Abstract
Cysteine proteinases of Entamoeba histolytica are considered to be one of the most important classes of molecules responsible for the parasite's ability to destroy human tissues. Interestingly, one particular cysteine proteinase, located on the surface of E. histolytica trophozoites and designated cysteine proteinase 5 (CP5), is not expressed in the closely related but nonpathogenic species Entamoeba dispar. By comparing the E. histolytica and E. dispar genomic loci containing the gene for CP5 (cp5), it was found that the position of cp5 within the genomic context is conserved between the two organisms, but that the gene is highly degenerated in E. dispar, as it contains numerous nucleotide exchanges, insertions, and deletions, resulting in multiple stop codons within the cp5 reading frame. An alignment of all available orthologous E. histolytica and E. dispar DNA sequences suggested that cp5 started to degenerate in E. dispar coincidently when the two organisms began to diverge from a common ancestor.
Entamoeba histolytica and Entamoeba dispar are genetically distinct but closely related protozoan species (6). Both colonize the human gut, but only E. histolytica is able to invade the tissues and cause disease such as hemorrhagic colitis and extraintestinal abscesses. As these amoeba species are highly similar in genetic background, cell biology, and host range (both can infect only humans and a few Old World monkey species), the comparison between E. histolytica and E. dispar constitutes an interesting area of research for identifying and analyzing factors which might be important for amoeba pathogenicity (9).
E. histolytica is characterized by its extraordinary capacity to invade and destroy human tissues. A number of molecules considered important for the tissue-damaging activity, including the galactose-inhibitable surface lectin (14), pore-forming peptides (known as amoebapores) (11), and cysteine proteinases (20), have been identified. Although quantitative differences have been observed, qualitatively, the various classes of molecules are present in both amoeba species. The most striking difference reported so far was found in the expression of cysteine proteinases. Compared to E. histolytica, E. dispar contains much less cysteine proteinase activity, apparently as a result of a lower number of cysteine proteinase-expressing genes (3, 21). So far, six genes (ehcp1 to ehcp6) encoding cysteine proteinases in E. histolytica, four of which (ehcp1, ehcp2, ehcp3, and ehcp5) are expressed in cultured trophozoites, have been identified. N-terminal sequencing of the respective purified enzymes revealed that EhCP1, EhCP2, and EhCP5 are responsible for at least 90% of the total cysteine proteinase activity in E. histolytica (3). Interestingly, functional genes homologous to two of the various ehcp genes are missing in E. dispar. Whereas genes homologous to ehcp2, ehcp3, ehcp4, and ehcp6 with high sequence similarity (about 95%) were found in E. dispar, the genes respectively encoding EhCP1 or EhCP5 were found to be absent in this amoeba species by Southern blot analysis (3). With regard to Entamoeba pathogenicity, the absence of an EhCP5 homologue in E. dispar seems to be of particular interest. In contrast to the other cysteine proteinases, which all are found within the amoeba granules, EhCP5 is exceptional in that it is the only one that is localized on the amoeba surface (10). As EhCP5 is presently the only structurally characterized member of the amoebic cysteine proteinase family that is exclusively present in E. histolytica and appears to be functionally unique, it is tempting to hypothesize that EhCP5 is an important factor for amoeba pathogenicity. However, the genetic basis for the lack of an EhCP5 homologue in E. dispar remains to be determined. Whereas the nucleotide sequences of the various cp genes identified so far differ between 15 and 60% within each group of amoebae, the interspecies differences of orthologous cp genes comprise only 4 to 8%. Therefore, it is most likely that the various cp genes had evolved before the two organisms diverged from a common ancestor. In addition, according to their close phylogenetic relationship, linkage of genes within the genomic context was found to be highly conserved (26). Thus, it is difficult to explain the absence of an ehcp5 homologue in E. dispar, as it would imply that the gene was lost after the separation of the two organisms, which should have resulted in a deletion of a particular genomic region.
Here, we report on the comparison of the respective genomic regions from E. histolytica and E. dispar and show that a sequence corresponding to ehcp5 is present and positionally conserved in E. dispar. However, the gene is highly degenerated and does not contain any overt open reading frame (ORF), suggesting that the gene has been nonfunctional for a considerable period of time during the evolution of the nonpathogenic amoeba species.
MATERIALS AND METHODS
Cultivation of parasites.
