Abstract
The 23-valent pneumococcal polysaccharide vaccine was formulated to prevent invasive infection in the elderly and other high-risk populations from the most prevalent Streptococcus pneumoniae serotypes. However, the immunogenicity of all 23 vaccine polysaccharides has not been fully characterized in elderly adults. We previously reported that whereas the majority of elderly subjects had vigorous immune responses to selected pneumococcal vaccine polysaccharides, a subset of elderly individuals responded to fewer than two of seven vaccine serotypes after immunization. To determine whether these elderly low responders have a general inability to respond to pneumococcal vaccine and to determine whether elderly low responders might be identified by their responses to a few polysaccharides, we measured antibody responses of elderly adults to all 23 vaccine polysaccharides after pneumococcal immunization. As a group, elderly subjects showed a significant rise after immunization in geometric mean antibody levels to all 23 vaccine serotypes. However, when individual rather than group immune responses were assessed, the 23-valent vaccine did not appear to be uniformly immunogenic in these elderly subjects. Eleven elderly subjects (20%) had twofold increases in specific antibody after vaccination to only 5 or fewer of the 23 vaccine polysaccharides, and they did not respond to the most prevalent serotypes causing invasive disease. Antibody responses to serotype 9N were found to reliably distinguish low vaccine responders from other elderly subjects. However, no particular group of vaccine polysaccharides could be used as a marker for adequate immune responses if only postvaccination sera were analyzed.
Effective prevention of Streptococcus pneumoniae infection has renewed priority in the present era, when the population of elderly adults at increased risk of pneumococcal pneumonia and invasive disease is expanding. Although the 23-valent pneumococcal polysaccharide (PPS) vaccine was formulated to prevent invasive infection in the elderly and other high-risk populations, the effectiveness of this vaccine for the growing population of adults over 65 years old remains controversial (3, 14, 21, 30–33). The variable efficacy of the pneumococcal vaccine in the elderly may reflect the variable immunogenicity of polysaccharide-based vaccines in this population. We have previously shown that the majority of elderly outpatients with stable, chronic illnesses monitored in a primary-care clinic had a vigorous immune response to pneumococcal vaccine that was comparable to that of healthy young adults (27). However, we identified a subset of elderly individuals who responded to fewer than two of seven serotypes tested at both 1 and 3 months after immunization. Presumably, if their lack of response to these particular seven PPSs indicates a general failure to respond to the majority of the 23 vaccine PPSs, these elderly low responders may be at particularly high risk for invasive pneumococcal infection with its attendant age-dependent mortality. Furthermore, if such elderly low responders could be easily identified, they should be the intended target of future efforts to develop a more immunogenic pneumococcal vaccine, whereas the current 23-valent PPS vaccine could be successfully used for the majority of elderly adults.
To date, the immunogenicity in elderly adults of all 23 PPSs included in the available pneumococcal vaccines is unknown. Previous reports of immune responses in the elderly have typically assayed only 6 to 10 of the 23 vaccine PPSs (11, 12, 15, 19, 25, 28), and many earlier studies were confounded by use of the 14-valent vaccine, use of radioimmunoassay methodology, or failure to adsorb antibodies to cell wall polysaccharides (1, 12, 15, 25, 26). Consequently, to determine whether a specific subset of elderly adults had poor immune responses to the majority of the vaccine PPSs and to determine whether such poor responders could be identified by their responses to a few PPSs, we measured the changes in capsular-polysaccharide-specific serum immunoglobulin G (IgG) to all 23 vaccine PPSs after pneumococcal immunization by using standardized enzyme-linked immunosorbent assay (ELISA) methods and reference standards.
MATERIALS AND METHODS
Subjects.
As described in detail previously (27), all 53 elderly subjects were male, with a mean age of 71 years (range, 65 to 84), and were receiving primary care at the Minneapolis Veterans Affairs Medical Center for chronic health problems. None were institutionalized or had acute illness at the time of vaccination. None had a history of pneumonia or previous vaccination. At the time of entry into the study, 20% were current or recent (had quit only within the last 2 years) smokers.
Immunization and collection of sera.
Each subject received an intradeltoid injection with 0.5 ml of a single lot of pneumococcal vaccine (Pnu-Immune 23; Lederle Laboratories [American Cyanamid], Pearl River, N.Y.) containing 25 μg of each of the following capsular polysaccharides (Danish nomenclature): types 1 to 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F. Blood for serum analysis was obtained prior to immunization. Equal aliquots of serum from blood samples obtained at 1 and 3 months after immunization, which were previously shown to have very similar levels for seven vaccine serotypes (27), were pooled and designated as postvaccination serum. Serum was stored at −70°C until it was assayed for capsule PPS-specific IgG.
Capsule PPS-specific IgG by ELISA.
