Abstract
Urinary tract infections (UTIs) are associated with approximately 27% of premature births. Escherichia coli is the most frequent causal agent of UTIs and expresses virulence factors, including surface adhesins that recognize specific host tissue receptors. We have reported that E. coli Dr adhesin recognizes decay-accelerating factor as the host tissue receptor and that these receptors are increased during pregnancy. Induction of pathogenesis is a cumulative effect of the host-pathogen relationship involving specific host factors and virulence characteristics of the invading organism. Recently, an experimental model of chronic pyelonephritis has been developed with E. coli bearing Dr adhesin (E. coli Dr+) in nonpregnant lipopolysaccharide hyporesponder C3H/HeJ mice. In this study, we investigated the role of E. coli Dr+ on the outcome of pregnancy in C3H/HeJ mice. Groups of pregnant mice were infected with E. coli Dr+ or its isogenic mutant which does not bear the Dr adhesin (E. coli Dr−) by urethral catheterization. Nearly 90% of pregnant mice infected with E. coli Dr+ delivered preterm (before 90% gestation) compared to 10% of mice infected with E. coli Dr− and none of the mice treated with phosphate-buffered saline (PBS). Also, there was a significant reduction in fetal birth weight in the E. coli Dr+-infected group compared to the E. coli Dr−- and PBS-treated groups (P = 0.003). This experimental model of E. coli Dr+-induced preterm delivery in mice may help in understanding the molecular mechanisms involved in UTI-induced preterm labor involving bacterial adhesins.
Approximately 1 of every 10 babies in the United States is born prematurely, and prematurity is the leading cause of death among infants before their first birthday (2). In addition to being small, premature babies are developmentally unprepared for life, which can result in physical and mental disabilities. Premature babies are more likely to have respiratory problems during childhood and a higher incidence of learning disabilities and problems with speech, hearing, and vision. The increased risk of preterm birth is very high, and the cost to society is overwhelming from financial and health perspectives (11).
Preterm labor has been associated with bacterial infection. The most common infections associated with preterm labor are intrauterine infections and urinary tract infections (UTIs) (4, 6, 12, 25, 43). The relationship between asymptomatic bacteriuria and preterm birth has been well documented (37), and recently, Schultz et al. (38) reported that UTIs are associated with approximately 27% of premature births. UTIs, including asymptomatic bacteriuria, cystis, and pyelonephritis are the most common and frequently encountered medical complications of pregnancy (28). Although UTIs may be asymptomatic in pregnancy, physiological changes that occur during pregnancy predispose women patients to a very high risk of developing acute pyelonephritis, resulting in considerable maternal morbidity and mortality. Additionally, UTIs during pregnancy may adversely affect the fetus (1). Preterm labor and low birth weight have been cited as the significant adverse outcomes in pregnant women with asymptomatic bacteriuria (24, 29, 43). A meta-analysis by Romero et al. (37) showed that nonbacteriuric patients had only two-thirds the risk of low-birth-weight infants and half the risk of preterm delivery compared to patients with untreated asymptomatic bacteriuria.
The most common pathogen isolated from pregnant women with UTIs is Escherichia coli; it has been reported to be the primary pathogen in 80% of UTI cases (17). An important factor in the pathogenesis of UTIs, as in any bacterial infection, is bacterial virulence. The ability of certain uropathogenic E. coli to adhere to uroepithelium is largely responsible for its virulence. This adherence is considered to be a prerequisite for the initiation of the disease process. E. coli's ability to adhere is mediated by structures called adhesins, which are present on the bacterial cell surface. Our previous studies and reports by other investigators indicate that E. coli and other bacteria express different surface adhesins that mediate the attachment of these pathogens to various human or animal cells through specific tissue receptors (3, 21, 31). Host-pathogen interactions through these specific receptors determine the extent of pathogenesis.
Considerable progress has been made in characterizing adhesins in E. coli (41, 42, 44). Studies of E. coli adhesins and their respective specific tissue receptors have established an adhesin-ligand-based mechanism of ascending UTI (32). In 1988, Nowicki et al. (31) reported that one such adhesin, isolated from a female patient with UTI, bound to the Dr blood group antigen; they proposed the term Dr hemagglutinin for this bacterial adhesin. Our earlier studies showed that decay-accelerating factor (DAF) also known as CD55, a complement regulatory protein, is expressed in human endometrium and may serve as the receptor for E. coli Dr adhesin (21). Studies have also shown that E. coli Dr adhesin may recognize DAF, located in the tubular basement membrane and Bowman's capsule, resulting in colonization of renal tissues (33–35, 45). Our earlier studies have shown that there is an upregulation of DAF during the third trimester of pregnancy (26). Also, it has been reported that there is a higher incidence of E. coli bearing Dr adhesin (E. coli Dr+) during the third trimester of pregnancy in women with gestational pyelonephritis (36).
Recently, Goluzsko et al. (15) reported the development of an experimental model of chronic pyelonephritis with E. coli Dr+ in nonpregnant lipopolysaccharide (LPS)-hyporesponder C3H/HeJ mice. Intrauterine infections with E. coli Dr+ have been reported to result in a high death rate in pregnant rats compared to that of nonpregnant rats (30).
There is an increasing emphasis on the role of E. coli Dr+ in the pathogenesis of UTIs, particularly during pregnancy. However, the precise role of Dr adhesin in UTI during pregnancy has not been investigated. Therefore, in this study, we investigated the role, if any, of E. coli Dr+ in the induction of preterm labor. Pregnant C3H/HeJ mice were experimentally infected with either E. coli Dr+ or its isogenic mutant (which does not express Dr adhesin) (E. coli Dr−), and their effects on the outcome of pregnancy and fetal birth weight were determined.
MATERIALS AND METHODS
Animals.
Timed pregnant LPS-hyporesponsive C3H/HeJ mice were obtained from Jackson Laboratories, Bar Harbor, Maine. Use of C3H/HeJ mice with decreased response to LPS made it possible to elucidate the role of non-LPS factors such as Dr adhesin in pathogenicity and its effect on the outcome of pregnancy. Timed pregnant mice arrived in our animal facility on day 5 of pregnancy. All animals were housed under pathogen-free conditions in the animal facility of the Minneapolis Medical Research Foundation (MMRF). Once infected, the mice were placed in separate cages in a clean room. All experimentation described in this study was approved by the MMRF Institutional Animal Care and Use Committee.
Bacterial strains.
Two bacterial strains of E. coli Dr+ IH11128 and Dr14 were used to infect the pregnant mice. Bacterial strain IH11128, which expresses Dr adhesin, has been isolated from a female patient with a symptomatic UTI (32). Dr14 is an isogenic mutant of wild-type IH11128 and does not express Dr adhesin (15). Both strains were kindly provided by Bogdan Nowicki of The University of Texas Medical Branch, Galveston.
