Background:
Functional defects in eye movements and reduced reading speed in neurodegenerative diseases represent a potential new biomarker to support clinical diagnosis. We investigated whether computer-based eye-tracking (ET) analysis of the King-Devick (KD) test differentiates persons with idiopathic normal pressure hydrocephalus (iNPH) from cognitively unimpaired [control (CO)] and persons with Alzheimer’s disease (AD).
Methods:
We recruited 68 participants (37 CO, 10 iNPH, and 21 AD) who underwent neurological examination, the Consortium to Establish a Registry for Alzheimer’s Disease neuropsychological test battery (CERAD-NB), and a Clinical Dementia Rating interview. The KD reading test was performed using computer-based ET. We analyzed the total time used for the reading test, number of errors, durations of fixation and saccade, and saccade amplitudes.
Results:
The iNPH group significantly differed from the CO group in the KD test mean total time (CO 69.3 s, iNPH 87.3 s; P≤0.009) and eye-tracking recording of the mean saccade amplitude (CO 3.6 degree, iNPH 3.2 degree; P≤0.001). The AD group significantly differed from the CO group in each tested parameter. No significant differences were detected between the iNPH and AD groups.
Conclusion:
For the first time, we demonstrated altered reading ability and saccade amplitudes in patients with iNPH.
Key Words: idiopathic normal pressure hydrocephalus (iNPH), Alzheimer’s disease (AD), biomarker, saccadic eye movements, eye tracking (ET), King-Devick (KD)
Recent studies demonstrate that several age-related progressive neurodegenerative diseases can be detected by monitoring patients’ eye movements.1,2 Typical reported changes include unintentional eye movements, decreased amplitude of saccades, and increased number of fixation points compared with age-matched healthy persons.3–6 Fixation means focusing the visual gaze on a stationary object, with the intent of keeping the object in the fovea.7 Saccadic eye movements are rapid jumps of the gaze from one point of fixation to another, during which no visual observations can be made.8 Saccades can be either reflexive or voluntary, with the latter including predictive saccades, antisaccades, and memory-guided saccades.8 Changes in unintentional eye movements are also related to decreased reading speed or increased number of reading errors during a reading task.2,4 Changes in all of these functions have been observed in patients with Alzheimer’s disease (AD),3–5 even in its prodromal stage,1,6,9,10 compared with in cognitively unimpaired persons.
There is a need for new easy-to-use and specific biomarkers for neurodegenerative disorders to help clinicians make a diagnosis as early as possible, and for accurate differential diagnostics. AD is a typical neurodegenerative disorder in which it is desirable to reach a diagnosis before the disease has progressed to involve massive neuronal loss of the brain, to enable early initiation of targeted medication and nonpharmacological interventions.11 However, it can be challenging to distinguish specific neurodegenerative disorders from each other in the early disease stages.12
Idiopathic normal pressure hydrocephalus (iNPH) is a progressive neurodegenerative disease with characteristic symptoms of gait disturbances, cognitive decline, and urinary incontinence.13,14 Differential diagnostics of iNPH with other neurodegenerative disorders may be challenging in the absence of typical iNPH-specific symptoms and findings, especially when persons with iNPH exhibit AD-like neuropathology.15,16
The King-Devick (KD) test is a relatively short, rapid number naming assessment, which involves measurements of eye movements, attention, and language. The test was originally developed to detect reading problems in children, but its use has expanded, mostly in research involving the detection of concussions, Parkinson’s disease (PD), multiple sclerosis and, most recently, AD.17
Eye tracking (ET) is a useful tool for detecting saccadic eye movements during a reading task. The control of saccades decreases with age.18 ET recordings have been used in research to find differences in oculomotor activity between cognitively unimpaired persons and patients with AD dementia.2,5 In addition to AD, ET technology has been used to screen for cognitive dysfunction in other neurological disorders, such as PD, amyotrophic lateral sclerosis, MS, and epilepsy.19
The combination of the KD test and ET constitutes an easy-to-use test battery, and a potential new biomarker for detecting a person with cognitive decline or to differentiate memory disorders. The current literature lacks reports of possible reading or eye movement changes in persons with iNPH. As iNPH is a neurodegenerative disorder, we hypothesize that affected persons may experience problems relating to unintentional eye movements and reading.
In the present study, we investigated whether it was possible to distinguish cognitively unimpaired controls, persons with iNPH or AD from each other based on changes in eye movements and reading problems as determined using computer-based ET recordings.
