Background:
Studies using animal models have shown that cerebral hypoperfusion causes hyperphosphorylation of tau protein, leading to neuronal damage. However, the relationship between hypoperfusion and tau deposition in humans is unclear. Hence, we aimed to determine whether cerebral hypoperfusion leading to decreased blood flow relative to metabolic demand [increased oxygen extraction fraction (OEF), misery perfusion] is associated with increased tau deposition in patients with atherosclerotic internal carotid artery or middle cerebral artery disease.
Methods:
We prospectively evaluated the distribution of tau aggregate deposition using positron emission tomography and 18F-florzolotau (PMPBB3 [1-fluoro-3-((2-((1E,3E)-4-(6-(methylamino)pyridine-3-yl)buta-1,3-dien-1-yl)benzo[d]thiazol-6-yl)oxy)propan-2-ol)]) in 8 patients with atherosclerotic disease of the internal carotid artery or middle cerebral artery. The standardized uptake value ratio of 18F-florzolotau at 100 to 110 minutes after injection was calculated using the cerebellar cortex as a reference region and was correlated with OEF obtained from 15O-gas positron emission tomography in the middle cerebral artery distributions.
Results:
Significant decreases in cerebral blood flow and cerebral metabolic rate of oxygen and increases in OEF were found in the hemisphere ipsilateral to the arterial lesion. 18F-florzolotau standardized uptake value ratio in this region was also greater than that in the contralateral hemisphere. In the ipsilateral hemisphere, 18F-florzolotau standardized uptake value ratio positively correlated with OEF values.
Conclusions:
This pilot study with a small sample size suggests that increases in OEF–misery perfusion–may be associated with increased tau aggregates deposition in atherosclerotic internal carotid artery or middle cerebral artery disease.
Keywords: cerebrovascular disorders, perfusion, positron emission tomography, tau protein
See related article, p e504
Studies using animal models have shown that cerebral ischemia causes hyperphosphorylation of tau protein.1 Tau hyperphosphorylation causes the dissociation of tau from microtubules and increases the propensity for self-oligomerization and fibril formation, which may lead to neuronal degeneration in the course of neurofibrillary tangle formation.1 However, the relationship between cerebral hypoperfusion and tau deposition in humans is unclear.
Recently, the development of tau ligands for positron emission tomography (PET) has enabled the in vivo visualization of tau aggregate deposition in humans. However, to the best of our knowledge, no studies have demonstrated the association of cerebral hypoperfusion with tau deposition. 18F-florzolotau, a second-generation tau ligand, has recently become available for tau imaging.2
In this study, we evaluated the degree of cerebral hypoperfusion and tau deposition in patients with atherosclerotic internal carotid artery or middle cerebral artery (MCA) disease using 15O-gas PET and 18F-florzolotau PET to determine whether an increased oxygen extraction fraction (OEF)–misery perfusion3–is associated with an increase in tau aggregate deposition.
Methods
Detailed methods are available in the Supplemental Material. This article follows the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting guideline (https:www.goodreports.org). Additional data can be made available via the corresponding author to qualified researchers upon reasonable request.
Subjects
We studied 8 male patients aged 69±6 years (mean±SD) with symptomatic atherosclerotic occlusion or stenosis of the internal carotid artery or MCA within an 18-month period (Table S1). We also studied the 10 healthy controls (5 men and 5 women) aged 55±11 years.
All protocols were approved by the Shiga General Hospital Institutional Review Board and the Human Study Committee (approval number: 20201020-01). All the participants provided written informed consent.
Positron Emission Tomography
We performed PET scans using a whole-body PET/computed tomography scanner, the Siemens True Point Biograph 16 (Siemens/CTI, Erlangen, Germany).4
Patients received 18F-florzolotau by slow intravenous injection.2 A 10-minute static PET acquisition was performed 100 minutes after injections. The standardized uptake value (SUV) for 18F-florzolotau was calculated as follows: SUV=C (kBq/mL)/ID (kBq)/body weight (g), where C represents the tissue activity concentration measured by PET and ID is the injected dose.
15O-gas experiments were performed the day after the 18F-florzolotau study.5 We calculated the cerebral blood flow (CBF), cerebral metabolic rate of oxygen (CMRO2), and OEF using the steady-state method.5
Data Analysis
For 18F-florzolotau PET scanning analysis, we employed a template-based predefined region of interest (ROI) approach using an in-house computed tomography template.4 The SUV ratio (SUVR) of each region that indicates tau deposition was calculated as follows: SUVR=SUV brain/SUV cerebellar cortex.2
To obtain quantitative regional SUVR values, we performed automated ROI analyses. The automated anatomical labeling atlas was used for the template-based predefined ROIs.6
The mean MCA values were calculated as the average of the SUVR value of the anatomical labeling atlas ROIs for the cerebral cortex within the MCA distribution. The ROIs including cerebral infarction were excluded from the analysis.
