Abstract
Background
Respiratory syncytial virus (RSV) is a substantial source of severe illnesses including acute lower respiratory infections (ALRIs) like pneumonia. However, its burden in older children remains less well understood.
Methods
Using a community-based prospective cohort, we assessed the burden of symptomatic reverse-transcription polymerase chain reaction–confirmed RSV among Nicaraguan children aged 0–14 years from 2011 to 2016. ALRI was defined as physician diagnosis of pneumonia, bronchiolitis, bronchitis, or bronchial hyperreactivity.
Results
Between 2011 and 2016, 2575 children participated in the cohort. Of these, 630 (24.5%) had at least 1 episode of symptomatic RSV and 194 (7.5%) had multiple episodes. Subtype was identified in 571 (69.3%) episodes with 408 (71.5%) RSV-A, 157 (27.5%) RSV-B, and 6 (1%) positive for both. Children aged <2 years displayed the highest incidence of symptomatic RSV, with 269.3 cases per 1000 person-years (95% confidence interval [CI], 242.1–299.5). Beyond 2 years, incidence (95% CI) of symptomatic RSV decreased rapidly: 145.6 (129.9–163.1), 37.9 (31.9–45.0), and 19.3 (14.9–25.0) cases per 1000 person-years among children aged 2–4, 5–9, and 10–14 years, respectively. Incidence of RSV-associated ALRI was highest in children aged <2 years (85.95 per 1000 person-years [95% CI, 71.30–103.61]): 2.1, 9.5, and 17.3 times that of participants aged 2–4, 5–9, and 10–14 years, respectively. Children <2 years old were significantly more likely to have an RSV-associated hospitalization (P < .001).
Conclusions
There is a substantial burden of symptomatic and severe RSV in children. While older children did present with RSV, the rates of symptomatic and severe RSV decreased by as much as 95% beyond age 5.
Keywords: child health, cohort study, incidence rate, Nicaragua, respiratory syncytial virus, RSV
Respiratory syncytial virus (RSV) is a significant source of morbidity among children, though incidence rates decrease by as much as 95% by age 5. While affecting older children, RSV's strong association with age suggests interventions will be most impactful if targeting young children.
Among children aged <5 years, respiratory syncytial virus (RSV) is the most common pathogen associated with acute lower respiratory infections (ALRIs) [1, 2]. In 2019, an estimated 33 million episodes of RSV-associated ALRI (RSV-ALRI) led to 101 400 deaths among children younger than 5 years, with >95% of RSV-ALRI and >97% of deaths occurring in low- and middle-income countries (LMICs) [3]. Most cases of severe RSV occur among previously healthy children [4, 5].
An estimated 97% of children are infected with RSV by the age of 2 years, but protection following infection is often incomplete and short in duration [6, 7]. Although symptoms in older children are less severe, these infections can be responsible for household transmission to younger siblings [8–10]. Children aged <6 months of age account for about 39% of hospitalizations and 45% of deaths among children aged <5 years [3]. These young children are more likely to experience serious respiratory disease due to their immature airway structure and immune system [11].
While there is currently no RSV vaccine available, many vaccine candidates and monoclonal antibodies are in clinical trials [12]. To optimize vaccine implementation and distribution, strategies will need to rely on a clear picture of RSV burden worldwide. There exists a high and widely unmeasured burden of community RSV among children in LMICs, particularly among children aged >5 years [13]. Addressing this gap is of particular importance given the substantial changes in RSV burden and seasonality that have been observed since the start of the coronavirus disease 2019 pandemic. Establishing the prepandemic burden of RSV, particularly in older children, will help us better understand the effects of this pandemic on other important respiratory viruses like RSV. We have previously shown a high incidence of symptomatic and severe RSV in Nicaragua, among children aged <2 years in a separate cohort [14]. We aim to add to our current understanding of RSV burden in LMICs by assessing RSV incidence, subtype, and disease severity among children aged 0–14 years in a community-based cohort in Nicaragua.
