Abstract
Oral candidiasis is the most common opportunistic fungal infection caused by commensal Candida species. Since there are various local and systemic predisposing factors for the disease, the treatment also varies from topical to systemic antifungal agents. Nystatin is a common antifungal agent used topically. The aim of this systematic review was to evaluate and compare the efficacy of different antifungal agents and the safety of nystatin in the treatment of oral candidiasis. Three electronic databases were searched for randomized controlled trials comparing nystatin with other anti-fungal therapies or placebo. Clinical and/or mycological cure was the outcome evaluation. A meta-analysis and descriptive study on the efficacy, treatment protocols, and safety of nystatin was also conducted. The meta-analysis included five studies, which compared the efficacy of nystatin suspensions with photodynamic therapy. A significant difference in the colony-forming units per milliliters (CFU/mL) of Candida species was observed at 60 days intervals for both palatal mucosa and denture surfaces, with both groups favoring nystatin with low heterogeneity at a 95% confidence interval. Nystatin and photodynamic therapy were found to be equally effective for the clinical remission of denture stomatitis as well as a significant reduction of CFU/mL of Candida species from dentures and palatal surfaces of the patients.
Keywords: nystatin, oral candidiasis, denture stomatitis, systematic review, meta-analysis, photodynamic therapy, treatment duration
1. Introduction
The normal oral microflora is a complex population of diverse microorganisms consisting of eubacteria, mycoplasmas, fungi, archaea, and protozoa [1,2]. Fungi are free-living, eukaryotic organisms that may be in round, filamentous, or dimorphic forms, amongst which the Candida species are most frequently encountered by dentists [2,3]. The opportunistic infections in humans afflicted by Candida albicans and other related species can clinically manifest ranging from the most common oral thrush to fatal, systemic superinfections in patients with local or systemic predisposing factors [4,5,6]. Typically, the infection due to Candida is opportunistic, occurring due to various factors, including compromised host defense, or a break in the normal oral mucosa. In addition to that, other external factors like the use of broad-spectrum antibiotics or poor oral hygiene especially in denture wearers increase the probability of Candida infection [2,7]. Use of dentures favors oral infections due to continuous local micro-trauma and increased time of contact with micro-organisms. Both partial and complete denture wearers harbor micro-organisms especially Candida species [2,8]. The use of soft lining materials to increase the comfort and the fit of removable dentures are penetrated by the fungal hyphae, predisposing to Candida infection [6,9]. Candida albicans is the most frequently encountered Candida species that reports for more than 90% of the isolates from the oral cavity [4,10,11]. Other species that have been identified to be pathogenic to humans are Candida parapsilosis, Candida tropicalis, Candida glabrata, Candida krusei, Candida guilliermondii, and Candida lusitaniae. It has been reported that Candida species are present in 26 to 75% of the healthy inhabitants in absence of any lesion as commensals in the oral cavity [4,12].
The management of oral candidiasis involves topical as well as systemic antifungal agents. For patients who are intolerant to or fail to respond to topical forms of treatment and are at higher peril of developing systemic infections, systemic antifungal agents like fluconazole, itraconazole, and miconazole are appropriate [13,14,15]. Because of various drug interactions as well as the reduced vulnerability of Candida species, except for Candida albicans towards azoles, the use of systemic antifungal agents is limited [14,15,16]. Therefore, for noninvasive cases of oral candidiasis, the first line of treatment is topical antifungal agents like nystatin, miconazole, amphotericin B, and clotrimazole [17,18]. Nystatin is produced by Streptomyces noursei strains, which is a membrane-active polyene macrolide. It is the most frequently prescribed topical antifungal drug by dentists and is available in various forms like topical creams, oral pastilles, and oral suspensions [17,18,19,20]. The drug is not absorbed from the gastrointestinal tract when orally administered eliminating the adverse effects [21]. Nystatin is usually administered in doses of 200,000–400,000 IU four times a day and 100,000–200,000 IU for infants and neonates for about 4 weeks, but there is no universally accepted dosage, formulation or duration of treatment for oral candidiasis [22,23,24,25,26]. Therefore, this study aims to summarize as well as evaluate the efficiency of different protocols of treatment (like dosage, formulations, and durations), along with the safety of nystatin in different inhabitants with oral candidiasis with the help of systematic review and meta-analysis.
2. Methods
The systematic review was performed under the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [27]. This systematic review was registered on PROSPERO as CRD42021290307, and the search was completed by October 2021.
2.1. Inclusion Criteria
This review was restricted to randomized controlled trials in English language comparing nystatin in all topical forms to all other antifungal therapies or placebo. The clinical diagnosis was the basis of diagnosing oral candidiasis with or without mycological test confirmation. Primary outcomes were the clinical response rate defined as the cure or improvement of signs and symptoms attributable to the oral lesion as well as mycological cure rate defined as the negative culture’s result. Secondary outcomes were the relapse rate, the incidence of systemic infections, and compliance. Adverse effects were also evaluated. Review papers, case series, editorials, monographs, animal studies, in vitro studies, uncontrolled trials, letters to editors, case reports were omitted.
2.2. Database and Search Strategies
Three electronic databases were searched by independent reviewers: PubMed, Cochrane library and Scopus. Combinations of various keywords like “oral candidiasis” OR “oral candidosis” OR “oropharyngeal candidiasis” OR “candidal stomatitis” AND “nystatin” OR “antifungal agents” OR “polyenes” were used to extract all pertinent studies. Manual searches had also been conducted as a supplement.
2.3. Data Extraction and Quality Assessment
The scanning of titles and abstracts, selection of studies, reading full reports, data extraction and quality assessment was independently done by the two review authors (A.R., S.P.). All the pertinent data of each included study, including author, year of publication, region, study design, risk factors, characteristics of the patients like age and gender, detailed interventions, recall periods, outcomes, and adverse effects, were extracted and summarized in a table format using Excel Spreadsheet (Microsoft, Redmond, WA, USA) for qualitative synthesis.
The quality of the included studies was assessed using the Cochrane Handbook for Systematic Review of Interventions and the Rev Man 5.4.1 software. The following assessment criteria were used to assess the quality of the studies: (1) random sequence generation (if the study did not use this method, it was considered to have a selection bias); (2) allocation concealment (selection bias); (3) blinding of participants and personnel (performance bias); (4) blinding of outcome assessment (detection bias); (5) incomplete outcome data (attrition bias); and (6) selective reporting (reporting bias). The Kappa coefficient was used to calculate inter-rater agreement with regard to study inclusion and quality assessment. A third reviewer (S.R.M.) made an assessment when the two review authors could not reach a consensus. Each of the six points in every included study was assessed and colored ‘green’ for low risk, ‘yellow’ for unclear, and ‘red’ for high risk were appraised. The risk of bias was categorized as low when the study was showing more and equal to 60% of the ‘green’ score and high when there was 40% of either ‘yellow’ or ‘red’.
2.4. Data Synthesis and Analysis
The efficacy of nystatin versus PDT was evaluated using the Rev Man 5.4.1 software. Results were expressed as standard deviation (SD) together with the 95% confidence interval (CI), and plotted on a forest plot. I2 test was performed on the eligible studies and the value of the test <30% was considered low, 30%–70% was considered moderate and >70% was considered high heterogeneity of the effect size among the studies. The data extracted were from the various demographics; therefore, the random effect model was employed. A descriptive study was conducted on studies evaluating the efficacy of nystatin versus other antifungal treatments due to the limited number of studies or marked heterogeneity in many aspects of the study characteristics.
