Abstract
We examined the immune responses of patients with active pulmonary tuberculosis (TB) and their healthy household contacts to short-term culture filtrate (ST-CF) of Mycobacterium tuberculosis or molecular mass fractions derived from it. Our goal was to identify fractions strongly recognized by donors and differences among the donor groups of possible relevance for vaccine development. The study population consisted of 65 human immunodeficiency virus-negative donors from the Hossana Regional Hospital, Hossana, Ethiopia. Peripheral blood leukocytes from the donors were stimulated with different antigens and immune responses were determined. Household contacts produced significantly higher levels of gamma interferon (IFN-γ) than the TB patients in response to antigens present in ST-CF and the 10 narrow-molecular-mass fractions. A similar difference in leukocyte proliferative responses to the antigens between the two groups was also found. In general, while all fractions stimulated immune responses, the highest activity was seen with the low-molecular-mass fractions, which include well-defined TB antigens such as ESAT-6. Leukocytes from contacts of TB patients with severe disease produced higher levels of antigen-specific IFN-γ than those from contacts of patients with minimal disease. Both groups of contacts exhibited higher cell-mediated responses than the patients themselves. The enhanced immune response of healthy contacts, especially those of patients with severe disease, to secreted mycobacterial antigens is suggestive of an early stage of infection by M. tuberculosis, which could in time result in overt disease or containment of the infection. This possibility is currently being investigated by follow-up studies of the household contacts.
The global burden of disease due to tuberculosis (TB) which is caused by Mycobacterium tuberculosis cannot be overemphasized. Today, we are witnessing a reemergence of TB driven by its association with human immunodeficiency virus (HIV) and AIDS (30), the increase of multidrug-resistant strains of M. tuberculosis (31), and the deterioration of socioeconomic conditions, especially in developing countries. A recent World Health Organization report estimates that about 4 million people will die of TB annually by the year 2005 (7). The widespread use of the Mycobacterium bovis BCG (BCG) vaccine was for many years believed to be the key to the worldwide control of TB, but its efficacy has been questioned after recent trials conducted in developing countries (14). There is therefore an urgent need, particularly in developing countries, for a vaccine with high efficacy to combat the TB epidemic. In recent years, research has focused on antigens released by live M. tuberculosis in culture medium, as these antigens are believed to be at least partially responsible for the efficacy of live vaccines (2, 21). Pools of such extracellular antigens have been tested for their vaccine potential in several laboratories and have been demonstrated to induce substantial levels of protection in animal models (1, 23, 26, 29). However, the composition of culture filtrates varies depending on cultivation time, temperature, shaking of the cultures, etc. (4), and for vaccine development, it is therefore important to identify defined protective antigens which can be produced in a standardized and reproducible manner.
In this study, a novel approach was used, whereby short-term culture filtrate (ST-CF) was separated into nonoverlapping narrow-molecular-mass fractions, and immune responses to these fractions in individuals with different levels of exposure to TB were characterized. The individuals are from Ethiopia, which has a high incidence of the disease (estimated at 100,000 cases annually in a population of approximately 60 million; the incidence is essentially identical in the area where this study was conducted). Since the induction of gamma interferon (IFN-γ) has been shown to be critical for protection against tuberculosis (18, 22) and both animal models (3, 17, 27, 33) and a previous patient study from Denmark (9) have shown that cells from infected individuals respond strongly to the low-molecular-mass fraction of ST-CF by the production of IFN-γ, proliferative and IFN-γ responses were studied in patients with minimal and severe TB as well as their healthy household contacts. The study has revealed a highly heterogeneous pattern of antigen recognition in all groups, with a slight trend towards stronger responses to the low-molecular-mass fractions of ST-CF, in agreement with previous reports (9). In particular, we have observed that leukocytes from healthy household contacts are more reactive to secreted mycobacterial antigens than those from patients. This is especially true of the contacts of patients with symptoms of severe TB, who might be expected to have the highest levels of exposure. While these data, taken together, suggest that many contacts may have active but subclinical infections, a cross-sectional study such as this cannot definitively resolve this issue. However, this study has identified important distinct donor groups which are currently the subject of follow-up studies.