METHODS
Study Population
The Nicaraguan Pediatric Influenza Cohort Study (NPICS) is an ongoing community-based prospective cohort study. Nicaragua is a tropical country in Central America of approximately 6.8 million people, of whom >1.2 million live in the Managua area [15]. Our analysis examines the period from January 2011 through December 2016. The study is described in detail by Gordon et al [16]. Participants were enrolled through the Health Center Sόcrates Flores Vivas (HCSFV) in District II of Managua, Nicaragua. Study participants are children aged 0–14 years. Initial enrollment was done through random selection of children 3–11 years old previously enrolled in the Nicaraguan Influenza Cohort Study conducted between 2007 and 2010 [17]. Additional children ≤2 years old were enrolled in NPICS from home visits in 2011. Since that time, infants aged ≤4 weeks have been enrolled on a continual basis. Children age out of the cohort at their 15th birthday.
Data Collection
Clinical history, sociodemographic surveys, and household surveys were collected at enrollment. Surveys were conducted, and a blood sample collected, in March/April of each year.
Respiratory Sample Collection and Testing
Parents agreed to bring their children in to HCSFV upon the first indication of fever/feverishness. A study physician recorded history of illness and current symptoms. Nasal and oropharyngeal swabs were collected from children ≥2 years of age if they had fever or reported fever along with rhinorrhea and/or cough. Swabs were collected for children <2 years of age if they had fever or reported fever. Respiratory samples were also collected from any participants presenting to the study clinic with severe respiratory symptoms. RSV was detected by reverse-transcription polymerase chain reaction (RT-PCR) using the QIAamp Viral RNA Mini Kit (Qiagen Corporation, Valencia, California) using validated Centers for Disease Control and Prevention RT-PCR protocol [18].
Samples positive for RSV were later subtyped using a validated protocol [19]. However, stored samples from 2016 and earlier were destroyed in accordance with Nicaraguan polio eradication procedures, so sufficient volume to subtype all samples was not available.
Clinical Definitions
RSV was considered in all cases where a sample was positive for RSV by RT-PCR and without a previous RSV positive in the prior 14 days. ALRI was defined as physician diagnosis of pneumonia, bronchiolitis, bronchitis, or bronchial hyperreactivity. Pneumonia was diagnosed according to Nicaraguan Ministry of Health criteria, based on the Integrated Management of Childhood Illness guidelines [20]. Specifically, pneumonia was diagnosed in the following cases: children <2 months old and ≥60 breaths per minute, children 2–11 months old and ≥50 breaths per minute, children 12–59 months old and ≥40 breaths per minute, or children ≥60 months old and ≥25 breaths per minute. Severe ALRI was defined as ALRI along with a separate diagnosis of severe or very severe illness by a study physician. A participant was considered hospitalized if they were transferred to a hospital for care. If a case of ALRI, severe ALRI, or a hospitalization occurred within 14 days of symptom onset of a laboratory-confirmed RSV diagnosis, we considered it to be RSV associated.
Statistical Analysis
We calculated person-time as the number of days between participant enrollment and their exit from the study (at their 15th birthday, or when withdrawn, or lost to follow-up). For symptomatic RSV, this did not include the 14 days following a laboratory-confirmed RSV diagnosis, when participants were not considered at risk. Incidence rates and corresponding 95% confidence intervals (CIs) were calculated using a Poisson distribution in SAS version 9.4 (SAS Institute, Cary, North Carolina). Figures were created with R version 4.1.2 software (R Foundation for Statistical Computing). Pearson χ2 and Fisher exact tests were used to compare the observed and expected distributions (assuming the viruses being compared were independent) of coinfections during periods of co-circulation and RSV subtype reinfections.
Patient Consent Statement
The Institutional Review Boards of the Nicaraguan Ministry of Health and the University of Michigan approved the study (HUM00088895). Parents/guardians of all participants provided written informed consent and verbal assent was obtained from children aged ≥6 years.
RESULTS
Between 2011 and 2016, 2575 children aged 0–14 years participated in the cohort (Table 1). About 4% of participants were withdrawn or lost to follow-up per year (Supplementary Table 1). The most common reasons for loss to follow-up were participants not showing up for annual sampling, inability to locate the participant's home, or the child had moved out of the study area. The mean follow-up time was 3.8 person-years (PY) and 49.3% of participants were male. A total of 6 children died while enrolled, with 1 of these deaths associated with an RSV infection—a 4-month-old child.
