Abstract
Although there appears to be little if any role for specific antibodies in protection against intracellular bacteria, such as the model pathogen F. tularensis live vaccine strain (LVS), the role of B cells themselves in primary and secondary infection with such bacteria has not been examined directly. We show here that mice deficient in mature B cells and antibodies (B-cell knockout mice) are marginally compromised in controlling primary sublethal infection but are 100-fold less well protected against secondary lethal challenge than are their normal counterparts. This defect in optimal specific protective immunity was readily reconstituted by the transfer of primed, and to a lesser degree, unprimed B cells, but not by the transfer of specific antibodies. The results indicate a previously unappreciated role for B cells in secondary immunity to intracellular pathogens through a function other than antibody production.
To better understand the mechanisms of protective immunity operative against intracellular pathogens, we have characterized the murine protective immune response to the intracellular bacterium Francisella tularensis live vaccine strain (LVS). As with fully virulent F. tularensis, LVS infects and replicates in macrophages and macrophage-like cells such as Kupffer cells in the spleen, liver, and lung (10, 16, 38). The histopathology of LVS infections in mice is also similar to that of human infections with fully virulent F. tularensis (38), and this model infection has several convenient features. For example, in normal mice the intradermal (i.d.) 50% lethal dose (LD50) with LVS is fairly high (about 105) for BALB/cByJ mice. On the other hand, mice are quite susceptible to intraperitoneal (i.p.) or intravenous (i.v.) infection, and the i.p. LD50 approaches a single bacterium (12, 16). Mice that survive and clear sublethal i.d. infection are solidly immune to subsequent lethal i.p. or i.v. infection a month later and can survive a lethal challenge of >107 LD50s (12, 16, 46). Since survival of sublethal infection leads to such a strong and easily measurable secondary protective immunity, we and others (39) have found the study of LVS infection in mice to be an informative in vivo model of immunity to intracellular pathogens.
As for other intracellular pathogens, the contribution of T cells to control of primary LVS infection has been studied by using athymic nu/nu mice, which lack mature T cells in the periphery; these mice arose as a result of a spontaneous mutation (33) and have been widely used to study the role of T cells in immune responses. While unprimed nu/nu mice, like normal mice, die within a week of lethal LVS infection i.p., nu/nu given 103 LVS i.d. survive initially but die after about 30 days (11). Thus, innate, non-T-cell-mediated immunity is able to control infection for several weeks, as is the case for other intracellular bacteria such as Listeria (13) and Salmonella (32) spp. We have also described a nonspecific, B-cell-dependent phase of innate immunity that leads to the generation of strong protective immunity against lethal challenge very quickly after the establishment of sublethal infection: normal mice, but not B-cell-deficient mice, given a sublethal dose of LVS i.d. on day 0 survive a lethal i.p. or i.v. challenge of over 106 LD50s given on day 3 (6, 8, 11). Similar early protective immunity is also operative in murine Listeria infection (9). This B-cell-dependent phase of innate immunity apparently wanes after about a month, since T-cell knockout or nu/nu mice that are primed and challenged 3 days later die after about 30 days (6, 11). Recent studies with either mice depleted of T-cell subpopulations or genetically defined T-cell knockout mice directly demonstrate that either CD4+ or CD8+, α/β+ T cells alone are sufficient for resolution of primary infection, as well as for the development and expression of secondary protective immunity (46).
Other studies, including those with CD4 knockout mice that have a very poor specific antibody response, strongly suggest that specific antibodies appear to have a limited role at best in long-term protection against LVS, as might be expected for an intracellular pathogen (34, 46). However, since no spontaneous mutant mice lacking B cells, comparable to nu/nu mice, have been identified to date, the role of B cells in immunity to intracellular pathogens has been much less well studied than that of T cells. The recent development of genetically deficient mice lacking B cells (24) and thus lacking the capability to make specific antibodies has made such studies feasible. Here we study the outcome of both primary and long-term secondary LVS infection in B-cell knockout (BKO) mice. We find that such mice are marginally defective in primary responses but, surprisingly, are significantly impaired in the quality of the secondary protective immune response to this pathogen. This defect appears to be due not to the absence of specific anti-LVS antibodies but to a function provided by B cells themselves.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free, male C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, Maine) or from the Biological Resources Branch, Frederick Cancer Research and Development Center, National Cancer Institute (Frederick, Md.). Male Igh6− (μMT; B cell negative [24]) knockout mice on a C57BL/6J background (>14 back-crosses) were purchased from the Induced Mutant Resource of the Jackson Laboratories. At least one mouse from each shipment of knockout mice was sacrificed and spleen cells were stained and assessed by flow cytometry (see below) to confirm the phenotype of the mutation; no discrepancies were found. All mice were housed in sterile microisolator cages in a barrier environment at the Center for Biologics Evaluation and Research (CBER), were fed autoclaved food and water ad libitum, and were routinely tested for common murine pathogens by a diagnostic service provided by the Division of Veterinary Services, CBER. In conducting the research described in this report, the investigators adhered to a protocol approved by the Animal Care and Use Committee of CBER.
Bacterial infections.