METHODS
Ethics Statement
This study adhered to the principles of the Declaration of Helsinki, and was evaluated by the Kuopio University Hospital Ethical Committee (Dnro: 482/2017 and 276/2016). All study participants read the information letter and signed an informed consent before the start of the study. Proxy consent was required from participants in the dementia stage of AD.
Study Design, Participants, and Study Protocol
For this study, we recruited a total of 68 volunteers, including 37 cognitively unimpaired persons [(control (CO) group], 21 persons with very mild or mild AD [Clinical Dementia Rating (CDR) =0.5 to 1.0; AD group], and 10 persons with iNPH (iNPH group) without other neurodegenerative disorders. The CO and AD groups were recruited from the Brain Research Unit, University of Eastern Finland (UEF). The iNPH group was recruited from the Kuopio NPH-registry (http://www.uef.fi/nph), which included 764 consecutive iNPH patients by the end of 2020.
All iNPH diagnoses were clinically evaluated by a neurosurgeon based on the ICD-10 criteria, including magnetic resonance imaging (MRI) brain scan.13,14 All persons with iNPH had a shunt and had no AD-related pathology detected in the right frontal cortical biopsy taken during shunt surgery. The persons with AD were diagnosed by neurologists, and all underwent a brain MRI or computed tomography (CT) scan, differential diagnostic laboratory tests, and a neuropsychological test battery. The revised National Institute on Aging and Alzheimer’s Association (NIA/AA) criteria were used.20 All persons with AD were treated according to standard clinical practices, and appropriate AD medication was initiated. Persons were accepted to the CO group if they performed all cognitive tests within normal limits, and exhibited no anamnestic cognitive decline or decline in daily functions based on the demographic and CDR interview.
All the study participants underwent clinical examination by an ophthalmologist, a demographic interview, and a neurological status examination. In addition, a Consortium to Establish a Registry for Alzheimer´s Disease neuropsychological test battery (CERAD-NB) was performed.21 Disease severity was assessed using the CDR global score (CDR global score).20 All study participants gave venous blood samples for detection of ApoE ε4 alleles.
All the participants performed the KD test. ET recordings was used to measure saccadic eye movements and fixations.
CERAD-NB
The CERAD-NB21 was used to evaluate cognition. The Finnish version of the CERAD-NB includes all subtests from the original English test battery: the Boston Naming Test (15-item version; range 0 to 15), category fluency (animals; range from 0 to no limit), Mini-Mental State Examination (MMSE, range 0 to 30), wordlist learning (range 0 to 30), wordlist recall (range 0 to 10), wordlist recognition (range 0 to 20), and constructional praxis (range 0 to 11). In addition, the clock drawing test (range 0 to 6) and constructional praxis delayed recall test (range 0 to 11) were added to the Finnish version.22
ApoE Genotyping
From venous blood samples, genomic DNA was extracted using the QIAamp DNA blood mini extraction kit (QIAGEN). ApoE gene alleles were determined using TaqMan genotyping assays [Applied Biosystems (ABI), Foster City, CA] for 2 single nucleotide polymorphisms (rs429358 and rs7412), and an allelic discrimination method on the ABI 7000 platform.23
KD
We used the standardized KD test to detect possible reading problems among the study participants.17 The KD test (1 to 2 min) includes 3 short reading tasks where a participant reads the items (numbers) as fast as possible from cue cards. The tasks gradually become more demanding, and the items more difficult to visually follow correctly. The total reading time (in seconds, s) and the number of errors during the task are counted.
ET Recordings and Apparatus
ET recordings were generated using a Windows PC with an USB web camera, microphone, and a Tobii TX300 display with an integrated ET unit from Tobii Technology AB, Sweden.24 The ET data were recorded in authentic hospital settings with fluorescent lighting and daylight in the room. All participants were seated 60 cm from the screen. Internal movement compensation by the Tobii TX300 unit was used to ensure data accuracy in the event of small head movements. The unit was calibrated for each participant using the regular 9-point calibration with the medium speed preset. In addition to ET data, the participants were audio and video recorded using Tobii Studio 3.2.1 software.25
Data Processing
ET results were obtained by exporting the recording in Tobii Studio 3.4.8 using the I-VT filter with the following standard default values: max gap length interpolation =75 ms, window length =20 ms, velocity threshold =30 deg/s, merging adjacent fixations between 75 ms and 0.5 deg, and discarding fixations <60 ms.26 Next, the exported data were further processed using custom Python 3.6.9 scripts, where task-specific medians were calculated for each participant for further eye-tracking analysis. The timing of the ET test slides was also used to calculate the total time used for KD test per participant.
