was revealed that minor modifications to the C-ring could help to alter the activity of these compounds, which may be used in the synthesis of anti-inflammatory analogues for the management of a variety of metabolic conditions [58]. Equol is an optically active molecule, owing to its non-planar structure, and the two phenolic groups present in its structure may be potential sites for tyrosinase activity, yielding quinones. These oxidation products have been observed to exert pro-oxidant activity [59].
4. Equol Production at a Laboratory and Industrial Scale
The industrial scaling-up of equol production has been limited due to the oxygen susceptibility and fastidiousness of the biosynthetic bacteria [60]. However, researchers have attempted to overcome this drawback by carrying out the cloning of equol-producing genes in certain microbes and expressed them in host organisms. A study by Vázquez et al. [61] explored the genes coding for major enzymes involved in equol production, in Adlercreutzia equolifaciens DSM19450T. This was followed by the cloning of the genes and expression in Escherichia coli hosts, offering an avenue for biotechnological advancement [61]. In addition to this, Lactobacillus intestinalis has been observed to effectively yield equol from daidzein, as it expresses the genes of the enzymes required in its production [62].
As previously discussed, the main barriers to scaling-up and the industrial production of equol include bacterial constraints; however, advances have led to the exploration of alternate strategies for equol production. In a study conducted by Kawada et al. [63], it was observed that a cluster of three genes is associated with the conversion of daidzein to S-equol in Eggerthella sp. YY7918, a bacterium found in human feces. It was observed that the gene sequence responsible for production was similar to that of Lactococcus, indicating the potential to explore other species for the industrial production of equol. A study conducted by Mustafa et al. [64] highlighted the effects of various physiological conditions such as pH, temperature, and inulin concentration on the production of isoflavones in soymilk by Bifidiobacterium sp.; temperature was found to be a key factor influencing daidzein production. This may be indicative of the fact that the optimization of temperature conditions in industrial settings may enhance isoflavone production. Drawbacks of traditional production techniques include harnessing non-productive strains found intestinally; however, the development of recombinant strains has paved the way for the effective aerobic production of S-equol. Although a major drawback included a limited yield owing to limited isoflavone solubility, a study undertaken by Lee et al. [65] explored the utilization of hydrophilic polymers and polar aprotic solvents to considerably improve the solubility of these compounds. This indicates that the inclusion of these compounds may enhance the production of these compounds and improve the outcomes on an industrial scale.
5. Equol as a Potent Anticancer Agent
5.1. Apoptotic and Cell Cycle Arrest Mechanisms
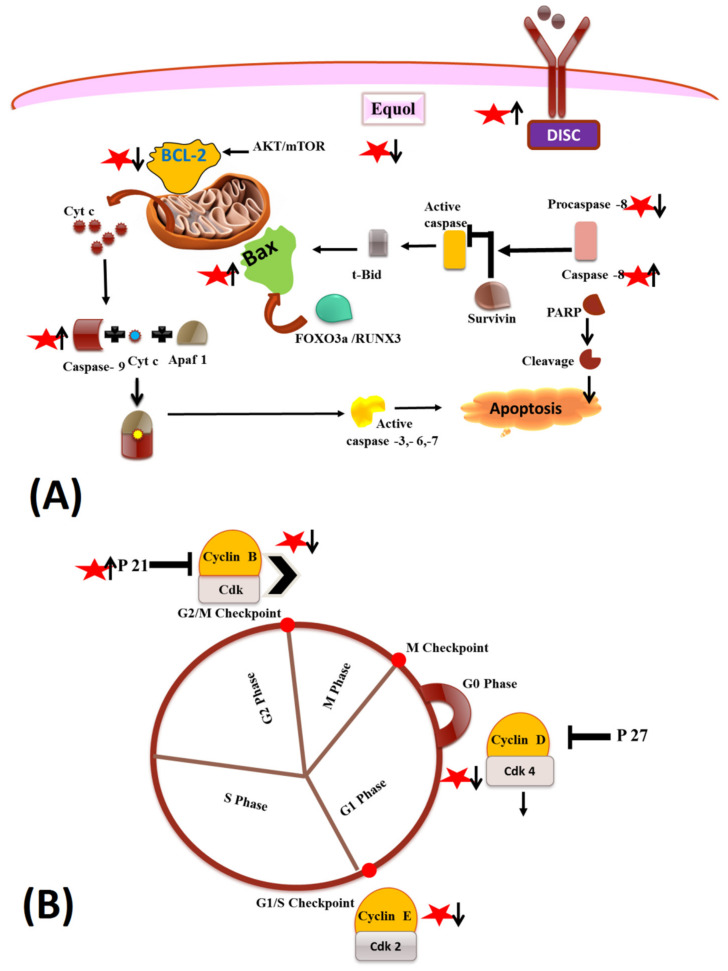
Equol is being consumed and marketed as a dietary nutraceutical agent with promising anticancer activities [66,67,68]. Numerous studies have highlighted the protective role of equol in different hormone-dependent and -independent cancer cells, such as breast, gastric, prostate cancers, etc. Studies have attributed the anti-cancerous properties of equol via its apoptotic and cell cycle arrest mechanisms. Apoptosis, being a fundamental process, is essential for normal development and in the maintenance of tissue homeostasis [69,70]. Studies have shown that targeting different signaling intermediates in apoptosis-inducing pathways may prove beneficial in cancer prevention and therapy [71]. Different studies have depicted the apoptotic and anti-proliferative properties of equol alone or in combination with available anticancer drugs/herbal formulations in in vitro and in vivo models of cancer [71,72]. Equol has been shown to induce apoptosis via both extrinsic and intrinsic pathways of apoptosis involving different biological mechanisms in divergent cancer models; further equol was shown to arrest cell cycle progression via the involvement of different cell cycle check points (Figure 2). The phytohormone property of equol has proved to be beneficial in many in vitro breast cancer studies as it is reported to bind to both estrogen receptors i.e., ERα and ERß, and is implicated in the inhibition of proliferation and induction of apoptosis in breast cancer cells. Further studies have also reported that equol induces apoptosis in ER-negative breast cancer cells [69,71,72,73]. In a study, [23] delved deep into the cytotoxic effect of equol in MCF-7 breast cancer cells, delineating the apoptotic pathways and proteins involved. The study depicted the involvement of cleaved caspases-9,7 and the subsequent release of cytochrome C into the cytosol and its subsequent action on cytosolic targets, thereby inducing apoptosis. Moreover, the combined effect of equol and tamoxifen induced a time-dependent reduction in bcl-2 expression, thereby the bcl-2: bax ratio was reduced by the combination of the two compounds. These findings are consistent with previous studies on breast cancer cell lines. Various studies have also reported the role of the equol-mediated intrinsic pathway of apoptosis in different in vivo and in vitro cancer models [23,74]. For instance, in vitro studies have depicted the inhibitory role of equol in a time- and dose-dependent manner to arrest cell cycles [75]. On human gastric carcinoma cells MGC-803, the role of equol was elucidated in inhibiting cell cycle proliferation at the G0/G1 phase by regulating CDK2/4, Cyclin D1/E1, and P21 expression. Further, equol induced apoptosis in the cells via the cleavage of PARP and caspase-3. The study also depicted the role of AKT-mediated cell cycle arrest and apoptosis. Several studies have shown the effect of different isoflavones and equol in regulating cell cycles via reducing the activity of the Cyclin B/CDK complex, thereby inhibiting cell proliferation. In different prostate cancer in vitro studies, equol was associated with the activation of FOXO3a, one of the forkhead-family factors of transcription involved in apoptosis via the protein kinase B (Akt)-specific signaling pathway [76]. The studies available on the role of equol in inducing apoptosis and cell cycle arrest on different types of cancer cells are promising but limited; there is a requirement of more delineated and elucidated investigations involving commonly occurring cancers and at different doses of equol alone or in combination with the commonly available chemotherapeutic agents are needed along with the approach of translational research from bench side to bed side [77,78,79,80].
Figure 2.
(A) The effect of equol (represented as red star) on apoptosis. (B) The effect of equol on regulation of cell cycle progression.
5.2. Antiestrogenic Action
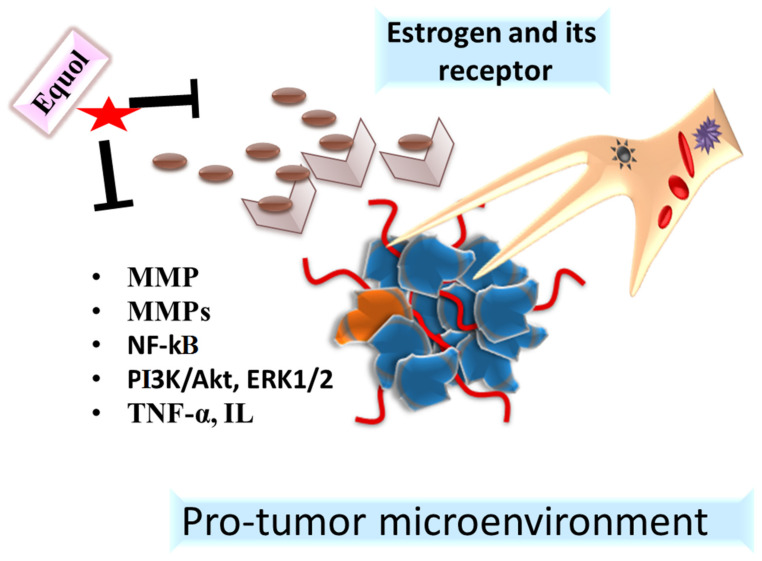
Estrogens are the hormones that play an important role in the development of the female reproductive system as well as secondary sex characters. However, when there is an excessive production of estrogens in the body, it may lead to problems such as fibroids, weight gain, and breast cancer. The excessive estrogen levels are known to cause interference with the DNA, leading to the development of cancerous cells [81,82]. Unbalanced production of estrogens may lead to the formation of catechol estrogens (4-hydroxyestrone, 4-hydroxyestradiol, 2-hydroxyestrone, and 2-hydroxyestradiol) and 16α-hydroxylation to 16α-hydroxyestrone [81,83]. The oxidation of 4-hydroxyestrone and 4-hydroxyestradiol to estrone-3,4-quinone and estradiol-3,4-quinone leads to the formation of depurinating adducts by interacting with the host DNA, which in turn leads to the generation of apurinic sites. These apurinic sites then lead to the error-prone base excision repair-causing mutations in the DNA, resulting in the onset of prostate and breast cancers [83]. However, anti-estrogenic metabolites such as equol have been known to reduce the risk of development of cancer by metabolizing the estrogens and facilitating their excretion through urine [84,85]. Equol is an isoflavone metabolite that is produced by the gut bacteria as a result of soy protein digestion [86,87]. However, the mechanism how its production differs in individuals is not understood completely [88]. It exhibits its anti-estrogenic activity by binding to the α and β estrogen receptors [84] and is known to provide relief from menopausal symptoms such as hot flashes [88,89]. In addition, the binding of equol with the ER receptor has been linked to the reduction in breast cancer cell growth and the activation of apoptosis. Lathrop et al., 2020, reported S-equol as a well-tolerated oral ER-Beta agonist in patients with triple negative breast cancer (TNBC), and inhibited proliferation via the downregulation of nucleoprotein Ki-67 [90]. The endogenously produced equol differs from the exogenously produced one as the gut bacteria produce S-equol, whereas the synthetically produced equol is the racemic R/S-(±) mixture [91]. The enantiomers of equol are distinguished based on their binding mechanism to estrogen receptors, where S-equol binds to α estrogen receptors and R-equol binds to β estrogen receptors [92,93]. In a study, the effect of R/S-(±) equol, S-equol, and soy isoflavone was explored on the reproductive tract of adult apoE-null mice population. It was found that an anti-estrogenic effect was observed in mice administrated with both R/S-(±) equol and S-equol, irrespective of the soy isoflavone dosage [88]. It was concluded from this work that the equol production in the mice was dependent on the gut bacteria rather than the soy isoflavone-dependent diet. However, in humans, the anti-estrogenic effect of equol was dependent on the soy consumed in the diet [94,95,96]. Thus, equol can be used for reducing the risk of development of various cancers such as breast cancer due to its anti-estrogenic properties (Figure 3).