Mice were given 0.5 ml i.p. or 0.1 ml i.d. of the indicated dilution of LVS; actual doses of bacteria inoculated were simultaneously determined by plate count. All materials, including bacteria, were diluted in phosphate-buffered saline (PBS; BioWhittaker, Walkersville, Md.) containing <0.01 ng of endotoxin per ml. LD50s were calculated by the method of Reed and Muench as discussed by Lennette (27). LD50 determinations, organ burden studies, and transfer experiments used groups of three to six mice, as indicated. Mean time to death (MTD) was calculated by arithmetic mean ± the standard deviation for all mice within a group that died; surviving mice were not included in this calculation.
Bacteria and growth conditions.
F. tularensis LVS (ATCC 29684; American Type Culture Collection, Rockville, Md.) was cultured on modified Mueller-Hinton (MH) agar plates or in modified MH broth (Difco Laboratories, Detroit, Mich.) supplemented with ferric pyrophosphate and IsoVitalex as previously described (Becton Dickinson, Cockeysville, Md. [2, 16]). Aliquots (1 ml) of bacteria were frozen in broth alone at −70°C and periodically thawed for use, and viable bacteria were quantified by plating serial dilutions on MHA plates. The number of CFU after thawing varied less than 5% over a 6-month period.
Determination of organ burdens in infected mice.
Experiments enumerating numbers of CFU in organs of various mice were performed as follows: mice were infected as indicated, and spleens, livers, lungs, and peritoneal cells (with 7 to 8 ml of PBS to lavage the peritoneal cavity) were removed aseptically. The organs were emulsified in a Stomacher (Tekmar, Cincinnati, Ohio) in 5 to 10 ml of sterile PBS, and appropriate dilutions of organ homogenates or peritoneal cells were plated on MH plates. Results are expressed as the mean ± the standard deviation of the mean for groups of three to four mice.
Reconstitution of B-cell knockout mice with lymphocyte subpopulations and antibody preparations.
Single cell suspensions were prepared from spleens from the indicated donor mice. Erythrocytes were lysed using ammonium chloride, and the resulting cell suspensions were counted under trypan blue. For depletion of T cells, spleen cells at a concentration of 108/ml were treated with a cocktail of 10 μg of anti-Thy1.2 (30-H12), anti-CD4 (RM4-5), anti-CD8 (53-6.7), and anti-γ/δ T-cell receptor (TCR) per ml TCR (GL3; all were purchased from Pharmingen, San Diego, Calif.), determined to be optimal in initial experiments, for 30 min on ice, followed by treatment with 1:10 rabbit complement (Pel-Freeze, Brown Deer, Wis.) for 30 min at 37°C. Aliquots of both the starting spleen cell populations and the final populations were analyzed by flow cytometry using a FACScan with gates set for viable lymphocytes and monocytes according to forward and side scatter profiles. Cells were stained using a panel of monoclonal antibodies, including fluorescein isothiocyanate–anti-B220, phycoerythrin (PE)–anti-CD4, PE–anti-CD8, PE–anti-CD11b, and PE–anti-γ/δ TCR (all purchased from Pharmingen and chosen to recognize different epitopes from those used in cytotoxicity where available), in both one- and two-color staining protocols. Optimal concentrations for staining with each lot of each fluorochrome-labeled antibody were determined in preliminary experiments. All B-cell-enriched populations used for transfer were >92% B220+; approximately 5 to 8% CD11b+; and <1% CD4+, CD8+, or γ/δ+. Serum used for reconstitution experiments was obtained by bleeding normal C57BL/6J mice from the lateral tail vein and pooling the resulting serum (normal mouse serum [NMS]). To obtain serum with very high amounts of anti-LVS antibodies, mice were then given a sublethal infection of 105 LVS i.d. and then given a second sublethal infection of 104 LVS i.d. on day 30, and sera were obtained and pooled 35 days later (immune mouse serum [IMS]). The endpoint anti-LVS antibody titers of the pools used in these experiments were <1:10 IgM and IgG for NMS, and 1:640 IgM and 1:10,240 IgG for IMS, as determined by enzyme-linked immunosorbent assay (ELISA) as described previously (34 [see also below]). All pools of LVS antibody were effective in vivo, in that normal BALB/cByJ mice given 0.5 ml of a 1:10 dilution of IMS and challenged i.p. immediately with 5 × 102 LVS survived, as previously described (34).
Characterization of antibody response.
Sera were obtained via the lateral tail vein and pooled from the indicated groups of mice both before (prebleed) and after LVS infection. Titers of specific anti-LVS serum antibodies were determined as previously described (34). Briefly, Immulon 1 plates were coated overnight with live LVS; the plates were washed and blocked the next day with 10% calf serum. Test serum samples and appropriate positive and negative controls were applied using twofold dilutions and incubated for 2 h at 37°C. After washing, enzyme-labeled antibodies (goat anti-mouse Ig; anti-IgM; or anti-IgG that detects IgG1, IgG2a, IgG2b, and IgG3), directly conjugated to horseradish peroxidase (Southern Biotech, Birmingham, Ala.), were added for detection. Optimal concentrations for use of the various lots of enzyme labeled antibodies (to ensure isotype specificity and to establish the limit of detection) were determined in separate experiments. For color development, ABTS Peroxidase Substrate (Kirkegaard and Perry Laboratories, Inc., Gaithersburg, Md.) was added; the reaction was stopped after 30 min by the addition of 1.5% KFl and read at an optical density at 405 nm (OD405) with an OD600 reference. The endpoint titer was defined as the lowest dilution of immune serum that had a mean OD405 value that was greater than the mean value of the matched dilution of normal prebleed mouse serum plus three standard deviations of the mean and also was >0.050 (34).
