Abstract
N6-methyladenosine (m6A) modification is a newly discovered regulatory mechanism in eukaryotes. As one of the most common epigenetic mechanisms, m6A’s role in the development of atherosclerosis (AS) and atherosclerotic diseases (AD) has also received increasing attention. Herein, we elucidate the effect of m6A on major risk factors for AS, including lipid metabolism disorders, hypertension, and hyperglycemia. We also describe how m6A methylation contributes to endothelial cell injury, macrophage response, inflammation, and smooth muscle cell response in AS and AD. Subsequently, we illustrate the m6A-mediated aberrant biological role in the pathogenesis of AS and AD, and analyze the levels of m6A methylation in peripheral blood or local tissues of AS and AD, which helps to further discuss the diagnostic and therapeutic potential of m6A regulation for AS and AD. In summary, studies on m6A methylation provide new insights into the pathophysiologic mechanisms of AS and AD, and m6A methylation could be a novel diagnostic biomarker and therapeutic target for AS and AD.
Keywords: N6-methyladenosine (m6A), atherosclerosis, atherosclerotic diseases, diagnostic biomarkers, targeted therapeutics
1. Introduction
Atherosclerosis (AS) is a chronic inflammatory disease with multiple pathological features, such as endothelial dysfunction, vascular inflammation, and cholesterol accumulation. AS can cause artery plaque and stenosis, leading to the occurrence of atherosclerotic diseases (AD), such as coronary artery disease, stroke, and other arterial diseases [1,2]. AD remain the leading causes of death worldwide, and have created a vital global burden, which is still increasing [3,4]. However, the pathogenesis of AS and AD is extremely complex and largely unclear. In a word, it is of great significance to investigate the new mechanism and potential therapeutic targets of AS and AD.
A growing number of studies [5,6] show that post-transcriptional epigenetic modifications are closely related to the processes of AS and AD. N6-methyladenosine (m6A) modifications (one of the common post-transcriptional epigenetic modifications) are involved in the occurrence and development of AS and AD, and are novel and potential therapeutic targets and diagnostic biomarkers for AS and AD [7,8,9].
m6A methylation is a post-transcriptional epigenetic modification at the RNA level, which is a process of methylation of adenine at the sixth nitrogen atom catalyzed by RNA methyltransferases. m6A methylation is the most prevalent and reversible type of modification in eukaryotic mRNA, and it also plays a role in noncoding RNAs such as microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs) [10,11,12,13]. m6A methylation can regulate RNA stability, positioning, transport, splicing, and translation [14,15], which will affect the structure and function of RNAs. It plays a crucial regulatory role in the pathogenesis of various diseases, such as tumors, cardiovascular, and cerebrovascular diseases, etc. [16,17]. Recent studies [7,8,9] have identified the significant role of m6A methylation in the occurrence and development of AS and AD. In this review, we describe the relationship between m6A and risk factors of AS, highlight its mechanism in the pathogenesis of AS, and elucidate the impact of m6A methylation on the development of AS and AD. We also discuss the diagnostic and therapeutic potential of m6A methylation regulators for AS and AD. Our review may provide novel insights into the pathophysiologic mechanisms, diagnostic biomarkers, and therapeutic targets for AS and AD.
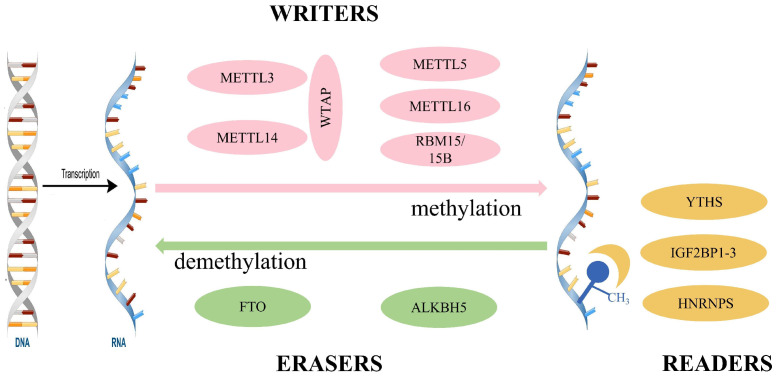
2. Regulators of m6A Methylation
Since its first discovery in the 1970s [18], m6A has been identified as the most common mRNA internal modification in most eukaryotic species [18,19,20,21,22,23]. Similar to DNA methylation and histone modification, RNA methylation is a dynamic and reversible modification that regulates gene expression. m6A modifications are mediated by three regulators: “writers” (methyltransferases), “erasers” (demethylases), and “readers” (m6A-binding proteins) (Figure 1). The m6A methylation site has a typical consensus sequence RRACH (R=G or A; H=A, C, or U), which is enriched in the coding sequence and 3′ untranslated region, particularly around stop codon regions [20,21]. Interactions among m6A-modified writers, readers, and erasers are involved in the regulation of the RNA life cycle, thereby affecting many physiological and pathological processes, such as cell differentiation, self-renewal, and apoptosis, and the development of cancer, cardiovascular, and metabolic diseases.
Figure 1.
Regulators of m6A methylation. MTTL3, methyltransferase-like 3; WTAP, Wilms tumor 1-associated protein; RBM15/15B, RNA binding motif protein 15/15B; FTO, fat mass and obesity-related protein; ALKBH5, alkylation repair homologous protein 5; YTHS, YTDF homeodomain family proteins; IGF2BP1-3, insulin-like growth factor 2 mRNA binding protein; HNRNPS, heterogeneous ribonucleoproteins.
2.1. Writers
The methyltransferases, also known as the codons or writers, are involved in the composition of the methyltransferase complex (MTC). MTC is composed of methyltransferase-like 3 (METTL3), METTL14, and other related regulators such as Wilms tumor 1-associated protein (WTAP), METTL5, METTL16, and RNA binding motif protein 15/15B (RBM15/15B) [24,25,26,27,28].
METTL3 was discovered in 1997 and it contains two S-adenosylmethionine binding sites called catalytically active methyltransferase domains [29]. METTL14, a homolog of METTL3, plays an important role in structurally supporting RNA binding by providing an RNA-binding scaffold [30,31]. WTAP can bind to the METTL3-METTL14 complex and plays an important role in regulating the localization of the METTL3-METTL14 complex to nuclear foci [32]. In addition, METTL5, METTL16, RBM15/15B, and ZC3H13 play integral roles in m6A methylation [25,26,27,28].
2.2. Erasers
In contrast to MTC, demethylases are called erasers. Their role is to remove m6A methylation [33]. Demethylases include fat mass and obesity-related protein (FTO) and alkylation repair homologous protein 5 (ALKBH5) [22,34]. Although these two enzymes have similar functions, they play different roles in the process of demethylation.
In 2007, Frayling et al. [30] discovered a genetic variation in a gene associated with obesity risk, which was officially named FTO. FTO is abundant in the brain, especially in neurons. Hence, it may play an important role in the brain. FTO-dependent m6A demethylation contributes to human obesity and regulates energy balance, which is critical for its biological role in the cardiovascular system [35]. ALKBH5 is another nuclear-localized m6A demethylase. Zheng et al. [22] found that m6A total RNA levels were reduced in ALKBH5-overexpressing cells, which have been shown to regulate mRNA export, RNA metabolism, and mRNA assembly in nuclear speckles. In addition, ALKBH5 also plays a key role in biological processes such as cell cycle, stress response, and apoptosis [36].
2.3. Readers
Similar to DNA methylation, the biological function of m6A methylation is mediated by the recognition of m6A sites by m6A “readers”. m6A readers include YTDF homeodomain family proteins (YTDF1, YTDF2, YTDF3, YTDC1, and YTDC2), insulin-like growth factor 2 mRNA binding protein (IGF2BP1-3), and heterogeneous ribonucleoproteins (HNRNPA2B1, HNRNPC, and HNRNPG) [37].
Different readers have different biological functions. YTHDF1 promotes the translation of m6A methylated mRNAs, YTHDF2 accelerates the decay of m6A methylated mRNAs, and YTHDF3, together with YTHDF1 and YTHDF 2, significantly enhances the metabolism of m6A methylated mRNAs in the cytoplasm [38]. In addition, IGF2BP expressed in the cytoplasm not only enhances mRNA stability but also increases translation efficiency [39]. Moreover, HNRNPG is involved in mRNA splicing and regulates pre-mRNA processing [40].
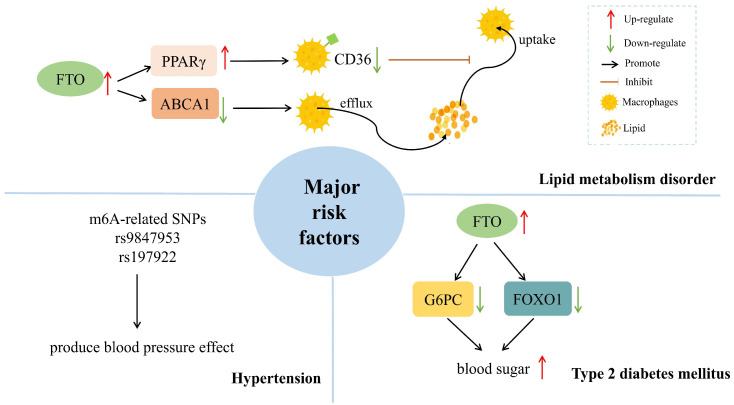
3. The Effects of m6A Methylation in AS Major Risk Factors
Dyslipidemia, hypertension, and diabetes are the most common risk factors for AS. We reviewed the effects of m6A methylation on the development of AS major risk factors as follows (Figure 2).
