Background:
The chin is important for facial appearance, affecting overall balance and harmony of the face. The purpose of this study was to evaluate effectiveness of the hyaluronic acid filler Restylane Defyne for chin augmentation and correction of chin retrusion versus a no-treatment control.
Methods:
Male and female subjects, aged 22 years or older, with mild to moderate chin retrusion, were randomized 3:1 to the hyaluronic acid filler Restylane Defyne (n = 107) or no treatment (n = 33). Assessments included live, blinded evaluations on a validated chin retrusion scale (Galderma Chin Retrusion Scale), aesthetic improvement (Global Aesthetic Improvement Scale), subject-reported FACE-Q Satisfaction with Chin, and safety follow-up.
Results:
Galderma Chin Retrusion Scale responder rate (≥1 grade improvement) was higher for the hyaluronic acid filler Restylane Defyne (81 percent) than for control (6 percent) (p < 0.001) at week 12, and remained higher at week 48 (74 percent versus 11 percent; p < 0.001). Aesthetic improvement rates were high throughout the study as reported by investigators (≥96 percent) and subjects (≥85 percent). Subject satisfaction was higher in the hyaluronic acid filler Restylane Defyne group than in the control group at week 12 (p < 0.001). In the individual FACE-Q scale items, 87 to 98 percent of subjects were satisfied at week 12. Treatment-related adverse events were mild to moderate.
Conclusions:
The hyaluronic acid filler Restylane Defyne was safe and effective for augmentation of the chin region to improve the chin profile and associated with high aesthetic improvement and subject satisfaction. Effectiveness was sustained throughout 48 weeks.
CLINICAL QUESTION/LEVEL OF EVIDENCE:
Therapeutic, II.
The chin is an important component of facial attractiveness,1,2 often overlooked in aesthetic analysis.3 Correction of a retruded chin or other chin deficiency can improve the balance and harmony of the face.4 Chin augmentation may also benefit patients seeking overall facial improvement, as many are unaware that they have this condition.5
Aesthetic treatments of the chin should consider the height and width of the chin, and the chin projection and shape,5 and should also take into account adjacent facial structures.4 Using a hyaluronic acid filler allows shaping of the chin three-dimensionally with individual adaptation to each patient.6 The choice of filler should match the patient’s needs, from subtle contouring to facial structural reshaping, and ideally also allow natural movement in the chin area.7,8
Restylane Defyne (Galderma, Uppsala, Sweden) is designed with XpresHAn Technology and is a balanced hyaluronic acid filler in terms of gel properties, with the highest firmness and support (G′) among XpresHAn gel products,9 with maintained flexibility (xStrain)10 and high tissue integration.11 It is therefore suitable for providing volume to improve the chin projection, profile, and shape. In clinical trials, Restylane Defyne has been proven effective for correcting lower face wrinkles while maintaining natural movement in various facial expressions.12,13
Restylane Defyne was approved in the European Union in 2010 and has been approved in the United States since 2016 for correction of moderate to severe, deep facial wrinkles and folds, such as nasolabial folds, in patients older than 21 years. This study aimed to evaluate the effectiveness and safety of Restylane Defyne when used for augmentation and correction of chin retrusion versus a no-treatment control.
PATIENTS AND METHODS
Study Design
This was a randomized, evaluator-blinded, parallel group, no-treatment control, 48-week study, performed at 11 centers in the United States, between August of 2018 and February of 2020. Subjects were healthy men and nonpregnant women aged 22 years or older, with baseline scores of mild (1) to moderate (2) chin retrusion as assessed by a blinded evaluator on the Galderma Chin Retrusion Scale,14 seeking augmentation therapy for chin retrusion. All other facial plastic surgical or cosmetic procedures were prohibited during the study. Exclusion criteria included known allergy to hyaluronic acid or lidocaine, and surgery or certain facial procedures near the area to be treated.
The study complied with Good Clinical Practice and the Declaration of Helsinki. All subjects provided signed informed consent.
Treatment
Subjects were randomized 3:1 to Restylane Defyne treatment or no-treatment control and received initial treatment on day 1. Subjects were treated to optimal chin augmentation, defined as a greater than or equal to one-point improvement on the Galderma Chin Retrusion Scale and the best correction that could be achieved in the opinion of the treating investigator and subject. Subjects who did not achieve this at initial treatment could be given an optional touch-up after 4 weeks. The maximum recommended volumes were 4 ml at initial treatment and 2 ml at touch-up. An optional re-treatment (or initial treatment for the control group) with Restylane Defyne was offered at 48 weeks.