The E. histolytica isolates HM-1:IMSS, HK-9, and 200:NIH, as well as the E. dispar isolates ERI1007, SAW 142, and SAW 760, were included in this study. HM-1:IMSS, HK-9, and 200:NIH were cultured under axenic conditions, SAW 760 was cultured under monoxenic conditions in the presence of Crithidia fasciculata, and ERI1007 and SAW 142 were cultured under polyxenic conditions in the presence of mixed intestinal flora. All isolates were cultured in TYI-S-33 or TYSGM9 medium, as previously described (7, 8). All isolates were classified as E. histolytica or E. dispar by zymodeme and DNA analyses (19, 22).
Molecular cloning of genomic DNA sequences of E. histolytica and E. dispar.
A genomic E. histolytica library, derived from the isolate HM-1:IMSS, a generous gift from John Samuelson (Harvard School of Public Health, Boston, Mass.) was screened with an ehcp5 cDNA probe previously identified in our laboratory (10). Independent overlapping clones were identified by restriction analysis with several enzymes. The corresponding E. dispar sequence was identified by screening a genomic library derived from the isolate SAW 760, a generous gift from Michael Duchene (Institute of Specific Prophylaxis and Tropical Medicine, Vienna, Austria). Screening of this library was performed with a 5′ fragment of the E. histolytica cation-transporting ATPase gene under moderate stringency. The various genomic E. histolytica and E. dispar DNA fragments were sequenced by the dideoxy chain-termination method with an ABI 377 sequencer.
PCR analysis.
A number of different genomic DNA fragments from the various E. histolytica and E. dispar isolates were amplified by PCR and subjected to DNA sequencing. PCR was performed under standard conditions (17) with template DNA isolated from cultured amoeba trophozoites by a commercially available DNA extraction kit (Invitrogen), according to the manufacturer's recommendation. DNA fragments were amplified with primer pairs, as follows: 496 bp containing the 388-bp sod-actin intergenic region (5′-GAG CTG CTT ACT TAG AAC ATT GGT GG and 5′-CCA GAT CCA TTA TCT ACA ACA AGT GC); 742 bp containing the 695-bp H4-nifR3 intergenic region (5′-CCA AGA GTT ACT CCC TTT CCT CC and 5′-CCA GAA TTT TCC ACT CTT TTA CAT TC); 538 bp containing part of the 5′ upstream region, the complete exon 1 (E1), the intron, and part of exon 2 (E2) of the cation-transporting ATPase gene (5′-CTG CAT TAT CTC AAT TTG TTC C and 5′-GGA TCT TCA TTA ATT CTA ATT G); and 564 bp containing part of the 5′ upstream region and part of the coding region of cp5 (for E. histolytica isolates, 5′-GTA AAA TTC AGA CGT ATT TAA TG and 5′-CAA CAG ATT CTG GTA CAT CTC CCC; for the corresponding sequence in E. dispar isolates, 5′-GTA ATA AAT TTC AGA GGT ATT AAT G and 5′-CAA ATG ATT CTG GTG TAT CTC TCC).
Northern blot analysis.
Amoeba RNA was isolated according to standard procedures (17). For Northern blot analysis, 10 μg of total RNA or 2 μg of poly(A)+ RNA were separated on a 1% agarose–formaldehyde gel and transferred to nylon membranes for hybridization with randomly primed probes.
Nucleotide sequence accession numbers.
Nucleotide sequence data reported in this paper have been submitted to the EMBL, GenBank, and DDBJ databases under the accession no. X91644 and AF118046.
RESULTS
Analysis of 10.5 kb of E. histolytica genomic sequence encompassing ehcp5.
