Abstract
To investigate the role of antibody responses to (glyco)lipids in immunity to schistosome infection, lipids extracted from Schistosoma mansoni eggs and adult worms were fractionated, and the antibody isotype profile reactive to the fractionated lipids in a well-characterized S. haematobium-infected population was investigated. In tests of 10 plasma samples it was found that immunoglobulin G (IgG) reactivity was highest to the fraction containing ceramidepolyhexosides, whereas IgE reactivity was most prominent to both cerebroside- and ceramidepolyhexoside-containing fractions. The fraction containing ceramidepolyhexosides was then tested for reactivity with IgG subclasses and IgE in plasma samples from 66 S. haematobium-infected patients. Considering IgG4 and IgE, isotypes of particular interest in helminth infections, we found that both isotypes recognized egg (glyco)proteins in more than 90% of the infected subjects. However, in the case of glycolipids, IgE reactivity was much more prominent than IgG4 reactivity (found in 80 and 41% of the subjects, respectively). Furthermore, worm glycolipid-specific IgE prior to treatment of the subjects with praziquantel was negatively correlated with egg counts at 2 years posttreatment, indicating that IgE directed towards glycolipids could play an important role in resistance to reinfection.
In recent years, the regulation of immune responses in schistosome infections has been a topic of great interest. In particular, the role of antibodies in resistance or susceptibility to reinfection has been studied intensively. Helminth infections are characterized by elevated levels of immunoglobulin E (IgE) and IgG4 antibodies, isotypes which are normally expressed at very low levels. In schistosomiasis, the balance between these two isotypes is thought to play a role in resistance or susceptibility to infection. Immunoepidemiological studies have established a clear age-related resistance to infection, with children being more susceptible than adults (7, 11, 12). IgE levels are low in children and high in adults, whereas for IgG4 the reverse has been found (7, 12). Therefore, IgE has been implicated in protection against reinfection, whereas IgG4 has been associated with susceptibility to reinfection (4, 7, 12). Furthermore, since IgG4 and IgE can display parallel specificity spectra (13, 17), it has been postulated that IgG4 acts as a blocking antibody for IgE-mediated protective effector functions (12).
Many antigenic determinants of various schistosome life cycle stages are carbohydrate in nature (for a review, see reference 2). So far, the detailed characterization of antigens that are recognized by IgE and IgG4 has involved studying protein or glycoprotein components of schistosomes either directly or by cloning (14, 21). However, carbohydrate moieties not only occur on proteins but also can be lipid bound. In fact, there is a remarkable overlap in the occurrence of immunogenic carbohydrate epitopes on proteins and lipids (22). Recent studies by Dennis et al. (5) have shown that glycolipids extracted from Schistosoma mansoni adult worms can be recognized by IgG antibodies in sera from S. mansoni- and Schistosoma haematobium-infected individuals, indicating that glycolipids may play an active role in shaping immune responses to schistosomes.
To characterize antibody responses to (glyco)lipids in immune responses mounted during a schistosome infection, we have examined the antibody isotype profile reactive to these moieties in a well-characterized S. haematobium-infected population, focusing on IgG4 and IgE. To further clarify the potential role of antibodies directed to glycolipids in protective immunity, we used plasma samples from individuals who were classified as resistant or susceptible to reinfection 2 years after treatment with praziquantel.
MATERIALS AND METHODS
Study population.
The study population was from an area in Lambaréné, Gabon, where S. haematobium is endemic and has previously been described (10, 11). Plasma samples used were from 66 infected individuals, 38 children (5 to 14 years) and 28 adults (15 to 48 years), who have been described in detail before (10, 11). The adults selected had egg output (geometric mean, 111 eggs/10 ml of urine; range, 4 to 2,073 eggs/10 ml of urine) at levels equivalent to those for the children (geometric mean, 107 eggs/10 ml of urine; range, 11 to 3,230 eggs/10 ml of urine). Thus, egg output was not a variable that could affect pretreatment antibody reactivity. Samples that were taken immediately prior to treatment with praziquantel and 2 years thereafter were used. Two years after treatment, 33 subjects were reinfected and 24 subjects remained uninfected; the other subjects had left the study area or refused to participate in the study, as detailed before (11). For the study described here, posttreatment plasma samples from 20 reinfected and 15 uninfected subjects were available. Viable eggs in urine were counted, the level of circulating anodic antigen in plasma was determined, and the combined reagent strip index was calculated as a marker for acute pathology in the lower urinary tract as described previously (10). The results of these tests are shown in Table 1.