Table 1.
Characteristics of Pediatric Cohort Participants
| Characteristic | Total (N = 2575) |
|---|---|
| Age at enrollment | |
| <2 y | 972 (37.8) |
| 2–4 y | 539 (20.9) |
| 5–9 y | 743 (28.9) |
| 10–14 y | 321 (12.5) |
| Male sex | 1269 (49.3) |
| Follow-up time, person-years, mean (SD) | 3.8 (2.0) |
| No. of persons in household, mean (SD) | 8.9 (4.7) |
| Dirt floor (n = 2252) | 306 (13.6) |
| Mothers with secondary or tertiary education (n = 2449) | 1833 (74.8) |
| Fathers with secondary or tertiary education (n = 2311) | 1651 (71.4) |
Data are presented as No. (%) unless otherwise indicated.
Incidence of RSV Illness
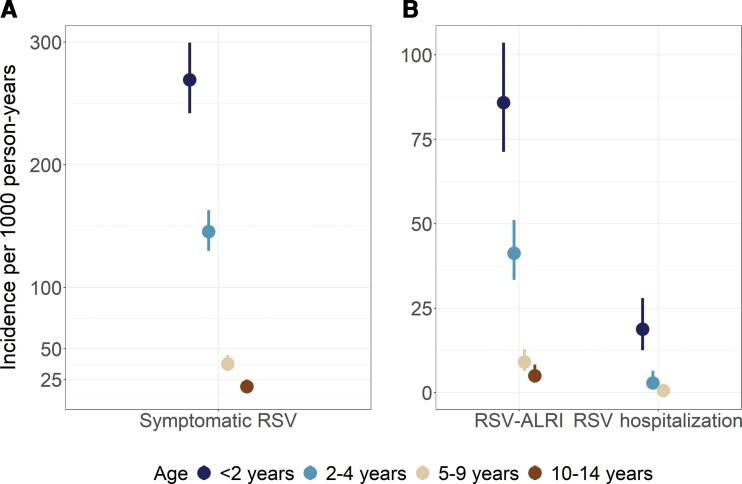
A total of 824 laboratory-confirmed RSV infections were identified, 340 of which were in children <2 years old (Table 2). Six hundred thirty participants had at least 1 episode of laboratory-confirmed RSV, while 151 participants had multiple episodes. Children aged <2 years displayed the highest incidence of symptomatic RSV with 269.3 cases per 1000 PY (95% CI, 242.1–299.5) (Figure 1A). The next highest incidence was seen in the age group 2–4 years (145.6 cases per 1000 PY [95% CI, 129.9–163.1]). The age groups 5–9 years and 10–14 years had successively lower RSV incidence at 37.9 (95% CI, 31.9–45.0) and 19.3 (95% CI, 14.9–25.0), respectively (Table 2).
Table 2.
Incidence of Crude and Severe Respiratory Syncytial Virus by Age Group
| Age | RSV | RSV ALRI | RSV Severe ALRI | RSV Hospitalization | ||||
|---|---|---|---|---|---|---|---|---|
| Cases | Incidencea | Cases | Incidencea | Cases | Incidencea | Cases | Incidencea | |
| <2 y | 340 | 269.3 (242.1–299.5) | 110 | 85.9 (71.3–103.6) | 15 | 11.7 (7.1–19.4) | 24 | 18.8 (12.6–28.0) |
| 2–4 y | 297 | 145.6 (129.9–163.1) | 85 | 41.3 (33.4–51.1) | 2 | 1.0 (.2–3.9) | 6 | 2.9 (1.3–6.5) |
| 5–9 y | 129 | 37.9 (31.9–45.0) | 31 | 9.1 (6.4–12.9) | 0 | 0 | 2 | 0.6 (.2–2.3) |
| 10–14 y | 58 | 19.3 (14.9–25.0) | 15 | 5.0 (3.0–8.3) | 0 | 0 | 0 | 0 |
Abbreviations: ALRI, acute lower respiratory infection; RSV, respiratory syncytial virus.
