Background:
Hyaluronic acid fillers are widely used in nonsurgical rhinoplasty.
Methods:
The authors performed a no-treatment control, multicenter, 12-month follow-up study to evaluate efficacy and safety of Restylane Lyft (Galderma, Uppsala, Sweden) in shaping the nasal dorsum and radix. Assignment to Restylane Lyft or no-treatment control was randomized (3:1). The Restylane Lyft group received a maximum of 1 ml of Restylane Lyft on day 1; the control group was offered a maximum of 1 ml of Restylane Lyft at month 6. Both groups were offered re-treatment (a maximum of 0.5 ml of Restylane Lyft) at month 12. Outcome assessments included blinded evaluation of three-dimensional photography measurements of change in volume (primary endpoint; month 6) and elevation of the nasal dorsum and radix, aesthetic improvement, adverse events, and diary-reported injection-site reactions.
Results:
One hundred thirty-two Chinese subjects were enrolled. The Restylane Lyft group had a greater increase in volume of the nasal dorsum and radix than the no-treatment control (mean difference at month 6, 0.71 ml; 95 percent confidence interval, 0.59 to 0.83 ml; p < 0.001). Restylane Lyft was also more effective than no-treatment control in achieving an elevation of the nasal dorsum and radix. The increase in volume and elevation persisted up to 12 months after injection and was supported by clinical assessments of aesthetic improvement. Treatment-related adverse events were mild to moderate, nonserious, and resolved during the study. Injection-site reactions were mostly mild to moderate and resolved within 1 week.
Conclusion:
Restylane Lyft injection was effective for aesthetic shaping of the nasal dorsum and radix and achieved aesthetic improvement for up to 12 months with an acceptable safety profile.
CLINICAL QUESTION/LEVEL OF EVIDENCE:
Therapeutic, I.
Since the introduction of the first hyaluronic acid dermal filler (Restylane) in Europe in 1996, the use of hyaluronic acid fillers has become widely established in aesthetic dermatology.1 The use has evolved from treatment of folds and wrinkles (i.e., replacement of lost volume and fullness)2,3 to also encompass enhancement of shape, contour, and definition.4,5 The desire to shape the contour of the nose is widespread, particularly in Asia, and minimally invasive nonsurgical rhinoplasty with injectable dermal fillers has become increasingly popular.6
To evaluate the efficacy and safety of a nonanimal stabilized hyaluronic acid filler in shaping the nasal dorsum and radix, we performed a randomized, no-treatment control, multicenter study with Restylane Lyft [also known as Restylane Perlane (Galderma, Uppsala, Sweden)] in the People’s Republic of China with follow-up extending to 12 months. The label for Restylane Lyft in the United States does not include use in the nose. This study extends knowledge on efficacy and safety of nonsurgical rhinoplasty using hyaluronic acid fillers because available data on clinical efficacy of nonsurgical rhinoplasty using hyaluronic acid fillers mainly derive from nonrandomized trials7–13 and case series (e.g., by Han et al.14 and Hedén15).
PATIENTS AND METHODS
Study Details
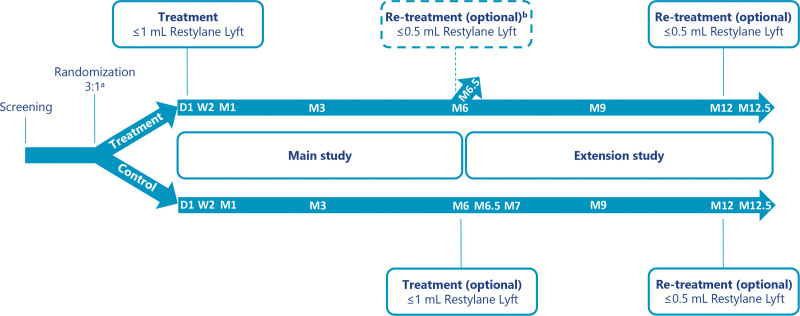
This open-label study (Fig. 1) was performed at three hospital clinics in China after obtaining approval from independent ethics committees. Assignment to Restylane Lyft or no-treatment control was randomized in a 3:1 ratio per center and was performed by the treating investigator after enrollment by opening sealed envelopes marked with subject number. One protocol amendment was made after the start of subject recruitment. This amendment affected subjects randomized to the Restylane Lyft group by extending the follow-up period from 6 months after treatment (main study) to 12 months after treatment (extension study). Subjects randomized to the Restylane Lyft group were asked to re-sign the informed consent form. Subjects that did not re-sign followed the original protocol (i.e., received the optional re-treatment at the 6-month visit and completed the study after that).
Fig. 1.
Study design and treatments. aTreatment (Restylane Lyft) or control (no treatment) was assigned randomly in a 3:1 ratio. bOnly subjects who did not consent to participate in the extension study. D1, day 1; W1, week 1; M1, month 1; M6, month 6; M6.5, month 6.5; M7, month 7; M9, month 9; M12, month 12; M12.5, month 12.5.
Subjects
Eligible subjects signed informed consent, were aged 18 years or older, were of Chinese origin, desired shaping of the nasal dorsum and/or radix, and (in the opinion of the investigator) could achieve a clinically meaningful aesthetic correction of their nose with a maximum of 1 ml of Restylane Lyft. Main exclusion criteria were previous nasal surgery, grafts, or implants in the nose area; history of chronic sinusitis or rhinitis; previous aesthetic dermal filler treatment in the forehead, glabellar, and/or nose area; and filler treatment around the nasal tip or in the glabellar region required to achieve a good aesthetic outcome.