TABLE 1.
Description of subjects resistant to and reinfected with S. haematobiuma
| Infection status | No. of females/no. of males | Median age (yr) (range) | Median no. of eggs/ml of urine posttreatment (range) | Median plasma CAA (pg/ml) posttreatment (range) | Median RSI score posttreatment (range) |
|---|---|---|---|---|---|
| Resistant | 10/5 | 24 (14–48) | 0 | 0 | 1 (0–4) |
| Reinfected | 8/12 | 11** (6–16) | 99* (11–939) | 749* (0–6,773) | 2* (0–6) |
CAA, circulating anodic antigen; RSI, reagent strip index. *, P < 0.05; **, P < 0.001 (compared to the resistant group).
Antigen preparation.
Although plasma samples from S. haematobium-infected individuals were used in this study, the limited availability of S. haematobium parasite material prompted us to use antigens from S. mansoni, since previous studies have indicated that there is extensive cross-reactivity between S. mansoni and S. haematobium glycoprotein and glycolipid antigens (5, 15, 19).
S. mansoni adult worms were collected by perfusion of golden hamsters 45 to 48 days after infection. S. mansoni eggs were isolated from livers of infected hamsters after treatment of the liver homogenate with trypsin (6). Adult worm antigens (AWA) and soluble egg antigens (SEA) were prepared as described previously (3).
Lipids of S. mansoni adult worms (12 g [wet weight]) and eggs (1.6 g [wet weight]) were extracted by the method described by Bligh and Dyer (1). The organic phase was dried by rotary evaporation, dissolved in 10 ml of chloroform, and applied to a 20-ml column of the anion exchanger TEAE-cellulose (Serva, Heidelberg, Germany) that was converted to the hydroxyl form. Lipids were eluted as described by Rouser et al. (18). According to this protocol, the fractions contain the following lipids: fraction 1, cholesterol, glycerides, and other neutral lipids; fraction 2, cerebrosides, glycerol diglycerides, phosphatidylcholine, and sphingomyelin; fraction 3, ceramidepolyhexosides; fraction 4, inorganic substances; fraction 5, phosphatidylethanolamine and free fatty acids; fraction 6, phosphatidylserine; fraction 7, none (washing step); and fraction 8, phosphatidic acid, cardiolipin, phosphatidylglycerol, phosphatidylinositol, and other acidic lipids. The presence of glycolipids in fractions 2 and 3 was confirmed by orcinol staining of the lipid fractions on HPTLC plates (20).
Antibody analysis.
PolySorp microtiter plates (Nunc, Roskilde, Denmark) were coated overnight at room temperature with SEA or AWA (5 μg/ml in 0.035 M phosphate-buffered saline–0.15 M NaCl [pH 7.6] [PBS]) or with methanol-dissolved lipids (0.1% of the worm fractions and 0.25% of the egg fractions per well; for fraction 3 this is equivalent to 33 ng per well for worm glycolipids and 17 ng per well for egg glycolipids). Lipid-coated plates were air dried overnight. The following incubations were at 37°C with shaking in a total volume of 100 μl per well, unless stated otherwise. Between each incubation, plates were washed five times with PBS–0.01% Tween 20. Plates were blocked by a 1-h incubation with 200 μl of blocking solution (0.07% [wt/vol] bovine nonfat dry milk in PBS). To control for nonspecific binding, plates coated with blocking solution (1 h) were tested. Plasma samples as well as detection antibodies were diluted in blocking solution. Plates were incubated with plasma dilutions (60 min at a 1/100 dilution for total IgG; 90 min at 1/20 for IgG2, IgG4, and IgE; and overnight at 1/100 at 4°C for IgG1) and then with horseradish peroxidase-conjugated anti-human IgG1 (120 min, 1/3,000; CLB, Amsterdam, The Netherlands) or with biotin-conjugated (i) goat anti-human total IgG (60 min, 1/10,000; Vector, Burlingame, Calif.), (ii) goat anti-human IgE (90 min, 1/1,000; Vector), (iii) monoclonal anti-human IgG4 (90 min, 1/3,000; CLB), or (iv) monoclonal anti-human IgG2 (90 min, 1/1,000; Sigma, St. Louis, Mo.). The plates (except those for IgG1) were further incubated for 1 h with streptavidin-horseradish peroxidase (1/10,000; Central Laboratory for Blood Transfusion, Amsterdam, The Netherlands). Assays were developed at room temperature with 3,3′,5,5′-tetramethylbenzidine as the substrate. Reactions were stopped by adding 2 M H2SO4, and absorbance was read at 450 nm in an automated plate reader. For analysis, the optical density (OD) of each plasma sample on the blocking solution-coated plates was subtracted from the OD of the antigen-coated plates. Plasma samples from healthy Dutch donors were used as negative controls to calculate the cutoff OD values (these values were not significantly different from values obtained when plasma samples with high levels of total IgE from Indonesian donors with intestinal helminth infections but without schistosomiasis were used). IgG3 was excluded from this study due to technical problems with the assay, as in our hands all anti-human IgG3 conjugates tested showed cross-reactivity with other isotypes.