Figure 3.
Anticancer action of equol through modulating its binding to estrogen receptors. Red  star indicated the Equol.
star indicated the Equol.
5.3. Anti-Angiogenic and Anti-Metastasis Activities
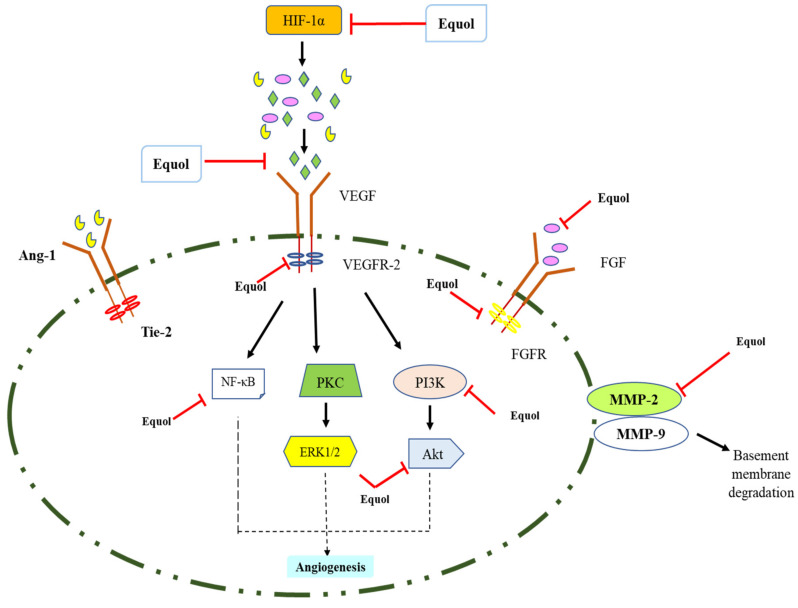
Cancer is one of the prime health issues in the world, requiring an effective strategy to battle its rising incidence and mortality rates [97]. In all forms of malignancy, there is a higher invasive potential and poor mortality relating to angiogenesis and metastasis [12]. The main characteristics of cancer are changes in vascular architecture and uncontrolled angiogenesis. Angiogenesis is the process of forming new blood vessels from existing ones, which is regulated by positive (angiogenic) and negative (anti-angiogenic) endogenous factors such as adult endothelial cells (ECs) [98]. Angiogenesis is linked to the development of numerous illnesses and plays an important part in normal physiological activities such as embryo development, wound healing, and the menstrual cycle [99]. The dysregulation of angiogenesis is widely established to cause the onset and progression of a variety of illnesses, including rheumatoid arthritis, psoriasis, diabetic retinopathy, malignant tumors, and age-related macular degeneration (AMD) [100]. Angiogenesis is essential for the beginning, growth, and spread of malignancies because tumor cells require oxygen, nutrition, and growth factors for proliferation and development [101]. Tumors maintain blood supply by generating chemical signals that cause angiogenesis to occur [102]. A vital equilibrium between numerous pro-angiogenic and anti-angiogenic factors is required for angiogenesis, and a shift in this balance can result in pro- or anti-angiogenic consequences [103]. Inflammation, ischemia, hypoxia, and other circumstances that act on cytokines, as well as angiogenic factors such as vascular endothelial growth factor (VEGF) or fibroblast growth factor (FGF) generated by tumors and other defective cells, can all trigger angiogenesis [104]. Pro-angiogenic factors such as vascular endothelial growth factor-A (VEGF-A), basic fibroblast growth factor (bFGF), thymidine phosphorylase (TP), metalloproteinase-9 (MMP-9), urokinase-type plasminogen activator (uPA), and adrenomedullin (ADM) have the main roles in angiogenesis (Figure 4) [105]. As a result, inhibiting angiogenesis appears to be a promising therapeutic strategy for the treatment of a variety of disorders, including cancer [106]. Although anti-angiogenic medications such as bevacizumab, pegaptanib, ranibizumab, sunitinib, sorafenib, regorafenib, and axitinib are available, they all have substantial side effects such as cardiovascular toxicity, bleeding risk, intraocular inflammation, ocular hemorrhage, and retinal detachment [107]. As a result, novel and effective medicines targeting angiogenesis with fewer adverse effects that complement and can be coupled with existing medications are needed to be developed/explored [108].
Figure 4.
Major signaling pathways targeted by equol in angiogenesis and metastasis processes.
Researchers have focused on potent, naturally occurring anti-angiogenic molecules because these inexpensive constituents have minimal toxicity—they have been used as a natural remedy for centuries in different parts of the world for curing various disorders [109]. Among the broad spectrum of natural products, soybean is one of them and daidzein is one of the potent isoflavones present in it. Daidzein can be metabolized by specific flora in the gut to produce equol [110]. Equol is a bioactive metabolite of daidzein with higher biological activity than daidzein. Equol has numerous pharmacological activities such as anticancer, neuroprotective, and anti-angiogenesis [111]. Bellou et al. [112] reported that 6-methoxyequol (6-ME) reduced the growth of human endothelial cells from umbilical vein (HUVEC) by downregulating the expression of VEGF- and FGF2 through the phosphorylation of MEK1/2 and ERK1/2 via the mitogenic MAPK pathway. 6-ME significantly reduced neovascularization and tumor volume in BALB/c nude mice A-431 xenograft tumors, showing that its anti-angiogenic potential is useful in cancer chemoprevention. Ref. [113] investigated that equol-treated MGC-803 cells revealed a significant downregulation of Ki67, CDK2/4 and Cyclin D1/Cyclin E1, whereas the upregulation of P21WAF1, PARP, caspase-3, and P-Akt (Ser473 and Thr308) led to arresting cells at G0/G1 phase, showing its anticancer and apoptosis-inducing potential. Ref. [23] reported that the combination of equol and tamoxifen significantly inhibited the proliferation of MCF-7 cells and induced apoptosis in MCF-7 more efficiently than each compound alone. The combination treatment in MCF-7 cells significantly upregulated the levels of PARP, caspase-9, caspase-7, and α-fodrin cleavage, and downregulated the bcl-2: bax ratio, showing the apoptosis-inducing potential of equol and tamoxifen. Ref. [114] investigated that equol and daidzein significantly inhibited the migration and invasion of prostate cancer cell lines (DU-145 and PC-3) by downregulating the MMP-2 and MMP-9 expression. Therefore, these results suggest that equol could inhibit the pathway of angiogenesis via associated signaling markers and might serve as a therapeutically potent antitumor agent.
5.4. Antioxidant and Anti-Inflammatory Effects of Equol
Equol has attracted the attention of many researchers due to its substantial antioxidant properties; its strong antioxidant effects have been proven in many experimental models [115]. Comparative studies with many compounds that are accepted to have antioxidant properties have shown that equol is a superior antioxidant and has a higher antioxidant capacity than antioxidant compounds such as vitamin C, vitamin E, quercetin, ascorbic acid, and genistein [116]. It has been determined that the macrophage-protective activity of equol against oxidative stress is achieved by decreasing the lipid peroxidation product malondialdehyde (MDA), increasing the activities of the antioxidant enzymes superoxide dismutase (SOD) and glutathione, and suppressing the effect of L-lactate dehydrogenase or oxLDL [117]. There are also many papers about equol showing antioxidant properties in other tissues and cells. For example, equol protects rats against focal cerebral ischemia by significantly reducing oxidative stress, and the other studies provided that equol causes an increase in SOD, catalase, acetylcholinesterase and glutathione peroxidase activities, and decreases MDA levels and superoxide production in mice [118,119,120]. In a paper examining the molecular mechanism of antioxidant activity of equol, it has been indicated that equol inhibits activator protein 1 (AP-1), a substantial molecule of the oxidative stress chain, and thus, exerts an anti-tumor effect by suppressing the cell transformation directed by the MEK signaling pathway [121,122]. Moreover, it has been reported that intracellular superoxide anion and hydrogen peroxide production is decreased by equol in phagocytic cells, toxic effects caused by increased ROS in neutrophils are eliminated, and the phosphorylation of NADPH oxidase regulatory proteins is decreased by equol [123,124]. Additionally, numerous studies offered that equol supports the expression of antioxidant genes, increases nuclear factor erythroid 2 (Nrf2) transcripts, increases SOD activities, shows protective activity against hydrogen peroxide-induced cell death, and prevents oxidative stress-induced apoptosis and vascular damage in epithelial and endothelial cells [125,126,127,128]. In primary cortical neuron cells, it was determined that equol decreased the amount of the reduced/oxidized glutathione [129].
The data obtained as a result of numerous studies have shown that continued oxidative stress causes chronic inflammation, resulting in many diseases, including cancers [130,131,132]. Known as a pro-inflammatory factor expressed in all cell types, NF-κB is involved in the oxidative stress mechanism by regulating the expression of many genes playing substantial roles in the pathology of inflammatory diseases [133,134]. Equol has been identified as a NF-κB inhibitor (Figure 5) along with the down-regulation of the production of various kinds of cytokines (e.g., TNF-α, IL-1, IL-6 and IL-8) and inflammatory biomarkers (e.g., prostaglandin E2, COX-1 and MCP-1) in macrophages, and suppresses the increase in free radicals such as nitric oxide [135,136,137,138,139]. Additionally, it was determined that equol suppresses the release of IL-6 and TNF-α in microglia cells and mediates the inhibition of MAPK, NF-κB, and Toll-like receptor 4 [140,141]. It has been also reported that equol decreases NO synthase expression and nitric oxide production in astrocytes [142]. On the other hand, there are published papers about the anti-inflammatory activities of the isomers of equol named racemic equol, S-equol, and R-equol; the studies indicate that S-equol is less potent on inflammation than racemic and R-equol, of which inhibit the inflammatory factors such as IL-1, IL-6 and COX-1 expressions [135,143]. Consequently, equol appears to have significant potential as an antioxidant and anti-inflammatory natural isoflavandiol estrogen, and more studies are clearly needed to gain a detailed understanding of the molecular targets and molecular activity mechanisms of equol and its isomers in oxidative stress formations and inflammatory processes.