MATERIALS AND METHODS
Donors.
A total of 65 donors were recruited from Hossana Regional Hospital, Hossana, Ethiopia. A chest X ray and the clinical status of each patient were evaluated. The severity of the disease was graded according to the criteria of the National Tuberculosis and Respiratory Disease Association (6). Briefly, patients classified as having severe TB had lesions visible on chest X ray that involved more than one segment of the lung. These patients typically presented with a history of up to 6 months of cough, fever, and cachexia. Patients classified as having minimal TB had only one lesion on X ray that did not involve more than one segment, and they usually had milder symptoms for a shorter period of time than patients with severe disease. Sputum samples were collected from all donors, and bacterial counts were determined by the direct examination of smears by Ziehl-Neelsen staining. Patients were scored thus. (i) Patients with no bacteria in 300 fields were considered negative (these patients were excluded from the study, since they could not be unequivocally defined as TB positive). (ii) Patients with one to two bacteria in 300 fields were scored +/− (these patients were retested, and sputum was cultured for bacterial growth to confirm their TB-positive status). (iii) Patients with one to two bacteria in 100 fields were scored +. (iv) Patients with one to two bacteria in 10 fields were scored ++. (v) Patients with one to two bacteria in one field were scored +++. (vi) Patients with 10 or more bacteria in one field were scored ++++. Patients with severe disease had a higher proportion (P < 0.05) classified as ++ or higher than patients with minmal disease. Infection was confirmed by a positive sputum culture for up to eight weeks in Lowenstein-Jensen medium after decontamination with sodium dodecyl sulfate and neutralization with bromocresol solution. Mantoux tests were not performed, due to the poor specificity of the test in this population and the unwillingness of most donors to remain at the clinic for several days for the test to be read.
Based on the above clinical and radiological presentations, the donors were divided into groups of 30 index cases, made up of 8 patients with minimal TB and 22 patients with severe TB. Each TB patient was matched with a healthy adult household contact (wherever possible, the person in closest contact with the index case, usually the spouse). Contacts were also categorized as minimal (n = 9) or severe (n = 22), based on the severity of the disease in their index cases. Active TB was excluded in all contacts by radiological and clinical examinations, and contacts were excluded if they had a previous history of TB infection. Although no reliable BCG vaccination data is available, vaccination levels are known to be low in the area where the study was conducted. Identification of BCG-vaccinated individuals by the presence of a vaccination scar has proven to be problematic, given the nature of the study population. A total of 13 of the TB patients were female and 17 were male, while 16 contacts were female and 19 were male. Samples were obtained from the patients prior to treatment. Only consenting donors were included in the study population and the study protocol was approved by the Armauer Hansen Research Institute-All Africa Leprosy Rehabilitation and Training Centre Institutional Research Committee, Addis Ababa, Ethiopia. Although the incidence of HIV is low in the study area (less than 5%), all blood samples were subjected to HIV screening and HIV-positive individuals were excluded from the study.
Leukocyte preparation.
Venous blood was drawn into syringes and dispensed into 50-ml centrifuge tubes (Falcon, Lincoln Park, N.J.) containing EDTA. Leukocytes were enriched from the blood samples within 24 h by centrifugation over Ficoll-Hypaque (Pharmacia Biotech), as previously described (10). Cell viability was determined by trypan blue staining before suspension in a freezing mixture containing 10% dimethyl sulfoxide in heat-inactivated fetal calf serum (Sigma) and freezing in liquid nitrogen. Frozen leukocytes were used for all assays. Internal controls to ensure the viability of leukocytes after thawing were included in all assays.
Antigen preparation.
