Abstract
To investigate whether helminth infections may affect the efficacy of vaccines by impairing the immune response to nonparasite vaccine antigens, we compared the antibody responses to tetanus toxoid (TT) after tetanus vaccination in 193 subjects with Onchocerca volvulus infection with 85 comparable noninfected controls. After vaccination, the proportions of subjects in each group attaining protective levels of antitetanus antibodies were similar (96.9% infected versus 97.6% noninfected). Postvaccination increases in antitetanus immunoglobulin G (IgG) and the predominant IgG isotype, IgG1, were equivalent in both groups, as were increases in specific IgG4 and IgE; however, significantly greater increases in specific IgG2 (P < 0.05) and IgG3 (P < 0.001) were observed in the noninfected group. Stratification of the O. volvulus-infected group into two groups representing light and heavy infections revealed a significantly impaired antitetanus IgG response in those with heavy infections compared to those with light infections (P < 0.01) or no infection (P < 0.05). The impact of concurrent intestinal helminth infections on the antitetanus response was also examined; an increased IgG4/IgE ratio was seen in those infected with Strongyloides stercoralis (P < 0.05) and when all helminth infections were combined as a single group (P < 0.05). These findings indicate that concurrent infection with O. volvulus does not prevent the development of a protective antitetanus response, although heavier O. volvulus infections are able to alter the magnitude of this response, and concurrent helminth infections (O. volvulus and intestinal helminths) may alter TT-specific antibody isotype responses.
Onchocerciasis, caused by the filarial helminth parasite Onchocerca volvulus, is a major cause of debilitating skin and eye disease in regions of Africa and the Americas where it is endemic. Infections with this parasite tend to be chronic, and clinical disease is thought to be caused by the cumulative effects of inflammatory reactions stimulated by the presence of the parasite larvae or microfilariae that invade the tissues.
Human helminth infections, including those caused by O. volvulus, are associated with a highly polarized, adaptive immune response to parasite antigens which is characterized by (i) impaired production of gamma interferon (IFN-γ) but prominent production of type 2 cytokines (e.g., interleukin-4 [IL-4] and IL-5) (22, 26), (ii) production of IL-10 both spontaneously and upon antigen stimulation (28), and (iii) high circulating levels of polyclonal and parasite-specific immunoglobulin E (IgE) and IgG4 (16, 27). Such a highly polarized immune environment might affect the immune response to nonparasite antigens. There is evidence from studies of experimental animal models (2, 25, 32) and humans (3, 7, 18, 20, 21, 33, 36) that chronic helminth infections may bias the cellular response to nonparasite antigens, leading to an inappropriate increase in Th2 cytokine production or a failure to produce the characteristic Th1 (IFN-γ) response.
In the case of onchocerciasis, a number of studies have demonstrated an impaired cellular immune response (7, 20, 21, 34) and humoral response (7, 20, 33) to a number of nonparasite antigens in infected individuals, suggesting that this phenomenon may be common to human helminth infections. Most human studies investigating the impact of helminth infections on vaccine-induced immune responses have examined rather crude markers of cellular immune responses (e.g., delayed-type hypersensitivity skin tests) (20, 21, 35) and humoral immune responses (e.g., antitetanus IgG) (18, 20, 33). Helminth infections may also have the potential to alter the immune response qualitatively to resemble the parasite-specific immune response. For example, a bystander effect of chronic and heavy infections might be an increase in the production of antigen-specific IgG4 and IgE.
Tetanus toxoid is a potent immunogen that induces long-lasting immunity in humans (8). Tetanus vaccination has had a dramatic impact on the incidence of tetanus infection in both adults and neonates worldwide (8). Vaccine-associated immunity to tetanus is associated with the production of neutralizing IgG antibodies to tetanus toxoid. Levels of these antibodies can be quantitated by using international standards and provide a useful model with which to investigate the impact of a helminth infection on the protective efficacy of vaccines.
We have demonstrated previously that, after tetanus vaccination, adults with O. volvulus have an impaired cellular and IgG antibody response to tetanus toxoid (TT) (7). These observations, however, were derived by using a group of chronically infected adults, and it is possible that relatively early or acute infections may cause different bystander effects on the response to TT.
The present study was designed to investigate the impact of O. volvulus infection on the quantitative (IgG) and qualitative (IgG isotypes and IgE) antitetanus antibody response after tetanus vaccination in a population sample that included both adults and children where O. volvulus is hyperendemic. As multiple geohelminth infections were also prevalent in the same population, we attempted to assess the impact of these other intestinal helminth infections on the same immune parameters.
MATERIALS AND METHODS
Study population and recruitment procedures.
The study was conducted in communities living along the Rio Cayapas in the Santiago River Basin of Esmeraldas Province, Ecuador. Studies were performed before the start of onchocerciasis control with ivermectin in the selected communities. The area studied included communities where onchocerciasis is hyperendemic (upper Cayapas) and a community (lower Cayapas) where there was thought to be no transmission. By using recently updated census data compiled by the Ecuadorian Onchocerciasis Control Programme, all seven communities were visited, and all healthy inhabitants aged 5 years and older were invited to enter the study. Informed consent was obtained from all subjects, and procedures were explained in the local language. The study was performed under protocols approved by The National Institutes of Health and Hospital Vozandes, Quito, Ecuador.
Vaccination.
Adsorbed TT (a kind gift of Pasteur Mérieux) was injected intramuscularly into the deltoid in two separate doses of 0.5 ml (5 Lf units of TT per dose), given 1 month apart.
Sample collection.