Incidence rate per 1000 person-years (95% confidence interval) using Poisson distribution.
Figure 1.
A, Incidence of symptomatic respiratory syncytial virus (RSV) by age group. B, Incidence of RSV-associated acute lower respiratory infection (ALRI) and hospitalizations by age group. No RSV-associated hospitalizations were observed in the age group 10–14 years. In both panels, vertical lines around point estimates are 95% confidence intervals, estimated using a Poisson distribution.
RSV-Associated ALRI Infections and Hospitalizations
Children <2 years old also displayed the highest incidence rates of RSV-associated outcomes: ALRI, severe ALRI, and hospitalizations (Table 2). Their incidence rate of RSV-ALRI was 85.9 per 1000 PY (95% CI, 71.3–103.6) compared to 41.3 per 1000 PY (95% CI, 33.4–51.1) in children aged 2–4 years (Figure 1B). Children aged 5–9 years and 10–14 years had the lowest incidence of RSV-ALRI at 9.1 (95% CI, 6.4–12.9) and 5.0 (95% CI, 3.0–8.3), respectively (Table 2). Similarly, cases of severe RSV-ALRI were also highest in children <2 years old (11.7 per 1000 PY [95% CI, 7.1–19.4]). There were no cases of severe RSV-ALRI in children aged ≥5 years throughout the entire study period. RSV-associated hospitalizations followed the same pattern with the highest incidence again seen in children <2 years old (18.8 per 1000 PY [95% CI, 12.6–28.0]). There were 24 hospitalizations among children <2 years old compared to 8 hospitalizations combined among children 2–14 years old. One RSV-associated death occurred in a child aged 4 months.
RSV Subtype and Seasonality
Among the 824 laboratory-confirmed RSV cases, 597 (72.5%) were successfully subtyped, with RSV-A identified in 408 (68.3%) cases and RSV-B identified in 184 (30.8%) cases (Supplementary Table 2). Both RSV-A and RSV-B were detected in 5 (0.8%) RSV cases. The odds of RSV-A were 1.6 times higher in initial RSV infections compared with reinfections for a single participant (95% CI, 1.02–2.27; P = .04; Supplementary Table 3). Among RSV-A cases, 23.3% had RSV-ALRI, 1.5% had severe RSV-ALRI, and 2.2% were hospitalized (Supplementary Table 2). Similarly, among RSV-B cases, 22.3% had RSV-ALRI, 1.9% had severe RSV-ALRI, and 2.5% were hospitalized.
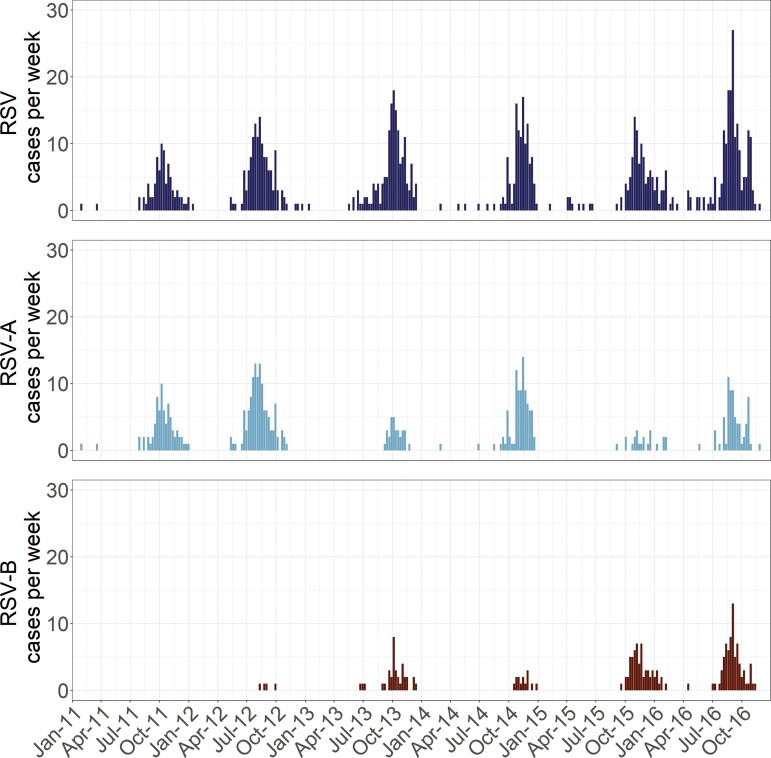
The number of RSV cases peaked annually starting between July and September, with epidemics lasting 4–6 months (Figure 2). RSV-A was the predominant subtype circulating during 2011, 2012, and 2014 (Supplementary Table 4). RSV-B was more common in 2015, while RSV-A and RSV-B co-circulated equally in 2016. In 2013, 65 of 160 PCR-confirmed RSV infections (40.6%) were subtyped, which was the most incomplete year. Between 66.3% (in 2015) and 91.5% (in 2011) of RSV infections were subtyped in the other 5 years.