RESULTS
Primary infection of BKO mice with F. tularensis LVS.
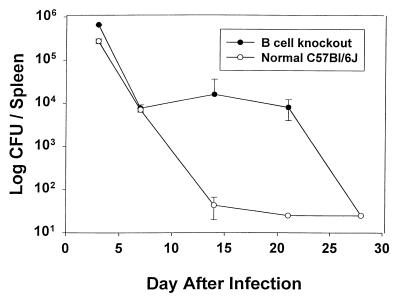
Two approaches were taken to study the nature of the primary response to infection with F. tularensis LVS. Since a single bacterium is lethal for normal mice when given i.p. or i.v., the LD50 in normal C57BL/6J mice was compared to BKO mice by using the less virulent i.d. route. These mice contain pre-B cells but do not express IgM and thus lack mature IgM+ B cells, as well as the capacity for antibody production (24). As shown in Table 1 , the i.d. LD50 for BKO mice was three- to fourfold lower than that for normal C57BL/6J mice in two separate determinations. However, this was a relatively modest difference that could be of minimal biological significance, and due to subtle remaining differences in the murine genetic backgrounds rather than to the absence of B cells. To examine whether such a difference might be reflected in the dissemination and clearance of bacteria, BKO and normal mice were given a maximal sublethal infection of 105 LVS i.d. At various days after i.d. infection, mice were sacrificed and bacterial burdens determined in spleen, liver, and lung. As shown in Fig. 1, the numbers of bacteria in spleens were similar in both strains at 3 to 7 days after infection. However, there was a 2-week delay in clearance of bacteria from spleens of infected BKO mice compared to normal C57BL/6J mice, and a substantial bacterial burden was found in spleens over these 2 weeks. This delay in clearance, with a similar time course, was also observed in the livers and lungs of infected BKO mice (data not shown). Thus, the absence of B cells results in a significant delay in clearance of LVS from B-cell-deficient mice, with a slight but measurable impact on overall susceptibility to sublethal infection and mortality.
TABLE 1.
Comparison of i.d. LD50 of LVS in BKO and normal micea
| Expt | No. of mice/group (total no.) | i.d. LD50 of LVS for:
|
|
|---|---|---|---|
| Normal C57BL/6J mice | BKO mice | ||
| 1 | 5 (42) | 1.8 × 107 | 4.4 × 106 |
| 2 | 4 (33) | 2.3 × 107 | 7.3 × 106 |
The i.d. LD50 values calculated from deaths of mice given doses of LVS i.d. ranging from 105 to 108 are shown; actual LVS doses were confirmed by plate count at the time of inoculation. Mice were observed for morbidity and mortality through day 30, at which time surviving mice were sacrificed to assess clearance of bacteria from spleens.
FIG. 1.
Normal C57BL/6J and C57.Igh6− (BKO) mice were given 105 LVS i.d.; actual doses were confirmed by plate count at the time of inoculation. On the indicated day mice of each type were sacrificed, and spleens, livers, and lungs were homogenized for enumeration of bacterial CFU. Only results for the spleen are shown. Error bars indicate the standard deviation for groups of three mice.
Secondary infection of BKO mice with F. tularensis LVS.
To determine the capacity of BKO mice to withstand maximal secondary challenges after primary infection, normal and BKO mice were given a sublethal (priming) dose of 105 LVS i.d. Previous results have clearly demonstrated that survival of sublethal LVS infection reliably results in the ability of normal mice to withstand a secondary challenge of >106 LD50s of LVS given a month later i.p., a route for which small numbers of bacteria, approaching a single bacterium, are lethal (12, 16, 46). As shown in Table 2, unprimed C57BL/6J mice did not survive a dose of 104 i.p. (approximately 1,000 LD50s for this mouse strain; 16, 46) but when primed with 105 LVS i.d. 35 days earlier, these mice survived a dose of up to 107 LVS i.p. This time point of challenge was chosen to minimize the contribution of nonspecific B-cell-dependent early protective immunity, which wanes about 1 month after i.d. infection (6, 11), and to maximize detection of conventional T-cell-dependent secondary protective immunity. In contrast, the majority of BKO mice survived a challenge of 104 LVS i.p. (16 of 17 total mice in four different repeated experiments using this challenge dose), but only about 50% survived a challenge dose of 2 × 105 i.p., and none survived challenges of ≥106 (data not shown). In seven different repeated experiments using a challenge dose of 5 × 105 or greater, 0 of 31 BKO mice survived. Thus, while secondary protective immunity developed in BKO mice, this immunity was not maximal; wild-type primed mice survived challenge doses at least 2 logs higher than the corresponding primed BKO mice. Further, those BKO mice that succumbed to challenge appeared to have an MTD shorter than that of wild-type mice (Table 2).
TABLE 2.
