Abstract
Shiga toxin-producing Escherichia coli (STEC) strains cause a wide spectrum of diseases in humans. In this study, we tested 206 STEC strains isolated from patients for potential virulence genes including stx, eae, and enterohemorrhagic E. coli hly. In addition, all strains were examined for the presence of another genetic element, the high-pathogenicity island (HPI). The HPI was first described in pathogenic Yersinia species and encodes the pesticin receptor FyuA and the siderophore yersiniabactin. The HPI was found in the genome of distinct clonal lineages of STEC, including all 31 eae-positive O26:H11/H− strains and 7 of 12 eae-negative O128:H2/H− strains. In total, the HPI was found in 56 (27.2%) of 206 STEC strains. However, it was absent from the genome of all 37 O157:H7/H−, 14 O111:H−, 13 O103:H2, and 13 O145:H− STEC isolates, all of which were positive for eae. Polypeptides encoded by the fyuA gene located on the HPI could be detected by using immunoblot analysis in most of the HPI-positive STEC strains, suggesting the presence of a functional yersiniabactin system. The HPI in STEC was located next to the tRNA gene asnT. In contrast to the HPI of other pathogenic enterobacteria, the HPI of O26 STEC strains shows a deletion at its left junction, leading to a truncated integrase gene int. We conclude from this study that the Yersinia HPI is disseminated among certain clonal subgroups of STEC strains. The hypothesis that the HPI in STEC contributes to the fitness of the strains in certain ecological niches rather than to their pathogenic potential is discussed.
Shiga toxin (Stx)-producing Escherichia coli (STEC) strains are a worldwide cause of human disease, the spectrum of which ranges from mild diarrhea to life-threatening hemolytic-uremic syndrome (HUS). In addition to expressing Stx, most of these strains possess other virulence characteristics such as the ability to cause attaching-and-effacing (A-E) lesions on mucosal epithelial cells of the large intestine (52), and they contain an approximately 90-kb plasmid (54). STEC strains which, in addition to Stx production, display the A-E activity may also be referred to as enterohemorrhagic E. coli (EHEC). Although most STEC strains belong to the serotype O157:H7, non-O157 STEC, mostly those of the serogroups O26, O111, O103, O145, and O128, are a significant cause of human disease in Europe (5, 8).
STEC produce one or more Stx. The E. coli Stx family consists of two major toxin types, Stx1 and Stx2, that display only 58% overall nucleotide sequence homology (31). The genes encoding Stx1 and Stx2 are located in the genomes of temperate lambdoid bacteriophages (30, 33, 46, 50), and this may facilitate the spread of the genes via transduction. The large plasmids of STEC O157 and non-O157 encode determinants that may serve as additional virulence factors (24), such as the EHEC hemolysin, which has the function of a pore-forming cytolysin (45). In STEC O157:H7, the genes encoding proteins involved in producing the A-E lesions are located on a 42-kb pathogenicity island (PAI) termed the locus of enterocyte effacement (LEE). The LEE consists of three functional domains: the eae and tir genes in the central region, a type III secretion system, and genes for other secreted proteins (esp loci) (for review, see reference 22).
Whereas most PAIs are species- and even pathotype-specific, e.g., PAIs encoding alpha-hemolysin and P fimbriae are found exclusively in extraintestinal E. coli (3, 16, 40), one PAI, termed the high-pathogenicity island (HPI) and first described in pathogenic Yersinia strains, is widespread among enterobacteria (49). The HPI region carries the gene fyuA, which is specific for the pesticin receptor and the irp (iron repressible protein) loci encoding the siderophore yersiniabactin. The HPI element is associated with asparagine-specific tRNA loci and carries an integrase gene, int, often associated with a phage genome (7, 38). It is of interest that the HPI is not only present in the genomes of the pathogenic Yersinia species, including Yersinia enterocolitica, Yersinia pseudotuberculosis, and Yersinia pestis, but is also a part of the genomes of other enterobacteria such as Klebsiella spp., Citrobacter spp., and E. coli (16, 17, 49).
In pathogenic E. coli, the HPI element is frequently found in the genomes of enteroaggregative E. coli and of extraintestinal E. coli strains associated with urinary tract infections and sepsis (49). The HPI has also been detected in more than 30% of E. coli strains from physiological intestinal microflora (49). In a previous publication, we reported that STEC strains of serotype O157:H7 did not possess the HPI element (49). In this report, we confirm this observation and show that the Yersinia HPI is a part of the genome of certain non-O157 STEC clonal lineages.
MATERIALS AND METHODS
Bacterial strains.
In total, 206 STEC strains isolated from patients were investigated. The strains belonged to the serotype O157:H7/H− and to 57 different non-O157 serotypes (Table 1). Most of them were isolated from German patients with HUS or diarrhea in our laboratory during routine diagnostic work between 1987 and 1998. Sixteen strains originated from patients with HUS or diarrhea in France, Italy, Canada, and the United States and were described elsewhere (6, 25, 27, 42, 47, 51). Enteroaggregative E. coli strain 17-2 (53) was a gift from J. P. Nataro (Center for Vaccine Development, Baltimore, Md.). Strains of Y. pestis KIM6+, Y. enterocolitica WA-314, Y. pseudotuberculosis O1, E. coli K-12 MG1655, and E. coli DH5α were described previously (1, 13, 34, 37).
TABLE 1.
Potential virulence factors of STEC strains used in this study
| Serotype | Total no. of strains | No. containing stx gene
|
No. containing eae | No. containing EHEC hly | ||
|---|---|---|---|---|---|---|
| stx1 | stx2 | stx1 plus stx2 | ||||
| O157:H7/H− | 37 | 2 | 25a | 10a | 37 | 37 |
| O26:H11/H− | 31 | 13 | 15 | 3 | 31 | 31 |
| O103:H2 | 13 | 12 | 0 | 1 | 13 | 13 |
| O111:H− | 14 | 9 | 0 | 5 | 14 | 13 |
| O145:H− | 13 | 3 | 9a | 1a | 13 | 13 |
| O128:H2/H− | 12 | 2 | 1b | 9b | 0 | 7 |
| Otherc | 86 | 37 | 28d | 21d | 21 | 45 |
| Total | 206 | 78 | 78 | 50 | 129 | 159 |
stx2 variant was stx2c.
stx2 variant was stx2d.
Fifty different serotypes within 39 O serogroups; the serotypes that comprised at least three strains were O8:H− (six strains), O62:H− (three strains), O78:H− (three strains), O113:H− (three strains), and O118:H− (three strains).
stx2 variant was stx2c (nine strains) or stx2d (22 strains).
Isolation and identification of STEC.