Treatment Procedure
Any cosmetics were removed before treatment, and the treatment area was cleaned with antiseptic solution. Local anesthesia was used at the investigators’ discretion. Figure 1 illustrates the timing of treatments and follow-up visits. Re-treatment was not to be done for subjects in case they had an ongoing treatment-related adverse event.
Day 1
Subjects in the treatment group received a single treatment with a maximum of 1 ml of Restylane Lyft slowly injected subcutaneously and/or in the supraperiosteal plane in the nasal dorsum and/or radix region. Injections were done using a co-packed 29-gauge thin-wall needle and an aseptic technique. Retrograde linear threading injection was recommended. Digital pressure was applied as applicable on both sides of the dorsum to avoid lateral spreading of the product. Gentle massage of the treatment area was allowed to smooth the contour to the surrounding tissues. To reduce swelling and discomfort, ice packs could be applied on the treatment site for approximately 15 minutes.
Month 6
Subjects in the no-treatment control group were offered optional treatment with a maximum of 1 ml of Restylane Lyft (administered as described for day 1 above). Subjects in the Restylane Lyft group were offered optional re-treatment with a maximum of 0.5 ml of Restylane Lyft at month 6 only if they did not consent to participate in the extension study, and if the treating investigator assessed that they had not maintained or achieved optimal aesthetic improvement. If re-treatment was done, subjects completed the study after follow-up at month 6.5.
Month 12
Subjects in the extension study who had not maintained or achieved optimal aesthetic improvement (as assessed by the treating investigator) were offered optional re-treatment with a maximum of 0.5 ml of Restylane Lyft. If re-treatment was done, subjects completed the study after follow-up at month 12.5. If re-treatment was not done, subjects completed the study at the month-12 visit.
Posttreatment Care
Subjects were asked to avoid intensive physical training and extensive heat/sun or extreme cold in the first 24 hours after treatment. To prevent infections, subjects were asked to avoid touching the treated area and to not apply creams or cosmetics before the skin had healed. Subjects were also asked not to use heavy glasses during the study.
Outcome Assessments
Efficacy
The primary endpoint was change in volume of the nasal dorsum and radix measured at month 6 by standardized three-dimensional photography using the LifeViz II camera system (QuantifiCare S.A., Biot, France). Other endpoints measured by standardized three-dimensional photography were as follows:
Change in volume measured at week 2, and months 6.5, 9, and 12.
Remaining change in volume (versus week 2 or month 6.5) at months 6, 9, and 12.
Elevation in the midsagittal plane at week 2, and months 6, 6.5, 9, and 12.
Remaining elevation (versus week 2 or month 6.5) in the midsagittal plane at months 6, 9, and 12.
Analysis of three-dimensional photographs was performed centrally at QuantifiCare, by a blinded independent evaluator, using a region of interest (for the analysis of change in volume) and the highest point of elevation in the midsagittal plane (for the analysis of elevation). In the main study, the screening photograph served as reference for obtaining volume change and elevation in both groups. In the extension study, the screening photograph served as reference for subjects in the Restylane Lyft group, whereas subjects in the control group had their reference photograph taken at month 6 (pretreatment).
In addition to three-dimensional photography measurements, clinical assessment of aesthetic improvement from baseline on the five-grade Global Aesthetic Improvement Scale (adapted from Narins et al.)2 was performed by subjects (using two-dimensional photographs) and a central blinded independent evaluator (using three-dimensional photographs) at week 2 and months 3, 6, 6.5, 9, 12, and 12.5.
Safety
Adverse events were collected from day 1 and throughout the study. Predefined injection-site reactions (bruising, redness, swelling, pain, tenderness, and itching) were recorded in subject diaries for 14 days after each treatment. Reactions that were ongoing on day 14 were reported as adverse events.
Statistical Analysis
Sample Size
Approximately 100 treated subjects were considered a reasonable number for investigating the safety of nasal shaping with Restylane Lyft. With an assumed dropout rate of 10 percent, 132 subjects were to be included in the study. With 90 subjects in the Restylane Lyft group, and 30 subjects in the control group, a two-sided t test at a significance level of 5 percent would have 90 percent power to detect differences between treatment means.
Analysis Populations
The safety population included all subjects who were treated with Restylane Lyft or randomized to control. The full analysis set included all subjects who were treated with Restylane Lyft or randomized to control and who had at least one efficacy variable measurement. All efficacy analyses were based on the full analysis set. The primary analysis was also performed on the per-protocol population as a sensitivity analysis. The per-protocol population included all subjects in the full analysis set with no protocol deviations that could affect evaluation of the primary endpoint.
Analyses
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, N.C.). The randomization list was generated by an independent statistician using SAS and predefined blocks [block size, four; block number, 45 (15 per site)]. Formal statistical testing was performed at a significance level of 5 percent (two-sided). No correction for multiplicity was done. Change in volume and elevation was analyzed using t tests. Other data were presented descriptively. For the primary endpoint, missing data were handled primarily using a multiple imputation method. Missing values were not imputed for other endpoints.
