Abstract
In the 1980s, Shiga toxin (Stx)-producing Escherichia coli O157:H7 (STEC) was identified as a cause of hemorrhagic colitis in the United States and was found to be associated with hemolytic uremic syndrome (HUS), a microangiopathic hemolytic anemia characterized by thrombocytopenia and renal failure. The precise way that Stxs cause hemorrhagic colitis and HUS is unclear. Stxs have been thought to cause disease by killing or irreversibly harming sensitive cells through a nonspecific blockade of mRNA translation, eventually resulting in cytotoxicity by preventing synthesis of critical molecules needed to maintain cell integrity. Because STEC is noninvasive, we have been exploring the host-toxin response at the level of the gastrointestinal mucosa, where STEC infection begins. We have found that Stx is capable of interleukin-8 (IL-8) superinduction in a human colonic epithelial cell line. Despite a general blockade of mRNA translation, Stx treatment results in increased IL-8 mRNA as well as increased synthesis and secretion of IL-8 protein. Our data suggest that an active Stx A subunit is required for this activity. Ricin, which has the same enzymatic activity and trafficking pathway as Stx, has similar effects. Exploration of the effects of other protein synthesis inhibitors (cycloheximide, anisomycin) suggests a mechanism of gene regulation that is distinct from a general translational blockade. Use of the specific p38/RK inhibitor SB202190 showed that blocking of this pathway results in decreased Stx-mediated IL-8 secretion. Furthermore, Stxs induced mRNA of the primary response gene c-jun, which was subsequently partially blocked by SB202190. These data suggest a novel model of how Stxs contribute to disease, namely that Stxs may alter regulation of host cell processes in sensitive cells via activation of at least one member of the mitogen-activated protein kinase family in the p38/RK cascade and induction of c-jun mRNA. Stx-induced increases in chemokine synthesis from intestinal epithelial cells could be important in augmenting the host mucosal inflammatory response to STEC infection.
Infection with Shiga toxin (Stx)-producing Escherichia coli (STEC) is a cause of bloody diarrhea and hemorrhagic colitis in humans and has been epidemiologically associated with the subsequent development of thrombotic microangiopathy resulting in hemolytic uremic syndrome (HUS) (19). The pathogenomonic hallmark of diarrhea-associated thrombotic microangiopathy is the presence of fibrin-platelet thrombi in the microvasculature of involved tissues (23, 36), but our understanding of the role of Stxs in the pathogenesis of this syndrome is limited. Stxs have been found in the gastrointestinal tract of humans infected with STEC (1). Because STEC is a noninvasive pathogen, it is proposed that Stxs gain access to the systemic vasculature from the gut lumen and subsequently cause damage to microvascular endothelial cells in target organs such as the kidney and the central nervous system. Indeed, we have previously demonstrated that Stxs can traverse polarized intestinal epithelial cell monolayers in tissue culture and remain biologically active (2).
Intestinal STEC infection is a complex relationship between host and bacteria. Bacterial surface and secreted proteins, as well as host factors that mediate the inflammatory response, are involved (24, 26). Although initial epidemiologic studies suggested that STEC did not commonly provoke a dramatic intestinal inflammatory response (10), it has since been demonstrated that humans with STEC infection frequently have fecal leukocytes as well as a peripheral leukocytosis (33). In the rabbit model of STEC-associated hemorrhagic colitis, histopathologic specimens show severe inflammatory colitis with polymorphonuclear infiltrates (22). STEC infection in rabbits causes intestinal epithelial cell disruption that is prevented by pretreatment with a monoclonal antibody to the leukocyte adhesion molecule CD18 (8), suggesting that the host response to infection plays a major role in STEC infection in this model and that intestinal epithelial cell damage is not simply due to the direct cytotoxic action of Stxs.
How this intestinal pathogen initiates the host inflammatory response is under investigation. Chemokines of the C-X-C subfamily, such as interleukin-8 (IL-8), are known to recruit polymorphonuclear cells into areas of infection. Furthermore, IL-8 not only acts as a neutrophil chemokine but can also stimulate several responses in neutrophils essential for microbial killing, such as granule content release and the respiratory burst. By releasing granule constituents such as proteases, toxic oxygen products, and other proinflammatory substances, neutrophils that migrate to the gastrointestinal tract in response to infection can subsequently damage the intestinal mucosa (11, 35).
Enteropathogenic E. coli strains, which share several virulence factors with STEC but do not express Stxs (24), can attach to intestinal epithelial cell monolayers, stimulate IL-8 production, and cause neutrophil transmigration which is subsequently attenuated by use of an anti-IL-8 antibody (31). We have found that STEC attachment to intestinal epithelial cell monolayers can also cause neutrophil transmigration (13). In a rabbit model, animals infected with a rabbit enteropathogenic E. coli strain engineered to produce Stx (RDEC H19B) developed more severe disease with increased inflammatory changes and elevated mucosal IL-1 bioactivity levels than rabbits infected with the non-Stx-producing parent strain (5). These findings suggest that Stx itself, either directly or indirectly, has a significant proinflammatory role in pathogenesis.
We are currently investigating the hypothesis that direct proinflammatory effects of Stxs on the intestinal epithelium contribute to the disruption of this important barrier by host defenses, allowing enhanced uptake of toxins into the underlying tissues. We describe the effects of Stx1 and Stx2 on IL-8 mRNA levels and IL-8 protein synthesis by a human intestinal epithelial cell line. We characterize the apparent paradox of enhanced IL-8 secretion and inhibition of protein synthesis by Stxs. We explore the specificity of this effect by using other protein synthesis inhibitors that inhibit translation via different mechanisms. We also present data suggesting that Stxs may induce IL-8 via the ribotoxic stress response, inducing c-jun mRNA and activating at least one mitogen-activated protein kinase (MAPK) family member in the p38/RK cascade.
MATERIALS AND METHODS
Materials.
Cell culture media and additives were purchased from Gibco BRL-Life Technologies (Grand Island, N.Y.). IL-8 enzyme-linked immunosorbent assay (ELISA) kits and tumor necrosis factor alpha (TNF-α) were purchased from Endogen (Woburn, Mass.). Lectin from Ricinus communis (ricin), cycloheximide, and lipopolysaccharide from E. coli serotype O111:B4 were purchased from Sigma (St. Louis, Mo.). Anisomycin and SB202190 were purchased from Calbiochem (La Jolla, Calif.). [3H]leucine was purchased from New England Nuclear (Boston, Mass.).
Cell culture, treatments, IL-8 secretion, and protein synthesis inhibition.