For each experiment, both strains of bacteria were grown overnight on Luria agar plates at 37°C. Several loopfuls of the culture were suspended in 10 ml portions of sterile phosphate-buffered saline (PBS) (pH 7.5), washed, and resuspended in sterile PBS to a final concentration that gave an optical density at 600 nm of 1.0; 0.05 ml of the inoculum was injected in each mouse bladder (15). The presence or absence of Dr adhesin in both bacterial strains was checked by hemagglutination (31).
Induction of experimental UTI in pregnant C3H/HeJ mice by bladder catheterization with E. coli Dr+.
Groups of pregnant C3H/HeJ mice were infected on day 7 of pregnancy (35% gestation) with E. coli Dr+ or E. coli Dr− by urethral catheterization and instillation of a single dose of bacterial suspension (0.05 ml of a suspension with an optical density at 600 nm of 1) into the urinary bladder, as previously described (15). The control group received sterile PBS. Animals were anesthetized with isoflurane in a sealed glass jar for 30 s. The inoculum of E. coli Dr+ or E. coli Dr− strains was instilled into the bladder through a soft polyethylene catheter with an outer diameter of 0.30 mm (Norton Performance Plastics, Akron, Ohio) adapted to a needle on a tuberculin syringe (0.4 by 20 mm). A 0.05-ml sample of the appropriate microbial inoculum was injected. The catheter was then immediately withdrawn without further manipulations. The mice were placed in individual cages and allowed free access to food and drink under a 12-h day-night cycle. The animals were monitored every 4 to 6 h for symptoms of preterm labor, such as bleeding, fetal loss, or other sickness. The animals were also monitored for changes in body weight during pregnancy. Any delivery occurring on or before day 18 of gestation was considered preterm (before 90% gestation). The survival of the pups delivered by these mice, either term or preterm, was monitored.
Determination of fetal birth weight in infected mice.
To investigate whether infection with E. coli Dr+ or E. coli Dr− affects the weight of the developing fetuses in pregnant C3H/HeJ mice, we performed a set of experiments in which 22 pregnant mice were infected with E. coli Dr+, E. coli Dr−, or PBS on day 7 of pregnancy (at 35% gestation), as described above. The mice were monitored every 6 to 8 h for symptoms of preterm labor. The mice were sacrificed on day 17 of pregnancy (at 85% gestation), and the fetuses were carefully removed and weighed.
Quantitative tissue culture.
Quantitative tissue culture was performed at two time points: (i) a group of infected mice completing delivery, preterm or term, that was sacrificed immediately after the delivery and (ii) another group of infected mice that was sacrificed, at an earlier time point, on day 17 (80% gestation). The fetus, spleen, and renal tissues were collected and subjected to bacteriological cultures, and the E. coli bacteria recovered were quantitated and typed for adhesin expression. The tissue from each mouse was homogenized separately in 0.5 ml of sterile PBS and plated on Luria agar and MacConkey plates for isolation of live bacteria. The bacterial counts were recorded as CFU per gram of tissue.
The E. coli bacteria recovered from the tissues were typed for the presence of Dr adhesin, and its specificity was tested by agglutination test, using human O erythrocytes and anti-DAF antibody (Wako Chemicals USA Inc., Richmond, Va.).
Histological analysis of tissues.
The kidneys and placental and fetal tissues collected for histological analysis were fixed overnight in formalin, embedded in paraffin, and sectioned for immunohistochemical analysis and stained with hematoxylin and eosin. Unstained paraffin-embedded tissue sections were stained with Gram stain for detection of bacteria.
Inflammation scores of 0 to 4+ were assigned based on the percentage involvement of the structure or tissue being examined on the basis of polymorphonuclear cell (PMN) or mononuclear cell (MNC) infiltration as follows: 1+, 5% involvement; 2+, 5 to 15% involvement; 3+, 15 to 25% involvement; and 4+, >25% involvement. The overall score for histopathological grading of pyelonephritis was based on pelvic, interstitial, and tubular involvement. Scoring for percentage tissue involvement in the placental or fetus was also determined as described above. Kidney, placental, or fetal tissue samples without lesions were graded as 0. All tissue samples were coded and read blind by one observer (J.T.C.).
Statistical analysis.
All data were recorded as means ± standard errors of the means per subgroup. Individual comparisons among the different groups were assessed by the Student's t test. Significance in the incidence of preterm birth among groups was evaluated by the Fisher exact test.
RESULTS
Induction of gestational UTI in pregnant mice and its effect on outcome of pregnancy, survival of pups, and dissemination of infection.
We observed that the group of mice infected with E. coli Dr+ showed signs of preterm labor. The mice in this group started delivering from day 11 on, and most delivered by day 18 of gestation. Table 1 gives the results on the outcome of pregnancy and survival of pups after the delivery in the E. coli-infected and control groups of mice. The mice undergoing preterm labor showed signs of vaginal bleeding followed by preterm parturition, delivering both live and dead pups. A significant number of mice infected with E. coli Dr+ delivered preterm compared to the E. coli Dr−-infected group or the control group. The difference among the groups was statistically significant. Thus, experimental infection with E. coli Dr+ in C3H/HeJ mice led to preterm labor.
TABLE 1.
Outcome of pregnancy in C3H/HeJ mice after experimental UTI with E. coli
| Group | No. of mice (%)
|
Day of delivery | No. of mice delivering | % Mortality of pups born (no. died/ total no.) | ||
|---|---|---|---|---|---|---|
| Total | Preterm | Term | ||||
| E. coli Dr+ | 21 | 19b (90) | 2 (10) | 11 | 1a | 53.6cd (37/69) |
| 12 | 1a | |||||
| 15 | 1a | |||||
| 16 | 1a | |||||
| 17 | 8a | |||||
| 18 | 7a | |||||
| 19 | 1 | |||||
| 20 | 1 | |||||
| E. coli Dr− | 10 | 1 (10) | 9 (90) | 18 | 1a | 18 (10/55) |
| 19 | 2 | |||||
| 20 | 7 | |||||
| Control | 10 | 0 (0) | 10 (100) | 19 | 2 | 9.5 (6/63) |
| 20 | 8 | |||||
Animals delivering preterm (on or before day 18 of pregnancy; before 90% gestation).
Significantly different from the values obtained for the E. coli Dr− and control groups (P = 0.03).
Significantly different from the value obtained for the E. coli Dr− group (P = 0.007).
Significantly different from the value obtained for the control group (P < 0.001).