ST-CF and the derived molecular mass fractions were produced as previously described (4). Briefly, M. tuberculosis H37Rv (8 × 106 CFU/ml) was grown in Sauton's medium without Tween 80 on an orbital shaker for 7 days. The culture supernatants were sterile filtered and concentrated with a YM3 membrane (Amicon, Danvers, Mass.). Multiple fractions, designated 1 to 10 in order of increasing molecular mass, were prepared from ST-CF with a whole-gel eluter as previously described (5). Protein concentration was determined by the Micro BCA assay (Pierce, Oud-Beijerland, The Netherlands) and each fraction was used in assays at a concentration of 1 μg/ml. Purified protein derivative (PPD RT23; Statens Seruminstitut, Copenhagen, Denmark) and phytohemagglutinin (Phaseolus vulgaris; Murex, Norcross, United Kingdom) was used at concentrations of 20 and 10 μg/ml, respectively.
Leukocyte cultures.
Frozen cell samples were thawed in a water bath at 37°C and washed three times in RPMI-1640 medium containing 10% fetal calf serum by centrifugation. Cell clumps were disrupted by incubation with 10 μg of RNase-free DNase per ml (Boehringer, Mannheim, Germany), and the cells were again washed, counted, and adjusted to the required density in complete medium (RPMI-1640 supplemented with 5% heat-inactivated pooled human AB serum [Blood Bank, Rigshopitallet, Copenhagen, Denmark], 1% l-glutamine, and 1% penicillin-streptomycin). Triplicate wells of 96-well round-bottomed (for antigen) or flat-bottomed (for mitogen) microtiter plates (Linbro), containing 1.5 × 105 cells per well, were stimulated with either antigen or mitogen in a final volume of 200 μl. Cell density and the concentrations of the mitogen and antigens used had been titrated in preliminary experiments and found to be optimum (data not shown). The plates were incubated at 37°C in a humidified atmosphere of 5% CO2. In experiments designed to study IFN-γ release, 100 μl of the supernatants from the cell cultures was harvested at day 2 (for phytohemagglutinin) or day 5 (for antigen) after stimulation and replaced with an equal volume of fresh complete medium. The supernatants were stored at −80°C until assayed. For proliferation assays, leukocytes were cultured with mitogen or antigen for 3 and 6 days, respectively, before pulsing with 1 μCi of [methyl-3H]thymidine (Amersham) for 18 to 20 h. The cells were thereafter harvested with a Skatron cell harvester (Lierbyen, Norway) onto filter mats, dried, and immersed in scintillation fluid {PPO [2,5-diphenyloxazole], POPOP [1,4-bis(5-phenyloxazolyl)benzene], and toluene}, before reading the incorporation of the radionuclide into the DNA on a beta liquid scintillation counter (1216 Rackbeta 11).
Enzyme-linked immunosorbent assay for IFN-γ.
A double-sandwich enzyme-linked immunosorbent assay was used to quantify the levels of IFN-γ in culture supernatants in duplicate wells, with a commercial kit for the IFN-γ assay, in accordance with the manufacturer's instructions (Mabtech, AB, Nacka, Sweden). Concentrations of IFN-γ in the samples were calculated with the standard curve generated from recombinant IFN-γ (Life Technologies), and results are expressed in picograms per milliliter. The difference between the duplicate wells was consistently less than 10% of the mean.
Statistics.
The data obtained were analyzed with the Mann-Whitney rank-sum test and a one-way analysis of variance of repeated measures.
RESULTS
Immune responses of TB patients and healthy household contacts to ST-CF antigens from M. tuberculosis.