The following samples were taken before tetanus vaccination and at 1, 3, and 6 months postvaccination (after the second vaccine dose). (i) Skin snips were taken from both iliac crests and examined for the presence of microfilariae after incubation in saline for 24 h. Skin snips negative for the presence of microfilariae were tested for the presence of O. volvulus DNA by using a highly sensitive and specific PCR-based assay as previously described (41). (ii) A 5-ml sample of venous blood was drawn into SST Vacutainer tubes, the tubes were centrifuged, and the serum was divided into aliquots immediately and stored in liquid nitrogen. (iii) Thick and thin blood films were stained by use of Giemsa staining (Sigma, St. Louis, Mo.) and examined for the presence of malaria parasites. (iv) Lastly, stool samples (preserved in 10% formaldehyde-saline) were examined for the presence and quantitation of intestinal helminth eggs and larvae by using the Formol-ether concentration method as previously described (40).
TT-specific antibodies.
Microtiter plates (Immulon 4; Dynatech Laboratories, Springfield, Va.) were coated with TT (Massachusetts Public Health Laboratory) at concentrations of 0.56 Lf units of TT per ml (for IgG and IgG isotypes) or 5.6 Lf units of TT per ml (for IgE) in carbonate buffer (0.045 M NaHCO3–0.02 M Na2CO3 at pH 9.6) overnight at 4°C. After blocking the plates with blocking buffer (5% bovine serum albumin [BSA]–0.05% Tween 20 in phosphate-buffered saline [PBS]), dilutions of serum samples in enzyme-linked immunosorbent assay diluent (1% BSA–0.05% Tween 20 in PBS) were added, and the plates were incubated at 37°C for 2 h with alkaline phosphatase-conjugated goat anti-human IgG Fc (Jackson ImmunoResearch, West Grove, Pa.) for IgG or mouse ascites-derived monoclonal antibodies directed against the Fc fragment of human IgG1, IgG2, IgG3, IgG4, and IgE. The procedures for the preparation of the monoclonal antibodies used in these studies have been described in detail previously (16). For IgE analysis, the IgG fraction was removed from serum samples by prior incubation with Gammabind G Sepharose (Pharmacia Biotech, Uppsala, Sweden) according to the manufacturer's instructions before addition to the plates. The IgG isotype and IgE plates were subsequently incubated for 2 h at 37°C with alkaline phosphatase-conjugated goat anti-mouse Fc (Jackson ImmunoResearch Laboratories) for IgG isotypes or biotin goat anti-mouse IgG Fc (Jackson ImmunoResearch Laboratories) for IgE. The plates for IgE detection were then incubated with alkaline phosphatase-conjugated streptavidin (Jackson ImmunoResearch Laboratories) for 1 h at 37°C. Plate development was performed with p-nitrophenyl phosphate in sodium carbonate buffer. Plates were read on a microtiter plate reader, and unknown values were interpolated from standard curves prepared by using a World Health Organization reference serum (Statens Serum Institute) for IgG (expressed as IU per milliliter) and pools of positive sera for each of the IgG isotypes and IgE. The sensitivity of the IgG assay was 0.01 IU/ml, and protective levels of tetanus antitoxin were defined as those exceeding 0.15 IU/ml (11).
Statistical analysis.
Skin infection intensities are expressed as the geometric mean number of microfilariae per milligram of skin over the four sampling time points. Geohelminth prevalence rates and infection intensities were calculated by using the results from all four study time points, as follows. (i) Geohelminth prevalence was defined as the presence of eggs or larvae in stool samples at at least one of the four time points. (ii) Geohelminth intensity was defined as the geometric mean number of eggs or larvae per gram of stool during the four observation times. Helminth infection intensities were also quantified as an increasing score of 1 to 4, representing the quartile in which an individual's parasite burden fell in the frequency distribution of infection intensities for each helminth. An overall helminth score was calculated for each individual as the sum of individual helminth scores (e.g., maximum of 20). Antibody data were normalized by loge transformation and were analyzed as follows. Comparisons of means from independent samples were calculated by using the Student t test and of paired data by using a paired t test. Comparisons of more than two means were performed by analysis of variance (ANOVA) (for independent samples and repeated measurements as appropriate). Bivariate analysis was performed by calculation of Spearman's rank correlation coefficients. Multivariate analysis was performed by using multiple linear or logistic regression as appropriate. Statistical significance is inferred from a P value of <0.05.
RESULTS
Demographic and parasitologic details of study population.
A total of 529 subjects were screened for the presence of O. volvulus infection, and 352 were entered into the study. Prevaccination samples were collected from all 352 subjects, and it was possible to follow-up completely (e.g., up to 6 months postvaccination) a total of 278 subjects (79%) of this latter group. Losses to follow-up were due to migration, death from other causes, and noncompliance. For the purpose of analysis, recruited subjects were divided into two groups based on the presence or absence of O. volvulus infection: a subject was classified as parasitologically negative if skin snips were negative microscopically and negative by PCR at all four observation times. Study recruits with “cryptic” O. volvulus infection (e.g., with negative skin snips at all four observation times, but with a positive PCR for O. volvulus on at least one occasion) were included in the O. volvulus-infected group. The O. volvulus-infected group was further stratified according to the burden of infection, as follows: light infections (positive PCR only or mean infection intensity of fewer than 5 microfilariae [mf]/mg) and heavier infections (microfilarial density of >5 mf/mg).
The age, sex, and microfilarial intensity of the O. volvulus-infected and noninfected study groups are shown in Table 1. The mean age was similar for both O. volvulus-infected and noninfected groups, and the two study populations did not differ with regard to age distribution (data not shown). The sex distribution of the two groups was similar also. The overall mean infection intensity in the O. volvulus-infected group was 3.1 mf/mg (range, 0 to 72.5 mf/mg). Cryptic O. volvulus infection (e.g., skin snip negative and PCR positive at at least one of the four time points) was seen in 38 of these individuals.
TABLE 1.