The pregnant mice delivering preterm remained very sick and eventually died after delivery. The mice infected with E. coli Dr− and those in the control group did not die after the delivery. There was a high mortality rate among the pups born to the mothers infected with E. coli Dr+. These pups were born dead or died within a few days of premature delivery. A significant number of the pups in the E. coli Dr+ group were born prematurely and were found dead compared to the E. coli Dr− group or the control group (Table 1). The difference in the mortality rate of pups born to the E. coli Dr+ group or E. coli Dr− group and the control group was significant.
To investigate the extent of E. coli infection, recovery of E. coli from kidney and fetal tissues was examined in a group of infected mice upon completion of delivery. The results of the bacterial tissue cultures are given in Table 2. The mean bacterial CFU/gram of kidney tissue was significantly higher in the E. coli Dr+ group than in the E. coli Dr− group (P = 0.004). The kidney tissues obtained from all the mice in E. coli Dr+- and E. coli Dr−-infected groups demonstrated positive bacterial cultures.
TABLE 2.
Quantitative bacterial tissue cultures and determination of fetal birth weight outcome in infected and control groups of C3H/HeJ mice
| Group | Mean no. (CFU/g) of bacteria ± SEM found by bacterial culture
|
Mean fetal wtb (g) ± SEM (no. of fetuses) | |||||
|---|---|---|---|---|---|---|---|
| After deliverya
|
At day 17 of gestationb
|
||||||
| Kidney | Fetus | Spleen | Kidney | Fetus | Spleen | ||
| E. coli Dr+ | 5 × 108 ± 1 × 108c | 3 × 108 ± 2 × 108d | ND | 8 × 109 ± 2 × 109e | 4 × 109 ± 2 × 109f | >108e (4/8)g | 0.644 ± 0.030h (6) |
| E. coli Dr− | 5 × 107 ± 2 × 107 | 7 × 106 ± 7 × 106 | ND | 5 × 109 ± 2 × 109 | 1 × 107 ± 1 × 107 | >108 (2/8) | 0.813 ± 0.021 (6) |
| Control | 0 | 0 | ND | 0 | 0 | 0 | 0.88 ± 0.10 (5) |
Day 7 E. coli-infected and control groups of mice were sacrificed after the delivery, and tissues were collected for E. coli bacterial cultures. These data were obtained from 10 mice in the E. coli Dr+ group and 12 mice in the E. coli Dr− group and 6 mice in the control group. ND, not determined.
Day 7 E. coli-infected and control groups of mice were sacrificed on day 17 of gestation, tissues were collected for bacterial cultures, and fetuses were removed and carefully weighed for each group of mice. This experiment included eight mice in each E. coli Dr+ or E. coli Dr− group and five mice in the control group.
Significantly different from the value obtained for the E. coli Dr− group (P = 0.004).
Significantly different from the value obtained for the E. coli Dr− group (P = 0.02).
Not significantly different from the value obtained for the E. coli Dr− group (P > 0.05).
Significantly different from the value obtained for the E. coli Dr− group (P = 0.05).
The number of mice with >108 CFU per g of spleen to the total number of mice in the group is shown in the parentheses (P > 0.05).
Significantly different from the values obtained for the E. coli Dr− and control groups (P = 0.003).
The mean bacterial CFU/gram of fetal tissue was significantly higher in the E. coli Dr+-infected group than in the E. coli Dr−-infected group (P ≤ 0.02). In the E. coli Dr+-infected group, all fetuses (10 of 10) showed positive bacterial cultures, compared to only 33% fetuses (4 of 12) in the E. coli Dr−-infected group. The remaining fetal cultures from the E. coli Dr− group were sterile, indicating limited dissemination of E. coli Dr− to the developing fetus compared to E. coli Dr+ group.
Effect of E. coli infection on fetal birth weight and dissemination of infection on day 17 of gestation.
E. coli Dr+ infection in pregnant mice affects the fetal birth weight outcome as demonstrated by the low fetal birth weight of the E. coli Dr+-infected group compared to that of the E. coli Dr− group or the control group. There was a significant difference in the fetal weights in the E. coli Dr+, E. coli Dr−, and control groups. The difference in fetal weight between the groups was statistically significant (P = 0.003). Table 2 provides the mean fetal weight and bacterial culture results found for the three groups.
All kidney tissues obtained from E. coli Dr+ and E. coli Dr−-infected mice sacrificed on day 17 (at 85% of gestation) exhibited positive bacterial cultures with growth of a significant number of bacteria (Table 2). However, the difference between the mean CFU/gram of E. coli in kidney tissue, at this time point, among the two E. coli-infected groups was not significant (P > 0.05).
A significantly higher number of E. coli bacteria were recovered from fetal tissues of the E. coli Dr+ group than from those of the E. coli Dr− group (P = 0.05). Fetal tissue from all six fetuses tested from the E. coli Dr+ group showed E. coli growth, compared to only two of seven fetuses in the E. coli Dr− group. The remaining fetal tissue cultures in the E. coli Dr− group were sterile.
Spleens recovered from mice in each group were cultured for bacterial counts. Four of 8 mice in the E. coli Dr+ group had >108 CFU per spleen, whereas only 2 of 8 mice in the E. coli Dr− group had >108 CFU per spleen. The difference between the two groups was not significant (P > 0.05).
Histopathological studies.
Histopathological evaluation of the kidney, fetus, and placenta was performed on infected mice, after sacrificing one group of animals on completion of delivery and another group on day 17 of gestation, to evaluate the evolution of pathogenicity after infection with E. coli.
(i) Kidney histopathology.