Statistical analysis.
The relationship between antibody levels and egg output was examined by a Spearman rank correlation test. The Wilcoxon matched-pairs signed-rank test was used for comparison of pre- and posttreatment measurements; a Mann-Whitney U test was used to compare data between uninfected and reinfected groups.
RESULTS
Recognition of S. mansoni lipid fractions by infection sera.
Lipids extracted from S. mansoni eggs were fractionated by TEAE-cellulose column chromatography, and antigenicity was analyzed by enzyme-linked immunosorbent assay (ELISA). Binding of IgG and IgE to the lipid fractions was monitored with plasma samples from 10 S. haematobium-infected individuals (Fig. 1). IgG reacted most strongly with fraction 3, containing ceramidepolyhexosides, whereas IgE reactivity was high against both fraction 3, containing ceramidepolyhexosides, and fraction 2, containing cerebrosides. In control plasma samples from 10 healthy Dutch donors antibody binding was low and no differences in reactivity to the various lipid fractions were observed. Results obtained with lipids extracted from S. mansoni adult worms were comparable (data not shown).
FIG. 1.
Binding of IgG and IgE to lipid fractions extracted from S. mansoni eggs in 10 plasma samples from S. haematobium-infected individuals. Antibody reactivities are plotted as ODs at 450 nm. The mean ODs plus two times the standard deviations for the healthy Dutch donors were 0.114 for IgG and 0.074 for IgE.
Subclass reactivity to glycolipids.
The reactivities of IgG1, IgG2, IgG4, and IgE to the fraction containing ceramidepolyhexosides (glycolipids) of S. mansoni eggs and adult worms were characterized by ELISA with plasma samples from 66 S. haematobium-infected subjects (Table 1). Reactivity to glycolipids was compared to binding of antibodies to (glyco)protein extracts of adult worms (AWA) and eggs (SEA). Ten plasma samples from healthy Dutch donors were used as negative controls. For each isotype the percentage of positive plasma samples was calculated, as shown in Table 2. Almost all plasma samples from infected subjects showed binding of IgG1 to proteins that were present in SEA, whereas only 56% of the samples showed IgG1 binding to the egg-derived glycolipids. In the case of IgG2, 58% of the samples contained IgG2 directed to SEA, and 41% contained IgG2 specific for egg glycolipids. The recognition pattern of IgG4 was comparable to that of IgG1; reactivity of IgG4 was particularly high to SEA and was substantial to AWA. In contrast, only 41 and 26% of the plasma samples contained IgG4 specific for glycolipids of eggs and worms, respectively. These findings indicate a limited reactivity of IgG1 and IgG4 to glycolipids. Remarkably, IgE reactivities to the protein extracts and to the glycolipids were comparable, being particularly high for egg antigens (>95%). In the case of adult worms, IgE reactivity to components of the protein extract and the glycolipid fraction was found in 59 and 55% of the samples, respectively. For all isotypes, subjects positive for antibodies to glycolipids were also positive for antibodies to the protein extracts.
TABLE 2.
