Abstract
Background
Infectious diseases physicians are leaders in assessing the health risks in a variety of community settings. An understudied area with substantial controversy is the safety of dental aerosols. Previous studies have used in vitro experimental designs and/or indirect measures to evaluate bacteria and viruses from dental surfaces. However, these findings may overestimate the occupational risks of dental aerosols. The purpose of this study was to directly measure dental aerosol composition to assess the health risks for dental healthcare personnel and patients.
Methods
We used a variety of aerosol instruments to capture and measure the bacterial, viral, and inorganic composition of aerosols during a variety of common dental procedures and in a variety of dental office layouts. Equipment was placed in close proximity to dentists during each procedure to best approximate the health risk hazards from the perspective of dental healthcare personnel. Devices used to capture aerosols were set at physiologic respiration rates. Oral suction devices were per the discretion of the dentist.
Results
We detected very few bacteria and no viruses in dental aerosols—regardless of office layout. The bacteria identified were most consistent with either environmental or oral microbiota, suggesting a low risk of transmission of viable pathogens from patients to dental healthcare personnel. When analyzing restorative procedures involving amalgam removal, we detected inorganic elements consistent with amalgam fillings.
Conclusions
Aerosols generating from dental procedures pose a low health risk for bacterial and likely viral pathogens when common aerosol mitigation interventions, such as suction devices, are employed.
Keywords: aerosols, COVID-19, dentistry, infection control, infection prevention, SARS-CoV-2
The coronavirus disease 2019 (COVID-19) pandemic has brought substantial awareness of the potential risks associated with dental aerosols. Several common dental procedures are known to generate aerosols, which may increase the risk of dental healthcare personnel (DHCP) being exposed to bacterial and viral pathogens. Laboratory models support these findings, with authors of in vitro experimental studies suggesting that special barriers [1, 2], decreased operatory occupancy [3], and other environmental changes may be necessary to protect DHCPs. However, studies that evaluate the impact of aerosol mitigation devices, such as intraoral suction, suggest that current aerosol mitigation interventions are helpful but may not provide complete protection [4–10]. These studies have generally been limited to the use of water sources that have been experimentally contaminated with bacteria or dyes to evaluate the effectiveness of mitigation strategies. However, this approach does not reflect the clinical practice setting and limits generalizability and accurate interpretation of health risks for DHCPs [9]. Other analyses are limited to indirect studies of dental aerosols with adenosine triphosphate, surface swabs [11], and settle plates [12].