Restylane Defyne (20 mg/ml hyaluronic acid and 3 mg/ml lidocaine hydrochloride) was injected using a 27-gauge, ½–inch, ultra-thin-wall needle into the chin and the area inferior to lower lip, between the two lines from oral commissures to prejowl sulcus. (See Figure, Supplemental Digital Content 1, which shows the chin injection area. Image used under license from Valentina Razumova/Shutterstock.com, modified by Galderma, http://links.lww.com/PRS/F460.) Injection techniques were chosen by the treating investigator and additional local anesthesia could be applied. Posttreatment care was according to the instructions for use.
Visits
Follow-up was conducted over 48 weeks, with visits at 72 hours (telephone call), and 2 and 4 weeks after each treatment, as well as at 12, 24, 36, and 48 weeks after the last injection or baseline (control). Additional safety follow-up was performed for 12 weeks after the optional re-treatment at week 48.
Effectiveness Assessments
The primary objective was to evaluate the effectiveness of Restylane Defyne versus no treatment for correction of chin retrusion using blinded live evaluations of the Galderma Chin Retrusion Scale at 12 weeks after last injection or baseline for control subjects.
The Galderma Chin Retrusion Scale is a validated,14 four-grade scale from no retrusion (0) to severe retrusion (3), assessed live with support of a photograph guide, on subjects with a neutral facial expression. Baseline Galderma Chin Retrusion Scale was evaluated at screening or on day 1 before treatment. A Galderma Chin Retrusion Scale responder was defined as having a greater than or equal to one-point improvement from baseline.
Secondary objectives were to evaluate efficacy and subject satisfaction up to 48 weeks after last injection or baseline (for controls). This included Galderma Chin Retrusion Scale evaluated by blinded evaluators and treating investigators, aesthetic improvement assessed by subjects and treating investigators using the Global Aesthetic Improvement Scale, the subject-reported FACE-Q Satisfaction with Chin scale, and three-dimensional photographic assessment of volume change in the chin. The Global Aesthetic Improvement Scale was a seven-grade scale scored from very much worse to very much improved. The FACE-Q scale consisted of 10 individual items, each graded on a four-point scale from very dissatisfied to very satisfied, which were Rasch-transformed to a total score of zero to 100 for each subject.
Safety Assessments
Adverse events were collected throughout the study. Predefined, injection-related events were collected in a 4-week subject diary after each treatment. Assessment of chin and lower lip sensation, lip and chin function, and changes in hair growth (male subjects only) was performed at all visits.
Statistical Analysis
The sample size was calculated to give 90 percent power to demonstrate the difference between a Galderma Chin Retrusion Scale responder rate of 70 percent in the Restylane Defyne group, and a responder rate of 35 percent in the no-treatment control group using a two-sided significance level of 0.05.
In the primary analysis, Galderma Chin Retrusion Scale responder rates for Restylane Defyne–treated and untreated subjects at week 12 were compared using Fisher exact test and presented as estimated responder rates with two-sided 95 percent confidence intervals and p value. A value of p < 0.05 for the treatment difference was considered significant.
To evaluate the consistency of the primary analysis results across subgroups, the analysis of Galderma Chin Retrusion Scale responder rate at week 12 was repeated in subgroups based on race (i.e., white, black, and other), ethnicity (i.e., Hispanic and non-Hispanic), Fitzpatrick skin types (i.e., I to III, IV, and V to VI), study site, age groups (i.e., 20 to 29 years, 30 to 50 years, and >50 years), and sex (i.e., men and women).
Secondary analyses included between-group comparisons of Galderma Chin Retrusion Scale responder rates at all visits using Fisher exact test, FACE-Q Rasch-transformed total scores at week 12 using t test, and three-dimensional volume change using a two-sample t test.
All other variables were analyzed descriptively. Efficacy results are presented for the intention-to-treat population (all randomized subjects) and safety results for the safety population (all treated subjects).
RESULTS
Subjects and Treatment
In total, 140 subjects were randomized to Restylane Defyne treatment (n = 107) or no treatment (n = 33). One subject randomized to Restylane Defyne was not treated, and 88 percent completed the study. No subjects withdrew because of adverse events.
Subjects within an age range of 20 to 73 years were included (mean age, 48.3 years in the Restylane Defyne group and 44.4 years in the no-treatment control group); 89 percent were female, and 76 percent were white. The treatment groups had comparable baseline characteristics, including representation of all Fitzpatrick skin types in both groups. (See Table, Supplemental Digital Content 2, which shows patient demographics, http://links.lww.com/PRS/F461.)