Percentages of S. haematobium-infected subjects positive for binding of IgG1 IgG2, IgG4, and IgE to SEA, glycolipids of eggs, AWA, and glycolipids of adult worms
| Ig | % Positivea for binding to:
|
|||
|---|---|---|---|---|
| SEA | Glycolipids of eggs | AWA | Glycolipids of worms | |
| IgG1 | 94 | 56 | NDb | ND |
| IgG2 | 58 | 41 | ND | ND |
| IgG4 | 97 | 41 | 73 | 26 |
| IgE | 97 | 80 | 59 | 55 |
Sera were characterized as positive when OD values were above the mean plus two times the standard deviation for sera from 10 control Dutch subjects.
ND, not determined.
Differential recognition of glycolipids by IgG4 and IgE.
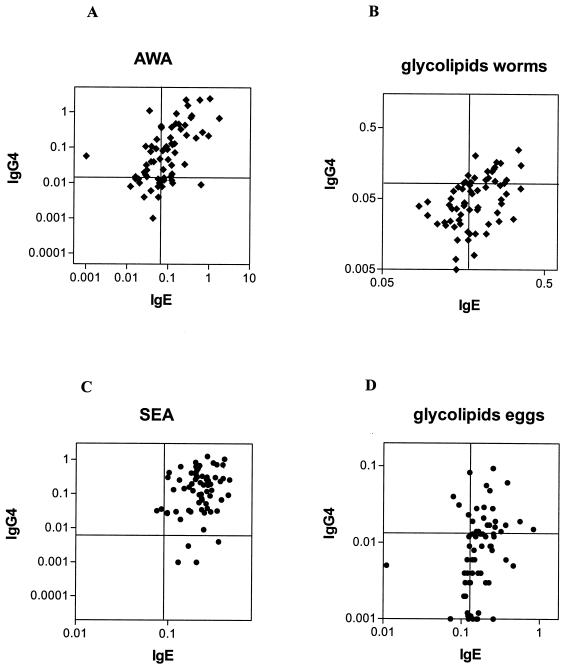
Given that IgE and IgG4 are the isotypes of most interest in helminth infections, we compared the reactivities of IgE and IgG4 to glycolipids and protein extracts (Fig. 2). For SEA and AWA, most plasma samples contained both IgE and IgG4 specific for components in these extracts. However, a substantial proportion of the samples was found to be positive for IgE directed to glycolipids but negative for IgG4 directed to these components, indicating preferential IgE binding to glycolipids relative to IgG4 binding.
FIG. 2.
Pretreatment IgE and IgG4 reactivities to AWA (A), glycolipids of adult worms (B), SEA (C), and glycolipids of eggs (D). Antibody reactivities are plotted as ODs at 450 nm. Each plot is divided by a horizontal line representing the mean plus two times the standard deviation for the healthy Dutch donors for IgG4 and by a vertical line representing the mean plus two times the standard deviation for the healthy Dutch donors for IgE.
Relationship between antibody isotypes and susceptibility to reinfection.
The study population described here was treated with praziquantel, and plasma samples were taken immediately prior to treatment and 2 years thereafter (10, 11). Previously, a correlation between pretreatment IgE specific for AWA and posttreatment egg counts was found, indicating that IgE directed to AWA may play a role in resistance to reinfection (7, 11, 12). To determine the predictive value of antibodies directed to glycolipid antigens in resistance to reinfection, the relationship between pretreatment antibody levels and posttreatment egg counts was determined. IgE reactivity to glycolipids of worms was significantly higher in individuals who were resistant to reinfection after treatment than in subjects who were reinfected at 2 years posttreatment (P = 0.025). Furthermore, pretreatment IgE specific for glycolipids derived from worms was negatively correlated with posttreatment egg counts (r = −0.371; P = 0.029) (Table 3). For AWA the same trend was observed; however, in the present study, where fewer plasma samples were available, the association did not reach statistical significance (r = −0.274; P = 0.111). No significant correlation with posttreatment egg counts was found for IgE to egg glycolipids and SEA or for other antibody isotypes directed to any of the antigen preparations.
TABLE 3.
Correlations between pretreatment antibody levels and egg counts at 2 years posttreatment
| Antibody isotype | Correlationa
|
|
|---|---|---|
| Glycolipids of worms | Glycolipids of eggs | |
| IgG1 | NDb | r = 0.200; P = 0.249 |
| IgG2 | ND | r = −0.102; P = 0.562 |
| IgG4 | r = −0.075; P = 0.671 | r = 0.229; P = 0.186 |
| IgE | r = −0.371; P = 0.028* | r = 0.211; P = 0.224 |
P values were determined by Spearman rank correlation. ∗, significant.