The purpose of this manuscript was to evaluate the occupational health risk of dental aerosols by directly capturing aerosols in a clinical setting with live patients and an experimental design that closely approximates the distance of dentists to aerosols, taking into account DHCP respiratory rate.
METHODS
Experimental Design
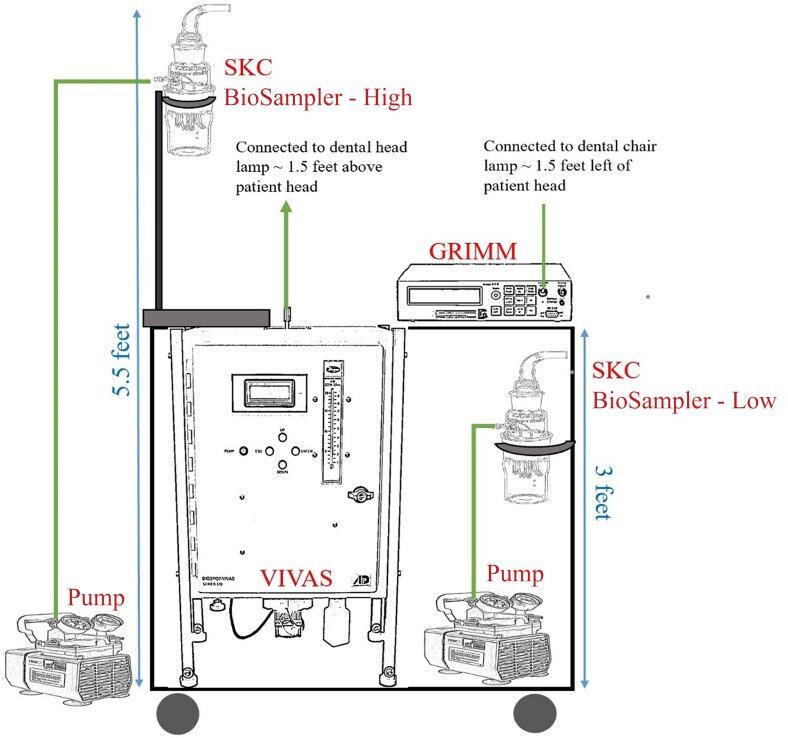
Between 8 June and 8 July 2021, we placed aerosol capturing equipment on a cart (Figure 1). The cart contained a viable virus aerosol sampler (VIVAS) (Aerosol Dynamics, Berkeley, California) and 2 SKC BioSamplers (SKC Inc, Eighty Four, Pennsylvania). VIVAS collects airborne particle by using water vapor condensation method, and previous studies have shown highly efficient (74 ± 12%) collection of particles in the size range from 10 nm to 10 µm [13]. The SKC BioSampler efficiently traps airborne particles into swirling liquid for subsequent analysis and is considered the industry-standard aerosol capture device [14]. One SKC BioSampler was placed 5.5 feet above the ground, and the second was placed approximately 3 feet from the ground. This was to determine if there was a qualitative and quantitative difference in bacterial specimen collection between low and high heights. The VIVAS and SKC BioSampler aerosol suction rate was set at 8 L per minute to approximate human respiration. The cart also included a GRIMM aerosol spectrometer (Model 11D, GRIMM Aerosol, Germany) to quantify the aerosol concentration and particle size during the procedure, similar to our previous studies [13].
Figure 1.
Aerosol sampling cart setup. Pumps were set at 8 L/minute. Abbreviation: VIVAS, viable virus aerosol sampler.
Operatory Layout and Equipment Placement
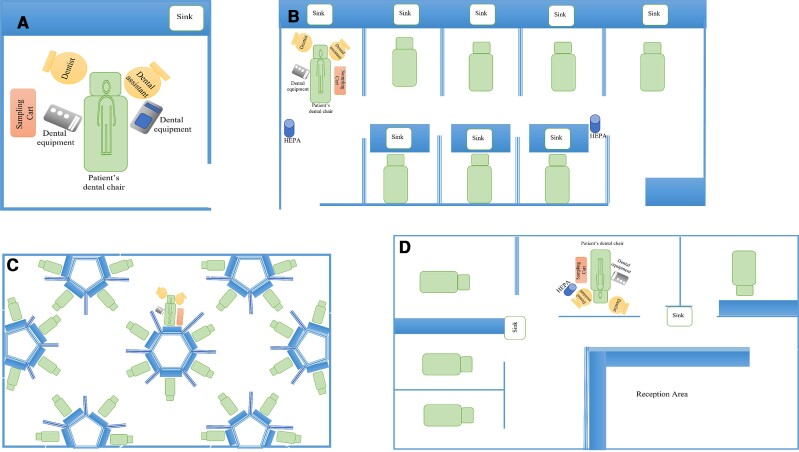
We placed the equipment cart in a variety of operatory layouts (eg, single dental chair with door closed, semi-open bay, and large multioperator space). Each layout is depicted in Figure 2A–D. We placed the equipment cart as close to the patient without disrupting clinical care in order to capture aerosols within close proximity to the DHCP. Specifically, we connected sterile tubing to the VIVAS aerosol intake port; the other side of the tubing was then taped to the dental overhead lamp to approximate the distance between the DHCP and the aerosol emission source.
Figure 2.
Experimental layouts. A, Operatory. B, Semi-open bay type 1. C, Large multioperator space. D, Semi-open bay type 2. Abbreviation: HEPA, high-efficiency particular air filter.