In the Restylane Defyne group, the mean total volume of product injected was 3.60 ml (range, 1.0 to 6.0 ml), including initial treatment (n = 106) and optional 4-week touch-up (n = 78) (Table 1). Injections in the pogonion (mean total volume, 1.94 ml) were mainly given supraperiosteally (>80 percent of subjects) and most commonly using depot, serial puncture, and/or linear retrograde techniques, whereas surrounding areas (mean total volume, 1.67 ml) were mainly injected subcutaneously (>60 percent of subjects) and mostly using linear retrograde and/or serial puncture techniques.
Table 1.
Volumes of Hyaluronic Acid Injected in the Restylane Defyne Group
| Initial Treatment | Touch-Up Treatment | Total | |
|---|---|---|---|
| No. of subjects treated | 106 | 78 | 106 |
| Total chin, ml* | |||
| Mean ± SD | 2.61 ± 0.957 | 1.35 ± 0.620 | 3.60 ± 1.419 |
| Median | 2.65 | 1.00 | 3.58 |
| Range | 0.8–4.0 | 0.4–3.2 | 1.0–6.0 |
| Pogonion, ml | |||
| Mean ± SD | 1.44 ± 0.557 | 0.81 ± 0.378 | 1.94 ± 0.834 |
| Median | 1.50 | 0.80 | 2.00 |
| Range | 0.3–3.1 | 0.1–1.8 | 0.4–4.4 |
| Surrounding area, ml | |||
| Mean ± SD | 1.20 ± 0.538 | 0.79 ± 0.388 | 1.67 ± 0.801 |
| Median | 1.00 | 0.80 | 1.60 |
| Range | 0.2–2.8 | 0.1–2.0 | 0.2–4.0 |
*Volumes presented for total chin includes the pogonion plus the surrounding area.
Chin Retrusion Improvement
The primary objective was met; chin retrusion was significantly improved in the Restylane Defyne group at week 12 after the last injection compared to the no-treatment control, as measured by Galderma Chin Retrusion Scale assessed live by a blinded evaluator (Table 2). The Galderma Chin Retrusion Scale responder rate, subjects achieving a greater than or equal to one-point improvement from baseline, was 81 percent versus 6 percent for Restylane Defyne versus no treatment at week 12, and the treatment difference was statistically significant (p < 0.001).
Table 2.
Chin Retrusion Responder Rates According to the Galderma Chin Retrusion Scale by Blinded Evaluator at Week 12
| HARD (%) | No Treatment (%) | Difference in Responder Rate* | p † | |
|---|---|---|---|---|
| No. | 107 | 33 | ||
| At least one-point improvement from baseline | 87 (81.3) | 2 (6.1) | 75.2 | <0.001 |
| 95% CI, % | 72.62–88.19‡ | 0.74–20.23‡ | 62.27–88.22§ |
HARD, hyaluronic acid filler Restylane Defyne.
*Difference = HARD responder rate − no-treatment responder rate.
†Fisher exact test for responder rate. Missing data were imputed using the baseline observation carried forward method.
‡Calculated using the Clopper-Pearson method.
§Calculated using the Wald approximation with a continuity correction.
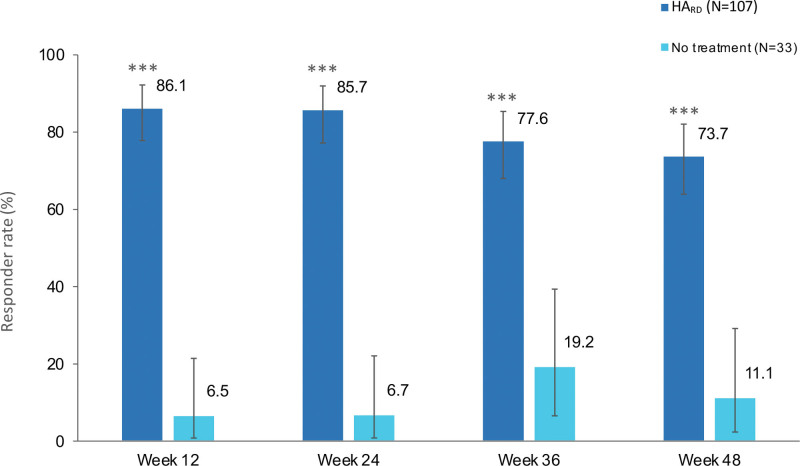
Subgroup analyses showed statistically significant higher Galderma Chin Retrusion Scale responder rates in the Restylane Defyne group at week 12 versus the no-treatment control group in all Fitzpatrick skin type categories (i.e., I to III, IV, V to VI), race categories (i.e., white, black, and other), ethnicity categories (i.e., Hispanic and non-Hispanic), age groups (i.e., 20 to 29 years, 30 to 50 years, and >50 years), and sex (i.e., men and women) (p < 0.05). (See Table, Supplemental Digital Content 3, which shows subgroup analyses of chin retrusion responder rates according to the Galderma Chin Retrusion Scale by blinded evaluator at week 12, http://links.lww.com/PRS/F462.) The Galderma Chin Retrusion Scale responder rate remained higher (p < 0.001) in the Restylane Defyne group compared to the no-treatment control group throughout the study (Fig. 1), with 74 percent responders in the Restylane Defyne group at week 48 after the last injection.