Screening for STEC in stool cultures was performed by PCR as described previously (23) using primer pair KS7 and KS8 (42) and either GK3 and GK4 (14) or LP43 and LP44 (10) complementary to the stx1 and stx2 genes (Table 2). The stx2c was demonstrated by restriction analysis of the stx2B PCR products with HaeIII and FokI as described (41). The strategy to detect the stx2d was that described by Piérard et al. (35). To identify STEC strains in PCR-positive samples, colony hybridization with 100 to 200 well-separated colonies was performed (44) by using digoxigenin-labeled stx1 and stx2 probes prepared as described (42, 44). The identified STEC strains were serotyped according to Bockemühl et al. (4).
TABLE 2.
PCR primers and conditions used in this study
| Primer designation | Nucleotide sequence of primers | Target | PCR conditions
|
Length of PCR product (bp) | Reference for PCR | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Denaturing
|
Annealing
|
Extension
|
||||||||
| Tempa | Tb | Tempa | Tb | Tempa | Tb | |||||
| KS7 | 5′-CCCGGATCCATGAAAAAAACATTATTAATAGC-3′ | stx1B | 94 | 30 | 52 | 60 | 72 | 40 | 285 | 42 |
| KS8 | 5′-CCCGAATTCAGCTATTCTGAGTCAACG-3′ | |||||||||
| GK3 | 5′-CCCGGATCCATGAAGAAGATGTTTATGGCG-3′ | stx2B | 94 | 30 | 52 | 60 | 72 | 40 | 260 | 14 |
| GK4 | 5′-CCCGAATTCTCAGTCATTTATTAAACTGCAC-3′ | stx2cB | ||||||||
| LP43 | 5′-ATCCTATTCCCGGGAGTTTACG-3′ | stx2A and variants | 94 | 30 | 57 | 60 | 72 | 60 | 584 | 10 |
| LP44 | 5′-GCGTCATCGTATACACAGGAGC-3′ | |||||||||
| VT2-cm | 5′-AAGAAGATATTTGTAGCGG-3′ | stx2d | 94 | 30 | 55 | 60 | 72 | 60 | 256 | 35 |
| VT2-f | 5′-TAAACTGCACTTCAGCAAAT-3′ | |||||||||
| SK1 | 5′-CCCGAATTCGGCACAAGCATAAGC-3′ | eae | 94 | 30 | 52 | 60 | 72 | 60 | 863 | 43 |
| SK2 | 5′-CCCGGATCCGTCTCGCCAGTATTCG-3′ | |||||||||
| Hly A1 | 5′-GGTGCAGCAGAAAAAGTTGTAG-3′ | EHEC hlyA | 94 | 30 | 57 | 60 | 72 | 90 | 1,551 | 45 |
| Hly A4 | 5′-TCTCGCCTGATAGTGTTTGGTA-3′ | |||||||||
| asnU | 5′-TTTCGCTGTTAAGATGTGCC-3′ | asnU/int | 94 | 30 | 53 | 60 | 72 | 60 | 1,500 | This studyc |
| int2 | 5′-TGCTTCCAGATAATCCGACCAC-3′ | |||||||||
| asnV | 5′-GACAGCAAACAAACAAAAA-3′ | asnV/int | 94 | 30 | 53 | 60 | 72 | 60 | 1,500 | This study |
| int2 | 5′-TGCTTCCAGATAATCCGACCAC-3′ | |||||||||
| ybtEup | 5′-GCAAGATAGACAAAAAACGCC-3′ | ybtE/fyuA | 94 | 60 | 52 | 60 | 72 | 60 | 359 | This study |
| fyuybtE (IX) | 5′-GCTGACAACGGTAGACGAGA-3′ | |||||||||
| fyuA FP | 5′-GCGACGGGAAGCGATTTA-3′ | fyuA | 94 | 60 | 57 | 60 | 72 | 60 | 780 | 49 |
| fyuA RP (X) | 5′-CGCAGTAGGCACGATGTTGTA-3′ | |||||||||
| 50A | 5′-ATTGATCCACCGTTTTACTC-3′ | IS100 | 94 | 60 | 50 | 60 | 72 | 60 | 100 | This study |
| 50B (XI) | 5′-CGAACGAAAGCATGAAACAA-3′ | |||||||||
| int5 | 5′-ATGGAATCGGGTTTATGGG-3′ | int/ybtS | 94 | 60 | 54 | 60 | 72 | 60 | 830 | This study |
| ybtSlp (XII) | 5′-GCTATTGCTGAACTGGAGG-3′ | |||||||||
| ybtSup | 5′-GAAACAGCACGGTAAACGCA-3′ | ybtS/ybtQ | 94 | 30 | 55 | 60 | 72 | 180 | 2,797 | This study |
| ybtQ3lp (XIII) | 5′-ACGCGGCAGGAGGTAGAAG-3′ | |||||||||
| ybtQup | 5′-GCCGCCAGTCTATCCACA-3′ | ybtQ/ybtA | 94 | 30 | 52 | 60 | 72 | 180 | 2,805 | This study |
| ybtA1lp (XIV) | 5′-GAATCGGCCACAATAGGA-3′ | |||||||||
| ybtAup | 5′-GGTATGGATATTTTGCTCTGG-3′ | ybtA/irp2 | 94 | 60 | 54 | 60 | 72 | 60 | 1,340 | This study |
| irp2 RP (XV) | 5′-TCGTCGGGCAGCGTTTCTTCT-3′ | |||||||||
| irp2-1up | 5′-ACCTCTTCACCCACCCTTCT-3′ | irp2/irp1 | 94 | 60 | 54 | 60 | 72 | 60 | 300 | This study |
| irp1-1lp (XVI) | 5′-TTCAGGAAAATGGCAGGCGT-3′ | |||||||||
| irp1-1up | 5′-TTCCGGTCCCCTGTCTCA-3′ | irp1/ybtT | 94 | 30 | 52 | 60 | 72 | 120 | 1,762 | This study |
| ybtTlp (XVII) | 5′-ATCCGCCAATGTCTATCA-3′ | |||||||||
| asnT | 5′-GACAGACAAGGTACCGCTAA-3′ | asnT/int | 94 | 60 | 52 | 60 | 72 | 60 | 1,500 | This study |
| int2 | 5′-TGCTTCCAGATAATCCGACCAC-3′ | |||||||||
| asnT1 | 5′-CACGATTCCTCTGTAGTTCA-3′ | asnT/int | 94 | 60 | 52 | 60 | 72 | 60 | 1,255 | This study |
| int3 (I) | 5′-TCCTTTTTCGTGTCGTAACCC-3′ | |||||||||
| asnT1 | 5′-CACGATTCCTCTGTAGTTCA-3′ | asnT/int | 94 | 60 | 52 | 60 | 72 | 60 | 1,500 | This study |
| int2 (II) | 5′-TGCTTCCAGATAATCCGACCAC-3′ | |||||||||