Levels of pneumococcal capsule-specific IgG were measured as previously described (27). A reference pooled human serum sample was standardized by comparing it with the international standard serum 89-SF (a gift of Carl Frasch, Food and Drug Administration, Bethesda, Md.), for which the concentrations of vaccine type-specific IgG antibody have been established for 15 of the 23 vaccine serotypes (6, 24). Concentrations of specific IgG antibody in serum 89-SF for serotypes without established standard values were determined by a cross-standardization method (6): PPS 8, 5.74 μg/ml; PPS 9N, 7.90 μg/ml; PPS 10A, 5.41 μg/ml; PPS 12F, 4.16 μg/ml; PPS 15B, 14.20 μg/ml; PPS 17F, 8.19 μg/ml; PPS 22F, 5.48 μg/ml; and PPS 33F, 7.69 μg/ml.
Purified capsular PPSs were bound to 96-well polystyrene microtiter plates (Nunc Maxisorp, PGC Scientifics Corp., Gaithersburg, Md.) at 0.5 μg per 100 μl of phosphate-buffered saline per well by overnight incubation at 4°C. Because inspection of standard curves suggested that PPS 3 did not bind reproducibly to polystyrene plates, as suggested by others, plates were first coated with methylated albumin prior to capture with PPS 3, as described (6). All sera and standards were preadsorbed with cell wall polysaccharide (10 μg/ml of serum; Statens Seruminstitut, Copenhagen, Denmark) at room temperature for 30 min. Sera were applied to plates at an initial dilution of 1:200 in 1% bovine serum albumin with 0.05% (vol/vol) Tween 20 in phosphate-buffered saline. IgG that was reactive with PPS was detected with affinity-purified horseradish peroxidase-conjugated goat anti-human IgG (Jackson Laboratories, Bar Harbor, Maine). Every plate included a positive laboratory reference and control serum sample that contained a known level of IgG for the relevant PPS to assess the coefficient of variation of measurement between plates; those with coefficients of variation greater than 12% were repeated. Results are reported in micrograms of IgG per milliliter, based upon comparison with the international standard serum 89-SF.
As a marker of overall immune response to the vaccine, serum IgG concentrations were also measured by using a semiquantitative assay in which plates were coated with 11.5 μg of the whole 23-valent vaccine as previously described (5). Values were calculated as ELISA units of specific IgG per milliliter by comparing them with the 89-SF reference serum, which was assigned a value of 10,000 ELISA U per ml.
Statistics.
Differences in geometric mean concentrations (GMC) of capsule-specific IgG in serum between prevaccination and postvaccination sera were calculated by paired t tests. Correlation coefficients were calculated by using the Pearson method for linearly related variables and the Kendall tau-b method for ordinal variables. Cluster analysis of antibody responses to the 23 vaccine PPSs was performed by using average linkage between groups by the Pearson correlation method. Statistics were calculated by using SPSS for Windows 6.1 (Chicago, Ill.), and all P values were two-tailed.
RESULTS
Prior to immunization, the group of elderly subjects had appreciable levels of PPS-specific IgG for all 23 vaccine PPSs, with the GMC in serum for serotypes ranging from 1.5 to 8.1 μg/ml (Table 1). Prevaccination GMC in serum for this group of elderly adults did not correlate with the prevalence of the respective serotypes isolated from adults in Minnesota from 1995 to 1998 (17) (P = 0.96). Although the highest mean levels of prevaccination PPS-specific IgG were found for the most common isolate, type 14, some of the lowest mean baseline antibody levels were detected for other prevalent serotypes (4, 3, 22F, and 8).
TABLE 1.
Prevaccination antibody levels for capsular PPSs in elderly subjects
| Serotypea | GMC of antibody (μg/ml) | 95% confidence interval | % of elderly with <1 μg/ml concn |
|---|---|---|---|
| 14 | 8.12 | 6.58–10.03 | 0 |
| 4 | 2.72 | 1.99–3.73 | 18.5 |
| 23F | 3.98 | 3.13–5.06 | 7.4 |
| 1 | 3.82 | 2.73–5.34 | 11.1 |
| 3 | 2.26 | 1.95–2.61 | 7.4 |
| 22F | 2.11 | 1.59–2.79 | 33.3 |
| 6B | 3.38 | 2.57–4.44 | 11.1 |
| 8 | 1.54 | 1.19–2.00 | 27.8 |
| 19A | 3.96 | 2.97–5.27 | 5.6 |
| 12F | 2.34 | 1.68–3.24 | 18.5 |
| 19F | 5.61 | 4.32–7.29 | 5.6 |
| 7F | 3.46 | 2.60–4.61 | 11.1 |
| 18C | 3.90 | 2.98–5.11 | 9.3 |
| 9V | 3.57 | 2.66–4.79 | 11.1 |
| 33F | 2.85 | 2.02–4.04 | 31.5 |
| 9N | 4.18 | 3.20–5.45 | 7.4 |
| 5 | 4.19 | 3.20–5.47 | 7.4 |
| 11A | 4.18 | 3.30–5.31 | 5.6 |
| 20 | 5.89 | 4.42–7.86 | 5.6 |
| 15B | 4.83 | 3.75–6.22 | 5.6 |
| 10A | 4.32 | 3.08–6.05 | 5.6 |
| 17F | 3.17 | 2.38–4.23 | 16.7 |
| 2 | 2.37 | 1.79–3.13 | 18.5 |
In order of decreasing prevalence of vaccine serotypes among 791 invasive S. pneumoniae isolates from adults over 35 years old in Minnesota from 1995 to 1998 (17).