RESULTS
Subject Disposition, Demographics, and Injection Characteristics
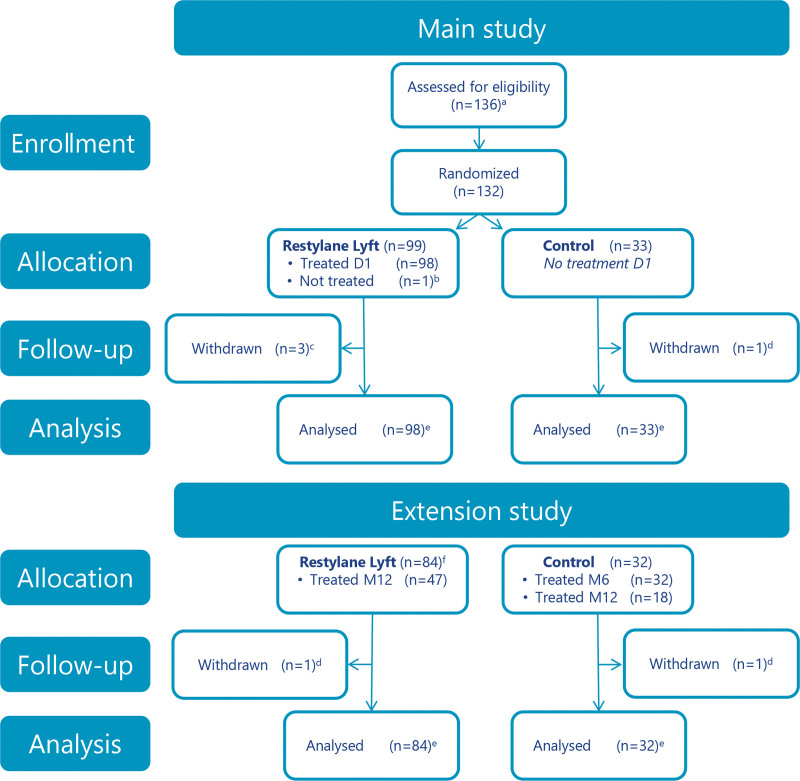
The first subject was enrolled on September 16, 2014; the last subject completed the study on June 17, 2016. Figure 2 shows subject disposition; Table 1 presents demographics. Mean follow-up time after treatment was 11.25 months for subjects treated with Restylane Lyft and approximately 6.25 months for the no-treatment controls. The most common injection depth was the supraperiosteal plane, both at initial treatment (Restylane Lyft, 97 percent of subjects; control, 100 percent of subjects) and at re-treatment (100 percent of subjects in both groups). All other injections were placed both subcutaneously and supraperiosteally to achieve optimal treatment results. Table 2 presents further injection characteristics.
Fig. 2.
Subject disposition. aFour subjects were not randomized (did not meet eligibility criteria). bWithdrew consent. cLost to follow-up (n = 2); withdrew consent (n = 1). dLost to follow-up. eFull analysis and safety analysis sets. fSubjects consenting to participate in the extension study. Twelve subjects did not consent to participate in the extension study; of these, eight subjects received optional re-treatment at month 6. D1, day 1; M6, month 6; M12, month 12.
Table 1.
Demographics*
| Main Study | Extension Study | |||
|---|---|---|---|---|
| Restylane Lyft (%)† | Control (%)† | Restylane Lyft (%)† | Control (%)† | |
| No. | 98 | 33 | 84 | 32 |
| Age, yr | ||||
| Mean ± SD | 34.2 ± 10.2 | 36.1 ± 9.8 | 34.2 ± 10.2 | 35.8 ± 9.8 |
| Median | 31.1 | 36.8 | 31.1 | 35.5 |
| Range | 20–58 | 22–60 | 20–58 | 22–60 |
| Sex | ||||
| Female | 94 (95.9) | 33 (100.0) | 81 (96.4) | 32 (100.0) |
| Male | 4 (4.1) | 0 | 3 (3.6) | 0 |
| Ethnicity | ||||
| Han Chinese | 95 (96.9) | 32 (97.0) | 81 (96.4) | 31 (96.9) |
| Other‡ | 3 (3.1) | 1 (3.0) | 3 (3.6) | 1 (3.1) |
*Safety population.
†% = (n/N) * 100.
‡Restylane Lyft: Dai Chinese, Hui Chinese and Zhuang Chinese. Control: Dai Chinese.
Table 2.
Injection Characteristics*
| Restylane Lyft | Control | |||||
|---|---|---|---|---|---|---|
| Day 1 | Mo 6/12† | Total | Mo 6 | Mo 12 | Total | |
| No. | 98 | 55 | 32 | 18 | ||
| Volume, ml | ||||||
| Mean ± SD | 0.8 ± 0.2 | 0.5 ± 0.1 | 1.1 ± 0.3 | 0.9 ± 0.2 | 0.5 ± 0.1 | 1.2 ± 0.4 |
| Minimum | 0.1–1.0 | 0.2–0.8 | 0.2–1.5 | 0.4–1.0 | 0.1–0.9 | 0.4–1.8 |
*Safety population.
†Eight subjects were re-treated at mo 6; 47 subjects were re-treated at mo 12.