Figure 2.
Number of all respiratory syncytial virus (RSV) cases (top), RSV-A cases (middle), and RSV-B cases (bottom) by study week, January 2011–December 2016.
Coinfections
We assessed coinfection patterns between RSV infections and influenza A, influenza B, human metapneumovirus (hMPV), and human coronaviruses (HCoVs). During weeks when pathogens were co-circulating, the number of RSV–influenza A (P < .0001, χ2 test), RSV–influenza B (P < .0001, χ2 test), and RSV-hMPV (P = .0002, χ2 test) coinfections were lower than expected (Table 3). RSV-HCoV (OC43, 229E, NL63, or HKU1) coinfections were no different than expected (P = .69, χ2 test) overall and among all 4 age groups.
Table 3.
Actual Versus Expected Coinfections by Age Group
| Coinfection | Age Group | ||||
|---|---|---|---|---|---|
| All Ages | <2 y | 2–4 y | 5–9 y | 10–14 y | |
| RSV-IA | |||||
| Samples | 3017 | 991 | 865 | 780 | 381 |
| Actual coinfections | 33 | 9 | 10 | 10 | 4 |
| Expected coinfections | 116 | 32 | 44 | 23 | 11 |
| P value | <.0001 | <.0001 | <.0001 | .0005 | .0052 |
| RSV-IB | |||||
| Samples | 1458 | 465 | 373 | 412 | 208 |
| Actual coinfections | 10 | 0 | 1 | 7 | 2 |
| Expected coinfections | 37 | 5 | 12 | 12 | 5 |
| P value | <.0001 | .0151 | <.0001 | .103 | .0664 |
| RSV-hMPV | |||||
| Samples | 2813 | 985 | 834 | 669 | 325 |
| Actual coinfections | 23 | 12 | 5 | 3 | 3 |
| Expected coinfections | 45 | 19 | 19 | 6 | 3 |
| P value | .0002 | .0722 | .0002 | .1613 | .7253a |
| RSV-HCoV | |||||
| Samples | 4067 | 1452 | 1189 | 974 | 452 |
| Actual coinfections | 42 | 25 | 9 | 6 | 2 |
| Expected coinfections | 44 | 23 | 14 | 6 | 2 |
| P value | .6934 | .7198 | .0862 | .9989 | 1a |
Total samples include samples collected during weeks where both pathogens of interest were circulating. Pearson χ2 and Fisher exact tests were used to compare the observed and expected distributions of coinfections.
Abbreviations: HCoV, human coronaviruses (OC43, 229E, NL63, HKU1); hMPV, human metapneumovirus; IA, influenza A; IB, influenza B; RSV, respiratory syncytial virus.
If observed counts <5.
DISCUSSION
This prospective, community-based cohort study conducted in Managua, Nicaragua, followed 2575 children between the ages of 0 and 14 years. To our knowledge, this is one of the few studies to assess the community burden of RSV among older children in an LMIC. We found children <2 years old to have the highest rates of symptomatic RSV, rates of RSV-ALRI, and rates of RSV hospitalizations. Among older age groups, RSV incidence, RSV-ALRI, and RSV hospitalizations decreased, with children aged 10–14 years demonstrating the lowest RSV burden.