| int1 | 5′-TCCCTTACCGACGCAAAAATCC-3′ | int | 94 | 60 | 58 | 60 | 72 | 60 | 1,203 | This study |
| int2 (III) | 5′-TGCTTCCAGATAATCCGACCAC-3′ | |||||||||
| ybtSup | 5′-GAAACAGCACGGTAAACGCA-3′ | ybtS | 94 | 60 | 52 | 60 | 72 | 60 | 160 | This study |
| ybtSlp (IV) | 5′-GCTATTGCTGAACTGGAGG-3′ | |||||||||
| ybtQup | 5′-CGGGCGGCCTCTTCTACCT-3′ | ybtQ | 94 | 30 | 59 | 60 | 72 | 90 | 797 | This study |
| ybtQ1lp (V) | 5′-GCGATGCGGCGACAAATGC-3′ | |||||||||
| ybtAup | 5′-GGTATGGATATTTTGCTCTGG-3′ | ybtA | 94 | 60 | 52 | 60 | 72 | 60 | 233 | This study |
| ybtAlp (VI) | 5′-GGTAATGCTCTCGAATCGG-3′ | |||||||||
| irp2 FP | 5′-AAGGATTCGCTGTTACCGGAC-3′ | irp2 | 94 | 60 | 60 | 60 | 72 | 60 | 280 | 49 |
| irp2 RP (VII) | 5′-TCGTCGGGCAGCGTTTCTTCT-3′ | |||||||||
| irp1up | 5′-TGAATCGCGGGTGTCTTATGC-3′ | irp1 | 94 | 60 | 56 | 60 | 72 | 60 | 240 | 34 |
| irp1lp (VIII) | 5′-TCCCTCAATAAAGCCCACGCT-3′ | |||||||||
| ybtTup | 5′-TGCAAAAACAGCCCAGTA-3′ | ybtT/fyuA | 94 | 30 | 50 | 60 | 72 | 180 | 2,518 | This study |
| fyuA1lp (XVIII) | 5′-CATTCCATCCCACATAGG-3′ | |||||||||
Temperature in degrees Celsius.
Time in seconds.
Detection of STEC virulence genes.
The presence of the STEC virulence genes, including the stx1, stx2 and stx2 variants (stx2c, stx2d), eae, and EHEC hly was detected by PCRs performed with the GeneAmp PCR System 9600 (Perkin-Elmer, Weiterstadt, Germany). Amplifications were carried out in a total volume of 50 μl containing 15 μl of bacterial suspension (106 cells), each deoxynucleoside triphosphate at a concentration of 200 μM, 30 pmol of each primer, 5 μl of 10-fold-concentrated polymerase synthesis buffer, 1.5 mM MgCl2, and 2.0 U of AmpliTaq DNA polymerase (Perkin-Elmer). The primer sequences and PCR conditions are shown in Table 2. After 30 cycles had been completed, a 5-μl volume of each PCR sample was analyzed by submarine gel electrophoresis on a 1.5% (wt/vol) agarose gel and was visualized by staining with ethidium bromide. Strains EDL933 (O157:H7; stx1+ stx2+ eae+, EHEC hly+) (32, 44, 45), E32511 (O157:H−; stx2c+ eae+, EHEC hly+) (48), and 4797/97 (O103:H−; stx2d+) from our collection were used as positive controls.
Detection of the Yersinia HPI genes in STEC strains.
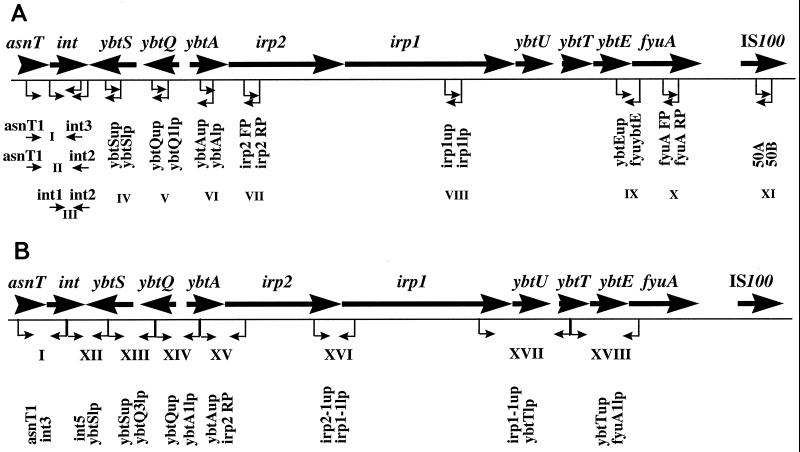
The irp2 and fyuA genes of the Yersinia HPI were detected by PCR as described by Schubert et al. (49) with small modifications. For a more detailed analysis of the HPI in STEC strains, several primer pairs targeting further genes described in the Yersinia HPI were designed, mostly according to sequences published for Y. pestis HPI (13). The target regions and the primers and PCR conditions are shown in Fig. 1 and Table 2, respectively.
FIG. 1.
Physical map of the HPI element of pathogenic yersiniae. Important genes are indicated by large black arrows and include the following: asnT and int boundary genes; ybtS, ybtQ, ybtA, irp2, irp1, ybtU, ybtT, and ybtE, constituting the siderophore yersiniabactin biosynthetic gene cluster; fyuA, encoding the receptor for yersiniabactin and pesticin; and IS100 insertion element (7, 13, 34, 38). PCR primers used to target single HPI genes (panel A, regions III to VIII, X, and XI) or to link consecutive genes (panel A, regions I, II, and IX, and panel B) are indicated by small arrows, and nucleotide sequences of the primers are given in Table 2.
Characterization and sequencing of the integrase gene.
The presence of the integrase gene in the HPI of STEC strains was demonstrated by PCR amplification using the primers and conditions shown in Table 2. In order to sequence the integrase gene of STEC strains, the amplified DNA PCR products obtained with primers asnT1 and int2 were purified with the QIAquick PCR purification kit (Qiagen, Hilden, Germany). For each sequencing reaction, 12 μl (100 ng) of DNA was subjected to the thermosequenase fluorescent-labeled primer cycle sequencing kit (Amersham, Pharmacia Biotech, Freiburg, Germany). Electrophoresis of the sequencing products was performed on a model 4000 automated sequencer (MWG-Biotech, Ebersberg, Germany).
Detection of FyuA by immunoblotting.