Change in Volume of Nasal Dorsum and Radix
Comparison with Control
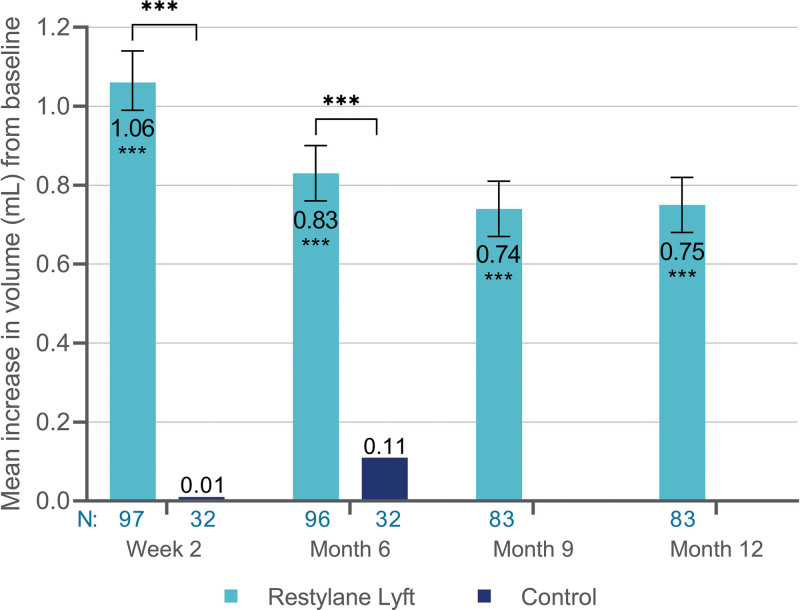
Treatment with Restylane Lyft achieved a greater increase in volume from baseline to week 2 and month 6 compared with no-treatment control (Fig. 3). Mean difference between Restylane Lyft and control was 1.05 ml (95 percent CI, 0.92 to 1.19 ml; p < 0.001) at week 2, and 0.71 ml (95 percent CI, 0.59 to 0.83 ml; p < 0.001) at month 6; the primary efficacy objective was thus met. Similar results were obtained for month-6 comparisons in the per-protocol population (data not shown), confirming the primary analysis.
Fig. 3.
Mean increase in volume (in milliliters) of the nasal dorsum and radix from baseline (full analysis set population). ***p < 0.001. N, Number of subjects with available data; 95 percent CI for Restylane Lyft: 0.99 to 1.14 ml (week 2), 0.76 to 0.90 ml (month 6), 0.67 to 0.81 ml (month 9), and 0.68 to 0.82 ml (month 12).
Comparison with Baseline
In the Restylane Lyft group, the volume increased from baseline to week 2 and persisted at month 12 (Fig. 3). In the control group, the volume also increased from baseline (month 6, pretreatment) to month 6.5 (mean change, 1.00 ml; 95 percent CI, 0.89 to 1.10 ml; p < 0.001) and month 12 (mean change, 0.65 ml; 95 percent CI, 0.55 to 0.75 ml; p < 0.001). This increase in volume was similar to that measured at week 2 and month 6 in the Restylane Lyft group (Fig. 3).
Remaining Change in Volume of Nasal Dorsum and Radix