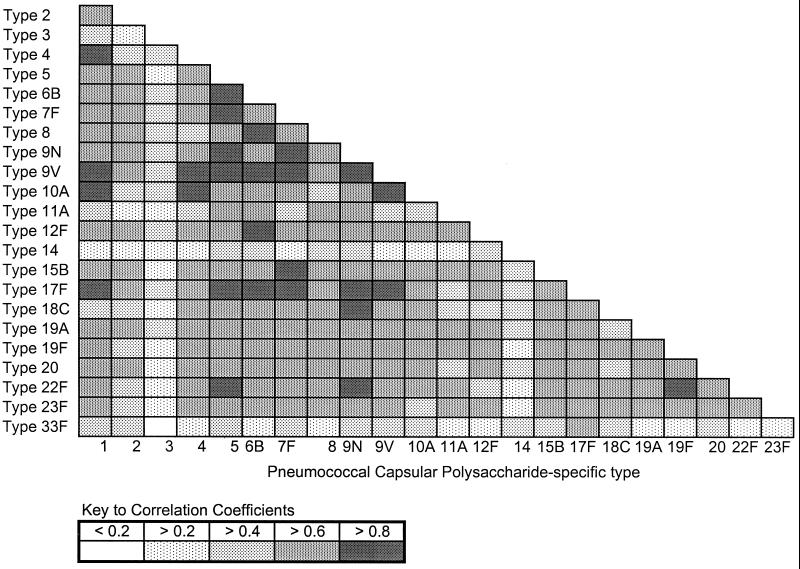
Comparison of each elderly subject's baseline antibody levels for the 23 vaccine PPSs by cluster analysis revealed a high correlation between the concentrations within individuals of PPS-specific IgG for 20 of the 23 serotypes (Fig. 1). In contrast, prevaccination antibody levels for PPSs 14, 3, and 33F correlated distinctly more poorly with those for other vaccine PPSs. In addition, there was no correlation in prevaccination antibody levels between these three distinct PPSs.
FIG. 1.
Correlation matrix of prevaccination antibody levels for 23 vaccine PPSs in the elderly. Baseline antibody levels of elderly subjects were compared by using the Pearson correlation for each pair of vaccine serotypes. Correlation coefficients for each comparison of each pair of PPSs are indicated by degree of shading.
Despite the prevalence of baseline antibody for vaccine PPSs, appreciable proportions of the elderly group had serum antibody levels for particular PPSs of less than 1 μg/ml, a level of serotype specific antibody reported to correlate with significant opsonophagocytic activity (34). Over 25% of the elderly subjects had low baseline antibody levels for serotypes 22F, 8, and 33F, and over 15% of the study group had low antibody levels for serotypes 4, 12F, 17F, and 2.
As a group, elderly subjects showed a statistically significant rise (P < 0.001) in GMC in serum of PPS-specific IgG to all vaccine serotypes after immunization (Table 2). GMC in serum of specific antibody increased after vaccination by greater than 1 μg/ml for all serotypes except type 3 (Table 2). Of note, immune responses after vaccination correlated inversely (P = 0.009) with the prevalence of the respective serotype as an invasive strain among adults in Minnesota. Increases in PPS-specific IgG determined as either change in GMC or as fold rise were generally smaller for more prevalent serotypes and greater for less prevalent types (Table 2). In addition, the immunogenicity of the 23 vaccine PPSs measured as an absolute change in GMC of specific antibody correlated (P = 0.04) with baseline antibody levels (Fig. 2). Serotypes for which these elderly subjects had the lowest baseline antibody levels generally elicited less increases in antibody after vaccination, and those for which they had higher baseline antibody levels generally produced greater increases. Absolute changes in specific antibody GMC after vaccination also were significantly correlated with immunogenicity as determined by fold rise in specific antibody concentrations for all serotypes (P < 0.001), even though this latter calculation was influenced by baseline antibody concentrations. In this elderly group, geometric mean fold rises in specific antibody after vaccination were greater than twofold for all serotypes except type 3. Thus, by these criteria, 21 of the 23 vaccine polysaccharides (excluding types 3 and 4) appeared to be highly immunogenic in this group of elderly subjects.
TABLE 2.