3. Results
3.1. Databases Search Results
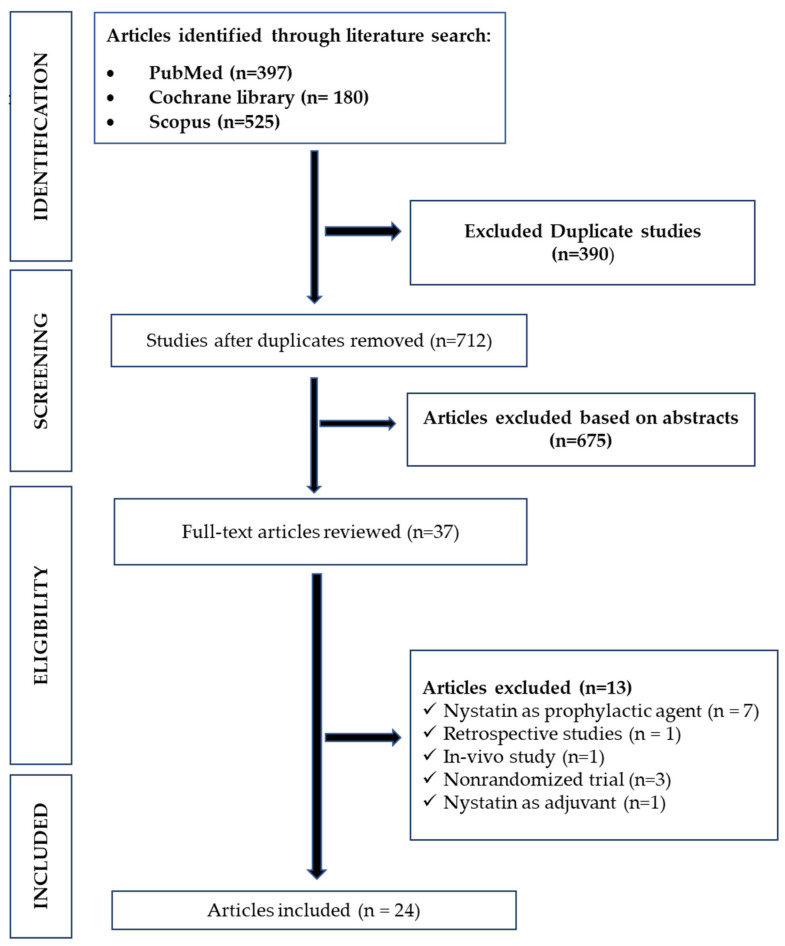
A total of 1102 abstracts were extracted from the three databases PubMed, Cochrane, Scopus (Figure 1).
Figure 1.
Study selection and PRISMA flowchart.
After screening, three hundred ninety duplicate studies and six hundred seventy-five irrelevant studies were removed. On the basis of the eligibility criteria, thirteen studies were excluded: seven studies with nystatin as prophylactic agent, three nonclinical studies, one retrospective, one in vivo study, and one study with nystatin as adjuvant.
Finally, only 24 studies with a total of 1746 patients were included in the present analysis, with the average age of participants ranging from 12 months to 70 years.
3.2. Characteristics of the Included Studies
Fifteen trials were performed in patients with denture stomatitis [28,29,30,31,32,33,34,35,36,37,38,39,40], in which one was on patients with respiratory disorders [41] and another with diabetes mellitus [42]; four trials were conducted on infants or children [43,44,45,46], three trials included Human Immunodeficiency (HIV) or Acquired immune deficiency syndrome (AIDS) patients [47,48,49]; one trial was on hospitalized cancer patients and one trial were performed in multigroup patients (having diverse diseases like erythematous candidiasis, HIV, Xerostomia, organ transplantation and denture stomatitis) [50] (Table 1). These studies have compared nystatin with different interventions like Fluconazole, photodynamic therapy (PDT), microwave, placebo, etc., and they have been designated as controls. Six trials had compared nystatin with two different controls [36,37,38,41,46,48], while the rest had a single control. Nystatin was used in the suspension, gel, and pastille forms; the dosage ranged from 100,000 IU to 1,100,000 IU, three to five times a day, and the treatment duration was 10 to 30 days.
Table 1.
Characteristics of the included studies.
| Author/Country | Risk Factor | Nystatin Group | Control Group | Nystatin Group | Control Group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (Mean) | Sex | N | Age (Mean) | Sex | N | Formulation | Dose | Frequency (Times/day) | Duration (Days) | Medication | Formulation | Dose | Frequency | Duration (Days) | ||
| Afroozi et al. 2019 [28], Iran |
Denture | 67.4 y | - | 33 | 67.6 y | - | 33 | Sol | 100,000 IU | 3 times a day | 15 | PDT | - | - | 2 sessions | 15 |
| Alrabiah et al. 2019 [29], Saudi Arabia |
Denture | - | - | 18 | - | - | 18 | Susp | 100,000 IU | 4 times a day | 14 | PDT | - | - | twice in one week | 30 |
| Alves et al. 2020 [30], Brazil |
Denture | 69 y | - | 35 | 70 y | F = 19 M = 11 |
30 | Susp | 100,000 IU/mL) | 4 times a day | 15 | PDT | - | - | 6 sessions | 15 |
| Araújo et al. 2021 [35], Brazil |
Denture | 57 y | - | 18 | - | - | 18 | Sol | 1,000,000 IU | 3 times a day | 15 | CZ | oral spray | - | 3 times a day | 15 |
| Bakhshi et al. 2012 [31], Iran |
Denture | 73.52 y | - | 20 | - | - | 20 | Sol | 100,000 IU/ml | 3 times a day | 30 | GE | Sol | 40 mg/ml | 3 times a day | 30 |
| Gonoudi et al. 2021 [32], Iran |
Denture | 60.93 y | - | 14 | 55.86 y | - | 14 | Susp | 100,000 IU | 4 times a day | 14 | ZM | Sol | 5 ml | 5 times a day | 14 |
| Johnson et al. 1989 [39], USA |
Denture | - | - | 8 | - | - | 8 | Pas | 200,000 IU | 5 | 14 | Placebo | Pastilles | 5 | 14 | |
| - | - | 8 | - | - | 8 | Pas | 400,000 IU | 5 | 14 | Placebo | Pas | 5 | 14 | |||
| Labban et al. 2021 [33], Saudi Arabia |
Denture | 56.9 y | - | 15 | 57.2 y | - | 15 | Susp | 100,000 IU/mL | 4 times a day | 15 | PDT | - | - | 6 sessions | 15 |
| Li et al. 2014 [34], China |
Denture | 64.84 y | F = 24 M = 7 |
31 | 62.72 y | F = 29 M = 5 |
34 | Paste | 2% | 3 | 30 | NYT + Pb | Paste + Lozenges | 3 | 30 | |
| Mima et al. 2012 [40], Brazil |
Denture | 62.45 y | - | 20 | 61.25 y | - | 20 | Susp | 100 000 IU | 4 times a day | 15 | PDT | - | - | 6 sessions | 15 |
| Nairn et al. 1975 [37], England |
Denture | - | - | 13 | - | - | 18 | Pas | 500,000 IU | 4 | 30 | AMB | Lozenges | 10 mg | 4 | 30 |