Audio recordings of tests were processed using the Aalto-ASR 1.1 module on CSC’s Taito supercluster.27 The resulting TextGrid files were then manually quality controlled in Elan 5.2, where erroneous words were tagged and necessary timing corrections were made. Next, the processed TextGrid files were utilized in custom Python 3.6.9 scripts to automatically calculate the number of errors made by each participant in the KD test.
Statistical Analyses
The demographic data and CERAD-NB subtests were analyzed using the IBM SPSS Statistics for Mac (Version 27.0; IBM, Armonk, NY). For categorical variables (sex and ApoE ε4 carrier), differences between the groups were analyzed using the χ2 test or Fisher exact test, when applicable. A 1-way ANOVA was used for a multiple comparison for continuous variables (age, education, and CERAD-NB subtest results), and Bonferroni correction was used for post hoc comparisons between the groups.
Data points for both eye-tracking and audio-based analysis were collated and processed using custom-made Python 3.6.9 scripts. For each participant, we calculated KD task-specific eye-tracking medians for fixation duration, saccade duration, and saccade amplitudes, as explained above. We preferred to use the medians of each participant’s performances (rather than means) to compensate for the interindividual variability in eye-tracking data quality (ie, sample success rate), as medians would not be affected by abnormal artificial outliers the same way as means would. The annotated audio transcripts were utilized for automated calculation of errors made during the KD test. The data were then imported to IBM SPSS Statistics 27 for statistical analysis. Between-group differences are presented in the results as means calculated based on each participant’s median performances (3 performances per participant). The data were evaluated with 1-way analysis of variance (ANOVA) followed by Bonferroni correction.
Before the study was conducted, we performed power calculations based on the study by Galetta et al17 With a power of 0.80, P=0.05, and a 3:1 enrollment ratio, the total sample size required was determined to be 44.
RESULTS
Table 1 presents the demographic characteristics of the study participants and the results of the CERAD-NB subtests. The CO and iNPH groups significantly differed in seven CERAD subtests (P <0.02), and the CO and AD groups significantly differed in 9 subtests (P≤0.02). In addition, compared with the AD group, the iNPH group performed significantly better on 3 subtests: wordlist learning, delayed constructional praxis, and global memory scores (P<0.05) (Table 1).
TABLE 1.
Demographic Characteristics of the Study Participants and the CERAD-NB Results
| CO | iNPH | AD | P CO-iNPH | P CO-AD | P iNPH-AD | |
|---|---|---|---|---|---|---|
| N | 37 | 10 | 21 | |||
| Demographics | ||||||
| Age in years | 71.0 (5.1) | 75.9 (5.7) | 71.1 (6.9) | 0.061 | 1.000 | 0.098 |
| Women | 20 (54.1) | 7 (70.0) | 13 (61.9) | 0.481 | 0.594 | 0.712 |
| Education in years | 12.6 (4.3) | 10.0 (3.5) | 12.5 (4.2) | 0.261 | 1.000 | 0.354 |
| ApoE ε4 carrier | 14 (37.8) | 0 (0.0) | 15 (78.9) | 0.022 | 0.005 | <0.001 |
| CERAD-NB (maximum score) | ||||||
| Verbal fluency (animals) | 25.1 (7.3) | 15.5 (5.2) | 15.9 (6.3) | <0.001 | <0.001 | 1.000 |
| Boston Naming Test (15) | 13.4 (1.7) | 12.7 (1.9) | 11.1 (3.3) | 1.000 | 0.002 | 1.000 |
| MMSE (30) | 28.4 (1.5) | 24.7 (3.6) | 23.9 (3.0) | <0.001 | <0.001 | 1.000 |
| Wordlist learning (30) | 23.1 (3.1) | 15.9 (2.0) | 13.3 (2.2) | <0.001 | <0.001 | 0.043 |
| Wordlist savings (%) | 95.1 (10.6) | 56.3 (24.5) | 38.6 (29.9) | <0.001 | <0.001 | 0.099 |
| Wordlist recognition (%) | 98.2 (3.6) | 83.5 (21.5) | 74.8 (11.6) | <0.001 | <0.001 | 0.106 |
| Constructional praxis (11) | 10.5 (1.0) | 9.2 (1.8) | 9.4 (2.7) | 0.133 | 0.092 | 1.000 |
| Constr. praxis savings (%) | 95.1 (10.1) | 87.8 (26.1) | 60.8 (39.3) | 1.000 | <0.001 | 0.020 |
| Clock drawing (6) | 5.5 (0.6) | 4.4 (1.2) | 4.2 (1.6) | 0.013 | <0.001 | 1.000 |
| Global Memory Score (30) | 27.8 (1.8) | 21.8 (2.7) | 16.9 (3.3) | <0.001 | <0.001 | <0.001 |
Values are presented as mean and SD, except for sex and ApoE ε4 carrier status, which are shown as number and percentage of the participants.