Secondary protective immunity to LVS is impaired in BKO micea
| Mice | LVS dose
|
No. of deaths/total | MTD | |
|---|---|---|---|---|
| i.d., day 0 | i.p., day 35 | |||
| C57BL/6J | PBS | 104 | 5/5 | 6.2 ± 0.8 |
| 105 | 106 | 0/5 | ||
| 105 | 107 | 1/5 | 4 | |
| C57.Igh6− | 105 | 104 | 1/4 | 3 |
| 105 | 2 × 105 | 2/4 | 3.5 ± 0.7 | |
| 105 | 106 | 4/4 | 3.0 ± 0.0 | |
The indicated mice (groups of five) were primed with PBS or 105 LVS i.d. on day 0 and challenged as indicated with 104 to 106 LVS i.p. on day 35. Actual priming and challenge doses were confirmed by plate count at the time of inoculation. Mice were observed for morbidity and mortality through day 30, at which time surviving mice were sacrificed to assess clearance of bacteria from spleens. MTD refers to deaths in relationship to the day of i.p. challenge. These experiments are representative of seven total experiments of similar design.
To examine the mechanism provided by B cells for the maximal expression of secondary protective immunity, a series of reconstitution experiments were performed. In the first series, wild-type or BKO mice were primed with a sublethal dose of LVS i.d., and 34 days later either LVS-primed B cells, NMS, or anti-LVS IMS (containing high titers of IgM and IgG anti-LVS antibodies) were given to primed mice i.v. One day later (day 35), all mice were challenged with a dose of 5 × 105 LVS i.p., which was routinely lethal for primed BKO mice but not for primed wild-type mice (see above). While all primed wild-type mice survived secondary challenge, none of the BKO mice, whether given anti-LVS antibodies or LVS-immune B cells, survived secondary challenge; the preparations of IMS used in this study were functional in vivo, since normal BALB/cByJ mice given 0.5 ml of a 1:10 dilution of IMS and challenged i.p. immediately with 5 × 102 LVS survived, as previously described (data not shown; see also reference 34). In the second series of experiments, the time of transfer was changed to day 28. As shown in Table 3, BKO mice that were primed were unable to survive a challenge dose of 5 × 105 LVS given i.p. on day 35, even when given anti-LVS antibodies i.v. 1 week earlier; the same preparation of anti-LVS antibodies given i.p. to BALB/cByJ mice in the same experiments protected against low-dose i.p. challenge given on the same day (day 28), however (data not shown). In contrast, the majority of BKO mice given primed B cells i.v. on day 28 survived subsequent lethal challenge on day 35. Although all control, unreconstituted BKO mice died following challenge with this dose of LVS, some BKO mice given unprimed B cells IV (4 of 14 total mice in three repeated experiments) survived lethal i.p. challenge, and there was a trend toward an increased MTD among those that died. Sera were obtained from all groups of mice on day 34 (1 day before challenge); when tested for LVS-specific Ig(M+G) antibody by ELISA, only sera from primed wild-type C57BL/6J mice contained detectable anti-LVS antibodies (titers of 1:800 and 1:1,280 for experiments 1 and 2, respectively). Thus B cells, but not antibodies, were able to reconstitute the impairment of BKO mice in secondary protective immunity.
TABLE 3.
Secondary protective immunity to LVS can be reconstituted in BKO mice with LVS primed B cellsa
| Mouse group | LVS i.d. dose (day 0) | Transfer, i.v. (day 28) | Ab titer (day 34) | LVS, i.p. (day 35) | No. of deaths/total |
|---|---|---|---|---|---|
| Expt 1 | |||||
| C57BL/6J | PBS | <1:10 | 5 × 105 | 5/5 | |
| 105 | 1:800 | 5 × 105 | 0/5 | ||
| C57.Igh6− | 105 | PBS | <1:10 | 5 × 105 | 5/5 |
| 105 | IMS | <1:10 | 5 × 105 | 5/5 | |
| 105 | 2 × 107 (primed B cells) | <1:10 | 5 × 105 | 2/5 | |
| Expt 2 | |||||
| C57BL/6J | PBS | <1:10 | 5 × 105 | 5/5 | |
| 105 | 1:1280 | 5 × 105 | 0/4 | ||
| C57.Igh6− | 105 | PBS | <1:10 | 5 × 105 | 5/5 |
| 105 | 3 × 107 (primed B cells) | <1:10 | 5 × 105 | 1/5 | |
| 105 | 3 × 107 (unprimed B cells) | <1:10 | 5 × 105 | 4/5 |
The indicated mice (groups of four or five) were primed with 105 LVS i.d. on day 0, given either PBS or the indicated number of normal C57BL/6J or LVS-primed spleen cells i.v. on day 28, bled for serum on day 34, and challenged with 5 × 105 LVS i.p. on day 35. Actual priming and challenge doses were confirmed by plate count at the time of inoculation. Mice were observed for morbidity and mortality through day 30, at which time surviving mice were sacrificed to assess the clearance of bacteria from the spleens, livers, and lungs. These experiments are representative of three total experiments of similar design. Ab, antibody.
