Abstract
Enteropathogenic Escherichia coli (EPEC) intimately adhere to epithelial cells producing cytoskeletal rearrangement with typical attaching and effacing lesions and altered epithelial barrier and transport function. Since EPEC and Shiga toxin-producing E. coli (STEC) share similar genes in the “locus for enterocyte effacement” (LEE) thought to cause these changes, it has been assumed that STEC shares similar pathogenic mechanisms with EPEC. The aims of this study were to compare the effects of EPEC and STEC on bacterial-epithelial interactions and to examine changes in epithelial function. T84 monolayers were infected with STEC O157:H7 (wild strain EDL 933 or non-toxin-producing strain 85/170), EPEC (strain E2348/69), or HB101 (nonpathogenic) and studied at various times after infection. EPEC bound more avidly than EDL 933, but both strains exhibited greater binding than HB101. Attaching and effacing lesions and severe disruption to the actin cytoskeleton were observed in EPEC by 3 h postinfection but not in EDL 933 or HB101 at any time point. EPEC and EDL 933 increased monolayer permeability to [3H]mannitol 5- to 10-fold. In contrast to EPEC, EDL 933 completely abolished secretagogue-stimulated anion secretion as assessed under voltage clamp conditions in Ussing chambers. Several other STEC strains induced changes similar to those of EDL 933. In conclusion, STEC impairs epithelial barrier function and ion transport without causing major disruption to the actin cytoskeleton. Pathogenic factors other than products of LEE may be operant in STEC.
Enteropathogenic Escherichia coli (EPEC) strains are prototypical adherent pathogens which intimately attach to the gastrointestinal epithelium. After colonization, EPEC cells attach loosely to the epithelium via the pili and subvert epithelial cell signal transduction mechanisms by secreting bacterial proteins via a type III secretion apparatus. These include products which are involved in modulating intracellular calcium, protein kinases, and phospholipase C. In addition, the bacteria secrete a transposable intimin receptor (Tir) which is inserted into the epithelial cell and incorporated into the apical membrane. Tir acts as a receptor for the bacterium-derived product intimin. The bacterial-epithelial interaction results in the development of epithelial cell ultrastructural alterations and the formation of the typical attaching and effacing (A/E) lesions which mediate intimate bacterial attachment (reviewed in reference 14). EPEC can also cause changes in epithelial cell function, including marked alterations in paracellular permeability, modulation of epithelial secretion (2, 29, 34), and altered immune modulatory signals from the cells, such as secretion of the chemokine interleukin-8 (13, 32). The bacterial products responsible for modulating epithelial cell signal transduction and for inducing the A/E lesions are derived from a pathogenicity island in the bacterial genome called the locus for enterocyte effacement (LEE) (19).
Shiga toxin-producing E. coli (STEC) strains are also adherent. Current evidence indicates that the Shiga-like toxins (Stx) are probably not involved in intestinal epithelial cell injury (16, 27, 35). Like EPEC, STEC strains express the LEE gene sequence, and recent studies indicate that they also produce Tir (26), although it is not phosphorylated on tyrosine residues as it is for EPEC. The implication of these findings has been that STEC strains share mechanisms with EPEC that are important for colonizing and inducing disease in the gastrointestinal tract (14). However, the spectrum of STEC disease is quite different from that seen with EPEC infection. STEC causes colitis without damaging the small intestine. The syndrome often progresses to hemorrhagic colitis and can be complicated by Stx-induced systemic manifestations such as hemolytic uremic syndrome. EPEC strains, on the other hand, colonize both the small and the large intestine, and infection is often associated with nonbloody diarrhea in infants (4). Several other observations suggest that the mechanisms of pathogenesis are quite different. Recent studies in a rabbits and pigs indicate that severe inflammation and colitis occurs with STEC strains lacking pathogenicity plasmid (16, 36). eae-Negative STEC strains have been reported in association with bloody diarrhea and hemolytic uremic syndrome in humans (5, 6), implying that intimin is not central to disease pathogenesis. In support of these epidemiological observations, the development of A/E lesions, a phenomenon central to the pathogenesis of EPEC, has not been observed in human STEC infection (9, 31), in some animal model studies (16, 23), and in cell culture models utilizing cell lines derived from gastrointestinal epithelia (12, 15, 20). Thus, a body of evidence implicates pathogenic mechanisms other than those caused by products of the LEE genes in STEC infection.
The aims of this study were to compare changes to cell structure and function over time in an in vitro model of EPEC and STEC infection in T84 colon cancer cells. The results indicate that STEC can cause disruption of epithelial cell function without inducing the typical A/E lesions and, the results also imply that bacterial factors other than products of LEE may be involved in disease pathogenesis.
MATERIALS AND METHODS
Bacterial strains and reagents.
The pathogenic strains of E. coli were kindly provided by Roy Robins-Brown, Royal Hospital for Children, Melbourne, Australia. The majority of experiments described here used EPEC E2348/69 (serotype O127:H6), the STEC strain EDL933 (serotype O157:H7), and the toxin-negative STEC strain 85/170 (serotype O157:H7). These strains have been previously described by this laboratory (16). A nonpathogenic laboratory strain of E. coli, HB101 (serotype O:rough), was used as a control for this study. In addition, we utilized several other strains of STEC derived from an outbreak of hemolytic-uremic syndrome (HUS) and from sporadic cases of diarrhea-associated HUS; these are presented in Table 1. Bacteria were cultured overnight in brain heart infusion (Oxoid, Hampshire, England), and then counting of viable bacteria was done by plating serial 10-fold dilutions of the culture. A total of 105 to 107 CFU of bacteria per ml was used, depending on the experiment, to infect the T84 monolayers.
TABLE 1.