Bold P values indicate significant differences between groups (P ≤ 0.05).
AD indicates Alzheimer’s disease; CERAD-NB, the Consortium to Establish a Registry for Alzheimer’s disease neuropsychological test battery; CO, control; iNPH, idiopathic normal pressure hydrocephalus; MMSE, Mini-Mental State Examination; N, number of participants.
KD Test
The total time used and total number of reading errors during the KD test are summarized in Table 2 and visualized in Fig. 1A, B. The CO group performed the test significantly faster (mean 69.3 s) than the iNPH group (mean 87.3 s; P≤0.009) and the AD group (mean 82.7 s; P≤0.016) (Table 2). The mean reading speed did not significantly differ between the iNPH and AD groups. The within-group variability in median reading speed (between the fastest and the slowest readers) was highest in the AD group (median time 83.6 s, range 48.1 to 123.8 s), followed by the CO group (median time 65.6 s, range 48.6 to9 107.8), and lowest in the iNPH group (median time 77.4 s, range 67.7 to 125.9) (Fig. 1A).
TABLE 2.
Results of the King-Devick Test and Simultaneous Eye-tracking Recording
| CO | iNPH | AD | P CO-iNPH | P CO-AD | P iNPH-AD | |
|---|---|---|---|---|---|---|
| King-Devick test | ||||||
| N | 34 | 10 | 19 | |||
| Total time, s | 69.3 (14.2) | 87.3 (21.1) | 82.7 (16.5) | 0.009 | 0.016 | 1.000 |
| Total number of errors | 0.62 (1.8) | 0.70 (0.9) | 2.74 (3.6) | 1.000 | 0.010 | 0.106 |
| Eye-tracking results | ||||||
| N | 34 | 9 | 16 | |||
| Fixation duration, ms | 248.9 (47.0) | 230.5 (44.7) | 241.0 (44.5) | 0.199 | 0.991 | 1.000 |
| Saccade duration, ms | 27.1 (4.3) | 25.0 (6.1) | 24.8 (4.4) | 0.111 | 0.016 | 1.000 |
| Saccade amplitude, deg | 3.6 (0.6) | 3.2 (0.6) | 3.2 (0.6) | 0.031 | 0.001 | 1.000 |
Values are presented as mean and SD.
Bold P values indicate significant differences between groups (P≤0.05).
AD indicates Alzheimer’s disease; CO, control; deg, degrees; iNPH, idiopathic normal pressure hydrocephalus; ms, milliseconds; N, number of participants, s, seconds.
FIGURE 1.
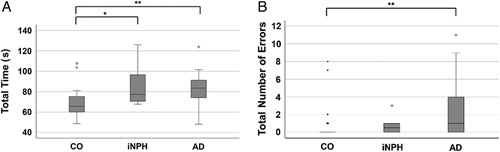
King-Devick test results presented as box plots. A, Total time used for the test in seconds. B, Number of errors made during the test. AD indicates Alzheimer’s disease; CO, control; iNPH, idiopathic normal pressure hydrocephalus; s, seconds. *P≤0.05, **P≤0.01, ***P≤0.001.
The mean number of reading errors (±SD) was significantly higher in the AD group than the CO group (2.74 ± 3.6 vs. 0.62 ± 1.8, respectively; P≤0.01). The mean number of reading errors (±SD) did not significantly differ between the iNPH group (mean 0.70 ± 0.9) and the CO group, or between the iNPH and the AD groups (Table 2). The within-group variability in number of reading errors was highest in the AD group (range 0 to 11), followed by the CO group (range 0 to 8), and lowest in the iNPH group (range 0 to 3). In all study groups, some participants performed the test without mistakes (Fig. 1B).
ET Recordings
The findings regarding fixation and saccade duration (ms) and saccade amplitude (degrees) in the ET recordings are presented in Table 2 and illustrated in Fig. 2A, B. As we expected, the mean saccade duration was longer in the CO group (27.1 ± 4.3 ms) than in the iNPH group (25.0 ± 6.1 ms) and AD group (24.8 ± 4.4 ms). The difference between the CO and AD groups was significant (P≤0.016). The range of saccade duration was 13.0 to 33.0 ms in the CO group, 17.0 to 37.0 ms in the iNPH group, and 17.0– to 38.5 ms in the AD group (Fig. 2A).