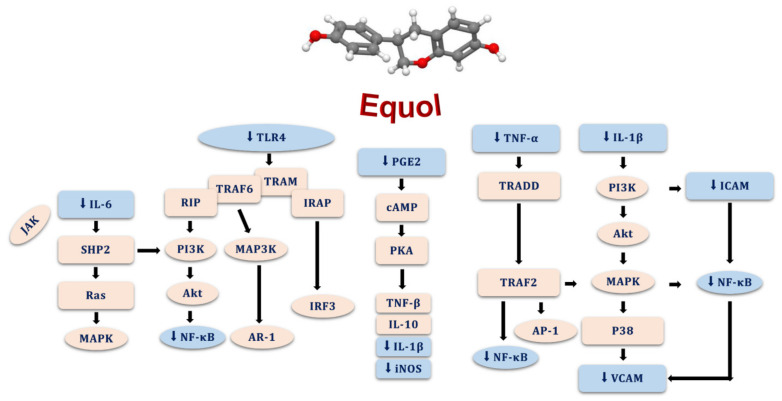
Figure 5.
Anti-inflammatory activity mechanisms of equol. Akt: protein kinase B, AP: activator protein, AR: androgen receptor, cAMP: cyclic adenosine monophosphate, ICAM: intercellular adhesion molecule, IL: interleukin, IRAP: insulin-responsive aminopeptidase, IRF: IFN regulatory factor, iNOS: nitric oxide system, JAK: janus kinase, MAPK or P38: mitogen-activated protein kinase, NF-κB: nuclear factor kappa B, PGE2: prostaglandin E2, PI3K: phosphoinositide 3-kinase, PKA: protein kinase A, SHP: Src homology-2 domain-containing protein tyrosine phosphatase, TLR: Toll-like receptor, TNF: tumor necrosis factor, TRADD: TNFR1-associated death domain protein, TRAF: TNFR-associated factor, TRAM: TRIF-related adaptor molecule, VCAM: vascular cell adhesion molecule.
6. Synergism with Anti-Cancer Agents
The effectiveness of therapeutic anticancer treatments is currently hampered by the prevalent incidence of multidrug resistance. Combinatorial therapy has, therefore, received a lot of interest in the field of cancer therapy, with researchers trying to understand how a combination of anticancer drugs might behave either additively or synergistically to offer increased antitumor activity at lower doses than single-drug therapy. Recent advances in medical research have made synergy assessments a crucial area for improving therapeutic efficacy and influencing multiple targets in addition to just one. Equol has shown promising anticancer capabilities, and various studies have been carried out to assess its potential to be combined with other chemotherapeutic drugs to provide synergistic effects.
Kim and Kim demonstrated that treating HeLa cells with TRAIL (Tumor necrosis factor-related apoptosis-inducing ligand) and equol made them more susceptible to TRAIL-mediated apoptosis. Caspases-3, -8, and -9 activation and BID cleavage by equol increased TRAIL-induced apoptosis. Additionally, DR4/Fc chimaera protein and DR5/Fc chimaera protein effectively decreased the caspases’ activation, BID cleavage, and apoptotic cell death brought on by equol and TRAIL co-treatment [144].
A study by Charalambous et al. [23] reported that 4-hydroxy-tamoxifen (4-OHT; >100 nM) and equol (>50 M) dramatically decreased the viability of MCF-7 cells. In addition, the induction of apoptosis was enhanced by the combination of equol (100 μM) and 4-OHT (10 μM) more potently than each compound alone, suggesting that equol increases the effectiveness of tamoxifen. Together, these findings lend credence to the hypothesis that equol and tamoxifen activate the intrinsic apoptotic pathway more effectively than each drug does on its own [144].
Together with adenoviral mutations, the compounds curcumin, genistein, epigallocatechin-gallate, equol, and resveratrol effectively killed both androgen receptor-positive (22Rv1) and -negative (PC-3, DU145) cell lines. The combination of the wild-type virus (Ad5) and AdΔΔ with the antioxidants equol and resveratrol was used to demonstrate synergistic cell death [145]. All three combination-treated cell lines showed a three- to eight-fold reduction in the EC50 values for viruses and phytochemicals, respectively. When combined with wild-type virus or AdΔΔ, equol and resveratrol significantly increased the level of apoptotic cell death in PC-3 and DU145 cells, despite the fact that they alone only slightly increased apoptosis in PC-3 and DU145 cells. AdΔΔ and equol or resveratrol were used in in vivo experiments at suboptimal levels, which resulted in less tumor growth without adverse effects on normal tissue. Overall, this study showed that AdΔΔ, an oncolytic adenoviral mutant that increases drug-induced apoptosis in cells, can also interact synergistically with dietary phytochemicals such as equol and resveratrol [145].
7. Role of Nanotechnology and Clinical Studies Using Equol
While the spectrum of therapeutic actions of phytochemicals continues to grow (Sak, 2022), it is essential to address certain drawbacks to optimum efficacy. These primarily include low bioavailability and a short residence time in the intestine due to extensive metabolism and instability of these phytoconstituents, thereby limiting their applications [146]. In order to combat these disadvantages, nanotechnological approaches may be applied to improve the solubility of these natural compounds, thereby increasing their bioavailability. Nanoparticles are a commonly used approach in drug delivery, and are widely used in cancer therapy. Studies conducted to analyze the effect of flavonoids with other synthetic anticancer drugs and compounds of natural origin have indicated promising results, thereby indicating more widespread applications in clinical settings [147].
As discussed, the spectrum of action of phytoconstituents and products of their metabolism, such as equol, remain limited. In addition to this, due to the contrasts in the pharmacokinetic profiles of different agents, their movement across barriers such as the blood–brain barrier (BBB) and intestinal barrier is variable [148]. This may be enhanced by implementing strategies such as loading onto nanostructured polymers, complexation, and nanostructured lipid carriers, thereby improving the stability and delivery of these agents to the target site [149]. In order to improve the aqueous solubility and thereby oral bioavailability of these agents, strategies such as the usage of carrier complexes and co-crystallization may be employed. This may be used to counter degradation and extensive metabolism for the improvement of the dissolution of these compounds, as well as facilitating target-specific delivery.
The antioxidant potential of polyphenols continues to be at the forefront of research, owing to their role in the amelioration of a wide variety of metabolic disorders. Keeping pace with the growing importance of environmentally friendly synthesis and drug delivery, green nanoparticles may be employed in therapy. They offer advantages such as uniformity in structure, as well as effective scavenging of reactive oxygen species (ROS) [150]. Nanotechnology may be employed in the delivery of phytoconstituents to overcome multi-drug resistance (MDR), utilizing the principle of controlled release in the management of pathogenic infections. Some of the strategies include the formulation of liposomes (for the delivery of smaller molecules and those with different solubility profiles), hydrogels (for drug entrapment and regulation of drug release), polymeric nanomicelles (for the lowering of toxicity and improved drug bioavailability), and nanofibers (improved biocompatibility and easy modifications of the solubility profile) [151]. While the therapeutic potentials of equol continue to be explored, greater advancements in nanotechnological delivery are required. An increased focus on the designing of nanoformulations, as well as undertaking pre-clinical and clinical studies, would help to further improve our understanding of this molecule, as well as to investigate newer avenues of application. Table 3 represents the current status of clinical studies using equol as a therapeutic molecule.
Table 3.
Clinical applicability of equol in the management of human health.
| Agent Administered, Dosage and Duration | Volunteers | Design | Outcome | Conclusion | Reference |
|---|---|---|---|---|---|
| 10 mg/day supplement containing 98% equol, for 3 months | 57 post-menopausal women | Single center, randomized, controlled clinical trial | Reduction in visceral fat, as well as the levels of LDL and total cholesterol, some indications of delayed skin ageing | Equol supplementation may be used for the management of excess visceral fat, and may be used for the alleviation of climacteric symptoms as well as metabolic disorders | [152] |
| 40 g low-fat milk powder + 63 mg daidzein, for a period of 6 months | 270 post-menopausal, equol-producing women | Double-blind, randomized, placebo controlled clinical trial | Lowering of T4 levels following daidzein supplementation, no disruption of thyroid functioning | Daidzein supplementation was found to be safe and did not hamper the levels of key markers of thyroid functioning, thereby establishing its safety profile | [153] |
| Supplement containing 10 mg equol + 10 mg resveratrol + 150 mg quercetin + 178 mg Passiflora, administered up to 8 months | 126 post-menopausal women | Clinical trial | Improved vaginal health index, stabilization of pH, improvement of dyspareunia | Equol supplementation may be used for relieving post-menopausal symptoms | [154] |
| 20 g/day soy isolate supplementation, containing 41 mg of isoflavones | 44–75-year-old men predisposed to prostate cancer recurrence, following prostatectomy | Randomized, placebo-controlled clinical trial | Slight improvement in hemoglobin and hematocrit levels, reduction in blood pressure in non-producers of equol | The observation from the trial helped to establish the relationship between the equol-producing status of volunteers and soy supplementation, suggesting that certain effects may be observed in each sub-type that may be different from the other, and may be used to enhance therapeutic outcomes | [155] |
| 50 and 100 mg supplementation of phytoSERM, containing genistein, daidzein and S-equol, for 12 weeks | 71 peri-menopausal women | Double-blinded, randomized, placebo-controlled clinical trial | Good tolerance of the formulation in volunteers, mild adverse effects | Potentials for the usage of equol in the management of post-menopausal symptoms, as well as mild improvement vasomotor and cognitive functioning | [156] |
| Oral isoflavone administration (150 mg extract), alone or in conjunction with probiotics or hormonal therapy | 60 post-menopausal women | Randomized, controlled clinical trial | Alleviation of urogenital complications, increase in the formation of metabolic intermediates, overall improvement of vaginal health | Isoflavone administration may be used in the management of urogenital symptoms and administration with probiotics may be linked with improvement of the synthesis of products of metabolism of isoflavones, which have been established to have therapeutic benefits | [157] |
| Oral isoflavone administration (consumption of a soy drink providing a dosage of 10–60 mg/day), over 12 weeks | 101 post-menopausal women | Randomized, controlled clinical trial | Reduction in the severity of vasomotor symptoms associated with post-menopausal complications | Soy drink supplementation, containing isoflavones, may be used in the management of vasomotor symptoms and may be provided as a natural therapeutic agent | [158] |
8. Conclusions and Future Perspectives
In this review article, different types of bioactivities of the degradation product of soy isoflavone daidzein, i.e., equol, are described in different types of malignant models. These bioactivities comprise the effects of equol on hormonal balance by binding to estrogen receptors, but its has also showed anti-inflammatory-, antiproliferative-, antimetastatic-, and apoptosis-inducing effects. All the results described in this review show the high potential of equol in the fight against cancerous neoplasms. Looking to the future, further studies should identify the bacterial species in the colon, which are involved in the biosynthesis of equol from its precursor, daidzein, thereby allowing to understand the mechanisms more thoroughly behind the bacterial production of equol and, if possible, including these species in some food products containing soy isoflavones. In addition, structure-activity analyses might be useful for further improving the anticancer properties of equol to make this molecule semi-synthetically even more potent for the use against tumors in the future.