Change in capsular polysaccharide-specific antibody after pneumococcal vaccination of elderly
| Serotypea | GMC antibody increase (μg/ml) | % with >1 μg/ml increase in concn | Mean fold rise | % with >2-fold rise |
|---|---|---|---|---|
| 14 | 11.07 | 77.8 | 2.36 | 51.9 |
| 4 | 3.18 | 66.7 | 2.17 | 44.4 |
| 23F | 6.37 | 75.9 | 2.60 | 53.7 |
| 1 | 5.74 | 83.3 | 2.50 | 46.3 |
| 3 | 1.00 | 50.0 | 1.44 | 31.5 |
| 22F | 3.79 | 66.7 | 2.80 | 51.9 |
| 6B | 6.18 | 75.9 | 2.83 | 53.7 |
| 8 | 3.52 | 70.4 | 3.28 | 63.0 |
| 19A | 7.18 | 75.9 | 2.82 | 55.6 |
| 12F | 3.22 | 64.8 | 2.38 | 53.7 |
| 19F | 8.82 | 85.2 | 2.57 | 63.0 |
| 7F | 10.68 | 83.3 | 4.09 | 63.0 |
| 18C | 9.34 | 88.9 | 3.39 | 63.0 |
| 9V | 8.20 | 79.6 | 3.30 | 64.8 |
| 33F | 15.90 | 90.7 | 6.57 | 77.8 |
| 9N | 11.83 | 87.0 | 3.83 | 66.7 |
| 5 | 9.83 | 83.3 | 3.35 | 63.0 |
| 11A | 4.75 | 81.5 | 2.13 | 44.4 |
| 20 | 13.73 | 88.9 | 3.33 | 64.8 |
| 15B | 14.88 | 88.9 | 4.08 | 75.9 |
| 10A | 9.54 | 79.6 | 3.21 | 55.6 |
| 17F | 4.48 | 68.5 | 2.41 | 51.9 |
| 2 | 7.74 | 83.3 | 4.27 | 72.2 |
In order of decreasing prevalence of vaccine serotypes among 791 invasive S. pneumoniae isolates from adults over 35 years old in Minnesota from 1995 to 1998 (17).
FIG. 2.
Antibody responses of elderly adults to 23 vaccine polysaccharides. GMC of polysaccharide-specific IgG were determined for elderly adults prior to pneumococcal vaccination (black bars) and after vaccination (total bar height). Serotypes are arranged in order of lowest to highest increase in antibody levels after vaccination, indicated by white portion of bars.
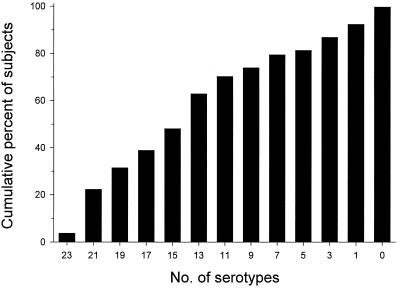
However, when individual rather than group immune responses were assessed, the 23-valent vaccine did not appear to be uniformly immunogenic in these elderly subjects. Vaccination produced a twofold rise in specific antibody in less than 50% of the elderly subjects to common serotypes 4, 1, and 3, as well as to the less common serotype 11A. Furthermore, even the most immunogenic serotypes, 2, 15B, and 33F, produced twofold rises in specific antibody in only 72 to 78% of subjects (Table 2). When classified by the number of vaccine serotypes for which they mounted a twofold rise in specific antibody, only 2 of 53 elderly subjects responded to all 23 vaccine serotypes (Fig. 3). The median number of serotypes to which they responded was 14, with 25% of elderly subjects responding to fewer than 8 of the 23 PPSs. Eleven elderly subjects (20%) had twofold increases in specific antibody after vaccination for only 5 or fewer of the 23 vaccine PPSs and, by these conservative criteria, were designated low responders. Four of these low responders did not have twofold increases in specific antibody for any PPS, and two low responders showed a twofold rise for only a single vaccine PPS. None of these low responders had twofold increases after vaccination in specific antibody for serotypes 14, 4, 23F, 22F, 6B, 19A, 12F, 9V, 9N, and 20, which include the most common invasive pneumococcal infection-causing serotypes in North America (4, 13, 18, 22, 23). Thus, one out of five ambulatory elderly adults showed consistently impaired antibody responses to the majority of vaccine serotypes.
FIG. 3.
Cumulative immune responses of elderly adults to 23 vaccine polysaccharides after vaccination. Bars indicate the cumulative percent of elderly subjects showing at least a twofold increase in polysaccharide-specific IgG to the indicated number of serotypes. For example, only 3.7% of the elderly had twofold increases in antibody for all 23 vaccine serotypes, 48% had twofold increases for 15 or more serotypes, and 80% responded to 6 or more serotypes.
