Abstract
Background: Candidaemia and invasive candidiasis are typically hospital-acquired. Genotyping isolates from patients admitted to different hospitals may be helpful in tracking clones spreading across hospitals, especially those showing antifungal resistance. Methods: We characterized Candida clusters by studying Candida isolates (C. albicans, n = 1041; C. parapsilosis, n = 354, and C. tropicalis, n = 125) from blood cultures (53.8%) and intra-abdominal samples (46.2%) collected as part of the CANDIMAD (Candida in Madrid) study in Madrid (2019–2021). Species-specific microsatellite markers were used to define the genotypes of Candida spp. found in a single patient (singleton) or several patients (cluster) from a single hospital (intra-hospital cluster) or different hospitals (widespread cluster). Results: We found 83 clusters, of which 20 were intra-hospital, 49 were widespread, and 14 were intra-hospital and widespread. Some intra-hospital clusters were first detected before the onset of the COVID-19 pandemic, but the number of clusters increased during the pandemic, especially for C. parapsilosis. The proportion of widespread clusters was significantly higher for genotypes found in both compartments than those exclusively found in either the blood cultures or intra-abdominal samples. Most C. albicans- and C. tropicalis-resistant genotypes were singleton and presented exclusively in either blood cultures or intra-abdominal samples. Fluconazole-resistant C. parapsilosis isolates belonged to intra-hospital clusters harboring either the Y132F or G458S ERG11p substitutions; the dominant genotype was also widespread. Conclusions: the number of clusters—and patients involved—increased during the COVID-19 pandemic mainly due to the emergence of fluconazole-resistant C. parapsilosis genotypes.
Keywords: Candida, microsatellite genotyping, blood culture, intra-abdominal samples, COVID-19, candidaemia, Madrid
1. Introduction
Invasive candidiasis, commonly presented as candidaemia and intra-abdominal infection, is typically hospital-acquired and mostly caused by Candida albicans, Candida parapsilosis, and Candida tropicalis [1,2]. Molecular epidemiology has proven useful in tracking the dynamics of Candida spp. infections (e.g., pinpointing the infection source and unraveling outbreaks) [3,4]. Some Candida genotypes, commonly referred to as clusters, have been found to cause candidaemia in different patients and could involve patients located in a single hospital ward (intra-ward clusters), patients cared for at a given hospital (intra-hospital clusters), or patients admitted to different hospitals, sometimes located in different cities (widespread clusters) [5,6].
Intra-ward and intra-hospital clusters may suggest active patient-to-patient hospital transmission. In fact, we recently proved that the implantation of a campaign to decrease the number of catheter-related infections correlated with a decrease, not only in the overall number of candidaemia episodes but also in the number of Candida clusters [7]. In contrast, widespread clusters involve unrelated patients (not admitted to the same hospital) and may represent genotypes prone to causing candidaemia and not necessarily active hospital transmission [6,8]. Some studies have shown an increase in the number of candidaemia cases during the COVID-19 pandemic, in some cases alongside an increase in patient-to-patient hospital transmission [9,10,11,12,13,14].
We recently conducted a three-year study (Candida in Madrid, CANDIMAD) to assess the epidemiology and antifungal susceptibility of a large number of Candida spp. isolates from blood cultures and intra-abdominal samples from patients admitted to 16 hospitals located in Madrid, Spain [15,16]. We found that the rate of echinocandin resistance remained low; contrarily, we detected the emergence of fluconazole-resistant C. parapsilosis across the region. Fluconazole resistance is a matter of concern in C. parapsilosis infections, given the intrinsic lower susceptibility of the species to echinocandins [17,18]. Several questions remained unresolved. First, since the study period (2019 to 2021) spanned a period prior to the COVID-19 pandemic and the pandemic’s first waves, it is unknown whether the number of clusters—and patients involved—rose due to the COVID-19 pandemic in Madrid. Moreover, it is unknown whether highly spread clusters may be found not just in blood cultures but also in intra-abdominal samples. Finally, we studied whether antifungal-resistant genotypes found in the blood may also be found in the intra-abdominal compartment and vice versa.
To elucidate these questions, we genotyped the C. albicans, C. parapsilosis, and C. tropicalis isolates collected in the CANDIMAD study.
2. Materials and Methods
2.1. Isolates Collected and Studied during the CANDIMAD Study
From 1 January 2019 to 31 December 2021, 1520 Candida isolates (C. albicans, n = 1041; C. parapsilosis, n = 354, and C. tropicalis, n = 125) from blood cultures (53.8%, n = 817) and intra-abdominal samples (46.2%, n = 703) were prospectively collected from 1452 patients cared for at 16 hospitals in Madrid, Spain. Details about the CANDIMAD study and participating hospitals were reported elsewhere [15,16], and the numbers of isolates per participating hospital are shown in Table 1.
Table 1.