Acknowledgments
The author, H.S.T., S.H. sincerely acknowledges MMDU India and Jazan University, Saudi Arabia for providing the access of the Digital Library for this work.
Author Contributions
H.S.T., A.K. and K.S.: Methodology, Conceptualization and Writing—original draft, D.A., D.S.G., G.K. and K.V.: Formal analysis, Investigation, Data curation, K.D., J.K., A.K.S. and M.V.: Visualization, writing and editing, E.C. and S.H.: Data curation, Supervision and proof reading. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Feng X., Li Y., Brobbey Oppong M., Qiu F. Insights into the Intestinal Bacterial Metabolism of Flavonoids and the Bioactivities of Their Microbe-Derived Ring Cleavage Metabolites. Drug Metab. Rev. 2018;50:343–356. doi: 10.1080/03602532.2018.1485691. [DOI] [PubMed] [Google Scholar]
- 2.Mayo B., Vázquez L., Flórez A.B. Equol: A Bacterial Metabolite from the Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients. 2019;11:2231. doi: 10.3390/nu11092231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rafii F. The Role of Colonic Bacteria in the Metabolism of the Natural Isoflavone Daidzin to Equol. Metabolites. 2015;5:56–73. doi: 10.3390/metabo5010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horiuchi H., Harada N., Adachi T., Nakano Y., Inui H., Yamaji R. S-Equol Enantioselectively Activates CAMP-Protein Kinase A Signaling and Reduces Alloxan-Induced Cell Death in INS-1 Pancreatic b-Cells. J. Nutr. Sci. Vitaminol. 2014;60:291–296. doi: 10.3177/jnsv.60.291. [DOI] [PubMed] [Google Scholar]
- 5.Yemelyanov A., Czwornog J., Gera L., Joshi S., Chatterton R.T., Jr., Budunova I. Novel steroid receptor phyto-modulator compound a inhibits growth and survival of prostate cancer cells. Cancer Res. 2008;68:4763–4773. doi: 10.1158/0008-5472.CAN-07-6104. [DOI] [PubMed] [Google Scholar]
- 6.Thulasidasan A.K., Retnakumari A.P., Shankar M., Vijayakurup V., Anwar S., Thankachan S., Pillai K.S., Pillai J.J., Nandan C.D., Alex V.V., et al. Folic acid conjugation improves the bioavailability and chemosensitizing efficacy of curcumin-encapsulated PLGA-PEG nanoparticles towards paclitaxel chemotherapy. Oncotarget. 2017;12:107374. doi: 10.18632/oncotarget.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahman M.S., Cao J. Estrogen receptors in gastric cancer: Advances and perspectives. World J. Gastroenterol. 2016;28:2475. doi: 10.3748/wjg.v22.i8.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michikawa T., Inoue M., Sawada N., Tanaka Y., Yamaji T., Iwasaki M., Shimazu T., Sasazuki S., Mizokami M., Tsugane S., et al. Plasma Isoflavones and Risk of Primary Liver Cancer in Japanese Women and Men with Hepatitis Virus Infection: A Nested Case–Control StudyPlasma Isoflavones and Primary Liver Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2015;24:532–537. doi: 10.1158/1055-9965.EPI-14-1118. [DOI] [PubMed] [Google Scholar]
- 9.Jeong H., Phan A.N.H., Choi J.W. Anti-Cancer Effects of Polyphenolic Compounds in Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor-Resistant Non-Small Cell Lung Cancer. Pharm. Mag. 2017;13:595–599. doi: 10.4103/pm.pm_535_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J., Ren L., Yu M., Liu X., Ma W., Huang L., Li X., Ye X. S-Equol Inhibits Proliferation and Promotes Apoptosis of Human Breast Cancer MCF-7 cells via Regulating MiR-10a-5p and PI3K/AKT Pathway. Arch. Biochem. Biophys. 2019;672:108064. doi: 10.1016/j.abb.2019.108064. [DOI] [PubMed] [Google Scholar]
- 11.Kim E.Y., Shin J.Y., Park Y.J., Kim A.K. Equol Induces Mitochondria-Mediated Apoptosis of Human Cervical Cancer Cells. Anticancer Res. 2014;34:4985–4992. [PubMed] [Google Scholar]
- 12.Fares J., Fares M.Y., Khachfe H.H., Salhab H.A., Fares Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020;5:28. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fatima A., Khan M.S., Ahmad M.d.W. Therapeutic Potential of Equol: A Comprehensive Review. Curr. Pharm. Des. 2020;26:5837–5843. doi: 10.2174/1381612826999201117122915. [DOI] [PubMed] [Google Scholar]
- 14.Hüser S., Guth S., Joost H.G., Soukup S.T., Köhrle J., Kreienbrock L., Diel P., Lachenmeier D.W., Eisenbrand G., Vollmer G., et al. Effects of Isoflavones on Breast Tissue and the Thyroid Hormone System in Humans: A Comprehensive Safety Evaluation. Arch. Toxicol. 2018;92:2703–2748. doi: 10.1007/s00204-018-2279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Y., Yang Z., Xie Y., Yang M., Zhang Y., Deng Z., Cai L. Investigation of Inhibition Effect of Daidzein on Osteosarcoma Cells Based on Experimental Validation and Systematic Pharmacology Analysis. PeerJ. 2021;9:e12072. doi: 10.7717/peerj.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han B.-J., Li W., Jiang G.-B., Lai S.-H., Zhang C., Zeng C.-C., Liu Y.-J. Effects of Daidzein in Regards to Cytotoxicity in Vitro, Apoptosis, Reactive Oxygen Species Level, Cell Cycle Arrest and the Expression of Caspase and Bcl-2 Family Proteins. Oncol. Rep. 2015;34:1115–1120. doi: 10.3892/or.2015.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang Y.-S., Qi W.-T., Guo W., Wang C.-L., Hu Z.-B., Li A.-K. Genistein and Daidzein Induce Apoptosis of Colon Cancer Cells by Inhibiting the Accumulation of Lipid Droplets. Food Nutr. Res. 2018;62:1–9. doi: 10.29219/fnr.v62.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai Y.F., Zhang H.M., Niu W.Y., Zou Y.Q., Ma D.F. Effects of Equol on Colon Cancer Cell Proliferation. J. Peking Univ. Health Sci. 2017;49:383–387. [PubMed] [Google Scholar]
- 19.Kumar V., Chauhan S.S. Daidzein Induces Intrinsic Pathway of Apoptosis along with ER α/β Ratio Alteration and ROS Production. Asian Pac. J. Cancer Prev. 2021;22:603–610. doi: 10.31557/APJCP.2021.22.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montalesi E., Cipolletti M., Cracco P., Fiocchetti M., Marino M. Divergent Effects of Daidzein and Its Metabolites on Estrogen-Induced Survival of Breast Cancer Cells. Cancers. 2020;12:167. doi: 10.3390/cancers12010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo J., Wang Q., Zhang Y., Sun W., Zhang S., Li Y., Wang J., Bao Y. Functional Daidzein Enhances the Anticancer Effect of Topotecan and Reverses BCRP-Mediated Drug Resistance in Breast Cancer. Pharm. Res. 2019;147:104387. doi: 10.1016/j.phrs.2019.104387. [DOI] [PubMed] [Google Scholar]
- 22.Bao C., Namgung H., Lee J., Park H.-C., Ko J., Moon H., Ko H.W., Lee H.J. Daidzein Suppresses Tumor Necrosis Factor-α Induced Migration and Invasion by Inhibiting Hedgehog/Gli1 Signaling in Human Breast Cancer Cells. J. Agric. Food Chem. 2014;62:3759–3767. doi: 10.1021/jf500231t. [DOI] [PubMed] [Google Scholar]
- 23.Charalambous C., Pitta C.A., Constantinou A.I. Equol Enhances Tamoxifen’s Anti-Tumor Activity by Induction of Caspase-Mediated Apoptosis in MCF-7 Breast Cancer Cells. BMC Cancer. 2013;13:238. doi: 10.1186/1471-2407-13-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi E.J., Kim G.-H. Daidzein Causes Cell Cycle Arrest at the G1 and G2/M Phases in Human Breast Cancer MCF-7 and MDA-MB-453 Cells. Phytomedicine. 2008;15:683–690. doi: 10.1016/j.phymed.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Kaushik S., Shyam H., Sharma R., Balapure A.K. Dietary Isoflavone Daidzein Synergizes Centchroman Action via Induction of Apoptosis and Inhibition of PI3K/Akt Pathway in MCF-7/MDA MB-231 Human Breast Cancer Cells. Phytomedicine. 2018;40:116–124. doi: 10.1016/j.phymed.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Rigalli J.P., Scholz P.N., Tocchetti G.N., Ruiz M.L., Weiss J. The Phytoestrogens Daidzein and Equol Inhibit the Drug Transporter BCRP/ABCG2 in Breast Cancer Cells: Potential Chemosensitizing Effect. Eur. J. Nutr. 2019;58:139–150. doi: 10.1007/s00394-017-1578-9. [DOI] [PubMed] [Google Scholar]
- 27.Ono M., Ejima K., Higuchi T., Takeshima M., Wakimoto R., Nakano S. Equol Enhances Apoptosis-Inducing Activity of Genistein by Increasing Bax/Bcl-XL Expression Ratio in MCF-7 Human Breast Cancer Cells. Nutr. Cancer. 2017;69:1300–1307. doi: 10.1080/01635581.2017.1367945. [DOI] [PubMed] [Google Scholar]
- 28.de la Parra C., Borrero-Garcia L.D., Cruz-Collazo A., Schneider R.J., Dharmawardhane S. Equol, an Isoflavone Metabolite, Regulates Cancer Cell Viability and Protein Synthesis Initiation via c-Myc and EIF4G. J. Biol. Chem. 2015;290:6047–6057. doi: 10.1074/jbc.M114.617415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magee P.J., Allsopp P., Samaletdin A., Rowland I.R. Daidzein, R-(+)Equol and S-(−)Equol Inhibit the Invasion of MDA-MB-231 Breast Cancer Cells Potentially via the down-Regulation of Matrix Metalloproteinase-2. Eur. J. Nutr. 2014;53:345–350. doi: 10.1007/s00394-013-0520-z. [DOI] [PubMed] [Google Scholar]
- 30.Jin S., Zhang Q.Y., Kang X.M., Wang J.X., Zhao W.H. Daidzein Induces MCF-7 Breast Cancer Cell Apoptosis via the Mitochondrial Pathway. Ann. Oncol. 2010;21:263–268. doi: 10.1093/annonc/mdp499. [DOI] [PubMed] [Google Scholar]