Figure 2.
Effects of m6A methylation on the development of AS major risk factors. PPARγ, proliferator-activated receptor γ; SNPs, single nucleotide polymorphisms; G6PC, glucose-6-phosphatase catalytic subunit; FOXO1; forkhead box protein O1.
3.1. Lipid Metabolism Disorder
Atherosclerotic lesions are based on lipid metabolism disorders [41]. Serum low-density lipoprotein levels are negatively correlated with m6A levels [42]. In addition, FTO catalyzes the demethylation of m6A methylation to alter mRNA processing, maturation, and translation of lipid-related genes [22,43,44].
Previous studies [45] have demonstrated that FTO inhibits the macrophage uptake of extracellular lipids, promotes intracellular lipid efflux, and inhibits macrophage lipid accumulation and foam cell formation. Scavenger receptor CD36, the primary transporter mediating extracellular lipid uptake by macrophages, is directly targeted by the oxisome proliferator-activated receptor γ (PPARγ). Mo et al. [46] subsequently observed that FTO-dependent m6A demethylation reduced PPARγ protein expression, resulting in downregulation of CD36 expression and decreased lipid uptake in RAW264.7 cells. Furthermore, Wu et al. showed that [47] FTO promotes AMPK phosphorylation and up-regulated ATP-binding cassette transporter A1 (ABCA1) in macrophages of mice. ABCA1 consumes ATP to mediate intracellular cholesterol efflux, which strongly prevents excessive lipid accumulation in macrophages. Reduced AMPK activity was shown to block FTO-induced upregulation of ABCA1. Additionally, FTO increases ABCA1 expression in an AMPK activity-dependent manner [46,48].
In conclusion, FTO is the key to regulating lipid homeostasis. m6A demethylation of FTO inhibits macrophage lipid influx by downregulating PPARγ protein expression and accelerates cholesterol efflux by phosphorylating AMPK, thereby preventing foam cell formation and development of AS.
3.2. Hypertension
Hypertension is one of the main risk factors for AS, but the mechanism by which hypertension promotes the occurrence of AS is unclear. However, one study showed [49] that epigenetics could influence the pathogenesis of hypertension.
Wu et al. [50] demonstrated by m6A high-throughput sequencing analysis that the average abundance of m6A was reduced in microvascular pericytes of spontaneously hypertensive rats. This means that m6A methylation may regulate hypertension in mammals. Genetic variation affects m6A expression by altering the RNA sequence of the target site, which can be referred to as m6A-related single nucleotide polymorphisms (SNPs) [51]. The study by Mo et al. [52] showed that many m6A-related SNPs, such as rs9847953 and rs197922, affect the expression of related genes, such as C1orf167, DOT1L, and thus produce blood pressure effects. These findings may shed light on the underlying mechanism of hypertension from the perspective of m6A modification.
3.3. Type 2 Diabetes Mellitus (T2DM)
T2DM is a common risk factor for AS. A previous study [53] showed that specific variants in FTO could predispose individuals to T2DM. Among these variants, the FTO rs9939609 (T > A) polymorphism is the most studied; for example, in the Oulu Project Elucidation of Atherosclerosis Risk study [54], it was shown that the FTO rs9939609 minor allele individuals with genetic variants had significantly higher rates of cardiovascular disease events or deaths. Yang et al. [55] showed that FTO positively regulates gluconeogenesis-related genes, such as the glucose-6-phosphatase catalytic subunit (G6PC) and forkhead box protein O1 (FOXO1), in an m6A-dependent manner. In T2DM patients, decreased m6A promotes hepatic gluconeogenesis, which leads to increased blood glucose by reducing the expression of gluconeogenesis-related genes. Furthermore, in a study of pancreatic islet cells from T2DM patients [56], m6A methylation was significantly reduced in β cells, but not in α cells, providing evidence that m6A methylation controls cellular insulin secretion. This evidence suggests that m6A methylation plays a vital role in T2DM.
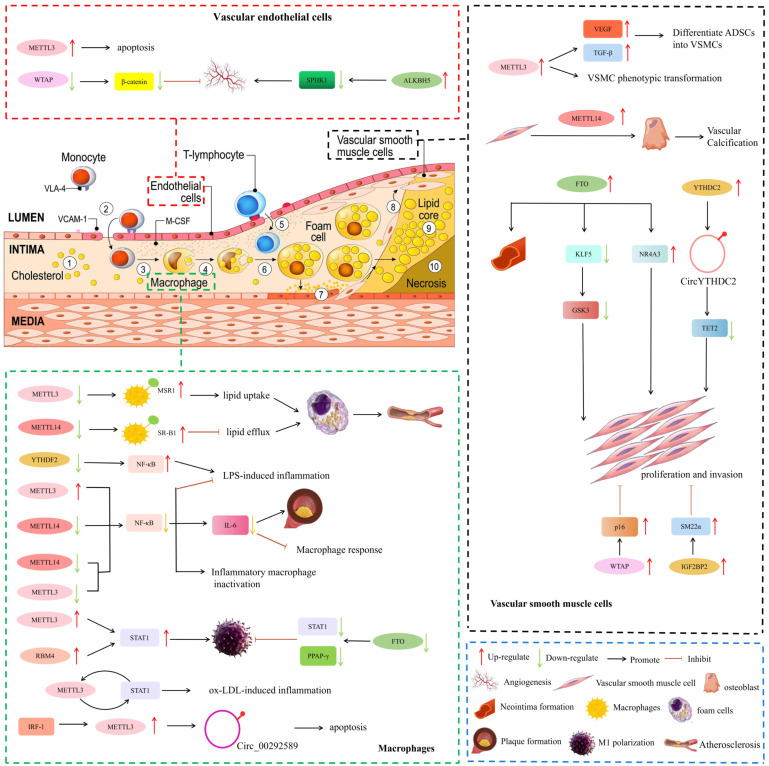
4. The Mechanisms of m6A Methylation in AS
Endothelial cells, macrophages, and smooth muscle cells are the most important initiating and developing cell types of AS. We summarized the mechanism by which m6A regulates these cell types to induce AS as follows (Figure 3).
Figure 3.
Potential mechanisms involved in m6A methylation-mediated regulation of AS. NR4A3, nuclear receptor subfamily 4 group A member 3; GSK3, glycogen synthase kinase 3; TET2, ten-eleven translocation 2; SM22α, smooth muscle 22α; ADSCs, adipose-derived stem cells; VSMCs, Vascular Smooth Muscle Cells; MSR1, macrophage scavenger receptor A; SR-B1, scavenger receptor B type 1; STAT1, signal transducer and activator of transcription 1; IRF-1, interferon regulatory factor-1.
4.1. Vascular Endothelial Cells
During the initial stages of AS, endothelial dysfunction and morphological damage occur, which lead to leukocyte adhesion, vasoconstriction, platelet aggregation, and thrombosis [57]. Vascular endothelial cell dysfunction is a key factor in the pathogenesis of AS.
m6A methylation plays a major role in post-transcriptional regulation in vascular endothelial cells. Zhu et al. [58] found that human cytomegalovirus (HCMV) infection could induce abnormally elevated m6A methylation, especially METTL3 and YTHDF3, leading to endothelial cell apoptosis. Wang et al. [59] used RNA transcriptome sequencing and found that in cerebral arteriovenous malformations, the expression levels of WTAP were significantly reduced but could inhibit endothelial cell angiogenesis.
m6A demethylation also plays important roles in endothelial cell angiogenesis. For example, Rajesh Kumari et al. [60] found that ALKBH5 levels were up-regulated after ischemia and correlated with the maintenance of ischemia-induced endothelial cell angiogenesis. ALKBH5 contributes to the maintenance of endothelial angiogenesis after acute ischemic stress by reducing SPHK1 m6A methylation and downstream eNOS-AKT signaling.
4.2. Macrophages Response and Inflammation
m6A methylation can influence AS progression by affecting macrophage cholesterol efflux and cell death. Cholesterol accumulation in macrophages, foam cell formation, and atherosclerotic lesions are all affected by macrophage cholesterol efflux capacity [61]. Zhao et al. [62] showed that during AS, oxidized low-density lipoprotein (ox-LDL) induced the expression of dead box protein 5 (DDX5) in macrophages and restricted the METTL3 function. METTL3 can transfer methyl groups to macrophage scavenger receptor A (MSR1) mRNA. Eventually, MSR1 mRNA stabilizes, and more MSR1 is synthesized. The uptake of more lipids further promotes the formation of foam cells, leading to the progression of AS. However, the specific mechanism of METTL3 inhibition by DDX5 is unclear. Park et al. [63] showed that MELL14 knockout attenuated cholesterol efflux and promoted foam cell formation by affecting m6A levels of scavenger receptor B type 1 (SR-B1) mRNA.