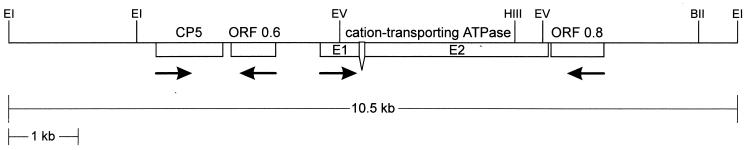
An ehcp5 cDNA sequence was used as a probe to isolate overlapping genomic clones covering a stretch of about 10.5 kb of contiguous E. histolytica genomic DNA of the isolate HM-1:IMSS. Sequence analysis revealed the presence of the entire 1 kb of the ehcp5 coding region flanked by an additional 2.1 kb of upstream sequences and 7.4 kb of downstream sequences (Fig. 1). Within the upstream sequence no overt coding regions or significant ORFs were detected. In contrast, 1.5 kb downstream of ehcp5, a gene (ehcta) was identified encoding a protein with a calculated molecular mass of 121 kDa, which exhibited significant sequence similarity (about 30 to 50%) to cation-transporting ATPases of various species. Comparison between the genomic ehcta sequence and a respective full-length cDNA clone indicated the presence of a small intron of 69 bp which contained the typical 5′ and 3′ sequences found in introns of other E. histolytica genes (12, 16, 24). Besides ehcta, sequence analysis indicated the presence of two further ORFs of about 0.6 and 0.8 kb, one located between ehcp5 and ehcta and the other located downstream of ehcta. Database searches indicated significant similarity between the ORF0.6-derived amino acid sequence and that of a protein encoded by ORF0.75 (accession no. X70851), a sequence previously identified upstream of the E. histolytica amoebapore A gene, whereas the ORF0.8 gene product was found to represent a homologue to the Proteus mirabilis NrpG protein (accession no. U46488) and the Bacillus brevis GSP protein (accession no. A55218). Relative to that of ehcp5, transcription of ehcta is in the same orientation, whereas transcription of ORF0.6 and ORF0.8 is in the reverse orientation.
FIG. 1.
Schematic description of the E. histolytica 10.5-kb genomic region containing the gene for CP5. Shown is a partial restriction map with the locations of ehcp5 (CP5), ORF0.6 (ORF 0.6), and ORF0.8 (ORF 0.8) as well as of the ehcta gene (cation-transporting ATPase) indicated. The latter consists of two exons (E1 and E2), which are separated by a 69-bp intron. Orientations of the various coding sequences are indicated by arrows. Cleavage sites of relevant enzymes are indicated. BII, BglII; EI, EcoRI; EV, EcoRV; HIII, HindIII.
Identification and analysis of an ehcp5 orthologous sequence in E. dispar.
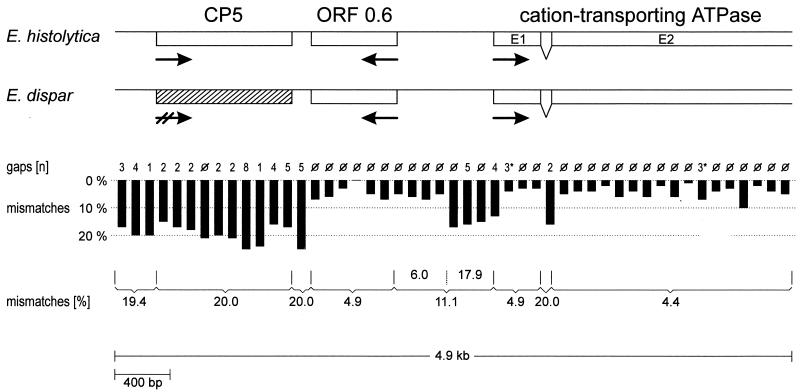
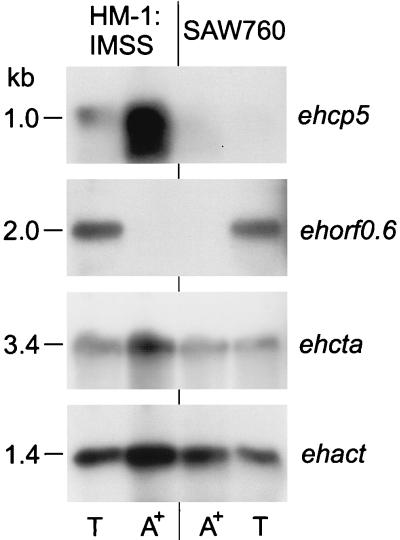
Southern blot analysis indicated single-copy representation of the cation-transporting ATPase gene within the genomes of E. histolytica and E. dispar (data not shown). Therefore, ehcta was used as a probe to clone the corresponding genomic region from a respective E. dispar library constructed from total genomic DNA of the isolate SAW 760. A 4.854-kb DNA fragment, which was found to represent the orthologous sequence corresponding to the stretch between nucleotide positions 1819 and 6693 of the 10.5-kb E. histolytica genomic region, was isolated (Fig. 2). The E. dispar sequence contained an entire cp5 and ORF0.6 homologue as well as about 2.1 kb of the 5′ coding region of the cation-transporting ATPase gene. Compared to those of E. histolytica, the various sequences, including that of the intron within the ATPase gene, were found to be positionally conserved. ORF0.6 as well as the two cta exons of both organisms exhibited more than 95% sequence identity, whereas cp5 and the adjacent upstream and downstream sequences, as well as the cta intron, revealed only 80% identity. The 700-bp intergenic region between ORF0.6 and cta showed an 11% difference but could be divided into two regions. The stretch of about 400 bp upstream adjacent to ORF0.6 revealed sequences with only 6% nucleotide exchanges, whereas an 18% difference was found within the 300 nucleotides preceding the cta translation initiation ATG. Despite the various nucleotide exchanges within the E. dispar ORF0.6 and cta homologues, the ORFs of both genes remained intact. In contrast, the sequence corresponding