To appropriately understand these age group differences, it is helpful to first compare our results in the context of previously reported RSV burden in younger children. Among children aged <5 years in LMICs, the incidence of RSV-associated lower respiratory tract infections per 1000 PY was found to be 30 in India, 34 in Indonesia, and 94 in Nigeria [21, 22]. A comprehensive, global review found RSV-ALRI incidence per 1000 PY among children under 5 years old to be 94 in low-income countries, 41 in lower-middle-income countries, and 85 in upper-middle-income countries [23]. These surveillance systems are not uniform between countries, so direct comparisons are difficult. However, our estimates of RSV-ALRI between 41.3 and 85.9 cases per 1000 PY for children aged <5 years are in line with or higher than previous studies in LMICs.
Interestingly, our ALRI findings among children <2 years old (85.9 RSV-ALRI cases per 1000 PY [95% CI, 71.3–103.6]) are slightly lower than the 119.9 RSV-ALRI cases per 1000 PY (95% CI, 103.2–139.4) we previously found in a Nicaraguan birth cohort among children <2 years old, despite similar overall RSV incidence rates: 248.1 symptomatic RSV cases per 1000 PY in our previous birth cohort and 269.3 symptomatic RSV cases per 1000 PY in our current study [14]. We suspect this is due to the previous birth cohort utilizing active surveillance (ie, weekly home visits), which resulted in more frequent visits to the clinic or home medical visits where ALRI was diagnosed.
Our combined RSV-associated hospitalization rate of 8.99 per 1000 PY for children <5 years old is slightly higher than prior worldwide findings. RSV-associated ALRI hospital admission rates, per 1000 children per year, among children <5 years old, have been estimated to be between 3.5 and 6.2 worldwide in low-income to lower-middle-income countries [3].
We also collected RSV subtype information to help describe differences between initial infections among infants and repeat infections. In line with previous studies [24, 25], we found no clinical differences between RSV-A and RSV-B infections. However, we did find that initial infections were more likely to result from RSV-A than repeat infections. While this may be the result of RSV-A being the dominant subtype circulating early in the study (when more initial infections occurred), further study is warranted to examine whether the strength and duration of protection following RSV infection differs by subtype.
RSV coinfections during weeks of co-circulation were found to be lower than expected for RSV–influenza A, RSV–influenza B, and RSV-hMPV, possibly suggesting viral interference. Previous studies have found that children aged <5 years, specifically children between 6 and 24 months of age, are the most common victims of viral coinfections [26, 27]. Additionally, Chan et al [28] found that a nonspecific innate immune response by influenza A can reduce or prevent infection and replication by RSV. Influenza also has a higher growth rate than RSV, which may keep RSV viral loads lower during coinfections, possibly below the detection level [29]. Our findings suggest that we are seeing similar resource competition between RSV and other respiratory viruses, leading to fewer detectable coinfections than expected during weeks of pathogen co-circulation.
This study has several strengths, most notably its community-based, longitudinal design, which allowed us to calculate the incidence of RSV and associated outcomes. This study also provides much needed data on the community-level burden of RSV among children in a tropical LMIC, specifically among children older than 5 years. Limitations include likely underestimates of the true burden of RSV, specifically among children aged <2 years, due to our inclusion of fever/feverishness in the criteria for sample collection. Multiple studies have found that inclusion of fever within RSV case definition results in an overall underestimate of infection [30–32]. However, our definition of feverishness does not require a measured fever, and our estimates are in line with other studies that did not use fever in the testing definition [21–23]. Finally, we had some differential loss to follow-up by parent education, which could have biased our results. We conducted a sensitivity analysis and observed minimal change in estimated incidence rates when accounting for parental education, suggesting this was unlikely a substantial source of bias.
This study shows lower RSV incidence, RSV-ALRI, RSV hospitalizations, and RSV mortality among children 5–14 years old compared to children 0–4 years old in Nicaragua. Although the burden of RSV is lower among older children, reinfection is common, and these older children likely play a key role in household transmission. RSV maternal vaccines and infant monoclonal antibodies are currently in phase 3 trials, which, if found effective, would greatly reduce RSV morbidity and mortality worldwide [33]. Given the lower burden of RSV among older children, particularly more severe manifestations, efforts to increase access to high-quality treatments and preventives would be most impactful if children <2 years of age are prioritized.