Summary of E. coli strains and their effects on cell cultures
| Strain | Serotype | Virulence factors | HeLa FASc | T84 FASc | G (msiemens/cm2)d | ΔIsce |
|---|---|---|---|---|---|---|
| EPEC 2348/69 | O126:H6 | + | + | ↑ | ↓ | |
| STEC/Sadlera | O111:H− | Stx 1,2, eae, EHf | + | + | ↑↑↑ | A |
| STEC/E41b | O113:H21 | Stx1, eae, EH | + | − | ↑↑↑ | A |
| STEC/E44b | O26:− | Stx1, eae, EH | + | − | ↑↑↑ | A |
| STEC/E45b | O111:H− (Canada) | Stx1,2, eae, EH | + | + | ↑↑↑ | A |
| STEC/E51b | O157:H− | Stx1, eae, EH? | + | − | ↑↑↑ | A |
| STEC/EDL 933b | O157:H7 | Stx1,2, eae, EH | + | − | ↑↑↑ | A |
Denotes strain derived from a mettwurst outbreak in South Australia.
Denotes strain from an area where diarrhea-associated HUS is endemic.
FAS staining of cells was performed at both 6 and 18 h postinfection. +, Positive staining; –, negative staining (at both time periods).
G, conductance.
ΔIsc, secretagogue-stimulated short circuit; A, abolished.
EH, enterohemolysin; Stx, Shiga-like toxin.
T84 and HeLa cell culture.
Human T84 colonic carcinoma cells (passage 57 to 67) were grown at 37°C with 5% CO2 in Dulbecco modified Eagle medium-F12 (DMEM-F12; 1:1 mixture) medium (GIBCO-BRL) supplemented with l-glutamine, 15 mM HEPES, 10% fetal bovine serum, and 1% penicillin-streptomysin (Trace Biosciences). Confluent T84 monolayers were subcultured every 7 to 10 days by trypsin-EDTA treatment in Ca2+- and Mg2+-free phosphate-buffered saline (PBS). Experiments that required infection of T84 cells were performed with antibiotic-free media.
HeLa cells (passage 35 to 40) were grown at 37°C with 5% CO2 in DMEM–F-12 (1:1 mixture) medium (GIBCO-BRL) with 2 mM l-glutamine, 10% fetal bovine serum, and 1% antibiotics. Confluent HeLa cells were trypsinized by using trypsin-EDTA (Trace Biosciences), washed in PBS, and seeded into chamber slides at a density of 105 cells per well. When the monolayers of HeLa cells reached confluence, the monolayers were washed with PBS five times and maintained in antibiotic-free DMEM–F-12 medium containing all of the essential nutrient ingredients. The HeLa cells were then used for infection experiments.
Bacterial binding to T84 cells.
T84 cells were grown in 6-well cell culture plates, seeded at a concentration of 5 × 105 cells/per well, and grown to confluence in 7 days. Bacterial binding was performed as previously described (12). Before the bacteria were inoculated, T84 monolayers were washed with PBS, and 5 ml of fresh antibiotic-free medium was added into each well. Ca. 107 CFU of bacteria was added into each well. Infected T84 monolayers were then incubated at 37°C and in 5% CO2 for 3, 6, or 18 h. The monolayers were washed in PBS five times to remove nonadherent bacteria, and the T84 cells were then lysed by vigorous pipetting, followed by 30 min of incubation at 4°C in sterile H2O. A serial 10-fold dilution of the cell lysate was done, and viable counts for all wells were obtained as described above. The bacterial adherence to T84 cells for each strain was assessed by normalizing the number of CFU per milliliter in cell lysate to the number of inoculated CFU per milliliter; results were expressed as a percentage of the inoculated CFU per milliliter.
Morphological assessment of microbial epithelial interactions.
The adherence of bacteria to T84 and HeLa cells was also assessed by F-actin staining (15). Cells were grown to confluence on coverslips or chamber slides. Bacteria were added into the apical media, and monolayers were incubated at 37°C in a 5% CO2 atmosphere for 3, 6, or 18 h. Nonadherent bacteria were removed by five washes with PBS, and the cells were fixed for 20 min in 3% buffered formalin at room temperature. Cells were washed three times in PBS, permeablized in 0.1% Triton X-100–PBS for 5 min, and washed again in PBS. Cells were then stained with fluorescein isothiocyanate (FITC)-phalloidin (Sigma-Aldrich, St. Louis, Mo.) as previously described (15) and were examined for F-actin rearrangement by using a confocal laser scanning microscope (Leica, Heerbrugg, Switzerland).
Microtubules were examined in a similar fashion in T84 cells. Cells infected with bacteria for 6 or 18 h were washed in PBS and fixed in freshly prepared 3% paraformaldehyde in PBS for 30 min. The cells were then permeabilized with 0.5% Triton X-100 in PBS for 10 min, postfixed in 95% ethanol at −20°C for 10 min, and subsequently blocked and rehydrated in 3% bovine serum albumin (Sigma-Aldrich) in PBS at room temperature for 30 min. The cells were then incubated at 37°C with mouse monoclonal immunoglobulin G1 (IgG1) anti-human β-tubulin (Sigma-Aldrich) for 1 h and incubated with an FITC-conjugated goat anti-mouse IgG (Sigma-Aldrich) at room temperature for 1 h. The cells were washed three times in PBS and once in deionized water, mounted in Immu-Mount (Shandon, Pittsburgh, Pa.), and examined by confocal microscopy.
Electron microscopy was also performed. T84 monolayers were grown to confluence on collagen-coated Costar inserts (0.4-μm, pore size; 24-mm diameter; Corning Costar, Cambridge, Mass.) and infected with bacteria for 6 or 18 h. After five washes in PBS to remove nonadherent bacteria, samples were fixed in 4% glutaraldehyde in PBS for 1.5 h. Samples were then washed in PBS, postfixed for 1 h in 0.5% osmium tetroxide in PBS, dehydrated, and embedded in resin. Ultrathin sections were cut, stained with uranyl acetate, and examined by transmission electron microscopy (Philips EM410).
Secretagogue-stimulated anion secretion and monolayer permeability.