- 31.Ju Y.H., Fultz J., Allred K.F., Doerge D.R., Helferich W.G. Effects of Dietary Daidzein and Its Metabolite, Equol, at Physiological Concentrations on the Growth of Estrogen-Dependent Human Breast Cancer (MCF-7) Tumors Implanted in Ovariectomized Athymic Mice. Carcinogenesis. 2006;27:856–863. doi: 10.1093/carcin/bgi320. [DOI] [PubMed] [Google Scholar]
- 32.Guo S., Wang Y., Li Y., Li Y., Feng C., Li Z. Daidzein-Rich Isoflavones Aglycone Inhibits Lung Cancer Growth through Inhibition of NF-ΚB Signaling Pathway. Immunol. Lett. 2020;222:67–72. doi: 10.1016/j.imlet.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Spagnuolo C., Russo G.L., Orhan I.E., Habtemariam S., Daglia M., Sureda A., Nabavi S.F., Devi K.P., Loizzo M.R., Tundis R., et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015;6:408–419. doi: 10.3945/an.114.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Z.-P., Zhao Y., Huang F., Chen J., Yao Y.-H., Li J., Wu X.-N. Equol Inhibits Proliferation of Human Gastric Carcinoma Cells via Modulating Akt Pathway. World J. Gastroenterol. 2015;21:10385–10399. doi: 10.3748/wjg.v21.i36.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang S., Hu J., Meng Q., Dong X., Wang K., Qi Y., Chu C., Zhang X., Hou L. Daidzein Induced Apoptosis via Down-Regulation of Bcl-2/Bax and Triggering of the Mitochondrial Pathway in BGC-823 Cells. Cell Biochem. Biophys. 2013;65:197–202. doi: 10.1007/s12013-012-9418-2. [DOI] [PubMed] [Google Scholar]
- 36.Gao L., Wang K.-X., Zhang N.-N., Li J.-Q., Qin X.-M., Wang X.-L. 1H Nuclear Magnetic Resonance Based Metabolomics Approach Reveals the Metabolic Mechanism of (-)-5-Hydroxy-Equol against Hepatocellular Carcinoma Cells in Vitro. J. Proteome. Res. 2018;17:1833–1843. doi: 10.1021/acs.jproteome.7b00853. [DOI] [PubMed] [Google Scholar]
- 37.Liang X.-L., Li M., Li J., Wang X.-L. Equol Induces Apoptosis in Human Hepatocellular Carcinoma SMMC-7721 Cells through the Intrinsic Pathway and the Endoplasmic Reticulum Stress Pathway. Anticancer Drugs. 2014;25:633–640. doi: 10.1097/CAD.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 38.Park H.J., Jeon Y.K., You D.H., Nam M.J. Daidzein Causes Cytochrome C-Mediated Apoptosis via the Bcl-2 Family in Human Hepatic Cancer Cells. Food Chem. Toxicol. 2013;60:542–549. doi: 10.1016/j.fct.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 39.Guo J.-M., Xiao B.-X., Dai D.-J., Liu Q., Ma H.-H. Effects of Daidzein on Estrogen-Receptor-Positive and Negative Pancreatic Cancer Cells in Vitro. World J. Gastroenterol. 2004;10:860–863. doi: 10.3748/wjg.v10.i6.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salama A.A.A., Allam R.M. Promising Targets of Chrysin and Daidzein in Colorectal Cancer: Amphiregulin, CXCL1, and MMP-9. Eur. J. Pharm. 2021;892:173763. doi: 10.1016/j.ejphar.2020.173763. [DOI] [PubMed] [Google Scholar]
- 41.Zou Y., Wang Y., Cai Y., Ma D. Effects of Equol on Proliferation of Colorectal Cancer HCT-15 Cell. Wei Sheng Yan Jiu. 2019;48:803–806. [PubMed] [Google Scholar]
- 42.He Y., Wu X., Cao Y., Hou Y., Chen H., Wu L., Lu L., Zhu W., Gu Y. Daidzein Exerts Anti-Tumor Activity against Bladder Cancer Cells via Inhibition of FGFR3 Pathway. Neoplasma. 2016;63:523–531. doi: 10.4149/neo_2016_405. [DOI] [PubMed] [Google Scholar]
- 43.Ranjithkumar R., Saravanan K., Balaji B., Hima S., Sreeja S., Timane S.R., Ram Pravin Kumar M., Kabilan S., Ramanathan M. Novel Daidzein Molecules Exhibited Anti-Prostate Cancer Activity through Nuclear Receptor ERβ Modulation, in Vitro and in Vivo Studies. J. Chemother. 2021;33:582–594. doi: 10.1080/1120009X.2021.1924935. [DOI] [PubMed] [Google Scholar]
- 44.Lu Z., Zhou R., Kong Y., Wang J., Xia W., Guo J., Liu J., Sun H., Liu K., Yang J., et al. S-Equol, a Secondary Metabolite of Natural Anticancer Isoflavone Daidzein, Inhibits Prostate Cancer Growth In Vitro and In Vivo, Though Activating the Akt/FOXO3a Pathway. Curr. Cancer Drug Targets. 2016;16:455–465. doi: 10.2174/1568009616666151207105720. [DOI] [PubMed] [Google Scholar]
- 45.Zheng W., Zhang Y., Ma D., Shi Y., Liu C., Wang P. (±)Equol Inhibits Invasion in Prostate Cancer DU145 Cells Possibly via down-Regulation of Matrix Metalloproteinase-9, Matrix Metalloproteinase-2 and Urokinase-Type Plasminogen Activator by Antioxidant Activity. J. Clin. Biochem. Nutr. 2012;51:61–67. doi: 10.3164/jcbn.11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng W., Zhang Y., Ma D., Li G., Wang P. Anti-Invasion Effects of R- and S-Enantiomers of Equol on Prostate Cancer PC3, DU145 Cells. Wei Sheng Yan Jiu. 2011;40:423–425. [PubMed] [Google Scholar]
- 47.Dai W., Wang F., He L., Lin C., Wu S., Chen P., Zhang Y., Shen M., Wu D., Wang C., et al. Genistein inhibits hepatocellular carcinoma cell migration by reversing the epithelial–mesenchymal transition: Partial mediation by the transcription factor NFAT1. Mol. Carcinog. 2015;54:301–311. doi: 10.1002/mc.22100. [DOI] [PubMed] [Google Scholar]
- 48.Hua F., Li C.-H., Chen X.-G., Liu X.-P. Daidzein Exerts Anticancer Activity towards SKOV3 Human Ovarian Cancer Cells by Inducing Apoptosis and Cell Cycle Arrest, and Inhibiting the Raf/MEK/ERK Cascade. Int. J. Mol. Med. 2018;41:3485–3492. doi: 10.3892/ijmm.2018.3531. [DOI] [PubMed] [Google Scholar]
- 49.Goris T., Cuadrat R.R.C., Braune A. Flavonoid-Modifying Capabilities of the Human Gut Microbiome—An in Silico Study. Nutrients. 2021;13:2688. doi: 10.3390/nu13082688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soukup S.T., Stoll D.A., Danylec N., Schoepf A., Kulling S.E., Huch M. Metabolism of Daidzein and Genistein by Gut Bacteria of the Class Coriobacteriia. Foods. 2021;10:2741. doi: 10.3390/foods10112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiaobin L., Jinglong X., Fang Z., Chenchen W., Kailun Y. Effect of the HXBM408 Bacteria on Rat Intestinal Bacterial Diversity and the Metabolism of Soybean Isoflavones. PLoS ONE. 2021;16:e0253728. doi: 10.1371/journal.pone.0253728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wada K., Ueno T., Uchiyama S., Abiru Y., Tsuji M., Konishi K., Mizuta F., Goto Y., Tamura T., Shiraki M., et al. Relationship of Equol Production between Children Aged 5–7 Years and Their Mothers. Eur. J. Nutr. 2017;56:1911–1917. doi: 10.1007/s00394-016-1233-x. [DOI] [PubMed] [Google Scholar]
- 53.Wang Q., Spenkelink B., Boonpawa R., Rietjens I.M.C.M., Beekmann K. Use of Physiologically Based Kinetic Modeling to Predict Rat Gut Microbial Metabolism of the Isoflavone Daidzein to S-Equol and Its Consequences for ERα Activation. Mol. Nutr. Food Res. 2020;64:1900912. doi: 10.1002/mnfr.201900912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iino C., Shimoyama T., Iino K., Yokoyama Y., Chinda D., Sakuraba H., Fukuda S., Nakaji S. Daidzein Intake Is Associated with Equol Producing Status through an Increase in the Intestinal Bacteria Responsible for Equol Production. Nutrients. 2019;11:433. doi: 10.3390/nu11020433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu A., Sunagawa S., Mende D.R., Bork P. Inter-Individual Differences in the Gene Content of Human Gut Bacterial Species. Genome Biol. 2015;16:82. doi: 10.1186/s13059-015-0646-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan J.P., Wang J.H., Liu X. Metabolism of Dietary Soy Isoflavones to Equol by Human Intestinal Microflora—Implications for Health. Mol. Nutr. Food Res. 2007;51:765–781. doi: 10.1002/mnfr.200600262. [DOI] [PubMed] [Google Scholar]
- 57.Kim I.-S. Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans. Antioxidants. 2021;10:1064. doi: 10.3390/antiox10071064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho H.W., Gim H.J., Li H., Subedi L., Kim S.Y., Ryu J.-H., Jeon R. Structure–Activity Relationship of Phytoestrogen Analogs as ERα/β Agonists with Neuroprotective Activities. Chem. Pharm. Bull. 2021;69:99–105. doi: 10.1248/cpb.c20-00706. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka H., Ito S., Ojika M., Nishimaki-Mogami T., Kondo K., Wakamatsu K. The Oxidation of Equol by Tyrosinase Produces a Unique Di-Ortho-Quinone: Possible Implications for Melanocyte Toxicity. Int. J. Mol. Sci. 2021;22:9145. doi: 10.3390/ijms22179145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu Z.T., Yao W., Zhu W.Y. Isolation and Identification of Equol-Producing Bacterial Strains from Cultures of Pig Faeces. FEMS Microbiol. Lett. 2008;282:73–80. doi: 10.1111/j.1574-6968.2008.01108.x. [DOI] [PubMed] [Google Scholar]
- 61.Vázquez L., Flórez A.B., Rodríguez J., Mayo B. Heterologous Expression of Equol Biosynthesis Genes from Adlercreutzia Equolifaciens. FEMS Microbiol. Lett. 2021;368:fnab082. doi: 10.1093/femsle/fnab082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heng Y., Kim M.J., Yang H.J., Kang S., Park S. Lactobacillus Intestinalis Efficiently Produces Equol from Daidzein and Chungkookjang, Short-Term Fermented Soybeans. Arch. Microbiol. 2019;201:1009–1017. doi: 10.1007/s00203-019-01665-5. [DOI] [PubMed] [Google Scholar]