to ehcp5 was found to be highly degenerated, as it contained numerous nucleotide insertions and deletions, resulting in multiple stop codons. To examine whether the sequence differences of the two genomic regions were due to particular properties of the isolates HM-1:IMSS and SAW 760 or whether they were indicative of genomic differences between E. histolytica and E. dispar in general, additional amoeba isolates were investigated. With appropriate pairs of primers and total genomic DNA of the E. histolytica isolates 200:NIH and HK9 as well as of the E. dispar isolates SAW 142 and ERI1007, two regions of about 550 bp within the 4.9-kb sequence were amplified by PCR. Sequence analysis revealed more than 99% sequence identity between the various isolates within each group of amoebae irrespective of whether coding or noncoding regions were investigated (data not shown). Northern blot analysis indicated that cp5 is expressed in E. histolytica but not in E. dispar, whereas ORF0.6 and cta are expressed in both species. Interestingly, ORF0.6 did not hybridize to poly(A)+ RNA but to a poly(A)− RNA species which appeared to be considerably larger than 0.6 kb (Fig. 3).
FIG. 2.
Comparison between the E. histolytica CP5 gene-containing genomic region and the orthologous sequence from E. dispar. Shown is a schematic description of the two sequences. Coding regions are represented by open boxes and their orientations are represented by arrows. The position of the degenerated sequence homologous to ehcp5 is shown as a hatched box. The vertical black bars each represent a stretch of 100 nucleotides and indicate the percentage of mismatches found. Numbers above each bar indicate the number of gaps that have to be introduced for optimal alignment. Note that the gaps within exon 1 (E1) and exon 2 (E2) of the cation-transporting ATPase gene constitute three consecutive nucleotides each and result in deletion of a single codon in the E. dispar sequence but do not disrupt the reading frame. Numbers below the bars represent percentages of mismatches found for the various coding and noncoding regions.
FIG. 3.
Northern blot analysis. Ten micrograms each of total (T) and poly(A)+ (A+) RNA from the E. histolytica isolate HM-1:IMSS and the E. dispar isolate SAW 760 were subjected to electrophoresis, blotted, and sequentially hybridized with respective coding regions derived from the E. histolytica genomic sequence. An E. histolytica actin probe served as the control.
Comparison between orthologous E. histolytica and E. dispar sequences.
To further determine the phylogenetic relationship between E. histolytica and E. dispar, a comparison was performed between all of the currently available orthologous DNA sequences of the two amoeba species. As only a limited number of respective noncoding sequences have been deposited in public databases, the intergenic regions of about 400 bp and 700 bp, respectively, located between the sod gene and an actin gene copy (4) and between the histone H4 and the nifR3 gene (1) were amplified by PCR with appropriate pairs of primers and total DNA of the various E. histolytica and E. dispar isolates. Sequence analysis of the amplified fragments again revealed more than 99% sequence identity within each group of amoebae, but between the two species, only 79.2% identity (400-bp sod-actin intergenic region) and 91.0% identity (700-bp H4-nifR3 intergenic region), respectively, were found. Together with the two intergenic regions, a total of 8 noncoding and 17 coding sequences were subjected to the alignment of orthologous sequences (Table 1). The results indicated sequence identity of 93% on average for coding regions, with results ranging from 78 to 99.5%, whereas for noncoding sequences, identity was about 87% on average, with results ranging from 79 to 96.5%. These values perfectly matched those recently found by an alignment of orthologous sequences of mouse and rat but are substantially higher than those found when mouse and human sequences were aligned (13).
TABLE 1.