For immunoblotting, ultrasonicated bacterial cell pellets were treated with Triton X-100, and the insoluble membrane material was purified and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis as described previously (18, 21). After electrotransfer to polyvinylidene difluoride membranes (Millipore, Eschborn, Germany), FyuA was detected by antiserum (anti-FyuA) raised against FyuA from Y. enterocolitica O8 strain Y1852 in rabbits (21). Goat anti-rabbit antibody conjugated to horseradish peroxidase was employed as a second antibody (ECL Western blotting detection reagents; Amersham Pharmacia Biotech). The membrane was soaked briefly in the detection reagent. This elicited a peroxidase-catalyzed oxidation of luminol and subsequently enhanced chemiluminescence when the horseradish peroxidase-labeled protein was bound to the antigen on the membrane. The chemiluminescence was detected by exposing the membrane to Kodak Bio Max MR film at room temperature (19).
Nucleotide sequence accession numbers.
The nucleotide sequences for the HPI integrase genes of E. coli O128:H2 (strain 3172/97) and E. coli O26:H− (strain 5720/96) have been entered into the EMBL database under the accession no. AJ245584 and AJ245585, respectively.
RESULTS
Analysis of STEC strains for chromosomal and plasmid-encoded determinants.
All STEC strains were tested by PCR with the primers specific for the stx, eae, and EHEC hly genes, respectively, shown in Table 2. The presence of the genes encoding potential virulence characteristics in 206 strains is shown in Table 1. All strains harbored one or more stx genes, including stx1, stx2, and stx2 variants (stx2c or stx2d). However, there were marked differences among the serotypes regarding the types of the stx genes. Whereas the majority of O157:H7/H− and O145:H− isolates harbored the stx2 and/or stx2c genes, all strains belonging to serotypes O103:H2 and O111:H− harbored stx1, usually as the only stx gene, and none of the strains of these two serotypes possessed the stx2 gene only. Within the serotype O26:H11/H−, isolates containing the stx1 and stx2 gene occurred with similar frequency. Ten of 12 isolates of serotype O128:H2/H− harbored genes encoding a new stx2 variant, stx2d. With the exception of one strain, the stx2d gene was generally present in combination with the stx1 gene. The stx2d gene was not found in any of the isolates belonging to the serotypes O157:H7/H−, O26:H11/H−, O103:H2, O111:H−, or O145:H−. In the heterogeneous group of 86 STEC strains comprising 50 different serotypes, more than one-third of the strains harbored the stx1 gene only; among 49 strains with the stx2 and/or the stx2 variant genes, 22 strains contained stx2d alone or in combination with stx1, and nine strains contained stx2c. The 22 stx2d-positive isolates belonged to 16 different serotypes.
The eae and EHEC hly genes were used as markers for the presence of the LEE PAI and the large EHEC plasmid, respectively. As demonstrated in Table 1, all strains of serotypes O157:H7/H−, O26:H11/H−, O103:H2, and O145:H−, and all but one strain of serotype O111:H−, harbored both eae and EHEC hly genes. One additional O111:H− strain possessed the eae but not the EHEC hly gene. In contrast, all 12 strains of serotype O128:H2/H− lacked the eae gene, and only seven contained the EHEC hly gene. Of the 86 strains belonging to 50 different serotypes, 21 and 45 isolates harbored the eae and EHEC hly genes, respectively, but only 13 isolates possessed both genes.
Presence of HPI in STEC strains.
In order to test whether STEC strains carry the HPI, PCRs specific for irp2 and fyuA genes were performed by using primers shown in Table 2. The distribution of the irp2 and fyuA genes in strains of different serotypes and the correlation of these genes with the presence of the eae gene are shown in Table 3. All 31 eae-positive O26:H11/H− STEC strains were positive for both irp2 and fyuA genes. Moreover, 7 of 12 eae-negative strains of serotype O128:H2/H− contained the HPI-specific genes. An additional 18 STEC strains that harbored irp2 and fyuA included four eae-positive and 14 eae-negative isolates that belonged to nine different serotypes (Table 3). In total, the HPI-specific genes were found in 56 (27.2%) of 206 STEC strains. However, none of the STEC strains of serotypes O157:H7/H−, O103:H2, O111:H−, and O145:H−, which were all eae-positive, harbored either irp2 or fyuA.
TABLE 3.
Presence of the HPI and LEE in STEC strains
| Serotype | Total no. of strains | No. of strains positive for:
|
|
|---|---|---|---|
| LEE (eae) | HPI (irp2, fyuA) | ||
| O157:H7/H− | 37 | 37 | 0 |
| O103:H2 | 13 | 13 | 0 |
| O111:H− | 14 | 14 | 0 |
| O145:H− | 13 | 13 | 0 |
| O26:H11/H− | 31 | 31 | 31 |
| O128:H2/H− | 12 | 0 | 7 |
| Others | 21 | 21 | 4a |
| 65 | 0 | 14b | |
| Total | 206 | 129 | 56 |
Serotypes O118:H (two strains), O4:H (one strain), and O121:H10 (one strain).
Six different serotypes, including O3:H2 (one strain), O3:H10 (one strain), O41:H− (one strain), O60:H− (one strain), O78:H− (three strains), and O152:H4 (one strain); three additional strains were not typeable with the O antisera used, and three strains were rough.
Two STEC strains, including 3172/97 (O128:H2) and 5720/96 (O26:H−), were subjected to 11 different PCRs with primers targeting the genes described to occur in the HPI of pathogenic yersiniae in order to determine whether these genes are present in the investigated E. coli strains. The target regions (I to XI) and the corresponding primer pairs are indicated in Fig. 1A and in Table 2, respectively. As demonstrated in Table 4, besides an asparagine tRNA gene and the integrase gene, sequences similar to ybtS, ybtQ, ybtA, irp2, irp1, ybtE, and fyuA could be detected in both E. coli strains. The sizes of the PCR products obtained from O128 STEC strain 3172/97 were close to the sizes of the corresponding HPI regions in Y. pestis and Y. enterocolitica determined from published sequence data (13, 34). In O26 STEC strain 5720/96, however, the sizes of PCR products with the primers homologous to the int gene were smaller (Table 4).
TABLE 4.
Comparison of the HPI in STEC and Y. pestis and Y. enterocolitica
| Strain | Serotype | Size of PCR product (bp) from regiona:
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIII | XIV | XV | XVI | XVII | XVIII | ||
| STEC 3172/97 | O128:H2 | 1,200 | 1,400 | 1,200 | 160 | 800 | 230 | 280 | 240 | 360 | 780 | —b | 800 | 2,800 | 2,800 | 1,300 | 300 | 1,700 | 2,500 |
| STEC 5720/96 | O26:H− | 900 | 1,100 | 900 | 160 | 800 | 230 | 280 | 240 | 360 | 780 | — | 800 | 2,800 | 2,800 | 1,300 | 300 | 1,700 | 2,500 |
| E. coli DH5α | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | |
| Y. pestis KIM6+ | 1,255 | 1,500 | 1,203 | 160 | 797 | 233 | 286 | 237 | 359 | 780 | 100 | 830 | 2,797 | 2,805 | 1,340 | 300 | 1,762 | 2,518 | |
| Y. enterocolitica WA-314 | O8 | 1,255 | 1,500 | 1,203 | 160 | 797 | 233 | 286 | 237 | 359 | 780 | NPc | 830 | 2,797 | 2,930 | 1,340 | 300 | 1,762 | 2,518 |
I to XI are regions shown in Fig. 1A; XII to XVIII are regions shown in Fig. 1B. The sizes of Yersinia PCR products derived from regions I to XVIII are according to the published HPI sequences (13, 34, 39); the sizes of E. coli PCR products derived from the corresponding regions of E. coli strains were determined after agarose gel electrophoresis.