DISCUSSION
Although no mouse strain naturally deficient in B-cell development has been identified, the recent development of genetically defined “knockout” mice lacking in mature B cells has made it possible to directly test the role of these cells, including the role of immunoglobulins, in immunity to pathogens. Previous work suggested that specific antibodies have little or no role in protection against intracellular pathogens, as might be expected from their inaccessible localization. Here we show that although primary infection with LVS is only marginally impaired in the absence of B cells (Table 1 and Fig. 1), secondary immunity is quantitatively diminished by over 2 logs (Table 2). This defect can be reconstituted by the transfer of B cells, but not serum from immune mice, shortly before challenge (Table 3). Thus, B cells provide a function other than antibody production that is critical to the development of optimal protective immunity against this intracellular bacterium. To our knowledge, this is the first demonstration of a role for B cells in secondary immunity to a classic facultative intracellular bacterium.
BKO mice have been utilized to productively study the role of these cells in other infectious diseases, particularly viral infections. Secondary immunity to rabies (44) and rotavirus (17) is clearly compromised in BKO mice, a defect attributed to absence of specific antibodies; however, defects in BKO mice in immunity to lymphocytic choriomeningitis virus (20, 40) and influenza (15, 18, 41) have been noted in some circumstances and may be due at least in part to impaired CD4+ T-cell function, not simply to the absence of antibodies. In primary infection with the parasite Schistosoma mansoni, BKO mice develop and maintain larger granulomas despite equivalent parasitic burden, a result apparently due to the absence of Fc receptor-mediated signalling (21), and in secondary immunity following vaccination with irradiated parasites BKO mice exhibited suboptimal immunity as well (22). A dual role for B cells has been proposed in malaria infection: BKO mice fail to clear a primary infection with Plasmodium chabaudi chabaudi, and have limited capacity to control a secondary challenge (42). The defects in malaria infection may be due to a combination of the absence of specific antibodies and a dysregulation of CD4+ T-cell function, particularly in terms of a Th1-Th2 switch (25). In diseases such as schistosomiasis and malaria, where the pathogen life cycle is complex and protective immunity is probably dependent on both humoral and cellular effector mechanisms, a significant role for B cells is not surprising. However, the role of B cells has been less well studied in intracellular bacterial infections. BKO mice infected with Bordetella pertussis, generally considered to be an extracellular bacterium, exhibit a chronic infection rather than clearance (31). Although responses to primary and secondary genital infections with the obligate intracellular bacterium Chlamydia trachomatis were only slightly impaired in BKO mice (37), BKO mice were compromised in their ability to control both primary and secondary intranasal C. trachomatis infection, corresponding with impaired cytokine production by splenic T cells (45). Consistent with the obligate intracellular location of chlamydiae, the role of antibody in this infection is limited, and thus it seems likely that there may be a non-antibody-mediated role for B cells in this infection as well. Two conflicting reports have examined the role of B cells in primary infection with Mycobacterium tuberculosis, a classic facultative intracellular bacterium. In one, using a low-dose aerosol infection with M. tuberculosis H37Rv, no differences in bacterial burden, pathology, or survival were found (23); in the other, however, using high-dose i.v. infection of M. tuberculosis H37Rv, higher numbers of bacteria were observed in the organs of infected BKO mice (43). Taken together, these studies, as well as the present one, suggest that B cells have an underappreciated role in regulating pathological changes from infection, T-cell function, and optimal protective immunity even to intracellular pathogens.
It is not clear why successful reconstitution of optimal protection was readily accomplished by transfer of cells on day 28 and not on day 34, 1 day before challenge. This may be simply a function of complete recirculation and recovery and is unlikely to reflect B-cell proliferation; study of the transfer of naive B cells into BKO mice indicated that the number of B220+ cells found in recipient mice was similar on days 1 and 3 after i.v. transfer but declined somewhat by day 9 (35). The possible role of particular B-cell subpopulations is also presently unknown. It is possible that B-cell-dependent optimal protection is due to B-1 B cells, CD5+ B cells, and/or the recently described “B/macrophages” cell (3, 36); however, this seems unlikely since the defect can be reconstituted by transfer of splenic B cells, and these populations are present in spleens only in very small numbers.
Previous studies (6, 34, 46), as well as the reconstitution data presented here (Table 3), strongly suggest that the impaired secondary immunity to LVS is not due to the absence of specific antibodies. This possibility will be tested directly using knockout mice that have mature B cells but lack the capacity to secrete antibodies (4); these studies are planned. The specific effector activities provided by B cells in optimal protective immunity to this intracellular bacterium is the subject of future studies, and there are several obvious candidates. These include a role in the appropriate development of specific T cells and/or in antigen presentation to immune T cells, B-cell production of cytokines, B-cell interaction with other cells such as macrophages or natural killer cells, and B-cell regulation of trafficking (e.g., through production of chemokines). Some of these possible functions may also be involved in appropriate development of innate, B-cell-dependent early protective immunity to LVS (6, 8, 11), and deficiencies in the innate response may result in suboptimal development of long-term protection. Obviously not all of these possibilities are mutually exclusive, and in fact it is likely that several different effector mechanisms contribute to optimal B-cell-dependent protective immunity. It is also possible that B cells directly kill LVS and/or macrophages infected with LVS; such studies are ongoing, but there is no precedent for this kind of B-cell function. Similarly, future studies will also consider the possibility that macrophages from BKO mice are defective in their capacity to control intracellular LVS growth, but to date no immunological or physiological differences between macrophages derived from BKO and wild-type mice have been described.