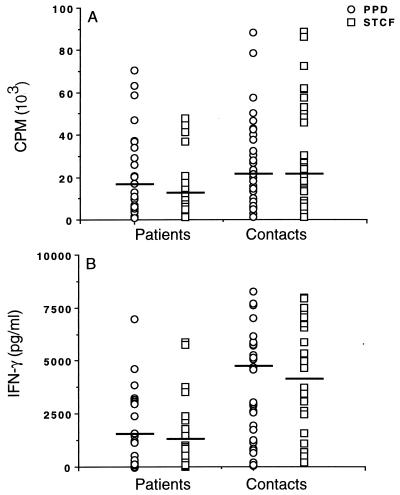
Previous studies have indicated a difference in T-cell responses between patients with minimal and severe TB in countries with a low endemicity of the disease (9). We wished to extend this work by analyzing immune responses in a country where TB is highly endemic. Moreover, it is known that a proportion of the contacts of TB patients will progress to having the disease, so we therefore compared the immune responses of TB patients and their healthy household contacts to ST-CF and PPD. Peripheral blood leukocytes (PBL) from patients and contacts were thus cultured in vitro in the presence of optimum concentrations of the antigens and were examined for proliferation and IFN-γ secretion. Median proliferative responses to both ST-CF and PPD were higher in the contacts than the patients, although the difference was only significant for ST-CF (Fig. 1A) (P < 0.005). This difference was augmented when we examined the levels of IFN-γ produced after stimulation with ST-CF and PPD, where the response was much higher in the contacts than the patients (Fig. 1B) (P < 0.002 for both antigens). No difference between ST-CF and PPD in their ability to stimulate responses from any of the groups tested was found.
FIG. 1.
Individual proliferative responses (A) and IFN-γ levels (B) stimulated by ST-CF or PPD in PBL of TB patients (n = 30) and healthy contacts (n = 35). Median values (solid bars) are indicated.
Immune responses of TB patients with severe or minimal disease and their contacts to antigens from M. tuberculosis.
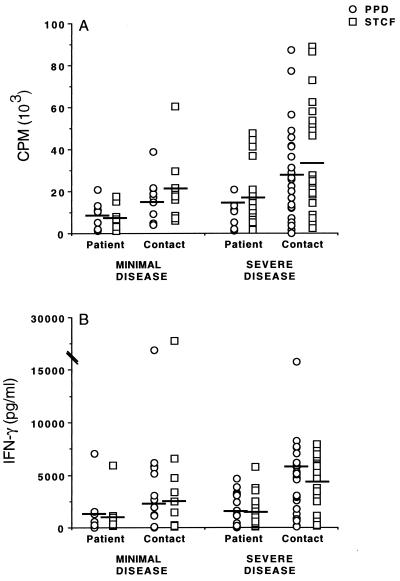
To further characterize responses, we divided patients on the basis of severity of disease and contacts on the basis of disease in their index cases, as outlined in Materials and Methods. In vitro analysis of leukocyte responses from patients revealed no significant differences in proliferation (Fig. 2A) or levels of IFN-γ produced (Fig. 2B) in response to either ST-CF or PPD between groups with different radiological findings. Likewise, in vitro proliferative responses to stimulation with ST-CF or PPD in contacts of patients with minimal or severe disease were not significantly different between the two groups (Fig. 2A). In contrast however, IFN-γ responses were significantly higher among the contacts of patients with severe TB, compared to the contacts of patients with minimal TB (Fig. 2B) (P < 0.05). Since patients with severe disease had, as a group, the highest levels of bacteria in their sputa (data not shown), these data are consistent with the likelihood that their contacts have had a high level of exposure to M. tuberculosis. Although there was no absolute correlation between an index case's sputum counts and a contact's level of response, this may reflect the cross-sectional nature of the study, since the length of exposure in contacts varied dramatically.
FIG. 2.
Individual proliferative responses (A) and IFN-γ levels (B) stimulated by ST-CF or PPD in PBL of TB patients and healthy contacts segregated on the basis of disease. Patients with minimal TB, n = 8; patients with severe TB, n = 22; contacts of patients with minimal TB, n = 9; contacts of patients with severe TB, n = 26. Median values (solid bars) are indicated.
Recognition of narrow-molecular-mass fractions derived from ST-CF.