Statistical Analysis
PET variable values between the 2 hemispheres were compared using Wilcoxon signed-rank tests. The relationships between the 2 variables were analyzed using Spearman correlation analysis. Multiple linear regression analysis (forward stepwise selection) was used to assess the independent predictive value of the CBF, CMRO2, and OEF with respect to the 18F-florzolotau SUVR. Statistical significance was set at P<0.05.
Results
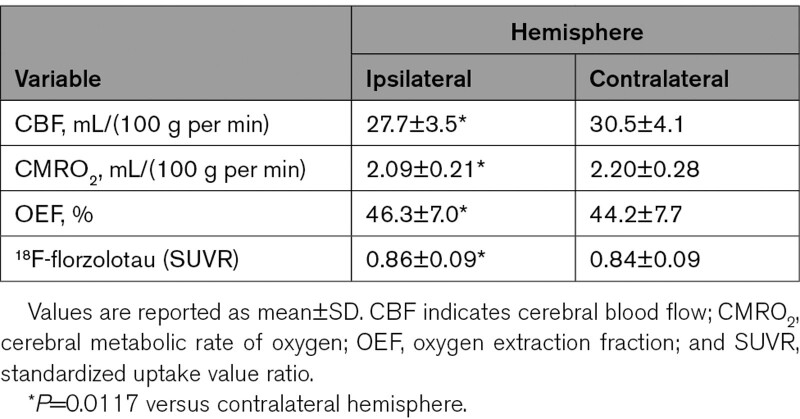
Significant decreases in CBF and CMRO2 along with increases in OEF were found in the hemisphere ipsilateral to the arterial lesion compared with the contralateral hemisphere. Also, 18F-florzolotau SUVR values were higher in this region than those in the contralateral hemisphere (Table).
Table.
Positron Emission Tomography Values in the Hemisphere Ipsilateral and Contralateral to the Diseased Artery
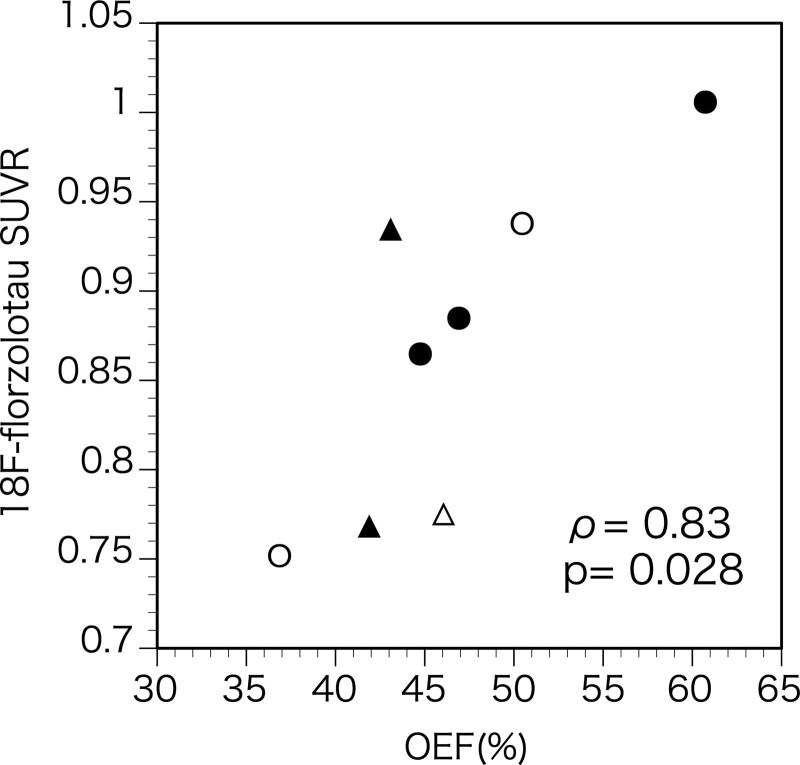
The 18F-florzolotau SUVR was positively correlated with OEF (ρ=0.83, P=0.027; Figures 1 and 2). One patient with the highest OEF value exhibited an increased SUVR value beyond the control range. Six patients exhibited increased SUVR ratios (ipsilateral to contralateral ratio) beyond the control range (median, 1.016, range, 1.0–1.121).