- 63.KAWADA Y., YOKOYAMA S., YANASE E., NIWA T., SUZUKI T. The production of S-equol from daidzein is associated with a cluster of three genes in Eggerthella sp. YY7918. Biosci. Microbiota. Food Health. 2016;35:113–121. doi: 10.12938/bmfh.2015-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mustafa S.E., Mustafa S., Abas F., Manap M.Y.A.B.D., Ismail A., Amid M., Elzen S. Optimization of Culture Conditions of Soymilk for Equol Production by Bifidobacterium Breve 15700 and Bifidobacterium Longum BB536. Food Chem. 2019;278:767–772. doi: 10.1016/j.foodchem.2018.11.107. [DOI] [PubMed] [Google Scholar]
- 65.Lee P.-G., Lee S.-H., Kim J., Kim E.-J., Choi K.-Y., Kim B.-G. Polymeric Solvent Engineering for Gram/Liter Scale Production of a Water-Insoluble Isoflavone Derivative, (S)-Equol. Appl. Microbiol. Biotechnol. 2018;102:6915–6921. doi: 10.1007/s00253-018-9137-8. [DOI] [PubMed] [Google Scholar]
- 66.Nazzaro F., Fratianni F., d’Acierno A., Coppola R. Advances in Food Biotechnology. John Wiley & Sons Ltd.; Chichester, UK: 2015. Gut Microbiota and Polyphenols: A Strict Connection Enhancing Human Health; pp. 335–350. [Google Scholar]
- 67.Mahalingam S., Gao L., Gonnering M., Helferich W., Flaws J.A. Equol Inhibits Growth, Induces Atresia, and Inhibits Steroidogenesis of Mouse Antral Follicles in Vitro. Toxicol. Appl. Pharm. 2016;295:47–55. doi: 10.1016/j.taap.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iglesias-Aguirre C.E., Cortés-Martín A., Ávila-Gálvez M.Á., Giménez-Bastida J.A., Selma M.V., González-Sarrías A., Espín J.C. Main drivers of (poly) phenol effects on human health: Metabolite production and/or gut microbiota-associated metabotypes? Food Funct. 2021;12:10324–10355. doi: 10.1039/D1FO02033A. [DOI] [PubMed] [Google Scholar]
- 69.Shi J., Ji A., Cao Z., Cao R., Li D., Yang R., Wang F. Equol Induced Apoptosis of Human Breast Cancer MDA-MB-231 Cell by Inhibiting the Expression of Nuclear Factor-KappaB. Wei Sheng Yan Jiu. 2011;40:95–98. [PubMed] [Google Scholar]
- 70.Hong S.H., Cha H.J., Hwang-Bo H., Kim M.Y., Kim S.Y., Ji S.Y., Cheong J., Park C., Lee H., Kim G.Y., et al. Anti-proliferative and pro-apoptotic effects of Licochalcone A through ROS-mediated cell cycle arrest and apoptosis in human bladder cancer cells. Int. J. Mol. Sci. 2019;20:3820. doi: 10.3390/ijms20153820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang H.-B., Zhang Y.-F., Yang J.-D., Lu K.-L. Study on Soy Isoflavone Consumption and Risk of Breast Cancer and Survival. Asian Pac. J. Cancer Prev. 2012;13:995–998. doi: 10.7314/APJCP.2012.13.3.995. [DOI] [PubMed] [Google Scholar]
- 72.Choi E.J., Ahn W.S., Bae S.M. Equol Induces Apoptosis through Cytochrome C-Mediated Caspases Cascade in Human Breast Cancer MDA-MB-453 Cells. Chem. Biol. Interact. 2009;177:7–11. doi: 10.1016/j.cbi.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 73.Taghizadeh B., Ghavami L., Nikoofar A., Goliaei B. Equol as a Potent Radiosensitizer in Estrogen Receptor-Positive and -Negative Human Breast Cancer Cell Lines. Breast Cancer. 2015;22:382–390. doi: 10.1007/s12282-013-0492-0. [DOI] [PubMed] [Google Scholar]
- 74.Setchell K.D.R., Clerici C. Equol: Pharmacokinetics and Biological Actions. J. Nutr. 2010;140:1363S–1368S. doi: 10.3945/jn.109.119784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Majeed R., Hamid A., Sangwan P.L., Chinthakindi P.K., Koul S., Rayees S., Singh G., Mondhe D.M., Mintoo M.J., Singh S.K., et al. Inhibition of phosphotidylinositol-3 kinase pathway by a novel naphthol derivative of betulinic acid induces cell cycle arrest and apoptosis in cancer cells of different origin. Cell Death Dis. 2014;5:e1459. doi: 10.1038/cddis.2014.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Itsumi M., Shiota M., Takeuchi A., Kashiwagi E., Inokuchi J., Tatsugami K., Kajioka S., Uchiumi T., Naito S., Eto M., et al. Equol Inhibits Prostate Cancer Growth through Degradation of Androgen Receptor by S-Phase Kinase-Associated Protein 2. Cancer Sci. 2016;107:1022–1028. doi: 10.1111/cas.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lund T.D., Blake C., Bu L., Hamaker A.N., Lephart E.D. IEquol an Isoflavonoid: Potential for Improved Prostate Health, in Vitro and in Vivo Evidence. Reprod. Biol. Endocrinol. 2011;9:4. doi: 10.1186/1477-7827-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hod R., Maniam S., Mohd Nor N.H. A systematic review of the effects of equol (soy metabolite) on breast cancer. Molecules. 2021;26:1105. doi: 10.3390/molecules26041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Onoda A., Ueno T., Uchiyama S., Hayashi S.-I., Kato K., Wake N. Effects of S-Equol and Natural S-Equol Supplement (SE5-OH) on the Growth of MCF-7 in Vitro and as Tumors Implanted into Ovariectomized Athymic Mice. Food Chem. Toxicol. 2011;49:2279–2284. doi: 10.1016/j.fct.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 80.Mitchell J.H., Duthie S.J., Collins A.R. Effects of phytoestrogens on growth and DNA integrity in human prostate tumor cell lines: PC-3 and LNCaP. Nutr. Cancer. 2000;38:223–228. doi: 10.1207/S15327914NC382_12. [DOI] [PubMed] [Google Scholar]
- 81.Thibodeau P.A., Kachadourian R., Lemay R., Bisson M., Day B.J., Paquette B. In Vitro Pro-and Antioxidant Properties of Estrogens. J. Steroid Biochem. Mol. Biol. 2002;81:227–236. doi: 10.1016/S0960-0760(02)00067-5. [DOI] [PubMed] [Google Scholar]
- 82.Caldon C.E. Estrogen signaling and the DNA damage response in hormone dependent breast cancers. Front. Oncol. 2014;14:106. doi: 10.3389/fonc.2014.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cavalieri E., Chakravarti D., Guttenplan J., Hart E., Ingle J., Jankowiak R., Muti P., Rogan E., Russo J., Santen R., et al. Catechol Estrogen Quinones as Initiators of Breast and Other Human Cancers: Implications for Biomarkers of Susceptibility and Cancer Prevention. Biochim. Biophys. Acta Rev. Cancer. 2006;1766:63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 84.Atkinson C., Frankenfeld C.L., Lampe J.W. Gut bacterial metabolism of the soy isoflavone daidzein: Exploring the relevance to human health. Exp. Biol. Med. 2005;230:155–170. doi: 10.1177/153537020523000302. [DOI] [PubMed] [Google Scholar]
- 85.Nettleton J.A., Greany K.A., Thomas W., Wangen K.E., Adlercreutz H., Kurzer M.S. The Effect of Soy Consumption on the Urinary 2:16-Hydroxyestrone Ratio in Postmenopausal Women Depends on Equol Production Status but Is Not Influenced by Probiotic Consumption 1. J. Nutr. 2005;135:603–608. doi: 10.1093/jn/135.3.603. [DOI] [PubMed] [Google Scholar]
- 86.Cassidy A., Brown J.E., Hawdon A., Faughnan M.S., King L.J., Millward J., Zimmer-Nechemias L., Wolfe B., Setchell K.D.R. Nutrient Physiology, Metabolism, and Nutrient-Nutrient Interactions Factors Affecting the Bioavailability of Soy Isoflavones in Humans after Ingestion of Physiologically Relevant Levels from Different Soy Foods 1. J. Nutr. 2006;136:45–51. doi: 10.1093/jn/136.1.45. [DOI] [PubMed] [Google Scholar]
- 87.Rowland I.R., Wiseman H., Sanders T.A.B., Adlercreutz H., Bowey E.A. Interindividual Variation in Metabolism of Soy Isoflavones and Lignans: Influence of Habitual Diet on Equol Production by the Gut Microflora. Nutr. Cancer. 2000;36:27–32. doi: 10.1207/S15327914NC3601_5. [DOI] [PubMed] [Google Scholar]
- 88.Dewi F.N., Wood C.E., Lampe J.W., Hullar M.A.J., Franke A.A., Golden D.L., Adams M.R., Cline J.M. Endogenous and Exogenous Equol Are Antiestrogenic in Reproductive Tissues of Apolipoprotein E-Null Mice. J. Nutr. 2012;142:1829–1835. doi: 10.3945/jn.112.161711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Uchiyama S. New Insights into “Equol”, a Novel Ingredient Derived from Soy. Nippon Shokuhin Kagaku Kogaku Kaishi. 2015;62:356–363. doi: 10.3136/nskkk.62.356. [DOI] [Google Scholar]
- 90.Lathrop K.I., Kaklamani V.G., Brenner A.J., Li R., Nazarullah A., Hackman S., Thomas C., Gelfond J., Rodriguez M., Elledge R. Novel Estrogen Receptor Beta Agonist S-Equol Decreases Tumor Proliferation in Patients with Triple Negative Breast Cancer (TNBC) J. Clin. Oncol. 2020;38 doi: 10.1200/JCO.2020.38.15_suppl.560. [DOI] [Google Scholar]
- 91.Setchell K.D.R., Zhao X., Jha P., Heubi J.E., Brown N.M. The Pharmacokinetic Behavior of the Soy Isoflavone Metabolite S-(−)Equol and Its Diastereoisomer R-(+)Equol in Healthy Adults Determined by Using Stable-Isotope-Labeled Tracers. Am. J. Clin. Nutr. 2009;90:1029–1037. doi: 10.3945/ajcn.2009.27981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Setchell K.D., Clerici C., Lephart E.D., Cole S.J., Heenan C., Castellani D., Wolfe B.E., Nechemias-Zimmer L., Brown N.M., Lund T.D., et al. S-Equol, a Potent Ligand for Estrogen Receptor β, Is the Exclusive Enantiomeric Form of the Soy Isoflavone Metabolite Produced by Human Intestinal Bacterial Flora. Am. J. Clin. Nutr. 2005;81:1072–1079. doi: 10.1093/ajcn/81.5.1072. [DOI] [PubMed] [Google Scholar]