FIGURE 2.
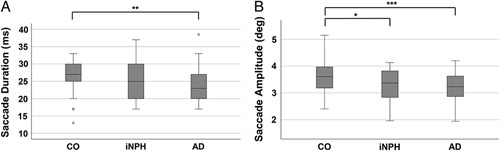
Eye-tracking results as box plots. A, Median saccade durations in milliseconds. B, Median saccade amplitudes in degrees. AD indicates Alzheimer’s disease; CO, control; deg, degrees; iNPH, idiopathic normal pressure hydrocephalus; ms, milliseconds. *P≤0.05, **P≤0.01, ***P≤0.001.
The mean saccade amplitude (±SD) was 3.6 ± 0.6 degrees in the CO group, 3.2 ± 0.6 degrees in the iNPH group, and 3.2 ± 06 degrees in the AD group. Saccade amplitudes were significantly lower in the iNPH group (P≤0.031) and AD group (P≤0.001) compared with the CO group (Fig. 2B). The iNPH and AD group did not significantly differ from each other.
The duration of fixation time (ms) was highest in the CO group and, was only slightly lower in the iNPH and AD groups. We found no statistically significant differences between any of the groups. Within-group variability in fixation duration was highest in the CO group and lowest in the iNPH group (Table 2).
DISCUSSION
To our knowledge, this is the first study that has used computer-based ET during the KD test to examine the reading ability and eye movements in persons with iNPH compared with persons with AD and cognitively unimpaired controls. The reading speed and accuracy, as well as eye saccades and fixations during the task, differed between the iNPH and CO groups, supporting our hypothesis, and were parallel between the iNPH and AD groups.
KD Test Results
The literature describes slower reading speeds in AD,4,5,9 but lacks reported reading speeds in persons with iNPH. Here, for the first time, we report altered reading performance of persons with iNPH. Reading speed was similarly decreased in iNPH as in persons with AD. These results indicate the potential usefulness of the KD reading task and ET recordings to differentiate persons with iNPH or AD from cognitively unimpaired individuals.
Although mean reading speeds were statistically similar between the AD and iNPH groups, the reading speed variation was greater in the AD group than in the iNPH group. Moreover, the AD group made more errors than the CO or iNPH groups, and the reading error variability was highest in the AD group and lowest in the iNPH group.
There are no previous results to compare with our present findings in the iNPH group. However, it has been reported that persons with AD show greater variability in raw reading speeds and make more errors than, for example, persons with mild cognitive impairment or controls.2,4,17 Persons with Lewy Body dementia are reportedly slower readers than persons with AD.28 Therefore, it would be interesting to further investigate differences between persons with iNPH and other neurodegenerative disorders.
ET Recordings
Age-related alterations of the frontal cortex are thought to be associated with the deterioration of saccade generation and saccadic eye movements in aging.18 We may assume that the change in saccadic eye movements is even more profound in neurodegenerative disorders. In the presently obtained ET recordings, the iNPH and AD groups showed similarly decreased mean saccade duration. Shorter saccade durations in AD have been previously reported.2,5 Notably, in our study, the within-group variability in saccade duration was lower in the iNPH group compared with the AD group. Furthermore, saccade amplitudes were similarly decreased in the iNPH and AD groups.
Fixation durations did not differ between the study groups, although the mean time was slightly longer in the CO group. In contrast, most previous studies have shown a longer mean fixation duration in persons with AD compared with cognitively unimpaired controls.29 This may be related to the study group selection. In our study, all of the persons with AD or iNPH had a disease stage involving very mild or mild dementia, and their performance, for example, in MMSE was better than in many previously published studies focusing on differences between AD and controls.29 Interestingly, the variability in fixation duration was lowest in the iNPH group. No previous studies have reported fixation duration in iNPH, and this finding must be further studied in a larger group of persons with iNPH.
The CERAD-NB was used to assess the participants’ cognitive performance and to form a CO group of cognitively healthy individuals. Persons with iNPH and AD were clearly different from controls and, given the sample size, the cognitive profile was parallel to previous research evidence.30 ApoE ε4 is a known genetic risk factor for AD,31 but is not associated with iNPH.32 The groups in our study had different ApoE ε4 carrier profiles, supporting the successful patient selection. ApoE ε4 carriers constituted 79% of the AD group, and 38% of the CO group, whereas there were no ApoE ε4 carriers in the iNPH group.