Differences in age, sex, and helminth infections between the two study groupsa
| Study group (n) | Sex (% male/ % female) | Mean age, yr (range) | MfS (range) | % Prevalence (geometric mean intensity [range]) of intestinal helminth
|
|||
|---|---|---|---|---|---|---|---|
| A. lumbricoides | T. trichiura | A. duodenale | S. stercoralis | ||||
| O. volvulus-infected (193) | 51/49 | 23 (5–70) | 3.1 (0–72.5) | 100.0 (553** [6–3,601]) | 98.7* (13.8** [0–101]) | 80.7** (3.6** [0–107]) | 49.0** (0.8** [0–21]) |
| Noninfected (85) | 54/46 | 25 (5–80) | 0 | 98.4 (306 [0–3,609]) | 91.8 (9.3 [0–201]) | 68.0 (2.0 [0–41]) | 28.3 (0.5 [0–8]) |
Geohelminth infection intensities are expressed as eggs or larvae/gram of stool. MfS, geometric mean dermal microfilarial intensity over the observation period. Statistical comparisons are between the two study groups: ∗, P < 0.05; ∗∗, P < 0.01.
Geohelminth infections were highly prevalent in both groups (Table 1). Although there was no difference in the prevalence of Ascaris lumbricoides between the two groups, the prevalence of Trichuris trichiura, Ancylostoma duodenale, and Strongyloides stercoralis was significantly greater (P < 0.05) in the O. volvulus-infected group. The infection intensities of all four helminths were greater in the infected group (P < 0.01).
Positive blood films for malaria parasites were seen in 14 subjects over the observation period. No subject had a positive film on more than one occasion, and the parasites observed were Plasmodium vivax (13 individuals) and Plasmodium falciparum (1 individual). Over the study period, these communities were the focus of an intensive community-based program of malaria control involving active case search and treatment, the use of permethrin-impregnated bed nets (repeated every 3 months), and mosquito larval control in standing water with larva-eating fish.
Changes in antitetanus antibody levels in the study population.
A protective antibody response after tetanus vaccination is defined as an antitetanus antibody level of >0.15 IU/ml. Pre- and postvaccination antibody levels and the proportions of each group with protective antibody levels are shown in Table 2. Before vaccination, mean levels of antitetanus IgG were 0.24 IU/ml (95% confidence intervals [CI], 0.21 to 0.29 IU/ml) in the study population, and levels rose significantly after vaccination, peaking at 3 months (mean, 1.35 IU/ml; 95% CI, 1.19 to 1.53 IU/ml; P < 0.001) and remained elevated at 6 months (mean, 0.93 IU/ml; 95% CI, 0.80 to 1.08 IU/ml; P < 0.001). Prevaccination, the proportion of those with protective antibody levels was 61.5%, and this proportion rose to 97.1% by 3 months after vaccination and remained high at 6 months (93.9%). Only eight individuals (2.9%) did not mount an adequate antitetanus response after two doses of TT. Pre- and postvaccination antibody levels were strongly negatively correlated with age (prevaccination, r = −0.253 and P < 0.05; at 3 months postvaccination, r = −0.433 and P < 0.01) but not sex. Changes in antitetanus antibody levels at the time of the peak IgG response (e.g., 3 months postvaccination) compared with the baseline are shown in Fig. 1 for IgG (mean, 642%; 95% CI, 546 to 753%; P < 0.001), the IgG isotypes, and IgE. The IgG response to TT was predominantly IgG1 before vaccination (data not shown), and this isotype was predominant in the postvaccination response. A mean increase in IgG1 levels of 540% (95% CI, 460 to 631%) (P < 0.001) was seen in the study group at the 3-month postvaccination time point. Postvaccination increases in the other IgG isotypes and IgE were more modest (mean, 95% CI) for IgG2 (176%, 157 to 197%, P < 0.001), IgG3 (105%, 96 to 114%, P < 0.05), IgG4 (130%, 114 to 147%, P < 0.001), and IgE (149%, 133 to 166%, P < 0.001). Increases (at 3 months) in IgG were strongly correlated with levels of antitetanus IgG1 (r = 0.592, P < 0.01) but not with changes in the levels of the other antibody isotypes. Changes in IgG4 were correlated with changes in the levels of IgE (r = 0.225, P < 0.05). Antibody levels (IgG and all isotypes) at 3 months postvaccination expressed as a percent change compared with the baseline were not correlated with either age or sex (data not shown). The presence of protective levels of antitetanus antibody levels (e.g., vaccinated previously) before vaccination correlated with subsequent changes in levels of IgG1 (P < 0.001) but not with any of the other isotypes.
TABLE 2.
Antitetanus IgG antibodies in the total population and the two study groups before vaccination and up to 6 months after tetanus vaccination
| Study group (n) | IU of TT-specific IgG/ml (95% CI interval), % protectiona
|
|||
|---|---|---|---|---|
| Prevaccination | 1 mo postvaccination | 3 mo postvaccination | 6 mo postvaccination | |
| O. volvulus-infected (193) | 0.26 (0.21–0.32), 62.7 | 1.41 (1.15–1.74), 90.7 | 1.32 (1.14–1.52), 96.9 | 0.94 (0.79–1.13), 94.8 |
| Noninfected (85) | 0.21 (0.15–0.28), 58.8 | 1.01 (0.84–1.44), 85.9 | 1.41 (1.15–1.74), 97.6 | 0.90 (0.68–1.19), 91.5 |
| Total (278) | 0.24 (0.21–0.29), 61.5 | 1.27 (1.05–1.52), 89.2 | 1.35 (1.19–1.53), 97.1 | 0.93 (0.80–1.08), 93.9 |
% Protection represents the percentage in each study group at each of the observation times that have protective levels of antitetanus antibodies (e.g., >0.15 IU/ml).
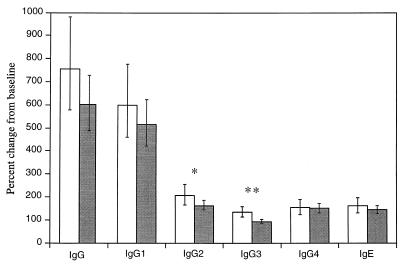
FIG. 1.