Equipment Preparation and Specimen Collection
Sterile SKC BioSampler impingers were loaded with 20 mL of sterile phosphate-buffered saline (PBS). For the VIVAS, 2 mL of sterile PBS was placed in the sterile collection plate. As the SKC BioSamplers capture aerosols via an air vortex, these were turned on only during aerosol-generating portions of the dental procedure (eg, high-speed drill or ultrasonic scaler use). In settings where the PBS was at risk of evaporating (∼30 minutes), old impingers were exchanged for a new impinger full of PBS, and fluid from the 2 impingers were consolidated into 1 specimen for analysis. The VIVAS, which uses a condensation approach to capture aerosols, continuously collected specimens from beginning to end of dental procedures. This allowed for a more accurate assessment of individual procedure–level risk for DHCPs. The samples were then transported in sealed and labeled containers within 4 hours for microbiological analysis. The detailed protocol is available in the Supplementary File. This process was repeated for each dental procedure. One baseline sample was collected from SKC BioSamplers and the VIVAS in each dental operatory layout while no procedure was being performed for comparison to procedure samples. Total sampling collection time was measured for both SKC BioSamplers and VIVAS. Aerosol mitigation interventions employed by dentists were documented for each procedure.
Microbiological Analysis
We plated each specimen onto (1) tryptic soy agar with 5% sheep's blood (BAP), (2) chocolate agar (CHOC), and (3) brucella blood agar (BBA). BAP and CHOC agars were incubated for up to 48 hours in a 35°C carbon dioxide incubator. BBA was incubated in anaerobic conditions for 5–7 days. After 24 hours of incubation for the BAB and CHOC agars and 48 hours for the BBA, the plates were examined for growth and the number of bacterial colonies was counted. Subculturing was performed if >1 bacterial colony type was observed. Colonies were identified at the genus and species level using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) with the VITEK MS MALDI-TOF MS (bioMérieux).
We performed viral analyses by sampling 300 µL each of the SKC BioSampler and VIVAS specimens, and testing with the BioFire Respiratory Pathogen Panel 2.1 (RP2.1; bioMérieux, Salt Lake City, Utah). The RP2.1 can detect up to 22 respiratory pathogens.
Chemical Analyses
During amalgam removal procedures, we collected aerosol particles on a stub with carbon tape by holding it 15 cm away from the patient's mouth. The weight of the stub was noted before and after collection. Scanning electron microscopy was performed on the sample with voltage 20 kV and working distance of 9.9 mm followed by energy dispersive X-ray (EDX) analysis to find the elemental composition of the area in focus.
This study was reviewed and approved by the Washington University Human Rights Protection Office.
RESULTS
We evaluated the bacterial and viral composition of dental aerosols for 12 procedures in 4 different dental office layouts. A description of each procedure and the microbiological results are presented in Table 1. Of note, individual dental procedures ran from 30 to 74 minutes and involved both anterior and posterior teeth. DHCPs utilized between 1 and 3 aerosol mitigation strategies, with high-volume evacuation the most common (large-bore dental suction devices), followed by saliva ejectors (standard-bore dental suction devices), high-efficiency particulate air (HEPA) filters, and rubber dams (rubber material that covers part of the mouth).
Table 1.
Bacterial and Viral Sampling Results for Common Dental Procedures in Real-World Settings
| Procedure Type | Sampling Time, min | Aerosol Mitigation Approach | Dental Space | Bacterial Culture | Respiratory Virus | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| VIVAS | SKC | HVE | Saliva Ejector | HEPA Filter | Rubber Dam | VIVAS | SKC | VIVAS and SKC | ||
| Implant | 45 | 17 | 1 | 1 | 0 | 0 | Operating room | NG | NG | … |
| Ultrasonic cleaning | 30 | 25 | 1 | 1 | 1 | 0 | Semi-open bay type 1 | NG | NG | … |
| Gingival flap with cavitron | 74 | 30 | 1 | 1 | 1 | 0 | Semi-open bay type 1 | NG | a | … |
| Root canal amalgam removal | 43 | 30 | 1 | 0 | 0 | 1 | Semi-open bay type 1 | NG | NG | … |
| Root canal drilling through composite | 40 | 30 | 1 | 0 | 1 | 1 | Semi-open bay type 1 | NG | NG | … |
| Root canal amalgam removed; temporary crown placement | … | 22 | 1 | 0 | 0 | 1 | Operating room | NG | b | … |
| Braces debonding | 31 | 30 | 0 | 1 | 0 | 0 | Large multioperator space | c | NG | … |
| Braces debonding | 59 | 30 | 1 | 1 | 0 | 0 | Large multioperator space | NG | NG | … |
| Braces debonding | 30 | 21 | 0 | 1 | 0 | 0 | Large multioperator space | d | NG | … |
| Amalgam removal and replacement | 30 | 14 | 1 | 0 | 0 | 0 | Semi-open bay type 2 | e | NG | … |
| Post and core CEREC crown | 30 | 26 | 1 | 0 | 1 | 0 | Semi-open bay type 2 | NG | f | … |
| Amalgam removal and composite filling | 30 | 16 | 1 | 0 | 1 | 0 | Semi-open bay type 2 | g | h | … |
SKC BioSampler run times were the same for high and low settings.