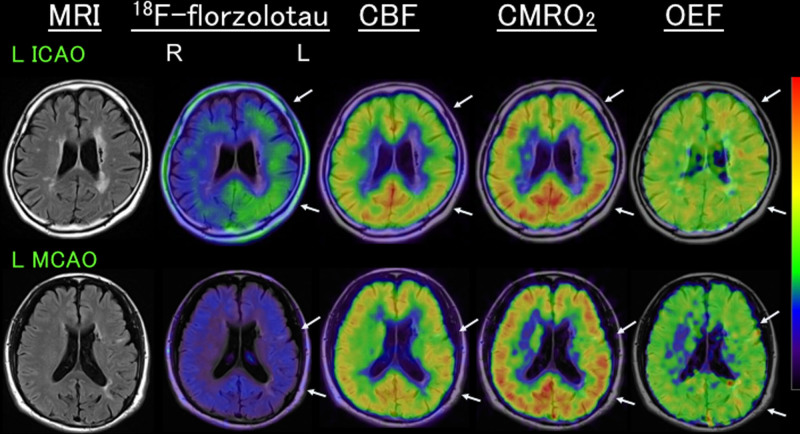
Figure 1.
Representative positron emission tomography images. Upper, Increased 18F-florzolotau standardized uptake value ratio (SUVR; 0.94, ipsilateral/contralateral, 1.12), with decreased cerebral blood flow (CBF) and increased oxygen extraction fraction (OEF; 50.5%), despite a reduction in cerebral metabolic rate of oxygen (CMRO2), in a patient with left (L) internal carotid artery occlusion (ICAO) (fetal type). Note a relatively lower 18F-florzolotau uptake in the anterior middle cerebral artery (MCA) region with a relatively lower CMRO2. Lower row: a low 18F-florzolotau SUVR (0.77, ipsilateral/contralateral, 1.02) in a patient with LMCA occlusion (O) with mild decreases in both CBF and CMRO2 and normal OEF (46.0%). Arrows indicate the affected regions. Both patients were negative for amyloid positron emission tomography (PET).7 MRI indicates magnetic resonance imaging; and R, right.
Figure 2.
Scatter diagram plotting the 18F-florzolotau standardized uptake value ratio (SUVR) against the value of oxygen extraction fraction (OEF) in the hemisphere with arterial diseases. Open symbols, left diseases; closed symbols, right diseases; circles, carotid artery diseases; triangles, middle cerebral artery diseases.
There was no significant correlation between 18F-florzolotau SUVR and CBF (ρ=−0.21, P=0.57) or CMRO2 (ρ=0.02, P=0.94). However, multiple linear regression analysis created a model including the values of OEF (%; coefficient: 0.012, P<0.005) and CMRO2 (mL/100 g per min; coefficient: 0.207, P<0.05) with a correlation coefficient of 0.92 for the 18F-florzolotau SUVR (P<0.01).
There was no correlation between 18F-florzolotau SUVR and the time elapsed from symptoms (ρ=−0.57, P=0.12) or from the diagnosis of artery diseases (ρ=0.43, P=0.25).
Discussion
To our knowledge, this is the first study showing an increase in tau deposition in patients with misery perfusion caused by atherosclerotic internal carotid artery or MCA disease. Increased 18F-florzolotau SUVR in the noninfarcted cerebral cortex was associated with increased OEF.
Hypoperfusion leading to decreased CBF relative to CMRO2 and, in turn, to an increase in OEF (misery perfusion) may be associated with an increase in neurofibrillary tau deposition. In the hemisphere ipsilateral to the arterial lesion with a reduction in CBF and CMRO2 and an elevation in OEF, 18F-florzolotau SUVR was increased compared with the contralateral hemisphere. The 18F-florzolotau SUVR was positively correlated with OEF, which suggested that misery perfusion, defined as an increased OEF, was associated with increased tau deposition. 18F-florzolotau SUVR was not correlated with CBF. This may be because the decrease in the CBF may reflect not only misery perfusion but also secondary CBF decreases due to the decreased CMRO2 by tissue damage or deafferentation.
The decrease in CMRO2 was correlated with a decrease in 18F-florzolotau SUVR. Misery perfusion may cause cortical neuronal damage, which may be accompanied by decreases in CMRO2.7 As shown in Figure 1, in the hemisphere with increased 18F-florzolotau uptake and OEF, a relatively lower 18F-florzolotau uptake was observed in the anterior MCA region with a relatively lower CMRO2. We speculate that increases in tau deposition due to misery perfusion might not have occurred or might have disappeared in the region with cortical neuronal loss.