Fig. 1.
Chin retrusion responder rates (±95 percent CI) according to the Galderma Chin Retrusion Scale, assessed by blinded evaluator. A responder was defined as a subject with at least a one-point improvement from baseline according to the Galderma Chin Retrusion Scale. HARD, hyaluronic acid filler Restylane Defyne. ***p < 0.001 HArd versus no-treatment control, Fisher exact test. The 95 percent CI was calculated using the Clopper Pearson method. Observed cases.
Global Aesthetic Improvement
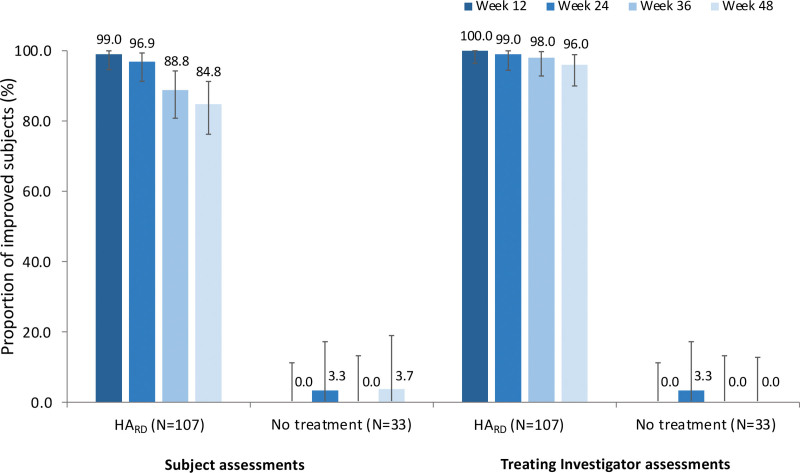
Aesthetic improvement, assessed by the Global Aesthetic Improvement Scale, was achieved in high proportions of subjects and maintained up to 48 weeks after the last injection with Restylane Defyne (Fig. 2). At week 12, greater than or equal to 99 percent of subjects in the Restylane Defyne group were assessed as improved, much improved, or very much improved on the Global Aesthetic Improvement Scale by both treating investigators and subjects. At week 48, 96 percent of subjects remained aesthetically improved according to the treating investigators and 85 percent according to the subjects. In the no-treatment control group, aesthetic improvement was reported in less than 4 percent of subjects. Representative photographs of subjects before and after treatment are shown in Figure 3.
Fig. 2.
Proportion of subjects with aesthetic improvement (±95 percent CI), assessed by subjects and treating investigators using the Global Aesthetic Improvement Scale. Improved subjects were defined as subjects recorded as improved, much improved, or very much improved on the seven-grade Global Aesthetic Improvement Scale. The 95 percent CI was calculated using the Clopper-Pearson method. HARD, hyaluronic acid filler Restylane Defyne.