Abbreviations: CEREC, chairside economical restoration of esthetic ceramics; HEPA, high-efficiency particular air; HVE, high-volume evacuator; NG, no growth; VIVAS, viable virus aerosol sampler.
One Micrococcus luteus, 2 Staphylococcus hominis.
One Cutibacterium acnes.
Seventeen Cutibacterium acnes.
One Streptococcus gordonii, 1 Actinomyces oris/viscosus.
One Prevotella oris.
One Stenotrophomonas maltophilia.
One Sphingomonas parapaucimobilis.
One mold.
Microbiological Analyses
Bacterial analysis showed that 5 of 13 procedures had no growth (Table 1). No differences were observed in specimen yield between high- and low-placed SKC BioSamplers (Supplementary Table 1). These results are supportive that we captured aerosols, as a lower placed device should more readily capture large droplets, which settle toward the floor quickly. No substantial differences in microbiological yield were observed between VIVAS and SKC BioSamplers. We identified gram-positive and gram-negative bacteria, and an environmental mold. Organisms were normal human oral flora (eg, Actinomyces oris, Prevotella oris, Streptococcus gordonii), common environmental flora (eg, Micrococcus luteus, Stenotrophomonas maltophilia, and Sphingomonas parapaucimobilis), or human skin flora (eg, Cutibacterium acnes and Staphylococcus hominis). None of the respiratory viral panel tests (from the VIVAS and both SKC BioSamplers) detected any pathogens. All baseline testing (negative controls) was negative for bacteria and viruses. Aerosol concentration and distribution was similar to our previous findings [15].
Chemical Analyses
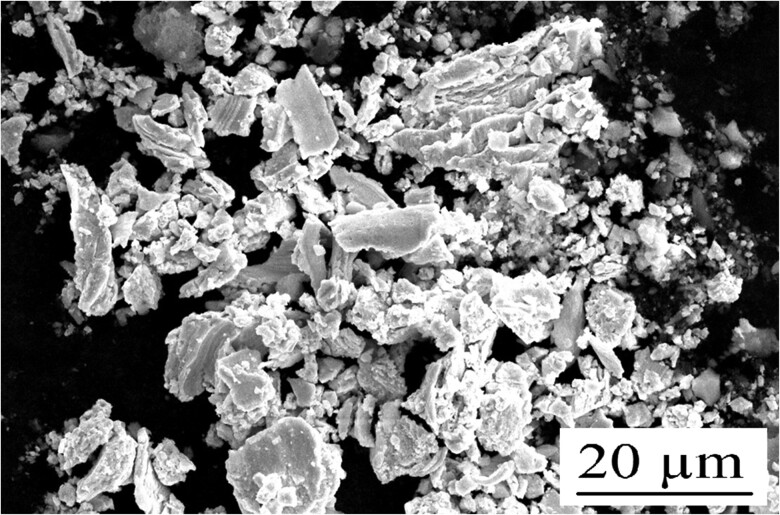
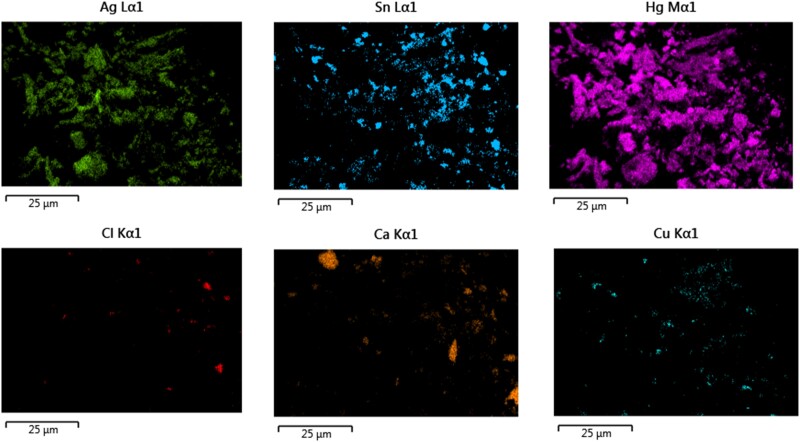
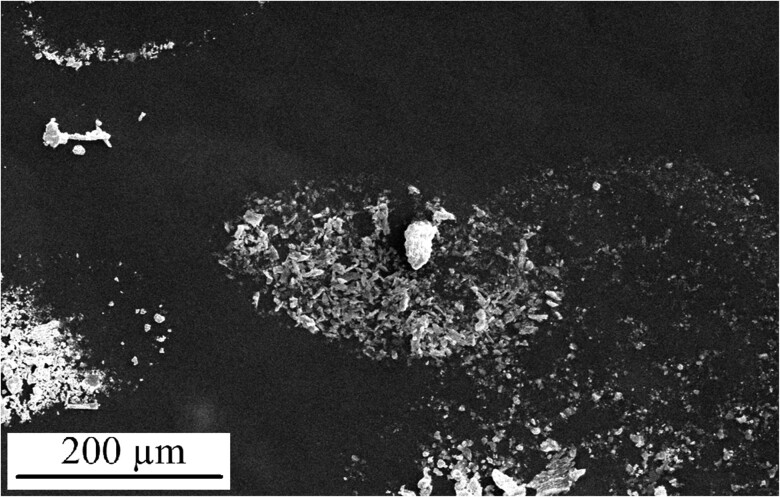
SEM analysis of a sample collected on a stub placed in close proximity to the dentist during amalgam removal is depicted in Figure 3. EDX analyses demonstrated that the sample primarily comprised of amalgam alloy metals (Figure 4). Additional SEM images from the stub demonstrate that the amalgam particles are very fine. However, these fine particles largely accumulated in oval- and circular-shaped droplets mixed with water (Figure 5).