Hct-8 cells, a human colonic carcinoma cell line, were obtained from the American Type Culture Collection (ATCC) and grown at 37°C in 5% CO2 in RPMI 1640 medium with l-glutamine, 10% fetal calf serum, 100 units of penicillin G sodium per ml, 100 μg of streptomycin sulfate per ml, 1 mM sodium pyruvate, and 10 mM HEPES to 95 to 100% confluency in 6-, 24-, or 96-well culture dishes. Preliminary data demonstrated that the 50% inhibitory dose (ID50) was variable and increased with increasing age and plating density of cells (data not shown). For this reason and to be consistent, cells were plated at 5 × 105 to 1 × 106 cells per ml and used at 24 to 48 h for all experiments. Confluent monolayers plated and used in this way were sensitive to Stxs with ID50s of approximately 1 μg/ml for Stx1 and 5 to 10 μg/ml for Stx2. Six-, 24-, or 96-well plates were seeded with 2 ml, 0.5 ml, or 0.1 ml of cells, respectively. Cells were exposed to the following agents for 18 to 24 h: TNF-α at 10 ng/ml, Stx1 and Stx2 at a range of concentrations from 1 ng/ml to 100 μg/ml, Stx1 and Stx2 inactivated by boiling for 4 h at a range of doses from 1 ng/ml to 1 μg/ml, ricin at a range of doses from 1 pg/ml to 1 ng/ml, cycloheximide at a range of doses from 10 μg/ml to 2.5 mg/ml, and anisomycin at a range of doses from 5 ng/ml to 10 μg/ml. For experiments using the p38 kinase inhibitor SB202190, cells were preincubated for 30 min with 3 μM inhibitor (approximately 10 times the 50% inhibitory concentration) or the inhibitor diluent dimethylsulfamethoxide (DMSO); then, Stx1 or anisomycin was added in the presence of either the inhibitor or DMSO for 18 h. Cell culture supernatants were collected, and IL-8 protein concentrations were determined by ELISA. The effects of Stx1, Stx2, ricin, cycloheximide, and anisomycin on protein synthesis were determined by measuring [3H]leucine incorporation in Hct-8 cells, as previously described for Vero cells (17). Briefly, following the various cell treatments, cell supernatants were removed and cells were washed and then incubated for 60 min in leucine-free medium to which 1 mCi of [3H]leucine per 100 ml was added. Incorporation of label into trichloroacetic acid-precipitable material was then measured. Boiled toxin controls were checked for biological activity and did not inhibit [3H]leucine incorporation in Vero cells.
RNA isolation, determination of IL-8 mRNA levels by semiquantitative RT-PCR, and Northern blotting.
Primer selections for IL-8 and β-actin genes were based on previously published sequences (18). Cells were treated with agents for 4 to 6 h. For experiments using the p38 kinase inhibitor SB202190, cells were preincubated for 30 min with 3 μM inhibitor or the inhibitor diluent DMSO; then, Stxs or anisomycin was added in the presence of either the inhibitor or DMSO for 4 h. Total RNA was recovered by one of the following two methods. The first method included lysis of cell monolayers in TriReagent (Sigma), followed by homogenization in QIAshredder spin columns (QIAGEN Inc., Santa Clarita, Calif.). Lysates underwent chloroform extraction, isopropanol precipitation, and washing with 70% ethanol in diethyl pyrocarbonate-treated water. The second method included lysis of cell monolayers in lysis reagent, homogenization in QIAshredder spin columns, and further processing according to the manufacturer's instructions (RNeasy; QIAGEN Inc.). The concentration of RNA was determined by measuring the optical density at 260 nm. One microgram of the extracted total RNA per 20-μl reaction volume was used for reverse transcription (RT) according to manufacturer's instructions (Reverse Transcription System kit; Promega, Madison, Wis.). Between 2 and 8 μl of cDNA was used for each PCR. The cycle conditions were 1 min at 94°C, 1 min at 66°C, and 1 min at 72°C for 27 to 30 amplification cycles. The number of amplification cycles used was chosen based on maintenance of linearity of the PCR product response. Verification that the amplified PCR products originated from RNA rather than DNA was accomplished by preparing duplicate RNA samples without the reverse transcriptase step, followed by PCR amplification and visualization on agarose gels. Lack of products ensured that potential DNA contamination was absent.
For Northern blotting, 10 to 20 μg of total RNA was separated on glyoxal agarose gels and transferred to nylon membranes according to the manufacturer's instructions (NorthernMax Gly; Ambion, Inc., Austin, Tex.). IL-8 template DNA, a kind gift from Edouard Vannier, corresponds to IL-8 gene exon sequences from +38 in exon 1 to the EcoRI site in exon 4. This template DNA was subcloned, and an RNA probe was synthesized by using T3 polymerase and modified CTP according to the manufacturer's instructions (Strip-EZ RNA; Ambion, Inc.). A glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe was isolated from ATCC plasmid 57090. A probe for c-jun mRNA was synthesized as a 352-bp product by using previously described primers (14). Probes were labeled by cross-linking with psoralen-biotin and detected by chemiluminescence according to the manufacturer's instructions (BrightStar labeling and detection kits; Ambion, Inc.). Blots were hybridized overnight with the IL-8 probe at 65°C, the c-jun probe at 52°C, or the GAPDH probe at 45°C, followed by washing and detection. Bands were quantitated by scanning densitometry and use of QuantityOne software (Bio-Rad, Hercules, Calif.).
RESULTS
Effects of Stx1 and Stx2 on secretion of IL-8 by Hct-8 cells.
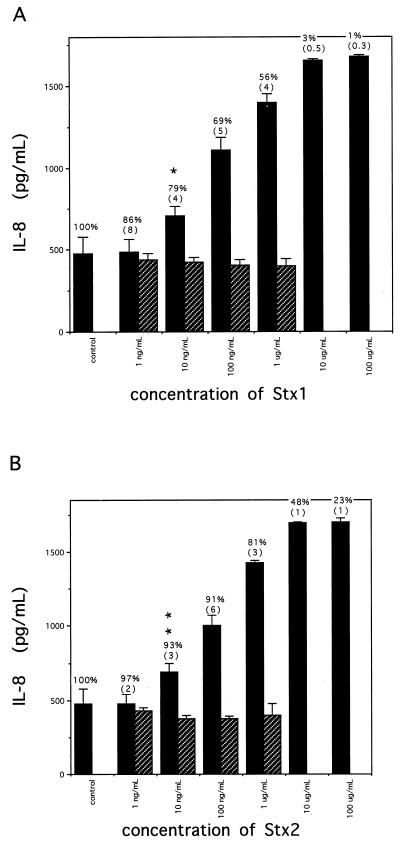
Addition of Stx1 or Stx2 to Hct-8 cells caused an increase in IL-8 secretion. The dose response of IL-8 protein secreted from Hct-8 cells incubated with various amounts of Stx1 or Stx2 for 18 h is shown in Fig. 1. The data shown are mean IL-8 levels and standard deviations (SD) for three to six wells per condition from a single representative experiment; at least three experiments demonstrated results similar to those shown in Fig. 1, though there was some variability in total IL-8 produced from experiment to experiment based on age and plating density of cells. In the experiment shown, unstimulated Hct-8 cells secreted 475 ± 101 pg of IL-8 per ml into cell supernatants after 18 h. Stx1 and Stx2 increased IL-8 secretion to a maximum of approximately 3.6-fold over control levels in a dose-dependent way. The potencies of Stx1 and Stx2 were similar; both stimulated increased IL-8 secretion into cell supernatants at doses of 10 ng/ml and higher. Exposure to Stx1 at 100 μg/ml and Stx2 at 100 μg/ml resulted in mean IL-8 levels in cell supernatants of 1,680 ± 9 and 1,702 ± 24 pg/ml, respectively. Stx1 and Stx2 inactivated by boiling did not stimulate IL-8 secretion above control levels. Purified Stx1 B (binding) subunit did not increase the amount of IL-8 protein found in conditioned media (data not shown).