Inflammation is one of the major and fundamental pathological processes for all stages of AS [64]. The transformation of macrophages into an inflammatory phenotype is closely related to the progression of AS. Signal transducer and activator of transcription 1 (STAT1) is a key transcription factor whose activation leads to signaling cascades activated by pro-inflammatory macrophages. Liu et al. [65] showed that METTL3 has been shown to directly methylate STAT1 mRNA to increase mRNA stability, thereby upregulating STAT1 expression and promoting the polarization of M1 macrophages. Huang et al. [66] demonstrated that RBM4 regulates M1 macrophage polarization by targeting STAT1-mediated glycolysis. This study shows that RBM4 may be a candidate for regulating M1 polarization and inflammatory responses in macrophages. In addition, Gu et al. [67] found that the FTO gene knockout of m6A demethylase inhibited the phosphorylation of key proteins in the NF-κB signaling pathway, and was involved in reducing the mRNA stability of STAT1 and PPARγ through YTHDF2, thereby hindering macrophages polarization of cells. Similarly, Li et al. [68] also showed that ox-LDL stimulation significantly increased m6A-modified mRNA levels in macrophages. METTL3 promotes ox-LDL-triggered inflammation by interacting with STAT1 protein and mRNA in macrophages. In summary, m6A via the STAT1 pathway plays an important role in macrophage inflammation in AS.
In addition, m6A via the NF/κB signaling pathway also plays a crucial role in macrophage inflammation in AS. Wang et al. [69] showed that METTL3 reduced lipopolysaccharide (LPS)-induced macrophage inflammatory response by inhibiting the NF-κB pathway. However, Yu et al. [70] showed that downregulation of YTHDF2 significantly increased the LPS-induced expression of pro-inflammatory cytokines, such as IL-6 and TNF-α, and activated the MAPK and NF-κB signaling pathways. In addition, Yu et al. [71] found that the inhibition of METTL14 and METTL3 expression in macrophages could abolish m6A methylation of NF-κB mRNA, affect the stability of NF-κB mRNA, and ultimately lead to the inactivation of inflammatory macrophages, thereby significantly alleviating the progression of AS. Zheng et al. [72] found that Mettl14 knockout significantly reduced macrophage inflammatory response and atherosclerotic plaque formation through the NF-κB/IL-6 signaling pathway.
Moreover, Zhang et al. [73] identified a role for METTL3 in promoting oxidized LDL-induced monocyte inflammation. Guo et al. [74] showed that overexpression of interferon regulatory factor-1 (IRF-1) promoted apoptosis and inflammatory responses in atherosclerotic macrophages by upregulating m6A methylation levels and METTL3 expression on circ_0029589.
4.3. Vascular Smooth Muscle Cell (VSMC)
During AS progression, contractile VSMCs undergo phenotypic transformation into proliferative synthetic cells that generate an extracellular matrix, form fibrous caps, and stabilize plaques [75]. Accumulating evidence suggests that m6A can affect the pathophysiological function of VSMCs.
The “writers” of m6A methylation are involved in VSMC proliferation and migration. Lin et al. [76] showed that hypoxia can affect METTL3 expression and further affect m6A modification of related factors such as VEGF and TGF-β, thereby inducing adipose-derived stem cells (ADSCs) to differentiate into VSMCs. Chen et al. [77] found that overexpressed METTL14 increased m6A methylation by promoting the transformation of VSMCs to osteoblasts, and played an important role in the pathological mechanism of vascular calcification. Furthermore, in the study by Zhu et al. [78], the expression of WTAP in VSMCs altered cell proliferation and migration. Total notoginseng saponins regulate p16 m6A methylation by promoting WTAP expression, thereby inhibiting intimal thickening.
The “erasers” of m6A demethylases have been reported to promote VSMC proliferation and migration. For example, Ma et al. [79] demonstrated that both FTO overexpression and Ang II-induced FTO expression promoted VSMC proliferation and migration. FTO promotes the expression of Kruppel-like factor 5 (KLF5) mRNA by reducing the m6A methylation of KLF5 mRNA, thereby upregulating the expression of downstream glycogen synthase kinase 3 (GSK3). Similarly, Huo et al. [80] also showed that FTO promoted Ang II-induced VSMC proliferation and inflammatory response by demethylating the m6A methylation of nuclear receptor subfamily 4 group A member 3 (NR4A3) mRNA. In addition, Deng et al. [81] established a rat carotid artery balloon injury model to confirm the role of the FTO in neointima formation.
Different m6A “readers” function differently in VSMC proliferation and migration. Yuan et al. [82] found that YTHDC2-mediated m6A modification stabilizes circYTHDC2, which promotes VSMC proliferation and migration by negatively regulating the expression of ten-eleven translocation 2 (TET2). However, Zhang et al. [83] showed that IGF2BP2 increased the stability of smooth muscle 22α (SM22α) mRNA by acting as a “reader” for m6A-modified SM22α, inhibited the proliferation and migration of VSMC, and inhibited intimal hyperplasia.
5. The Role of m6A Methylation in AS and AD
m6A methylation not only causes the most common atherosclerotic diseases (such as coronary heart disease (CHD) and ischemic stroke (IS) through AS. Moreover, m6A also plays an important role in the injury and repair of CHD and IS. In Table 1, we reviewed the progress of m6A methylation regulator-guided epigenetic modification in AS and AD.
Table 1.
m6A methylation regulator-guided epigenetic modification in AS and AD.
| Atherosclerotic Process | m6A Regulators | Expression | Target Gene | Main Function | Reference |
|---|---|---|---|---|---|
| AS | METTL3 | ↑ | LRP6 and DVL1 | Enhances translation of LRP6 and DVL1, modulates Wnt signaling, and thus exerts angiogenic effects | [84] |
| METTL3 | ↑ | PGC-1α mRNA | Promotes mitochondrial dysfunction and ox-LDL-induced inflammation | [73] | |
| METTL3 | ↓ | JAK2/STAT3 | Alleviates ox-LDL-induced endothelial cell dysfunction, prevents in vivo angiogenesis of developing embryos, and hinders progression in AS mice models | [85] | |
| METTL3 | ↑ | NLRP1 and KLF4 | Up-regulates NLRP1, down-regulates KLF4, hypermethylates m6A, and triggers atherosclerotic response | [86] | |
| METTL3 | ↑ | miR-375-3p/PDK1 | Makes AS plaques more vulnerable | [87] | |
| METTL3 | ↑ | EGFR | Promotes EGFR degradation and alleviates endothelial atherogenic progression | [88] | |
| METTL14 | ↑ | FOXO1 | Increases FOXO1 m6A methylation, aggravates endothelial inflammation and AS | [89] | |
| METTL14 | ↓ | miR-19a | Inhibits the proliferation and invasion of ASVEC | [90] | |
| METTL14 | ↑ | LncRNA ZFAS1 | Plays a vital role in AS | [91] | |
| METTL14 | ↓ | p65 mRNA | Relieves the development of AS | [92] | |
| METTL14 | ↓ | NF-κB/IL-6 | Reduces the inflammation response of macrophages and the development of AS plaques | [72] | |
| FTO | ↑ | Not known | Modulates neointima formation in vivo | [81] | |
| FTO | ↓ | NR4A3 | Alleviates AngII-induced VSMC proliferation and inflammatory response | [80] | |
| ALKBH5 | ↓ | HIF1α | Inhibits the expression of MIAT induced by ox-LDL | [42] | |
| CHD | METTL3 | ↑ | TFEB | Promotes cardiomyocyte apoptosis | [8] |
| WTAP | ↑ | ATF4 | Promotes endoplasmic reticulum stress and apoptosis, aggravates myocardial I/R injury | [93] | |
| FTO | ↑ | SERCA2A MYH6/7 RYR2 | Reverses ischemic damage | [94] | |
| FTO | ↑ | MHRT | Inhibits cardiomyocyte apoptosis | [95] | |
| ALKBH5 | ↓ | TFEB | Promotes cardiomyocyte apoptosis | [8] | |
| ALKBH5 | ↑ | WNT5A | Regulates angiogenesis after ischemia | [96] | |
| IS | METTL3 | ↑ | miR-335 | Promotes formation of SG and reduces damage of IS | [97] |
| YTHDC1 | ↑ | Not known | Protects rats from brain damage | [98] |
↑, high expression; ↓, low expression; AS, atherosclerosis; METTL3, methyltransferase-like 3; LRP6, lipoprotein receptor-related protein 6; DVL1, dishevelled 1; ox-LDL, oxidized low-density lipoprotein; NLRP1, NOD-like receptor protein 1; KLF4, Kruppel-like factor 4; EGFR, epidermal growth factor receptor; FOXO1, forkhead box protein O1; ASVEC, atherosclerotic vascular endothelial cell; LncRNA, long non-coding RNA; ZFAS1, Zinc finger NFX type 1 antisense RNA 1; FTO, fat mass and obesity-related protein; NR4A3, nuclear receptor subfamily 4 group A member 3; ALKBH5, alkylation repair homologous protein 5; HIF1α, hypoxia-inducible factor 1α; CHD, coronary artery heart disease; MIAT, myocardial infarction-associated transcript; TFEB, transcription factor EB; WTAP, Wilms tumor 1-associated protein; ATF4, activated transcription factor 4; SERCA2A, sarcoplasmic reticulum Ca2+-ATPase; MYH6/7, myosin heavy chain 6/7; RYR2, ryanodine receptor 2; MHRT, myosin heavy chain-related RNA transcript; WNT5A, WNT family member 5A; IS, ischemic stroke; SG, stress granule; YTHDC1, YTH Domain Containing 1.
5.1. AS
AS is the main cause of CHD and IS [99]. Quiles Jiménez et al. [6] used mass spectrometry to analyze m6A methylation levels in tissue from non-atherosclerotic arterial and carotid atherosclerotic patients, which showed the changes in the expression levels of m6A writers, erasers, and readers in atherosclerotic tissue. The findings of Wu et al. [42] showed that m6A methylation levels were significantly reduced in peripheral blood leukocytes of atherosclerotic patients and mice. The bioinformatic analysis indicated that differentially methylated genes were involved in the pathogenesis of AS. These findings suggest that m6A methylation is involved in the occurrence and progression of AS.