Figure 3.
Scanning electron microscopy image of the emissions during amalgam removal. The black and white photo represents all elements.
Figure 4.
Energy dispersive X-ray elemental analysis of the aerosols emitted during amalgam removal. Elements are color coded and labeled by periodic table name. Abbreviations: Ag, silver; Ca, calcium; Cl, chlorine; Cu, copper; Hg, mercury; Sn, tin.
Figure 5.
Scanning electron microscopy image showing amalgam particles concentrating within dried water droplets during routine amalgam removal. Water droplets can be identified by accumulations of fine aerosol materials in a circular or ovoid pattern.
DISCUSSION
Our study identified relatively few viable bacteria and no viruses in dental aerosols when DHCPs were applying common aerosol mitigation techniques. In comparison to other studies, our comparatively low yield of bacteria and viruses is notable. Indeed, the number of bacteria we captured based on colony counts is far lower than what one would expect from standard saliva. We suspect that this is because the majority of dental aerosols captured were comprised of small amounts of saliva diluted in large volumes of water from water cooled instruments, such as high-speed drills. Previous work using 16S RNA gene sequencing confirms these findings with saliva contributing to a median of 0% of the total aerosol microbiota [11]. Our findings demonstrate that a substantial proportion of captured bacteria are from a nonoral source, such as the environmental water and sloughed human skin cells. The particularly low overall yield of our findings suggest that dental aerosols do not pose a substantial microbiological health hazard to DHCPs. These results should be reassuring to DHCPs and patients. Indeed, with the colony counts our team observed, we believe that when standard aerosol mitigation strategies are employed, standard surgical masks should provide adequate protection against bioaerosols for DHCPs during aerosol-generating procedures.