In general, we found that, in line with AD group, the iNPH group showed impaired results on the reading tests and in saccade generation. Persons with AD develop progressive attentional, visuoperceptual, language, and oculomotor alterations that may impact their reading ability.2 Certain saccade pursuit errors and horizontal prosaccade latency are thought to be linked to posterior brain regions that typically show degeneration in AD.33 Theoretically, the degenerative process related to parietal and posterior temporal regions in AD may explain the impaired oculomotor movements that are also associated with visuospatial function.33 We may speculate that these changes could also explain the changes observed in iNPH. Psychomotor slowing and attention impairment play a greater role in iNPH than in AD,34 and are also observed in other functions, such as gait.35 Therefore, it would be reasonable to investigate differences between iNPH and AD by further examining the reading ability and oculomotor function.
Strengths and Limitations
The sample size for this study was calculated based on a previous study in which the KD test was able to distinguish persons with AD from cognitively healthy controls.17 We hypothesized that the effect size would be approximately the same in persons with iNPH due to cognitive characteristics of the disease, which proved to be correct. On the basis of eye movements, our test arrangement was able to distinguish both neurodegenerative diseases, iNPH and AD, from controls, but not from each other. This results may have been influenced by the small sizes of 2 disease groups, if the statistical power of our study was not sufficient to make this distinction. One strength of our study was the strict exclusion criteria. Various eye diseases that impair vision and cause reading difficulties become more common with aging.36 Each participant in our study underwent clinical examination by an ophthalmologist, to avoid errors in the results caused by decreased visual acuity. In addition, this study excluded persons having any brain disorders other than iNPH or AD. Participants underwent thorough assessment of cognitive level, along with daily functioning, to ensure comprehension and cooperation before the KD test.
Implications and Future Studies
INPH and AD are progressive neurodegenerative disorders in which early diagnosis is crucial for initiating early treatment and maintaining affected persons’ quality of life.37–39 Our present results, together with previous evidence, suggest that the KD test combined with a computer-based ET device could be a potential tool for diagnosis of neurodegenerative diseases. It is even possible that studying changes in these tests could facilitate the identification of individuals who are at risk of developing AD.1,3,6,10 Because of its ease of use and cost-effectiveness, this type of testing could become a valuable tool for primary health care units, for identification of neurodegenerative diseases. Further studies with larger sample size are needed to confirm our findings in iNPH. It would also be interesting to further investigate which reading or oculomotor function tests can distinguish iNPH from AD.
CONCLUSIONS
In this study, we were able to distinguish the iNPH group from cognitively unimpaired persons by using the KD test and ET recordings. The iNPH patient group significantly differed from cognitively unimpaired persons in terms of reading ability and oculomotor functions, and showed performance similar to the AD group on several tests. We demonstrated that iNPH was associated with decreased reading speed, saccade durations, and saccade amplitudes.
ACKNOWLEDGMENTS
The authors thank all study participants, statistician Tuomas Selander and study nurses Ulla Vanhanen, Kati Mönttinen, Kristiina Holopainen, Tarja Lappalainen, and Marita Parviainen. The authors are grateful to the Juho and Lempi Pitkänen foundation, the Maire Taponen Foundation, Maud Kuistila Foundation, Finnish Cultural Foundation, Finnish Brain Foundation and Finnish Government Research Funding for financial support.
Footnotes
J.M.L. and V.K. contributed equally and shared first authorship.
Study design: A.M.K., L.H., M.H., T.H., V.L., K.K., and R.B. Data collection: J.M.L., S.H., S.A., and V.Ko. Data analysis: J.M.L., S.A., and V.K. Monitoring of the data collection and confirmation of diagnoses: A.M.K., M.H., and V.L. Interpretation of study analysis: A.M.K., L.H., T.H., M.H., K.K., R.B., and V.L. Manuscript drafting: J.M.L. and V.K., A.M.K. supported the manuscript drafting as supervisor, and S.A. and T.S. supported the writing of the Methods and Results sections. A.M.K. is the primary investigator of the study. Project administration: A.M.K., M.H., and V.K. Funding acquisition A.M.K., M.H., and V.K.
The authors declare no conflicts of interest.
Contributor Information
Juha-Matti Lehtola, Email: juha-matti.Lehtola@uef.fi.
Virve Kärkkäinen, Email: virve.karkkainen@kuh.fi.
Sami Andberg, Email: sami.andberg@uef.fi.
Sanna Hannonen, Email: sanna.hannonen@kuh.fi.