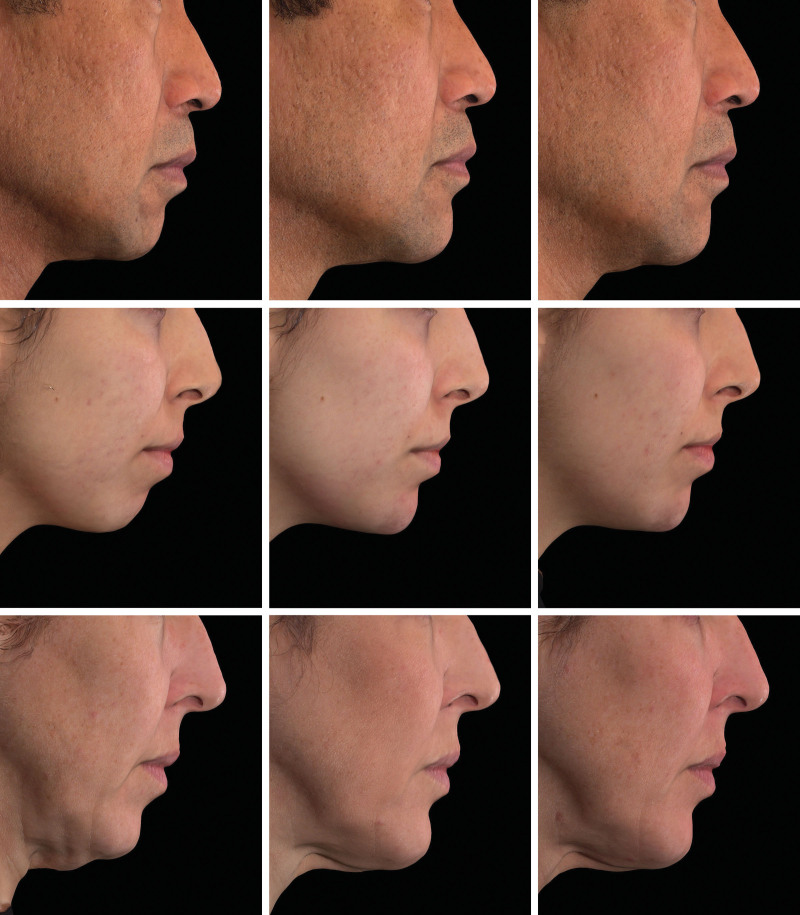
Fig. 3.
Representative photographs of male and female subjects before and after injection of Restylane Defyne in the chin. The subject above was injected with 2 ml at initial treatment (1 ml in the pogonion and 1 ml in the surrounding area) and 2 ml at touch-up (1 ml in the pogonion and 1 ml in the surrounding area). The subject in the center was injected with 2 ml at initial treatment (2 ml in the pogonion and 0 ml in the surrounding area) and 1.2 ml at touch-up (0.4 ml in the pogonion and 0.8 ml in the surrounding area). The subject shown below was injected with 2.9 ml at initial treatment (1.8 ml in the pogonion and 1.1 ml in the surrounding area) and 1.8 ml at touch-up (0.8 ml in the pogonion and 1 ml in the surrounding area). (Above) A 52-year-old man on day 1 before injection, with a Galderma Chin Retrusion Scale score of 2 (above, left); at week 12, a Galderma Chin Retrusion Scale score of 0 (above, center); and at week 48, a Galderma Chin Retrusion Scale score of 1 (above, right). (Center) A 22-year-old woman on day 1 before injection, with a Galderma Chin Retrusion Scale score of 2 (center, left); at week 12, with a Galderma Chin Retrusion Scale score of 0 (center, center); and at week 48, with a Galderma Chin Retrusion Scale score of 0 (center, right). (Below) A 44-year-old woman on day 1 before injection, with a Galderma Chin Retrusion Scale score of 2 (below, left); at week 12, with Galderma Chin Retrusion Scale score of 1 (below, center); and at week 48, with a Galderma Chin Retrusion Scale score of 1 (below, right).
Satisfaction with Chin (FACE-Q)
The FACE-Q Satisfaction with Chin scale mean Rasch-transformed total scores (a 100-point scale, with higher scores representing greater satisfaction) showed significantly improved subject satisfaction with chin appearance after treatment with Restylane Defyne (78.6) compared to the no-treatment control (35.1) at week 12. Both groups had similar mean scores at baseline: 37.4 for Restylane Defyne versus 34.6 for the control. The treatment difference in change from baseline to week 12 was statistically significant: 40.7 (95 percent CI, 33.7 to 47.8; p < 0.001, t test, baseline observation carried forward).
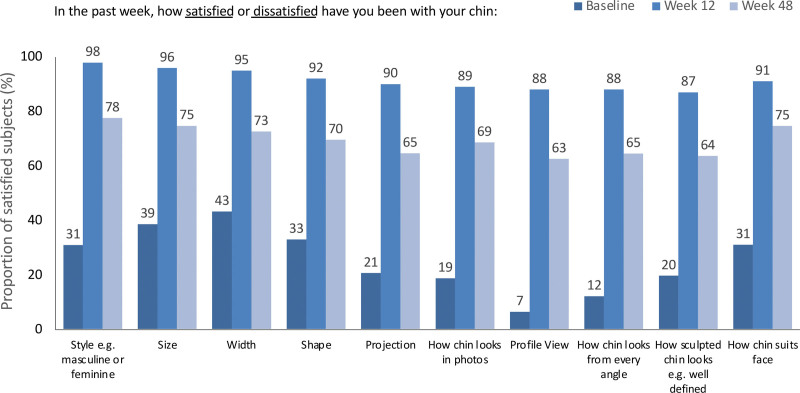
On review of the individual items of the FACE-Q Satisfaction with Chin scale, greater than or equal to 87 percent of subjects in the Restylane Defyne group reported being very satisfied or somewhat satisfied with each of the 10 items at week 12 (Fig. 4). Greater than or equal to 95 percent of subjects were satisfied with each of the items (i.e., style, size, and width) of their chin at week 12. At week 48, 63 to 78 percent remained satisfied with each of the 10 items of the scale.