To our knowledge, this is the first study to measure the inorganic composition of dental aerosols. Although we used indirect approaches to measure the composition of dental droplets and aerosols for our inorganic analyses, SEM images reveal that metal particles may be aerosolized during high-speed drilling. This is concerning because standard surgical masks may not fully capture finer amalgam particles. However, the patterns of the particles suggest that these pieces of amalgam may collect in larger droplets, which would be more likely to be filtered out with standard personal protective equipment. Furthermore, these results suggest that “wet” cutting (using high-speed drills with water cooling) would increase the size and number of droplets and aerosols would thus have a protective effect. Studies evaluating urinary mercury levels among dentists are variable. Recent studies suggest that dentists have similar mercury levels to nondentists [16, 17] and may be decreasing over time. In the future, real-time monitoring of amalgam particles could provide detailed information about particles suspended in the air for periods longer than the duration of the dental procedure.
We believe that our analyses provides a more realistic assessment for DHCPs than prior studies. To our knowledge, this is the second study to directly evaluate the bacterial composition of dental aerosols [18] with the use of aerosol mitigation strategies. This is likely to provide a more accurate evaluation of pathogen inhalational risk compared to prior analyses. This is because larger particles, such as splatter and droplets, are likely to fall to the floor or get caught by a face shield or a mask [18]. Our team's experimental design included live patients, placed aerosol collection devices in close proximity to DHCPs, and used suction rates that approximate human respiration to provide the most accurate assessment of occupational health risks. Indeed, the use of specimen collection in real-world clinical settings for the duration of aerosol-generating procedures allowed us to determine the quantity of bacteria an unmasked DHCP would inhale during a dental procedure. Furthermore, we used multiple aerosol collection methods to confirm our findings. Although our results are similar to those of Tan et al, who found that root canal treatments and dental scaling procedures were associated with an increase in oral bacteria in the air, we observed very few total bacteria compared to their study. This discrepancy is likely because Tan et al used supraphysiologic suction rates to capture dental aerosols in their experimental design [18].
There are several limitations to our study. First, due to the real-world nature of these data, we were unable to assess the impact of aerosol mitigation strategies on the bacterial, viral, and inorganic chemical composition of dental aerosols. However, our results reflect the true risks for dentists who overwhelmingly use aerosol mitigation approaches during routine care. Second, due to the small sample size and low bacterial/viral yield of our study, we were unable to properly assess if certain dental clinic layouts or aerosol mitigation strategies have a marginally more protective effect than others. However, the overall results of our study suggest that the marginal benefits would likely be low. Third, our bacterial analyses relied on standard culture approaches. This may underestimate the measurement of bacterial materials in the aerosols when compared to 16S ribosomal RNA gene sequencing. However, we believe that this approach provides a more realistic evaluation of viable microorganisms, which better reflects occupational health hazards for DHCPs. Fourth, our relatively small sample size limited our ability to draw firm conclusions on the viral occupational health risks for dentists. It is quite possible that none of our patients had a viral infection during our sampling time. Furthermore, even if one of the patients did, the performance characteristics for the respiratory panel we used are based on nasopharyngeal swabs rather than aerosols. We anticipate that this would reduce the sensitivity of our viral analyses. However, we believe that our bacterial assessments can provide useful surrogate data, as every participant would have viable bacteria in their saliva. The low bioburden of bacteria in the aerosols compared to saliva demonstrates that substantial dilution of saliva is occurring. This indirectly suggests that the same would be the case for viral pathogens present in the saliva. Fifth, our studies were limited to capturing aerosols during individual aerosol-generating procedures. DHCPs will also be exposed to pathogens from unmasked patients who may be coughing or gagging during non-aerosol-generating procedures, which we did not measure during this study. Similarly, these data are evaluating dental aerosols from an oral source. For example, patients with a lower respiratory tract infection, such as tuberculosis, may still be able to transmit an infection via standard respiration. However, we would assume that patients with common acute lower respiratory tract infections, such as pneumonia would defer dental care while acutely ill. Sixth, this study was performed when the amount of circulating severe acute respiratory syndrome coronavirus 2 infections were lower in the community. This may have reduced the likelihood of capturing an asymptomatic patient with coronavirus disease 2019. Finally, the bacterial estimates presented here are likely a substantial overestimate of the procedure specific bacterial and viral exposures DHCPs would receive, as our experimental design used an unfiltered tube to mimic normal human respiration, when DHCPs would be wearing a mask and/or face shield during aerosol-generating procedures.