| - | - | 13 | - | - | 15 | Pas | 500,000 IU | 4 | 30 | Placebo | ||||||
| Pinelli et al. 2013 [38], Brazil |
Denture | 81.4 y | - | 10 | - | - | 10 | Sol | 100,000 IU | 4 times a day | 30 | RC | Sol | - | - | 30 |
| 81.4 y | - | 10 | - | - | 10 | Sol | 100,000 IU | 4 times a day | 30 | MIC | Gel | 4 times a day | 30 | |||
| Silva et al. 2012 [36], Brazil |
Denture | 62.5 y | - | 20 | 59.5 y | - | 20 | Susp | 100,000 IU/ml | 4 | 14 | DM | Irr | Once per week | 14 | |
| 62.5 y | - | 20 | 56.8 y | - | 20 | Susp | 100,000 IU/ml | 4 | 14 | DM | Irr | 3 times per week | 14 | |||
| Sanita et al. 2012 [42], Brazil |
Denture in diabetic patients | 62.6 y | - | 10 | 62.2 y | - | 10 | Susp | 100,000 IU/m | 4 | 14 | DM | Irr | 3 times per week | 14 | |
| Thompson et al. 1986 [41], England |
Respiratory disease | 59 y | - | 18 | - | - | 18 | Pas | 100,000 IU | 4 | 7 | NYT | Susp | 100 000 units | 4 | 7 |
| Goins et al. 2002 [44], USA |
Infants | 1–12 mon | - | 28 | 1–12 mon | - | 17 | Susp | 100,000 IU | 4 | 10 | FLC | Susp | 1 per day | 7 | |
| Hoppe 1997 [46], Multicenter study |
Infants | 130 days | F = 0 M = 77 |
85 | 132 days | F = 0 M = 95 |
98 | Susp | 100,000 IU | 4 | 12 | MIC | Gel | 4 | 12 | |
| Hoppe et al. 1996 [45], Multicenter study |
Infants | 5 months | - | 35 | 5 mon | - | 27 | Gel | 250,000 IU | 4 | 14 | MIC | Gel | 4 | 14 | |
| 5 months | - | 35 | 5 mon | - | 27 | Gel | 100,000 IU | 4 | 14 | MIC | Gel | 4 | 14 | |||
| Flynn et al. 1995 [43], USA |
Infants Children | 6 months–13 y | - | 88 | 6 mon–13 y | - | 94 | Susp | 400,000 IU | 4 | 14 | FLC | Susp | 14 | ||
| Meunier et al. 1990 [51], Belgium |
Cancer patients | - | F = 10 M = 14 |
24 | - | F = 8 M = 10 |
18 | Susp + Pas | 1000,000 IU + 100,000 IU | 3 | 10 to 12 | KCZ | Tab | 10 to 12 | ||
| Mukherjee et al. 2017 [47], Multicenter study |
HIV | - | F = 66 M = 45 |
111 | - | F = 62 M = 48 |
110 | Susp | 500,000 IU | 4 | 14 | GV | Sol | 14 | ||
| Pons et al. 1997 [49], USA |
HIV, AIDS | 38 y | - | 84 | 38 y | - | 83 | Susp | 500,000 IU | 4 | 14 | FLC | Susp | 14 | ||
| Nyst et al. 1992 [48], Zaire |
AIDS | 35.4 y | - | 47 | 34.5 y | - | 49 | Susp | 200,000 IU | 4 | 14 | GV | Susp | 14 | ||
| 35.4 y | - | 47 | 34.5 y | - | 45 | Susp | 200,000 IU | 4 | 14 | KCZ | Troche | 14 | ||||
| Blomgren et al. 1998 [50], Sweden |
Multigroup patients | 60.7 y | - | 33 | 58.4 y | - | 34 | Sol | 100,000 IU | 4 | 21 | FLC | Cap | 7 | ||
HIV = human immunodeficiency virus; AIDS = acquired immunodeficiency syndrome; PDT—Photodynamic therapy; CZ—Cinnamomum zeylanicum; GE—Garlic extract; ZM—Zataria multiflora; NYT—nystatin; Pb—probiotics; AMB—Amphotericin B; RC—Ricinus communis; MIC—Miconazole; DM—Denture microwave; FLC—Fluconazole; KCZ—Ketoconazole; GV—Gentain violet; Susp—Suspension; Sol—Solution; Tab—Tablet; Cap—Capsule; Irr—Irradiation; Pas—Pastilles.
3.3. Risk of Bias and Quality of the Included Studies
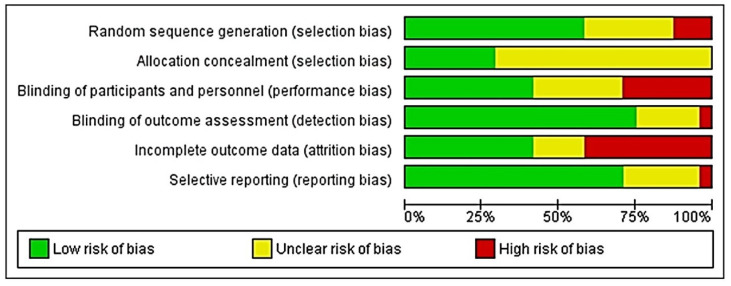
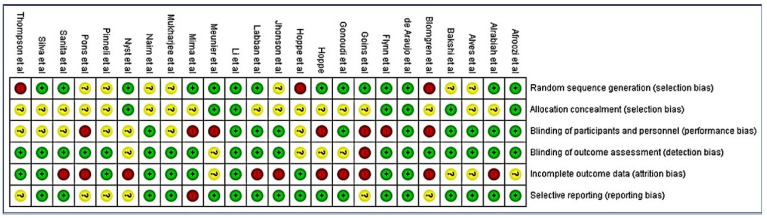
Only two of the included studies met all the seven assessment criteria [34,35]. Most studies were found to have an unclear risk of selection, performance and attrition bias, and a moderate risk of other biases. The overall risk of each bias is presented in Figure 2, and the risk of each bias in each of the studies separately is presented in Figure 3. A 100% agreement was achieved on study quality among the reviewers.
Figure 2.
Depicting the plot of the distribution of review authors’ judgments across studies for each risk of bias item.
Figure 3.
A summary table of review authors’ judgments for each risk of bias item for each study.
3.4. Potency Evaluation
The clinical and mycologic cure rates associated with both nystatin and the different controls have been summarized in Table 2.
Table 2.
Clinical and mycologic potency of nystatin and the control treatments.
| Authors | Risk Factors | Clinical Cure Rates | Mycological Cure Rates | ||
|---|---|---|---|---|---|
| Nystatin | Controls | Nystatin | Controls | ||
| Afroozi et al. 2019 [28] | Denture | 89.30% | 53.60% | - | - |
| Alrabiah et al. 2019 [29] | Denture | - | - | - | - |