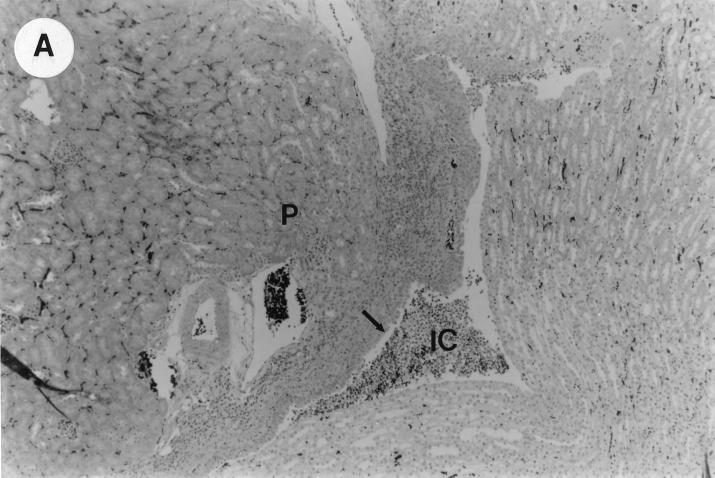
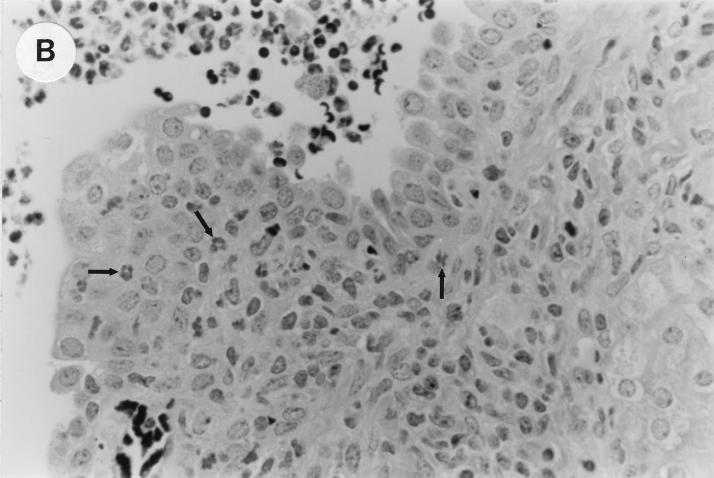
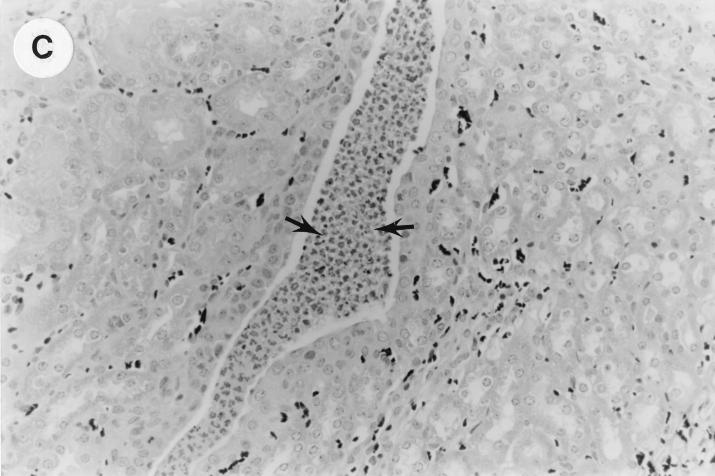
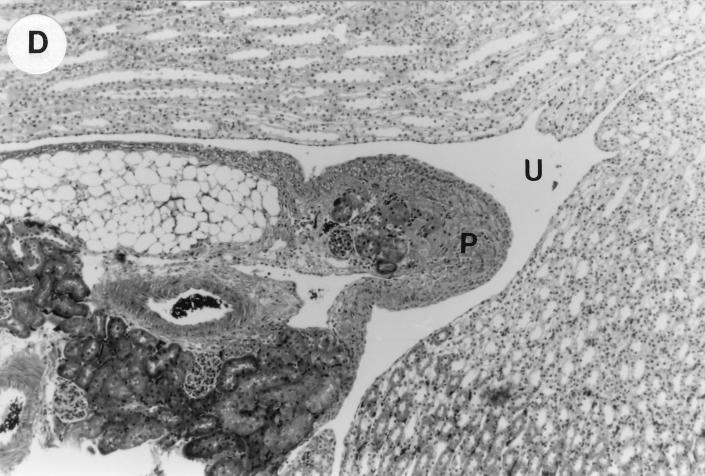
The kidney histopathology inflammatory scores in infected and control group of mice are listed in Table 3, and representative histopathology of the E. coli Dr+ group of mice is shown in Fig. 1A, B, and C and representative histopathology of the E. coli Dr− or control group is shown in Fig. 1D.
TABLE 3.
Histopathology data showing mean inflammatory scores of kidney, placental, and fetal tissues of E. coli Dr+- and E. coli Dr−-infected and control mice
| Groupa | Inflammatory score (mean ± SEM) in tissueb
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Kidney
|
Placental tissue | Fetal tissue | ||||||
| Renal pelvis
|
Tubular involvement
|
Interstitial involvement
|
||||||
| Acute | Chronic | Acute | Chronic | Acute | Chronic | |||
| Sacrificed after delivery | ||||||||
| E. coli Dr+ | 2.12 ± 0.44c | 1.50 ± 0.32c | 0.62 ± 0.41 | 0 | 1.62 ± 0.32 | 1.12 ± 0.51 | 2.37 ± 0.32d | 3.25 ± 0.25d |
| E. coli Dr− | 0.25 ± 0.16 | 0.25 ± 0.25 | 0 | 0 | 0 | 0 | 0.62 ± 0.26 | 0.50 ± 0.26 |
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sacrificed on day 17 of gestation | ||||||||
| E. coli Dr+ | 0.75 ± 0.25e | 1.87 ± 0.35e | 0 | 0 | 0.25 ± 0.16e | 0.37 ± 0.18e | ND | 0.66 ± 0.42 |
| E. coli Dr− | 0.62 ± 0.41 | 1.25 ± 0.16 | 0 | 0 | 0.12 ± 0.12 | 0.37 ± 0.26 | ND | 0 |
| Control | 0 | 0 | 0 | 0 | 0 | 0 | ND | 0 |
Histopathology results in mice sacrificed after delivery were obtained from eight mice in each E. coli Dr+ and E. coli Dr− group and six mice in the control group. Group sacrificed on day 17 of gestation included eight mice in the E. coli Dr+ group, eight mice in the E. coli Dr− group, and five mice in the control group.
Histopathologic evaluation was performed and scores were given as described in Materials and Methods under “Histological analysis of tissues.” ND, not determined.
Significantly different from the value obtained for the E. coli Dr− group (P ≤ 0.001).
Significantly different from the value obtained for the E. coli Dr− group (P ≤ 0.0001).
Not significantly different from the value obtained for the E. coli Dr− group (P > 0.05).
FIG. 1.
UTI by E. coli Dr+ in C3H/HeJ mice leads to acute and chronic inflammation of kidneys. (A) Representative renal pathology in kidney sections obtained from mice infected with E. coli Dr+ at 35% gestation and sacrificed after preterm delivery revealed 1 to 2+ acute (PMN infiltration) and chronic (MNC infiltration) in the pelvis by hematoxylin and eosin staining. The arrow points to renal pelvis with inflammatory cells. Abbreviations: IC, inflammatory cells; P, renal pelvis. Magnification, ×64. (B) Higher-power view of Fig. 1A focussed on renal pelvic urothelium showing neutrophils (indicated by the arrows). Magnification, ×400. (C) Higher-power view of Fig. 1A showing renal tubular involvement. The arrows point to renal tubule with large number of PMNs within one tubular lumen. Magnification, ×200. (D) Normal histopathological appearance of a kidney section from the E. coli Dr− group or control group with absence of MNC infiltration. Abbreviations: U, urinary space; P, renal pelvis. Magnification, ×80.