CONCLUSIONS
To our knowledge, we have provided the most detailed characterization and accurate picture of the true risks of aerosols among dental healthcare providers to date. The bacterial composition of dental aerosols appears to be extremely low during routine dental procedures. Our findings suggest that commonly employed aerosol mitigation interventions and standard personal protective equipment likely allows for the safe practice of dentistry, regardless of operatory layout or procedure type. These findings suggest that community leaders in infectious diseases and infection control should not advocate for substantial changes in community dental clinic design or ventilation systems. However, due to the small sample size of our study, additional studies confirming these findings for patients with known viral infections would be beneficial.
Supplementary Material
Contributor Information
Shruti Choudhary, Aerosol and Air Quality Research Laboratory, Department of Chemical, Environmental and Material Engineering, University of Miami, Miami, Florida, USA.
Tracey Bach, Division of Infectious Disease, Washington University School of Medicine, St Louis, Missouri, USA.
Meghan A Wallace, Department of Pathology and Immunology, Washington University School of Medicine, St Louis, Missouri, USA.
Daniel C Stoeckel, St Louis University Center for Advanced Dental Education, St Louis University, Missouri, USA.
Martin H Thornhill, School of Clinical Dentistry, University of Sheffield, Sheffield, United Kingdom; Department of Oral Medicine/Oral and Maxillofacial Surgery, Carolinas Medical Center–Atrium Health, Charlotte, North Carolina, USA.
Peter B Lockhart, Department of Oral Medicine/Oral and Maxillofacial Surgery, Carolinas Medical Center–Atrium Health, Charlotte, North Carolina, USA.
Jennie H Kwon, Division of Infectious Disease, Washington University School of Medicine, St Louis, Missouri, USA.
Stephen Y Liang, Division of Infectious Disease, Washington University School of Medicine, St Louis, Missouri, USA; Department of Emergency Medicine, Washington University School of Medicine, St Louis, Missouri, USA.
Carey-Ann D Burnham, Department of Pathology and Immunology, Washington University School of Medicine, St Louis, Missouri, USA.
Pratim Biswas, Aerosol and Air Quality Research Laboratory, Department of Chemical, Environmental and Material Engineering, University of Miami, Miami, Florida, USA.
Heidi M Steinkamp, St Louis University Center for Advanced Dental Education, St Louis University, Missouri, USA; Department of Pediatric Dentistry, University of Iowa College of Dentistry, Iowa City, Iowa, USA.
Michael J Durkin, Division of Infectious Disease, Washington University School of Medicine, St Louis, Missouri, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. S. C. contributed to conception, design, data acquisition, analysis and interpretation, drafted and critically revised the manuscript. T. B. contributed to experiment setup, data acquisition, sample transport, and equipment purchases. M. A. W. contributed to bacterial and viral sample analyses. P. B. contributed to conception, design, and interpretation and critically revised the manuscript. M. J. D., S. Y. L., J. H. K., H. M. S., and C.-A. D. B. contributed to grant acquisition, conception, and draft writing and critically revised the manuscript. D. C. S., H. M. S., M. H. T., P. B. L., and T. B. contributed to interpretation and critically revised the manuscript.
Acknowledgments. We thank Drs Dena Fisher, Gregg Gilbert, and Mary Ann McBurnie and the National Dental Practice Based Research Network Practitioner Executive Committee for their support.
Patient consent. This study was approved by the Washington University Human Rights Protection Office and the St Louis University Institutional Review Board as non–human subjects research because no identifiable patient information was collected and the study was performed during routine patient care. As such, this study did not require verbal or written consent from patients.
Financial support. This project was funded by the National Institutes of Health (award number U19DE028717, subaward X01 DE031119).