METTL3-dependent m6A methylation was recently shown to play an important role in AS. For example, Yao et al. [84] demonstrated that METTL3 promotes the translation of low-density lipoprotein receptor-related protein 6 (LRP6) and dishevelled 1 (DVL1) in human umbilical vein endothelial cells (HUVEC) under hypoxic stress in a YTHDF1-dependent manner, thereby exerting an angiogenesis effect. Zhang et al. [73] demonstrated that METTL3 plays a role in ox-LDL-induced monocyte inflammation, in which METTL3 and YTHDF2 synergistically modify PGC-1α mRNA, mediate its degradation, and reduce PGC-1α protein levels, thereby enhancing the inflammatory response. This study provides new insights into the role of METTL3-dependent m6A methylation of PGC-1α mRNA in the inflammatory response of monocytes. In addition, Dong et al. [85] explored the role and molecular mechanism of m6A-METTL3 in AS progression from an in vivo perspective using an AS mouse model and an in vivo chick embryo chorioallantoic membrane assay. The results indicated that METTL3 knockout prevented AS progression through IGF2BP1 inhibition of the JAK2/STAT3 pathway. Furthermore, Chien et al. [86] showed that METTL3 up-regulated NOD-like receptor protein 1 (NLRP1) and down-regulated Kruppel-like factor 4 (KLF4) in an m6A-dependent manner. METTL3 exerts pro-inflammatory effects in HUVEC or mouse aortic endothelial cells exposed to pro-atherosclerotic oscillatory stress or TNF-α stimulation, thereby promoting inflammatory cell adhesion and AS pathogenesis. In a recent study, Chen et al. [87] found that silencing METTL3 alleviated AS progression in mice. Silencing METTL3 suppressed m6A levels and decreased the binding of DGCR8 to pri-miR-375, further limiting the expression of miR-375-3p. miR-375-3p targets PDK1 transcription. Ultimately silencing METTL3 plays a role in stabilizing AS plaques. However, Li et al. [88] reported a protective role of METTL3 in AS. The authors found that METTL3 promotes m6A-dependent degradation of epidermal growth factor receptor (EGFR) mRNA, a molecule associated with vascular endothelial cell (EC) dysfunction, thereby attenuating the progression of endothelial atherosclerosis.
Similarly, METTL14 also plays a pivotal role in the process of AS. Jian et al. [89] constructed a model of EC inflammation induced by TNF-α. With an increase in the expression of METTL14 in endothelial cells stimulated with TNF-α, METTL14 increases the m6A methylation of FOXO1, promoting its expression, which triggers endothelial inflammatory responses and the development of AS. Subsequent in vivo experiments showed that METTL14 knockout could inhibit AS plaque development in an m6A-dependent manner in METTL14 knockout mice. Furthermore, Zhang et al. [90] pointed out that METTL14 promoted the production of mature miR-19a by increasing the expression of m6A in miR-19a, thereby accelerating the invasion and proliferation of cardiovascular ECs. Chen et al. [100] showed that the m6A methylation of Zinc finger NFX type 1 (ZNFX1) antisense RNA 1 (ZFAS1) was significantly higher in AS patients than in controls, and that m6A methylation in ZFAS1 was regulated by METTl14. Tang et al. [91] found that METTl14 affects the expression of downstream ADAM10/RAB22A by affecting the m6A methylation of LncRNA ZFAS1, thereby participating in cholesterol metabolism and vascular inflammation, and ultimately regulating the occurrence and development of AS. Liu et al. [92] demonstrated that silencing METTL14 attenuates the development of AS through the m6A methylation of p65 mRNA by establishing an in vitro atherosclerotic cell model and an in vivo high-fat diet mouse model. Zheng et al. [72] showed that Mettl14 plays a crucial role in macrophage inflammation in AS through the NF-κB/IL-6 signaling pathway. METTL14 knockout significantly reduced the macrophage inflammatory response and atherosclerotic plaque formation.
In addition, demethylases also play an important role in AS. For example, Deng et al. [81] established a rat carotid artery balloon injury model, which confirmed that FTO could induce neointima formation. Huo et al. [80] used Ang II to construct vascular smooth muscle cells (VSMC) and vascular inflammation models in vitro and in vivo, and confirmed that the FTO/NR4A3 axis plays a key role in Ang II-induced VSMC proliferation and inflammation. Wu et al. [42] found that ox-LDL-induced ALKBH1 promotes myocardial infarction-associated transcript (MIAT) transcription by promoting the binding of hypoxia-inducible factor 1α (HIF1α). In addition, ALKBH1 knockdown inhibited ox-LDL-induced MIAT expression. All in all, the present findings suggest that the ALKBH1-m6A axis may control atherosclerotic plaque progression by regulating MIAT expression.
5.2. AD
5.2.1. CHD
CHD, also known as ischemic cardiomyopathy, refers to the clinical syndrome of long-term myocardial ischemia caused by coronary atherosclerosis, resulting in diffuse myocardial fibrosis [101].
Mathiyalagan et al. [94] demonstrated for the first time that mRNA m6A methylation was significantly higher in ischemic myocardium than in non-ischemic regions. Song et al. [8] demonstrated that m6A RNA methylation is involved in the development of myocardial hypoxia/reperfusion injury by regulating autophagy. Deng et al. [81] identified differentially methylated m6A sites in mRNAs and lncRNAs between peripheral blood mononuclear cells of the CHD group and control group. These studies suggest that m6A RNA methylation plays a crucial role in CHD.
Song et al. [8] established a mice model of hypoxia-reperfusion and ischemia-reperfusion and found that the m6A methylation levels in mice cardiomyocytes increased, and METTL3 is the main cause of abnormal modification of m6A methylation. Silencing METTL3 enhances autophagic flux and inhibits cardiomyocyte apoptosis in hypoxic/reoxygenated cardiomyocytes. However, the overexpression of METTL3 or inhibition of m6A demethylase ALKBH5 promoted cardiomyocyte apoptosis. This suggests that METTL3 is a negative regulator of autophagy. Similarly, WTAP promotes endoplasmic reticulum (ER) stress and apoptosis by increasing mRNA m6A levels of activated transcription factor 4 (ATF4), a transcription factor that controls the expression of ER-related genes, and up-regulates its expression, thereby aggravating myocardial I/R injury [93].
FTO-mediated m6A demethylation is also associated with myocardial I/R injury. FTO can selectively demethylate sarcoplasmic reticulum Ca2+-ATPase (SERCA2A), myosin heavy chain 6/7 (MYH6/7), ryanodine receptor 2 (RYR2), and other mRNAs that affect cardiac calcium homeostasis, myofibril synthesis, and contractile function. It increases the transcription and translation of the above genes through an m6A-dependent pathway, thereby reversing ischemic injury [94]. Myosin heavy chain-related RNA transcript (MHRT) is a heart-specific lncRNA derived from the antisense strand of the MYH7 gene. Shen et al. [95] demonstrated that overexpression of FTO inhibited apoptosis in I/R-treated cardiomyocytes by reducing the m6A modification of MHRT.
In addition, overexpression of ALKBH5 can reverse the damaging effects of METTL3 on cardiomyocytes [8]. Another study explored the effect and mechanism of ALKBH5 on angiogenesis after ischemia. Zhao et al. [96] demonstrated that ALKBH5 negatively regulates angiogenesis after ischemia by reducing the mA levels of WNT family member 5A (WNT5A) mRNA and by promoting its degradation in cardiac microvascular endothelial cells.
5.2.2. IS
The stenosis of the cerebral arterial lumen often occurs due to AS, resulting in ensuing thrombosis and IS. Emerging evidence [9,102] suggests that m6A methylation is involved in the injury and repair of IS.
Si et al. [97] established an oxygen–glucose deprivation/reperfusion model in primary cortical neurons and PC12 cells by using a middle cerebral artery occlusion model in rats to explore m6A methylation of potential mechanisms involved in stress granule (SG) formation in the early stages of acute ischemic stroke. Both in vitro and in vivo results showed that METTL3 protein, m6A levels, and miR-335 expression were significantly decreased with prolonged reperfusion time. The finding suggests that METTL3-mediated m6A methylation plays an important role in promoting SG formation and reducing IS damage in the early stages of disease.
Similar to METTL3, YTHDC1 also plays a protective role in the pathological process of IS. Zhang et al. [98] found that the knockout of YTHDC1 aggravated ischemic brain injury, while the overexpression of YTHDC1 protected rats from brain injury; mechanistically, YTHDC1 promotes PTEN mRNA degradation to increase Akt phosphorylation, thereby promoting neuronal survival, especially after ischemia.
6. Potential Diagnostic Biomarkers and Therapeutic Targets of m6A for AS and AD
The above findings provide new information for understanding the molecular pathogenesis of AS and exploring potential diagnostic biomarkers and therapeutic targets for AS and AD.