We believe that it is unlikely that the observed defect is due to poor T-cell function for several reasons. A number of studies have suggested that T-cell priming or function, particularly CD4+ T-cell activity, may be suboptimal in BKO mice (4, 20, 29, 30, 40); however, others have not (1, 7, 14), and our previous studies have demonstrated that CD8+ (or CD4+) T cells are sufficient for optimal protection against LVS (46). Further, optimal protection in BKO mice can be reconstituted by the transfer of B cells on day 28 and only a week before challenge, a time point at which bacteria are cleared (Fig. 1) and thus not available for further antigenic stimulus of the T-cell population. It is therefore difficult to envision further T-cell stimulation and maturation of effector function in the short remaining time before challenge. This conclusion is further supported by other results, using an in vitro system with LVS-infected bone marrow macrophages that indicate T cells derived from primed BKO mice 4 weeks after sublethal infection control bacterial growth in macrophages and produce IFN-γ and IL-12, as well as T cells derived from wild-type mice (3a).
However, the delay in clearance of primary infection (Fig. 1) is also unlikely to be due to the absence of antibodies and may indeed reflect a delay in the development of specific effector T cells. Of interest in this regard is the role of germinal centers in the appropriate maturation of memory T cells, since germinal centers are essentially absent in BKO mice.
B cells may contribute to antigen presentation, as suggested by many other studies (4, 5, 19, 47); this is of particular interest since primed B cells appear to reconstitute optimal protection more readily than naive B cells. Similarly, B cells may be significant sources of cytokines and/or chemokines, and primed cells may be more efficient and/or more numerous upon secondary antigen stimulation than unprimed cells for cytokine production. Although we are not aware of a cytokine whose production is unique to B cells, B cells have been reported to produce alpha interferon (IFN-α), interleukin-4 (IL-4), IL-10, IL-6, and IL-12 (28). Of these, IL-12 and/or IL-18 appear to be the most likely candidates for participation in the development of optimal protective immunity, since optimal protection against LVS can be demonstrated in mice depleted of IL-4 (26) or knockout mice lacking in IL-6 (7a). Experiments defining the role of IL-12 in LVS infection by using IL-12 depleted and IL-12 knockout mice are in progress.
Cytokine or chemokine production may be a component of B-cell interaction with other cell types or regulate the appropriate trafficking of cells to the sites of infection. B-cell interaction with natural killer (NK) cells, both in terms of upregulation of NK cell production of IFN-γ and in terms of increasing IgG2a production, has been studied extensively (48); since NK cells are clearly an important source of early IFN-γ production in LVS infection (10), B cells may serve to amplify NK cell activity that is important even in secondary infections. Similarly, B cells may amplify macrophage-mediated control of bacterial replication, a possibility supported by the preliminary observation that bacterial burdens are much higher in spleens and livers of LVS-primed and -challenged BKO mice compared to wild-type mice as soon as 1 day after challenge; further, neutrophil infiltration in the spleens of such BKO mice appears to be much greater than in wild-type mice (3a). It is curious that LVS-primed and -challenged BKO mice reproducibly exhibit a shorter mean time to death, usually between 3 and 4 days after challenge, than wild-type mice challenged with LVS, which usually die between 5 and 7 days after LVS infection (Table 2 [see also reference 6]). Consistent with the apparently normal specific T-cell activity in BKO mice, death in BKO mice may well be due to early unrestrained bacterial replication, shock following a neutrophil or macrophage overreaction to unrestrained infection, or a combination of the two and not to a failure in specific immunity. Taken together, the results presented here demonstrate a surprising non-antibody-mediated B-cell role in optimal protective immunity to an intracellular bacterium and underscore the complex nature of in vivo protective immunity.
ACKNOWLEDGMENTS
We thank Susan Colombini for excellent technical assistance and our CBER colleagues Marjorie Shapiro, Dorothy Scott, Suzanne Epstein, and Jerry Weir for critical readings of the manuscript.
This work was supported in part by the National Vaccine Program.
Footnotes
This work is dedicated to the memory of Roberta D. Shahin, our friend and colleague, whose insight, encouragement, and companionship were instrumental throughout the progression of these and many other studies.
REFERENCES
- 1.Asano M S, Ahmend R. CD8 T cell memory in B cell-deficient mice. J Exp Med. 1996;183:2165–2174. doi: 10.1084/jem.183.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker C N, Hollis D G, Thornsberry C. Anti-microbial susceptibility testing of Francisella tularensis with a modified Mueller-Hinton broth. J Clin Microbiol. 1985;22:212–215. doi: 10.1128/jcm.22.2.212-215.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrello M A, Phipps R P. The B/macrophage cell: an elusive link between CD5+ T lymphocytes and macrophages. Immunol Today. 1996;17:471–475. doi: 10.1016/0167-5699(96)20031-b. [DOI] [PubMed] [Google Scholar]
- 3a.Bosio, C. M., and K. L. Elkins. Unpublished data.