| Alves et al. 2020 [30] | Denture | 54.20% | 53.30% | - | - |
| Bakhshi et al. 2012 [31] | Denture | - | - | - | - |
| Araújo et al. 2021 [35] | Denture | 89% | 61% | 83% | 33% |
| Gonoudi et al. 2021 [32] | Denture | - | - | - | - |
| Johnson et al. 1989 [39] | Denture | 28.60% | 0 | 57.10% | 0 |
| 14.30% | 0 | 71.40% | 0 | ||
| Labban et al. 2021 [33] | Denture | - | - | - | - |
| Li et al. 2014 [34] | Denture | - | - | 30.77% | 20% |
| Mima et al. 2012 [40] | Denture | 53% | 45% | - | - |
| Nairn et al. 1975 [37] | Denture | 76.90% | 88.80% | 40% | 6.25% |
| 76.90% | 40% | 40% | 20% | ||
| Pinelli et al. 2013 [38] | Denture | - | - | - | - |
| Silva et al. 2012 [36] | Denture | 18.75% | 23.53% | - | - |
| 18.75% | 22.22% | - | - | ||
| Sanita et al. 2012 [42] | Denture in diabetic patients | 20% | 25% | - | - |
| Thompson et al. 1986 [41] | Respiratory disease and dentures | 87% | 80% | - | - |
| Goins et al. 2002 [44] | Infants | 28.60% | 100% | 5.60% | 73.30% |
| Hoppe et al. 1996 [45] | Infants | 42.80% | 85.10% | 20% | 29.60% |
| 48.50% | 85.10% | 3.00% | 29.60% | ||
| Hoppe 1997 [46] | Infants | 54.10% | 99% | 8.20% | 54.10% |
| Flynn et al. 1995 [43] | Infants, Children | 51% | 91% | 11% | 76% |
| Meunier et al. 1990 [51] | Cancer patients | 72% | 87% | 24% | 61% |
| Mukherjee et al. 2017 [47] | HIV | 67.80% | 68.50% | - | - |
| Pons et al. 1997 [49] | HIV, AIDS | 52% | 87% | 6% | 60% |
| Nyst et al. 1992 [48] | AIDS | 9% | 42% | 13% | 62% |
| 9% | 43% | 13% | 57% | ||
| Blomgren et al. 1998 [50] | Multigroup patients | 16.70% | 30% | - | - |
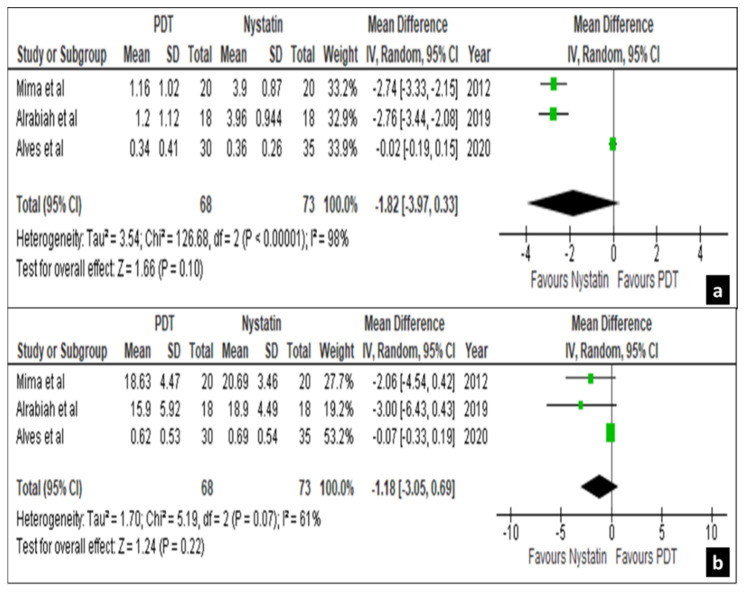
Only four out of the twenty-four trials comparing the efficacy of nystatin suspension with photodynamic therapy (PDT) were eligible for meta-analysis with mycological cure rates on the palatal mucosa and denture surfaces as an outcome [29,30,33,40]. Since, Labban et al. [33] used two forms of PDT (one used rose Bengal mediated and the other using curcumin), the studies were marked as Labban et al. A and B, respectively. Then, the results of mycological cure rates at different follow-up periods were expressed into proportions and mean depending on the data extracted from the individual studies. On day 15, three out of four studies, i.e., Mima et al. [40], Alrabiah et al. [29] and Alves et al. [30] showed a non-significant difference in the treatment effects on palatal mucosa and denture surfaces with a mean difference of −1.82 [−3.97, 0.33] and −1.18 [−3.05, 0.69], respectively, at a confidence interval of 95% (Figure 4a,b).
Figure 4.
Forest plot comparing the efficacy of nystatin and PDT on palatal mucosa (a) and denture surface (b) in 15 days.
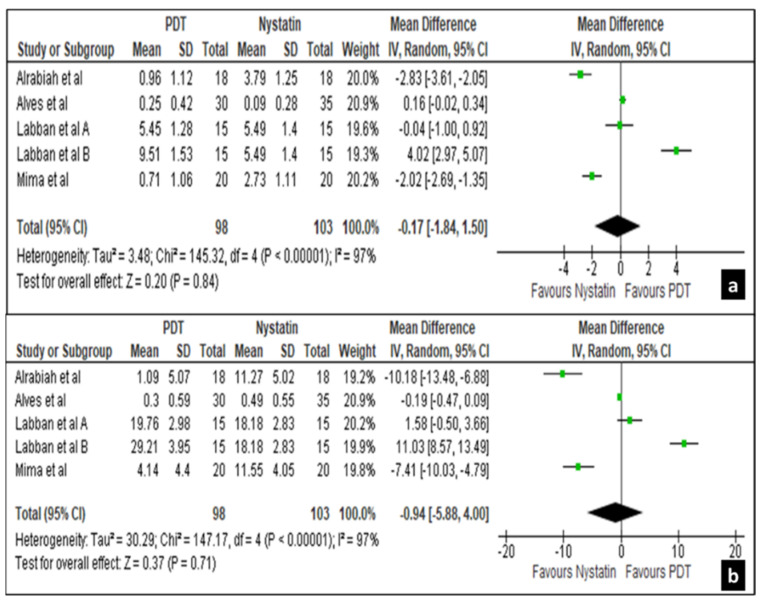
Between 30 to 45 days intervals all 4 studies revealed a non-significant difference in the treatment effects in mycological cure rates for both palatal mucosa and denture surfaces with a mean difference of −0.17 [−1.84, 1.50] and −0.94 [−5.88, 4.00], respectively, at 95% confidence interval (Figure 5a,b).
Figure 5.
Forest plot comparing the efficacy of nystatin and PDT on palatal mucosa (a) and denture surface (b) in 30 to 45 days.
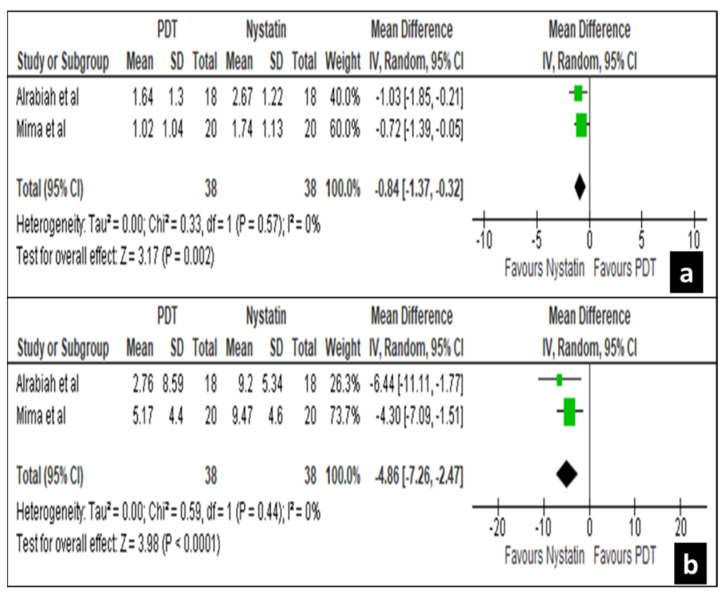
At 60 days interval two studies by Alrabiah et al. [29] and Mima et al. [40] revealed significant treatment effects for both palatal mucosa and denture surfaces with a mean difference of −0.84 [−1.37, −0.32] and −4.86 [−7.26, −2.47] at a confidence interval of 95, respectively (Figure 6a,b).
Figure 6.
Forest plot comparing the efficacy of nystatin and PDT on palatal mucosa (a) and denture (b) surface in 60 days.
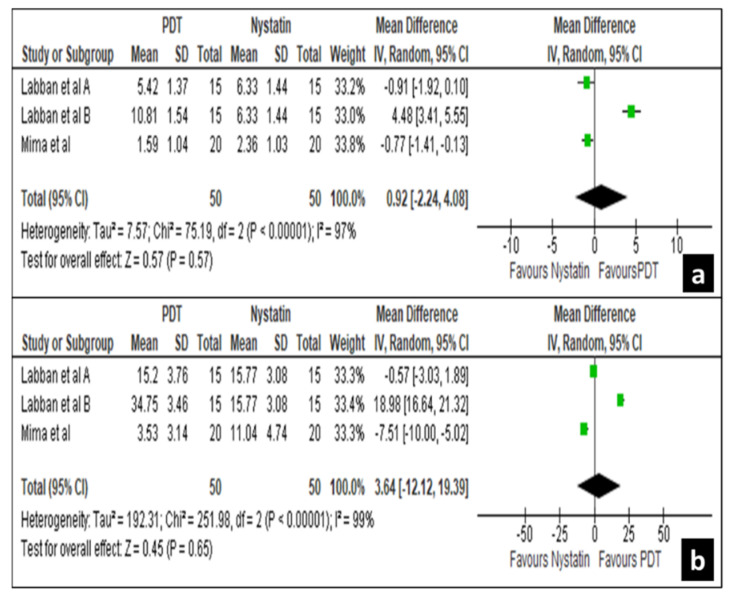
Again between 80 to 90 days intervals, two studies, i.e., Labban et al. [33] and Mima et al. [40] revealed a non-significant difference in the treatment effects for both palatal mucosa and denture surfaces with a mean difference of −0.92 [−2.24, 4.08] and 3.64 [−12.12, 19.39] at a confidence interval of 95% (Figure 7a,b). These results show that nystatin suspension has similar clinical efficacy as that of photodynamic therapy.