In the Restylane Lyft group, the median remaining increase in volume (from week 2) was 78 percent (month 6), 63 percent (month 9), and 62 percent (month 12). Two- and three-dimensional photographs are shown in Figures 4 through 6. The median remaining increase in volume (from month 6.5) was slightly lower in the control group (66 percent at month 12; i.e., 6 months after the first treatment).
Fig. 4.
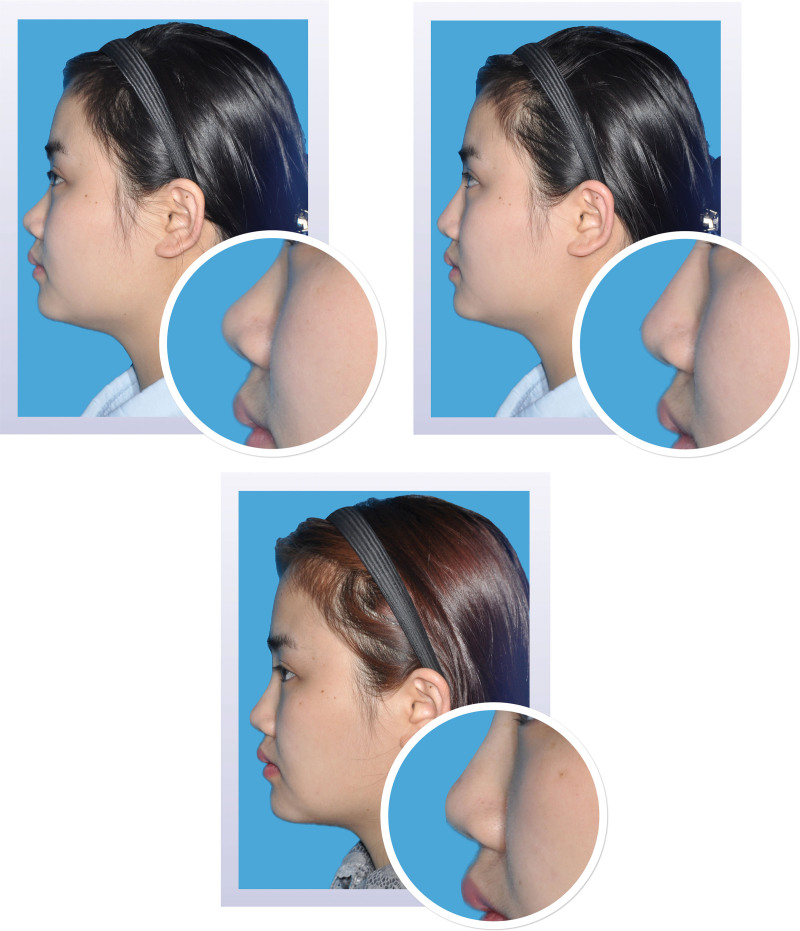
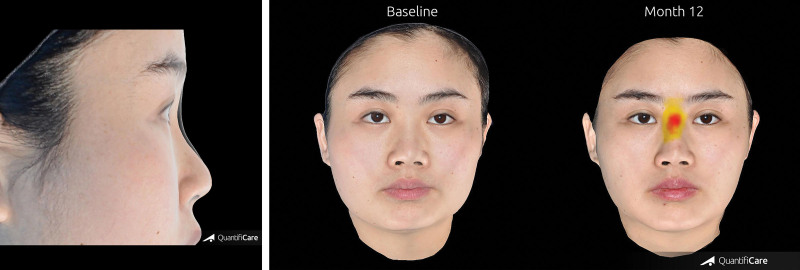
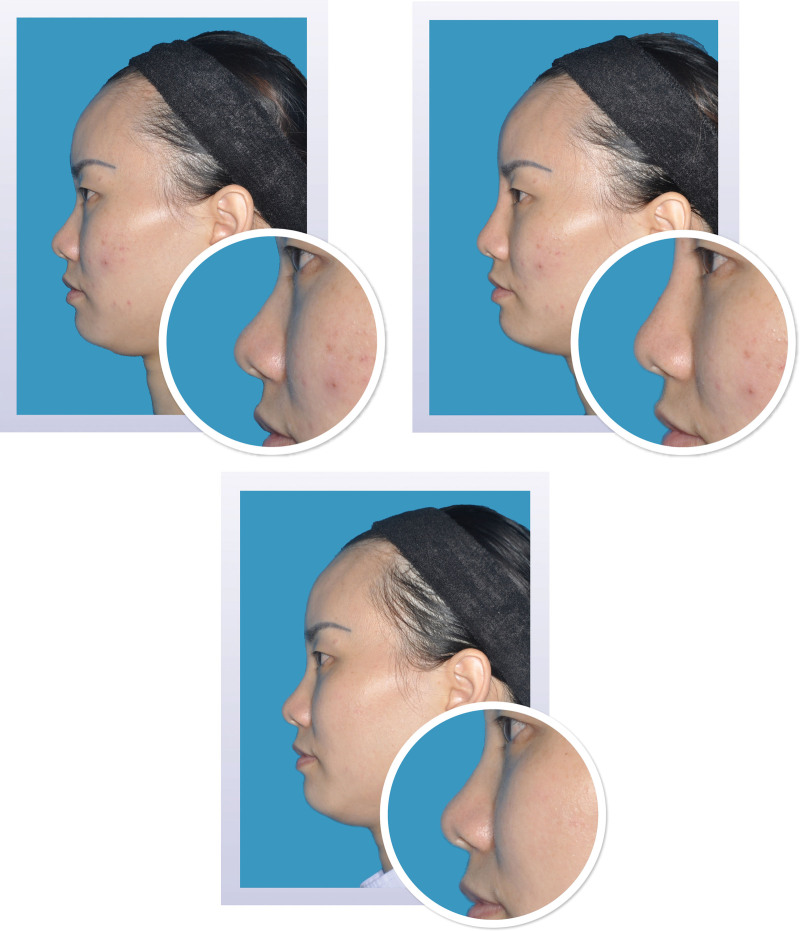
Two-dimensional photographs before and after treatment. Appearance of a 25-year-old woman on day 1 before (above, left) and after (above, right) injection of 1.0 ml Restylane Lyft in the nasal dorsum and radix. At month 12 (below), the change from baseline in volume of the nasal dorsum and radix was 1.00 ml; the change from baseline in elevation was 1.55 mm.
Fig. 6.
Three-dimensional photographs before and after treatment. (Left) Overlay of the appearance of a 25-year-old woman at baseline and at month 12 after injection with 1.0 ml of Restylane Lyft in the nasal dorsum and radix. At month 12, the change from baseline in volume of the nasal dorsum and radix was 1.00 ml; the change from baseline in elevation was 1.55 mm. (Right) Volume change from baseline shown in color at month 12.
Fig. 5.
Two-dimensional photographs before and after treatment. Appearance of a 31-year-old woman on day 1 before (above, left) and after (above, right) injection of 1.0 ml Restylane Lyft in the nasal dorsum and radix. At month 12 (below), the change from baseline in volume of the nasal dorsum and radix was 0.94 ml; the change from baseline in elevation was 1.15 mm.