Fig. 4.
FACE-Q Satisfaction with Chin scale, individual items: proportion of subjects satisfied with their chin before and after treatment with Restylane Defyne. Satisfied subjects include those who responded “very satisfied” or “somewhat satisfied.”
Volume Change
A statistically significant volume increase in the chin was measured using three-dimensional photography in subjects treated with Restylane Defyne. The mean increase from baseline was between 2.5 and 3.0 ml across all time points in the Restylane Defyne group, whereas subjects in the no-treatment group had a volume decrease of between 0.3 and 0.6 ml. The treatment difference in favor of Restylane Defyne was statistically significant at all time points (p < 0.001, data not shown).
Safety
In total, 14 percent of subjects had treatment-related adverse events after initial Restylane Defyne treatment (Table 3). Treatment-related adverse events had a median duration of 4 days, and all events were mild, except one event of implant-site pain, which was moderate. The most common treatment-related adverse events after initial treatment were implant-site pain (5 percent of subjects), implant-site bruising (2 percent), and implant-site swelling (2 percent). There were two events of implant-site nodule: one with delayed onset (53 days after treatment), reported as “irregular resorption of product causing a 6-mm nodule” that resolved after 74 days, following treatment with hyaluronidase; and one with onset on the day of treatment, reported as “filler nodule on chin,” which resolved after 112 days without intervention. There were no serious treatment-related adverse events in the study.
Table 3.
Adverse Events
| Initial Treatment* with HARD (n = 129) | ||
|---|---|---|
| No. of Subjects (%) | No. of Events | |
| All AEs | ||
| Any | 41 (31.8) | 71 |
| Serious† | 1 (0.8) | 1 |
| Treatment-related AEs | ||
| Any | 18 (14.0) | 24 |
| Mild | 17 (94.4) | 23 |
| Moderate | 1 (5.6) | 1 |
| Most common treatment-related AEs occurring in ≥2 subjects | ||
| Implant-site pain | 6 (4.7) | 8 |
| Implant-site bruising | 3 (2.3) | 3 |
| Implant-site swelling | 3 (2.3) | 3 |
| Implant-site erythema | 2 (1.6) | 2 |
| Implant-site hemorrhage | 2 (1.6) | 2 |
| Implant-site nodule | 2 (1.6) | 2 |
HARD, hyaluronic acid filler Restylane Defyne; AEs, adverse events.
*Includes subjects who were initially treated with HARD on day 1 and subjects randomized to no treatment who received optional treatment at wk 48.
†The serious adverse event was not related to treatment.
Lower lip function, sensation, and movement and chin function and sensation were normal in all subjects at all visits after treatment, and there were no changes in hair growth on the chin of male subjects (data not shown). The predefined, injection-related events reported by the subjects in the 4-week diary were considered tolerable and mostly resolved within 14 days. The most common symptoms reported in the diaries after initial treatment were tenderness (90 percent of subjects), pain (74 percent), and swelling (74 percent).
DISCUSSION
This trial was specifically designed to evaluate nonpermanent correction of chin retrusion using Restylane Defyne filler in a randomized, controlled setting. The specific and validated chin retrusion scale (Galderma Chin Retrusion Scale)14 captured projection changes of the chin, the most important measure of chin retrusion, and this was also the primary endpoint in the study. Galderma Chin Retrusion Scale assessments were performed by blinded evaluators to minimize bias. Other aspects of chin appearance (e.g., shape and width), which are also important for chin attractiveness,4,5 were captured in the FACE-Q Satisfaction with Chin scale, further complemented by evaluation of overall aesthetic improvement by subjects and investigators, and objective measurements of chin volume change.
As is common for dermal filler treatments, a first injection was given at baseline, followed by a touch-up injection 4 weeks later to obtain optimal correction. Subjects were followed for 48 weeks, enabling evaluation of short- and long-term effects of hyaluronic acid filler injections in the chin against a no-treatment control.
The primary objective was met. High effectiveness was shown using the Galderma Chin Retrusion Scale (81 percent responder rate in the Restylane Defyne group at week 12), and statistically significant effects (Galderma Chin Retrusion Scale response) persisted for up to 48 weeks in 74 percent of subjects, demonstrating long-lasting correction of chin retrusion in most subjects.