—, no PCR product obtained.
NP, not present.
In addition, a second set of primer pairs was used to analyze the order of the HPI-specific genes in both STEC strains. For this purpose, primer pairs derived from sequences of Y. pestis HPI were constructed that enabled us to link consecutively arranged genes. The location of the target regions (XII to XVIII) and the primers used are shown in Fig. 1B and in Table 2, respectively. As seen from Table 4, PCR products were obtained from all investigated regions of both STEC strains; the sizes of these PCR products closely corresponded to the sizes of the respective regions in the Y. pestis and Y. enterocolitica HPIs determined according to the published sequence analysis (13, 34).
Determination of the integration site of HPI in STEC.
The HPI element in all Yersinia species tested is located in the vicinity of an asparagine-specific tRNA gene. A recent study (7) demonstrated that the HPI in Y. pseudotuberculosis is located not only adjacent to asnT, as is the case in Y. enterocolitica and Y. pestis, but also adjacent to two other asn tRNA loci, asnU and asnV. In order to analyze the location of the HPI in STEC strains, PCRs specific for each of the three asparagine-specific tRNA location sites were performed. In 17 representative irp2-fyuA-positive STEC strains of different serotypes, the insertion site of the HPI is next to the tRNA gene asnT, as demonstrated by employing primer pair asnT and int2 in PCR. A PCR product was detected in all E. coli strains. Whereas all five E. coli O26:H11/H− strains and an O60:H− strain demonstrated a 1,200-bp product, a 1,500-bp product was obtained from strains of other serotypes, including 128:H2, O3:H10, ONT:H−, ONT:H8, and Orough:H− (data not shown). No PCR products were obtained with primer pairs asnU and int2 and asnV and int2 specific for a probable HPI insertion adjacent to the tRNA genes asnU and asnV, respectively.
Characterization of boundary genes in STEC.
The 17 representative irp2-fyuA-positive STEC isolates were further used to characterize HPI boundary genes in STEC. PCRs performed with the primer pair asnT1 and int2 specific for the left junction of the HPI, including asnT and the integrase gene int, revealed products of 1,100 bp in all O26:H11/H− strains and the O60:H− strain and products of 1,400 bp in all STEC strains of the other serotypes (data not shown). Sequence analysis of the 1,400-bp PCR product found in O128 STEC strain 3172/97 revealed that the integrase gene was intact, as seen in other E. coli isolates and Y. pseudotuberculosis and Y. pestis strains (7, 13). The integrase PCR product of strain 3172/97 showed 94.5% identity with the corresponding sequence from Y. pestis strain 6/69 (7). In O26 STEC strain 5720/96, however, a deletion of 347 bp was found in the integrase gene which resulted in a frameshift introducing a premature stop codon 36 bp downstream. Figure 2 shows an alignment of the deduced amino acid sequences of the integrases of the two STEC strains as compared with those of Y. pestis and Y. pseudotuberculosis.
FIG. 2.
Alignment of the deduced amino acid sequences of the integrases of Y. pestis (first line), Y. pseudotuberculosis (second line), STEC strain 3172/97 (third line), and STEC strain 5720/96 (fourth line). Translation of the latter sequence was performed without consideration of the frameshift resulting from the deletion of 347 bp. Bold letters represent differences in the amino acid sequence from the sequence of Y. pestis in the first line. Dashes in the last line indicate amino acid residues that are not present in this sequence (deletions). The deduced amino acid sequences of the Y. pestis and Y. pseudotuberculosis integrases are based on references 7 and 13.
Expression of fyuA gene in non-O157 STEC strains.
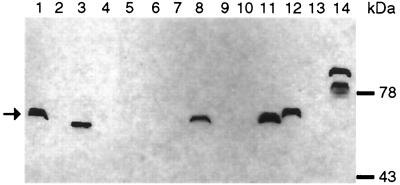
In order to analyze the expression of fyuA in non-O157 STEC strains carrying the Yersinia HPI, immunoblotting of outer membrane proteins was performed. As shown in Fig. 3, FyuA was detectable in four out of seven HPI-positive STEC strains, whereas none of the HPI-negative strains revealed expression of fyuA. In accordance with previous results, FyuA from three E. coli strains appeared to be the same size as Yersinia FyuA (67 kDa) (15, 20). However, in one E. coli isolate, two polypeptides were detected, both larger than the expected FyuA (Fig. 3). Polypeptides of apparently larger size have been previously observed in certain Y. pseudotuberculosis strains (36).
FIG. 3.
Immunoblot of outer membrane proteins probed with anti-FyuA rabbit serum. The arrow indicates the FyuA protein band. Lane 1, Y. pseudotuberculosis O1 (HPI+); lane 2, E. coli K-12 MG1655 (HPI−); lane 3, EAEC strain 17-2 (HPI+); lane 4, STEC strain O157:H7 3268/90 (HPI−); lane 5, STEC strain O62:H− 4595/97 (HPI−); lane 6, STEC strain O40:H− 4828/97 (HPI−); lane 7, STEC strain O103:H− 4797/97 (HPI−); lane 8, STEC strain O128:H2 3115/97 (HPI+); lane 9, STEC strain O128:H2 3172/97 (HPI+); lane 10, STEC strain ONT:H− 4941/97 (HPI+); lane 11, STEC strain O3:H10 5726/96 (HPI+); lane 12, STEC strain O60:H− 3357/98 (HPI+); lane 13, STEC strain Orough:H− 0512E015 (HPI+); lane 14, STEC strain O26:H11 6061/96 (HPI+). Molecular mass is shown on the right.
DISCUSSION
Horizontal gene transfer represents a key genetic mechanism in the evolution of pathogens (2, 12, 15, 26, 29). Genes encoding important virulence factors are often located on mobile genetic elements such as phages, plasmids, or transposons and can therefore be transferred from one cell to another. PAIs are discrete genetic units preferentially located in the chromosomes of pathogens which also carry virulence genes. Those genes may have been introduced into the genome of pathogens recently via lateral gene transfer (15, 17). Gene transfer processes such as these lead to a mosaic pattern of pathogenicity in many infectious agents. The STEC strains represent an example par excellence of pathogen development by lateral gene transfer. Important virulence factors such as Stx, the adherence factor intimin, and the EHEC hemolysin are encoded by phages, the LEE PAI, and the large plasmid, respectively (11, 30, 33, 45, 50). STEC strains are a heterogeneous group of pathogenic organisms with respect to their serotypes, stx genes, and the presence of additional virulence factors.