T84 cells were seeded onto collagen-coated Transwell inserts (0.4 μm, pore size; 24-mm diameter; Corning Costar) at a density of 106/well. The cells were grown for approximately 14 days, by which time they were exhibiting vectorial transport. Bacteria (105 CFU per insert) were added apically and incubated at 37°C and in 5% CO2 for 6 or 18 h. The monolayers were then mounted in modified Ussing chambers, with an exposed surface of 1.43 cm2, and bathed in oxygenated Krebs buffer containing 115 mM NaCl, 8 mM KCl, 1.2 mM MgCl2, 1.25 mM CaCl2, 2 mM KH2PO4, 25 mM NaHCO3, 5 mM glucose, and 5 mM mannitol at pH 7.4. Details of this technique have been described elsewhere (24). The spontaneous transepithelial potential difference (PD) was measured, and the tissue was clamped at zero voltage by continuously introducing a short-circuit current (Isc) with an automatic voltage clamp (DVC1000; World Precision Instruments, New Haven, Conn.). Open-circuit PD (in millivolts) was measured every 5 min. Monolayer conductance (G [millisiemens per square centimeter]) was calculated after voltage clamping of the cells at 10 mV and recording of the resulting Isc. Conductance was calculated from the ΔPD and ΔIsc by using Ohm's law.
Monolayer permeability was assessed by measuring the mucosal-to-serosal flux of [3H]mannitol (ICN, Costa Mesa, Calif.). First, 10 μCi of [3H]mannitol was added into the mucosal reservoir immediately after the Isc had stabilized. Then, after equilibration for 10 min, the samples were taken every 10 min for 30 min from both the mucosal and serosal sides of the Ussing chambers. The radioactivity of each sample was measured by using a liquid scintillation analyzer (Packard), and fluxes of [3H]mannitol (in micromoles per square centimeter per hour) were calculated as described previously (22).
At the end of the flux period, either 3-isobutyl-1-methylxanthine (IBMX; Sigma-Aldrich), at a final concentration of 30 μM or calcium inophore A23187 (Sigma-Aldrich) at a final concentration of 4 μM was added into both sides of the monolayers. Secretagogue-stimulated Isc (ΔIsc), a marker of Cl− secretion in T84 cells, was recorded and depicted as microamperes per square centimeter of monolayer surface area.
Assessment of T84 cell viability.
The viability of T84 cells after bacterial infection was assessed by measuring lactate dehydrogenase (LDH) release into the culture medium (29). Briefly, the medium samples for LDH assay were collected from control and infected T84 cell cultures. The T84 cells were lysed by incubation in 1.5 ml of 0.1% Triton X-100 for 25 min and by pipetting vigorously after removal of the culture medium. The LDH activity in the culture medium and the total cellular LDH activity in cell lysate were then determined (29). LDH release into the medium was expressed as a percentage of the total LDH (i.e., LDH present in medium and monolayer).
Other STEC strains.
To determine whether some of the initial observations could be extrapolated to all STEC strains, we examined several different strains of STECs obtained from epidemic and sporadic cases of diarrhea-associated HUS. In these studies, phalloidin-actin staining (FAS) was examined in both HeLa cells and T84 cells, and the electrical conductivity and secretagogue-stimulated short-circuit current values were measured in T84 cells as described above.
Statistical analysis.
All data are presented as the means ± the standard errors. Statistical comparison of the data among multiple groups was carried out by using one-way analysis of variance. Post-hoc testing of differences between means was performed by using the Student-Newman-Keuls test. Student t tests were used if only two groups were compared. The level of statistical significance was defined as a P of <0.05.
RESULTS
Time course of bacterial adherence to T84 monolayers.
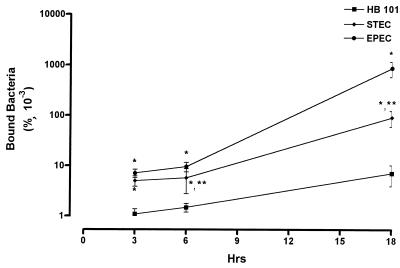
All strains of E. coli adhered to T84 cells (Fig. 1). However, there were major differences in the degree of binding and in the presence of A/E lesions. Both EPEC and O157:H7 strain EDL 933 exhibited significantly increased numbers of bound bacteria at 3, 6, and 18 h postinfection compared to HB101. However, EPEC bacteria bound more extensively than EDL 933 to the epithelial cells at both 6 and 18 h. At 18 h a 10-fold-greater number of EPEC cells bound to the monolayers than did EDL 933.
FIG. 1.
Binding of HB101, EPEC, and STEC to T84 cells. Values are the means ± the standard error of the percentage of the inoculation dose that bound to the monolayers at 3, 6, and 18 h postinfection. For each group, n = 6. Significance values: ∗, P < 0.01 compared to HB101; ∗∗, P < 0.01 compared to EPEC.
Cytoskeletal disruption.