FIG. 1.
Secretion of IL-8 by Hct-8 cells treated with Stxs for 18 h. For each concentration of Stx1 (A) or Stx2 (B), IL-8 measured in cell culture supernatants is shown (means ± SD from three to six wells). Solid bars represent native toxin; hatched bars represent boiled toxin. Inserted above each solid bar is mean protein synthesis expressed as a percentage of control (no toxin) levels, as determined by [3H]leucine incorporation at the end of the incubation. (SD are reported in parentheses.) Comparisons between Stx-induced IL-8 levels and control levels were calculated. P values are as follows: ∗, 0.009; ∗∗, 0.012.
Association between inhibition of protein synthesis by Stxs in Hct-8 cells and IL-8 secretion.
To investigate whether synthesis of IL-8 by Hct-8 cells was correlated with the known mechanism of action of Stx (inhibition of protein synthesis), we measured IL-8 synthesis by Hct-8 cells as a function of protein synthesis inhibition, using a range of doses as well as several doses over time. Figure 1 shows the percentage of protein synthesis inhibition achieved by a given amount of Stx1 or Stx2 as measured at the end of incubation (18 h after addition of toxin). Increasing amounts of Stx1 and Stx2 result in increasing inhibition of protein synthesis. As shown in Fig. 1A, Stx1 at a dose of 1 ng/ml inhibited protein synthesis to 86% of control levels but did not increase the levels of IL-8 secreted into cell supernatants. Stx1 began to affect IL-8 secretion into cell supernatants at a dose of 10 ng/ml, which reduced protein synthesis to 79% of control levels. As protein synthesis was further inhibited by increasing amounts of Stx1, increasing amounts of IL-8 were secreted into cell supernatants. In this representative experiment, the highest amounts of IL-8 were secreted into cell supernatants when protein synthesis was reduced to just 1 to 3% of control levels for Stx1; in other experiments, the effect of Stx1 on protein synthesis at these doses was less, in the 30 to 40% range, but with an increase in IL-8 secretion similar to that shown in Fig. 1A.
Stx2 had similar effects, as shown in Fig. 1B. Stx2 at 1 ng/ml had no significant effect on protein synthesis (97% ± 2% of control levels), and no increased IL-8 was demonstrated in cell supernatants. At 10 ng/ml, Stx2 inhibited protein synthesis to 93% of control levels and an increase in IL-8 in cell supernatants was demonstrated. At 10 μg of Stx2 per ml, protein synthesis was inhibited to 48% of control levels and a 3.6-fold increase in IL-8 protein secreted into cell supernatants was seen.
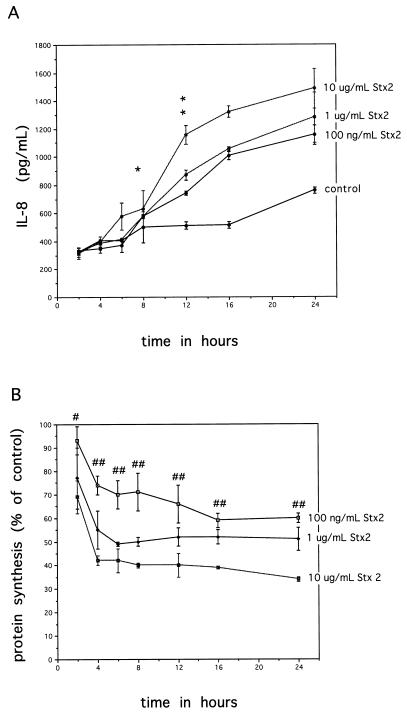
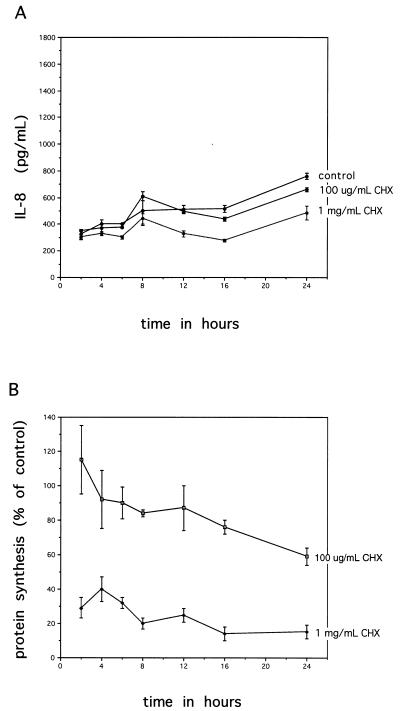
The phenomenon of increasing amounts of IL-8 synthesis despite reductions in overall protein synthesis due to Stxs raised the question of whether Stxs were inducing IL-8 expression soon after toxin exposure and IL-8 was being secreted by cells prior to an effect on protein synthesis. A time course experiment was therefore undertaken with several doses of Stx. As the single-time-point experiments showed no difference between Stx1 and Stx2 and the appearance of IL-8 in the medium was similar for both toxins, we used Stx2 in the time course experiment. At various times during incubation, cell supernatants were harvested for IL-8 protein level determinations, and protein synthesis inhibition was measured by [3H]leucine incorporation. These data are shown in Fig. 2. Figure 2A shows that IL-8 secretion by Stx2-treated Hct-8 cells begins to diverge from control levels by 12 h and thereafter increases steadily over time throughout the remainder of the incubation. As before, we saw the toxin dose response effect on IL-8 secretion. Figure 2B shows the percentage of protein synthesis inhibition following exposure to three doses of Stx2. By 4 h of exposure, all doses of Stx2 significantly reduced protein synthesis, and this preceded the increase of IL-8 in cell supernatants.
FIG. 2.
Association between inhibition of protein synthesis with Stx2 in Hct-8 cells and IL-8 synthesis over time. (A) IL-8 secretion into cell supernatants over time with various doses of Stx2. Comparisons were made between Stx2-induced IL-8 levels and control levels. P values are as follows: ∗, NS; ∗∗ (for 10 μg/ml), <0.0001; ∗∗ (1 μg/ml), 0.0002; ∗∗ (100 ng/ml), 0.0002. (B) Inhibition of protein synthesis over time following exposure to various doses of Stx2. Each point represents mean ± SD from three wells. #, significant P value of 0.02 for 10-μg/ml dose of Stx2; ##, P values for all doses at this time point are <0.03. P values for 1 μg/ml and 100 ng/ml are not significant.
Effects of Stxs on TNF-α-stimulated IL-8 protein and mRNA levels in Hct-8 cells.