Alignment of orthologous DNA and protein sequences
| Sequences compared | Value for sequences that were:
|
||||
|---|---|---|---|---|---|
| Coding
|
Noncoding
|
||||
| % Identity
|
Aligned length (bp) | % Identity | Aligned length (bp) | ||
| Protein | DNA | ||||
| Mouse and humana | |||||
| Mean (SD) | 86.4 (12.3) | 85.2 (6.5) | 1,425 (1,164) | 71.0 (12.2) | 416 (432) |
| Median | 89.0 | 86.2 | 1,175 | 69.4 | 263 |
| Range | 41.1–100 | 60.7–97.6 | 135–13,365 | 31.1–100 | 40–3,478 |
| Mouse and rata | |||||
| Mean (SD) | 94.5 (6.3) | 93.8 (3.2) | 1,293 (951) | 86.3 (8.9) | 392 (391) |
| Median | 96.6 | 94.3 | 1,104 | 87.7 | 235 |
| Range | 62.4–100 | 75.4–98.9 | 154–8,250 | 48.8–100 | 43–2,996 |
| E. histolytica and E. dispar | |||||
| Mean (SD) | 93.0 (5.6) | 93.5 (3.6) | 886 (549) | 86.9 (6.5) | 366 (179) |
| Median | 94.2 | 94.4 | 710 | 87.6 | 350 |
| Range | 78.0–99.5 | 86.0–99.4 | 310–1,920 | 79.2–96.5 | 135–600 |
Data were obtained from reference 13.
DISCUSSION
In an attempt to characterize the molecular basis for the failure to express a CP5-analogous enzyme in E. dispar, the cp5-containing genomic regions from E. histolytica and E. dispar were compared. Consistent with recent observations, which indicated high conservation of gene linkage groups between the two amoeba species (26), it was found that a sequence corresponding to cp5 is present and positionally conserved in E. dispar. However, the gene is highly degenerated and does not possess a functional reading frame. Interestingly, this degeneration is limited to the cp5 locus and does not extend to adjacent genes. Like other amoeba genes, cp5 is closely linked to other expressed ORFs (1, 4, 15), supporting the notion that coding sequences are clustered on the amoeba chromosomes (25), with intergenic sequences of less than 1,500 bp (4). One of the adjacent genes was identified as an intron-containing sequence encoding a cation-transporting ATPase. To our knowledge this is only the fourth intron-containing gene of E. histolytica so far reported and the second identified in E. dispar. As all of the various introns contain at their 5′ splice sites the unusual hexanucleotide motif 5′-GTTTGT (12, 16, 24), this motif will facilitate the identification of introns within other E. histolytica or E. dispar genomic sequences.
Besides the cation-transporting ATPase gene, an ORF of about 0.6 kb (ORF0.6) was found to be highly conserved between the two amoeba species. As ORF0.6 hybridized, not to poly(A)+ RNA, but to poly(A)− RNA of about 2 kb, processing and function of the ORF0.6 transcript will be of interest. With respect to recent findings for the related amoeba species Entamoeba invadens, which indicated that the histone H2B RNA is present in both the trophozoite and the encysting stage but is preferentially polyadenylated during encystation (18), it might be speculated that the ORF0.6 transcript is differentially polyadenylated during amoeba-stage conversion. Unfortunately, at present it will be difficult to prove or disprove this hypothesis, as all attempts have failed so far to induce in vitro cyst formation in E. histolytica or E. dispar.
The most interesting and unexpected finding was the identification of a DNA sequence in E. dispar with 80% identity to ehcp5. Previous Southern blot analyses had suggested that such a sequence is missing in E. dispar (3). However, the failure to detect a cp5 homologue was obviously due to the specific hybridization conditions used, which were designed to identify sequence differences of up to 15% only. These conditions had been selected to avoid cross-hybridization between the various cp genes within one species, which differ in their nucleotide sequences by 15 to 60%. On the other hand, interspecies variation of orthologous cp genes as far as identified did not exceed 8%. These differences are limited to nucleotide exchanges and do not alter the cp reading frames. In contrast, the differences between the two cp5 sequences of about 20%, which are caused by nucleotide exchanges as well as nucleotide insertions and deletions, resulted in destruction of a functional gene in E. dispar. This most likely indicates that the E. dispar sequence underwent random mutations for a considerable period of time after the two organisms diverged from a common ancestor.