The kidney sections of E. coli Dr+ group of mice, sacrificed upon completion of delivery, showed significant increased infiltration of acute (P < 0.001) and chronic inflammatory cells in the renal pelvis (Fig. 1B) compared to the infiltration seen in renal pelvis of E. coli Dr− group. This inflammation also extended to the tubules and interstitium of the kidney (Fig. 1C). Gram staining of the tissue sections also revealed bacterial infiltration in the tubules and glomeruli of the kidney. Tubular and interstitial involvement of the kidney tissue was absent in the E. coli Dr− group of mice. The majority of the animals in the E. coli Dr− group had cleared the infection and did not show any MNC infiltration (Fig. 1D). Also, no inflammatory cells were observed in kidney sections obtained from control animals.
In the groups of animals sacrificed on day 17 gestation after infection, the mean inflammatory score of acute and chronic inflammation in renal pelvis of the E. coli Dr+ group of mice was higher than that seen in the E. coli Dr− group where only a few mice had inflammatory cells. However, on comparison between the two groups, a statistically significant level was not achieved. None of the groups showed renal tubular involvement at this time point. Interstitial involvement of the kidney was observed in five of eight animals of the E. coli Dr+ group and in only two of eight animals of the E. coli Dr− group. The difference between the mean inflammatory score of the interstitium in the E. coli Dr+ and E. coli Dr− groups was not statistically significant. Kidney tissue sections from the control group of mice showed no inflammation.
(ii) Placental histopathology.
The placental histopathology in the E. coli Dr+ group sacrificed after delivery revealed infiltration of PMNs and bacteria in the placental tissue of all eight animals and the mean inflammatory score were significantly higher (P < 0.0001) than observed in the E. coli Dr− group where only four of eight animals showed a few inflammatory cells in the placenta (Table 3). In the severely infected placenta, very few villi were left (Fig. 2). A majority of the animals in the E. coli Dr− group had placental histology comparable to the animals in the control group. Histology was not performed on the placenta of mice sacrificed on day 17 of gestation.
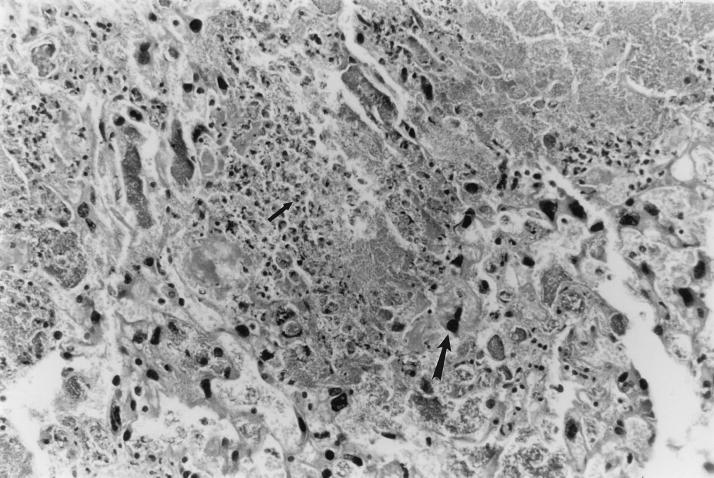
FIG. 2.
UTI by E. coli Dr+ in C3H/HeJ mice leads to massive inflammatory response in placenta. Representative placental pathology in sections obtained from mice infected at 35% gestation with E. coli Dr+ and undergoing preterm labor revealed massive infiltration of PMNs. In severely infected placenta, very few villi were left. The small arrow points to a PMN; the large arrow points to a trophoblastic cell. Magnification, ×200.
(iii) Fetal histopathology.
All fetal cross sections obtained from all eight mice sacrificed after the delivery in the E. coli Dr+ group showed higher infiltration of PMNs and bacteria which is shown by the higher mean inflammatory scores compared to the scores obtained from the E. coli Dr− group. The differences between the two groups were statistically significant (P < 0.0001). All the fetuses in the E. coli Dr+ group also showed impaired development of the organs (Fig. 3A). Only three of eight fetuses in the E. coli Dr− group showed PMN infiltration, while the rest of the fetal sections revealed no inflammation or impaired development. The fetuses of the control group showed no inflammation or impaired organ development (Fig. 3B).
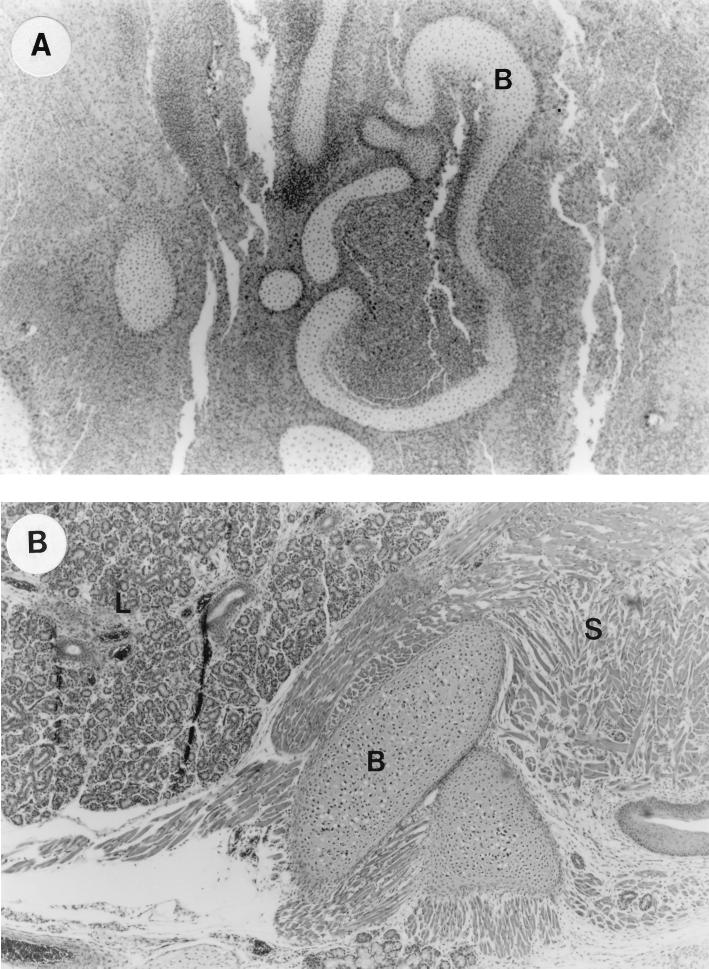
FIG. 3.