Figure 7.
Forest plot comparing the efficacy of nystatin and PDT on palatal mucosa (a) and denture (b) surface in 80 to 95 days.
Due to marked heterogeneity in many aspects of the study characteristics in all other included studies meta-analysis was not conducted. The efficacy of nystatin suspension was compared to other antifungal agents in five trials [43,44,46,47,48]. The results showed that the clinical, as well as mycological efficacy of nystatin suspension was 9%–67.8% and 5.6%–13%, respectively, whereas the clinical and mycological efficacy was higher with fluconazole (87%–100% and 60%–76%, respectively) [43,44,49,50], miconazole (99% and 54.1%, respectively) [45], and ketoconazole (43% and 57%, respectively) [51] in infants and children for the treatment of oral candidiasis. The clinical and mycological efficacy was higher with miconazole (99% and 54.1%, respectively) [46], and gentian violet (42%–68.5% and 62%, respectively) [47] in HIV/AIDS patients with oral candidiasis. Two studies compared the efficacy of nystatin pastilles to placebo and observed 14.3%–76.9% of clinical cure rates and 40%–71.4% of mycological cure rates [37,39]. One of the trials compared the efficacy of nystatin pastilles and amphotericin B and the results showed that both have similar clinical efficacy of 76.9% and 88.8%, respectively [37]. Another study [39] demonstrated a comparatively higher clinical cure rate for nystatin pastilles (87%) than that of nystatin suspension (80%). Two studies [36,42] compared the clinical efficacy of nystatin suspension to denture irradiation and the results showed that nystatin suspension had a clinical cure rate of 18.75%–20%, whereas the clinical cure rates of denture irradiation were 22.22%–25%. The results of the above trials point towards the higher clinical and mycological efficacy of fluconazole, miconazole, gentian violet, and ketoconazole as compared to that of nystatin.
3.5. Duration, Dosage, Formulations and Adverse Effects
There was a lot of diversity in the study designs and the treatment protocols (dose, duration of treatment, formulation used) of the included trials. Hence, only four trials qualified for meta-analysis, for the rest only descriptive study was performed. Nystatin was used in pastille, suspension, solution, gel and a combination of pastille and suspension forms in the twenty-four studies. Amongst the denture stomatitis patients, the clinical cure rate with solution form was 89%, with suspension form was 54% and with pastille was 28%. On increasing the dosage from 200,000–400,000 IU to 500,000 IU the clinical cure rate increased up to 77% while using pastilles. The mycologic cure rate with the paste form was 30.7%. The clinical cure rate was high on using pastilles form in respiratory patients at 87% and 87.5% in cancer patients when using both pastille and suspension forms. Meunier et al. [51] observed that the clinical and mycologic cure rates were 87.5% and 66% of cancer patients, respectively, with oral Candida infection on using a combination of nystatin pastilles and suspension. Trials conducted on infants, children, HIV and AIDS patients with oral candidiasis exhibited a clinical cure rate of 9%–63.5% and the mycologic cure rate was 6–13% with the use of suspension form. It was observed that pastilles worked better than suspension or solution (Table 3).
Table 3.
Summary of the use and potency of nystatin.
| Risk Factor | Formulation | Dose | Frequency (Times/Day) | Duration (Days) | Clinical Cure Rates (%) | Mycological Cure Rates (%) |
|---|---|---|---|---|---|---|
| Denture | Susp | 100,000 IU | 4 | 15 | 54.2 | |
| Denture | Sol | 100,000 IU | 3 | 15–30 | 89.3 | |
| Denture | Pas | 200,000–400,000 IU | 5 | 14 | 28.6–14.3 | 57.10–71.4 |
| Denture | Pas | 500,000 IU | 4 | 30 | 76.9 | 40 |
| Denture | Paste | 2% | 3 | 30 | 30.77 | |
| Denture in diabetic patients | Susp | 100,000 IU | 4 | 14 | 20 | - |
| Respiratory disease | Pas | 100,000 IU | 4 | 7 | 87 | - |
| Infants and children | Susp | 100,000–400,000 IU | 4 | 10 to 14 | 28.6–54.1 | 5.6 – 11 |
| Infants and children | Gel | 250,000 IU | 4 | 14 days | 42.8/48.5 | 20/3.0 |
| Cancer | Susp + Pas | 1,000,000 IU + 100,000 IU | 3 | 10 to 12 | 87.5 | 66 |
| HIV/AIDS | Susp | 100,000–500,000 IU | 4 | 14 | 9–67.8 | 6–13 |
| Multigroup | Susp | 100,000 IU | 4 | 21 | 16.7 | - |
Susp—Suspension; Sol—Solution; Pas—Pastille.
Six trials [31,37,43,45,49,50] out of twenty-four reported adverse effects after the administration of nystatin, while the remaining eighteen trials did not mention any adverse effects as secondary outcomes. Nausea, vomiting, unpleasant taste and diarrhea were the most common adverse effects in both nystatin as well as the control group (Table 4).
Table 4.
Adverse effects of nystatin and controls.
| Author | Risk Factor | Adverse Effects in Nystatin Group |
Adverse Effects in Control Group |
|---|---|---|---|
| Bakhshi et al. 2012 [31] | Denture | nausea in 6, vomiting in 1, diarrhea in 5, anorexia in 1, burning in 1 | itching in 1 |
| Nairn et al. 1975 [37] | Denture | unpleasant taste in eight patients | unpleasant taste in five patients |
| Hoppe et al. 1996 [45] | Infants | vomiting in one patient | vomiting in two patients |
| Flynn et al. 1995 [43] | Infants, Children | three patients (vomiting, nausea, diarrhea, anorexia, abdominal pain), one patient (rash, headache) | six patients (vomiting, nausea, diarrhea, anorexia, abdominal pain), one patient (rash, headache) |
| Pons et al. 1997 [49] | HIV, AIDS | nausea, vomiting and diarrhea | nausea in one patient, and elevated liver enzyme concentrations in two patients |
| Nyst et al. 1992 [48] | AIDS | - | irritation and small superficial oral ulcers in two patients |
| Blomgren et al. 1998 [50] | Multigroup patients | nausea in one patient | - |
HIV = human immunodeficiency virus; AIDS = acquired immunodeficiency syndrome.
4. Discussion
Oral candidiasis happens to be the most prevalent opportunistic infection in the oral cavity, often known as the “disease of the diseased” [6,52]. It commonly affects infants and aged individuals, especially denture wearers [53]. However, it can affect individuals of any age having local or systemic factors which predispose them to Candida infection [54]. Often uncontrolled diabetes mellitus, xerostomia due to different systemic causes, long-term glucocorticoid therapy, immunodeficiency states, patients with hematologic disorders or patients with oral malignancies on radiotherapy or chemotherapy develop oral candidiasis [55]. Even local factors like poor oral hygiene, oral sepsis or any mucosal alterations like ulceration/growth and tissue abuse habits like smoking can predispose to oral candidiasis [20]. It is imperative to manage candidiasis of the oral cavity on time before it spreads to contiguous mucosa like the upper respiratory tract, esophagus, blood, or even spread to the central nervous system [12,17]. It can also cause systemic infection which may be life-threatening. Besides the Candida, metabolites can lead to Id reaction which is a type of hypersensitivity reaction leading to eczema, bronchial asthma, and gastric irritation [3].