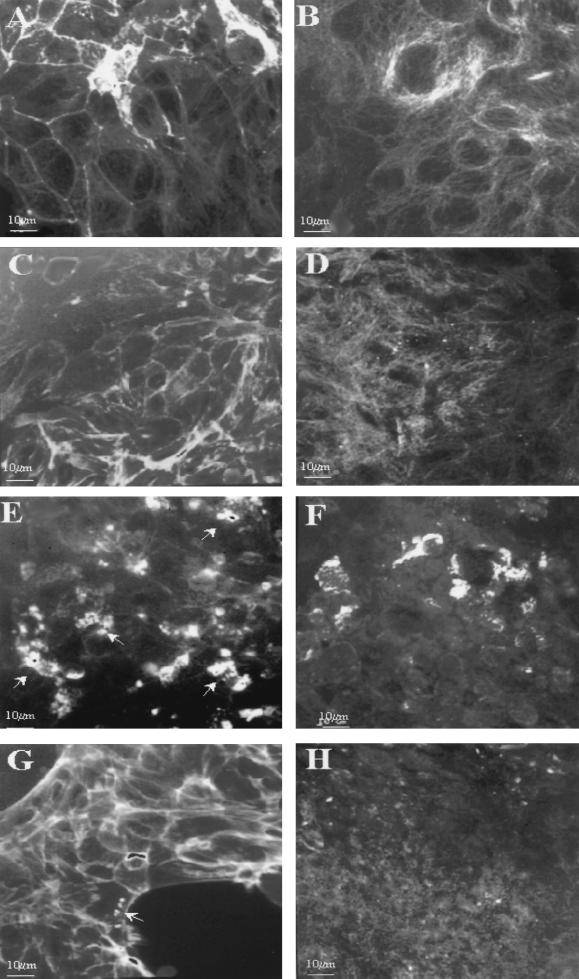
Changes in epithelial morphology were induced by infection with all pathogenic strains, but the pattern of disruption was different in EPEC compared to EDL 933. Representative micrographs of control and cells infected with HB101, EPEC, and EDL 933 for 18 h are depicted in Fig. 2. Fluorescent staining of the actin cytoskeleton by phalloidin revealed many sites of actin clumping with EPEC infection that are consistent with the development of A/E lesions (Fig. 2E). These were present by 3 h postinfection and persisted throughout the time course (not shown). Moreover, cortical actin staining was completely disorganized and cytoplasmic actin fibers were absent after infection for 6 and 18 h, indicating a major disruption of the cytoskeleton (Fig. 2A and E). In contrast, EDL 933 exhibited very few areas of actin clumping, and these were usually only observed at the edge of the spreading monolayers and only at 18 h postinfection (Fig. 2G). Actin clumping was not observed in any section at any time point in cells infected with the nonpathogenic strain, HB101 (Fig. 2C) or in control monolayers (Fig. 2A). EDL 933 infection, in contrast to EPEC, caused only minor disruption of cortical actin fibers in a way similar to that observed in monolayers infected with HB101. However, in both EDL 933 and HB101 infection, epithelial cells did exhibit some disruption of cytoplasmic actin cables.
FIG. 2.
Immunofluorescent micrographs of actin cytoskeleton (A, C, E, and G) and microtubules (B, D, F, and H) at 18 h after infection. Actin cytoskeleton was stained with FITC-phalloidin and microtubules with anti-β-tubulin monoclonal antibody. Control monolayers (A and B) were compared with monolayers infected with BH101 (C and D), EPEC (E and F), and STEC (G and H). Arrows depict the sites of actin rearrangement under adherent bacteria. Bar, 10 μm.
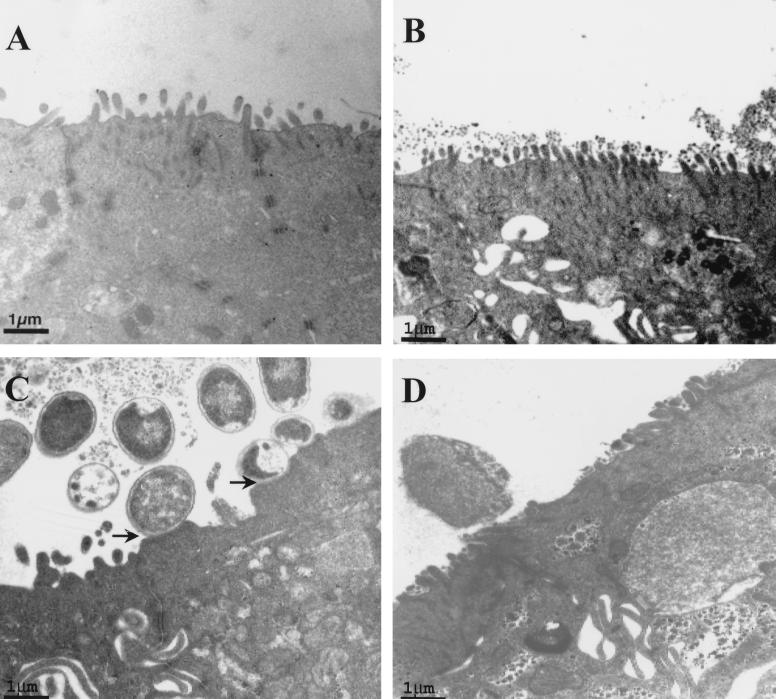
Typical EPEC-induced A/E lesions were confirmed by electron microscopy (Fig. 3C). While occasional bacteria were observed in close association with the apical membrane (Fig. 3D), typical A/E lesions were not identified at any time point with EDL 933. No evidence of physical microbial-epithelial interactions was observed with HB101 infection (Fig. 3B). Despite the lack of intimate attachment, EDL 933 infection caused disruption to the microvilli, which was also obvious in EPEC- but not in HB101-infected cells.
FIG. 3.
Electron micrographs of control (A) and HB101 (B)-, EPEC (C)-, and STEC (D)-infected T84 cells. Arrows in panel C depict A/E lesions of EPEC.
All strains of pathogenic E. coli tested induced A/E lesions in HeLa cells (as identified by FAS), including STEC strain EDL 933 (see Table 1), indicating that they were able to induce A/E lesions. In contrast, only two of the seven STEC strains tested caused A/E lesions in T84 cells, and these were both of the O111:H− serotype.