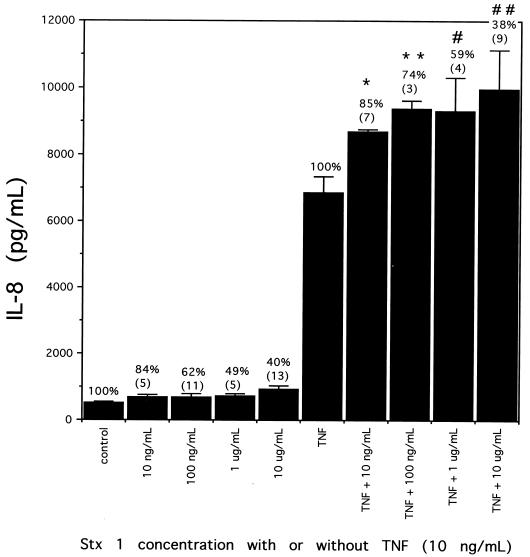
TNF-α is a potent inducer of the chemokine IL-8 from many cells, including Hct-8 cells. We treated cells with Stx1 at a range of doses, TNF-α at a single dose of 10 ng/ml, or a combination of Stx1 with TNF-α to determine if Stx1 was capable of causing an enhancement in IL-8 secretion above that seen with TNF-α alone. TNF-α alone caused a much higher IL-8 level in cell supernatants than did Stx1 alone (Fig. 3). When TNF-α and Stx1 were added together, the treatment resulted in higher levels of IL-8 secreted into culture supernatants than those expected from additive effects of either substance alone (Fig. 3). For example, cells treated with Stx1 alone at 1 μg/ml secreted 774 pg of IL-8 per ml and cells treated with TNF-α alone secreted 6,855 pg of IL-8 per ml, but cells treated with both Stx1 at 1 μg/ml and TNF-α secreted 9,320 pg of IL-8 per ml. Stx-mediated protein synthesis inhibition measured at the end of incubation was not affected by the presence of TNF-α (Fig. 3).
FIG. 3.
Secretion of IL-8 by Hct-8 cells treated with Stx1, TNF-α, or both. For each concentration of Stx1 alone, TNF-α alone, or Stx1 plus TNF-α, IL-8 measured in cell culture supernatants is shown (mean amounts from three to six samples with SD). Inserted above each solid bar is protein synthesis expressed as a percentage of control levels, as determined by [3H]leucine incorporation (SD are reported in parentheses). Comparisons were made between TNF-α-induced IL-8 levels and Stx1-plus-TNF-α-induced IL-8 levels. P values are as follows: ∗, 0.0002; ∗∗, <0.0001; #, 0.001; ##, 0.0005.
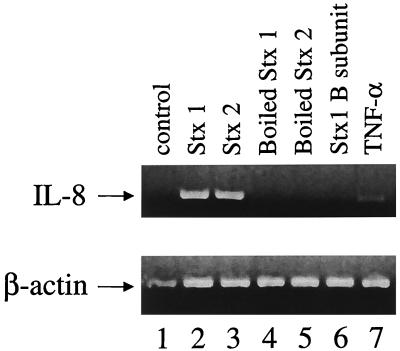
We used semiquantitative RT-PCR to determine whether IL-8 mRNA levels were increased in cells treated with Stxs. Total cellular RNA was extracted from Hct-8 cells exposed to either Stx1 (100 ng/ml), Stx2 (100 ng/ml), Stx1 B subunit (100 ng/ml), TNF-α, heat-inactivated Stxs (100 ng/ml), or control for 6 h, as described in Materials and Methods. Equal amounts of total RNA from each sample were subjected to RT-PCR analysis with specific human IL-8 and β-actin primers, generating 289- and 661-bp fragments, respectively. Figure 4 shows that IL-8 mRNA was present in increased amounts in cells treated with either Stx1 or Stx2. As expected, TNF-α increased IL-8 mRNA levels above control levels, and heat-inactivated Stx1 or Stx2 and Stx1 B subunit did not have a significant effect.
FIG. 4.
Effects of Stx1, Stx2, and TNF-α on IL-8 mRNA. Shown is an ethidium bromide-stained 1.5% agarose gel following RT-PCR analysis of total cellular RNA extracted from Hct-8 cells exposed to either Stx1 (100 ng/ml), Stx2 (100 ng/ml), heat-inactivated Stx1 and Stx2 at similar concentrations, Stx1 B subunit (100 ng/ml), TNF-α (10 ng/ml), or control (no additives) for 6 h, as described in Materials and Methods. Human IL-8 and β-actin primers generated fragments of 289 and 661 bp, respectively. Lanes are as follows: 1, control; 2, Stx1; 3, Stx2; 4, heat-inactivated Stx1; 5, heat-inactivated Stx2; 6, Stx1 B subunit; 7, TNF-α. The image shown was scanned with an Agfa Arcus II scanner and Fotolook SA.
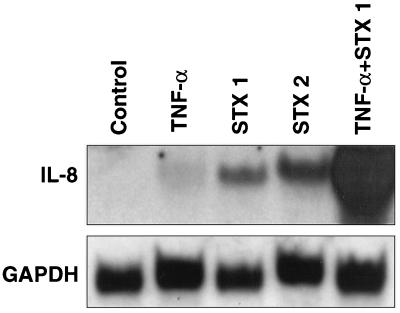
Figure 5 shows IL-8 mRNA as detected by Northern blotting. IL-8 mRNA levels increased over control levels after 6 h of incubation with TNF-α. When cells were stimulated with TNF-α in the presence of Stx1, levels of IL-8 mRNA increased dramatically over levels seen with TNF-α alone. Significant induction of IL-8 mRNA was also observed when cells were treated with Stx1 and Stx2 alone; it was approximately 5- to 10-fold over IL-8 mRNA induction due to TNF-α alone. Interestingly, although both Stxs and TNF-α increase IL-8 message, TNF-α results in a much greater increase in IL-8 protein levels (Fig. 3).
FIG. 5.
Superinduction of IL-8 mRNA with TNF-α and Stx1. Shown is a Northern blot of total cellular RNA extracted from Hct-8 cells exposed to TNF-α (10 ng/ml), Stx1 (1 μg/ml), Stx2 (1 μg/ml), or TNF-α and Stx1 (10 ng/ml and 1 μg/ml, respectively). The upper panel shows the results with an IL-8 probe; the lower panel shows the results with a GAPDH probe as a housekeeping gene.
Association between inhibition of protein synthesis by cycloheximide in Hct-8 cells and IL-8 secretion.
To determine if the effect of increased IL-8 secretion with increasing protein synthesis inhibition was specific to Stxs or if inhibition of protein synthesis resulted in cells releasing stored IL-8, another protein synthesis inhibitor that acts on the ribosome, cycloheximide, was used to inhibit protein synthesis to similar levels in a time course experiment (Fig. 6). Two doses of cycloheximide were used (100 μg/ml and 1 mg/ml). Figure 6A shows that both doses of cycloheximide resulted in decreased amounts of IL-8 secreted into cell supernatants, as would be typically expected from blockade of translation. The degrees of protein synthesis inhibition with the two doses of cycloheximide (Fig. 6B) were similar to those with the Stx doses used in the experiment shown in Fig. 2B. Cycloheximide begins to affect cells within 2 h of exposure, and IL-8 levels in the cell supernatants decrease to levels below control by 12 to 16 h and remain lower than control levels throughout the remainder of incubation. Doses of cycloheximide (5 mg/ml) that resulted in complete cell death and obvious disruption of the monolayer (as visualized by microscopy) had extremely low levels of IL-8 (data not shown).
FIG. 6.
Association between inhibition of protein synthesis with cycloheximide (CHX) in Hct-8 cells and IL-8 synthesis over time. (A) IL-8 secretion into cell supernatants over time with two doses of cycloheximide. (B) Inhibition of protein synthesis over time following exposure to the same two doses of cycloheximide. Each point represents the mean ± SD from three wells.