UTI by E. coli Dr+ in C3H/HeJ mice leads to inflammatory response in fetus and poor development of fetal organs. (A) Representative fetal pathology in sections obtained from mice infected at 35% gestation with E. coli Dr+ and sacrificed after preterm delivery showed 3+ chronic infiltration of PMNs and poor development of the fetal organs. Abbreviations: B, immature bone. Magnification, ×50. (B) Normal histopathological appearance of fetal section of the E. coli Dr− or control group showing normal organ development with no infiltrating PMNs. Abbreviations: B, bone; S, skeletal muscle; L, lung. Magnification, ×50.
None of the fetuses obtained from E. coli Dr+, E. coli Dr−, or control group of mice, sacrificed on day 17 of gestation revealed any inflammation with PMN infiltration or poor organ development. However, only two of eight fetuses in the E. coli Dr+-infected group that had delivered preterm revealed inflammation and poor organ development.
DISCUSSION
An appreciable number of pregnancies that culminate in preterm labor are associated with infectious processes. The molecular mechanisms that regulate the inflammatory response to bacterial infection have only recently become the focus of study (7–9). The information about the bacterial properties involved in this process, the nature of the inflammatory mediators, and the functional consequences of the response are very limited. To understand the pathophysiology of infection-induced preterm labor, various in vivo animal model studies have used LPS to induce preterm labor. Numerous reports describe deleterious effect of dose-dependent bacterial LPS in pregnant animals resulting in fetal death or abortion (5, 20, 36, 39). In contrast to the findings in animal studies, the quantity of endotoxin required to stimulate prostaglandin production in human amnion has not been found in the amniotic fluid of women with intra-amniotic infections. This suggests that other factors may be involved in the initiation of preterm labor (13). Thus, studies so far do not completely explain the relationship between infections, such as ascending UTI, and preterm labor. Several pieces of evidence suggest that Dr adhesin, present on the uropathogenic E. coli plays an important role in the pathogenesis of UTIs. Dr adhesins occur more frequently in UTI-associated E. coli isolates than in fecal isolates (10, 19), and the presence of Dr adhesin at the time of first UTI has been associated with an increased risk of a second UTI (14). Experimental ascending UTI with E. coli Dr+ leads to chronic pyelonephritis in C3H/HeJ mice, and E. coli Dr+ bacteria are found to colonize the interstitial space of the mouse kidney, an area that is not directly available for bacteria colonizing the renal pelvis (15). Also, recently it has been reported that E. coli Dr+ possess slow-invasive properties in the HeLa epithelial cell line infected in vitro by this bacteria (16).
This study describes, for the first time, the role of adhesin-based gestational UTI induced in LPS hyporesponder animals using E. coli Dr+ where animals infected with E. coli Dr+ delivered preterm and animals infected with E. coli Dr− were protected and delivered at term. Bacterial tissue cultures revealed overwhelming infiltration by bacteria and a systemic spread in pregnant animals infected with E. coli Dr+. Excessive colonization in the kidneys, placentae, fetuses, and spleens of E. coli Dr+-infected animals indicate chronic colonization and a suppressed clearing mechanism in these animals. The findings of increased isolation of bacterial cultures in the placentae and fetuses of the E. coli Dr+-infected animals indicate the transplacental transfer of virulent bacteria to the developing fetus. We also observed that the congenital infection in the pups of the animals that were infected with E. coli Dr+ leads to low fetal birth weight and poor organ development, thereby causing an increase in the fetal death rate. The restricted growth of the fetus and the poor development of the organs observed in fetal sections of the E. coli Dr+ group may be a result of infection-induced placental changes that left few placental villi to support fetal development. We need to further understand this mechanism. This model may provide an opportunity to understand the molecular mechanisms and clinical outcomes of UTIs during pregnancy.
Histopathological studies done on kidney, placenta, and fetus confirmed the differences in clinical outcome observed between the E. coli Dr+, E. coli Dr−, and control groups. Significant differences in the histopathology of the kidney and fetus were observed between E. coli Dr+- and E. coli Dr−-infected pregnant mice. Histopathology of the placental sections of the E. coli Dr+ group showed massive infiltration of bacteria, causing increased inflammation with very few villi left. In the E. coli Dr− group, a majority of the animals showed no inflammation and only a few showed minimal inflammation in the placenta. However, this minimal inflammation did not affect the pregnancy outcome or fetal birth weight in this group of mice.
In addition to virulence factors of the invading pathogen, host factors such as genetic background, LPS hyporesponsivness, or cytokine responses may also be critical for induction of pathogenicity. We believe host responses to infection with E. coli Dr+ differ from the host responses generated as a result of infection with E. coli Dr−. Clearance of bacteria and minimal pathology seen in the tissues from animals infected with E. coli Dr− strain may be results of optimum protective responses generated in response to this bacterium. Recently, we have observed increased levels of proinflammatory cytokine responses (in splenic and local [kidney and placental] tissues) in the E. coli Dr+-infected group compared to the E. coli Dr−-infected group of pregnant animals (23). These increased inflammatory responses in pregnant animals seem to be responsible for the adverse outcomes observed in these animals. Smith et al. (40) reports that increased apoptosis occurs in patients with intra-uterine growth retardation (40). It remains to be seen whether proinflammatory cytokines generated in response to E. coli Dr+ infection in this model are involved in apoptosis of trophoblastic cells leading to fetal loss.
Recently, we observed a differential pregnancy outcome in LPS-responder C3H/HeN mice and LPS-nonresponder C3H/HeJ mice when infected with E. coli Dr+. The majority of C3H/HeJ mice developed preterm labor, whereas none of the C3H/HeN mice developed preterm labor (22). Thus, the host factor LPS response may also be an important factor for the generation of this acute response in E. coli Dr+-infected C3H/HeJ mice leading to preterm delivery. This model raises some important questions that may have implications in human pregnancy, for example, it may be significant to investigate whether there is a transient LPS hyporesponsiveness in patients delivering preterm in response to bacterial infections, particularly in light of the recent report about LPS hyporesponse in surgical patients (18).