The majority of PAIs detected in enterobacteria are specific for particular species or even pathotypes. Thus, the LEE island, encoding virulence factors in diarrheagenic E. coli, has not been described in pathotypes other than STEC and EPEC (28). The so-called HPI, first described in pathogenic Yersinia (9), however, represents an exception, because the HPI element has been detected in many enterobacterial species and pathotypes, including both enteroaggregative and extraintestinal E. coli (49). In addition, more than 30% of E. coli isolates from physiological intestinal microflora also carry this island (49). The mobility of the HPI elements may be associated with an intact integrase gene located at the left junction of the HPI. The gene product, integrase, may be involved in the excision and mobilization of the HPI element (7, 16, 17).
It has been shown recently that the HPI elements are not present in the genome of STEC strains of serotype O157:H7/H− (49). We therefore analyzed 206 STEC strains to investigate the possibility that HPI elements are present in STEC strains of other serotypes. Although we could confirm that O157 strains do not carry the HPI element, it became apparent that STEC strains of other clonal lineages were HPI positive, including the O26:H11/H− group, which is currently regarded as the most common non-O157 group of STEC strains in Germany and in other European countries (5, 8). Detailed analysis of the HPI in two representative STEC strains demonstrated that with the exception of the IS100 insertion element, all investigated genes were present and arranged in the order that was demonstrated for the HPI of pathogenic yersiniae (13, 34).
For each of the HPI-positive STEC strains, the presence of both fyuA and irp2 genes was demonstrated. However, the yersiniabactin receptor FyuA was expressed in only about 60% of these strains. This may be due to partial deletions of the fyuA gene as has been previously shown for certain E. coli isolates (49). The fact that HPI-positive E. coli strains lack expression of FyuA may indicate that the yersiniabactin siderophore system is not the primary advantage of possessing the HPI. This hypothesis is supported by the observation that, in E. coli, deletions of the HPI are reported to affect solely the fyuA segment. However, the reason for the different expression of fyuA remains to be clarified.
The HPI of STEC shares common features with the HPI elements of other enterobacteria, including pathogenic yersiniae. It encodes FyuA proteins which may act as receptors for pesticin and the siderophore yersiniabactin (20, 21, 34). Other genes located on the HPI encode this particular iron uptake system. From an evolutionary point of view, the high degree of sequence identity between the homologous HPI-specific genes of various pathotypes and species including STEC suggests a recent transfer of the HPI from one species to another. As also shown for other enterobacteria, the HPI in STEC is located next to the tRNA gene asnT. The asnT locus is linked to a gene which is highly homologous with a phage-derived integrase determinant termed int. In Y. pseudotuberculosis, Y. pestis, and extraintestinal E. coli, the int gene seems to be intact, whereas in Y. enterocolitica the open reading frame has been destroyed by a frameshift mutation. In some STEC strains, the int open reading frame is intact, but in strains of the O26 group, a deletion in int has led to a truncation of the integrase. It therefore seems that the HPI of the STEC O26 group represents a new and unique type of HPI with a partially deleted int. The deletion in the int gene may result in a nonfunctional integrase and subsequent fixation of the HPI in the genome of this STEC clonal lineage. In pathogenic yersiniae, the HPIs are flanked by two direct repeats of 16 bp which may be involved in HPI mobility. In E. coli, however, only one direct repeat is present. Therefore, insertion and excision events may be prevented, even when the integrase gene is intact.
The HPI elements code for a particular iron uptake system, termed yersiniabactin. Iron uptake in general increases the metabolic fitness of bacteria and does not directly contribute to host damage and infection. The question arises whether the HPI elements in E. coli indeed represent PAIs or whether they contribute to the survival of the strains in certain ecological niches. Although, at the present time, we are unable to answer this question in regard to the STEC strains analyzed here, the fact that the HPI-positive as well as HPI-negative STEC strains differ little in their pathogenic potential supports the view that the HPI in E. coli is a form of fitness island rather than a PAI. This idea is corroborated by the fact that more than 30% of nonpathogenic fecal E. coli strains are also HPI positive (49). From an evolutionary point of view, and because of the occurrence of examples like these, we can assume that all these genetic elements are variations of genomic islands (17). Since genomic islands show similar structural features, it is likely that they have been transferred in recent times by horizontal processes. The genomic islands may contribute to the fitness (fitness islands) or metabolic flexibility (metabolic islands) of the organisms, or they may increase their pathogenic potential (PAIs). The particular function of an island will thus depend strongly on the genetic background of the individual strains. Further experiments are necessary in order to define the exact role of the HPI element in the life cycle of STEC strains.
ACKNOWLEDGMENTS
We thank M. Bielaszewska (Würzburg) and J. Heesemann (München) for discussions.
The work was supported by the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 479 (Projects A1 and A3), and by the Bavarian Ministry for Environmental Protection.