- 4.Constant S, Sant-Angelo D, Pasqualini T, Taylor T, Levin D, Flavell R, Bottomly K. Peptide and protein antigens require distinct antigen-presenting cell subsets for the priming of CD4+ T cells. J Immunol. 1995;154:4915–4923. [PubMed] [Google Scholar]
- 5.Constant S, Schweitzer N, West J, Ranney P, Bottomly K. B lymphocytes can be competent antigen-presenting cells for priming CD4+ T cells to protein antigens in vivo. J Immunol. 1995;155:3734–3741. [PubMed] [Google Scholar]
- 6.Culkin S J, Rhinehart-Jones T, Elkins K L. A novel role for B cells in early protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J Immunol. 1997;158:3277–3284. [PubMed] [Google Scholar]
- 7.DiRosa F, Matzinger P. Long-lasting CD8 T cell memory in the absence of CD4 T cells or B cells. J Exp Med. 1996;183:2153–2163. doi: 10.1084/jem.183.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Elkins, K. L. Unpublished data.
- 8.Elkins K L, Leiby D A, Winegar R K, Nacy C A, Fortier A H. Rapid generation of specific protective immunity to Francisella tularensis. Infect Immun. 1992;60:4571–4577. doi: 10.1128/iai.60.11.4571-4577.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elkins K L, MacIntyre A T, Rhinehart-Jones T R. Nonspecific early protective immunity in Francisella and Listeria infections can be dependent on lymphocytes. Infect Immun. 1998;66:3467–3469. doi: 10.1128/iai.66.7.3467-3469.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkins K L, Rhinehart-Jones T R, Culkin S J, Yee D, Winegar R K. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect Immun. 1996;64:3288–3293. doi: 10.1128/iai.64.8.3288-3293.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elkins K L, Rhinehart-Jones T, Nacy C A, Winegar R K, Fortier A H. T-cell-independent resistance to infection and generation of immunity to Francisella tularensis. Infect Immun. 1993;61:823–829. doi: 10.1128/iai.61.3.823-829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkins K L, Winegar R K, Nacy C A, Fortier A H. Introduction of Francisella tularensis at skin sites induces resistance to infection and generation of protective immunity. Microb Pathog. 1992;13:417–421. doi: 10.1016/0882-4010(92)90085-3. [DOI] [PubMed] [Google Scholar]
- 13.Emmerling P, Finger H, Bockemuhl J. Listeria monocytogenes infection in nude mice. Infect Immun. 1975;12:437–439. doi: 10.1128/iai.12.2.437-439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epstein M M, Di Rosa F, Jankovic D, Sher A, Matzinger P. Successful T cell priming in B cell-deficient mice. J Exp Med. 1995;182:915–922. doi: 10.1084/jem.182.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein S L, Lo C Y, Misplon J A, Bennick J R. Mechanism of protective immunity against influenza virus infection in mice without antibodies. J Immunol. 1998;160:322–327. [PubMed] [Google Scholar]
- 16.Fortier A H, Slayter M V, Ziemba R, Meltzer M S, Nacy C A. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun. 1991;59:2922–2928. doi: 10.1128/iai.59.9.2922-2928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franco M A, Greenberg H B. Role of B cells and cytotoxic T lymphocytes in clearance of and immunity to rotavirus infection in mice. J Virol. 1995;69:7800–7806. doi: 10.1128/jvi.69.12.7800-7806.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham M B, Braciale T J. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J Exp Med. 1997;186:2063–2068. doi: 10.1084/jem.186.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodgkin P D, Basten A. B cell activation, tolerance and antigen-presenting function. Curr Opin Immunol. 1995;7:121–129. doi: 10.1016/0952-7915(95)80037-9. [DOI] [PubMed] [Google Scholar]
- 20.Homann D, Tishon A, Berger D P, Weigle W O, von Herrath M G, Oldstone M B A. Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistant virus infection after adoptive immunotherapy with virus-specific memory cells from μMT/μMT mice. J Virol. 1998;72:9208–9216. doi: 10.1128/jvi.72.11.9208-9216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jankovic D, Cheever A W, Kullberg M C, Wynn T A, Yap G, Caspar P, Lewis F A, Clynes R, Ravetch J V, Sher A. CD4+ T cell-mediated granulomatous pathology in schistosomiasis is downregulated by a B cell-dependent mechanism requiring Fc receptor signaling. J Exp Med. 1998;187:619–629. doi: 10.1084/jem.187.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jankovic D, Wynn T A, Kullberg M C, Hieny S, Caspar P, James S, Cheever A W, Sher A. Optimal vaccination against Schistosoma mansoni requires the induction of both B cell- and IFN-γ-dependent effector mechanisms. J Immunol. 1999;162:345–351. [PubMed] [Google Scholar]