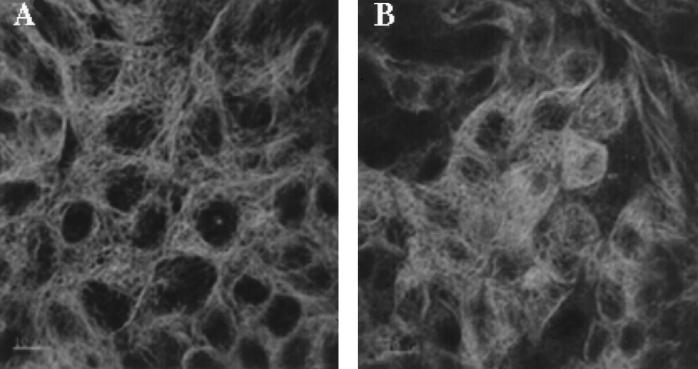
Examination of the microtubule system by fluorescent staining of β-tubulin also revealed major differences between bacterial strains. Characteristic microtubule staining was observed in control monolayers, which exhibited discrete fibers coursing throughout the cytoplasm (Fig. 2B). At 6 h after infection, a similar pattern of staining was observed in cells infected with HB101. In contrast, EPEC-infected cells exhibited partially disrupted microtubular arrays with a loss of the radiation of fibers (Fig. 4B). EDL 933-infected cells (Fig. 4A) demonstrated a pattern similar to that of the control (Fig. 2B) and HB101-infected monolayers. Extensive disruption of the microtubule system was evident at 18 h in both EPEC- and EDL 933-infected cells (Fig. 2F and H). Discrete microtubules could not be seen in either preparation. In contrast, infection with HB101 (Fig. 2D) did induce some minor disruption of the organization, though discrete microtubules were evident. The effect on HB101-infected cells is likely to be due to the cobblestone organization of the epithelial cells induced by HB101 rather than to specific disruption of the microtubules.
FIG. 4.
Immunofluorescent micrographs of microtubules at 6 h after infection with STEC (A) and EPEC (B). Bar, 10 μm.
Epithelial secretion and permeability.
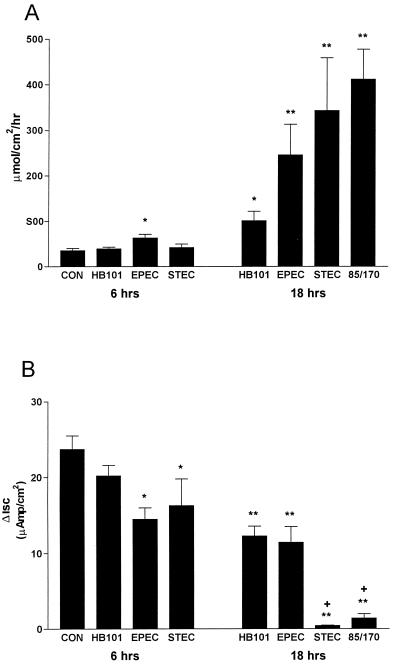
Mucosal-to-serosal flux of [3H]mannitol was used as a measure of paracellular permeability. Data are depicted in Fig. 5A. Compared to controls, only EPEC infection caused a significant increase in mannitol permeability at 6 h. However, both EPEC and EDL 933 induced a 5- to 10-fold increase in mannitol permeability at 18 h compared to HB101-infected and control cells. Permeability in cells infected with strain EDL 933, which express both Stx1 and Stx2, was not different from strain 85/170 (Stx negative), indicating that Shiga-like toxins did not play a role in the increased epithelial permeability.
FIG. 5.
Mucosal-to-serosal flux of [3H]mannitol (A) and IBMX-stimulated short-circuit current (ΔIsc) (B) in T84 cells infected for either 6 or 18 h. Values are the means ± the standard error (n = 8 to 16 monolayers in each group). Significance: ∗, P < 0.01 compared to control; and ∗∗, P < 0.001 compared to HB101 in panel A; ∗, P < 0.05 compared to control; ∗∗, P < 0.001 compared to control; and +, P < 0.001 compared to HB101 in panel B.
Infection also caused changes in secretagogue-stimulated anion secretion (Fig. 5B). IBMX-stimulated short-circuit current (ΔIsc) was significantly decreased in EDL 933- and EPEC-infected monolayers compared to control and HB101-infected cells by 6 h postinfection. By 18 h after infection, EPEC and HB101 significantly reduced ΔIsc to ca. 50% of the level in control monolayers, and EDL 933 infection abolished ΔIsc. As in the mannitol permeability studies, infection of T84 cells with the toxin-negative STEC strain, 85/170, had a similar effect on IBMX ΔIsc compared to STEC strain EDL 933, implying that toxins were not involved in the inhibition of anion secretion. To determine whether the inhibition of secretion by EDL 933 was restricted to cyclic AMP (cAMP)-dependent secretion or whether it also involved Ca2+-stimulated secretion, EDL 933-infected cells were treated at 18 h with the Ca2+ ionophore A23187, which increases intracellular Ca2+ and stimulates chloride secretion in T84 cells. A23187 reversed the Isc in seven of eight infected monolayers, suggesting that this manipulation stimulated secretion of a cation such as potassium. The mean level of A23187-stimulated Isc in EDL 933-infected cells (−6.1 ± 4.8 μAmp/cm2, n = 8) was significantly less than that for controls (35.3 ± 11, n = 3, P < 0.02).
T84 cell viability.
The release of LDH by the T84 cells into the tissue culture medium did not differ in any group at either 6 or 18 h postinfection. At 18 h the controls released 4.2 ± 0.4% of total LDH into the culture medium; HB101 released 5.5 ± 2.1%, EPEC released 5.3 ± 1.9%, EDL 933 released 5.1 ± 1.8%, and strain 85/170 released 5.8 ± 1.1% (n = 8 to 10 in each group).
DISCUSSION
This study utilized T84 cells to compare the effect of EPEC and STEC O157:H7 on epithelial cell structure and function. Although EPEC and STEC strains share some virulence determinants, several observations imply that the current paradigm for STEC-induced disease does not parallel that for EPEC and that other, unrecognized factors may contribute to disease pathogenesis. STEC O157:H7 had less impact on epithelial cell structure than did EPEC. However, this strain of STEC caused major alterations to epithelial barrier function and cell anion secretion. Importantly, these changes were not dependent on the development of A/E lesions, which are considered central to EPEC pathogenesis. Examination of several other pathogenic STEC strains demonstrated similar changes though the observations cannot be generalized for all STEC strains, since two strains of O111:H− did cause A/E lesions in the T84 cells.