REFERENCES
- 1.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of E. coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 2.Blum G, Ott M, Lischewski A, Ritter A, Imrich H, Tschäpe H, Hacker J. Excision of large DNA regions termed pathogenicity islands from tRNA-specific loci in the chromosome of an Escherichia coli wild-type pathogen. Infect Immun. 1994;2:606–614. doi: 10.1128/iai.62.2.606-614.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blum-Oehler G, Dobrindt U, Weiß N, Janke B, Schubert S, Rakin A, Heesemann J, Marre R, Hacker J. Pathogenicity islands of uropathogenic Escherichia coli: implications for the evolution of virulence. Zentbl Bakteriol Suppl. 1998;29:380–386. [Google Scholar]
- 4.Bockemühl J, Aleksic S, Karch H. Serological and biochemical properties of Shiga-like toxin (verocytotoxin)-producing strains of Escherichia coli, other than O-group 157, from patients in Germany. Zentbl Bakteriol. 1992;276:189–195. doi: 10.1016/s0934-8840(11)80005-8. [DOI] [PubMed] [Google Scholar]
- 5.Bockemühl J, Karch H, Tschäpe H. Zur situation der infektionen des menschen durch enterohämorrhagische Escherichia coli (EHEC) in Deutschland 1997. Bundesgesundheitsblatt. 1998;41:2–5. doi: 10.1007/s00103-002-0458-4. [DOI] [PubMed] [Google Scholar]
- 6.Bokete T N, Whittam T S, Wilson R A, Clausen C R, O'Callahan C M, Moseley S L, Fritsche T R, Tarr P I. Genetic and phenotypic analysis of Escherichia coli with enteropathogenic characteristics isolated from Seattle children. J Infect Dis. 1997;175:1382–1389. doi: 10.1086/516470. [DOI] [PubMed] [Google Scholar]
- 7.Buchrieser C, Brosch R, Bach S, Guiyoule A, Carniel E. The high pathogenicity island of Yersinia pseudotuberculosis can be inserted into any of the three chromosomal asn tRNA genes. Mol Microbiol. 1998;30:965–978. doi: 10.1046/j.1365-2958.1998.01124.x. [DOI] [PubMed] [Google Scholar]
- 8.Caprioli A, Tozzi A E, Rizzoni G, Karch H. Non-O157 Shiga toxin-producing Escherichia coli infections in Europe. Emerg Infect Dis. 1997;3:578–579. doi: 10.3201/eid0304.970425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carniel E, Guilvout I, Prentice M. Characterization of a large chromosomal “high pathogenicity island” in biotype 1B Yersinia enterocolitica. J Bacteriol. 1996;178:6743–6751. doi: 10.1128/jb.178.23.6743-6751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cebula T A, Payne W L, Feng P. Simultaneous identification of strains of Escherichia coli serotype O157:H7 and their Shiga-like toxin type by mismatch amplification mutation assay-multiplex PCR. J Clin Microbiol. 1995;33:248–250. doi: 10.1128/jcm.33.1.248-250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott S J, Wainwright L A, McDaniel T K, Jarvis K G, Deng Y K, Lai L C, McNamara B P, Donnenberg M S, Kaper J K. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol Microbiol. 1998;28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 12.Finlay B B, Falkow S. Common themes in microbial pathogenicity revised. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gehring A M, DeMoll E, Fetherston J D, Mori I, Meyhew G F, Blattner F R, Walsh T C, Perry R D. Iron acquisition in plaque: modular logic in enzymatic biogenesis of yersiniabactin by Yersinia pestis. Chem Biol. 1998;5:573–586. doi: 10.1016/s1074-5521(98)90115-6. [DOI] [PubMed] [Google Scholar]
- 14.Gunzer F, Böhm H, Rüssmann H, Bitzan M, Aleksic S, Karch H. Molecular detection of sorbitol-fermenting Escherichia coli O157 in patients with hemolytic-uremic syndrome. J Clin Microbiol. 1992;30:1807–1810. doi: 10.1128/jcm.30.7.1807-1810.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hacker J, Blum-Oehler G, Mühldorfer I, Tschäpe H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 16.Hacker J, Blum-Oehler G, Janke B, Nagy G, Goebel W. Pathogenicity islands of extraintestinal Escherichia coli. In: Kaper J, Hacker J, editors. Pathogenicity islands and other mobile virulence elements. Washington, D.C: ASM Press; 1999. pp. 59–76. [Google Scholar]
- 17.Hacker J, Kaper J. The concept of pathogenicity islands. In: Kaper J, Hacker J, editors. Pathogenicity islands and other mobile virulence elements. Washington, D.C: ASM Press; 1999. pp. 1–11. [Google Scholar]
- 18.Hames B D. Introduction to polyacrylamide gel electrophoresis. In: Hames B D, Rickwood D, editors. Gel electrophoresis of proteins: a practical approach. Washington, D.C: IRL Press; 1987. pp. 1–91. [Google Scholar]
- 19.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 20.Heesemann J. Chromosomal-encoded siderophores are required for mouse virulence of enteropathogenic Yersinia species. FEMS Microbiol Lett. 1987;48:229–233. [Google Scholar]
- 21.Heesemann J, Hantke K, Vocke T, Saken E, Rakin A, Stojiljkovic I, Berner R. Virulence of Yersinia enterocolitica is closely associated with siderophore production, expression of an iron-repressible outer membrane protein of 65000 Da and pesticin sensitivity. Mol Microbiol. 1993;8:397–408. doi: 10.1111/j.1365-2958.1993.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 22.Kaper J B, Elliott S, Sperandio V, Perna N T, Mayhew G F, Blattner F R. Attaching-and-effacing intestinal histopathology and the locus of enterocyte effacement. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C: American Society for Microbiology; 1998. pp. 163–182. [Google Scholar]
- 23.Karch H, Huppertz H-I, Bockemühl J, Schmidt H, Schwarzkopf A, Lissner R. Shiga toxin-producing Escherichia coli infections in Germany. J Food Prot. 1997;11:1454–1457. doi: 10.4315/0362-028X-60.11.1454. [DOI] [PubMed] [Google Scholar]
- 24.Karch H, Schmidt H, Brunder W. Plasmid-encoded determinants of Escherichia coli O157:H7. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C: American Society for Microbiology; 1998. pp. 183–194. [Google Scholar]
- 25.Karmali M A, Petric M, Louie S, Cheung R. Antigenic heterogeneity of Escherichia coli verotoxins. Lancet. 1986;1:164–165. doi: 10.1016/s0140-6736(86)92307-x. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence J G, Ochman H. Molecular archaeology of the Escherichia coli genome. Proc Natl Acad Sci USA. 1998;95:9413–9417. doi: 10.1073/pnas.95.16.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariani Kurkdjian P, Denamur E, Milon A, Picard B, Cave H, Lambert Zechovsky N, Loirat C, Goullet P, Sansonetti P J, Elion J. Identification of a clone of Escherichia coli O103:H2 as a potential agent of hemolytic-uremic syndrome in France. J Clin Microbiol. 1993;31:296–301. doi: 10.1128/jcm.31.2.296-301.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDaniel T K, Jarvis K G, Donnersberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacteria pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mühldorfer I, Hacker J. Genetic aspects of Escherichia coli virulence. Microb Pathog. 1994;16:171–181. doi: 10.1006/mpat.1994.1018. [DOI] [PubMed] [Google Scholar]
- 30.Newland J W, Strockbine N A, Miller S F, O'Brien A D, Holmes R K. Cloning of Shiga-like toxin structural genes from a toxin converting phage of Escherichia coli. Science. 1985;230:179–181. doi: 10.1126/science.2994228. [DOI] [PubMed] [Google Scholar]
- 31.Newland J W, Strockbine N A, Neill R J. Cloning of genes for production of Escherichia coli Shiga-like toxin type II. Infect Immun. 1987;55:2675–2680. doi: 10.1128/iai.55.11.2675-2680.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Brien A O, Lively T A, Chen M E, Rothman S W, Formal S B. Escherichia coli O157:H7 strains associated with haemorrhagic colitis in the United States produce a Shigella dysenteriae 1 (SHIGA) like cytotoxin. Lancet. 1983;1:702. doi: 10.1016/s0140-6736(83)91987-6. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien A D, Newland J W, Miller S F, Holmes R K, Smith H W, Formal S B. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science. 1984;226:694–696. doi: 10.1126/science.6387911. [DOI] [PubMed] [Google Scholar]
- 34.Pelludat C, Rakin A, Jacobi C A, Schubert S, Heesemann J. The yersiniabactin biosynthetic gene cluster of Yersinia enterocolitica: organization and siderophore-dependent regulation. J Bacteriol. 1998;180:538–546. doi: 10.1128/jb.180.3.538-546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piérard D, Muyldermans G, Moriau L, Stevens D, Lauwers S. Identification of new verocytotoxin type 2 variant B-subunit genes in human and animal Escherichia coli isolates. J Clin Microbiol. 1998;36:3317–3322. doi: 10.1128/jcm.36.11.3317-3322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rakin A, Heesemann J. Yersiniabactin/pesticin receptor: a component of an iron uptake system of highly pathogenic Yersinia. Contrib Microbiol Immunol. 1995;13:244–247. [PubMed] [Google Scholar]
- 37.Rakin A, Urbitsch P, Heesemann J. Evidence for two evolutionary lineages of highly pathogenic Yersinia species. J Bacteriol. 1995;177:2292–2298. doi: 10.1128/jb.177.9.2292-2298.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rakin, A., S. Schubert, C. Pelludat, D. Brem, and J. Heesemann. The high-pathogenicity island of Yersinia. In J. Kaper and J. Hacker (ed.), Pathogenicity islands and other mobile virulence elements, in press. ASM Press, Washington, D.C.