Changes in antitetanus IgG and specific isotypes at 3 months after tetanus vaccination. Levels are expressed as the percent change compared to prevaccination levels (e.g., 100% represents no change). Clear bars represent findings in the noninfected group, and shaded bars represent findings in the O. volvulus-infected group. The geometric mean changes and 95% CI values are shown. Statistical comparisons were made between the two study groups: ∗, P < 0.05; ∗∗, P < 0.001.
Impact of O. volvulus infection on antitetanus responses: presence or absence of O. volvulus infection.
O. volvulus infection status did not affect the humoral response to TT when compared as either antibody levels or the proportions achieving and maintaining protective antibody levels (e.g., antitetanus IgG) over the 6-month observation period (Table 2). Moreover, no differences were found between the study groups and the percent changes in levels (mean [95% CI]) of tetanus-specific IgG (O. volvulus infected, 598% [489 to 727%]; noninfected, 756% [577 to 982%]), IgG1 (O. volvulus, 515% [422 to 625%]; noninfected, 599% [459 to 775%]), IgG4 (O. volvulus, 150% [129 to 173%]; noninfected, 154% [123 to 188%]), and IgE (O. volvulus, 144% [126 to 163%]; noninfected, 161% [130 to 195%]) (Fig. 1). Significantly greater increases in noninfected subjects were seen in the levels (mean [95% CI]) of IgG2 (O. volvulus, 163% [143 to 186%]; noninfected, 208% [165 to 257%]; P < 0.05) and IgG3 (O. volvulus, 93% [84 to 102%]; noninfected, 135% [113 to 158%]; P < 0.001). Strong correlations were seen between changes in IgG1 and antitetanus IgG (infected, r = 0.603 and P < 0.01; noninfected, r = 0.559 and P < 0.01). A significantly positive correlation was observed between IgG4 and IgE in the infected group (r = 0.261, P < 0.01) but not in the noninfected group (r = 0.168, P > 0.05). These correlations remained statistically significant after controlling for potential confounding variables of (e.g., age, sex, and prevaccination protective antibody status) by multiple logistic regression. Since IgG4 antibodies may act as blocking antibodies to prevent IgE-mediated immunopathology, we compared the ratio of changes in IgG4 and IgE levels between the two study groups; there was no difference between groups (mean ratios of 0.90 [95% CI, 0.69 to 1.18] in the noninfected group and 0.99 [95% CI, 0.83 to 1.17] in the infected group).
Effect on antitetanus antibody responses of “light” versus “heavy” O. volvulus infections.
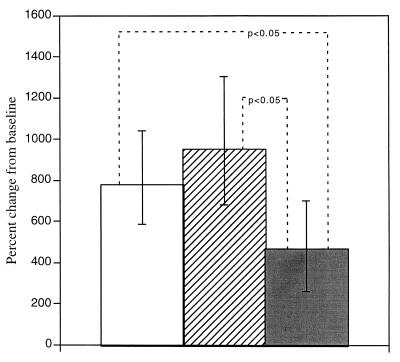
As with other helminth infections, the parasite burden in onchocerciasis is correlated with the presence and the severity of clinical disease. Study subjects were stratified into three groups representing their status as either noninfected, lightly (“light”) infected, or more heavily (“heavy”) infected (Fig. 2). The mean ages of the three groups were as follows: noninfected, 24.5 years; light, 19.9 years; and heavy, 30.6 years. To control for intergroup differences in age, changes in antibody levels for each of the isotypes were compared. Changes in antitetanus IgG differed significantly between the three groups (ANOVA; P < 0.05). Postvaccination increases (mean, 95% CI) in the more heavily infected group (466%, 262 to 699%) were less than either the noninfected group (780%, 586 to 1,039%) (P < 0.05) or the lightly infected group (949%, 678 to 1,301%) (P < 0.01). Significant differences between the three groups (by ANOVA) were seen in changes in levels of IgG1 (P < 0.05) and IgG3 (P < 0.001). IgG1 levels closely paralleled those of IgG (data not shown), and IgG3 levels (mean [95% CI]) were reduced in both infected groups (light, 95% [86 to 105%]; heavy, 85% [66 to 106%]) compared with the noninfected group (135% [113 to 158%]). No significant intergroup differences were seen for the other antibody isotypes.
FIG. 2.
Stratification of changes in antitetanus IgG by O. volvulus parasite burden. Levels are expressed as the percent change at 3 months postvaccination compared to prevaccination levels. Groups are as follows: no infection (clear bar), light O. volvulus infection (hatched bar), and heavy O. volvulus infection (shaded bar). The geometric mean changes and 95% CI values are shown.
Relationship between geohelminth infections and antitetanus responses.
Many inhabitants of areas where onchocerciasis is endemic harbor multiple helminth infections. In studies investigating the impact of O. volvulus infection on the immune response to vaccines, it is important to consider the effects of concurrent geohelminth infections on the vaccine-induced immune response. The prevalence and intensity of geohelminth infections in the study population are shown in Table 1. Geohelminth infections were highly prevalent, although infection intensities were low. Even though geohelminth infection intensities were greater in the O. volvulus-infected group (Table 1), none of the geohelminth infection intensities were significantly correlated with O. volvulus infection intensity but were strongly correlated with each other. A. lumbricoides was correlated with T. trichiura (r = 0.485, P < 0.01) and S. stercoralis (r = 0.208, P < 0.05) infections; T. trichiura was correlated with A. duodenale (r = 0.226, P < 0.05) infection; and A. duodenale was correlated with S. stercoralis (r = 0.333, P < 0.01) infection. Since the immune responses to human helminth infections bear striking similarities to one another (10), it is probable that other coendemic helminth infections may be capable of inducing bystander effects that may be additive. Therefore, the relationship between individual helminth infections and changes in antibody isotype levels were examined for each helminth infection and for all helminth infections combined (calculated as a score as described in Materials and Methods). After controlling for potential confounding variables by multiple linear regression (e.g., age, sex, and prevaccination antibody status), the findings were that (i) individual or all helminth infections were not correlated with changes in levels of antitetanus IgG; (ii) O. volvulus infection (P < 0.001), A. lumbricoides infection (P < 0.05), and all helminth infections combined (P < 0.05) were negatively correlated with changes in IgG3 levels; (iii) S. stercoralis infection was negatively correlated with changes in IgE levels (P < 0.05); and (iv) the ratio of IgG4 to IgE was greater for individual infections with S. stercoralis (P < 0.05) and all helminth infections combined (P < 0.05).