Effects of other protein synthesis inhibitors on IL-8 secretion and overall protein synthesis.
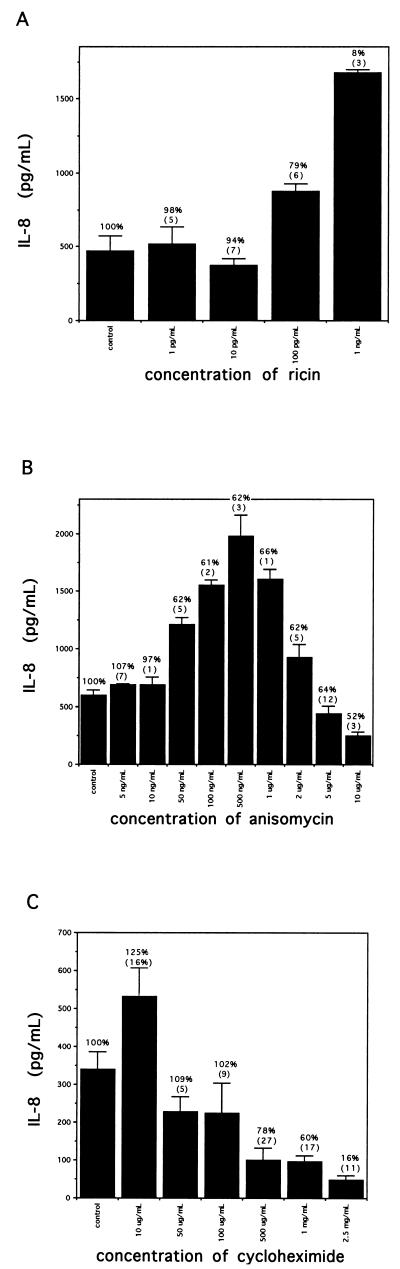
The effects of 18 h of exposure to various protein synthesis inhibitors on IL-8 secretion are shown in Fig. 7. Ricin has the same enzymatic action as Stx; at doses of 1 and 10 pg/ml, ricin had no effect on IL-8 secretion (Fig. 7A). At a dose of 100 pg/ml, which reduced protein synthesis to 78% of control, IL-8 secreted into cell supernatants was elevated 1.8-fold over baseline levels. When ricin was increased to 1 ng/ml, which inhibited protein synthesis to 8% ± 3% of control levels, IL-8 secreted into cell supernatants was elevated 3.5-fold above control levels. Like Stxs, increasing doses of ricin result in increasing amounts of IL-8 being secreted into cell supernatants, despite a concurrent inhibition of total protein synthesis. Ricin is more potent than Stx1 or Stx2 in inhibiting protein synthesis, with an ID50 of 0.1 to 1 ng/ml, compared to Stx1, which has an ID50 of approximately 1 μg/ml, but achieved a similar increase in IL-8 secretion over control levels (3.5-fold versus 3.6-fold over control) at comparable levels of protein synthesis (8% versus 1%).
FIG. 7.
Secretion of IL-8 by Hct-8 cells treated with various protein synthesis inhibitors. The data shown are from a single representative experiment; several experiments undertaken with each inhibitor showed similar results (two experiments for ricin [A], three for anisomycin [B], and three for cycloheximide [C]). For each protein synthesis inhibitor, IL-8 (mean amounts from three to six wells with SD) measured in cell culture supernatants is shown after 18 h of exposure to the different inhibitors. Control experiments were performed with no drug. Inserted above each solid bar is mean protein synthesis expressed as a percentage of control levels, as determined by [3H]leucine incorporation (SD are reported in parentheses).
Anisomycin at 5 and 10 ng/ml had a minimal effect on protein synthesis inhibition (Fig. 7B) and slightly but significantly increased IL-8 secretion into cell supernatants over control levels (P of 0.003 and 0.01, respectively). Anisomycin at 50 ng/ml resulted in increased IL-8 secretion by a 2.0-fold increase over control levels and inhibited protein synthesis to 62% of control levels. Increasing doses of anisomycin resulted in a further increase of IL-8 secretion into cell supernatants without further reducing overall protein synthesis inhibition. The maximum effect on IL-8 secretion was at a dose of 500 ng/ml, which resulted in IL-8 secretion into cell supernatants of 1,979 pg/ml (3.3-fold over control) and protein synthesis inhibition to 62% of baseline levels. Increasing doses beyond this point resulted in a progressive decline from the maximum effect on IL-8 secreted into supernatants with a minimal fall in overall protein synthesis (Fig. 7B).
Figure 7C shows results for various doses of cycloheximide. The control cells secreted 340 pg of IL-8 per ml into cell supernatants. In this example, any dose of cycloheximide that inhibited protein synthesis decreased the amount of IL-8 secreted into cell supernatants in a dose-dependent fashion, until doses that killed the Hct-8 epithelial cell monolayers were reached (in this case, a dose of 5 mg/ml [not shown]). There was a paradoxical effect of cycloheximide at 10 μg/ml, which appeared to increase IL-8 secretion. In the experiment shown, protein synthesis appeared to be stimulated to 124% of control, but this ranged from 86 to 124% for the stimulatory doses of cycloheximide in the three experiments undertaken. However, low-dose cycloheximide significantly increased IL-8 expression in all three experiments, with increases ranging from 1.3- to 1.6-fold over control and P values ranging from 0.02 to 0.04.
Effect of SB202190 on Stx-induced IL-8 secretion.
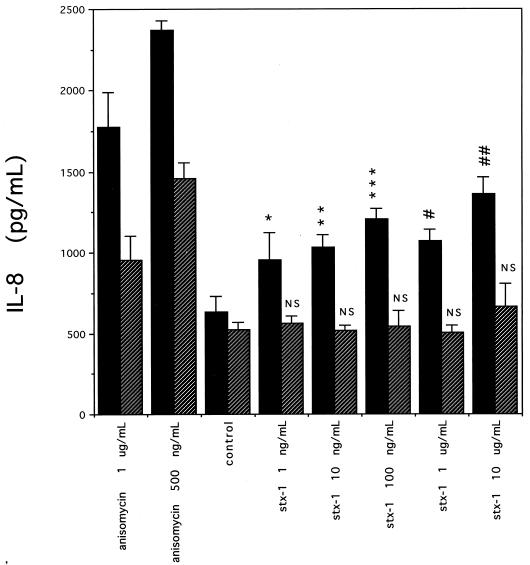
Anisomycin has been associated with induction of primary response genes in mammalian cells by activation of several members of MAPK family cascades (reviewed in reference 12). We used the specific p38 kinase inhibitor SB202190 (21) to determine if the p38/RK kinase pathway may be involved in Stx-induced IL-8 secretion. Figure 8 is representative of at least three different experiments and shows the effect of SB202190 on IL-8 secretion from Stx1-treated Hct-8 cells. IL-8 was slightly but significantly decreased in SB202190-treated controls compared to medium-only controls. As expected, SB202190 decreased anisomycin-induced IL-8 secretion significantly, but not completely, compared to SB202190-only controls. This is consistent with previous reports that anisomycin activates multiple MAPKs, including SAPK/JNK1 and multiple kinases in the p38/RK cascade (12). As treatment with a specific p38 inhibitor does not affect SAPK/JNK1 activity, a smaller amount of IL-8 is secreted in the presence of anisomycin plus SB202190. In contrast, at Stx1 doses of 10 μg/ml and below, SB202190 decreased IL-8 secretion to levels that were not significantly different from SB202190-only controls. In some experiments using 10 μg of Stx1 per ml plus inhibitor, IL-8 secretion was diminished but still significantly higher than control levels, similar to our observations with anisomycin (data not shown). In the absence of SB202190, Stx1 increased IL-8 to levels significantly above medium-only controls (P values ranging from 0.02 to 0.0003). Measurements of inhibition of protein synthesis by Stxs in the presence or absence of SB202190 were not significantly different (data not shown).