Elevation in the Midsagittal Plane of the Nasal Dorsum and Radix
Comparison with Control
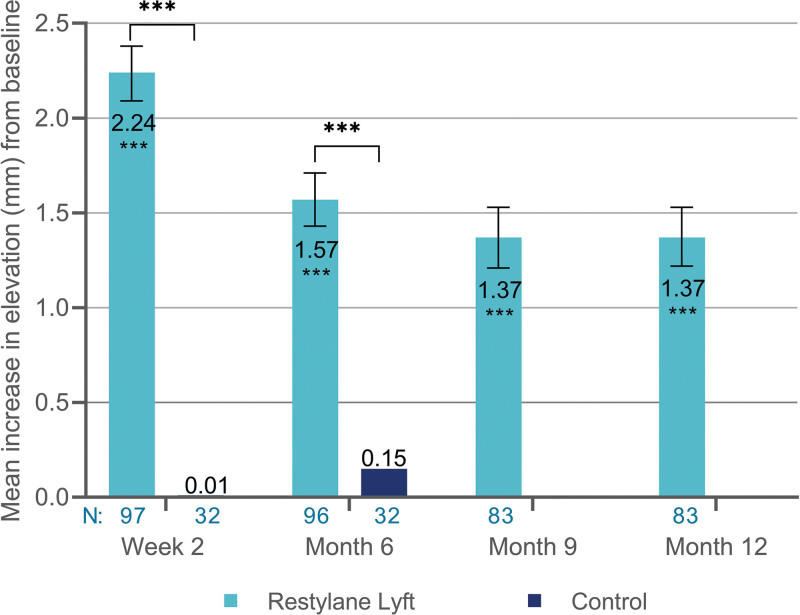
Treatment with Restylane Lyft achieved a greater elevation from baseline to week 2 and month 6 compared with the no-treatment control (Fig. 7). The mean difference between Restylane Lyft and no-treatment control was 2.22 mm (95 percent CI, 1.95 to 2.49 mm; p < 0.001) at week 2 and 1.43 mm (95 percent CI, 1.17 to 1.68 mm; p < 0.001) at month 6.
Fig. 7.
Mean increase in elevation (in millimeters) of nasal dorsum and radix from baseline (full analysis set population). ***p < 0.001. N, Number of subjects with available data; 95 percent CI for Restylane Lyft: 2.09 to 2.38 mm (week 2), 1.43 to 1.71 mm (month 6), 1.21 to 1.53 mm (month 9), and 1.22 to 1.53 mm (month 12).
Comparison with Baseline
In the Restylane Lyft group, the elevation increased from baseline to week 2 and persisted at month 12 (Fig. 7). In the control group, the elevation also increased from baseline (month 6, pretreatment) to month 6.5 (mean change, 2.28 mm; 95 percent CI, 2.05 to 2.51 mm; p < 0.001) and month 12 (mean change, 1.46 mm; 95 percent CI, 1.23 to 1.69 mm; p < 0.001). This increase was similar to that measured at week 2 and month 6 in the Restylane Lyft group (Fig. 7).
Remaining Elevation in the Midsagittal Plane of the Nasal Dorsum and Radix
In the Restylane Lyft group, the median remaining increase in elevation (from week 2) was 67 percent (month 6), 57 percent (month 9), and 55 percent (month 12). Two- and three-dimensional photographs are shown in Figures 4 through 6. The median remaining increase in elevation (from month 6.5) was similar in the control group (61 percent at month 12; i.e., 6 months after the first treatment).
Global Aesthetic Improvement Scale
Subjects’ Assessment
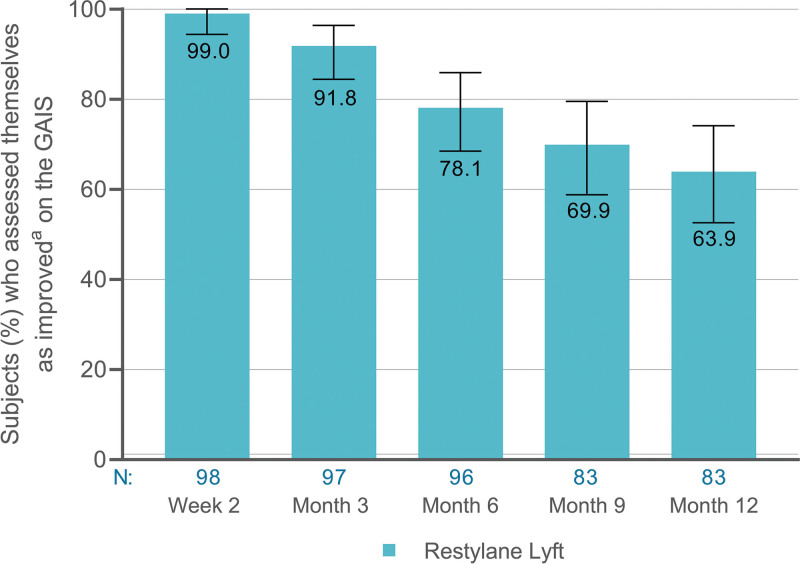
Most subjects in the Restylane Lyft group were improved (somewhat, much, or very much) up to 12 months after treatment (Fig. 8). Most subjects in the control group also assessed themselves as improved after treatment with Restylane Lyft (at month 6); 100 percent at month 6.5, 94 percent at month 9, and 88 percent at month 12. These data are similar to those in the Restylane Lyft group at week 2, month 3, and month 6 (Fig. 8).
Fig. 8.
Improvement on the Global Aesthetic Improvement Scale (GAIS) as assessed by subjects (Restylane Lyft group, full analysis set population). aSomewhat, much, or very much improved. N, Number of subjects with available data; 95 percent CI, 94.4 to 100 (week 2), 84.4 to 96.4 (month 3), 68.5 to 85.9 (month 6), 58.8 to 79.5 (month 9), and 52.6 to 74.1 (month 12).
Independent Evaluator’s Assessment
All or almost all subjects in the Restylane Lyft group were improved after treatment; 100 percent of subjects (week 2 and month 3), 97 percent (month 6), 95 percent (month 9), and 96 percent (month 12). A larger proportion of subjects were improved in the Restylane Lyft group than in the no-treatment control group at week 2, month 3, and month 6.