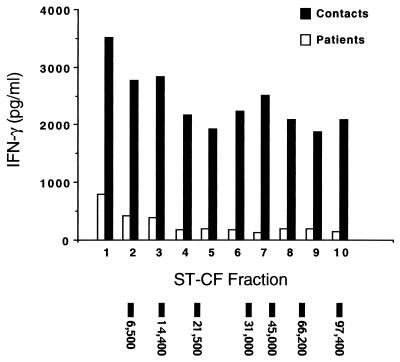
To better characterize the pattern of antigen recognition to ST-CF in contacts and TB patients, responses to individual antigenic fractions were assessed. Multiple fractions of ST-CF were separated on the basis of molecular mass and designated 1 to 10 in order of increasing size (5). These fractions were then used to stimulate PBL from patients and contacts. Results for the fractions are presented as bar graphs (Fig. 3) to simplify comparison between antigenic fractions and patient or contact groups. Values presented are medians, since the data within groups did not approximate normal distributions, consistent with the hypothesis that both groups contained antigen-responsive (potentially infected) and antigen-unresponsive (perhaps uninfected) individuals. As described above for the unfractionated antigen preparations, the IFN-γ response was higher in the group of contacts than in the group of patients—an observation that held true for each of the 10 individual fractions (Fig. 3) (P < 0.05). As observed with the unfractionated ST-CF, no significant difference in response between patients classified as having severe or minimal disease was seen (data not shown).
FIG. 3.
Median IFN-γ levels in response to stimulation with 10 molecular mass fractions derived from ST-CF in PBL of TB patients (open bars; n = 30) or healthy contacts (solid bars; n = 35). The migration of molecular mass markers has been indicated at the bottom of the figure.
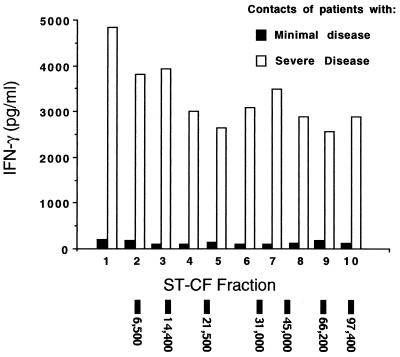
However, when the contact groups were compared on the basis of the severity of the disease to which they had been exposed, it was plain that there were significant differences in the responses observed between the contacts of patients with minimal or severe disease (Fig. 4). Both groups of donors recognized each of the 10 ST-CF fractions by proliferation (data not shown) and production of IFN-γ, although the magnitude of response varied. While it was clear that the contacts of patients with severe disease made both stronger proliferative responses (data not shown) and higher levels of IFN-γ in response to rechallenge with all fractions of ST-CF than did the contacts of patients with minimal disease (Fig. 4) (P < 0.05), the high levels of IFN-γ produced to each fraction by both groups indicated a broad range of antigenic recognition by most of these individuals. Although the difference was not significant, the low-molecular-mass fraction (fraction 1) which contains the strong, defined T-cell antigens ESAT-6 and CFP-10 (8) consistently induced a slightly higher level of IFN-γ than other fractions in all groups tested.
FIG. 4.
Median IFN-γ levels in response to stimulation with 10 molecular mass fractions derived from ST-CF in PBL of healthy contacts, divided on the basis of patients' severity of disease. Patients with minimal TB, n = 8; patients with severe TB, n = 22; contacts of patients with minimal TB, n = 9; contacts of patients with severe TB, n = 26. The migration of molecular mass markers has been indicated at the bottom of the figure.
DISCUSSION
Ideally, an improved vaccine against TB would limit (if not actually prevent) infection, which implies that it should contain antigens that are recognized during the early phases of infection. It has been suggested that secretory antigens are recognized in the first phase of the immune response following tuberculosis infection, prior to the presentation of antigens from dead and degraded mycobacteria, thereby leading to an early macrophage activation and intracellular killing of bacilli (3, 25). We have therefore analyzed the immune responses to ST-CF, a complex mixture of secreted proteins which induces strong production of IFN-γ in different animal TB models and in TB patients on in vitro challenge (1, 9, 23, 26, 29) and which is therefore a potential source for the identification and purification of molecules for the development of a subunit vaccine against TB.