6.1. Potential Diagnostic Biomarkers of m6A for AS and AD
The levels of m6A methylation in the tissue of AS patients are significantly lower than that of non-AS patients and that of early AS patients. The expression levels of WTAP, METTL3, YTHDF2, and FTO are significantly lower than those of non-AS patients [6]. In addition, m6A levels in peripheral blood leukocytes are negatively correlated with carotid plaque size and thickness [42]. Thus, these biomarkers may potentially be used in the future for the early diagnosis of AS. In addition, the m6A levels in peripheral blood mononuclear cells of CHD patients are significantly lower than that of the control group, and the expression levels of FTO, METTL14, and ALKBH5 in the CHD patients are also lower than those of the control group [81]. Moreover, global m6A levels in ipsilateral cortical tissue around cerebral infarction are significantly elevated after transient focal ischemia, and FTO levels are significantly reduced after stroke [9]. Taken together, these findings indicate that m6A may be a potential biomarker for the diagnosis of AD. In summary, m6A levels in RNA may prove to be a valuable diagnostic biomarker for AS and AD. However, the relationship between m6A and AS or AD, and its diagnostic specificity and sensitivity, would need to be confirmed by clinical studies with easy access to specimens such as peripheral blood.
6.2. Potential Therapeutic Targets of m6A for AS and AD
Numerous studies have shown that methyltransferases are associated with aberrant m6A modification and lead to the development of AS and AD, which indicate that m6A methylation may be the potential therapeutic target. METTL3 promotes ox-LDL-triggered inflammation by interacting with STAT1 protein and mRNA in macrophages [68]. METTL14-mediated m6A modification of ZFAS1/RAB22A may play an important role in AS [100], and METTL14 plays a crucial role in macrophage inflammation in AS through the NF-κB/IL-6 signaling pathway [72]. In addition, experiments in vivo have shown that silencing METTL3 can stabilize atherosclerotic plaques [87]. Moreover, METTL14 knockout can inhibit the development of AS plaques [89], and silencing METTL14 can alleviate the development of AS [92]. These indicated that METTL3 and METTL14 may be promising therapeutic targets for the clinical treatment of AS. Likewise, ALKBH5 can negatively regulate angiogenesis after ischemia [96]. Thus, targeting ALKBH5 may be a potential therapeutic option for CHD. Additionally, a key link between METTL3-ALKBH5 and autophagy provides a new direction for m6A methylation therapy in CHD [8]. Moreover, in IS, METTL3-mediated m6A modification plays an important role in reducing damage in the early stages of stroke [97]. Furthermore, YTHDC1 is a novel regulator of neuronal survival [98]. However, further experimental and clinical evidence is needed to confirm these potential therapeutic targets.
Based on the above therapeutic targets, exploratory research on the treatment of AS with chemical drugs and botanical drugs are ongoing. In terms of chemical drugs, Zhu et al. [58] showed that vitamin D3 inhibited HCMV-induced vascular endothelial cell apoptosis by correcting m6A modification of mitochondrial calcium transporter mRNA, which was regulated by METTL3 and YTHDF3. This study highlighted the significance of vitamin D3 supplementation in HCMV-induced AS. For botanical drugs, several studies have also shown that Chinese herbal medicine could significantly delay the onset and progression of AS [71,103,104]. Hua Tuo Zai Zao Wan (HTZZW) was the most frequently studied traditional Chinese medicine. In the study by Yu et al. [71], positive effects were observed in AS mice treated with HTZZW. HTZZW exerts its effect through epigenetic regulation, which can regulate the expression of METTL14 and METTL3 in macrophages, thereby eliminating the m6A modification of NF-κB mRNA, and finally leading to the inactivation of macrophages, which has the effect of preventing AS.
In conclusion, m6A modifications are potential therapeutic targets for AS and AD, and drugs that affect m6A methylation are expected to be explored in AS and AD treatment in the future.
7. Discussion and Perspectives
This review expounds on the impact of m6A methylation on the main risk factors for AS, such as lipid metabolism disorders, hypertension, and hyperglycemia. We also describe the m6A methylation mechanisms that may contribute to the development of AS, including vascular endothelial cell injury, macrophage responses, inflammation, and the proliferation and migration of smooth muscle cells. We then summarized the pathophysiological role of m6A methylation in AS and AD, and discussed m6A methylation and its regulators as diagnostic biomarkers and treatment targets.
In terms of mechanism, the study of m6A revealed a potential link between this epigenetic modification and AS and AD. However, the mechanism of AS is very complex, and the specific association between m6A methylation and AS remains to be elucidated. Studies of m6A methylation in AS have mainly focused on METTL3 and METTL14 expression. Future studies should also explore how other m6A regulators, such as erasers and readers, regulate the expression of downstream proteins, and the interactions between m6A writers, erasers, and readers. In addition, it is unclear whether m6A methylation crosstalk with other epigenetics, such as non-coding RNA, DNA methylation, and histone modification, occurs in the development of AS.
In terms of clinical application, m6A methylation is dynamic and reversible, and has important implications for the diagnosis, prevention, and treatment of AS and AD. For diagnostic application, most studies on differential expression of m6A levels in AS and AD were performed in animal, and most of them are from local tissues (such as artery, heart, or brain), which is difficult to obtain clinically. In addition, a comprehensive study of the relationship between m6A methylation from peripheral blood and the disease in human is badly needed, which will help identify potential biomarkers for the diagnosis of AS and AD. All in all, it is a beautiful blueprint to use a simple detection method to determine the level of m6A or related proteins in peripheral blood to achieve the purpose of diagnosing AS and AD. For therapeutic application, m6A inhibitors or agonists for the treatment of AS are still at the stage of animal experiments, and more effective drugs and new therapeutic strategies related to m6A remain to be discovered.
8. Conclusions
Studies on m6A methylation provide new insights into the pathophysiologic mechanisms of AS and AD, and m6A methylation will be a novel diagnostic biomarker and therapeutic target for AS and AD in the near future.
Acknowledgments
Not applicable.
Author Contributions
Conceptualization, J.Y., Y.L. and Q.T.; writing—original draft preparation, Q.T.; writing—review and editing, Q.T., Y.L., S.H., X.L., D.Z., F.M., J.H., K.C., H.J. and J.Y.; funding acquisition, J.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China (82171295), Chengdu Science and Technology Bureau (2020-GH02-00057-HZ), and Science & Technology Department of Sichuan Province (2021YFS0376).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rohde L.E., Lee R.T. Pathophysiology of Atherosclerotic Plaque Development and Rupture: An Overview. Semin. Vasc. Med. 2003;3:347–354. doi: 10.1055/s-2004-815692. [DOI] [PubMed] [Google Scholar]
- 2.Chen L., Zheng J., Xue Q., Zhao Y. YKL-40 promotes the progress of atherosclerosis independent of lipid metabolism in apolipoprotein E−/− mice fed a high-fat diet. Heart Vessel. 2019;34:1874–1881. doi: 10.1007/s00380-019-01434-w. [DOI] [PubMed] [Google Scholar]
- 3.Björkegren J.L.M., Lusis A.J. Atherosclerosis: Recent developments. Cell. 2022;185:1630–1645. doi: 10.1016/j.cell.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collaborators G.B.D.S. Global, regional, and national burden of stroke and its risk factors, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrington W., Lacey B., Sherliker P., Armitage J., Lewington S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ. Res. 2016;118:535–546. doi: 10.1161/CIRCRESAHA.115.307611. [DOI] [PubMed] [Google Scholar]
- 6.Quiles-Jiménez A., Gregersen I., de Sousa M.M.L., Abbas A., Kong X.Y., Alseth I., Holm S., Dahl T.B., Skagen K., Skjelland M., et al. N6-methyladenosine in RNA of atherosclerotic plaques: An epitranscriptomic signature of human carotid atherosclerosis. Biochem. Biophys. Res. Commun. 2020;533:631–637. doi: 10.1016/j.bbrc.2020.09.057. [DOI] [PubMed] [Google Scholar]