After the first treatment with Restylane Lyft in the control group (month 6), all subjects were improved (months 6.5, 9, and 12). These data are similar to those in the Restylane Lyft group at week 2 and months 3 and 6.
Adverse Events
Sixteen subjects (both groups combined) had 19 treatment-related adverse events. Fifteen of these adverse events occurred after first treatment [in 13 subjects (10 percent)]; four occurred after re-treatment [in three subjects (4 percent)]; none was serious. All treatment-related adverse events were mild (n = 12) or moderate (n = 7), and resolved during the study.
Implant-site pain was the most commonly reported event: nine events in nine subjects (7 percent) occurred after the first treatment, and three events in three subjects (4 percent) occurred after re-treatment. Other treatment-related adverse events were pain, epistaxis, tenderness, and nasal pruritus (each occurring in three or fewer subjects).
Predefined Injection-Site Reactions
All subjects recorded at least one injection-site reaction. The most commonly reported injection-site reactions were tenderness (≥93 percent of subjects), pain (≥85 percent), and swelling (≥76 percent). Most subjects (≥85 percent) assessed the injection-site reactions as mild or moderate. Reactions generally lasted 2 to 6 days.
DISCUSSION
This randomized, no-treatment control, multicenter, 12-month study with Restylane Lyft for aesthetic shaping of the nasal dorsum and radix in a Chinese population extends current knowledge on efficacy and safety of nonsurgical rhinoplasty with hyaluronic acid fillers. The primary efficacy objective was to establish whether Restylane Lyft was more effective than no-treatment control in changing the volume of the nasal dorsum and radix at month 6. This was assessed using three-dimensional photography measurements. Our primary efficacy analysis showed that subjects in the Restylane Lyft group had a greater increase in volume of the nasal dorsum and radix than subjects in the no-treatment control group at month 6. As this result was also confirmed in the per-protocol population, the primary month 6 efficacy objective was considered to be met.
Our study also showed that in most subjects, the increase in volume of the nasal dorsum and radix persisted up to 12 months after a single initial injection with a maximum of 1 ml of Restylane Lyft. Restylane Lyft was also more effective than no-treatment control in achieving an increased elevation in the midsagittal plane of the nasal dorsum and radix. Similar to the increase in volume, the increase in elevation also persisted up to 12 months in most subjects after Restylane Lyft injection. Duration of effect was further shown by the secondary endpoints that addressed remaining volume and elevation versus week 2, which was the anticipated time point for optimal treatment effect. At 6 to 12 months after Restylane Lyft injection, the median remaining increase in volume was greater than or equal to 62 percent of the assumed optimal treatment effect (measured at week 2); the median remaining increase in elevation was greater than or equal to 55 percent.
We also found that the three-dimensional photography measurements were supported by clinical assessments (i.e., Global Aesthetic Improvement Scale data). Both the subjects themselves and a qualified professional (i.e., the independent evaluator) recognized the aesthetic result achieved after Restylane Lyft injection to shape the nasal dorsum and radix; 78 percent or more of subjects were improved at month 6, and 64 percent or more were still improved at month 12. Liew et al.12 also reported good aesthetic results after nonsurgical rhinoplasty where the hyaluronic acid filler was injected into the anterior nasal spine, columella, and dorsum, although they used a slightly different assessment scale and a higher injection volume (mean baseline injection volume, 1.36 ml; mean 4-week touch-up injection volume, 0.37 ml) than in our study. Our results are also in line with previous clinical experience with hyaluronic acid filler rhinoplasty.14,15
In our study, subjects’ Global Aesthetic Improvement Scale ratings were lower than those of the independent evaluator. This may be a reflection of the fact that subjects become used to the posttreatment appearance of their nose, or that they wish for a longer duration of effect. Also, in contrast to the subjects, the independent evaluator is a qualified professional experienced in assessing aesthetic treatment results and may thus be able to recognize more subtle improvements.
Limitations of our study include that the injection volume was restricted (maximum, 1.0 ml at initial treatment and 0.5 ml at re-treatment). However, the total mean volume injected (1.1 ml in the Restylane Lyft group and 1.2 ml in the control group) shows that optimal aesthetic improvement was achieved for most subjects with less than the 1.5 ml allowed in the study. A no-treatment control group was used (according to the same principle as for U.S. dermal filler registration studies), as no hyaluronic acid or biodegradable filler was approved for this indication in China at study start. Because the no-treatment control group was not treated, the changes in volume are very small and further analysis would be irrelevant. As in any study, the choice of endpoints can be discussed. We chose a three-dimensional imaging method (analyzed by a blinded independent evaluator) for assessment of the primary endpoint to minimize the risk of bias and any placebo effect.
Minimally invasive nonsurgical rhinoplasty with injectable fillers provides immediately visible results with no or minimal downtime. Compared with permanent implants and semipermanent injectable fillers, advantages with hyaluronic acid fillers include their nonpermanent properties and the possibility to use hyaluronidase for product removal.6,15 However, as a consequence of the natural degradation of hyaluronic acid fillers over time, re-treatment is required to maintain the initial effect.