Despite the limited information about surrogate markers of protection in human tuberculosis, recent reports have suggested that activation pathways leading to the generation of IFN-γ are critical for protection against tuberculosis (18, 22). This is in agreement with the murine model of TB, where protection also requires the induction of a Th1-like immune response (3, 12, 16, 24). In the present study, levels of IFN-γ release discriminated between groups of donors with different levels of exposure to disease, implying that multiple fractions of ST-CF contain strongly recognized (and potentially protective) antigens. While in accordance with previous reports (3, 9, 17, 27, 33) we have observed a trend throughout this study toward stronger recognition of the low-molecular-mass fractions of ST-CF which contain proteins such as the strongly recognized antigen ESAT-6 (3, 11, 25, 28); the frequent recognition of each individual fraction by all groups indicates strong responses to multiple antigens present in ST-CF. Thus, these data may not reflect the stronger immunogenicity of individual antigens at the molar level.
Based on the levels of IFN-γ produced, our results show clearly that contacts of TB patients have a greatly enhanced response to a number of antigens present in ST-CF. Both in vitro leukocyte proliferation and IFN-γ secretion were enhanced approximately twofold in household contacts, compared to TB patients; this difference was most marked in those individuals with the highest levels of exposure. The lower response by TB patients is suggestive of a depressed immune status, which may be due to factors such as the differential distribution of antigen-reactive lymphocytes or overproduction of cytokines such as transforming growth factor-β and interleukin-10 (19), a hypothesis which is supported by the observation that mitogen responses were also lower in patients than in contacts (data not shown). We are currently examining blood and pleural fluids from patients and contacts to assess the levels of these cytokines.
An interesting observation made in the course of these studies was that no significant differences in the response to crude antigen preparations were seen when the patients were subdivided on the basis of their radiological findings. While this at first appears to contradict previous results (9), it should be remembered that both this and the previous study are cross-sectional analyses and cannot therefore provide information on the progress of the disease. In countries with low TB endemicity, TB is normally detected at a relatively early stage, which is emphatically not the case in most countries with high rates of TB transmission. In a Danish study, not all patients had TB-positive sputum (9). In contrast, all patients in this study (including those classified as having minimal disease on the basis of chest X rays) were positive for TB by microscopy. Moreover, all patients in the present study had a history of illness extending from 2 to 8 months prior to presentation at the hospital. Thus, even patients with minimal radiological findings may have had relatively advanced disease compared to those in the Danish study, a conclusion which is compatible with the apparent depression of TB-specific immune responses in many patients in this study and our inability to separate the two patient groups on the basis of IFN-γ production. These data therefore suggest that categorization on the basis of radiological criteria should be used with caution when comparing countries with greatly differing levels of health care and further that culture status may be a valuable addition to radiological observations when assessing the condition of patients.
The finding that contacts of TB patients had a response to the antigens in ST-CF implies that they are likely to be at an early stage of infection, with exposure to secretory antigens from M. tuberculosis. Moreover, when the contacts are subdivided on the basis of the severity of disease in the index case, it appears that the highly exposed contacts both mount the most pronounced IFN-γ response to M. tuberculosis antigens and are more likely to score positively in immunological analyses (Fig. 2 and 4). Preliminary and ongoing follow-up studies have indicated that a number of these donors, classified as contacts, were already in the early stages of the disease, as they developed disease within a few months of the study described here (data not shown). The high levels of IFN-γ observed with in vitro restimulation of leukocytes from the contact group (particularly among contacts of patients with severe disease) are thus consistent with the hypothesis that these contacts in fact have the disease in its earliest stages, which correlated with the production of high levels of IFN-γ, while those with more advanced levels of disease (represented here by the patient group, as discussed above) produced lower levels of IFN-γ (9, 32).