FIG. 8.
Secretion of IL-8 by Hct-8 cells in the presence of the p38/RK inhibitor SB202190. The data shown are from a single representative experiment; at least three experiments showed similar results. For each dose of Stx1 or anisomycin, IL-8 (mean amounts from three to six wells with SD) measured in cell culture supernatants is shown after 18 h of exposure in the presence or absence of SB202190. Hatched bars show results with SB202190; solid bars show results without SB202190. Control experiments used no Stx or anisomycin. P values are as follows: ∗, 0.02; ∗∗, 0.001; ∗∗∗, 0.003; #, 0.0003; ##, 0.002; NS, not significant.
Effects of Stxs on induction of c-jun mRNA.
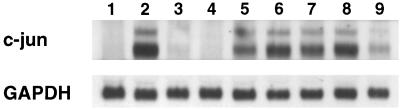
As both anisomycin and ricin are associated with induction of SAPK/JNK1 and induction of mRNA from the primary response genes c-fos and c-jun (15), we hypothesized that Stxs may also induce c-jun mRNA. We used Northern blotting to determine whether c-jun mRNA was increased in cells treated with Stxs. Hybridization of total RNA with a c-jun specific probe detects two bands as a result of alternate polyadenylation signals utilized in the processing of primary c-jun transcripts (4). Figure 9 shows that c-jun mRNA is present in increased amounts in cells treated with either Stx1 or Stx2 at 1 μg/ml (lanes 2 and 8, respectively) compared to control (lane 1). Heat-inactivated Stx1 at 1 μg/ml (lane 3) did not have a significant effect. Treatment with the p38/RK inhibitor SB202190 diminished the induction of c-jun mRNA in response to Stx1 and Stx2 (lanes 5 and 9, respectively). SB202190 alone did not have a significant effect on c-jun mRNA (lane 4). As expected, anisomycin also induced c-jun mRNA (lane 6), an effect that does not appear to be significantly diminished by treatment with SB202190 (lane 7).
FIG. 9.
Effects of Stx1 and Stx2 on c-jun mRNA in the presence and absence of SB202190. Shown is a Northern blot of total cellular RNA extracted from Hct-8 cells exposed to Stx1 (1 μg/ml), heat-inactivated Stx1 (1 μg/ml), Stx2 (1 μg/ml), or anisomycin (500 ng/ml) in the presence and absence of SB202190 or SB202190 diluent. The upper panel shows the results with a c-jun probe; the lower panel shows the results with a GAPDH probe as a housekeeping gene. Lanes are as follows: 1, diluent control; 2, Stx1; 3, heat-inactivated Stx1; 4, SB202190; 5, Stx1 + SB202190; 6, anisomycin; 7, anisomycin + SB202190; 8, Stx2; 9, Stx2 + SB202190.
DISCUSSION
Stxs have been thought to cause disease by killing or irreversibly harming sensitive cells by a nonspecific blockade of mRNA translation, eventually resulting in cytotoxicity as critical molecules needed to maintain cell integrity are no longer produced. The present data suggest a novel and different model of how Stxs may contribute to disease, namely, that Stxs alter regulation of host cell processes in sensitive intestinal epithelial cells. We have found that despite increasing general blockade of mRNA translation, Stx treatment results in the apparent paradox of increased synthesis and secretion of IL-8 protein by a human colonic epithelial cell line without affecting cell integrity. This response is not due to contaminating lipopolysaccharide in the Stx preparations, as Stxs inactivated by boiling or Stx1 B subunit alone did not stimulate IL-8 secretion above baseline. Thus, an enzymatically active Stx A subunit appears to be required for this response, an idea that is supported by recent work from Yamasaki et al. (37), who show that Stxs increase IL-8 from Caco-2 cells and that this is dependent on the toxin being biologically active. The increase in secretion of IL-8 protein into cell supernatants is not due to cell destruction with subsequent release of stored IL-8, as doses of cycloheximide that disrupted cell monolayers did not result in increased levels of IL-8 protein. This is consistent with the data demonstrating that IL-8 production is generally not constitutive but inducible and regulated at the level of gene transcription (25). These data suggest that the IL-8 secreted into cell supernatants is newly synthesized. How Stxs may exert an inhibitory effect on overall protein synthesis and yet allow for synthesis of the chemokine IL-8 is unknown. We (3), as well as others (16, 37), have shown previously that Stxs can stimulate chemokine synthesis in different intestinal epithelial cell lines (T84, Caco-2), suggesting that IL-8 synthesis by colonic epithelial cells exposed to Stx is not a unique property of the Hct-8 epithelial cell line described and utilized in these experiments.
Stxs increase IL-8 mRNA levels, as well as secreted IL-8 protein, in Hct-8 cells. As demonstrated by Northern blotting, the IL-8 mRNA levels found in response to Stxs is increased above amounts seen with the known IL-8 inducer TNF-α. Moreover, following exposure to Stxs there is a higher relative IL-8 mRNA/IL-8 protein ratio than that following TNF-α stimulation. This finding suggests that Stxs are either causing a partial translational block, enhancing mRNA stability, or both. As demonstrated by Northern blotting, Stx1 augmented the amount of IL-8 mRNA as well as IL-8 protein secreted by Hct-8 cells in response to TNF-α, reaching levels above those expected from additive effects of either substance alone. It is clear from these results that in Hct-8 cells, Stxs are capable of induction and superinduction of IL-8, at both the mRNA and protein levels.
The superinduction phenomenon has been described previously with other protein synthesis inhibitors, whereby a protein synthesis inhibitor added along with an inducing factor can lead to massive overaccumulation of mRNA transcripts of certain primary response genes, including cytokines (7, 29). It is also known that protein synthesis inhibitors alone can independently stimulate primary response gene expression. Several hypotheses have been proposed to explain how protein synthesis inhibitors cause induction and superinduction effects (9, 38), but the exact mechanism(s) by which protein synthesis inhibitors cause these effects is unknown, and multiple mechanisms may contribute. In one study, cycloheximide increased IL-8 mRNA levels in lung epithelial cells by modifying both transcriptional activator activity and mRNA stability (29). These questions are currently under study in our laboratory.