Infection with EPEC induced the expected alterations in the epithelial cells (14). These included rapid attachment of the bacteria to the apical membrane, with the development of typical A/E lesions. This process was associated with extensive disruption of both the actin cytoskeleton and the microtubular network within 6 h of infection. Epithelial permeability increased markedly by 18 h postinfection. The effect of EPEC on paracellular permeability is thought to result from altered structure of the tight junctions and involves phosphorylation of myosin light-chain kinase and altered intracellular calcium (34, 38). EPEC also caused a slight but significant reduction in cAMP-mediated chloride secretion, which has been previously reported by others (29). However, at both 6 and 18 h postinfection, the reduction was not different from that caused by E. coli HB101 infection, suggesting a nonspecific effect of the infected cell culture medium.
EDL 933 infection produced markedly different alterations in the epithelial cells. The time course of bacterial binding was similar, although EDL 933 bound less avidly than EPEC at both 6 and 18 h postinfection. A/E lesions were detected only occasionally by FAS, but this usually occurred in nonconfluent monolayers in the leading edge of the spreading cells. Electron microscopic examination failed to demonstrate typical A/E lesions despite the presence of numerous bacteria in close proximity to the T84 cells. This apparent lack of A/E lesions has previously been observed in STEC-infected T84 cells (12, 37). EDL 933 did cause some disruption to the cytoskeleton, though the pattern of actin disruption was quite different from that observed in EPEC strains. Cells infected with EDL 933 demonstrated preservation of the cortical actin structure, but with some disruption of the cytoplasmic actin cables. Like EPEC, EDL 933 completely disrupted the microtubular network. It is unclear whether this latter effect is due to disruption of the cytosolic actin network or whether these organisms produce specific toxins or substances capable of disrupting microtubules.
STEC infection also induced elevations in electrical conductivity of the monolayers (data not shown) which paralleled the increased permeability to mannitol. Philpott et al. have recently reported similar alterations in infected T84 cells (28). These authors described alterations to the paracellular permeability, which appeared to be modulated by elevated protein kinase C and involved calmodulin. The activity of myosin light-chain kinase was central to the changes in permeability, and associated morphological changes included the disruption of ZO-1, a tight-junction associated protein. Thus, these changes in epithelial barrier function can be ascribed to altered structure of the tight junction and permeability of the paracellular pathway. Recent studies of Helicobacter pylori infection have demonstrated similar effects on paracellular permeability (25). These data raise the possibility that altered paracellular permeability is induced by luminal bacteria as a mechanism for delivering essential nutrients, such as iron, from the extracellular fluid (25).
An unexpected finding of this study was the effect of infection on epithelial anion secretion. EPEC did reduce cAMP-dependent anion secretion by 6 h after infection, but this effect appears to be nonspecific since the nonpathogenic HB101 exhibited similar responses. Philpott and others recently described an inhibition of cAMP-mediated anion secretion by EPEC in experiments similar to our own (29), though they observed differences between EPEC and HB101. Another study reported increased anion secretion in Caco-2 cells, but the studies were performed within minutes of infection with EPEC and the effect was transient (3). It is surprising that the EPEC-infected T84 monolayers exhibited any vectorial transport capabilities considering the extent of damage to both actin cytoskeleton and microtubules, particularly at 18 h. Both structural components have been previously shown to play a major role in the regulation of apical and basolateral anion channels important in vectorial transport (7, 17, 18, 33), and microtubules are important in regulating endocytic cycling of epithelial chloride channels to and from the apical membrane (1, 21).
STEC infection completely abolished cAMP-dependent secretion at 18 h. Calcium-dependent chloride secretion was also inhibited. Indeed, raising intracellular calcium caused a reversal of the short-circuit current in the majority of the monolayers infected with strain EDL 933. This ability of STEC strains to inhibit chloride secretion may have some benefit for the organism in the early phases of infection since secretion of water, electrolytes, mucus provides an effective epithelial defense against laminal pathogens. The inhibition of secretion may provide a window of opportunity for effective microbial adhesion prior to the involvement of the mucosal immune system.
The mechanism(s) whereby STEC inhibited secretion is not known. Lack of cell viability is unlikely since LDH secretion was not different from controls and since cells were not disrupted, as evidenced by electron microscopy. The effect does not require the development of A/E lesions or complete disruption of the actin cytoskeleton as was seen with EPEC. Clearly, these data are not consistent with the proposed effect of secreted proteins (in both EPEC and STEC), which elevate the intracellular calcium level or activate protein kinase C (14). These changes would be expected to stimulate rather than inhibit chloride secretion in T84 cells. However, the observation that elevating intracellular calcium with calcium ionophore A23187 caused a reversal of the short-circuit current (i.e., secretion of a cation) suggests a possible mechanism. The observed effect is consistent with stimulating basolateral potassium secretion in the absence of apical chloride channels and is a well-recognized phenomenon in cystic fibrosis epithelial cells, which lack functional apical chloride channels (8, 22). It is possible that an STEC-secreted protein is directly damaging apical ion channels. This notion would be supported by the ultrastructural studies, which demonstrated obvious disruption of the microvilli. Abnormalities of apical sodium transport have also been observed previously in studies utilizing an infant rabbit model of STEC colitis (16). Three days after infection, colonic mucosa exhibited a significant reduction of aldosterone-sensitive sodium absorption despite the absence of mucosal damage or inflammation, suggesting that surface bacteria somehow modulated apical membrane ion transporters. Malabsorptive diarrhea has also been reported in rabbit diarrheagenic E. coli infection in rabbits (35). In this study, infection inhibited cecal NaCl absorption rather than inducing active secretion. Defective apical salt absorption was thought to be due to direct surface damage by the attaching E. coli. An alternative explanation is that disruption of the cytoskeleton could block cAMP-dependent chloride secretion (7, 10). This could occur by several mechanisms, including the inhibition of endocytic cycling of chloride channels to and from the apical membrane and the inhibition of basolateral channels such as potassium-activated chloride secretion, sodium potassium ATPase, or NaK2Cl cotransporter. This latter explanation is less likely because the calcium ionophore experiments imply that these transporters are intact (i.e., basolateral potassium secretion is dependent on intact sodium potassium ATPase and NaK2Cl cotransporter activities). These hypotheses require further testing since, to our knowledge, the inhibition of epithelial secretion by STEC has not been described for any other enteric pathogen. Moreover, this concept runs counter to the current disease paradigm that implicates a key role for chloride secretion in infectious diarrhea (30).