As noted, IFN-γ is known to be a powerful activator of macrophages, thus enabling the cells to kill intracellular mycobacteria (15, 20, 22). For this reason, the presence of IFN-γ is currently thought to correlate with protection against tuberculosis. But the presence of IFN-γ per se does not seem to provide complete protection, as patients with advanced TB also produced significant levels of IFN-γ in response to ST-CF antigens. This supports the hypothesis that levels of IFN-γ, in balance with the upregulation of anti-inflammatory cytokines and perhaps another unidentified factor(s), act in concert to control the infection (13).
The spectrum of disease caused by M. tuberculosis in humans offers a good opportunity for the identification of relevant antigens involved in protection. The current method of disease classification is adequate for the clinician involved in disease management. However, for rational vaccine development, more studies are required to elucidate the correlation between the immune response and clinical symptoms. Using the present cross-sectional study as groundwork, we have now commenced a longitudinal study to follow the household contacts of severe and minimal TB patients to see which of them will develop clinical tuberculosis and which will contain the infection and to correlate this with their immunological response profiles. While other variables, such as HLA genotype and nutritional status, are likely to play a role, we believe these studies will offer an indication of which immune parameters are associated with protection.
ACKNOWLEDGMENTS
This work was supported by grants from the Third Research and Development Programme Life Science and Technologies for Developing Countries, the European Commission DRXII Contract TS3(CT 940313), and the AHRI core budget. P.R. is the recipient of a DANIDA grant.
We thank Alemayehu Kifle, Misrak Sisay, and Elias Mersha for technical assistance.
REFERENCES
- 1.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen P. Host responses and antigens involved in protective immunity to Mycobacterium tuberculosis. Scand J Immunol. 1997;45:115–131. doi: 10.1046/j.1365-3083.1997.d01-380.x. [DOI] [PubMed] [Google Scholar]
- 3.Andersen P, Andersen A B, Sorensen A L, Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol. 1995;154:3359–3372. [PubMed] [Google Scholar]
- 4.Andersen P, Askgaard D, Ljungqvist L, Bennedsen J, Heron I. Proteins released from Mycobacterium tuberculosis during growth. Infect Immun. 1991;59:1905–1910. doi: 10.1128/iai.59.6.1905-1910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen P, Heron I. Simultaneous electroelution of whole SDS-polyacrylamide gels for the direct cellular analysis of complex protein mixtures. J Immunol Methods. 1993;161:29–39. doi: 10.1016/0022-1759(93)90195-d. [DOI] [PubMed] [Google Scholar]
- 6.Anonymous. Diagnostic standards and classification of tuberculosis. New York, N.Y: National Tuberculosis and Respiratory Disease Association; 1969. [Google Scholar]
- 7.Anonymous. WHO global tuberculosis programme. WHO fact sheet 104. Geneva, Switzerland: World Health Organization; 1996. [Google Scholar]
- 8.Berthet F X, Rasmussen P B, Rosenkrands I, Andersen P, Gicquel B. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10) Microbiology. 1998;144:3195–3203. doi: 10.1099/00221287-144-11-3195. [DOI] [PubMed] [Google Scholar]
- 9.Boesen H, Jensen B N, Wilcke T, Andersen P. Human T-cell responses to secreted antigen fractions of Mycobacterium tuberculosis. Infect Immun. 1995;63:1491–1497. doi: 10.1128/iai.63.4.1491-1497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyum A. Separation of leukocytes from blood and bone marrow. Introduction. Scand J Clin Lab Investig. 1968;97(Suppl.):7. [PubMed] [Google Scholar]
- 11.Brandt L, Oettinger T, Holm A, Andersen A B, Andersen P. Key epitopes on the ESAT-6 antigen recognized in mice during the recall of protective immunity to Mycobacterium tuberculosis. J Immunol. 1996;157:3527–3533. [PubMed] [Google Scholar]
- 12.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellner J J. Immunosuppression in tuberculosis. Infect Agents Dis. 1996;5:62–72. [PubMed] [Google Scholar]