The ability of Hct-8 cells to secrete IL-8 after treatment with protein synthesis inhibitors appears to be generalizable to other protein synthesis inhibitors acting on different steps in translation of mRNA: elongation factor-associated steps (Stx and ricin), peptide bond formation (anisomycin), or multiple steps (cycloheximide, acting on initiation, elongation, and termination). The similarities between IL-8 secretion due to Stxs, anisomycin, and ricin led us to explore the effects of Stxs as possible inducers of MAPK family cascades. Previously, anisomycin and ricin have been associated with a response termed the “ribotoxic stress response” (15). The ribotoxic stress response is induced following damage to the 28S rRNA by either anisomycin or ricin, resulting in induction of SAPK/JNK1 and induction of mRNAs of the primary response genes c-fos and c-jun. Anisomycin has also been associated with induction of primary response genes in mammalian cells by activation of the p38/RK cascade (reviewed in reference 12). We have shown that Stxs can induce and superinduce IL-8. Stx-mediated induction of IL-8 protein occurs via a mechanism that is strongly inhibited by the p38/RK specific inhibitor SB202190. Furthermore, we have demonstrated that Stxs can induce c-jun mRNA and that this induction can be diminished by SB202190. These data suggest that Stxs act to induce mRNAs from primary response genes via a mechanism similar to the ribotoxic stress response involving activation of the p38/RK cascade. Our data with residual IL-8 induction by Stx1 at high doses (10 μg/ml) in the presence of SB202190 and residual c-jun induction by Stx1 and Stx2 in the presence of SB202190 suggest that other MAPK cascades may be involved in this response at high doses. There are data to suggest that, in the case of anisomycin, the SAPK/JNK1 cascade is strongly implicated in induction of c-jun but that the ERK1/2 cascade is not (6). We are presently exploring Stx effects on SAPK/JNK1 activity.
The presence of proinflammatory cytokines such as TNF-α (20) and IL-8 (34) in the serum of some patients with diarrhea-associated HUS has been demonstrated. Proinflammatory cytokines appear to be locally produced in colitis due to Shigella species (27), and stool TNF-α and IL-8 levels were found to be higher in patients infected with the Stx-producing Shigella dysenteriae type 1 than in patients with dysentery due to the non-Stx-producing Shigella flexneri (28). Stxs have recently been reported to activate TNF-α gene transcription and nuclear translocation of NF-κB and AP-1 in human monocytes (30). On the basis of these data, it has been suggested that Stxs may engage in cell-signaling activities with various human cells, resulting in proinflammatory cytokine synthesis. In our study, cytokine synthesis was triggered by doses of Stxs that may be attained in the gastrointestinal tract during human infection (1). It may be that the inflammatory response to STEC is initiated in the gastrointestinal mucosa and submucosa where host cells are first exposed to the high levels of Stxs found in the intestine. We know that certain enteropathogenic E. coli strains can stimulate activation of NF-κB, production of IL-8, and neutrophil migration (32). A critical role of Stxs may be to augment the cytokine synthesis response to infection in the milieu of the submucosa, from which STEC strains are excluded. Chemokines synthesized in the submucosal areas may allow enhanced recruitment and activation of inflammatory cells with subsequent compromise of the intestinal barrier, thereby potentially increasing systemic absorption of Stxs and subsequent initiation of systemic complications.
In summary, these data show that Stxs can alter regulation of host cell processes, leading to a paradoxical increase in the synthesis of specific proteins. The mechanism by which this is mediated is not yet known, but it appears to include activation of the p38/RK cascade and induction of c-jun mRNA. We hypothesize that Stxs act in a fashion similar to anisomycin and ricin, damaging the 28S rRNA and inducing the ribotoxic stress response with subsequent initiation of signal transduction, activation of a MAPK family member(s), and induction of mRNAs of immediate-early genes such as c-jun and IL-8.
ACKNOWLEDGMENTS
Research support for this study includes the following grants from the National Institutes of Health (NIH), Bethesda, Md.: HL-55660, A1-16242, A1-39067, and P 30 DK-34928 for the Center for Gastroenterology Research on Absorptive and Secretory Processes. C. M. Thorpe was supported by NIH training grant T32-A1-07329.
We thank Thao N. Ngo and Jonathan L. Rubinstein for expert technical assistance.
REFERENCES
- 1.Acheson D W K, De Breucker S D, Donahue-Rolfe A, Kozak K, Yi A, Keusch G T. Development of a clinically useful diagnostic enzyme immunoassay for enterohemorrhagic Escherichia coli infection. In: Karmali M A, Goglio A G, editors. Recent advances in verocytotoxin-producing E. coli infections. Amsterdam, The Netherlands: Elsevier Science; 1994. pp. 109–112. [Google Scholar]
- 2.Acheson D W K, Moore R, De Breucker S, Lincicome L, Jacewicz M, Shutelsky E, Keusch G T. Translocation of Shiga toxins across polarized intestinal cells in tissue culture. Infect Immun. 1996;64:3294–3300. doi: 10.1128/iai.64.8.3294-3300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acheson D W K. Effect of Shiga toxins on cytokine production from intestinal epithelial cells. In: Keusch G T, Kawakami M, editors. Cytokines, cholera, and the gut. Amsterdam, The Netherlands: IOS Press; 1996. pp. 67–73. [Google Scholar]
- 4.Angel P, Allegretto E A, Okino S T, Hattori K, Boyle W J, Hunter T, Karin M. Oncogene jun encodes a sequence-specific trans-activator similar to AP-1. Nature. 1988;332:166–171. doi: 10.1038/332166a0. [DOI] [PubMed] [Google Scholar]
- 5.Blake D C I, Russell R G, Santini E, Bowen T, Boedeker E C. Pro-inflammatory mucosal cytokine responses to Shiga-like toxin-1 (SLT-1) In: Keusch G T, Kawakami M, editors. Cytokines, cholera, and the gut. Amsterdam, The Netherlands: IOS Press; 1996. pp. 75–82. [Google Scholar]
- 6.Cano E, Hazzalin C A, Mahadevan L C. Anisomycin-activated protein kinases p45 and p55 but not mitogen-activated protein kinases ERK-1 and -2 are implicated in the induction of c-fos and c-jun. Mol Cell Biol. 1994;14:7352–7362. doi: 10.1128/mcb.14.11.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards D R, Mahadevan L C. Protein synthesis inhibitors differentially superinduce c-fos and c-jun by three distinct mechanisms: lack of evidence for labile repressors. EMBO J. 1992;11:2415–2424. doi: 10.1002/j.1460-2075.1992.tb05306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott E, Zhe L, Bell C, Stiel D, Buret A, Wallace J, Brzuszczak I, O'Loughlin E. Modulation of host response to Escherichia coli O157:H7 infection by anti-CD18 antibody in rabbits. Gastroenterology. 1994;106:1554–1561. doi: 10.1016/0016-5085(94)90410-3. [DOI] [PubMed] [Google Scholar]
- 9.Faggioli L, Constanzo C, Merola M, Furia A, Palmieri M. Protein synthesis inhibitors cycloheximide and anisomycin induce interleukin-6 gene expression and activate transcription factor NF-κB. Biochem Biophys Res Commun. 1997;233:507–513. doi: 10.1006/bbrc.1997.6489. [DOI] [PubMed] [Google Scholar]
- 10.Griffin P M, Ostroff S M, Tauxe R V, Greene K D, Wells J G, Lewis J H, et al. Illnesses associated with Escherichia coli O157:H7 infections. A broad clinical spectrum. Ann Intern Med. 1988;109:705–712. doi: 10.7326/0003-4819-109-9-705. [DOI] [PubMed] [Google Scholar]
- 11.Grisham M B, Granger D N. Neutrophil-mediated mucosal injury: role of reactive oxygen metabolites. Dig Dis Sci. 1988;33:6S–15S. doi: 10.1007/BF01538126. [DOI] [PubMed] [Google Scholar]
- 12.Hazzalin C A, Le Panse R, Cano E, Mahadevan L C. Anisomycin selectively desensitizes signalling components involved in stress kinase activation and fos and jun induction. Mol Cell Biol. 1998;18:1844–1854. doi: 10.1128/mcb.18.4.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurley, B. P., C. M. Thorpe, and D. W. K. Acheson. Unpublished data.