- 93.Muthyala R.S., Ju Y.H., Sheng S., Williams L.D., Doerge D.R., Katzenellenbogen B.S., Helferich W.G., Katzenellenbogen J.A. Equol, a Natural Estrogenic Metabolite from Soy Isoflavones: Convenient Preparation and Resolution of R- and S-Equols and Their Differing Binding and Biological Activity through Estrogen Receptors Alpha and Beta. Bioorg. Med. Chem. 2004;12:1559–1567. doi: 10.1016/j.bmc.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 94.Xu W.H., Zheng W., Xiang Y.B., Ruan Z.X., Cheng J.R., Dai Q., Gao Y.T., Shu X.O. Soya Food Intake and Risk of Endometrial Cancer among Chinese Women in Shanghai: Population Based Case-Control Study. Br. Med. J. 2004;328:1285–1288. doi: 10.1136/bmj.38093.646215.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Horn-Ross P.L., John E.M., Canchola A.J., Stewart S.L., Lee M.M. Phytoestrogen Intake and Endometrial Cancer Risk. J. Natl. Cancer Inst. 2003;95:1158–1164. doi: 10.1093/jnci/djg015. [DOI] [PubMed] [Google Scholar]
- 96.Bandera E.V., Williams M.G., Sima C., Bayuga S., Pulick K., Wilcox H., Soslow R., Zauber A.G., Olson S.H. Phytoestrogen Consumption and Endometrial Cancer Risk: A Population-Based Case-Control Study in New Jersey. Cancer Causes Control. 2009;20:1117–1127. doi: 10.1007/s10552-009-9336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shah S.C., Kayamba V., Peek R.M., Heimburger D. Cancer Control in Low-and Middle-Income Countries: Is It Time to Consider Screening? J. Glob. Oncol. 2019;5:1–8. doi: 10.1200/JGO.18.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aspriţoiu V.M., Stoica I., Bleotu C., Diaconu C.C. Epigenetic Regulation of Angiogenesis in Development and Tumors Progression: Potential Implications for Cancer Treatment. Front. Cell Dev. Biol. 2021;9:2462. doi: 10.3389/fcell.2021.689962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Akbarian M., Bertassoni L.E., Tayebi L. Biological Aspects in Controlling Angiogenesis: Current Progress. Cell. Mol. Life Sci. 2022;79:349. doi: 10.1007/s00018-022-04348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhong H., Sun X. Contribution of Interleukin-17A to Retinal Degenerative Diseases. Front. Immunol. 2022;13:847937. doi: 10.3389/fimmu.2022.847937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singh P.G., Basalingappa K.M., Gopenath T.S., Sushma B.V. Tumor Angiogenesis and Modulators. IntechOpen; London, UK: 2022. Tumour Angiogenesis in Breast Cancer. [Google Scholar]
- 102.Sadhukhan S., Dey S. Cancer Diagnostics and Therapeutics. Springer Singapore; Singapore: 2022. Biology, Chemistry, and Physics of Cancer Cell Motility and Metastasis; pp. 81–109. [Google Scholar]
- 103.Unterleuthner D., Neuhold P., Schwarz K., Janker L., Neuditschko B., Nivarthi H., Crncec I., Kramer N., Unger C., Hengstschläger M., et al. Cancer-Associated Fibroblast-Derived WNT2 Increases Tumor Angiogenesis in Colon Cancer. Angiogenesis. 2020;23:159–177. doi: 10.1007/s10456-019-09688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aguilar-Cazares D., Chavez-Dominguez R., Carlos-Reyes A., Lopez-Camarillo C., Hernadez de la Cruz O.N., Lopez-Gonzalez J.S. Contribution of Angiogenesis to Inflammation and Cancer. Front. Oncol. 2019;9:1399. doi: 10.3389/fonc.2019.01399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shin S.S., Yoon M. Regulation of Obesity by Antiangiogenic Herbal Medicines. Molecules. 2020;25:4549. doi: 10.3390/molecules25194549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fallah A., Sadeghinia A., Kahroba H., Samadi A., Heidari H.R., Bradaran B., Zeinali S., Molavi O. Therapeutic Targeting of Angiogenesis Molecular Pathways in Angiogenesis-Dependent Diseases. Biomed. Pharmacother. 2019;110:775–785. doi: 10.1016/j.biopha.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 107.Cohen R.B., Oudard S. Antiangiogenic Therapy for Advanced Renal Cell Carcinoma: Management of Treatment-Related Toxicities. Investig. New Drugs. 2012;30:2066–2079. doi: 10.1007/s10637-012-9796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bhise N.S., Shmueli R.B., Sunshine J.C., Tzeng S.Y., Green J.J. Drug Delivery Strategies for Therapeutic Angiogenesis and Antiangiogenesis. Expert Opin. Drug Deliv. 2011;8:485–504. doi: 10.1517/17425247.2011.558082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fridlender M., Kapulnik Y., Koltai H. Plant Derived Substances with Anti-Cancer Activity: From Folklore to Practice. Front. Plant Sci. 2015;6:799. doi: 10.3389/fpls.2015.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miura A., Sugiyama C., Sakakibara H., Simoi K., Goda T. Bioavailability of isoflavones from soy products in equol producers and non-producers in Japanese women. J. Nutr. Intermed. Metab. 2016;6:41–47. doi: 10.1016/j.jnim.2016.08.001. [DOI] [Google Scholar]
- 111.Lu K., Bhat M., Basu S. Plants and Their Active Compounds: Natural Molecules to Target Angiogenesis. Angiogenesis. 2016;19:287–295. doi: 10.1007/s10456-016-9512-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bellou S., Karali E., Bagli E., Al-Maharik N., Morbidelli L., Ziche M., Adlercreutz H., Murphy C., Fotsis T. The Isoflavone Metabolite 6-Methoxyequol Inhibits Angiogenesis and Suppresses Tumor Growth. Mol. Cancer. 2012;11:35. doi: 10.1186/1476-4598-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang Z., Zhao Y., Yao Y., Li J., Wang W., Wu X. Equol induces mitochondria-dependent apoptosis in human gastric cancer cells via the sustained activation of ERK1/2 pathway. Mol. Cells. 2016;39:742–749. doi: 10.14348/molcells.2016.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leiva B., Carrasco I., Montenegro I., Gaete L., Lemus I., Tchernitchin A., Bustamante R., Párraga M., Villena J. Boletín Latinoamericano y Del Caribe de Equol and Daidzein Decrease Migration, Invasion and Matrix Metalloproteinase (MMPs) Gene Expression in Prostate Cancer Cell Lines, DU-145 and PC-3. Bol. Latinoam. Caribe Plant Med. Aromat. 2015;14:251–262. [Google Scholar]
- 115.Lu C., Gao R., Zhang Y., Jiang N., Chen Y., Sun J., Wang Q., Fan B., Liu X., Wang F. S-equol, a metabolite of dietary soy isoflavones, alleviates lipopolysaccharide-induced depressive-like behavior in mice by inhibiting neuroinflammation and enhancing synaptic plasticity. Food Funct. 2021;12:5770–5778. doi: 10.1039/d1fo00547b. [DOI] [PubMed] [Google Scholar]
- 116.Setchell K.D.R., Clerici C. Equol: History, Chemistry, and Formation. J. Nutr. 2010;140:1355S–1362S. doi: 10.3945/jn.109.119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mitchell J.H., Gardner P.T., Mcphail D.B., Morrice P.C., Collins A.R., Duthie G.G. Antioxidant Efficacy of Phytoestrogens in Chemical and Biological Model Systems. Arch. Biochem. Biophys. 1998;360:142–148. doi: 10.1006/abbi.1998.0951. [DOI] [PubMed] [Google Scholar]
- 118.Ma Y., Sullivan J.C., Schreihofer D.A. Dietary Genistein and Equol (4=, 7 Isoflavandiol) Reduce Oxidative Stress and Protect Rats against Focal Cerebral Ischemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:871–877. doi: 10.1152/ajpregu.00031.2010. [DOI] [PubMed] [Google Scholar]
- 119.Liu T.H., Tsai T.Y. Effects of Equol on Deoxycorticosterone Acetate Salt-Induced Hypertension and Associated Vascular Dementia in Rats. Food Funct. 2016;7:3444–3457. doi: 10.1039/C6FO00223D. [DOI] [PubMed] [Google Scholar]
- 120.Jackman K.A., Woodman O.L., Chrissobolis S., Sobey C.G. Vasorelaxant and Antioxidant Activity of the Isoflavone Metabolite Equol in Carotid and Cerebral Arteries. Brain Res. 2007;1141:99–107. doi: 10.1016/j.brainres.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 121.Nam J.K., Ki W.L., Rogozin E.A., Cho Y.Y., Heo Y.S., Bode A.M., Hyong J.L., Dong Z. Equol, a Metabolite of the Soybean Isoflavone Daidzein, Inhibits Neoplastic Cell Transformation by Targeting the MEK/ERK/P90RSK/Activator Protein-1 Pathway. J. Biol. Chem. 2007;282:32856–32866. doi: 10.1074/jbc.M701459200. [DOI] [PubMed] [Google Scholar]
- 122.Hong S., Ryu K.S., Oh M.S., Ji I., Ji T.H. Roles of Transmembrane Prolines and Proline-Induced Kinks of the Lutropin/Choriogonadotropin Receptor. J. Biol. Chem. 1997;272:4166–4171. doi: 10.1074/jbc.272.7.4166. [DOI] [PubMed] [Google Scholar]
- 123.Avantaggiato A., Bertuzzi G., Vitiello U., Iannucci G., Pasin M., Pascali M., Cervelli V., Carinci F. Role of Antioxidants in Dermal Aging: An In Vitro Study by q-RT-PCR. Aesthetic Plast Surg. 2014;38:1011–1016. doi: 10.1007/s00266-014-0380-9. [DOI] [PubMed] [Google Scholar]