Minna Rusanen, Email: minna.rusanen@kuh.fi.
Toni Saari, Email: toni.saari@uef.fi.
Ville Korhonen, Email: villekor91@gmail.com.
Laura Hokkanen, Email: laura.hokkanen@helsinki.fi.
Merja Hallikainen, Email: merja.hallikainen@uef.fi.
Tuomo Hänninen, Email: tuomo.hanninen@kuh.fi.
Kai Kaarniranta, Email: kai.kaarniranta@kuh.fi.
Roman Bednarik, Email: roman.bednarik@uef.fi.
Ville Leinonen, Email: ville.leinonen@kuh.fi.
Anne M. Koivisto, Email: anne.koivisto@kuh.fi.
REFERENCES
- 1. Wilcockson TDW, Mardanbegi D, Xia B, et al. Abnormalities of saccadic eye movements in dementia due to Alzheimer’s disease and mild cognitive impairment. Aging (Albany NY). 2019;11:5389–5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fernandez G, Manes F, Politi LE, et al. Patients with mild Alzheimer’s disease fail when using their working memory: evidence from the eye tracking technique. J Alzheimers Dis. 2016;50:827–838. [DOI] [PubMed] [Google Scholar]
- 3. Molitor RJ, Ko PC, Ally BA. Eye movements in Alzheimer’s disease. J Alzheimers Dis. 2015;44:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lueck KL, Mendez MF, Perryman KM. Eye movement abnormalities during reading in patients with Alzheimer disease. Neuropsychiatry Neuropsychol Behav Neurol. 2000;13:77–82. [PubMed] [Google Scholar]
- 5. Fernandez G, Mandolesi P, Rotstein NP, et al. Eye movement alterations during reading in patients with early Alzheimer disease. Invest Ophthalmol Vis Sci. 2013;54:8345–8352. [DOI] [PubMed] [Google Scholar]
- 6. Holden JG, Cosnard A, Laurens B, et al. Prodromal Alzheimer’s disease demonstrates increased errors at a simple and automated anti-saccade task. J Alzheimers Dis. 2018;65:1209–1223. [DOI] [PubMed] [Google Scholar]
- 7. Krauzlis RJ, Goffart L, Hafed ZM. Neuronal control of fixation and fixational eye movements. Philos Trans R Soc Lond B Biol Sci. 2017;372:20160205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McDowell JE, Dyckman KA, Austin BP, et al. Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain Cogn. 2008;68:255–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heuer HW, Mirsky JB, Kong EL, et al. Antisaccade task reflects cortical involvement in mild cognitive impairment. Neurology. 2013;81:1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pereira MLGF, Camargo MVZA, Bellan AFR, et al. Visual search efficiency in mild cognitive impairment and Alzheimer’s disease: an eye movement study. J Alzheimers Dis. 2020;75:261–275. [DOI] [PubMed] [Google Scholar]
- 11. Porteinsson AP, Isaacson RS, Knox S, et al. Diagnosis of early Alzheimer’s disease: clinical practice in 2021. J Prev Alzheimers Dis. 2021;3:371–386. [DOI] [PubMed] [Google Scholar]
- 12. Simrèn J, Ashton NJ, Blennow K, et al. An update on fluid biomarkers for neurodegenerative diseases: recent success and challenges ahead. Curr Opin Neurobiol. 2020;61:29–39. [DOI] [PubMed] [Google Scholar]
- 13. Relkin N, Marmarou A, Klinge P, et al. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57:S4–S16; discussion ii–v. [DOI] [PubMed] [Google Scholar]
- 14. Mori E, Ishikawa M, Kato T, et al. Guidelines for management of idiopathic normal pressure hydrocephalus: second edition. Neurol Med Chir (Tokyo). 2012;52:775–809. [DOI] [PubMed] [Google Scholar]
- 15. Koivisto AM, Kurki MI, Alafuzoff I, et al. High risk of dementia in ventricular enlargement with normal pressure hydrocephalus related symptoms1. J Alzheimers Dis. 2016;52:497–507. [DOI] [PubMed] [Google Scholar]
- 16. Luikku AJ, Hall A, Nerg O, et al. Predicting development of Alzheimer’s disease in patients with shunted idiopathic normal pressure hydrocephalus. J Alzheimers Dis. 2019;71:1233–1243. [DOI] [PubMed] [Google Scholar]
- 17. Galetta KM, Chapman KR, Essis MD, et al. Screening utility of the King-Devick test in mild cognitive impairment and Alzheimer disease dementia. Alzheimer Dis Assoc Disord. 2017;31:152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peltsch A, Hemraj A, Garcia A, et al. Age-related trends in saccade characteristics among the elderly. Neurobiol Aging. 2011;32:669–679. [DOI] [PubMed] [Google Scholar]