To determine whether their antibody responses to a few selected PPSs could distinguish low responders from other elderly adults with adequate responses to the pneumococcal vaccine, we first grouped the fold rises in specific IgG for the 23 vaccine PPSs among the elderly subjects by using cluster analysis. In these elderly subjects, immune responses to vaccine PPSs were highly correlated within groups of serotypes: group 1, serotypes 1, 19A, and 19F; group 2, serotypes 12F, 17F, 5, 7F, 8, 9N, 9V, 2, 20, 15B, and 33F; group 3, serotypes 18C and 4; and group 4, serotypes 22F, 23F, 14, 6B, 11A, and 3. Within these groups, a twofold increase in antibody levels for a single PPS, serotype 9N, could most efficiently discriminate the low responders from the adequate responders among these elderly adults. All of the elderly low responders were included among the 15 elderly subjects with less than a twofold response to serotype 9N. Furthermore, a twofold response to serotype 9N clearly delineated elderly adults with twofold responses to 12 or fewer of the 23 vaccine PPSs from those with twofold responses to more than 12 of the 23 vaccine PPSs.
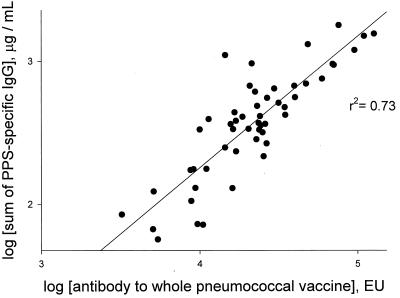
Although having a marker PPS for discriminating low from adequate responses to pneumococcal immunization is of great utility in research studies, these measurements require that the measurement of changes in specific IgG in sera be obtained before and after vaccination. However, in clinical application, patients do not routinely have serum drawn prior to pneumococcal immunization. Thus, we investigated whether the absolute levels of specific IgG for any group of vaccine PPSs or for the whole 23-valent pneumococcal vaccine measured in sera collected after vaccination could similarly distinguish low from adequate vaccine responders among elderly adults. Measurement of postvaccination antibody levels for the whole 23-valent vaccine appeared to be a useful estimate of overall antibody responses to the 23 vaccine PPSs, because for each subject antibody levels for the whole vaccine correlated significantly with the sum of the antibody levels for the 23 individual PPSs (Fig. 4). However, because of the variability between elderly subjects in levels of postvaccination PPS-specific IgG, no group of marker PPSs could be identified, nor were the results obtained by using the whole pneumococcal vaccine itself as the capture antigen predictive. Thus, although changes in antibody levels for serotype 9N appeared to be useful in research studies of pneumococcal vaccine immunogenicity in elderly adults, measurement of postimmunization antibody levels for a small group of PPSs or the total pneumococcal vaccine cannot be used clinically to distinguish low from more robust vaccine responders among the elderly.
FIG. 4.
Correlation of postvaccination antibody levels for whole pneumococcal vaccine and sum of antibody levels for 23 vaccine polysaccharides. For each elderly subject, the sums of postvaccination antibody levels for the 23 vaccine polysaccharides were calculated and plotted against their postvaccination antibody levels for the whole pneumococcal vaccine, as described in Materials and Methods. EU, ELISA unit.
DISCUSSION
This first comprehensive evaluation of antibody responses in the elderly adults older than 65 years to all 23 capsular polysaccharides comprising the pneumococcal vaccine revealed several important findings. First, the majority of elderly adults had detectable levels of natural antibody for vaccine PPSs prior to immunization, presumably reflecting antibodies acquired during life as a result of colonization or previous infection with S. pneumoniae or cross-reacting organisms. Second, pneumococcal vaccination significantly raised mean antibody levels for the 23 vaccine PPSs in this group of elderly subjects. Third, when individual rather than group immune responses were considered, uniform immunogenicity of all 23 vaccine PPSs was the exception rather than the rule, and a substantial proportion of elderly subjects had minimal antibody responses to the vaccine. Fourth, although changes in antibody levels for serotype 9N reliably distinguished elderly subjects who responded to the majority of vaccine PPSs from those responding more poorly, measurement of antibody levels only after vaccination did not allow discrimination of adequate from poor vaccine responders.
Our finding of appreciable preimmunization antibody levels for vaccine PPSs in most elderly subjects is generally in agreement with previous reports (8, 11, 15, 25, 26), although some studies have suggested that natural antibody levels decrease with aging (28, 29). Of note, several reports have suggested that among the elderly women may have lower prevaccination antibody levels for PPSs and that, on the other hand, smoking is associated with higher antibody levels (25, 28, 29). Consequently, the prevaccination antibody levels in our study group may have been relatively higher than those in some previous studies because our study population was comprised entirely of elderly men, 20% of whom had active or recent tobacco use. Although some earlier reports of high prevaccination antibody levels for PPSs in the elderly were confounded by failure to adsorb antibodies to cell wall polysaccharide, which may increase with aging, all samples and standards in our study were preadsorbed with cell wall polysaccharide before analysis by ELISA. Of note, prevaccination antibody levels for 20 of the 23 vaccine PPSs were highly correlated for elderly subjects. In agreement with a recent similar study in young healthy adults (7), prevaccination antibody levels for PPS 14 showed the lowest correlation with those to other vaccine PPSs. However, in contrast to this previous study, we found that antibody levels for PPSs 3 and 33F also correlated poorly with those to other vaccine PPSs in the elderly. Interestingly, in our study, these three serotypes represented the PPSs with the highest baseline antibody level (type 14), as well as the most immunogenic PPS (type 33F) and the least immunogenic PPS (type 3).