Supplementary Material
Contributor Information
Matthew Smith, Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
John Kubale, ICPSR, Institute for Social Research, University of Michigan, Ann Arbor, Michigan, USA.
Guillermina Kuan, Health Center Sócrates Flores Vivas, Ministry of Health, Managua, Nicaragua; Sustainable Sciences Institute, Managua, Nicaragua.
Sergio Ojeda, Health Center Sócrates Flores Vivas, Ministry of Health, Managua, Nicaragua; Sustainable Sciences Institute, Managua, Nicaragua.
Nivea Vydiswaran, Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
Nery Sanchez, Health Center Sócrates Flores Vivas, Ministry of Health, Managua, Nicaragua; Sustainable Sciences Institute, Managua, Nicaragua.
Lionel Gresh, Sustainable Sciences Institute, Managua, Nicaragua.
Krista Latta, Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
Roger Lopez, Sustainable Sciences Institute, Managua, Nicaragua; Centro Nacional de Diagnóstico y Referencia, Ministry of Health, Managua, Nicaragua.
May Patel, Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
Angel Balmaseda, Sustainable Sciences Institute, Managua, Nicaragua; Centro Nacional de Diagnóstico y Referencia, Ministry of Health, Managua, Nicaragua.
Aubree Gordon, Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the study staff at the Health Health Center Sόcrates Flores Vivas, the Centro Nacional de Diagnóstico y Referencia, and the Sustainable Sciences Institute for conducting the study, and the children and their families for participating in the study.
Financial support. This work was supported by the National Institutes of Health (grant number U01AI088654 and contract number HHSN272201400006C).
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Shi T, Balsells E, Wastnedge E, et al. Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: systematic review and meta-analysis. J Glob Health 2015; 5:020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2017 Causes of Death Collaborators . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392:1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022; 399:2047–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Butt ML, Symington A, Janes M, Elliott L, Steele S, Paes BA. The impact of prophylaxis on paediatric intensive care unit admissions for RSV infection: a retrospective, single-centre study. Eur J Pediatr 2011; 170:907–13. [DOI] [PubMed] [Google Scholar]
- 5. Geoghegan S, Erviti A, Caballero MT, et al. Mortality due to respiratory syncytial virus. Burden and risk factors. Am J Respir Crit Care Med 2017; 195:96–103. [DOI] [PubMed] [Google Scholar]
- 6. Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986; 140:543–6. [DOI] [PubMed] [Google Scholar]
- 7. Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis 1991; 163:693–8. [DOI] [PubMed] [Google Scholar]
- 8. Jacoby P, Glass K, Moore HC. Characterizing the risk of respiratory syncytial virus in infants with older siblings: a population-based birth cohort study. Epidemiol Infect 2017; 145:266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heikkinen T, Valkonen H, Waris M, Ruuskanen O. Transmission of respiratory syncytial virus infection within families. Open Forum Infect Dis 2015; 2:ofu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crowcroft NS, Zambon M, Harrison TG, Mok Q, Heath P, Miller E. Respiratory syncytial virus infection in infants admitted to paediatric intensive care units in London, and in their families. Eur J Pediatr 2008; 167:395–9. [DOI] [PubMed] [Google Scholar]
- 11. Anderson J, Do LAH, Wurzel D, et al. Severe respiratory syncytial virus disease in preterm infants: a case of innate immaturity. Thorax 2021; 76:942–50. [DOI] [PubMed] [Google Scholar]
- 12. Shang Z, Tan S, Ma D. Respiratory syncytial virus: from pathogenesis to potential therapeutic strategies. Int J Biol Sci 2021; 17:4073–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang X, Li Y, Mei X, Bushe E, Campbell H, Nair H. Global hospital admissions and in-hospital mortality associated with all-cause and virus-specific acute lower respiratory infections in children and adolescents aged 5–19 years between 1995 and 2019: a systematic review and modelling study. BMJ Glob Health 2021; 6:e006014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kubale J, Kuan G, Gresh L, et al. Assessing the incidence of symptomatic respiratory syncytial virus illness within a prospective birth cohort in Managua, Nicaragua. Clin Infect Dis 2020; 70:2029–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. United Nations . World population prospects 2022, online edition. New York: Department of Economic and Social Affairs Population Division; 2022. [Google Scholar]