DISCUSSION
Onchocerciasis is a leading cause of severe eye and skin disease and is the target of international control efforts. Infections with O. volvulus may have other less-recognized effects on the health of populations where the disease is endemic; the most widely relevant of these is the potential to impair the protective efficacy of parenterally administered vaccines. Onchocerciasis is not alone in this effect; a number of other parasite infections have been associated with impaired immunity to vaccines, including Schistosoma mansoni (3, 9, 36) and acute Plasmodium falciparum (8, 12). Because a large proportion of the world's population is estimated to be infected with parasites—helminths alone infect approximately three billion humans worldwide—impaired vaccine-induced immunity secondary to concurrent parasitic infections is of huge potential public health significance.
To investigate the bystander effects of concurrent O. volvulus infection on the immune response directed against a nonparasite antigen, we chose TT as the model antigen. The use of TT as an experimental antigen with which to conduct prospective studies of nonparasite protein-specific responses offers several advantages. (i) TT is one of the most widely evaluated and efficacious vaccines available. (ii) The cellular and humoral immune response is well defined. (iii) The levels of tetanus-specific IgG can be measured easily and are a widely recognized correlate of protective immunity (8).
Concurrent O. volvulus infection has been shown to affect the immune response to tetanus (18, 20, 33), Mycobacterium bovis BCG (21, 35), and yellow fever vaccines (5). The studies that have shown an impact of O. volvulus on tetanus seroconversion rates, however, have suffered from suboptimal immunization regimens (33) and excessively short (18, 33) or noncomparable follow-up observations in infected and control subjects. Thus, the clinical relevance of these observations remains unclear. One well-designed vaccination study, which evaluated infected and noninfected children and adults from a rain forest onchocerciasis focus in Liberia (20), showed comparable rates of seroconversion (i.e., protective antibody levels) in infected and control groups (81 versus 82%, respectively) 4 months after they had received two doses of TT. In contrast, we have found previously that subjects with chronic O. volvulus infection showed impaired antitetanus IgG responses compared to comparable noninfected controls (7). In the present larger study, which included both adults and children, we were unable to demonstrate an impaired IgG response to tetanus vaccination in the O. volvulus-infected group compared with controls. The reasons for these apparently conflicting results lie in important differences between the two O. volvulus-infected groups in the three studies. Only chronically infected adults were assessed in the previous study (7), while in this study and in the Liberian study (20) the O. volvulus-infected group included adults and children. The use of a single unstratified infection group to examine the impact of O. volvulus on the immune response to TT may mask real differences between those with early and relatively light infections (i.e., acute) and those with heavier (and more likely chronic) infections.
The different clinical phenotypes (i.e., acute and chronic) are associated with distinct immunologic profiles. Typical features of chronic filarial infections are the impaired production of IFN-γ, the enhanced production of the immunosuppressive cytokine IL-10, and the high circulating levels of polyclonal and parasite-specific IgG4 antibodies. These latter immune parameters are poorly developed in infections with low parasite burdens (e.g., acute or even postpatent), which are characterized by a more mixed immune response with the production of both type 1 (e.g., IFN-γ) and type 2 cytokines (29, 38). Acute and chronic O. volvulus infections have the potential to induce different bystander effects on the response to nonparasite antigens.
The definition of whether an O. volvulus-infected subject had acute or chronic infection was based on the mean parasite burden over the observation period. Since transmission is likely to be unstable in the Rio Cayapas region (13, 14), we chose a relatively low microfilarial intensity to distinguish between light and heavy infections, with <5 mf/mg representing light or acute infection and >5 mf/mg representing heavy or chronic infection. Despite the crudeness of this approach, we were able to detect real differences in the antitetanus antibody responses between the two infection groups. The more heavily infected group had significantly diminished responses compared to both noninfected and lightly infected groups. Interestingly, the lightly infected group had the highest responses of all three groups. A similar nonsignificant trend was seen when the three groups were stratified according to the presence or absence of previous tetanus sensitization. These findings indicate that impaired antitetanus responses associated with concurrent O. volvulus infection are seen only in chronic or heavier infections. In contrast, light infections that are associated with hyperresponsive antiparasite immune responses (24, 29) do not have the same effect and may enhance the IgG response to TT.
These observations also suggest that filarial infections may subvert the immune response to nonparasite antigens to resemble more closely the antiparasite response. We have demonstrated previously that chronic O. volvulus infection is associated with impaired lymphocyte blastogenesis and production of IFN-γ in response to TT and with the antigen-specific production of IL-10 (7). We were able to show also that IL-10 levels were negatively correlated with parameters of the cellular responses (e.g., lymphocyte proliferation in response to TT) and antibody responses (e.g., TT-specific IgG) (7). In fact, many of these findings are typical of the parasite-specific response in chronic infection and provide a potential mechanism by which O. volvulus (or other filarial infections) might affect the immune response to TT: the induction of TT-specific IL-10 secretion in an environment of excessive parasite-induced IL-10, resulting in impairment of both cellular and IgG antibody (perhaps via reduced T-cell help) arms of the immune response.