- 14.Ikawa T, Ikeda M, Yamaguchi A, Tsai W C, Tamura N, Seta N, Trucksess M, Raybourne R B, Yu D T Y. Expression of arthritis-causing HLA-B27 on HeLa cells promotes induction of c-fos in response to in vitro invasion by Salmonella typhimurium. J Clin Investig. 1998;101:263–272. doi: 10.1172/JCI471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iordanov M, Pribnow D, Magun J L, Dinh T-H, Pearson J A, Chen S L, Magun B E. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the α-sarcin/ricin loop in the 28S rRNA. Mol Cell Biol. 1997;17:3373–3381. doi: 10.1128/mcb.17.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ismaili A, Philpott D J, McKay D M, Perdue M H, Sherman P M. Epithelial cell responses to Shiga toxin-producing Escherichia coli infection. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C: American Society for Microbiology; 1998. pp. 213–225. [Google Scholar]
- 17.Jacewicz M, Feldman H A, Donohue-Rolfe A, Balasubramanian K A, Keusch G T. Pathogenesis of Shigella diarrhea. XIV. Analysis of Shiga toxin receptors on cloned HeLa cells. J Infect Dis. 1989;159:881–889. doi: 10.1093/infdis/159.5.881. [DOI] [PubMed] [Google Scholar]
- 18.Jung H C, Eckmann L, Yang S, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Investig. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karmali M A, Petric M, Lim C, Fleming P C, Arbus G S, Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verocytotoxin-producing Escherichia coli. J Infect Dis. 1985;151:775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- 20.Karpman D, Andreasson A, Thysell H, Kaplan B S, Svanborg C. Cytokines in childhood hemolytic uremic syndrome and thrombotic thrombocytopenia purpura. Pediatr Nephrol. 1995;9:694–699. doi: 10.1007/BF00868714. [DOI] [PubMed] [Google Scholar]
- 21.Lee J C, Young P R. Role of CSBP/p38/RK stress response kinase in LPS and cytokine signalling mechanisms. J Leukoc Biol. 1996;59:152–157. doi: 10.1002/jlb.59.2.152. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Bell C, Buret A, Robins-Browne R, Stiel D, O'Laughlin E V. The effect of enterohemorrhagic Escherichia coli O157:H7 on intestinal structure and solute transport in rabbits. Gastroenterology. 1993;104:467–474. doi: 10.1016/0016-5085(93)90415-9. [DOI] [PubMed] [Google Scholar]
- 23.Lieberman E, Heuser E, Donnell G N, Landing B H, Hammond G D. Hemolytic uremic syndrome. Clinical and pathological considerations. N Engl J Med. 1966;275:227–236. doi: 10.1056/NEJM196608042750501. [DOI] [PubMed] [Google Scholar]
- 24.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okamoto S, Mukaida N, Yasumoto K, Horiguchi H, Matsushima K. Molecular mechanism of interleukin-8 gene expression. In: Lindley I J D, et al., editors. The chemokines. New York, N.Y: Plenum Press; 1993. pp. 87–97. [DOI] [PubMed] [Google Scholar]
- 26.Paton J C, Paton A W. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev. 1998;11:450–479. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raqib R, Lindberg A A, Wretlind B, Bardhan P K, Andersson U, Andersson J. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infect Immun. 1995;63:289–296. doi: 10.1128/iai.63.1.289-296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raqib R, Wretlind B, Andersson J, Lindberg A A. Cytokine secretion in acute shigellosis is correlated to disease activity and directed more to stool than to plasma. J Infect Dis. 1995;171:376–384. doi: 10.1093/infdis/171.2.376. [DOI] [PubMed] [Google Scholar]
- 29.Roger T, Out T, Mukaida N, Matsushima K, Jansen H, Lutter R. Enhanced AP-1 and NF-κB activities and stability of interleukin 8 (IL-8) transcripts are implicated in IL-8 mRNA superinduction in lung epithelial H292 cells. Biochem J. 1998;330:429–435. doi: 10.1042/bj3300429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakiri R, Ramegowda B, Tesh V L. Shiga toxin type 1 activates tumor necrosis factor-α gene transcription and nuclear translocation of the transcriptional activators nuclear factor-κB and activator protein-1. Blood. 1998;92:558–566. [PubMed] [Google Scholar]
- 31.Savkovic S D, Koutsouris A, Hecht G. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect Immun. 1996;64:4480–4487. doi: 10.1128/iai.64.11.4480-4487.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savkovic S D, Koutsouris A, Hecht G. Activation of NF-κB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am J Physiol. 1997;273:C1160–C1167. doi: 10.1152/ajpcell.1997.273.4.C1160. [DOI] [PubMed] [Google Scholar]
- 33.Slutsker L, Ries A A, Greene K D, Wells J G, Hutwagner L, Griffin P M. Escherichia coli O157:H7 diarrhea in the United States: clinical and epidemiologic features. Ann Intern Med. 1997;126:505–513. doi: 10.7326/0003-4819-126-7-199704010-00002. [DOI] [PubMed] [Google Scholar]
- 34.van Setten P, van Hinsbergh V, van den Heuvel L, Preyers F, Dijkman H, Assmann K, van der Velden T, Monnens L. Monocyte chemoattractant protein-1 and interleukin-8 levels in urine and serum of patients with hemolytic uremic syndrome. Pediatr Res. 1998;43:759–767. doi: 10.1203/00006450-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Wallace J L, Higa A, McKnight G W, MacIntyre D E. Prevention and reversal of experimental colitis by a monoclonal antibody which inhibits leukocyte adherence. Inflammation. 1992;16:343–354. doi: 10.1007/BF00917626. [DOI] [PubMed] [Google Scholar]
- 36.Whitington P F, Friedman A L, Chesney R W. Gastrointestinal disease in the hemolytic uremic syndrome. Gastroenterology. 1979;76:728–733. [PubMed] [Google Scholar]
- 37.Yamasaki C, Natori Y, Zeng X-T, Ohmura M, Yamasaki S, Takeda Y, Natori Y. Induction of cytokines in a human colon epithelial cell line by Shiga toxin 1 (Stx1) and Stx2 but not by non-toxic mutant Stx1 which lacks N-glycosidase activity. FEBS Lett. 1999;442:231–234. doi: 10.1016/s0014-5793(98)01667-6. [DOI] [PubMed] [Google Scholar]
- 38.Zinck R, Cahill M A, Kracht M, Sachsenmaier C, Hipskind R A, Nordheim A. Protein synthesis inhibitors reveal differential regulation of mitogen-activated protein kinase and stress-activated protein kinase pathways that converge on Elk-1. Mol Cell Biol. 1995;15:4930–4938. doi: 10.1128/mcb.15.9.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]