- 14.Fine P E. The BCG story: lessons from the past and implications for the future. Rev Infect Dis. 1989;11(Suppl. 2):S353–S359. doi: 10.1093/clinids/11.supplement_2.s353. [DOI] [PubMed] [Google Scholar]
- 15.Flesch I, Kaufmann S H. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J Immunol. 1987;138:4408–4413. [PubMed] [Google Scholar]
- 16.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haslov K, Andersen A, Nagai S, Gottschau A, Sorensen T, Andersen P. Guinea pig cellular immune responses to proteins secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:804–810. doi: 10.1128/iai.63.3.804-810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Havlir D V, Wallis R S, Boom W H, Daniel T M, Chervenak K, Ellner J J. Human immune response to Mycobacterium tuberculosis antigens. Infect Immun. 1991;59:665–670. doi: 10.1128/iai.59.2.665-670.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsch C S, Ellner J J, Blinkhorn R, Toossi Z. In vitro restoration of T cell responses in tuberculosis and augmentation of monocyte effector function against Mycobacterium tuberculosis by natural inhibitors of transforming growth factor beta. Proc Natl Acad Sci USA. 1997;94:3926–3931. doi: 10.1073/pnas.94.8.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holland S M, Eisenstein E M, Kuhns D B, Turner M L, Fleisher T A, Strober W, Gallin J I. Treatment of refractory disseminated nontuberculous mycobacterial infection with interferon gamma. A preliminary report. N Engl J Med. 1994;330:1348–1355. doi: 10.1056/NEJM199405123301904. [DOI] [PubMed] [Google Scholar]
- 21.Horwitz M A, Lee B W, Dillon B J, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin M, Newport M J, D'Souza S, Kalabalikis P, Brown I N, Lenicker H M, Agius P V, Davies E G, Thrasher A, Klein N, Blackwell J M. Familial disseminated atypical mycobacterial infection in childhood: a human mycobacterial susceptibility gene? Lancet. 1995;345:79–83. doi: 10.1016/s0140-6736(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 23.Lindblad E B, Elhay M J, Silva R, Appelberg R, Andersen P. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect Immun. 1997;65:623–629. doi: 10.1128/iai.65.2.623-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orme I M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance, in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988;56:3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orme I M, Roberts A D, Griffin J P, Abrams J S. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993;151:518–525. [PubMed] [Google Scholar]
- 26.Pal P G, Horwitz M A. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect Immun. 1992;60:4781–4792. doi: 10.1128/iai.60.11.4781-4792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollock J M, Andersen P. Predominant recognition of the ESAT-6 protein in the first phase of infection with Mycobacterium bovis in cattle. Infect Immun. 1997;65:2587–2592. doi: 10.1128/iai.65.7.2587-2592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravn P, Boesen H, Pedersen B K, Andersen P. Human T cell responses induced by vaccination with Mycobacterium bovis bacillus Calmette-Guerin. J Immunol. 1997;158:1949–1955. [PubMed] [Google Scholar]
- 29.Roberts A D, Sonnenberg M G, Ordway D J, Furney S K, Brennan P J, Belisle J T, Orme I M. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology. 1995;85:502–508. [PMC free article] [PubMed] [Google Scholar]
- 30.Selwyn P A, Hartel D, Lewis V A, Schoenbaum E E, Vermund S H, Klein R S, Walker A T, Friedland G H. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 31.Snider D J, Dooley S W. Nosocomial tuberculosis in the AIDS era with an emphasis on multidrug-resistant disease. Heart Lung. 1993;22:365–369. [PubMed] [Google Scholar]
- 32.Sodhi A, Gong J, Silva C, Qian D, Barnes P F. Clinical correlates of interferon gamma production in patients with tuberculosis. Clin Infect Dis. 1997;25:617–620. doi: 10.1086/513769. [DOI] [PubMed] [Google Scholar]
- 33.Sørensen A L, Nagai S, Houen G, Andersen P, Andersen Å B. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:1710–1717. doi: 10.1128/iai.63.5.1710-1717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]