This study demonstrates the association of gestational UTI by E. coli Dr+ with preterm delivery, low birth weight, and fetal mortality in C3H/HeJ mice. This model could serve as a means of investigating the biomolecular processes involved in infection-induced preterm parturition. The survival of the fetomaternal unit depends on a complex network of mechanisms that can be disrupted by any infection. It has been reported that the infection of the reproductive tract can have a direct effect of the infection on the placenta (43). However, this study shows that gestational UTI can also have a detrimental effect on the fetus. Both local and systemic responses of the host and the fetus in response to infection may contribute to the complications of preterm delivery.
ACKNOWLEDGMENTS
We thank Bogdan Nowicki of the Department of Obstetrics and Gynecology of The University of Texas Medical Branch, Galveston, Texas, for providing E. coli isolates and Bharat Kachroo for assistance with E. coli cultures. We also thank Deborah Sowells and Kristen Schmidt for assistance in the preparation of the manuscript.
This work was supported in part by grants by Hennepin Faculty Associates Research Award (to M.G.M. and V.R.L.) and National Kidney Foundation Young Investigator Award (to R.K. in 1998).
REFERENCES
- 1.Andrews W W, Cox S M, Gilstrap L C. Urinary tract infections in pregnancy. Int Urogynecol J. 1990;1:155–163. [Google Scholar]
- 2.Barbara L. Preventing preterm birth. New York, N.Y: Times Books; 1995. [Google Scholar]
- 3.Beachey E H. Bacterial adherence/adhesin receptor interactions mediating the attachment of bacteria of mucosal surfaces. J Infect Dis. 1981;143:325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- 4.Cousins L M, Habel C J, Chang R J, Okada D M, Marshall J R. Serum progesterone and estradiol-17β in premature and term labor. Am J Obstet Gynecol. 1977;127:612–615. doi: 10.1016/0002-9378(77)90359-3. [DOI] [PubMed] [Google Scholar]
- 5.Cox S M, MacDonald P C, Casey M L. Assay of bacterial endotoxin (LPS) in human amniotic fluid: potential usefulness in diagnosis and treatment of preterm labor. Am J Obstet Gynecol. 1988;159:99–106. doi: 10.1016/0002-9378(88)90501-7. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham F G, MacDonald P C, Grant N F, Leveno K J, Gilstrap L C., III . Williams obstetrics. 19th ed. Norwalk, Conn: Appleton and Lange; 1993. Renal and urinary tract disease; pp. 1128–1130. [Google Scholar]
- 7.de Man P, Van Kooten C, Aarden L, Svanborg-Eden C, Engberg I, Linder H. Interleukin-6 induced by gram-negative bacterial infection at mucosal surfaces. Infect Immun. 1989;57:3383–3388. doi: 10.1128/iai.57.11.3383-3388.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Man P, Jodal U, Svanborg-Eden C. Dependence among host response parameters used to diagnose urinary tract infection. J Infect Dis. 1991;163:331–335. doi: 10.1093/infdis/163.2.331. [DOI] [PubMed] [Google Scholar]
- 9.de Man P, Jodal U, Lincoln K, Svanborg-Eden C. Bacterial attachment and inflammation in the urinary tract. J Infect Dis. 1998;158:29–35. doi: 10.1093/infdis/158.1.29. [DOI] [PubMed] [Google Scholar]
- 10.Donnenberg M S, Welch R A. Virulence determinants of uropathogenic Escherichia coli. In: Mobley H L T, Warren J W, editors. Urinary tract infection: molecular pathogenesis and clinical management. Washington, D.C: American Society for Microbiology; 1996. pp. 135–174. [Google Scholar]
- 11.Dyson D C, Danbe K H, Bamber J A, Crites Y M, Field D R, Maier J A, Newman L A, Ray D A, Walton D L, Armstrong M A. Monitoring women at risk for preterm labor. N Engl J Med. 1998;338:15–19. doi: 10.1056/NEJM199801013380103. [DOI] [PubMed] [Google Scholar]
- 12.Fidel P, Jr, Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, Cotton D B. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol. 1994;170:1467–1475. doi: 10.1016/s0002-9378(94)70180-6. [DOI] [PubMed] [Google Scholar]
- 13.Fortunato S, Menon R P, Swan K F, Menon R. Inflammatory cytokine (interleukins 1, 6, and 8 and tumor necrosis factor-α) release from cultured human fetal membranes in response to endotoxic lipopolysaccharide mirrors amniotic fluid concentrations. Am J Obstet Gynecol. 1996;174:1855–1862. doi: 10.1016/s0002-9378(96)70221-1. [DOI] [PubMed] [Google Scholar]
- 14.Foxman B, Shang L, Tallman P, Palin K, Rode C, Bloch C, Gillespie B, Marrs C F. Virulence characteristics of Escherichia coli causing first urinary tract infection predict risk of second infection. J Infect Dis. 1995;172:1536–1541. doi: 10.1093/infdis/172.6.1536. [DOI] [PubMed] [Google Scholar]
- 15.Goluszko P, Moseley S L, Truong L D, Kaul A, Willford J R, Selvarangan R, Nowicki S, Nowicki B. Development of experimental model of chronic pyelonephritis with Escherichia coli O75:K5:H-bearing Dr fimbriae. J Clin Invest. 1997;99:1662–1672. doi: 10.1172/JCI119329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goluszko P, Popov V, Selvarangan R, Nowicki S, Pham T, Nowicki B J. Dr fimbriae operon of uropathogenic Escherichia coli mediate microtubule-dependent invasion to the HeLa epithelial cell line. J Infect Dis. 1997;176:158–167. doi: 10.1086/514018. [DOI] [PubMed] [Google Scholar]
- 17.Harris R E, Gilstrap L C. Cystitis during pregnancy: a distinct clinical entity. Obstet Gynecol. 1981;57:578–580. [PubMed] [Google Scholar]
- 18.Haupt W, Riese J, Mehler C, Weber K, Zowe M, Hohenberger W. Monocyte function before and after surgical trauma. Dig Surg. 1998;15:102–104. doi: 10.1159/000018601. [DOI] [PubMed] [Google Scholar]
- 19.Johnson J R. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaga N, Katsuki Y, Obata M, Shibutani Y. Repeated administration of low-dose LPS induces preterm delivery in mice: a model for human preterm parturition and for assessment of the therapeutic ability of drugs against preterm delivery. Am J Obstet Gynecol. 1996;174:754–759. doi: 10.1016/s0002-9378(96)70460-x. [DOI] [PubMed] [Google Scholar]