- 16. Gordon A, Kuan G, Aviles W, et al. The Nicaraguan Pediatric Influenza Cohort Study: design, methods, use of technology, and compliance. BMC Infect Dis 2015; 15:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gordon A, Saborío S, Videa E, et al. Clinical attack rate and presentation of pandemic H1N1 influenza versus seasonal influenza A and B in a pediatric cohort in Nicaragua. Clin Infect Dis 2010; 50:1462–7. [DOI] [PubMed] [Google Scholar]
- 18. Fry AM, Chittaganpitch M, Baggett HC, et al. The burden of hospitalized lower respiratory tract infection due to respiratory syncytial virus in rural Thailand. PLoS One 2010; 5:e15098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuypers J, Wright N, Morrow R. Evaluation of quantitative and type-specific real-time RT-PCR assays for detection of respiratory syncytial virus in respiratory specimens from children. J Clin Virol 2004; 31:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization (WHO) . Guidance for sentinel influenza surveillance in humans. Copenhagen: WHO Regional Office for Europe; 2011. [Google Scholar]
- 21. Krishnan A, Kumar R, Broor S, et al. Epidemiology of viral acute lower respiratory infections in a community-based cohort of rural north Indian children. J Glob Health 2019; 9:010433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robertson SE, Roca A, Alonso P, et al. Respiratory syncytial virus infection: denominator-based studies in Indonesia, Mozambique, Nigeria and South Africa. Bull World Health Organ 2004; 82:914–22. [PMC free article] [PubMed] [Google Scholar]
- 23. Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390:946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gamiño-Arroyo AE, Moreno-Espinosa S, Llamosas-Gallardo B, et al. Epidemiology and clinical characteristics of respiratory syncytial virus infections among children and adults in Mexico. Influenza Other Respir Viruses 2017; 11:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fletcher JN, Smyth RL, Thomas HM, Ashby D, Hart CA. Respiratory syncytial virus genotypes and disease severity among children in hospital. Arch Dis Child 1997; 77:508–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goka E, Vallely P, Mutton K, Klapper P. Influenza A viruses dual and multiple infections with other respiratory viruses and risk of hospitalisation and mortality. Influenza Other Respir Viruses 2013; 7:1079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martin ET, Kuypers J, Wald A, Englund JA. Multiple versus single virus respiratory infections: viral load and clinical disease severity in hospitalized children. Influenza Other Respir Viruses 2012; 6:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chan KF, Carolan LA, Korenkov D, et al. Investigating viral interference between influenza A virus and human respiratory syncytial virus in a ferret model of infection. J Infect Dis 2018; 218:406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pinky L, Dobrovolny HM. Coinfections of the respiratory tract: viral competition for resources. PLoS One 2016; 11:e0155589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nyawanda BO, Mott JA, Njuguna HN, et al. Evaluation of case definitions to detect respiratory syncytial virus infection in hospitalized children below 5 years in rural western Kenya, 2009–2013. BMC Infect Dis 2016; 16:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saha S, Pandey BG, Choudekar A, et al. Evaluation of case definitions for estimation of respiratory syncytial virus associated hospitalizations among children in a rural community of northern India. J Glob Health 2015; 5:010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rha B, Dahl RM, Moyes J, et al. Performance of surveillance case definitions in detecting respiratory syncytial virus infection among young children hospitalized with severe respiratory illness—South Africa, 2009–2014. J Pediatric Infect Dis Soc 2019; 8:325–33. [DOI] [PubMed] [Google Scholar]
- 33. Srikantiah P, Vora P, Klugman KP. Assessing the full burden of respiratory syncytial virus in young infants in low- and middle-income countries: the importance of community mortality studies. Clin Infect Dis 2021; 73:S177–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.