An increased 18F-florzolotau SUVR value beyond the control range was found in only one patient, who showed an SUVR value slightly above the reported threshold for healthy controls and patients with Alzheimer disease.2 Although 6 patients exhibited increased SUVR hemispheric ratios, the magnitude of the increases was small. However, the distribution of 18F-florzolotau SUVR values was heterogenous, and the mean MCA values may miss regional increases in SUVR values.
The mechanisms of tau aggregation in the face of hypoperfusion are yet to be elucidated. Chronic hypoperfusion induces tau hyperphosphorylation and deposition.1 The pathological changes of tau may be caused by oxidative stress, autophagy, excitotoxicity, inflammation, endothelium and angiogenesis, and mitochondrial dysfunction, in ischemic stroke.1 Increased tau deposition on PET in this study may reflect other processes than neurodegenerative tauopathy.
We acknowledge some limitations. First, the sample size is small, and the patients included only men and a mixture of internal carotid artery and MCA diseases. Furthermore, patients and controls were not matched or age and sex. Additional studies with a larger number of patients are needed to confirm our results. Second, we did not perform correction of multiple comparisons in the statistical analyses since this was an exploratory study, and the use of multiple regression analysis would be inappropriate due to the small sample size.
Third, the assessment of amyloid uptake would be valuable for the correct interpretation of the results. However, we could assess amyloid uptake only in 2 patients (both negative).
Lastly, we could not correct for partial-volume effects. This effect might lead to the underestimation of 18F-florzolotau SUVR.
Article Information
Acknowledgements
The precursor of florzolotau was provided by APRINOIA Therapeutic, Inc (Tokyo, Japan).
Sources of Funding
This study was funded by Japan Society for the Promotion of Science KAKENHI (Grant Number: 22K07683).
Disclosures
None.
Supplemental Material
Supplemental Methods
Table S1
STROBE Statement
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CBF
- cerebral blood flow
- CBV
- cerebral blood volume
- CMRO2
- cerebral metabolic rate of oxygen
- MCA
- middle cerebral artery
- OEF
- oxygen extraction fraction
- PET
- positron emission tomography
- ROI
- region of interest
- SUVR
- standardized uptake value ratio
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.122.040493.
For Sources of Funding and Disclosures, see page e503.
Contributor Information
Shinya Kagawa, Email: kagawa@res.med.shiga-pref.jp.
Kuninori Kusano, Email: kusano@res.med.shiga-pref.jp.
Miki Ito, Email: m.ito@res.med.shiga-pref.jp.
Chio Okuyama, Email: okuyama@res.med.shiga-pref.jp.
References
- 1.Chen X, Jiang H. Tau as a potential therapeutic target for ischemic stroke. Aging (Albany NY). 2019;11:12827–12843. doi: 10.18632/aging.102547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tagai K, Ono M, Kubota M, Kitamura S, Takahata K, Seki C, Takado Y, Shinotoh H, Sano Y, Yamamoto Y, et al. High-contrast in vivo imaging of tau pathologies in alzheimer’s and non-alzheimer’s disease tauopathies. Neuron. 2021;109:421–58.e8. doi: 10.1016/j.neuron.2020.09.042 [DOI] [PubMed] [Google Scholar]
- 3.Baron JC, Bousser MG, Rey A, Guillard A, Comar D, Castaigne P. Reversal of focal “misery-perfusion syndrome” by extra-intracranial arterial bypass in hemodynamic cerebral ischemia. A case study with 15O positron emission tomography. Stroke. 1981;12:454–459. doi: 10.1161/01.str.12.4.454 [DOI] [PubMed] [Google Scholar]
- 4.Higashi T, Nishii R, Kagawa S, Kishibe Y, Takahashi M, Okina T, Suzuki N, Hasegawa H, Nagahama Y, Ishizu K, et al. (18)F-FPYBF-2, a new F-18-labelled amyloid imaging PET tracer: first experience in 61 volunteers and 55 patients with dementia. Ann Nucl Med. 2018;2018:206–216. doi: 10.1007/s12149-018-1236-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okazawa H, Yamauchi H, Sugimoto K, Takahashi M, Toyoda H, Kishibe Y, Shio H. Quantitative comparison of the bolus and steady-state methods for measurement of cerebral perfusion and oxygen metabolism: Positron emission tomography study using 15O gas and water. J Cereb Blood Flow Metab. 2001;21:793–803. doi: 10.1097/00004647-200107000-00004 [DOI] [PubMed] [Google Scholar]
- 6.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- 7.Baron JC, Yamauchi H, Fujioka M, Endres M. Selective neuronal loss in ischemic stroke and cerebrovascular disease. J Cereb Blood Flow Metab. 2014;34:2–18. doi: 10.1038/jcbfm.2013.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.