Potential bystander effects of concurrent O. volvulus helminth infection on the subclass response to a nonparasite antigen have not been examined previously. IL-4 induces antibody subclass switching to IgE and IgG4 (22), and the strong IL-4 responses observed in helminth infections (10) are probably responsible for the secretion of high levels of polyclonal and parasite-specific IgG4 and IgE (23). Parasite-specific IgE may be important in larval killing reactions in O. volvulus, since it is a key mediator of immediate hypersensitivity reactions that induce effector cell migration and eosinophil recruitment and degranulation at the sites of microfilarial sequestration (1, 6). In contrast, parasite antigen-specific IgG4, which often recognizes the same epitopes as IgE (16), may act as a blocking antibody to prevent the induction of damaging inflammatory reactions (31). The likely role of parasite-specific IgE in the pathogenesis of allergic phenomena in lymphatic filariasis (e.g., tropical pulmonary eosinophilia) (30) and the relative absence of allergic disease in patients with asymptomatic microfilaremia in whom a high proportion of IgG is of the IgG4 isotype (15) suggest that the parallel regulation of IgE and IgG4 (22) may be an important mechanism in chronic infections that serves to limit host pathology yet maintain a parasite-killing ability (e.g., at sites of high antigen concentration). In fact, as Wuchereria bancrofti microfilarial intensities increase, there may be a dissociation in parasite-specific IgG4 and IgE production resulting in relatively greater IgG4 levels (27).
We examined also the impact of individual geohelminth infections on TT-specific IgE and IgG4 responses. There was evidence for a relatively greater increase in IgG4 compared with IgE, as helminth infection intensities increased, but this was significant only for S. stercoralis infection. Helminth infections tend to induce a predominant Th2-like cytokine response (e.g., IL-4) (10, 26), and most humans living in an area where O. volvulus is endemic are likely to be coinfected with one or more geohelminth infections. Multiple helminth infections, therefore, might have additive or even synergistic effects on TT-specific IgE and IgG4 responses. In the present study, infection intensities for each helminth were expressed as a score based on the frequency distribution of infection intensities for each parasite (so that none of the helminths would be weighted disproportionately), and the relationship between changes in the IgG4/IgE ratio and the total score (e.g., sum of scores) was examined. A significant association was seen between increasing helminth score and an increasing IgG4/IgE ratio. These findings suggest that multiple helminth infections do indeed have additive effects on the response to TT. The mechanism for this is not clear, but the data suggest that there is a dissociation between IgG4 and IgE with increasing helminth burdens, as has been described for the parasite-specific response (27). Further, since parasite infection intensities for all the five helminth species examined were low, it is possible this divergence may be more marked in heavily infected populations.
Antitetanus IgG3 antibody levels were reduced in the O. volvulus infection group compared with noninfected controls (data not shown). After tetanus vaccination IgG3 levels did not change in the infected group, while a modest increase was observed in the control group. Interestingly, the absence of an IgG3 response was noted in both acute and chronic O. volvulus infection groups (data not shown). Further, a significant negative correlation was observed between changes in TT-specific IgG3 levels and A. lumbricoides infection intensities, as well as the combined helminth infection intensities. Increased levels of parasite-specific IgG3 have been observed in individuals who appear to be resistant to infection despite exposure to the parasite (as demonstrated by the presence of parasite-specific IgG) (4, 39) and have been attributed a protective role either by complement fixation (17) or by their ability to mediate antibody-dependent cellular cytotoxicity reactions (19). Of course, this putatively immune or endemic normal group would describe many of those in the noninfected control group in this study who mounted an IgG3 response against the nonparasite antigen TT. This observation points to a relative inability of subjects with O. volvulus infections to mount an IgG3 response against nonparasite antigens.
This study was designed to measure the impact of O. volvulus infection on the postvaccination antitetanus response. It is difficult, however, to investigate helminth infections in isolation since populations where they are endemic are inevitably coinfected with other helminth species. Therefore, we have attempted to investigate the potential effects of infections with single and multiple helminth species on the antitetanus response. A note of caution is warranted in interpreting the results of multiple additional post posteriori analyses (i.e., correlations between antitetanus responses and geohelminth infections) at a P value of <0.05, and it is suggested that these relationships deserve further investigation in future hypothesis-driven studies.
We were unable to demonstrate an impact of concurrent O. volvulus infection on the development of a protective immune response (e.g., TT-specific IgG) after tetanus vaccination, although we showed that chronic infection did impair this immune response quantitatively and qualitatively. TT is a highly potent vaccine requiring a booster every 10 years after the primary vaccination series (8). Vaccine titers tend to decrease exponentially after vaccination (37), and it is possible that chronic O. volvulus infection may have an effect on either the rate of antibody decline because of reduced T-cell help or a more rapid decline toward nonprotective levels. In fact, we have shown that a subgroup of adults with chronic O. volvulus who develop protective antibody levels by 3 months postvaccination, but who produce few if any cytokines (Th1 and Th2) in response to TT, have nonprotective antibody levels at 12 months (data not shown). An acceleration of the decline in antibody levels will require more frequent booster vaccinations. Further, protective responses to less immunogenic vaccines, such as pneumococcal and meningococcal vaccines, might be more greatly affected by concurrent helminth infections. More recently, TT has been used as a conjugate for molecules, which alone are poorly immunogenic (e.g., Haemophilus influenzae type b vaccine), and the potential impact of concurrent O. volvulus or other parasite infections on the immune response-potentiating role of TT should be taken into account before decisions to use these vaccines in areas where this organism is endemic are made.
ACKNOWLEDGMENTS
We thank the Ecuadorian physicians, nurses, and community health workers who assisted in patient vaccination and health care provision, particularly Tamara Mancero, Raquel Lovato, Carlos Sandoval, Gregorio Montano, Daniela Montano, and Alfonso Anapa. We also thank the study communities for their cooperation and Pasteur Mérieux, Lyon, France, for the generous donation of tetanus vaccine. The assistance of Kenneth Farr (U.S. Agency for International Development, Quito, Ecuador) is also gratefully acknowledged.
This research was supported in part by the Edna McConnell Clark Foundation.