Antibody responses in the elderly to the vaccine PPSs correlated with the level of prevaccination antibody titers. Serotypes 33F, 15B, 9N, and 20, previously unstudied in the elderly, appeared to be the most immunogenic, both by fold rise and absolute rise criteria. In contrast, consistent with previous reports, types 4 and 3 were poorly immunogenic in our elderly subjects (2, 9, 11, 12, 28), the latter eliciting the lowest absolute and fold rise increases in specific antibody. Of note, serotypes 3 and 4 account for many of the vaccine failures seen in clinical efficacy trials (2, 9), and serotype 4 is a leading cause of invasive pneumococcal infection in North America (4, 13, 18, 22, 23) and in Finland (28). Thus, our findings suggest that future pneumococcal vaccines redesigned to improve immunogenicity in elderly adults should include PPSs 3 and 4.
Our direct study of antibody responses in elderly adults confirms many of the conclusions derived from an earlier meta-analysis of pneumococcal vaccine studies by Go and Ballas (10). In both reports, antibody responses to vaccine PPSs correlated with baseline antibody titers, implying that preexisting antibodies did not neutralize the vaccine antigens and preclude immunologic responses (10). Both reports conclude that elderly subjects rarely mounted a twofold response to all vaccine PPSs. Finally, we also found that the wide overlap of postvaccination antibody titers precluded establishing a biologically meaningful absolute postvaccination antibody titer as a cutoff level for vaccine responsiveness. In contrast, the conclusions from the previous meta-analysis that serotypes 3 and 1 were consistently immunogenic were not supported by our study, highlighting potential limitations of meta-analyses.
Analysis of antibody responses to all 23 vaccine PPSs confirmed previous observations that an appreciable proportion (20%) of elderly adults respond poorly to pneumococcal immunization (27). All elderly low responders failed to mount twofold responses to 10 particular vaccine PPSs, including those serotypes responsible for the majority of invasive pneumococcal infection in North America (4, 13, 16, 18, 22, 23). Whether such limited responses to a broad range of capsular polysaccharides in this subgroup has a genetic basis (20) or is age-related is under investigation. The importance of assessing individual rather than group immune responses among the elderly to pneumococcal vaccination was highlighted by the pneumococcal vaccine efficacy trial of Simberkoff et al. (32). In this large prospective Veterans Affairs Cooperative study, the pneumococcal vaccine recipients who subsequently had pneumococcal respiratory infection with vaccine serotypes were those who did not make or sustain serum antibodies against their infecting serotype (32). Thus, elderly low responders may represent those with the highest risk for pneumococcal infection, despite having received pneumococcal immunization. Unfortunately, our findings further suggest that low vaccine responders among elderly adults cannot be identified simply by measuring antibody levels for any selected group of vaccine PPSs after immunization. The recent trend toward determining functional immune responses (e.g., antibody avidity and antibody-mediated opsonophagocytosis) in addition to antibody levels after pneumococcal vaccination may yield assays that can more readily discriminate low vaccine responders and groups at increased risk for serious pneumococcal infection among the elderly.
ACKNOWLEDGMENTS
We thank Catherine Lexau, Ruth Lynfield, and Richard Danila, all from the Minnesota Department of Health, for providing unpublished prevalence data of invasive pneumococcal serotypes in Minnesota.
This work was supported by the Department of Veterans Affairs Research Service and by grants from the NIH (AI042240 to J.B.R. and AI39445 and HL 57880 to E.N.J.).