ND, not determined.
In a previous study by our group, a correlation between pretreatment SEA-specific IgG4 responses and pretreatment egg counts was found (10). To determine if this association also exists for antibodies directed to glycolipids, correlations between pretreatment egg counts and pretreatment specific IgG4 responses were calculated. Pretreatment egg counts were found to correlate positively with pretreatment IgG4 specific for glycolipids of eggs (r = 0.325; P = 0.008) and SEA (r = 0.374; P = 0.002). In addition, we found that individuals infected at 2 years posttreatment had significantly higher levels of IgG4 to egg glycolipids and SEA than uninfected subjects (P = 0.035 for egg glycolipids; P = 0.025 for SEA). For AWA, there was no significant difference in IgG4 to AWA and adult worm glycolipids between these groups. Moreover for IgG1, IgG2, and IgE, no differences in antibody levels to proteins or glycolipids derived from eggs or adult worms were found between uninfected and reinfected subjects.
DISCUSSION
Several immunoepidemiological studies have examined antibody isotype responses to schistosomal protein extracts. In this study we have extended this analysis to antibody isotypes reactive to schistosome lipids and glycolipids, using plasma samples from a well-characterized S. haematobium-infected population. It was clearly shown that the ceramidepolyhexoside-containing fraction of lipids extracted from schistosomes is a prime target of IgG antibody responses. The peak IgE reactivity was equally distributed over the cerebroside- and ceramidepolyhexoside-containing fractions. To evaluate the role of glycolipids in the immune responses mounted to schistosomes, we measured specific IgG subclasses (IgG1, IgG2, and IgG4) and IgE antibodies directed to schistosome glycolipids and (glyco)protein extracts in plasma samples from S. haematobium-infected subjects before and 2 years after treatment. Reactivity of IgG1 was prominent to SEA and lower to glycolipids of eggs, whereas IgG2 reactivities to SEA and glycolipids were equivalent. This implies that for binding of IgG1, peptide epitopes are important, while IgG2 recognizes mainly epitopes that are carbohydrate in nature. When considering IgG4 and IgE, it was striking that IgE reactivity to glycolipids extracted from schistosome eggs or adult worms was more prominent than IgG4 responses. This pattern was distinct from responses to SEA or to AWA, where IgG4 responses were comparable or more predominant. Thus, in our study subjects, glycolipids are preferentially recognized by IgE antibodies. In the study on antibody responses to glycolipids performed by Dennis et al., no IgE reactivity to a comparable glycolipid fraction extracted from S. mansoni and S. haematobium adult worms was found (5). To some extent this may be attributable to higher serum dilutions used in the ELISA in that study (1/40) compared to ours (1/20). In the same study, in 1 of 10 plasma samples IgG4 was found to be a prominent part of the IgG reactivity to schistosome glycolipids at a plasma dilution of 1/1,500, whereas in the study presented here, we did not find high IgG4 reactivity to glycolipids in any of the 66 samples with a 1/20 plasma dilution. This finding cannot be explained by the sensitivity of the ELISA used here being too low to measure glycolipid-specific IgG4, because high levels of IgG4 directed to SEA and AWA could be detected. Thus, it remains possible that either methodological or patient-related differences account for the difference in IgG4 reactivity between the study presented here and the study performed by Dennis et al. (5). It should be realized however, that the neutral glycolipid fraction used by Dennis et al. is equivalent to the glycolipid fractions used by us. In our study the neutral glycolipids were not in a single fraction but were further separated into fractions 2 and 3.
The preferential reactivity of IgE with glycolipids is of particular interest in two ways. Regulation of IgG4 and IgE is thought to be coordinated via Th2 cytokines interleukin-4 and interleukin-13 (9). So far, switch studies have shown that the presence of these cytokines will drive a switch to both IgG4, produced in large amounts, and IgE, synthesized at more modest levels (16). The existence of conditions where IgE responses are produced preferentially has to imply that glycolipids stimulate T cells that are particularly adapted for providing switch signals for IgE, possibly in a CD1-restricted manner. Interestingly, it was reported recently by Fujieda et al. that a CD1-restricted T-cell interaction with antigen can influence the antibody isotype production (8). Alternatively, glycolipids could suppress the development of IgG4-producing B cells. In addition, since IgE antibodies are considered to play an important role in protective immunity in schistosomiasis, whereas IgG4 is thought to block protective effector functions of IgE, a closer characterization of structures that preferentially bind IgE becomes important when considering preventive measures. Indeed, the importance of IgE antibodies directed to glycolipids is further strengthened by the finding that pretreatment levels of IgE specific for adult worm glycolipids were negatively correlated with posttreatment egg counts, indicating that IgE directed to these glycolipids may play a role in resistance to reinfection.