That EPEC and STEC strains exhibit divergent signal transduction responses to infection of epithelial cells has recently been suggested by Ismaili et al. (11, 12). These investigators observed that STEC strains do not induce rearrangement of epithelial cell tyrosine phosphorylated proteins, which are usually associated with A/E lesions in EPEC and are considered to be central to EPEC pathogenesis. Their data suggest that STEC cells form adhesion pedestals, which were observed in a Hep-2 cell model system, by mechanisms that are distinct from those observed in EPEC. Our data reporting divergence with regard to effects on cell ultrastructure and anion secretion extend these earlier observations and further suggest that STEC strains possess hitherto-unrecognized pathogenic mechanisms. Whether these effects are independent of LEE remains to be determined.
In conclusion, while EPEC and STEC share similar pathogenicity genes, STEC infection of T84 epithelial cells causes major disruption to epithelial cell function without consistently inducing the typical morphological alterations characteristic of EPEC infection. These effects occur independently of Stx production and imply that pathogenic mechanisms other than those related to LEE may be operating in STEC infections.
ACKNOWLEDGMENTS
This work was supported by grants from the National Health and Medical Research Council of Australia and The Children's Hospital Fund.
We thank Roy Robins-Browne for providing the E. coli strains and for a critique of the manuscript.
REFERENCES
- 1.Bradbury N A, Jilling T, Berta B, Sorscher E J, Bridges R J, Kirk K L. Regulation of plasma membrane recycling by CFTR. Science. 1992;256:530–531. doi: 10.1126/science.1373908. [DOI] [PubMed] [Google Scholar]
- 2.Canil C, Rosenshine I, Ruschkowski S, Donnenberg M S, Kaper J B, Finlay B B. Enteropathogenic Escherichia coli decreases the transepithelial electrical resistance of polarized epithelial monolayers. Infect Immun. 1993;61:2755–2762. doi: 10.1128/iai.61.7.2755-2762.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collington G K, Booth I W, Knutton S. Rapid modulation of electrolyte transport in Caco-2 cell monolayers by enteropathogenic Escherichia coli (EPEC) infection. Gut. 1998;42:200–207. doi: 10.1136/gut.42.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli. Infect Immun. 1992;60:3953–3961. doi: 10.1128/iai.60.10.3953-3961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dytoc M T, Ismaili A, Philpott D J, Soni R, Brunton J L, Sherman P M. Distinct binding properties of eaeA-negative verocytotoxin-producing Escherichia coli of serotype O113:H21. Infect Immun. 1994;62:3494–3505. doi: 10.1128/iai.62.8.3494-3505.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott E, Henning P, Hogg G, Knight J, O'Loughlin E V, Powell H, Redmond D, Robins-Browne R. Haemolytic uraemic syndrome in Australia. J Gastroenterol Hepatol. 1995;10:A39. doi: 10.1136/adc.85.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuller C M, Bridges R J, Benos D J. Forskolin- but not ionomycin-evoked CT secretion in colonic epithelia depends on intact microtubules. Am J Physiol. 1994;266:C661–C668. doi: 10.1152/ajpcell.1994.266.3.C661. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein J L, Shapiro A B, Rao M C, Layden T J. In vivo evidence of altered chloride but not potassium secretion in cystic fibrosis rectal mucosa. Gastroenterology. 1991;101:1012–1019. doi: 10.1016/0016-5085(91)90728-4. [DOI] [PubMed] [Google Scholar]
- 9.Grifin P M, Olmstead L C, Petras R E. Escherichia coli O157:H7 associated colitis. A clinical and histological study of 11 cases. Gastroenterology. 1990;99:142–149. doi: 10.1016/0016-5085(90)91241-w. [DOI] [PubMed] [Google Scholar]
- 10.Gromol T, Van Dyke R W. Prostaglandin and theophylline induced Cl secretion in rat distal colon is inhibited by microtubule inhibitors. Dig Dis Sci. 1998;37:1709–1717. doi: 10.1007/BF01299864. [DOI] [PubMed] [Google Scholar]
- 11.Ismaili A, McWhirter E, Handelsman M Y, Brunton J L, Sherman P M. Divergent signal transduction responses to infection with attaching and effacing Escherichia coli. Infect Immun. 1998;66:1688–1696. doi: 10.1128/iai.66.4.1688-1696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ismaili A, Philpott D J, Dytoc M T, Sherman P M. Signal transduction responses following adhesion of verocytotoxin-producing Escherichia coli. Infect Immun. 1995;63:3316–3326. doi: 10.1128/iai.63.9.3316-3326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung H C, Eckmann L, Yang S-K, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Investig. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaper J B. EPEC delivers the goods. Trends Microbiol. 1998;6:169–173. doi: 10.1016/s0966-842x(98)01266-9. [DOI] [PubMed] [Google Scholar]
- 15.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Bell C, Buret A, Robins-Browne R, Stiel D, O'Loughlin E V. The effect of enterohemorrhagic Escherichia coli O157:H7 on intestinal structure and solute transport in rabbits. Gastroenterology. 1993;104:467–474. doi: 10.1016/0016-5085(93)90415-9. [DOI] [PubMed] [Google Scholar]