Previous comparisons of amoeba rRNA sequences had suggested that E. histolytica and E. dispar are as different as human and mice (5). Although rRNA analysis has been widely used as a powerful tool for identifying and characterizing prokaryotic and eukaryotic strains and species, many questions remain regarding the validity of evolutionary conclusions based on rRNA analysis (2). Our analysis of all available orthologous sequences indicates that most likely the two amoeba species are substantially more closely related than humans and mice. Unfortunately, only a limited number of sequences could be introduced into the comparison and the selection of sequences might not be representative. For a definite conclusion about the degree of sequence divergency between the two amoeba species, a larger number of sequences have to be analyzed. Nevertheless, despite the small number of comparable sequences, it seems noteworthy that the degree of differences between corresponding noncoding regions did not exceed that found between E. histolytica and E. dispar cp5 sequences, irrespective of whether cp5 coding sequences or the adjacent upstream or downstream sequences were compared. This suggests that the cp5 gene started to degenerate in E. dispar coincidently when the two organisms began to diverge from a common ancestor. If this holds true, it might be speculated that during evolution, the loss of CP5 activity in cp5-defective amoeba mutants provided the basis for the colonization of a new host and subsequently resulted in the development of two separate species. However, it remains to be determined whether the loss of CP5 activity was primarily due to inactivation of the cp5 gene or whether the loss was secondary to other mutational events. As cysteine proteinases have to be converted from their inactive pre-pro forms into active mature enzymes, and CP5 has to be targeted to specific compartments, such as the digestive vacuoles and the amoeba membrane (10), it might be possible that preceding the degeneration of the cp5 gene, E. dispar lost a specific, functional accessory molecule that is important for proper targeting or processing of CP5. Recent advances in DNA-mediated gene transfer of E. histolytica (23) may help to prove or disprove this hypothesis.
ACKNOWLEDGMENTS
We thank Heidrun Buß for skillful technical assistance and John Samuelson of the Harvard School of Public Health, Boston, Mass., and Michael Duchene of the Institute of Specific Prophylaxis and Tropical Medicine, Vienna, Austria, for providing the genomic libraries.
This work was supported by the Deutsche Forschungsgemeinschaft (TA 110/4-1).
REFERENCES
- 1.Binder M, Ortner S, Plaimauer B, Födinger M, Wiedermann G, Scheiner O, Duchene M. Sequence and organization of an unusual histone H4 gene in the human parasite Entamoeba histolytica. Mol Biochem Parasitol. 1995;71:243–247. doi: 10.1016/0166-6851(94)00044-n. [DOI] [PubMed] [Google Scholar]
- 2.Brown J R, Doolittle E F. Archaea and the prokaryote-to-eukaryote transition. Microbiol Mol Biol Rev. 1997;61:456–502. doi: 10.1128/mmbr.61.4.456-502.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruchhaus I, Jacobs T, Leippe M, Tannich E. Entamoeba histolytica and Entamoeba dispar: differences in numbers and expression of cysteine proteinase genes. Mol Microbiol. 1996;22:255–263. doi: 10.1046/j.1365-2958.1996.00111.x. [DOI] [PubMed] [Google Scholar]
- 4.Bruchhaus I, Leippe M, Lioutas C, Tannich E. Unusual gene organization in the protozoan parasite Entamoeba histolytica. DNA Cell Biol. 1993;12:925–933. doi: 10.1089/dna.1993.12.925. [DOI] [PubMed] [Google Scholar]
- 5.Clark C G, Diamond L S. Ribosomal RNA genes of pathogenic and nonpathogenic Entamoeba histolytica are distinct. Mol Biochem Parasitol. 1991;49:297–302. doi: 10.1016/0166-6851(91)90073-f. [DOI] [PubMed] [Google Scholar]
- 6.Diamond L S, Clark C G. A redescription of Entamoeba histolytica Schaudinn, 1903 (emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. J Eukaryot Microbiol. 1993;40:340–344. doi: 10.1111/j.1550-7408.1993.tb04926.x. [DOI] [PubMed] [Google Scholar]