Glycoconjugates, both glycoproteins and glycolipids, are present in all life cycle stages of schistosomes. It was shown by Weiss et al. that a carbohydrate epitope recognized by a monoclonal antibody that was raised against the cercarial glycocalyx was present on glycoproteins and glycolipids of various schistosome life cycle stages (22). It is uncertain whether the production of the anticarbohydrate antibodies measured in the present study was stimulated by glycolipids, glycoproteins, or both. So far, our results indicate that proteins alone do not constitute the major binding targets of IgE but that this isotype is substantially directed towards carbohydrate moieties that can be linked to the ceramide portion of glycolipids or proteins present in SEA or AWA. Moreover, this study shows that IgE antibodies reactive with glycolipids may play a role in immunity to schistosomes. It should be noted that the glycolipid extract used here presumably consists of a mixture of glycolipids which can vary both in the carbohydrate part and in the lipid part. Purification and characterization of the structures that preferentially induce IgE antibodies may provide insight into the mechanisms that are important in regulation of IgE and possible resistance to infection.
ACKNOWLEDGMENTS
We thank P. Kremsner and J. Grogan for their help with collection of plasma samples and A. Deelder, D. Kornelis, and M. Schmitz for providing S. mansoni material.
This work was supported by the Netherlands Foundation for Chemical Research (SON) and the Earth and Life Science Foundation (ALW), with financial support from The Netherlands Organization for Scientific Research (NWO).
REFERENCES
- 1.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 2.Cummings R D, Nyame A K. Glycobiology of schistosomiasis. FASEB J. 1996;10:838–848. doi: 10.1096/fasebj.10.8.8666160. [DOI] [PubMed] [Google Scholar]
- 3.Deelder A M, Kornelis D, Makbin M, Noordpool H N, Codfried R M, Rotmans J P, Oostburg B F J. Applicability of different antigen preparations in the enzyme-linked immunosorbent assay for schistosomiasis mansoni. Am J Trop Med Hyg. 1980;29:401–410. doi: 10.4269/ajtmh.1980.29.401. [DOI] [PubMed] [Google Scholar]
- 4.Demeure C E, Rihet P, Abel L, Ouattara M, Bourgois A, Dessein A J. Resistance to Schistosoma mansoni in humans: influence of the IgE/IgG4 balance and IgG2 in immunity to reinfection after chemotherapy. J Infect Dis. 1993;168:1000–1008. doi: 10.1093/infdis/168.4.1000. [DOI] [PubMed] [Google Scholar]
- 5.Dennis R D, Baumeister S, Lauer G, Richter R, Geyer E. Neutral glycolipids of Schistosoma mansoni as feasible antigens in the detection of schistosomiasis. Parasitology. 1996;112:295–307. doi: 10.1017/s0031182000065811. [DOI] [PubMed] [Google Scholar]
- 6.Dresden M H, Payne D C. A sieving method for the collection of schistosome eggs from mouse intestines. J Parasitol. 1981;67:450–452. [PubMed] [Google Scholar]
- 7.Dunne D W, Butterworth A E, Fulford A J C, Kariuki H C, Langley J G, Ouma J H, Capron A, Pierce R J, Sturrock R F. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol. 1992;22:1483–1494. doi: 10.1002/eji.1830220622. [DOI] [PubMed] [Google Scholar]
- 8.Fujieda S, Sieling P A, Modlin R L, Saxon A. CD1-restricted T-cells influence IgG subclass and IgE production. J Allergy Clin Immunol. 1998;101:545–551. doi: 10.1016/s0091-6749(98)70362-8. [DOI] [PubMed] [Google Scholar]