- 17.Matthews J B, Awtrey C S, Madara J L. Microfilament-dependent activation of Na+/K+/2Cl− cotransport by cAMP in intestinal epithelial monolayers. J Clin Investig. 1992;90:1608–1613. doi: 10.1172/JCI116030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews J B, Smith J A, Tally K J, Awtrey C S, Nguyen H, Rich J, Madara J L. Na-K-2Cl cotransport in intestinal epithelial cells. Influence of chloride efflux and F-actin on regulation of cotransporter activity and bumetanide binding. J Biol Chem. 1994;269:15703–15709. [PubMed] [Google Scholar]
- 19.McDaniel T K, Jarvis K G, Donnenberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKee M L, Melton-Celsa A R, Moxley R A, Francis D H, O'Brien A D. Enterohemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infect Immun. 1995;63:3739–3744. doi: 10.1128/iai.63.9.3739-3744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris A P, Cunningham S A, Tousson A, Benos D J, Frizzell R A. Polarization-dependent apical membrane CFIR targeting underlies cAMP-stimulated Cl− secretion in epithelial cells. Am J Physiol. 1994;266:C254–C268. doi: 10.1152/ajpcell.1994.266.1.C254. [DOI] [PubMed] [Google Scholar]
- 22.O'Loughlin E V, Hunt D M, Gaskin K J, Stiel D, Bruzuszcak I M, Martin H C O, Bambach C, Smith R. Abnormal epithelial transport in cystic fibrosis jejeunum. Am J Physiol. 1991;260:G758–G763. doi: 10.1152/ajpgi.1991.260.5.G758. [DOI] [PubMed] [Google Scholar]
- 23.Pai C H, Kelly J K, Meyers G L. Experimental infection of infant rabbits with verotoxin-producing Escherichia coli. Infect Immun. 1986;51:16–23. doi: 10.1128/iai.51.1.16-23.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pang G, Buret A, O'Loughlin E, Smith A, Batey R, Clancy R. Immunologic, functional, and morphological characterization of three new human small intestinal epithelial cell lines. Gastroenterology. 1996;111:8–18. doi: 10.1053/gast.1996.v111.pm8698229. [DOI] [PubMed] [Google Scholar]
- 25.Papini E, Satin B, Norais N, de Bernard M, Telford J L, Rappuoli R, Montecucco C. Selective increase of the permeability of polarized epithelial cell monolayers by Helicobacter pylori vacuolating toxin. Infect Immun. 1998;102:813–820. doi: 10.1172/JCI2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paton A E, Manning P A, Woodrow M C, Paton J C. Translocated intimin receptors (Tir) of shiga-toxigenic Escherichia coli isolates belonging to serogroups O26, O111, and O157 react with sera from patients with hemolytic uremic syndrome and exhibit marked sequence heterogeneity. Infect Immun. 1998;66:5580–5586. doi: 10.1128/iai.66.11.5580-5586.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Philpott D J, Ackerley C A, Kiliaan A J, Karmali M, Perdue M H, Sherman P M. Translocation of verotoxin-1 across T84 monolayers: mechanism of bacterial toxin penetration of epithelium. Am J Physiol. 1997;273:G1349–G1358. doi: 10.1152/ajpgi.1997.273.6.G1349. [DOI] [PubMed] [Google Scholar]
- 28.Philpott D J, McKay D M, Mak W, Perdue M H, Sherman P M. Signal transduction pathways involved in enterohemorrhagic Escherichia coli induced alterations in T84 epithelial permeability. Infect Immun. 1998;66:1680–1687. doi: 10.1128/iai.66.4.1680-1687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philpott D J, McKay D M, Sherman P M, Perdue M H. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am J Physiol. 1996;270:G634–G645. doi: 10.1152/ajpgi.1996.270.4.G634. [DOI] [PubMed] [Google Scholar]
- 30.Powell D W. New paradigms for the pathophysiology of infectious diarrhea. Gastroenterology. 1994;106:1705–1707. doi: 10.1016/0016-5085(94)90430-8. [DOI] [PubMed] [Google Scholar]
- 31.Riley L W, Remis R S, Helgerson S D, McGee H B, Wells J G, Davis B R, Hebert R J, Olcott E S, Johnson L M, Hargrett N T, Blake P A, Cohen M L. Hemorrhagic colitis associated with a rare Escherichia coli serotype. New Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 32.Savkovic S D, Koutsouris A, Hecht G. Activation of NF-κB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am J Physiol. 1998;273:C1160–C1167. doi: 10.1152/ajpcell.1997.273.4.C1160. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro M, Matthews J, Hecht G, Delp C, Madara J L. Stabilization of F-actin prevents cAMP-elicited Cl− secretion in T84 cells. J Clin Investig. 1991;87:1903–1909. doi: 10.1172/JCI115215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spitz J, Yuhan R, Koutsouris A, Blatt C, Alverdy J, Hecht G. Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am J Physiol. 1995;268:G374–G379. doi: 10.1152/ajpgi.1995.268.2.G374. [DOI] [PubMed] [Google Scholar]
- 35.Tai Y-H, Gage T, McQueen C, Formal S, Boedeker E. Electrolyte transport in rabbit cecum. 1. Effect of RDEC-1 infection. Am J Physiol. 1989;256:G721–G726. doi: 10.1152/ajpgi.1989.256.4.G721. [DOI] [PubMed] [Google Scholar]
- 36.Tzipori S, Karch H, Wachsmuth K I, Robins-Browne R M, O'Brien A D, Lior H, Cohen M L, Smithers J, Levine M L. Role of a 60-megadalton plasmid and shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect Immun. 1987;55:3117–3125. doi: 10.1128/iai.55.12.3117-3125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winsor D K, Ashkenazi S, Chiovetti R, Cleary T G. Adherence of enterohemorrhagic Escherichia coli strains to a human colonic epithelial cell line (T84) Infect Immun. 1992;60:1613–1617. doi: 10.1128/iai.60.4.1613-1617.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuhan R, Koutsouris A, Savkovic S D, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113:1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]