REFERENCES
- 1.Ackerman S J, Kephart G M, Francis H, Awadzi K, Gleich G J, Ottesen E A. Eosinophil degranulation: an immunologic determinant in the pathogenesis of the Mazzotti reaction in human onchocerciasis. J Immunol. 1990;144:3961–3969. [PubMed] [Google Scholar]
- 2.Actor J K, Shirai M, Kullberg M C, Buller R M L, Sher A, Berzofsky J A. Helminth infection results in decreased virus-specific CD8+ cytotoxic virus-specific T-cell and Th-1 cytokine responses as well as delayed virus clearance. Proc Natl Acad Sci USA. 1993;90:948–952. doi: 10.1073/pnas.90.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassily S, Hyams K C, El-Ghorab N, Mansour M, El-Masry N A, Dunn M A. Immunogenicity of hepatitis B vaccine in patients infected with S. mansoni. Am J Trop Med Hyg. 1987;36:549–553. doi: 10.4269/ajtmh.1987.36.549. [DOI] [PubMed] [Google Scholar]
- 4.Boyer A E, Tsang V C W, Eberhard M L, Zea-Flores G, Hightower A, Pilcher J B, Zea-Flores R, Zhou W, Reimer C B. Guatemalan human onchocerciasis. II. Evidence for IgG3 involvement in acquired immunity to Onchocerca volvulus and identification of possible immune-associated antigens. J Immunol. 1991;146:4001–4010. [PubMed] [Google Scholar]
- 5.Buck A A, Anderson R I, Sasaki T T, Kawata K. Health and disease in Chad: epidemiology, culture, and environment in 5 villages. Baltimore, Md: The Johns Hopkins Press; 1970. [Google Scholar]
- 6.Butterworth A E. Cell-mediated damage to helminths. Adv Parasitol. 1984;23:143–235. doi: 10.1016/s0065-308x(08)60287-0. [DOI] [PubMed] [Google Scholar]
- 7.Cooper P J, Espinel I, Paredes W, Guderian R H, Nutman T B. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a probable role for IL-10. J Infect Dis. 1998;178:1133–1138. doi: 10.1086/515661. [DOI] [PubMed] [Google Scholar]
- 8.Dietz V, Galazka A, van Loon F, Cochi S. Factors affecting the immunogenicity and potency of tetanus toxoid: implications for the elimination of neonatal and non-neonatal tetanus as public health problems. Bull W H O. 1997;75:81–93. [PMC free article] [PubMed] [Google Scholar]
- 9.El Ghorab N M, Bassily S, Fouad S, El Masry N A, Boctor F, Kamal K A. Immunological response to diphtheria/tetanus vaccine in Schistosomiasis [sic] mansoni patients. J Egypt Soc Parasitol. 1992;22:747–765. [PubMed] [Google Scholar]
- 10.Finkelman F D, Shea-Donohue T, Goldhill J, Sullivan C A, Morris S C, Madden K B, Gause W C, Urban J F. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 11.Gergen P J, McQuillan G M, Kiely M, Ezzati-Rice T M, Sutter R W, Virella G. A population-based serologic survey of immunity to tetanus in the United States. N Engl J Med. 1995;332:761–766. doi: 10.1056/NEJM199503233321201. [DOI] [PubMed] [Google Scholar]
- 12.Greenwood B M, Bradley-Moore A M, Palit A, Bryceson A D M. Immunosuppression in children with malaria. Lancet. 1972;i:169–172. doi: 10.1016/s0140-6736(72)90569-7. [DOI] [PubMed] [Google Scholar]
- 13.Guderian R H, Beck B J, Proano R. Onchocerciasis in Ecuador: infection in children in the Santiago Basin focus, province of Esmeraldas. Trans R Soc Trop Med Hyg. 1990;84:109–112. doi: 10.1016/0035-9203(90)90396-v. [DOI] [PubMed] [Google Scholar]
- 14.Guderian R H, Anselmi M, Espinel M, Mancero T, Rivadeneira G, Proano R, Calvopina H M, Vieira J C, Cooper P. Successful control of onchocerciasis with community-based ivermectin distribution in the Rio Santiago focus in Ecuador. Trop Med Int Health. 1997;2:982–988. doi: 10.1046/j.1365-3156.1997.d01-158.x. [DOI] [PubMed] [Google Scholar]
- 15.Hussain R, Poindexter R W, Ottesen E A. Control of allergic reactivity in human filariasis: predominant localization of blocking antibodies to the IgG4 subclass. J Immunol. 1991;148:2731–2737. [PubMed] [Google Scholar]
- 16.Hussain R, Poindexter R W, Ottesen E A, Reimer C B. Use of monoclonal antibodies to quantify subclasses of human IgG. II. Enzyme immunoassay to define antigen specific (anti-filarial) IgG subclass antibodies. J Immunol Methods. 1986;94:80. doi: 10.1016/0022-1759(86)90217-6. [DOI] [PubMed] [Google Scholar]
- 17.Jefferis R, Walker M R. The biological significance of the specific antibody IgG subclass profiles. Monogr Allergy. 1988;23:73–77. [PubMed] [Google Scholar]
- 18.Kawabata M, Izui S, Anan S, Kondo S, Fukumoto S, Flores G Z, Kobayakawa T. Circulating immune complexes and their possible relevance to other immunological parameters in Guatemalan onchocerciasis. Int Arch Allergy Appl Immunol. 1983;72:128–133. doi: 10.1159/000234854. [DOI] [PubMed] [Google Scholar]
- 19.Khalife J, Dunne D W, Richardson B A, Mazza G, Thorne K J, Capron A, Butterworth A E. Functional role of human IgG subclasses in eosinophil-mediated killing of schistosomula of Schistosoma mansoni. J Immunol. 1989;142:4422–4427. [PubMed] [Google Scholar]
- 20.Kilian H D, Nielsen G. Cell-mediated and humoral immune responses to tetanus vaccinations in onchocerciasis patients. Trop Med Parasitol. 1989;40:285–291. [PubMed] [Google Scholar]
- 21.Kilian H D, Nielsen G. Cell-mediated and humoral immune responses to BCG and rubella vaccinations and to recall antigens in onchocerciasis patients. Trop Med Parasitol. 1989;40:445–483. [PubMed] [Google Scholar]