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Zhu M, Medina M, Nalliah R, et al. Experimental evaluation of aerosol mitigation strategies in large open-plan dental clinics. J Am Dent Assoc 2022; 153:208–20. [DOI] [PubMed] [Google Scholar]
- 2. Chestsuttayangkul Y, Lertsooksawat W, Horsophonphong S. Efficacy of dental barriers in aerosols and splatters reduction during an ultrasonic scaling: an in-vitro study. J Int Soc Prev Community Dent 2022; 12:71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. D’Antonio N, Newnum J, Kanellis M, Howe B, Anthony TR. Assessment of respirable aerosol concentrations using local ventilation controls in an open multi-chair dental clinic. J Occup Environ Hyg 2022; 19:1–10. [DOI] [PubMed] [Google Scholar]
- 4. Onoyama K, Matsui S, Kikuchi M, et al. Particle size analysis in aerosol-generating dental procedures using laser diffraction technique. Front Oral Health 2022; 3:804314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yuan C, Yang H, Zheng S, et al. Spatiotemporal distribution and control measure evaluation of droplets and aerosol clouds in dental procedures [manuscript published online ahead of print 31 January 2022]. Infect Control Hosp Epidemiol 2022. 10.1017/ice.2021.511 [DOI] [PubMed] [Google Scholar]
- 6. Suwandi T, Nursolihati V, Sundjojo M, Widyarman AS. The efficacy of high-volume evacuators and extraoral vacuum aspirators in reducing aerosol and droplet in ultrasonic scaling procedures during the COVID-19 pandemic [manuscript published online ahead of print 11 January 2022]. Eur J Dent 2022. 10.1055/s-0041-1739448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eames I, D’Aiuto F, Shahreza S, et al. Removal and dispersal of biofluid films by powered medical devices: modeling infectious agent spreading in dentistry. iScience 2021; 24:103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horsophonphong S, Chestsuttayangkul Y, Surarit R, Lertsooksawat W. Efficacy of extraoral suction devices in aerosol and splatter reduction during ultrasonic scaling: a laboratory investigation. J Dent Res Dent Clin Dent Prospects 2021; 15:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noordien N, Mulder-van Staden S, Mulder R. In vivo study of aerosol, droplets and splatter reduction in dentistry. Viruses 2021; 13:1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ou Q, Placucci RG, Danielson J, et al. Characterization and mitigation of aerosols and spatters from ultrasonic scalers. J Am Dent Assoc 2021; 152:981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meethil AP, Saraswat S, Chaudhary PP, Dabdoub SM, Kumar PS. Sources of SARS-CoV-2 and other microorganisms in dental aerosols. J Dent Res 2021; 100:817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vernon JJ, Black EVI, Dennis T, et al. Dental mitigation strategies to reduce aerosolization of SARS-CoV-2. J Dent Res 2021; 100:1461–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lednicky J, Pan M, Loeb J, et al. Highly efficient collection of infectious pandemic influenza H1N1 virus (2009) through laminar-flow water based condensation. Aerosol Sci Technol 2016; 50:i–iv. [Google Scholar]
- 14. Fabian P, McDevitt JJ, Houseman EA, Milton DK. Airborne influenza virus detection with four aerosol samplers using molecular and infectivity assays: considerations for a new infectious virus aerosol sampler. Indoor Air 2009; 19:433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choudhary SD, Durkin MJ, Stoeckel DC, et al. Comparison of aerosol mitigation strategies and aerosol persistence in dental environments [manuscript published online ahead of print 20 April 2022]. Infect Control Hosp Epidemiol 2022 10.1017/ice.2022.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tucek M, Busova M, Cejchanova M, Schlenker A, Kapitan M. Exposure to mercury from dental amalgam: actual contribution for risk assessment. Cent Eur J Public Health 2020; 28:40–3. [DOI] [PubMed] [Google Scholar]
- 17. Anglen J, Gruninger SE, Chou HN, et al. Occupational mercury exposure in association with prevalence of multiple sclerosis and tremor among US dentists. J Am Dent Assoc 2015; 146:659–68.e1. [DOI] [PubMed] [Google Scholar]
- 18. Tan KS CR, Allen PF, Epub YV. Aerosol-generating dental procedures: a reappraisal of analysis methods and infection control measures. J Hosp Infect 2021; 117:81–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.