Since the incidence and the prevalence of oral candidiasis have been on the rise in recent years, the use of antifungals has been on the rise [6]. Both topical and systemic antifungal therapies for the patients have been advocated depending on the severity of the disease, though topical therapies are preferred over systemic ones due to the renal and hepatotoxicity associated with the latter [20,21]. Even with the availability of newer antifungal drugs, polyenes like itraconazole, voriconazole, posaconazole isavuconazole and echinocandins like caspofungin, micafungin, anidulafungin which have lesser side effects and more clinical options, topical antifungal therapy using nystatin is the treatment of choice owing to its efficacy, cost and less adverse effects [14].
Nystatin, a polyene antibiotic, interacts with the ergosterol in the fungal cell membrane making it porous and vulnerable to lysis, thus exerting its antifungal effect [56].
The clinical practice guidelines for the management of oral candidiasis were given by the Infectious Diseases Society of America recommending the usage of nystatin suspension having a concentration of 100,000 IU/mL with a dosage of 4–6 mL four times a day or one to two nystatin pastilles (200,000 IU) to be given four times a day for one to two weeks for mild oral/oropharyngeal candidiasis [57]. World Health Organization (WHO) has recommended topical therapies having nystatin either in the form of suspension or pastilles are good substitutes to orally administered fluconazole for treating oral and oropharyngeal candidiasis even in HIV-positive individuals [58]. However, the usage of nystatin, its availability and its administration vary in different countries and populations.
The duration of the antifungal treatment plays a vital role in its efficacy. Short term therapies of nystatin did not yield good results in case of infants or HIV/AIDS patients. Nystatin administered at a concentration of 0.25 to 1 times the minimum inhibitory concentration value for half an hour results has been reported to have better antifungal effect up to 6 h in Candida isolates [59]. Since Candida species adhere to the oral mucosal cells and colonize, topical antifungals like nystatin getting absorbed into the oral epithelium are more effective as compared to oral antifungals. Like in previous studies, a treatment duration of 4 weeks was considered clinically more effective in our descriptive analysis.
The present systematic review points towards the efficacy of nystatin being comparable to different other topical therapies in decreasing the mycological loads of Candida on the palatal and denture surfaces in oral candidiasis. Because of the heterogenicity of the data present in various studies, meta-analysis was possible for only four studies. The clinical efficacy of nystatin was significantly increased over PDT at a treatment duration of 60 days on both the palatal mucosa and denture surfaces while it was at par during treatment intervals of 15, 30 to 45 and 80 to 90 days. Insignificant results were shown by only one of the studies, i.e., Alves et al. [30] which was included in the meta-analysis. The higher heterogeneity observed could be attributed to the fact that the study by Alves et al. [30] had a larger sample size and did not use a secondary culture medium, i.e., sabouraud dextrose agar unlike the others. Nystatin and photodynamic therapy are equally effective for the clinical remission of denture stomatitis as well as in significant reduction of CFU/mL of Candida species from dentures and palatal surfaces of the patients. Additionally, both the therapies have equal effects against Candida albicans, which had the highest prevalence among all species in denture stomatitis. The principal advantage of photodynamic therapy is that, unlike antifungal agents, development of resistance to photodynamic therapy seems unlikely due to its mechanism of action and has no toxic adverse effects. In immunocompromised states like HIV/AIDS where the continuous use of antifungal regimens has led to drug resistance and poor patient tolerability, PDT could be effective as it is not toxic to the host cells and be used for recurrent oral candidiasis [33].
A similar systematic review was conducted in 2016 [25] assessing the efficacy of different antifungal agents taking into account the various formulations, dosages and duration for treating oral candidiasis. They had searched four databases and ultimately included only eleven trials for the qualitative analysis. A meta-analysis was conducted on two studies comparing the efficacy of nystatin pastilles to placebo in the treatment of denture stomatitis which showed nystatin pastilles were superior to placebo. Another meta-analysis included three trials which compared the efficacy of nystatin suspension with fluconazole in treating oral/oropharyngeal candidiasis in infants/children found fluconazole was significantly superior to nystatin. Finally, descriptive analysis was conducted on seven studies which were not included in meta-analysis due to limited sample size or marked heterogeneity. Their results indicated that nystatin suspension was at par in clinical efficacy when compared to interventions like PDT and sodium benzoate but inferior to miconazole, ketoconazole and gentian violet in infants, children and HIV/AIDS patients. Nystatin pastilles were also similar in efficacy in comparison with amphotericin B and ketoconazole.
Like the present study, even in the systematic review conducted by Lyu et al. [25], it was found that nystatin was superior to placebo in treating denture stomatitis, but in infants and immunocompromised patients fluconazole was more effective than nystatin suspension for oral candidiasis. They also concluded that a combination of nystatin pastilles and suspension for two weeks had a better clinical and mycologic cure rate compared to the usage of nystatin suspension alone. The common adverse effects reported by them were also bitter taste and gastrointestinal upset akin to the present study.
There are several limitations to this meta-analysis. First, very few clinical trials with homogeneity were available. Second, several studies were considered to be at a high risk of selection, performance and attrition bias, and a moderate risk of other biases. The majority of the studies did not provide enough information about allocation concealment. The inconsistent quality of the included studies would impact the credibility of the results. These deficiencies indicate that well-designed and high-quality randomized controlled trial studies are needed in the future.
5. Conclusions
Antifungal therapies have evolved over time. Nowadays, newer triazoles and echinocandins have high range of antifungal activity with better patient acceptance in terms of ease of dosage and better tolerability, especially in recurrent/recalcitrant cases. Yet, topical therapy using nystatin is still the mainstay for the treatment of oral candidiasis, because of its increased efficacy, low cost, and less side effects. Photodynamic therapy is an expensive option, though it is quite efficacious. Therefore, nystatin suspensions can be the treatment regimen of choice for denture stomatitis, as the present descriptive analysis highlights the equal efficacy of 100,000 IU of nystatin suspension and six sessions of PDT.