- 22.King C L, Nutman T B. Regulation of the immune response in lymphatic filariasis and onchocerciasis. Immunol Today. 1991;12:54–58. doi: 10.1016/S0167-5699(05)80016-7. [DOI] [PubMed] [Google Scholar]
- 23.King C L, Nutman T B. IgE and IgG subclass regulation by IL-4 and IFN-gamma in human helminth infections. Assessment by B cell precursor frequencies. J Immunol. 1993;151:458–465. [PubMed] [Google Scholar]
- 24.Klion A D, Massougbodji A, Sadeler B-C, Ottesen E A, Nutman T B. Loiasis in endemic and nonendemic populations: immunologically mediated differences in clinical presentation. J Infect Dis. 1991;163:1318–1325. doi: 10.1093/infdis/163.6.1318. [DOI] [PubMed] [Google Scholar]
- 25.Kullberg M C, Pearce E J, Hieny S E, Sher A, Berzofsky J A. Infection with Schistosoma mansoni alters Th1/Th2 cytokine responses to a nonparasite antigen. J Immunol. 1992;148:3264–3270. [PubMed] [Google Scholar]
- 26.Mahanty S, King C L, Kumaraswami V, Regunathan J, Maya A, Jayaraman K, Abrams J S, Ottesen E A, Nutman T B. IL-4- and IL-5-secreting lymphocyte populations are preferentially stimulated by parasite-derived antigens in human tissue invasive nematode infections. J Immunol. 1993;151:3704–3711. [PubMed] [Google Scholar]
- 27.Mahanty S, Day K P, Alpers M P, Kazura J W. Antifilarial IgG4 antibodies in children from filaria-endemic areas correlate with duration of infection and are dissociated from antifilarial IgE antibodies. J Infect Dis. 1994;170:1339–1343. doi: 10.1093/infdis/170.5.1339. [DOI] [PubMed] [Google Scholar]
- 28.Mahanty S, Nutman T B. Immunoregulation in human lymphatic filariasis: the role of interleukin-10. Parasite Immunol. 1995;17:385–392. doi: 10.1111/j.1365-3024.1995.tb00906.x. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy J S, Ottesen E A, Nutman T B. Onchocerciasis in endemic and nonendemic populations: differences in clinical presentation and immunologic findings. J Infect Dis. 1994;170:736–741. doi: 10.1093/infdis/170.3.736. [DOI] [PubMed] [Google Scholar]
- 30.Nutman T B, Vijayan V K, Pinkston P, Kumaraswami V, Steel C, Crystal R, Ottesen E A. Tropical pulmonary eosinophilia: analysis of antifilarial antibody localized to the lung. J Infect Dis. 1989;160:1042–1050. doi: 10.1093/infdis/160.6.1042. [DOI] [PubMed] [Google Scholar]
- 31.Ottesen E A, Kumaraswami V, Paranjape R, Poindexter R W, Tripathy S P. Naturally occurring blocking antibodies modulate immediate hypersensitivity responses in human filariasis. J Immunol. 1981;127:2014–2020. [PubMed] [Google Scholar]
- 32.Pearlman E, Kazura J W, Hazlett F E, Jr, Boom W H. Modulation of murine cytokine responses to mycobacterial antigens by helminth-induced T helper 2 cell responses. J Immunol. 1993;151:4857–4864. [PubMed] [Google Scholar]
- 33.Prost A, Schlumberger M, Fayet M T. Response to tetanus immunization in onchocerciasis patients. Ann Trop Med Parasitol. 1983;77:83–85. doi: 10.1080/00034983.1983.11811675. [DOI] [PubMed] [Google Scholar]
- 34.Rougemont A, Mattei X, Discamps C, Loreal E, Zander N, Mazelet J R. Ultrastructural aspects of the degeneration of the dermal microfilaria O. volvulus under the effect of diethylcarbamazine in human onchocerciasis. C R Seances Soc Biol Fil. 1978;172:397–402. [PubMed] [Google Scholar]
- 35.Rougemont A, Boisson-Pontal M E, Pontal P G, Gridel F, Sangare S. Tuberculin skin tests and BCG vaccination in a hyperendemic area of onchocerciasis. Lancet. 1977;i:309. doi: 10.1016/s0140-6736(77)91857-8. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 36.Sabin E A, Araujo M I, Carvalho E M, Pearce E J. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis. 1996;173:269–272. doi: 10.1093/infdis/173.1.269. [DOI] [PubMed] [Google Scholar]
- 37.Simonsen O, Badsberg J H, Kjeldsen K, Moller-Madsen B, Heron I. The fall-off in serum concentration of tetanus antitoxin after primary and booster vaccination. Acta Pathol Microbiol Immunol Scand. 1986;94:77–82. doi: 10.1111/j.1699-0463.1986.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 38.Soboslay P T, Geiger S M, Weiss N, Banla M, Luder C G, Dreweck C M, Batchassi E, Boatin B A, Stadler A, Schulz-Key H. The diverse expression of immunity in humans at distinct states of Onchocerca volvulus infection. Immunology. 1997;90:592–599. doi: 10.1046/j.1365-2567.1997.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart G R, Elson L, Araujo E, Guderian R, Nutman T B, Bradley J E. Isotype-specific characterization of antibody responses to Onchocerca volvulus in putatively immune individuals. Parasitol Immunol. 1995;17:371–380. doi: 10.1111/j.1365-3024.1995.tb00904.x. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. Diagnostic techniques for intestinal parasitic infections (IPI) applicable to primary health care (PHC) services. Document WHO/PDP/85.2. Geneva, Switzerland: World Health Organization; 1985. [Google Scholar]
- 41.Zimmerman P A, Guderian R, Araujo E, Elson L, Phadke P, Kubofcik J, Nutman T B. Polymerase chain reaction-based diagnosis of Onchocerca volvulus infection: improved detection of patients with onchocerciasis. J Infect Dis. 1994;169:686–689. doi: 10.1093/infdis/169.3.686. [DOI] [PubMed] [Google Scholar]