REFERENCES
- 1.Ammann A J, Schiffman G, Austrian R. The antibody response to pneumococcal capsular polysaccharides in aged individuals. Proc Soc Exp Biol Med. 1980;164:312–316. doi: 10.3181/00379727-164-40868. [DOI] [PubMed] [Google Scholar]
- 2.Bolan G, Broome C V, Facklam R R, Plikaytis B D, Fraser D W, Schlech W F., III Pneumococcal vaccine efficacy in selected populations in the United States. Ann Intern Med. 1986;104:1–6. doi: 10.7326/0003-4819-104-1-1. [DOI] [PubMed] [Google Scholar]
- 3.Butler J C, Breiman R F, Campbell J F, Lipman H B, Broome C V, Facklam R R. Pneumococcal polysaccharide vaccine efficacy. An evaluation of current recommendations. JAMA. 1993;270:1826–1831. [PubMed] [Google Scholar]
- 4.Butler J C, Hofmann J, Cetron M S, Elliott J A, Facklam R R, Breiman R F. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention's Pneumococcal Sentinel Surveillance System. J Infect Dis. 1996;174:986–993. doi: 10.1093/infdis/174.5.986. [DOI] [PubMed] [Google Scholar]
- 5.Carson P J, Schut R L, Simpson M L, O'Brien J, Janoff E N. Antibody class and subclass responses to pneumococcal polysaccharides following immunization of human immunodeficiency virus-infected patients. J Infect Dis. 1995;172:340–345. doi: 10.1093/infdis/172.2.340. [DOI] [PubMed] [Google Scholar]
- 6.Concepcion N, Frasch C E. Evaluation of previously assigned antibody concentrations in pneumococcal polysaccharide reference serum 89SF by the method of cross-standardization. Clin Diagn Lab Immunol. 1998;5:199–204. doi: 10.1128/cdli.5.2.199-204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coughlin R T, White A C, Anderson C A, Carlone G M, Klein D L, Treanor J. Characterization of pneumococcal specific antibodies in healthy unvaccinated adults. Vaccine. 1998;16:1761–1767. doi: 10.1016/s0264-410x(98)00139-x. [DOI] [PubMed] [Google Scholar]
- 8.Fata F T, Herzlich B C, Schiffman G, Ast A L. Impaired antibody responses to pneumococcal polysaccharide in elderly patients with low serum vitamin B12 levels. Ann Intern Med. 1996;124:299–304. doi: 10.7326/0003-4819-124-3-199602010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Forrester H L, Jahnigen D W, LaForce F M. Inefficacy of pneumococcal vaccine in a high-risk population. Am J Med. 1987;83:425–430. doi: 10.1016/0002-9343(87)90751-0. [DOI] [PubMed] [Google Scholar]
- 10.Go E S, Ballas Z K. Anti-pneumococcal antibody response in normal subjects: a meta-analysis. J Allergy Clin Immunol. 1996;98:205–215. doi: 10.1016/s0091-6749(96)70244-0. [DOI] [PubMed] [Google Scholar]
- 11.Hedlund J U, Kalin M E, Ortqvist A B, Henrichsen J. Antibody response to pneumococcal vaccine in middle-aged and elderly patients recently treated for pneumonia. Arch Intern Med. 1994;154:1961–1965. [PubMed] [Google Scholar]
- 12.Hilleman M R, Carlson A J, Jr, McLean A A, Vella P P, Weibel R E, Woodhour A F. Streptococcus pneumoniae polysaccharide vaccine: age and dose responses, safety, persistence of antibody, revaccination, and simultaneous administration of pneumococcal and influenza vaccines. Rev Infect Dis. 1981;3(Suppl.):S31–S42. doi: 10.1093/clinids/3.supplement_1.s31. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann J, Cetron M S, Farley M M, Baughman W S, Facklam R R, Elliott J A, Deaver K A, Breiman R F. The prevalence of drug-resistant Streptococcus pneumoniae in Atlanta. N Engl J Med. 1995;333:481–486. doi: 10.1056/NEJM199508243330803. [DOI] [PubMed] [Google Scholar]
- 14.Koivula I, Stén M, Leinonen M, Mäkelä P H. Clinical efficacy of pneumococcal vaccine in the elderly: a randomized, single-blind population-based trial. Am J Med. 1997;103:281–290. doi: 10.1016/s0002-9343(97)00149-6. [DOI] [PubMed] [Google Scholar]
- 15.Konradsen H B. Quantity and avidity of pneumococcal antibodies before and up to five years after pneumococcal vaccination of elderly persons. Clin Infect Dis. 1995;21:616–620. doi: 10.1093/clinids/21.3.616. [DOI] [PubMed] [Google Scholar]
- 16.Lee C J, Banks S D, Li J P. Virulence, immunity, and vaccine related to Streptococcus pneumoniae. Crit Rev Microbiol. 1991;18:89–114. doi: 10.3109/10408419109113510. [DOI] [PubMed] [Google Scholar]
- 17.Lexau, C., R. Lynfield, and R. Danila. (Minnesota Department of Health). Personal communication.