- 23.Johnson C M, Cooper A M, Frank A A, Bonorino C B C, Wysoki L J, Orme I M. Mycobacterium tuberculosis aerogenic rechallenge infections in B cell-deficient mice. Tubercle Lung Dis. 1997;78:257–261. doi: 10.1016/s0962-8479(97)90006-x. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 25.Langhorne J, Cross C, Seixas E, Li C, von der Weid T. A role for B cells in the development of T cell helper function in a malaria infection in mice. Proc Natl Acad Sci USA. 1998;95:1730–1734. doi: 10.1073/pnas.95.4.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leiby D A, Fortier A H, Crawford R M, Schreiber R D, Nacy C A. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun. 1992;60:84–89. doi: 10.1128/iai.60.1.84-89.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lennette E H. General principles underlying laboratory diagnosis of virus and rickettsial infections. In: Lennette E H, Schmidt N J, editors. Diagnostic procedures of virus and rickettsial disease. New York, N.Y: American Public Health Association; 1964. p. 45. [Google Scholar]
- 28.Liles W C, Van Voorhis W C. Review: nomenclature and biologic significance of cytokines involved in inflammation and the host immune response. J Infect Dis. 1995;172:1573–1580. doi: 10.1093/infdis/172.6.1573. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Wu Y, Ramarathinam L, Guo Y, Huszar D, Trounstine M, Zhao M. Gene-targeted B-deficient mice reveal a critical role for B cells in the CD4 T cell response. Int Immunol. 1995;7:1353–1362. doi: 10.1093/intimm/7.8.1353. [DOI] [PubMed] [Google Scholar]
- 30.Macaulay A E, DeKruyff R H, Umetsu D T. Antigen-primed T cells from B cell-deficient JHD mice fail to provide B cell help. J Immunol. 1998;160:1694–1700. [PubMed] [Google Scholar]
- 31.Mahon B P, Sheahan B J, Griffin F, Murphy G, Mills K H G. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-γ receptor or immunoglobulin μ chain genes. J Exp Med. 1997;186:1843–1851. doi: 10.1084/jem.186.11.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Brien A D, Metcalf E S. Control of early Salmonella typhimurium growth in innately Salmonella-resistant mice does not require functional T cells. J Immunol. 1982;129:1349–1351. [PubMed] [Google Scholar]
- 33.Raff M C. O-bearing lymphocytes in nude mice. Nature. 1973;246:350–351. doi: 10.1038/246350a0. [DOI] [PubMed] [Google Scholar]
- 34.Rhinehart-Jones T R, Fortier A H, Elkins K L. Transfer of immunity against lethal murine Francisella infection by specific antibody depends on host gamma interferon and T cells. Infect Immun. 1994;62:3129–3137. doi: 10.1128/iai.62.8.3129-3137.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roth R, Mamula M J. Trafficking of adoptively transferred B lymphocytes in B-lymphocyte-deficient mice. J Exp Biol. 1997;200:2057–2062. doi: 10.1242/jeb.200.14.2057. [DOI] [PubMed] [Google Scholar]
- 36.Stall A M, Wells S M, Lam K-P. B-1 B cells: unique origins and functions. Semin Immunol. 1996;8:45–59. doi: 10.1006/smim.1996.0007. [DOI] [PubMed] [Google Scholar]
- 37.Su H, Feilzer K, Caldwell H D, Morrison R P. Chlamydia trachomatis genital tract infection of antibody-deficient knockout mice. Infect Immun. 1997;65:1993–1999. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tärnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989;11:440–451. [PubMed] [Google Scholar]
- 39.Tärnvik A, Eriksson M, Sandström G, Sjöstedt A. Francisella tularensis—a model for studies of the immune response to intracellular bacteria in man. Immunology. 1992;76:349–354. [PMC free article] [PubMed] [Google Scholar]
- 40.Thomsen A R, Johansen J, Marker O, Christensen J P. Exhaustion of CTL memory and recrudescence of viremia in lymphocytic choriomeningitis virus-infection MHC class II-deficient mice and B cell-deficient mice. J Immunol. 1996;157:3074–3080. [PubMed] [Google Scholar]
- 41.Topham D J, Doherty P C. Clearance of an influenza A virus by CD4+ T cells is inefficient in the absence of B cells. J Virol. 1998;72:882–885. doi: 10.1128/jvi.72.1.882-885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von der Weid T, Kitamura D, Rajewsky K, Langhorne J. A dual role for B cells in Plasmodium chabaudi chabaudi (AS) infections? Res Immunol. 1994;145:412–419. doi: 10.1016/s0923-2494(94)80170-3. [DOI] [PubMed] [Google Scholar]
- 43.Vordermeier H M, Venkataprasad N, Harris D P, Ivanyi J. Increase of tuberculous infection in the organs of B cell-deficient mice. Clin Exp Immunol. 1996;106:312–316. doi: 10.1046/j.1365-2249.1996.d01-845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiang Z Q, Knowles B B, McCarrick J W, Ertl H C J. Immune effector mechanisms required for protection to rabies virus. Virology. 1995;214:398–404. doi: 10.1006/viro.1995.0049. [DOI] [PubMed] [Google Scholar]
- 45.Yang X, Brunham R C. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J Immunol. 1998;161:1439–1446. [PubMed] [Google Scholar]
- 46.Yee D, Rhinehart-Jones T R, Elkins K L. Loss of either CD4+ or CD8+ T cells does not affect the magnitude of protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J Immunol. 1996;157:5042–5048. [PubMed] [Google Scholar]
- 47.Yewdell J W, Bennink J R. The binary logic of antigen processing and presentation to T cells. Cell. 1990;62:203–206. doi: 10.1016/0092-8674(90)90356-j. [DOI] [PubMed] [Google Scholar]
- 48.Yuan D, Koh C Y, Wilder J A. Interactions between B lymphocytes and NK cells. FASEB J. 1994;8:1012–1018. doi: 10.1096/fasebj.8.13.7926365. [DOI] [PubMed] [Google Scholar]