The long treatment effect was confirmed by the Global Aesthetic Improvement Scale results, where almost all subjects (≥96 percent according to treating investigators, greater than or equal to 85 percent according to subjects) had aesthetic improvement up to the week-48 time point. Long-term effect confirmation was also seen in the objective measurements of volume increases in the chin that remained at similar levels from week 24 to week 48 after injection of Restylane Defyne.
Subject-reported FACE-Q results showed the highest increase in subject satisfaction with their chin after treatment at 12 weeks. There were only minor differences in the responses to the individual items constituting the FACE-Q scale at each time point, indicating improved satisfaction across a wide range of chin qualities, most notably for style (e.g., masculine or feminine), size, and width of the chin, for which greater than or equal to 95 percent of subjects reported satisfaction at week 12.
The present study included subjects representing diverse races, ethnicities, and Fitzpatrick skin types, and men and women of different ages. The subgroup analyses confirmed that the treatment efficacy shown in the primary endpoint was replicated in all of these subgroups, indicating that comparable chin retrusion correction can be expected across different skin types, subject populations, genders, and age groups with Restylane Defyne.
The use of hyaluronic acid fillers to augment and correct the chin’s shape and projection may be preferred for subjects who seek a less invasive treatment compared to chin implant surgery or who have not yet decided to make permanent changes to their facial appearance.4
With regard to choice of hyaluronic acid filler, products other than Restylane Defyne could also be expected to provide suitable projection to the chin, such as gels produced using the nonanimal stabilized hyaluronic acid technology (e.g., Restylane Lyft), which have greater firmness (higher G′) than Restylane Defyne.10 However, the firmer nonanimal stabilized hyaluronic acid gels tend to provide less flexibility,10 and because the chin participates in lower face movement,7,8 a hyaluronic acid filler capable of providing both adequate projection and high flexibility was selected for this study.10 In addition, Restylane Defyne can also be used to correct other areas of the lower face, such as the nasolabial folds.15
The injections of Restylane Defyne in the chin region were well tolerated in this study, in line with previous experience in other facial areas with this product.12,13,15,16 The adverse event profile reported in this study was also similar to prior experience from hyaluronic acid injection in the chin area.17
CONCLUSIONS
Injection of Restylane Defyne was safe and effective for augmentation of the chin region to improve the chin profile for up to 48 weeks, as assessed by blinded evaluation. High aesthetic improvement was demonstrated both by the treating investigators’ and by the subjects’ assessments throughout the study. The rates of subject-reported satisfaction with the chin remained high up to 48 weeks after the last Restylane Defyne injection.
ACKNOWLEDGMENT
The authors acknowledge the contributions of scientific writer Anna-Karin Berg in preparation of the manuscript.
Supplementary Material
Footnotes
This trial is registered under the name “A Study to Evaluate the Safety and Efficacy of Restylane Defyne for Chin Augmentation and Correction of Chin Retrusion,” ClinicalTrials.gov identification no. NCT03624816 (https://clinicaltrials.gov/ct2/show/NCT03624816).
Presented at the American Society for Dermatologic Surgery Virtual Annual Meeting, October 9 through 11, 2020; Maui Derm, in Maui, Hawaii, January 25 through 29, 2021; the ODAC Dermatology, Aesthetic and Surgical Conference, in Orlando, Florida, January 14 through 17, 2021; the Winter Clinical Dermatology Conference, January 15 through 24, 2021; and the Aesthetic and Anti-Aging Medicine World Congress, in Monaco, September 16 through 18, 2021.
Related digital media are available in the full-text version of the article on www.PRSJournal.com.
Disclosure: Galderma R&D, LLC, funded the authors’ study and provided study product and medical writing assistance. Dr. Marcus is an investigator for Galderma, a speaker for Galderma, Allergan, and Evolus, a trainer for Galderma and Merz, and an advisory board member for Galderma, Allergan, Merz, and Evolus. Dr. Moradi is an investigator, consultant, and advisory board member for Galderma, Allergan, and Merz, and faculty for Allergan. Dr. Kaufman-Janette is a paid advisory board consultant, clinical trial investigator, and speaker for Galderma. Drs. Ablon, Chapas, and Rivkin are investigators for Galderma. Dr. Donofrio is an investigator for Galderma, Allergan, and Revance, and an advisory board member and consultant for Galderma. Dr. Kazin is an investigator and trainer for Galderma. Dr. Rohrich is an investigator for Galderma and Allergan and a medical monitor for Merz, and has received grants from Galderma and Allergan. Dr. Weiss is an investigator for Galderma, Allergan, Merz, Evolus, Revance, and Croma, an advisory board member for Galderma, Allergan, Revance, and BTL Aesthetic, and a consultant for BTL Aesthetics and Canfield Scientific, and has received grants from Galderma. Dr. George is an investigator and consultant for Galderma, Allergan, and Merz.