- 7.Liu M., Xu K., Saaoud F., Shao Y., Zhang R., Lu Y., Sun Y., Drummer C., Li L., Wu S., et al. 29 m6A-RNA Methylation (Epitranscriptomic) Regulators Are Regulated in 41 Diseases including Atherosclerosis and Tumors Potentially via ROS Regulation—102 Transcriptomic Dataset Analyses. J. Immunol. Res. 2022;2022:1–42. doi: 10.1155/2022/1433323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song H., Feng X., Zhang H., Luo Y., Huang J., Lin M., Jin J., Ding X., Wu S., Huang H., et al. METTL3 and ALKBH5 oppositely regulate m6A modification of TFEB mRNA, which dictates the fate of hypoxia/reoxygenation-treated cardiomyocytes. Autophagy. 2019;15:1419–1437. doi: 10.1080/15548627.2019.1586246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chokkalla A.K., Mehta S.L., Kim T., Chelluboina B., Kim J., Vemuganti R. Transient Focal Ischemia Significantly Alters the m(6) A Epitranscriptomic Tagging of RNAs in the Brain. Stroke. 2019;50:2912–2921. doi: 10.1161/STROKEAHA.119.026433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He P.C., He C. m(6) A RNA methylation: From mechanisms to therapeutic potential. EMBO J. 2021;40:e105977. doi: 10.15252/embj.2020105977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das Mandal S., Ray P.S. Transcriptome-wide analysis reveals spatial correlation between N6-methyladenosine and binding sites of microRNAs and RNA-binding proteins. Genomics. 2021;113:205–216. doi: 10.1016/j.ygeno.2020.12.027. [DOI] [PubMed] [Google Scholar]
- 12.Di Timoteo G., Dattilo D., Centrón-Broco A., Colantoni A., Guarnacci M., Rossi F., Incarnato D., Oliviero S., Fatica A., Morlando M., et al. Modulation of circRNA Metabolism by m6A Modification. Cell Rep. 2020;31:107641. doi: 10.1016/j.celrep.2020.107641. [DOI] [PubMed] [Google Scholar]
- 13.Yang D., Qiao J., Wang G., Lan Y., Li G., Guo X., Xi J., Ye D., Zhu S., Chen W., et al. N6-Methyladenosine modification of lincRNA 1281 is critically required for mESC differentiation potential. Nucleic Acids Res. 2018;46:3906–3920. doi: 10.1093/nar/gky130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng H.-X., Zhang X.-S., Sui N. Advances in the profiling of N6-methyladenosine (m6A) modifications. Biotechnol. Adv. 2020;45:107656. doi: 10.1016/j.biotechadv.2020.107656. [DOI] [PubMed] [Google Scholar]
- 15.Zhao B.S., Roundtree I.A., He C. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 2017;18:31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun T., Wu R., Ming L. The role of m6A RNA methylation in cancer. Biomed. Pharmacother. 2019;112:108613. doi: 10.1016/j.biopha.2019.108613. [DOI] [PubMed] [Google Scholar]
- 17.Xu Z., Lv B., Qin Y., Zhang B. Emerging Roles and Mechanism of m6A Methylation in Cardiometabolic Diseases. Cells. 2022;11:1101. doi: 10.3390/cells11071101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desrosiers R., Friderici K., Rottman F. Identification of Methylated Nucleosides in Messenger RNA from Novikoff Hepatoma Cells. Proc. Natl. Acad. Sci. USA. 1974;71:3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yue Y., Liu J., He C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 2015;29:1343–1355. doi: 10.1101/gad.262766.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer K.D., Saletore Y., Zumbo P., Elemento O., Mason C.E., Jaffrey S.R. Comprehensive Analysis of mRNA Methylation Reveals Enrichment in 3′ UTRs and near Stop Codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dominissini D., Moshitch-Moshkovitz S., Schwartz S., Salmon-Divon M., Ungar L., Osenberg S., Cesarkas K., Jacob-Hirsch J., Amariglio N., Kupiec M., et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 22.Jia G., Fu Y., Zhao X., Dai Q., Zheng G., Yang Y., Yi C., Lindahl T., Pan T., Yang Y.-G., et al. N6-Methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen T., Hao Y.-J., Zhang Y., Li M.-M., Wang M., Han W., Wu Y., Lv Y., Hao J., Wang L., et al. m6A RNA Methylation Is Regulated by MicroRNAs and Promotes Reprogramming to Pluripotency. Cell Stem Cell. 2015;16:289–301. doi: 10.1016/j.stem.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Vu L.P., Pickering B.F., Cheng Y., Zaccara S., Nguyen D., Minuesa G., Chou T., Chow A., Saletore Y., Mackay M., et al. The N6-methyladenosine (m6A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat. Med. 2017;23:1369–1376. doi: 10.1038/nm.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aoyama T., Yamashita S., Tomita K. Mechanistic insights into m6A modification of U6 snRNA by human METTL16. Nucleic Acids Res. 2020;48:5157–5168. doi: 10.1093/nar/gkaa227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alderman M.H., 3rd, Xiao A.Z. N6-Methyladenine in eukaryotes. Cell. Mol. Life Sci. 2019;76:2957–2966. doi: 10.1007/s00018-019-03146-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu D., Zhou J., Zhao J., Jiang G., Zhang X., Zhang Y., Dong M. ZC3H13 suppresses colorectal cancer proliferation and invasion via inactivating Ras–ERK signaling. J. Cell. Physiol. 2019;234:8899–8907. doi: 10.1002/jcp.27551. [DOI] [PubMed] [Google Scholar]
- 28.Patil D.P., Chen C.-K., Pickering B.F., Chow A., Jackson C., Guttman M., Jaffrey S.R. m6A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537:369–373. doi: 10.1038/nature19342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bokar J.A., Shambaugh M.E., Polayes D., Matera A.G., Rottman F.M. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA. 1997;3:1233–1247. [PMC free article] [PubMed] [Google Scholar]
- 30.Wang P., Doxtader K.A., Nam Y. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol. Cell. 2016;63:306–317. doi: 10.1016/j.molcel.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koh C.W.Q., Goh Y.T., Goh W.S.S. Atlas of quantitative single-base-resolution N(6)-methyl-adenine methylomes. Nat. Commun. 2019;10:5636. doi: 10.1038/s41467-019-13561-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J., Yue Y., Han D., Wang X., Fu Y., Zhang L., Jia G., Yu M., Lu Z., Deng X., et al. A METTL3–METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frayling T.M., Timpson N.J., Weedon M.N., Zeggini E., Freathy R.M., Lindgren C.M., Perry J.R., Elliott K.S., Lango H., Rayner N.W., et al. A Common Variant in the FTO Gene Is Associated with Body Mass Index and Predisposes to Childhood and Adult Obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng G., Dahl J.A., Niu Y., Fedorcsak P., Huang C.-M., Li C.J., Vågbø C.B., Shi Y., Wang W.-L., Song S.-H., et al. ALKBH5 Is a Mammalian RNA Demethylase that Impacts RNA Metabolism and Mouse Fertility. Mol. Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J., Loos R.J.F., Powell J.E., Medland S.E., Speliotes E.K., Chasman D.I., Rose L.M., Thorleifsson G., Steinthorsdottir V., Mägi R., et al. FTO genotype is associated with phenotypic variability of body mass index. Nature. 2012;490:267–272. doi: 10.1038/nature11401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah A., Rashid F., Awan H.M., Hu S., Wang X., Chen L., Shan G. The DEAD-Box RNA Helicase DDX3 Interacts with m6A RNA Demethylase ALKBH5. Stem Cells Int. 2017;2017:8596135. doi: 10.1155/2017/8596135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H., Wu H., Wang Q., Ning S., Xu S., Pang D. Dual effects of N6-methyladenosine on cancer progression and immunotherapy. Mol. Ther. Nucleic Acids. 2021;24:25–39. doi: 10.1016/j.omtn.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi H., Wang X., Lu Z., Zhao B.S., Ma H., Hsu P.J., Liu C., He C. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 2017;27:315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang H., Weng H., Sun W., Qin X., Shi H., Wu H., Zhao B.S., Mesquita A., Liu C., Yuan C.L., et al. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 2018;20:285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geuens T., Bouhy D., Timmerman V. The hnRNP family: Insights into their role in health and disease. Hum. Genet. 2016;135:851–867. doi: 10.1007/s00439-016-1683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neeland I.J., Ross R., Després J.-P., Matsuzawa Y., Yamashita S., Shai I., Seidell J., Magni P., Santos R.D., Arsenault B., et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019;7:715–725. doi: 10.1016/S2213-8587(19)30084-1. [DOI] [PubMed] [Google Scholar]
- 42.Wu L., Pei Y., Zhu Y., Jiang M., Wang C., Cui W., Zhang D. Association of N6-methyladenine DNA with plaque progression in atherosclerosis via myocardial infarction-associated transcripts. Cell Death Dis. 2019;10:909. doi: 10.1038/s41419-019-2152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin L., Hales C.M., Garber K., Jin P. Fat mass and obesity-associated (FTO) protein interacts with CaMKII and modulates the activity of CREB signaling pathway. Hum. Mol. Genet. 2014;23:3299–3306. doi: 10.1093/hmg/ddu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Araujo T.M., Velloso L.A. Hypothalamic IRX3: A New Player in the Development of Obesity. Trends Endocrinol. Metab. 2020;31:368–377. doi: 10.1016/j.tem.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Gleissner C.A., Leitinger N., Ley K. Effects of Native and Modified Low-Density Lipoproteins on Monocyte Recruitment in Atherosclerosis. Hypertension. 2007;50:276–283. doi: 10.1161/HYPERTENSIONAHA.107.089854. [DOI] [PubMed] [Google Scholar]