- 124.Pandel R., Poljšak B., Godic A., Dahmane R. Skin Photoaging and the Role of Antioxidants in Its Prevention. ISRN Derm. 2013;2013:1–11. doi: 10.1155/2013/930164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Velarde M.C., Flynn J.M., Day N.U., Melov S., Campisi J. Mitochondrial Oxidative Stress Caused by Sod2 Deficiency Promotes Cellular Senescence and Aging Phenotypes in the Skin. Aging. 2012;4:3–12. doi: 10.18632/aging.100423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cheng C., Wang X., Weakley S.M., Kougias P., Lin P.H., Yao Q., Chen C. The Soybean Isoflavonoid Equol Blocks Ritonavir-Induced Endothelial Dysfunction in Porcine Pulmonary Arteries and Human Pulmonary Artery Endothelial Cells. J. Nutr. 2010;140:12–17. doi: 10.3945/jn.109.110981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chung J.E., Kim S.Y., Jo H.H., Hwang S.J., Chae B., Kwon D.J., Lew Y.O., Lim Y.T., Kim J.H., Kim E.J., et al. Antioxidant Effects of Equol on Bovine Aortic Endothelial Cells. Biochem. Biophys. Res. Commun. 2008;375:420–424. doi: 10.1016/j.bbrc.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 128.Kamiyama M., Kishimoto Y., Tani M., Utsunomiya K., Kondo K. Effects of Equol on Oxidized Low-Density Lipoprotein-Induced Apoptosis in Endothelial Cells. J. Atheroscler. Thromb. 2009;16:239–249. doi: 10.5551/jat.1057. [DOI] [PubMed] [Google Scholar]
- 129.Bar-Or D., Bar-Or R., Rael L.T., Brody E.N. Oxidative Stress in Severe Acute Illness. Redox. Biol. 2015;4:340–345. doi: 10.1016/j.redox.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Khansari N., Shakiba Y., Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009;3:73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 131.Landskron G., De la Fuente M., Thuwajit P., Thuwajit C., Hermoso M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gupta S.C., Sundaram C., Reuter S., Aggarwal B.B. Inhibiting NF-ΚB Activation by Small Molecules as a Therapeutic Strategy. Biochim. Biophys. Acta Gene Regul. Mech. 2010;1799:775–787. doi: 10.1016/j.bbagrm.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hayden M.S., Ghosh S. NF-ΚB, the First Quarter-Century: Remarkable Progress and Outstanding Questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lephart E.D. Protective Effects of Equol and Their Polyphenolic Isomers against Dermal Aging: Microarray/Protein Evidence with Clinical Implications and Unique Delivery into Human Skin. Pharm. Biol. 2013;51:1393–1400. doi: 10.3109/13880209.2013.793720. [DOI] [PubMed] [Google Scholar]
- 136.Kang J.S., Yoon Y.D., Han M.H., Han S.B., Lee K., Kang M.R., Moon E.Y., Jeon Y.J., Park S.K., Kim H.M. Estrogen Receptor-Independent Inhibition of Tumor Necrosis Factor-α Gene Expression by Phytoestrogen Equol Is Mediated by Blocking Nuclear Factor-ΚB Activation in Mouse Macrophages. Biochem. Pharm. 2005;71:136–143. doi: 10.1016/j.bcp.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 137.Kang J.S., Yoon Y.D., Han M.H., Han S.B., Lee K., Park S.K., Kim H.M. Equol Inhibits Nitric Oxide Production and Inducible Nitric Oxide Synthase Gene Expression through Down-Regulating the Activation of Akt. Int. Immunopharmacol. 2007;7:491–499. doi: 10.1016/j.intimp.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 138.Blay M., Espinel A.E., Delgado M.A., Baiges I., Bladé C., Arola L., Salvadó J. Isoflavone Effect on Gene Expression Profile and Biomarkers of Inflammation. J. Pharm. Biomed. Anal. 2010;51:382–390. doi: 10.1016/j.jpba.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 139.Gopaul R., Knaggs H.E., Lephart E.D. Biochemical Investigation and Gene Analysis of Equol: A Plant and Soy-Derived Isoflavonoid with Antiaging and Antioxidant Properties with Potential Human Skin Applications. BioFactors. 2012;38:44–52. doi: 10.1002/biof.191. [DOI] [PubMed] [Google Scholar]
- 140.Johnson S.L., Kirk R.D., Dasilva N.A., Ma H., Seeram N.P., Bertin M.J. Polyphenol Microbial Metabolites Exhibit Gut and Blood–Brain Barrier Permeability and Protect Murine Microglia against Lps-Induced Inflammation. Metabolites. 2019;9:78. doi: 10.3390/metabo9040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Subedi L., Ji E., Shin D., Jin J., Yeo J.H., Kim S.Y. Equol, a Dietary Daidzein Gut Metabolite Attenuates Microglial Activation and Potentiates Neuroprotection in Vitro. Nutrients. 2017;9:207. doi: 10.3390/nu9030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moriyama M., Hashimoto A., Satoh H., Kawabe K., Ogawa M., Takano K., Nakamura Y. S-Equol, a Major Isoflavone from Soybean, Inhibits Nitric Oxide Production in Lipopolysaccharide-Stimulated Rat Astrocytes Partially via the GPR30-Mediated Pathway. Int. J. Inflam. 2018;2018:8496973. doi: 10.1155/2018/8496973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lephart E.D. Equol’s Anti-Aging Effects Protect against Environmental Assaults by Increasing Skin Antioxidant Defense and ECM Proteins While Decreasing Oxidative Stress and Inflammation. Cosmetics. 2018;5:16. doi: 10.3390/cosmetics5010016. [DOI] [Google Scholar]
- 144.Kim E.Y., Kim A.K. Combination Effect of Equol and TRAIL against Human Cervical Cancer Cells. Anticancer Res. 2013;33:903–912. [PubMed] [Google Scholar]
- 145.Adam V., Ekblad M., Sweeney K., Müller H., Busch K.H., Johnsen C.T., Kang N.R., Lemoine N.R., Halldén G. Synergistic and Selective Cancer Cell Killing Mediated by the Oncolytic Adenoviral Mutant AdΔΔ and Dietary Phytochemicals in Prostate Cancer Models. Hum. Gene. 2012;23:1003–1015. doi: 10.1089/hum.2012.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Teng H., Zheng Y., Cao H., Huang Q., Xiao J., Chen L. Enhancement of Bioavailability and Bioactivity of Diet-Derived Flavonoids by Application of Nanotechnology: A Review. Crit. Rev. Food Sci. Nutr. 2021;19:1–16. doi: 10.1080/10408398.2021.1947772. [DOI] [PubMed] [Google Scholar]
- 147.Aiello P., Consalvi S., Poce G., Raguzzini A., Toti E., Palmery M., Biava M., Bernardi M., Kamal M.A., Perry G., et al. Dietary Flavonoids: Nano Delivery and Nanoparticles for Cancer Therapy. Semin. Cancer Biol. 2021;69:150–165. doi: 10.1016/j.semcancer.2019.08.029. [DOI] [PubMed] [Google Scholar]
- 148.Maan G., Sikdar B., Kumar A., Shukla R., Mishra A. Role of Flavonoids in Neurodegenerative Diseases: Limitations and Future Perspectives. Curr. Top Med. Chem. 2020;20:1169–1194. doi: 10.2174/1568026620666200416085330. [DOI] [PubMed] [Google Scholar]
- 149.Mishra P.K. Nano-Engineered Flavonoids for Cancer Protection. Front. Biosci. 2019;24:4771. doi: 10.2741/4771. [DOI] [PubMed] [Google Scholar]
- 150.Yang P., Zhang J., Xiang S., Jin Z., Zhu F., Wang T., Duan G., Liu X., Gu Z., Li Y. Green Nanoparticle Scavengers against Oxidative Stress. ACS Appl. Mater. Interfaces. 2021;13:39126–39134. doi: 10.1021/acsami.1c12176. [DOI] [PubMed] [Google Scholar]
- 151.Turuvekere Vittala Murthy N., Agrahari V., Chauhan H. Polyphenols against Infectious Diseases: Controlled Release Nano-Formulations. Eur. J. Pharm. Biopharm. 2021;161:66–79. doi: 10.1016/j.ejpb.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 152.Yoshikata R., Myint K.Z.Y., Ohta H., Ishigaki Y. Effects of an Equol-Containing Supplement on Advanced Glycation End Products, Visceral Fat and Climacteric Symptoms in Postmenopausal Women: A Randomized Controlled Trial. PLoS ONE. 2021;16:e0257332. doi: 10.1371/journal.pone.0257332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu Z., Zhang D., Li G., Ho S.C., Chen Y., Ma J., Huang Q., Li S., Ling W. The 6-month effect of whole soy and purified isoflavones daidzein on thyroid function—A double-blind, randomized, placebo controlled trial among Chinese equol-producing postmenopausal women. Phytother. Res. 2021;35:5838–5846. doi: 10.1002/ptr.7244. [DOI] [PubMed] [Google Scholar]
- 154.Caruso S., Cianci S., Fava V., Rapisarda A.M.C., Cutello S., Cianci A. Vaginal Health of Postmenopausal Women on Nutraceutical Containing Equol. Menopause. 2018;25:430–435. doi: 10.1097/GME.0000000000001061. [DOI] [PubMed] [Google Scholar]
- 155.Bosland M.C., Enk E., Schmoll J., Schlicht M.J., Randolph C., Deaton R.J., Xie H., Zeleniuch-Jacquotte A., Kato I. Soy Protein Supplementation in Men Following Radical Prostatectomy: A 2-Year Randomized, Placebo-Controlled Clinical Trial. Am. J. Clin. Nutr. 2021;113:821–831. doi: 10.1093/ajcn/nqaa390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schneider L.S., Hernandez G., Zhao L., Franke A.A., Chen Y.-L., Pawluczyk S., Mack W.J., Brinton R.D. Safety and Feasibility of Estrogen Receptor-β Targeted PhytoSERM Formulation for Menopausal Symptoms: Phase 1b/2a Randomized Clinical Trial. Menopause. 2019;26:874–884. doi: 10.1097/GME.0000000000001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ribeiro A.E., Monteiro N.E.S., de Moraes A.V.G., Costa-Paiva L.H., Pedro A.O. Can the Use of Probiotics in Association with Isoflavone Improve the Symptoms of Genitourinary Syndrome of Menopause? Results from a Randomized Controlled Trial. Menopause. 2019;26:643–652. doi: 10.1097/GME.0000000000001279. [DOI] [PubMed] [Google Scholar]
- 158.Furlong O.N., Parr H.J., Hodge S.J., Slevin M.M., Simpson E.E., McSorley E.M., McCormack J.M., Magee P.J. Consumption of a Soy Drink Has No Effect on Cognitive Function but May Alleviate Vasomotor Symptoms in Post-Menopausal Women; a Randomised Trial. Eur. J. Nutr. 2020;59:755–766. doi: 10.1007/s00394-019-01942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.