Author Contributions
Conceptualization, A.R. and S.P.; methodology, S.P.; software, A.R.; validation, S.P., L.M. and B.L.; formal analysis, A.R.; investigation, S.R.M. and L.M.; resources, R.D.; data curation, G.S.; writing—original draft preparation, S.R.M. and R.D.; writing—review and editing, L.M. and B.L.; visualization, A.R.; supervision, S.R.M.; project administration, R.D.; funding acquisition, B.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tuganbaev T., Yoshida K., Honda K. The effects of oral microbiota on health. Science. 2022;376:934–936. doi: 10.1126/science.abn1890. [DOI] [PubMed] [Google Scholar]
- 2.Baker J.L., Bor B., Agnello M., Shi W., He X. Ecology of the Oral Microbiome: Beyond Bacteria. Trends Microbiol. 2017;25:362–374. doi: 10.1016/j.tim.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arkell S., Shinnick A. Update on oral candidosis. Nurs. Times. 2003;99:52–53. [PubMed] [Google Scholar]
- 4.Millsop J.W., Fazel N. Oral candidiasis. Clin. Dermatol. 2016;34:487–494. doi: 10.1016/j.clindermatol.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Sherman R.G., Prusinski L., Ravenel M.C., Joralmon R.A. Oral Candidosis. Quintessence Int. 2001;33:521–532. doi: 10.12968/denu.2001.28.3.132. [DOI] [PubMed] [Google Scholar]
- 6.Vila T., Sultan A.S., Montelongo-Jauregui D., Jabra-Rizk M.A. Oral Candidiasis: A Disease of Opportunity. J. Fungi. 2020;6:15. doi: 10.3390/jof6010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson M.D., Warnock D.W. Fungal Infection: Diagnosis and Management. Blackwell Publishing; Oxford, UK: 2012. [Google Scholar]
- 8.Bulad K., Taylor R.L., Verran J., McCord J.F. Colonization and penetration of denture soft lining materials by Candida albicans. Dent. Mater. 2004;20:167–175. doi: 10.1016/S0109-5641(03)00088-5. [DOI] [PubMed] [Google Scholar]
- 9.Nowakowska-Toporowska A., Raszewski Z., Wieckiewicz W. Color change of soft silicone relining materials after storage in artificial saliva. J. Prosthet. Dent. 2016;115:377–380. doi: 10.1016/j.prosdent.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Richardson M.D., Jones B.L. Therapeutic Guidelines in Systemic Fungal Infections. 3rd ed. Remedica Publishing; London, UK: 2003. [Google Scholar]
- 11.Pankhurst C.L. Candidiasis (Oropharyngeal) BMJ Clin. Evid. 2013;2013:1304. [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnan P. Fungal infections of the oral mucosa. Indian J. Dent. Res. 2012;23:650–659. doi: 10.4103/0970-9290.107384. [DOI] [PubMed] [Google Scholar]
- 13.Taudorf E., Jemec G., Hay R., Saunte D.M.L. Cutaneous candidiasis—An evidence-based review of topical and systemic treatments to inform clinical practice. J. Eur. Acad. Dermatol. Venereol. 2019;33:1863–1873. doi: 10.1111/jdv.15782. [DOI] [PubMed] [Google Scholar]
- 14.Samaranayake L.P., Leung W.K., Jin L. Oral mucosal fungal infections. Periodontology 2000. 2009;49:39–59. doi: 10.1111/j.1600-0757.2008.00291.x. [DOI] [PubMed] [Google Scholar]
- 15.Hoare A., Marsh P.D., Diaz P.I. Ecological Therapeutic Opportunities for Oral Diseases. Microbiol. Spectr. 2017;5 doi: 10.1128/microbiolspec.BAD-0006-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakaguchi H. Treatment and Prevention of Oral Candidiasis in Elderly Patients. Med. Mycol. J. 2017;58:J43–J49. doi: 10.3314/mmj.17.004. [DOI] [PubMed] [Google Scholar]
- 17.Lewis M.A.O., Williams D.W. Diagnosis and management of oral candidosis. Br. Dent. J. 2017;223:675–681. doi: 10.1038/sj.bdj.2017.886. [DOI] [PubMed] [Google Scholar]
- 18.Xiao Y., Yuan P., Sun Y., Xu Y., Deng X., Wang X., Liu R., Chen Q., Jiang L. Comparison of topical antifungal agents for oral candidiasis treatment: A systematic review and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022;133:282–291. doi: 10.1016/j.oooo.2021.10.023. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed A.A., Lu X.-L., Mounmin F.A. Diagnosis and Treatment of Esophageal Candidiasis: Current Updates. Can. J. Gastroenterol. Hepatol. 2019;2019:3585136. doi: 10.1155/2019/3585136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Černáková L., Rodrigues C.F. Microbial interactions and immunity response in oral Candida species. Futur. Microbiol. 2020;15:1653–1677. doi: 10.2217/fmb-2020-0113. [DOI] [PubMed] [Google Scholar]
- 21.Akpan A. Oral candidiasis. Postgrad. Med. J. 2002;78:455–459. doi: 10.1136/pmj.78.922.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quindos G., Gil-Alonso S., Marcos-Arias C., Sevillano E., Mateo E., Jauregizar N., Eraso E. Therapeutic tools for oral candidiasis: Current and new antifungal drugs. Med. Oral Patol. Oral Y Cir. Buccal. 2019;24:e172–e180. doi: 10.4317/medoral.22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang J., Huang B., Ding Z. Efficacy of antifungal drugs in the treatment of oral candidiasis: A Bayesian network meta-analysis. J. Prosthet. Dent. 2021;125:257–265. doi: 10.1016/j.prosdent.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 24.Mundula T., Ricci F., Barbetta B., Baccini M., Amedei A. Effect of Probiotics on Oral Candidiasis: A Systematic Review and Meta-Analysis. Nutrients. 2019;11:2449. doi: 10.3390/nu11102449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyu X., Zhao C., Yan Z.M., Hua H. Efficacy of Nystatin for the Treatment of Oral Candidiasis: A Systematic Review and Meta-Analysis. Drug Des. Devel. Ther. 2016;10:1161–1171. doi: 10.2147/DDDT.S100795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pérez-Sayáns M., Beiro-Fuentes R., Otero-Rey E.M., Chamorro-Petronacci C.M., Gándara-Vila P., Somoza-Martín J.M., García-García A., Blanco-Carrión A. Efficacy of different formulations of nystatin in an experimental model of oral candidiasis in sialoadenectomized rats. J. Dent. Sci. 2021;16:123–130. doi: 10.1016/j.jds.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015 statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afroozi B., Zomorodian K., Lavaee F., Shahrabadi Z.Z., Mardani M. Comparison of the efficacy of indocyanine green-mediated photodynamic therapy and nystatin therapy in treatment of denture stomatitis. Photodiagnosis Photodyn. Ther. 2019;27:193–197. doi: 10.1016/j.pdpdt.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Alrabiah M., Alsahhaf A., Alofi R.S., Al-Aali K.A., Abduljabbar T., Vohra F. Efficacy of photodynamic therapy versus local nystatin in the treatment of denture stomatitis: A randomized clinical study. Photodiagnosis Photodyn. Ther. 2019;28:98–101. doi: 10.1016/j.pdpdt.2019.08.028. [DOI] [PubMed] [Google Scholar]
- 30.Alves F., Carmello J.C., Alonso G.C., Mima E.G.D.O., Bagnato V.S., Pavarina A.C. A randomized clinical trial evaluating Photodithazine-mediated Antimicrobial Photodynamic Therapy as a treatment for Denture stomatitis. Photodiagnosis Photodyn. Ther. 2020;32:102041. doi: 10.1016/j.pdpdt.2020.102041. [DOI] [PubMed] [Google Scholar]
- 31.Bakhshi M., Taheri J.-B., Basir Shabestari S., Tanik A., Pahlevan R. Comparison of therapeutic effect of aqueous extract of garlic and nystatin mouthwash in denture stomatitis. Gerodontology. 2012;29:e680–e684. doi: 10.1111/j.1741-2358.2011.00544.x. [DOI] [PubMed] [Google Scholar]
- 32.Gonoudi E., Rezai M., Farrokhnia T., Goudarzi M., Sima A. Comparison of Antifungal Efficacy of Zataria Multiflora and Nystatin for Treatment of Denture Stomatitis: A Randomized Clinical Trial. J. Dent. 2021;22:60–66. doi: 10.30476/DENTJODS.2020.84181.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labban N., Al Taweel S.M., Alrabiah M.A., Alfouzan A.F., Alshiddi I.F., Assery M.K. Efficacy of Rose Bengal and Curcumin mediated photodynamic therapy for the treatment of denture stomatitis in patients with habitual cigarette smoking: A randomized controlled clinical trial. Photodiagnosis Photodyn. Ther. 2021;35:102380. doi: 10.1016/j.pdpdt.2021.102380. [DOI] [PubMed] [Google Scholar]