- 46.Mo C., Yang M., Han X., Li J., Gao G., Tai H., Huang N., Xiao H. Fat mass and obesity-associated protein attenuates lipid accumulation in macrophage foam cells and alleviates atherosclerosis in apolipoprotein E-deficient mice. J. Hypertens. 2017;35:810–821. doi: 10.1097/HJH.0000000000001255. [DOI] [PubMed] [Google Scholar]
- 47.Wu Y.R., Shi X.Y., Ma C.Y., Zhang Y., Xu R.X., Li J.J. Liraglutide improves lipid metabolism by enhancing cholesterol efflux associated with ABCA1 and ERK1/2 pathway. Cardiovasc. Diabetol. 2019;18:146. doi: 10.1186/s12933-019-0954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li D., Wang D., Wang Y., Ling W., Feng X., Xia M. Adenosine Monophosphate-activated Protein Kinase Induces Cholesterol Efflux from Macrophage-derived Foam Cells and Alleviates Atherosclerosis in Apolipoprotein E-deficient Mice. J. Biol. Chem. 2010;285:33499–33509. doi: 10.1074/jbc.M110.159772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang M. Epigenetic Mechanisms and Hypertension. Hypertension. 2018;72:1244–1254. doi: 10.1161/HYPERTENSIONAHA.118.11171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Q., Yuan X., Han R., Zhang H., Xiu R. Epitranscriptomic mechanisms of N6-methyladenosine methylation regulating mammalian hypertension development by determined spontaneously hypertensive rats pericytes. Epigenomics. 2019;11:1359–1370. doi: 10.2217/epi-2019-0148. [DOI] [PubMed] [Google Scholar]
- 51.Zheng Y., Nie P., Peng D., He Z., Liu M., Xie Y., Miao Y., Zuo Z., Ren J. m6AVar: A database of functional variants involved in m6A modification. Nucleic Acids Res. 2018;46:D139–D145. doi: 10.1093/nar/gkx895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mo X.-B., Lei S.-F., Zhang Y.-H., Zhang H. Examination of the associations between m6A-associated single-nucleotide polymorphisms and blood pressure. Hypertens. Res. 2019;42:1582–1589. doi: 10.1038/s41440-019-0277-8. [DOI] [PubMed] [Google Scholar]
- 53.Scott L.J., Mohlke K.L., Bonnycastle L.L., Willer C.J., Li Y., Duren W.L., Erdos M.R., Stringham H.M., Chines P.S., Jackson A.U., et al. A Genome-Wide Association Study of Type 2 Diabetes in Finns Detects Multiple Susceptibility Variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Äijälä M., Ronkainen J., Huusko T., Malo E., Savolainen E.-R., Savolainen M.J., Salonurmi T., Bloigu R., Kesäniemi Y.A., Ukkola O. The fat mass and obesity-associated (FTO) gene variant rs9939609 predicts long-term incidence of cardiovascular disease and related death independent of the traditional risk factors. Ann. Med. 2015;47:655–663. doi: 10.3109/07853890.2015.1091088. [DOI] [PubMed] [Google Scholar]
- 55.Yang Y., Shen F., Huang W., Qin S., Huang J.-T., Sergi C., Yuan B.-F., Liu S.-M. Glucose Is Involved in the Dynamic Regulation of m6A in Patients With Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2019;104:665–673. doi: 10.1210/jc.2018-00619. [DOI] [PubMed] [Google Scholar]
- 56.De Jesus D.F., Zhang Z., Kahraman S., Brown N.K., Chen M., Hu J., Gupta M.K., He C., Kulkarni R.N. m6A mRNA Methylation Regulates Human beta-Cell Biology in Physiological States and in Type 2 Diabetes. Nat. Metab. 2019;1:765–774. doi: 10.1038/s42255-019-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chatzizisis Y.S., Coskun A.U., Jonas M., Edelman E.R., Feldman C.L., Stone P.H. Role of Endothelial Shear Stress in the Natural History of Coronary Atherosclerosis and Vascular Remodeling: Molecular, Cellular, and Vascular Behavior. J. Am. Coll. Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 58.Zhu W., Zhang H., Wang S. Vitamin D3 Suppresses Human Cytomegalovirus-Induced Vascular Endothelial Apoptosis via Rectification of Paradoxical m6A Modification of Mitochondrial Calcium Uniporter mRNA, Which Is Regulated by METTL3 and YTHDF3. Front. Microbiol. 2022;13:861734. doi: 10.3389/fmicb.2022.861734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang L.J., Xue Y., Li H., Huo R., Yan Z., Wang J., Xu H., Wang J., Cao Y., Zhao J.Z. Wilms’ tumour 1-associating protein inhibits endothelial cell angiogenesis by m6A-dependent epigenetic silencing of desmoplakin in brain arteriovenous malformation. J. Cell. Mol. Med. 2020;24:4981–4991. doi: 10.1111/jcmm.15101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumari R., Dutta R., Ranjan P., Suleiman Z.G., Goswami S.K., Li J., Pal H.C., Verma S.K. ALKBH5 Regulates SPHK1-Dependent Endothelial Cell Angiogenesis Following Ischemic Stress. Front. Cardiovasc. Med. 2021;8:817304. doi: 10.3389/fcvm.2021.817304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li J., Meng Q., Fu Y., Yu X., Ji T., Chao Y., Chen Q., Li Y., Bian H. Novel insights: Dynamic foam cells derived from the macrophage in atherosclerosis. J. Cell. Physiol. 2021;236:6154–6167. doi: 10.1002/jcp.30300. [DOI] [PubMed] [Google Scholar]
- 62.Zhao W., Wang Z., Sun Z., He Y., Jian D., Hu X., Zhang W., Zheng L. RNA helicase DDX5 participates in oxLDL-induced macrophage scavenger receptor 1 expression by suppressing mRNA degradation. Exp. Cell Res. 2018;366:114–120. doi: 10.1016/j.yexcr.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 63.Park M.H., Jeong E., Choudhury M. Mono-(2-Ethylhexyl)phthalate Regulates Cholesterol Efflux via MicroRNAs Regulated m6A RNA Methylation. Chem. Res. Toxicol. 2020;33:461–469. doi: 10.1021/acs.chemrestox.9b00367. [DOI] [PubMed] [Google Scholar]
- 64.Ketelhuth D.F.J., Lutgens E., Bäck M., Binder C.J., Van den Bossche J., Daniel C., Dumitriu I.E., Hoefer I., Libby P., O’Neill L., et al. Immunometabolism and atherosclerosis: Perspectives and clinical significance: A position paper from the Working Group on Atherosclerosis and Vascular Biology of the European Society of Cardiology. Cardiovasc. Res. 2019;115:1385–1392. doi: 10.1093/cvr/cvz166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y., Liu Z., Tang H., Shen Y., Gong Z., Xie N., Zhang X., Wang W., Kong W., Zhou Y., et al. The N6-Methyladenosine (m6A)-Forming Enzyme METTL3 Facilitates M1 Macrophage Polarization through the Methylation of STAT1 mRNA. Am. J. Physiol. Cell. Physiol. 2019;317:C762–C775. doi: 10.1152/ajpcell.00212.2019. [DOI] [PubMed] [Google Scholar]
- 66.Huangfu N., Zheng W., Xu Z., Wang S., Wang Y., Cheng J., Li Z., Cheng K., Zhang S., Chen X., et al. RBM4 regulates M1 macrophages polarization through targeting STAT1-mediated glycolysis. Int. Immunopharmacol. 2020;83:106432. doi: 10.1016/j.intimp.2020.106432. [DOI] [PubMed] [Google Scholar]
- 67.Gu X., Zhang Y., Li D., Cai H., Cai L., Xu Q. N6-methyladenosine demethylase FTO promotes M1 and M2 macrophage activation. Cell. Signal. 2020;69:109553. doi: 10.1016/j.cellsig.2020.109553. [DOI] [PubMed] [Google Scholar]
- 68.Li Z., Xu Q., Huangfu N., Chen X., Zhu J. Mettl3 promotes oxLDL-mediated inflammation through activating STAT1 signaling. J. Clin. Lab. Anal. 2022;36:e24019. doi: 10.1002/jcla.24019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J., Yan S., Lu H., Wang S., Xu D. METTL3 Attenuates LPS-Induced Inflammatory Response in Macrophages via NF-kappaB Signaling Pathway. Mediat. Inflamm. 2019;2019:3120391. doi: 10.1155/2019/3120391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu R., Li Q., Feng Z., Cai L., Xu Q. m6A Reader YTHDF2 Regulates LPS-Induced Inflammatory Response. Int. J. Mol. Sci. 2019;20:1323. doi: 10.3390/ijms20061323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu Z., Zheng X., Wang C., Chen C., Ning N., Peng D., Liu T., Pan W. The Traditional Chinese Medicine Hua Tuo Zai Zao Wan Alleviates Atherosclerosis by Deactivation of Inflammatory Macrophages. Evid.-Based Complement. Altern. Med. 2022;2022:2200662. doi: 10.1155/2022/2200662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng Y., Li Y., Ran X., Wang D., Zheng X., Zhang M., Yu B., Sun Y., Wu J. Mettl14 mediates the inflammatory response of macrophages in atherosclerosis through the NF-kappaB/IL-6 signaling pathway. Cell. Mol. Life Sci. 2022;79:311. doi: 10.1007/s00018-022-04331-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang X., Li X., Jia H., An G., Ni J. The m6A methyltransferase METTL3 modifies PGC-1alpha mRNA promoting mitochondrial dysfunction and oxLDL-induced inflammation in monocytes. J. Biol. Chem. 2021;297:101058. doi: 10.1016/j.jbc.2021.101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo M., Yan R., Ji Q., Yao H., Sun M., Duan L., Xue Z., Jia Y. IFN regulatory Factor-1 induced macrophage pyroptosis by modulating m6A modification of circ_0029589 in patients with acute coronary syndrome. Int. Immunopharmacol. 2020;86:106800. doi: 10.1016/j.intimp.2020.106800. [DOI] [PubMed] [Google Scholar]