- 7.Diamond L S, Harlow D R, Cunnick C C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72:431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- 8.Diamond L S. A new liquid medium for xenic cultivation of Entamoeba histolytica and other lumen-dwelling protozoa. J Parasitol. 1982;68:958–959. [PubMed] [Google Scholar]
- 9.Horstmann R D, Leippe M, Tannich E. Recent progress in the molecular biology of Entamoeba histolytica. Trop Med Parasitol. 1992;43:213–218. [PubMed] [Google Scholar]
- 10.Jacobs T, Bruchhaus I, Dandekar T, Tannich E, Leippe M. Isolation and molecular characterization of a surface-bound proteinase of Entamoeba histolytica. Mol Microbiol. 1998;27:269–276. doi: 10.1046/j.1365-2958.1998.00662.x. [DOI] [PubMed] [Google Scholar]
- 11.Leippe M. Amoebapores. Parasitol Today. 1997;13:178–183. doi: 10.1016/s0169-4758(97)01038-7. [DOI] [PubMed] [Google Scholar]
- 12.Lohia A, Samuelson J. Cloning of the Ehcdc2 gene from Entamoeba histolytica encoding a protein kinase p34cdc2 homologue. Gene. 1993;127:203–207. doi: 10.1016/0378-1119(93)90720-n. [DOI] [PubMed] [Google Scholar]
- 13.Makalowski W, Boguski M S. Evolutionary parameters of the transcribed mammalian genome: an analysis of 2,820 orthologous rodent and human sequences. Proc Natl Acad Sci USA. 1998;95:9407–9412. doi: 10.1073/pnas.95.16.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCoy J J, Mann B J, Petri W A. Adherence and cytotoxicity of Entamoeba histolytica or how lectins let parasites stick around. Infect Immun. 1994;62:3045–3050. doi: 10.1128/iai.62.8.3045-3050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petter R, Rozenblatt S, Nuchamowitz Y, Mirelman D. Linkage between actin and ribosomal protein L21 genes in Entamoeba histolytica. Mol Biochem Parasitol. 1992;56:329–333. doi: 10.1016/0166-6851(92)90182-j. [DOI] [PubMed] [Google Scholar]
- 16.Plaimauer B, Ortner S, Wiedermann G, Scheiner O, Duchene M. An intron-containing gene coding for a novel 39-kilodalton antigen of Entamoeba histolytica. Mol Biochem Parasitol. 1994;66:181–185. doi: 10.1016/0166-6851(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 18.Sanchez L B, Enea V, Eichinger D. Increased levels of polyadenylated histone H2B mRNA accumulate during Entamoeba invadens cyst formation. Mol Biochem Parasitol. 1994;67:137–146. doi: 10.1016/0166-6851(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 19.Sargeaunt P G, Williams J E. The differentiation of invasive and non-invasive Entamoeba histolytica by isoenzyme electrophoresis. Trans R Soc Trop Med Hyg. 1978;72:519–521. doi: 10.1016/0035-9203(78)90174-8. [DOI] [PubMed] [Google Scholar]
- 20.Scholze H, Tannich E. Cysteine endopeptidases of Entamoeba histolytica. Methods Enzymol. 1994;244:512–523. doi: 10.1016/0076-6879(94)44037-9. [DOI] [PubMed] [Google Scholar]
- 21.Tannich E, Scholze H, Nickel R, Horstmann R D. Homologous cysteine proteinases of pathogenic and nonpathogenic Entamoeba histolytica: differences in structure and expression. J Biol Chem. 1991;266:4798–4803. [PubMed] [Google Scholar]
- 22.Tannich E, Burchard G D. Differentiation of pathogenic from nonpathogenic Entamoeba histolytica by restriction fragment analysis of a single gene amplified in vitro. J Clin Microbiol. 1991;29:250–255. doi: 10.1128/jcm.29.2.250-255.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tannich E. Recent advances in DNA-mediated gene transfer of Entamoeba histolytica. Parasitol Today. 1996;12:198–200. doi: 10.1016/0169-4758(96)10008-9. [DOI] [PubMed] [Google Scholar]
- 24.Urban B, Blasig C, Förster B, Hamelmann C, Horstmann R D. Putative serine/threonine protein kinase expressed in complement-resistant forms of Entamoeba histolytica. Mol Biochem Parasitol. 1996;80:171–178. doi: 10.1016/0166-6851(96)02684-9. [DOI] [PubMed] [Google Scholar]
- 25.Willhoeft U, Tannich E. The electrophoretic karyotype of Entamoeba histolytica. Mol Biochem Parasitol. 1999;99:41–53. doi: 10.1016/s0166-6851(98)00178-9. [DOI] [PubMed] [Google Scholar]
- 26.Willhoeft, U. Unpublished results.