- 39.Rakin A, Noelting C, Schubert S, Heesemann J. Common and specific characteristics of the high-pathogenicity island of Yersinia enterocolitica. Infect Immun. 1999;67:5265–5274. doi: 10.1128/iai.67.10.5265-5274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ritter A, Gally D, Olsen P B, Dobrindt U, Friedrich A, Klemm P, Hacker J. The Pai-associated leuX specific tRNA5Leu affects type 1 fimbriation in pathogenic Escherichia coli by control of FimB recombinase expression. Mol Microbiol. 1997;25:871–882. doi: 10.1111/j.1365-2958.1997.mmi517.x. [DOI] [PubMed] [Google Scholar]
- 41.Rüssmann H, Schmidt H, Heesemann J, Caprioli A, Karch H. Variants of Shiga-like toxin II constitute a major toxin component in Escherichia coli O157 strains from patients with haemolytic uraemic syndrome. J Med Microbiol. 1994;40:338–343. doi: 10.1099/00222615-40-5-338. [DOI] [PubMed] [Google Scholar]
- 42.Rüssmann H, Kothe E, Schmidt H, Franke S, Harmsen D, Caprioli A, Karch H. Genotyping of Shiga-like toxin genes in non-O157 Escherichia coli strains associated with haemolytic uraemic syndrome. J Med Microbiol. 1995;42:404–410. doi: 10.1099/00222615-42-6-404. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt H, Plaschke B, Franke S, Rüssmann H, Schwarzkopf A, Heesemann J, Karch H. Differentiation of virulence patterns of Escherichia coli possessing eae genes. Med Microbiol Immunol. 1994;183:23–31. doi: 10.1007/BF00193628. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt H, Rüssmann H, Schwarzkopf A, Aleksic S, Heesemann J, Karch H. Prevalence of attaching and effacing Escherichia coli in stool samples from patients and controls. Zentbl Bakteriol. 1994;281:201–213. doi: 10.1016/s0934-8840(11)80571-2. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt H, Beutin L, Karch H. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL933. Infect Immun. 1995;63:1055–1061. doi: 10.1128/iai.63.3.1055-1061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt H, Scheef J, Janetzki-Mittermann C, Datz M, Karch H. An ileX tRNA gene is located close to the Shiga toxin II operon in enterohemorrhagic Escherichia coli O157 and non-O157 strains. FEMS Microbiol Lett. 1997;149:39–44. doi: 10.1111/j.1574-6968.1997.tb10305.x. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt H, Geitz C, Tarr P I, Frosch M, Karch H. Non-O157:H7 pathogenic Shiga toxin-producing Escherichia coli: phenotypic and genetic profiling of virulence traits and evidence for clonality. J Infect Dis. 1999;179:115–123. doi: 10.1086/314537. [DOI] [PubMed] [Google Scholar]
- 48.Schmitt C K, McKee M L, O'Brien A D. Two copies of Shiga-like toxin II-related genes common in enterohemorrhagic Escherichia coli strains are responsible for the antigenic heterogeneity of the O157:H− strain E32511. Infect Immun. 1991;59:1065–1073. doi: 10.1128/iai.59.3.1065-1073.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schubert S, Rakin A, Karch H, Carniel E, Heesemann J. Prevalence of the “high pathogenicity island” of Yersinia species among Escherichia coli strains that are pathogenic to humans. Infect Immun. 1998;66:480–485. doi: 10.1128/iai.66.2.480-485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scotland S M, Smith H R, Willshaw G A, Rowe B. Vero cytotoxin production in strain of Escherichia coli is determined by genes carried on bacteriophage. Lancet. 1983;2:216. doi: 10.1016/s0140-6736(83)90192-7. [DOI] [PubMed] [Google Scholar]
- 51.Tarr P I, Fouser L S, Stapleton A E, Wilson R A, Kim H H, Vary J C, Jr, Clausen C R. Hemolytic-uremic syndrome in a six-year-old girl after a urinary tract infection with Shiga-toxin-producing Escherichia coli O103:H2. N Engl J Med. 1996;335:635–638. doi: 10.1056/NEJM199608293350905. [DOI] [PubMed] [Google Scholar]
- 52.Tzipori S, Wachsmuth I K, Chapman C, Birner R, Brittingham J, Jackson C, Hogg J. The pathogenesis of hemorrhagic colitis caused by Escherichia coli O157:H7 in gnotobiotic piglets. J Infect Dis. 1986;154:712–716. doi: 10.1093/infdis/154.4.712. [DOI] [PubMed] [Google Scholar]
- 53.Vial P A, Robins-Browne R, Lior H, Prado V, Kaper J B, Nataro J P, Maneval D, Elsayed A, Levine M M. Characterization of enteroadherent-aggregative Escherichia coli, a putative agent of diarrheal disease. J Infect Dis. 1988;158:70–79. doi: 10.1093/infdis/158.1.70. [DOI] [PubMed] [Google Scholar]
- 54.Wells J G, Davis B R, Wachsmuth I K, Riley L W, Remis R S, Sokolow R, Morris G K. Laboratory investigation of hemorrhagic colitis outbreaks associated with a rare Escherichia coli serotype. J Clin Microbiol. 1983;18:512–520. doi: 10.1128/jcm.18.3.512-520.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]