REFERENCES
- 1.Naini FB, Donaldson AN, McDonald F, Cobourne MT. Assessing the influence of chin prominence on perceived attractiveness in the orthognathic patient, clinician and layperson. Int J Oral Maxillofac Surg. 2012;41:839–846. [DOI] [PubMed] [Google Scholar]
- 2.Grammer K, Fink B, Møller AP, Thornhill R. Darwinian aesthetics: Sexual selection and the biology of beauty. Biol Rev Camb Philos Soc. 2003;78:385–407. [DOI] [PubMed] [Google Scholar]
- 3.Danahey DG, Dayan SH, Benson AG, Ness JA. Importance of chin evaluation and treatment to optimizing neck rejuvenation surgery. Facial Plast Surg. 2001;17:91–97. [DOI] [PubMed] [Google Scholar]
- 4.Chin OY, Sykes JM. Optimizing the chin and jawline appearance: Does surgery or injection make sense? Facial Plast Surg. 2019;35:164–171. [DOI] [PubMed] [Google Scholar]
- 5.Vanaman Wilson MJ, Jones IT, Butterwick K, Fabi SG. Role of nonsurgical chin augmentation in full face rejuvenation: A review and our experience. Dermatol Surg. 2018;44:985–993. [DOI] [PubMed] [Google Scholar]
- 6.Sykes JM, Fitzgerald R. Choosing the best procedure to augment the chin: Is anything better than an implant? Facial Plast Surg. 2016;32:507–512. [DOI] [PubMed] [Google Scholar]
- 7.Gerstner GE, Lafia C, Lin D. Predicting masticatory jaw movements from chin movements using multivariate linear methods. J Biomech. 2005;38:1991–1999. [DOI] [PubMed] [Google Scholar]
- 8.Kohler CG, Turner T, Stolar NM, et al. Differences in facial expressions of four universal emotions. Psychiatry Res. 2004;128:235–244. [DOI] [PubMed] [Google Scholar]
- 9.Segura S, Anthonioz L, Fuchez F, Herbage B. A complete range of hyaluronic acid filler with distinctive physical properties specifically designed for optimal tissue adaptations. J Drugs Dermatol. 2012;11(Suppl):s5–s8. [PubMed] [Google Scholar]
- 10.Öhrlund Å. Evaluation of rheometry amplitude sweep cross-over point as an index of flexibility for HA fillers. J Cosmet Dermatol Sci Appl. 2018;8:47–54. [Google Scholar]
- 11.Lundgren B, Sandkvist U, Bordier N, Gauthier B. Using a new photo scale to compare product integration of different hyaluronan-based fillers after injection in human ex vivo skin. J Drugs Dermatol. 2018;17:982–986. [PubMed] [Google Scholar]
- 12.Philipp-Dormston WG, Wong C, Schuster B, Larsson MK, Podda M. Evaluating perceived naturalness of facial expression after fillers to the nasolabial folds and lower face with standardized video and photography. Dermatol Surg. 2018;44:826–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solish N, Bertucci V, Percec I, Wagner T, Nogueira A, Mashburn J. Dynamics of hyaluronic acid fillers formulated to maintain natural facial expression. J Cosmet Dermatol. 2019;18:738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moradi A, Lin X, Allen S, Fagien S, Norberg M, Smith S. Validation of photonumeric assessment scales for temple volume deficit, infraorbital hollows, and chin retrusion. Dermatol Surg. 2020;46:1148–1154. [DOI] [PubMed] [Google Scholar]
- 15.Baumann L, Weiss RA, Grekin S, et al. Comparison of hyaluronic acid gel with (HARDL) and without lidocaine (HAJUP) in the treatment of moderate-to-severe nasolabial folds: A randomized, evaluator-blinded study. Dermatol Surg. 2018;44:833–840. [DOI] [PubMed] [Google Scholar]
- 16.Ascher B, Bayerl C, Kestemont P, Rzany B, Edwartz C, Podda M. A 12-month follow-up, randomized comparison of effectiveness and safety of two hyaluronic acid fillers for treatment of severe nasolabial folds. Dermatol Surg. 2017;43:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang SH, Tsai TF. Safety and effectiveness of hyaluronic acid fillers with lidocaine for full-face treatment in Asian patients. J Drugs Dermatol. 2020;19:836–842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.