- 9.Gascan H, Gauchat J F, Roncarolo M G, Yssel H, Spits H, de Vries J E. Human B cell clones can be induced to proliferate and to switch to IgE and IgG4 synthesis by interleukin 4 and a signal provided by activated CD4+ T cell clones. J Exp Med. 1991;173:747–750. doi: 10.1084/jem.173.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grogan J L, Kremsner P G, van Dam G J, Metzger W, Mordmuller B, Deelder A M, Yazdanbakhsh M. Antischistosome IgG4 and IgE responses are affected differentially by chemotherapy in children versus adults. J Infect Dis. 1996;173:1242–1247. doi: 10.1093/infdis/173.5.1242. [DOI] [PubMed] [Google Scholar]
- 11.Grogan J L, Kremsner P G, van Dam G J, Deelder A M, Yazdanbakhsh M. Anti-schistosome IgG4 and IgE at 2 years after chemotherapy: infected versus uninfected individuals. J Infect Dis. 1997;176:1344–1350. doi: 10.1086/514131. [DOI] [PubMed] [Google Scholar]
- 12.Hagan P, Blumenthal U J, Dunn D, Simpson A J G, Wilkins H A. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991;349:243–245. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- 13.Hussain R, Ottesen E A. IgE responses in human filariasis. IV. Parallel antigen recognition by IgE and IgG4 subclass antibodies. J Immunol. 1986;136:1859–1863. [PubMed] [Google Scholar]
- 14.Li Z, King C L, Ogundipe J O, Licate L S, Blanton R E. Preferential recognition by human IgE and IgG4 of a species-specific Schistosoma haematobium serine protease inhibitor. J Infect Dis. 1995;171:416–422. doi: 10.1093/infdis/171.2.416. [DOI] [PubMed] [Google Scholar]
- 15.Norden A P, Strand M. Schistosoma mansoni, S. haematobium and S. japonicum: identification of genus-, species-, and gender-specific antigenic worm glycoproteins. Exp Parasitol. 1984;57:110–123. doi: 10.1016/0014-4894(84)90070-5. [DOI] [PubMed] [Google Scholar]
- 16.Punnonen J, Aversa G, Cooks B G, McKenzie A N J, Menon S, Zurawski G, de Waal Malefyt R, de Vries J E. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. J Immunol. 1993;90:3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rihet P, Demeure C E, Dessein A J, Bourgois A. Strong serum inhibition of specific IgE correlated to competing IgG4, revealed by a new methodology in subjects from a S. mansoni endemic area. Eur J Immunol. 1992;22:2063–2070. doi: 10.1002/eji.1830220816. [DOI] [PubMed] [Google Scholar]
- 18.Rouser G, Kritchevsky G, Yamamoto A, Simon G, Galli C, Bauman A J. Diethylaminoethyl and triethylaminoethyl cellulose column chromatographic procedures for phospholipids, glycolipids, and pigments. Methods Enzymol. 1969;14:272–317. [Google Scholar]
- 19.Simpson A J G, Hackett F, Kelly C, Knight M, Payares G, Omer-Ali P, Lillywhite J, Fleck S L, Smithers S R. The recognition of Schistosoma mansoni surface antigens by antibodies from patients infected with S. mansoni and S. haematobium. Trans R Soc Trop Med Hyg. 1986;80:261–270. doi: 10.1016/0035-9203(86)90032-5. [DOI] [PubMed] [Google Scholar]
- 20.Svennerholm L. Quantitative estimation of cerebrosides in nervous tissue. J Neurochem. 1956;1:42–53. doi: 10.1111/j.1471-4159.1956.tb12053.x. [DOI] [PubMed] [Google Scholar]
- 21.Webster M, Fulford A J C, Braun G, Ouma J H, Kariuki H C, Havercroft J C, Gachuhi K, Sturrock R F, Butterworth A E, Dunne D W. Human immunoglobulin E responses to a recombinant 22.6-kilodalton antigen from Schistosoma mansoni adult worms are associated with low intensities of reinfection after treatment. Infect Immun. 1996;64:4042–4046. doi: 10.1128/iai.64.10.4042-4046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss J B, Magnani J L, Strand M. Identification of Schistosoma mansoni glycolipids that share immunogenic carbohydrate epitopes with glycoproteins. J Immunol. 1986;136:4275–4282. [PubMed] [Google Scholar]