- 75.Bennett M.R., Sinha S., Owens G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016;118:692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin J., Zhu Q., Huang J., Cai R., Kuang Y. Hypoxia Promotes Vascular Smooth Muscle Cell (VSMC) Differentiation of Adipose-Derived Stem Cell (ADSC) by Regulating Mettl3 and Paracrine Factors. Stem Cells Int. 2020;2020:2830565. doi: 10.1155/2020/2830565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen J., Ning Y., Zhang H., Song N., Gu Y., Shi Y., Cai J., Ding X., Zhang X. METTL14-dependent m6A regulates vascular calcification induced by indoxyl sulfate. Life Sci. 2019;239:117034. doi: 10.1016/j.lfs.2019.117034. [DOI] [PubMed] [Google Scholar]
- 78.Zhu B., Gong Y., Shen L., Li J., Han J., Song B., Hu L., Wang Q., Wang Z. Total Panax notoginseng saponin inhibits vascular smooth muscle cell proliferation and migration and intimal hyperplasia by regulating WTAP/p16 signals via m6A modulation. Biomed. Pharmacother. 2020;124:109935. doi: 10.1016/j.biopha.2020.109935. [DOI] [PubMed] [Google Scholar]
- 79.Ma D., Liu X., Zhang J.J., Zhao J.J., Xiong Y.J., Chang Q., Wang H.Y., Su P., Meng J., Zhao Y.-B. Vascular Smooth Muscle FTO Promotes Aortic Dissecting Aneurysms via m6A Modification of Klf5. Front. Cardiovasc. Med. 2020;7:592550. doi: 10.3389/fcvm.2020.592550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huo Y.B., Gao X., Peng Q., Nie Q., Bi W. Dihydroartemisinin alleviates AngII-induced vascular smooth muscle cell proliferation and inflammatory response by blocking the FTO/NR4A3 axis. Inflamm. Res. 2022;71:243–253. doi: 10.1007/s00011-021-01533-3. [DOI] [PubMed] [Google Scholar]
- 81.Deng K., Ning X., Ren X., Yang B., Li J., Cao J., Chen J., Lu X., Chen S., Wang L. Transcriptome-wide N6-methyladenosine methylation landscape of coronary artery disease. Epigenomics. 2021;13:793–808. doi: 10.2217/epi-2020-0372. [DOI] [PubMed] [Google Scholar]
- 82.Yuan J., Liu Y., Zhou L., Xue Y., Lu Z., Gan J. YTHDC2-Mediated circYTHDC2 N6-Methyladenosine Modification Promotes Vascular Smooth Muscle Cells Dysfunction Through Inhibiting Ten-Eleven Translocation 2. Front. Cardiovasc. Med. 2021;8:686293. doi: 10.3389/fcvm.2021.686293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang B.-F., Wu Z.H., Deng J., Jin H.J., Chen W.B., Zhang S., Liu X.J., Wang W.-T., Zheng X.-T. m6A methylation-mediated elevation of SM22α inhibits the proliferation and migration of vascular smooth muscle cells and ameliorates intimal hyperplasia in type 2 diabetes mellitus. Biol. Chem. 2022;403:317–329. doi: 10.1515/hsz-2021-0296. [DOI] [PubMed] [Google Scholar]
- 84.Yao M.-D., Jiang Q., Ma Y., Liu C., Zhu C.-Y., Sun Y.-N., Shan K., Ge H.-M., Zhang Q.-Y., Zhang H.-Y., et al. Role of METTL3-Dependent N6-Methyladenosine mRNA Modification in the Promotion of Angiogenesis. Mol. Ther. 2020;28:2191–2202. doi: 10.1016/j.ymthe.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dong G., Yu J., Shan G., Su L., Yu N., Yang S. N6-Methyladenosine Methyltransferase METTL3 Promotes Angiogenesis and Atherosclerosis by Upregulating the JAK2/STAT3 Pathway via m6A Reader IGF2BP1. Front. Cell. Dev. Biol. 2021;9:731810. doi: 10.3389/fcell.2021.731810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chien C.-S., Li J.Y.-S., Chien Y., Wang M.-L., Yarmishyn A.A., Tsai P.-H., Juan C.-C., Nguyen P., Cheng H.-M., Huo T.-I., et al. METTL3-dependent N 6 -methyladenosine RNA modification mediates the atherogenic inflammatory cascades in vascular endothelium. Proc. Natl. Acad. Sci. USA. 2021;118:e2025070118. doi: 10.1073/pnas.2025070118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen J., Lai K., Yong X., Yin H., Chen Z., Wang H., Chen K., Zheng J. Silencing METTL3 Stabilizes Atherosclerotic Plaques by Regulating the Phenotypic Transformation of Vascular Smooth Muscle Cells via the miR-375-3p/PDK1 Axis. Cardiovasc. Drugs Ther. 2022 doi: 10.1007/s10557-022-07348-6. [DOI] [PubMed] [Google Scholar]
- 88.Li B., Zhang T., Liu M., Cui Z., Zhang Y., Liu M., Liu Y., Sun Y., Li M., Tian Y., et al. RNA N6-methyladenosine modulates endothelial atherogenic responses to disturbed flow in mice. eLife. 2021;11:e69906. doi: 10.7554/eLife.69906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jian D., Wang Y., Jian L., Tang H., Rao L., Chen K., Jia Z., Zhang W., Liu Y., Chen X., et al. METTL14 aggravates endothelial inflammation and atherosclerosis by increasing FOXO1 N6-methyladeosine modifications. Theranostics. 2020;10:8939–8956. doi: 10.7150/thno.45178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang B.Y., Han L., Tang Y.F., Zhang G.X., Fan X.-L., Zhang J.J., Xue Q., Xu Z.Y. METTL14 regulates m6A methylation-modified primary miR-19a to promote cardiovascular endothelial cell proliferation and invasion. Eur. Rev. Med. Pharmacol. Sci. 2022;24:7015–7023. doi: 10.26355/eurrev_202006_21694. [DOI] [PubMed] [Google Scholar]
- 91.Tang X., Yin R., Shi H., Wang X., Shen D., Wang X., Pan C. LncRNA ZFAS1 confers inflammatory responses and reduces cholesterol efflux in atherosclerosis through regulating miR-654-3p-ADAM10/RAB22A axis. Int. J. Cardiol. 2020;315:72–80. doi: 10.1016/j.ijcard.2020.03.056. [DOI] [PubMed] [Google Scholar]
- 92.Liu Y., Luo G., Tang Q., Song Y., Liu D., Wang H., Ma J. Methyltransferase-like 14 silencing relieves the development of atherosclerosis via m6A modification of p65 mRNA. Bioengineered. 2022;13:11832–11843. doi: 10.1080/21655979.2022.2031409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang J., Zhang J., Ma Y., Zeng Y., Lu C., Yang F., Jiang N., Zhang X., Wang Y., Xu Y., et al. WTAP promotes myocardial ischemia/reperfusion injury by increasing endoplasmic reticulum stress via regulating m6A modification of ATF4 mRNA. Aging. 2021;13:11135–11149. doi: 10.18632/aging.202770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mathiyalagan P., Adamiak M., Mayourian J., Sassi Y., Liang Y., Agarwal N., Jha D., Zhang S., Kohlbrenner E., Chepurko E., et al. FTO-Dependent m6A Regulates Cardiac Function During Remodeling and Repair. Circulation. 2019;139:518–532. doi: 10.1161/CIRCULATIONAHA.118.033794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shen W., Li H., Su H., Chen K., Yan J. FTO overexpression inhibits apoptosis of hypoxia/reoxygenation-treated myocardial cells by regulating m6A modification of Mhrt. Mol. Cell. Biochem. 2021;476:2171–2179. doi: 10.1007/s11010-021-04069-6. [DOI] [PubMed] [Google Scholar]
- 96.Zhao Y., Hu J., Sun X., Yang K., Yang L., Kong L., Zhang B., Li F., Li C., Shi B., et al. Loss of m6A demethylase ALKBH5 promotes post-ischemic angiogenesis via post-transcriptional stabilization of WNT5A. Clin. Transl. Med. 2021;11:e402. doi: 10.1002/ctm2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Si W., Li Y., Ye S., Li Z., Liu Y., Kuang W., Chen D., Zhu M. Methyltransferase 3 Mediated miRNA m6A Methylation Promotes Stress Granule Formation in the Early Stage of Acute Ischemic Stroke. Front. Mol. Neurosci. 2020;13:103. doi: 10.3389/fnmol.2020.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Z., Wang Q., Zhao X., Shao L., Liu G., Zheng X., Xie L., Zhang Y., Sun C., Xu R. YTHDC1 mitigates ischemic stroke by promoting Akt phosphorylation through destabilizing PTEN mRNA. Cell Death Dis. 2020;11:977. doi: 10.1038/s41419-020-03186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., Chiuve S.E., Cushman M., Delling F.N., Deo R., et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 100.Chen L., Yao H., Hui J.-y., Ding S.-h., Fan Y.-l., Pan Y.-h., Chen K.-h., Wan J.-q., Jiang J.-y. Global transcriptomic study of atherosclerosis development in rats. Gene. 2016;592:43–48. doi: 10.1016/j.gene.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 101.Mahmoudi M., Yu M., Serpooshan V., Wu J.C., Langer R., Lee R.T., Karp J.M., Farokhzad O.C. Multiscale technologies for treatment of ischemic cardiomyopathy. Nat. Nanotechnol. 2017;12:845–855. doi: 10.1038/nnano.2017.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diao M.Y., Zhu Y., Yang J., Xi S.S., Wen X., Gu Q., Hu W. Hypothermia protects neurons against ischemia/reperfusion-induced pyroptosis via m6A-mediated activation of PTEN and the PI3K/Akt/GSK-3beta signaling pathway. Brain Res. Bull. 2020;159:25–31. doi: 10.1016/j.brainresbull.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 103.Liu Q., Li J., Hartstone-Rose A., Wang J., Li J., Janicki J.S., Fan D. Chinese Herbal Compounds for the Prevention and Treatment of Atherosclerosis: Experimental Evidence and Mechanisms. Evid.-Based Complement. Altern. Med. 2015;2015:752610. doi: 10.1155/2015/752610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shen D.-Z., Xin S.-L., Chen C., Liu T. Effect of atorvastatin on expression of TLR4 and NF-κB p65 in atherosclerotic rabbits. Asian Pac. J. Trop. Med. 2013;6:493–496. doi: 10.1016/S1995-7645(13)60081-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.