- 34.Li D., Li Q., Liu C., Lin M., Li X., Xiao X., Zhu Z., Gong Q., Zhou H. Efficacy and safety of probiotics in the treatment ofCandida-associated stomatitis. Mycoses. 2014;57:141–146. doi: 10.1111/myc.12116. [DOI] [PubMed] [Google Scholar]
- 35.de Araújo M.R.C., Maciel P.P., Castellano L.R.C., Bonan P.R.F., Alves D.D.N., de Medeiros A.C.D., de Castro R.D. Efficacy of essential oil of cinnamon for the treatment of oral candidiasis: A randomized trial. Spéc. Care Dent. 2021;41:349–357. doi: 10.1111/scd.12570. [DOI] [PubMed] [Google Scholar]
- 36.Silva M.M., Mima E.G.D.O., Colombo A.L., Sanitá P.V., Jorge J.H., Massucato E.M.S., Vergani C.E. Comparison of denture microwave disinfection and conventional antifungal therapy in the treatment of denture stomatitis: A randomized clinical study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012;114:469–479. doi: 10.1016/j.oooo.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Nairn R. Nystatin and amphotericin B in the treatment of denture-related candidiasis. Oral Surg. Oral Med. Oral Pathol. 1975;40:68–75. doi: 10.1016/0030-4220(75)90348-5. [DOI] [PubMed] [Google Scholar]
- 38.Pinelli L.A.P., Montandon A.A.B., Corbi S.C.T., Moraes T.A., Fais L.M.G. Ricinus communis treatment of denture stomatitis in institutionalised elderly. J. Oral Rehabil. 2013;40:375–380. doi: 10.1111/joor.12039. [DOI] [PubMed] [Google Scholar]
- 39.Johnson G.H., Taylor T.D., Heid D.W. Clinical evaluation of a nystatin pastille for treatment of denture-related oral candidiasis. J. Prosthet. Dent. 1989;61:699–703. doi: 10.1016/S0022-3913(89)80045-9. [DOI] [PubMed] [Google Scholar]
- 40.Mima E.G., Vergani C.E., Machado A.L., Massucato E.M.S., Colombo A.L., Bagnato V.S., Pavarina A.C. Comparison of Photodynamic Therapy versus conventional antifungal therapy for the treatment of denture stomatitis: A randomized clinical trial. Clin. Microbiol. Infect. 2012;18:E380–E388. doi: 10.1111/j.1469-0691.2012.03933.x. [DOI] [PubMed] [Google Scholar]
- 41.Thompson P.J., Wingfield H.J., Cosgrove R.F., Hughes B.O., Turner-Warwick M.E. Assessment of oral candidiasis in patients with respiratory disease and efficacy of a new nystatin formulation. BMJ Br. Med. J. 1986;292:1699–1700. doi: 10.1136/bmj.292.6537.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanita P.V., Machado A.L., Pavarina A.C., Massucato E.M.S., Colombo A.L., Vergani C.E. Microwave denture disinfection versus nystatin in treating patients with well-controlled type 2 diabetes and denture stomatitis: A randomized clinical trial. Int. J. Prosthodont. 2012;25:232–244. [PubMed] [Google Scholar]
- 43.Flynn P.M., Cunningham C.K., Kerkering T., Jorge A.R.S., Peters V.B., Pitel P.A., Harris J., Gilbert G., Castagnaro L., Robinson P. Oropharyngeal candidiasis in immunocompromised children: A randomized, multicenter study of orally administered fluconazole suspension versus nystatin. The Multicenter Fluconazole Study Group. J. Pediatr. 1995;127:322–328. doi: 10.1016/S0022-3476(95)70321-7. [DOI] [PubMed] [Google Scholar]
- 44.Goins R.A., Ascher D., Waecker N., Arnold J., Moorefield E. Comparison of fluconazole and nystatin oral suspensions for treatment of oral candidiasis in infants. Pediatr. Infect. Dis. J. 2002;21:1165–1167. doi: 10.1097/00006454-200212000-00017. [DOI] [PubMed] [Google Scholar]
- 45.Hoppe J.E., Hahn H. The Antimycotics Study Group Randomized comparison of two nystatin oral gels with miconazole oral gel for treatment of oral thrush in infants. Infection. 1996;24:136–139. doi: 10.1007/BF01713319. [DOI] [PubMed] [Google Scholar]
- 46.Hoppe J.E. Treatment of oropharyngeal candidiasis in immunocompetent infants: A randomized multicenter study of miconazole gel vs. nystatin suspension. The Antifungals Study Group. Pediatr. Infect. Dis. J. 1997;16:288–293. doi: 10.1097/00006454-199703000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Mukherjee P.K., Chen H., Patton L., Evans S., Lee A., Kumwenda J., Hakim J., Masheto G., Sawe F., Pho M.T., et al. Topical gentian violet compared with nystatin oral suspension for the treatment of oropharyngeal candidiasis in HIV-1-infected participants. AIDS. 2017;31:81–88. doi: 10.1097/QAD.0000000000001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nyst M.J., Perriens J.H., Kimputu L., Lumbila M., Nelson A.M., Piot P. Gentian Violet, Ketoconazole and Nystatin in Oropharynge-al and Esophageal Candidiasis in Zairian AIDS Patients. Ann. Soc. Belg. Med. Trop. 1992;72:45–52. [PubMed] [Google Scholar]
- 49.Pons V., Greenspan D., Lozada-Nur F., McPhail L., Gallant J.E., Tunkel A., Johnson C.C., McCarty J., Panzer H., Levenstein M., et al. Oropharyngeal Candidiasis in Patients with AIDS: Randomized Comparison of Fluconazole Versus Nystatin Oral Suspensions. Clin. Infect. Dis. 1997;24:1204–1207. doi: 10.1086/513664. [DOI] [PubMed] [Google Scholar]
- 50.Blomgren J., Berggren U., Jontell M. Fluconazole versus nystatin in the treatment of oral candidosis. Acta Odontol. Scand. 1998;56:202–205. doi: 10.1080/00016359850142790. [DOI] [PubMed] [Google Scholar]
- 51.Meunier F., Aoun M., Gerard M. Therapy for Oropharyngeal Candidiasis in the Immunocompromised Host: A Randomized Double-Blind Study of Fluconazole vs. Ketoconazole. Rev. Infect. Dis. 1990;12:S364–S368. doi: 10.1093/clinids/12.Supplement_3.S364. [DOI] [PubMed] [Google Scholar]
- 52.Chaubal T., Bapat R. Oral Thrush. Am. J. Med. 2018;131:e371–e372. doi: 10.1016/j.amjmed.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 53.Hellstein J.W., Marek C.L. Candidiasis: Red and White Manifestations in the Oral Cavity. Head Neck Pathol. 2019;13:25–32. doi: 10.1007/s12105-019-01004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Firoozi P., Farshidfar N., Fekrazad R. Efficacy of antimicrobial photodynamic therapy compared to nystatin therapy in reducing Candida colony count in patients with Candida-associated denture stomatitis: A systematic review and meta-analysis. Evid. -Based Dent. 2021;23:34862461. doi: 10.1038/s41432-021-0208-9. [DOI] [PubMed] [Google Scholar]
- 55.Iversen D.B., Hellfritzsch M., Stage T.B., Aabenhus R.M., Lind B.S., Pottegård A. Antimycotic Treatment of Oral Candidiasis in Warfarin Users. Am. J. Med. 2020;134:e308–e312. doi: 10.1016/j.amjmed.2020.10.018. [DOI] [PubMed] [Google Scholar]
- 56.Kinoshita H., Yoshioka M., Ihara F., Nihira T. Cryptic antifungal compounds active by synergism with polyene antibiotics. J. Biosci. Bioeng. 2016;121:394–398. doi: 10.1016/j.jbiosc.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 57.Pappas P.G., Kauffman C.A., Andes D., Benjamin D.K., Jr., Calandra T.F., Edwards J.E., Jr., Filler S.G., Fisher J.F., Kullberg B.J., Ostrosky-Zeichner L., et al. Clinical Practice Guidelines for the Management of Candidiasis: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009;48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.WHO . Guidelines on the Treatment of Skin and Oral HIV-Associated Conditions in Children and Adults. World Health Organization; Geneva, Switzerlan: 2014. [PubMed] [Google Scholar]
- 59.Haro-Reyes T., Díaz-Peralta L., Galván-Hernández A., Rodríguez-López A., Rodríguez-Fragoso L., Ortega-Blake I. Polyene Antibiotics Physical Chemistry and Their Effect on Lipid Membranes; Impacting Biological Processes and Medical Applications. Membranes. 2022;12:681. doi: 10.3390/membranes12070681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.