- 18.Lovgren M, Spika J S, Talbot J A. Invasive Streptococcus pneumoniae infections: serotype distribution and antimicrobial resistance in Canada, 1992–1995. Can Med Assoc J. 1998;158:327–331. [PMC free article] [PubMed] [Google Scholar]
- 19.Musher D M, Groover J E, Graviss E A, Baughn R E. The lack of association between aging and postvaccination levels of IgG antibody to capsular polysaccharides of Streptococcus pneumoniae. Clin Infect Dis. 1996;22:165–167. doi: 10.1093/clinids/22.1.165. [DOI] [PubMed] [Google Scholar]
- 20.Musher D M, Groover J E, Watson D A, Pandey J P, Rodriguez-Barradas M C, Baughn R E, Pollack M S, Graviss E A, deAndrade M, Amos C L. Genetic regulation of the capacity to make immunoglobulin G to pneumococcal capsular polysaccharides. J Investig Med. 1997;45:57–68. [PubMed] [Google Scholar]
- 21.Örtqvist Å, Hedlund J, Burman L Å, Elbel E, Höfer M, Leinonen M, Lindblad I, Sundelöf B, Kalin M, Swedish Pneumococcal Vaccination Study Group Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Lancet. 1998;351:399–403. doi: 10.1016/s0140-6736(97)07358-3. [DOI] [PubMed] [Google Scholar]
- 22.Parkinson A J, Davidson M, Fitzgerald M A, Bulkow L R, Parks D J. Serotype distribution and antimicrobial resistance patterns of invasive isolates of Streptococcus pneumoniae: Alaska 1986–1990. J Infect Dis. 1994;170:461–464. doi: 10.1093/infdis/170.2.461. [DOI] [PubMed] [Google Scholar]
- 23.Plouffe J F, Moore S K, Davis R, Facklam R R. Serotypes of Streptococcus pneumoniae blood culture isolates from adults in Franklin County, Ohio. J Clin Microbiol. 1994;32:1606–1607. doi: 10.1128/jcm.32.6.1606-1607.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quataert S A, Kirch C S, Quackenbush Wiedl L J, Phipps D C, Strohmeyer S, Cimino C O, Skuse J, Madore D V. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin Diagn Lab Immunol. 1995;2:590–597. doi: 10.1128/cdli.2.5.590-597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roghmann K J, Tabloski P A, Bentley D W, Schiffman G. Immune response of elderly adults to pneumococcus: variation by age, sex, and functional impairment. J Gerontol. 1987;42:265–270. doi: 10.1093/geronj/42.3.265. [DOI] [PubMed] [Google Scholar]
- 26.Ruben F L, Uhrin M. Specific immunoglobulin-class antibody responses in the elderly before and after 14-valent pneumococcal vaccine. J Infect Dis. 1985;151:845–849. doi: 10.1093/infdis/151.5.845. [DOI] [PubMed] [Google Scholar]
- 27.Rubins J B, Puri A K G, Loch J, Charboneau D, MacDonald R, Opstad N, Janoff E N. Magnitude, duration, quality and function of pneumococcal vaccine responses in elderly adults. J Infect Dis. 1998;178:431–440. doi: 10.1086/515644. [DOI] [PubMed] [Google Scholar]
- 28.Sankilampi U, Honkanen P O, Bloigu A, Herva E, Leinonen M. Antibody response to pneumococcal capsular polysaccharide vaccine in the elderly. J Infect Dis. 1996;173:387–393. doi: 10.1093/infdis/173.2.387. [DOI] [PubMed] [Google Scholar]
- 29.Sankilampi U, Isoaho R, Bloigu A, Kivela S L, Leinonen M. Effect of age, sex and smoking habits on pneumococcal antibodies in an elderly population. Int J Epidemiol. 1997;26:420–427. doi: 10.1093/ije/26.2.420. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro E D, Berg A T, Austrian R, Schroeder D, Parcells V, Margolis A, Adair R K, Clemens J D. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325:1453–1460. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro E D, Clemens J D. A controlled evaluation of the protective efficacy of pneumococcal vaccine for patients at high risk of serious pneumococcal infections. Ann Intern Med. 1984;101:325–330. doi: 10.7326/0003-4819-101-3-325. [DOI] [PubMed] [Google Scholar]
- 32.Simberkoff M S, Cross A P, Al-Ibrahim M, Baltch A L, Geiseler P J, Nadler J, Richmond A S, Smith R P, Schiffman G, Shepard D S, Van Eeckhout J P. Efficacy of pneumococcal vaccine in high-risk patients: results of a Veterans Administration Cooperative study. N Engl J Med. 1986;315:1318–1327. doi: 10.1056/NEJM198611203152104. [DOI] [PubMed] [Google Scholar]
- 33.Sims R V, Steinmann W C, McConville J H, King L R, Zwick W C, Schwartz J S. The clinical effectiveness of pneumococcal vaccine in the elderly. Ann Intern Med. 1988;108:653–657. doi: 10.7326/0003-4819-108-5-653. [DOI] [PubMed] [Google Scholar]
- 34.Viõarsson G, Jónsdóttir I, Jónsson S, Valdimarsson H. Opsonization and antibodies to capsular and cell wall polysaccharides of Streptococcus pneumoniae. J Infect Dis. 1994;170:592–599. doi: 10.1093/infdis/170.3.592. [DOI] [PubMed] [Google Scholar]