- 19. Tao L, Wang Q, Liu D, et al. Eye tracking metrics to screen and assess cognitive impairment in patients with neurological disorders. Neurol Sci. 2020;41:1697–1704. [DOI] [PubMed] [Google Scholar]
- 20. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. [DOI] [PubMed] [Google Scholar]
- 22. Hänninen T, Pulliainen V, Salo J, et al. Suomen muistitutkimusyksiköiden asiantuntijaryhmä. Kognitiiviset testit muistihäiriöiden ja alkavan dementian varhaisdiagnostiikassa: CERAD-tehtäväsarja. Suom Lääkäril. 1999;54:1967–1975. [Google Scholar]
- 23. De la Vega FM, Lazaruk KD, Rhodes MD, et al. Assessment of two flexible and compatible SNP genotyping platforms: TaqMan SNP Genotyping Assays and the SNPlex Genotyping System. Mutat Res. 2005;573:111–135. [DOI] [PubMed] [Google Scholar]
- 24. Tobii TX300 Eye Tracker. Tobii Technology. August 12, 2013. Available at: https://www.tobiipro.com/siteassets/tobii-pro/brochures/tobii-pro-tx300-brochure.pdf. Accessed February 23, 2022.
- 25. Tobii Studio User’s Manual. editor. Tobii Technology. January 18, 2016. Available at: https://www.tobiipro.com/siteassets/tobii-pro/user-manuals/tobii-pro-studio-user-manual.pdf. Accessed February 23, 2022.
- 26. Olsen A, Matos R. Identifying parameter values for an I-VT fixation filter suitable for handling data sampled with various sampling frequencies. ETRA’, 12 2012:317–320. [Google Scholar]
- 27. Kurimo M: Aalto-yliopiston automaattinen puheentunnistin. 2016. AaltoASR. Available at: https://github.com/aalto-speech/AaltoASR. Accessed February 23, 2022.
- 28. Andersson M, Wiig EH, Minthon L, et al. A Quick Test for Cognitive Speed: a measure of cognitive speed in dementia with Lewy bodies. Am J Alzheimers Dis Other Demen. 2007;22:313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Readman MR, Polden M, Gibbs MC, et al. The potential of naturalistic eye movement tasks in the diagnosis of Alzheimer’s disease: a review. Brain Sci. 2021;11:1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nerg O, Junkkari A, Hallikainen I, et al. The CERAD neuropsychological battery in patients with idiopathic normal pressure hydrocephalus compared with normal population and patients with mild Alzheimer’s disease. J Alzheimers Dis. 2021;81:1117–1130. [DOI] [PubMed] [Google Scholar]
- 31. Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. [DOI] [PubMed] [Google Scholar]
- 32. Pyykko OT, Helisalmi S, Koivisto AM, et al. APOE4 predicts amyloid-β in cortical brain biopsy but not idiopathic normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2012;83:1119–1124. [DOI] [PubMed] [Google Scholar]
- 33. Lage C, Lopez-Garcia S, Bejanin A, et al. Distinctive oculomotor behaviors in Alzheimer’s disease and frontotemporal dementia. Front Aging Neurosci. 2021;12:603790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ogino A, Kazui H, Miyoshi N, et al. Cognitive impairment in patients with idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord. 2006;21:113–119. [DOI] [PubMed] [Google Scholar]
- 35. Sirkka J, Parviainen M, Jyrkkanen HK, et al. Upper limb dysfunction and activities in daily living in idiopathic normal pressure hydrocephalus. Acta Neurochir (Wien). 2021;163:2675–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Akpek EK, Smith RA. Overview of age-related ocular conditions. Am J Manag Care. 2013;19:S67–S75. [PubMed] [Google Scholar]
- 37. Andren K, Wikkelso C, Tisell M, et al. Natural course of idiopathic normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2014;85:806–810. [DOI] [PubMed] [Google Scholar]
- 38. Junkkari A, Sintonen H, Nerg O, et al. Health-related quality of life in patients with idiopathic normal pressure hydrocephalus. Eur J Neurol. 2015;22:1391–1399. [DOI] [PubMed] [Google Scholar]
- 39. Atri A. The Alzheimer’s disease clinical spectrum: diagnosis and management. Med Clin North Am. 2019;103:263–293. [DOI] [PubMed] [Google Scholar]