- 21.Kaul A K, Nowicki B J, Martens M G, Golusko P, Hart A, Nagamani M, Kumar D, Pham T Q, Nowicki S. Decay-accelerating factor is expressed in the human endometrium and may serve as the attachment ligand for Dr pilli of E. coli. Am J Reprod Immunol. 1994;32:194–199. doi: 10.1111/j.1600-0897.1994.tb01114.x. [DOI] [PubMed] [Google Scholar]
- 22.Kaul R, Khan S, Martens M, Crosson J, Kaul A. Pregnancy outcome in LPS responder and non-responder mice infected with Dr adhesin bearing Escherichia coli. Am J Reprod Immunol. 1999;41:389. . (Abstr. H-0-15.) [Google Scholar]
- 23.Kaul R, Khan S, Martens M, Crosson J, Kachroo B, Kaul A. Role of cytokines in experimental model of gestational pyelonephritis leading to preterm delivery. Clin Immunol. 1999;90:449–450. . (Abstr. H-86.) [Google Scholar]
- 24.Krieger J N. Complications and treatment of urinary tract infections during pregnancy. Urol Clin N Am. 1986;13:685–693. [PubMed] [Google Scholar]
- 25.Krohn M A, Thwin S S, Rabe L K, Brown Z, Hellier S L. Vaginal colonization of E. coli as a risk factor for very low birth weight delivery and other perinatal complications. J Infect Dis. 1997;175:606–610. doi: 10.1093/infdis/175.3.606. [DOI] [PubMed] [Google Scholar]
- 26.Martens M, Kaul A K, Nowicki S, Goluszko P. Presence of receptors for Dr-hemagglutinin of uropathogenic E. coli in the uterus of pregnant rats. Post; 1993. er presented at the 3rd World Congress for Infectious Diseases in OB/GYN, Acapulco, Mexico. [Google Scholar]
- 27.Mazor M, Hershkovitz R, Chaim W, Levy J, Sharony Y, Leiberman J, Glezerman M. Human preterm birth is associated with systemic and local changes in progesterone/17β-estradiol ratios. Am J Obstet Gynecol. 1994;1:221–236. doi: 10.1016/0002-9378(94)90474-x. [DOI] [PubMed] [Google Scholar]
- 28.Millar L K, Cox S M. Urinary tract infections complicating pregnancy. Infect Dis Clin N Am. 1996;11:13–26. doi: 10.1016/s0891-5520(05)70339-1. [DOI] [PubMed] [Google Scholar]
- 29.Naeye R L. Urinary tract infections and the outcome of pregnancy. Adv Nephrol. 1986;15:95–102. [PubMed] [Google Scholar]
- 30.Nowicki B, Faug L, Singhal J, Nowicki S, Yallampalli C. Lethal outcome of uterine infection in pregnant but not in nonpregnant rats and increased death ratio with inhibition of nitric oxide. Am J Reprod Immunol. 1997;38:309–312. doi: 10.1111/j.1600-0897.1997.tb00521.x. [DOI] [PubMed] [Google Scholar]
- 31.Nowicki B, Moulds J, Hull R, Hull S. A hemagglutinin of uropathogenic Escherichia coli recognizes the Dr blood group antigen. Infect Immun. 1988;56:1057–1060. doi: 10.1128/iai.56.5.1057-1060.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nowicki B J. In vitro models for the study of uropathogens in urinary tract infections. In: Mobley H L T, Warren J W, editors. Urinary tract infections: molecular pathogenesis and clinical management. Washington, D.C: American Society for Microbiology; 1996. pp. 341–376. [Google Scholar]
- 33.Nowicki B J, Hart A, Coyne K E, Lublin D M, Nowicki S. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of a cell-cell interaction. J Exp Med. 1993;178:2115–2121. doi: 10.1084/jem.178.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nowicki B J, Holthofer H, Saraneva T, Rhen M, Vaisasen-Rhen V, Korhonen T K. Location of adhesion sites for P-fimbriated and for O75X-positive Escherichia coli in the human kidney. Microb Pathog. 1986;1:169–180. doi: 10.1016/0882-4010(86)90019-7. [DOI] [PubMed] [Google Scholar]
- 35.Nowicki B J, Truong L, Moulds J, Hull R. Presence of Dr receptor in normal human tissues and its possible role in the pathogenesis of ascending urinary tract infection. Am J Pathol. 1988;133:1–4. [PMC free article] [PubMed] [Google Scholar]
- 36.Nowicki B J, Martens M G, Hart A, Nowicki S. Gestational age dependent distribution of E. coli fimbriae in pregnant patients with pyelonephritis. Ann N Y Acad Sci. 1994;730:290–291. doi: 10.1111/j.1749-6632.1994.tb44268.x. [DOI] [PubMed] [Google Scholar]
- 37.Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins J C, Bracken M. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol. 1989;73:576–582. [PubMed] [Google Scholar]
- 38.Schultz R, Read A W, Straton J, Stanley F J, Morich P. Genito urinary tract infection in pregnancy and low birth weight: case control study in Australian Aboriginal women. Brit Med J. 1991;303:1369–1373. doi: 10.1136/bmj.303.6814.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silver R M, Edwin S S, Trautman M S. Bacterial LPS mediated fetal death: production of a newly-recognized form of inducible cycloxygenase (COX-2) in murine decidua in response to LPS. J Clin Invest. 1995;95:725–731. doi: 10.1172/JCI117719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith S C, Baker P N, Symonds E M. Increased placental apoptosis in intrauterine growth restriction. Am J Obstet Gynecol. 1997;177:1395–1401. doi: 10.1016/s0002-9378(97)70081-4. [DOI] [PubMed] [Google Scholar]
- 41.Svanborg C, Godaly G. Bacterial virulence in urinary tract infection. Infect Dis Clin N Am. 1997;11:513–529. doi: 10.1016/s0891-5520(05)70371-8. [DOI] [PubMed] [Google Scholar]
- 42.Svanborg E C. Bacterial adherence in urinary tract infections caused by E. coli. Scand J Urol Nephrol. 1986;20:81–88. doi: 10.3109/00365598609040553. [DOI] [PubMed] [Google Scholar]
- 43.Sweet R L, Gibbs R S. Infectious diseases of the female genital tract. Baltimore, Md: Williams & Wilkins; 1990. pp. 442–445. [Google Scholar]
- 44.Warren J W. Clinical presentation and epidemiology of urinary tract infections. In: Mobley H L T, Warren J W, editors. Urinary tract infections: molecular pathogenesis and clinical management. Washington, D.C: American Society for Microbiology; 1996. pp. 3–27. [Google Scholar]
- 45.Westerlund B, Kuusela P, Risteli J, Vartio T, Rauvala H, Virkola R, Korhonen T K. The O75X adhesin of uropathogenic Escherichia coli is a type IV collagen-binding protein. Mol Microbiol. 1989;3:329–337. doi: 10.1111/j.1365-2958.1989.tb00178.x. [DOI] [PubMed] [Google Scholar]