No serious complications occurred in our study. Treatment-related adverse events were recorded for 10 percent of subjects after initial injection and for 4 percent of subjects after re-treatment. Thus, re-treatment did not increase the incidence of treatment-related adverse events. Implant-site pain was the most commonly reported treatment-related adverse event after both initial treatment and re-treatment. All treatment-related adverse events were mild or moderate, and resolved during the study. Injection-site reactions captured in subject diaries after Restylane Lyft injection in our study were mostly mild or moderate, and resolved within 1 week. Tenderness, pain, and swelling were most commonly recorded. Taking both treatment-related adverse events and injection-site reactions into account, the safety profile for Restylane Lyft in our study when used for nasal shaping was thus deemed acceptable.
CONCLUSION
Restylane Lyft injection was effective for aesthetic shaping of the nasal dorsum and radix and achieved clinically meaningful aesthetic improvement for up to 12 months with an acceptable safety profile.
ACKNOWLEDGMENTS
The authors acknowledge the contributions of Prof. Yongguang Ma, who initially served as a principal investigator in the study at Peking University Third Hospital, Beijing, People’s Republic of China. Medical writing assistance was provided by Carolina Edwartz, Ph.D., Galderma. Assistance with preparing the threedimensional images for Figure 6 was provided by Nicolas Dapis, QuantifiCare S.A.
PATIENT CONSENT
Patients provided written informed consent for the use of their images.
Footnotes
This trial is registered under the name “Restylane Perlane to Shape the Nasal Dorsum and/or Nasal Root,” ClinicalTrials.gov identification no. NCT02216851 (https://clinicaltrials.gov/ct2/show/NCT02216851).
Poster presented at the 22nd International Master Course on Aging Science World Congress, in Paris, France, January 30 through February 1, 2020; and the 18th Aesthetic & Antiaging Medicine World Congress, in Monte Carlo, Monaco, November 5 through 7, 2020.
Disclosure: Galderma funded the study and provided the study product and medical writing assistance. Galderma also contributed toward analysis and interpretation of data. The authors have no other financial interests or conflicts of interest to declare.
REFERENCES
- 1.American Society of Plastic Surgeons. 2017 Plastic surgery statistics report. Available at: https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-full-report-2017.pdf. Accessed August 16, 2018.
- 2.Narins RS, Brandt F, Leyden J, Lorenc ZP, Rubin M, Smith S. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg. 2003;29:588–595. [DOI] [PubMed] [Google Scholar]
- 3.Carruthers A, Carey W, De Lorenzi C, Remington K, Schachter D, Sapra S. Randomized, double-blind comparison of the efficacy of two hyaluronic acid derivatives, Restylane Perlane and Hylaform, in the treatment of nasolabial folds. Dermatol Surg. 2005;31:1591–1598; discussion 1598. [DOI] [PubMed] [Google Scholar]
- 4.Molina B, David M, Jain R, et al. Patient satisfaction and efficacy of full-facial rejuvenation using a combination of botulinum toxin type A and hyaluronic acid filler. Dermatol Surg. 2015;41(Suppl 1):S325–S332. [DOI] [PubMed] [Google Scholar]
- 5.Weiss RA, Moradi A, Bank D, et al. Effectiveness and safety of large gel particle hyaluronic acid with lidocaine for correction of midface volume deficit or contour deficiency. Dermatol Surg. 2016;42:699–709. [DOI] [PubMed] [Google Scholar]
- 6.Wang LL, Friedman O. Update on injectables in the nose. Curr Opin Otolaryngol Head Neck Surg. 2017;25:307–313. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka Y, Matsuo K, Yuzuriha S. Westernization of the Asian nose by augmentation of the retropositioned anterior nasal spine with an injectable filler. Eplasty 2011;11:e7. [PMC free article] [PubMed] [Google Scholar]
- 8.Xue K, Chiang CA, Liu K, Gu B, Li Q. Multiplane hyaluronic acid rhinoplasty. Plast Reconstr Surg. 2012;129:371e–372e. [DOI] [PubMed] [Google Scholar]
- 9.Liapakis IE, Englander M, Vrentzos NP, Derdas SP, Paschalis EI. Secondary rhinoplasty fixations with hyaluronic acid. J Cosmet Dermatol. 2013;12:235–239. [DOI] [PubMed] [Google Scholar]
- 10.Mashiko T, Mori H, Kato H, et al. Semipermanent volumization by an absorbable filler: Onlay injection technique to the bone. Plast Reconstr Surg Glob Open 2013;1:e4–e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Li SR, Yu P, Wang ZX. Comparison of Artecoll, Restylane and silicone for augmentation rhinoplasty in 378 Chinese patients. Clin Invest Med. 2014;37:E203–E210. [DOI] [PubMed] [Google Scholar]
- 12.Liew S, Scamp T, de Maio M, et al. Efficacy and safety of a hyaluronic acid filler to correct aesthetically detracting or deficient features of the Asian nose: A prospective, open-label, long-term study. Aesthet Surg J. 2016;36:760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rauso R, Colella G, Zerbinati N, Salti G. Safety and early satisfaction assessment of patients seeking nonsurgical rhinoplasty with filler. J Cutan Aesthet Surg. 2017;10:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han X, Hu J, Cheng L, Li F. Multiplane hyaluronic acid (EME) in female Chinese rhinoplasty using blunt and sharp needle technique. J Plast Reconstr Aesthet Surg. 2015;68:1504–1509. [DOI] [PubMed] [Google Scholar]
- 15.Hedén P. Nasal reshaping with hyaluronic acid: An alternative or complement to surgery. Plast Reconstr Surg Glob Open 2016;4:e1120. [DOI] [PMC free article] [PubMed] [Google Scholar]