Number of isolates studied (per species and source) collected during the CANDIMAD study at each of the 16 participant hospitals.
| Hospital Code |
Clinical Source and No. Isolates | ||||||
|---|---|---|---|---|---|---|---|
| Blood Cultures | Intra-Abdominal Samples | Overall | |||||
| C. albicans | C. parapsilosis | C. tropicalis | C. albicans | C. parapsilosis | C. tropicalis | ||
| 1 | 90 | 32 | 16 | 88 | 18 | 12 | 256 |
| 2 | 34 | 20 | 5 | 104 | 9 | 16 | 188 |
| 3 | 42 | 22 | 2 | 85 | 9 | 8 | 168 |
| 4 | 75 | 57 | 6 | 18 | 4 | 1 | 161 |
| 5 | 30 | 13 | 3 | 44 | 13 | 4 | 107 |
| 6 | 21 | 4 | 3 | 67 | 4 | 10 | 109 |
| 7 | 57 | 36 | 8 | 5 | 2 | 0 | 108 |
| 8 | 25 | 5 | 2 | 57 | 11 | 4 | 104 |
| 9 | 41 | 35 | 4 | 19 | 5 | 3 | 107 |
| 10 | 20 | 8 | 2 | 39 | 6 | 4 | 79 |
| 11 | 21 | 12 | 2 | 6 | 0 | 2 | 43 |
| 12 | 11 | 9 | 2 | 7 | 0 | 1 | 30 |
| 13 | 4 | 7 | 1 | 8 | 1 | 1 | 22 |
| 14 | 6 | 6 | 1 | 2 | 0 | 1 | 16 |
| 15 | 5 | 5 | 0 | 2 | 1 | 1 | 14 |
| 16 | 7 | 0 | 0 | 1 | 0 | 0 | 8 |
| Overall | 489 | 271 | 57 | 552 | 83 | 68 | 1520 * |
* A total of 62 isolates (4.1%; C. albicans, n = 10; C. parapsilosis, n = 49; and C. tropicalis, n = 3) were found to be antifungal resistant and sourced from either blood cultures (n = 51; C. albicans, n = 5; C. parapsilosis, n = 45; C. tropicalis, n = 1) or intra-abdominal samples (n = 11; C. albicans, n = 5; C. parapsilosis, n = 4; C. tropicalis, n = 2) [15,16]. Hospital codes were assigned based on the number of isolates collected at each participating hospital, with Hospital 1 being the hospital contributing the highest numbers and Hospital 16 contributing the lowest numbers.
Briefly, one incident isolate per species, patient, and compartment (blood culture and/or any intra-abdominal samples) was studied. Most patients (95.7%, n = 1389) contributed a single isolate, but 4.3% (n = 63) contributed ≥2 isolates.
2.2. Microsatellite Genotyping
Species-specific microsatellite markers were used to genotype isolates of C. albicans (CDC3, EF3, HIS3 CAI, CAIII, and CAVI) [19,20], C. parapsilosis (CP1, CP4a, CP6, and B) [21,22], and C. tropicalis (Ctrm1, Ctrm10, Ctrm12, Ctrm21, Ctrm24, and Ctrm28) [23]. Capillary electrophoresis using the ABI 3130xl (Applied Biosystems-Life Technologies Corporation, Carlsbad, CA, USA) analyzer was performed on the PCR products, and electropherograms were analyzed with the GeneMapper v.4.0 software (Applied Biosystems-Life Technologies Corporation, Carlsbad, CA, USA). A molecularly identified control strain from each species was used in each run to ensure size accuracy and avoid run-to-run variations. The allele results were converted to binary data by scoring the presence or absence of each allele. The data were treated as categorical, and the genetic relationship between genotypes was examined by constructing a minimum spanning tree (BioNumerics version 7.6, Applied Maths, Sint-Martens-Latem, Belgium). The isolates were considered to have identical genotypes when they presented the same alleles at all loci. Different genotypes were encoded as follows: CA-X (C. albicans), CP-X (C. parapsilosis), and CT-X (C. tropicalis), X representing the internal code of the genotype in our collection.
Definitions were adopted from a previous study [6]. Briefly, a singleton was defined as a genotype found in a single patient; cluster as a genotype found in samples from ≥2 patients; intra-hospital cluster as involving patients admitted to the same hospital (independently of the time elapsed between the first and last isolate within each cluster); and widespread cluster as involving patients admitted to different hospitals. The genotypes were clonally related when there was only one microsatellite locus difference; a group of clonally related genotypes was referred to as a clonal complex.
We compared proportions using a standard binomial method for the calculation of 95% confidence intervals (Epidat v.4.2 Consellería de Sanidade, Xunta de Galicia, Spain).
3. Results
Overall, we detected 1107 genotypes exclusively found in either blood cultures (n = 528) or intra-abdominal samples (n = 479) or in both compartments (n = 100).
3.1. Genotypes Found in Blood Cultures and Comparisons with Intra-Abdominal Genotypes
We detected 628 genotypes in the blood, of which 545 (86.8%) were singleton and 83 (13.2%) were clusters. The proportion of singletons versus clusters per species was: 86.7% versus 13.3% (C. albicans); 86.8% versus 13.2% (C. parapsilosis); and 87.2% versus 12.8% (C. tropicalis); no differences reaching statistical significance in the proportion of clusters among species were found (p > 0.05) (Table 2).
Table 2.
Number of isolates, patients, and genotypes found in blood per species, and genotype analysis.
| C. albicans | C. parapsilosis | C. tropicalis | |
|---|---|---|---|
| Isolates/patients | 489 | 271 | 57 |
| Genotypes | 399 | 182 | 47 |
| Singleton, n (%) | 346 (86.7%) | 158 (86.8%) | 41 (87.2%) |
| Cluster, n (%) | 53 (13.3%) | 24 (13.2%) | 6 (12.8%) |
| Isolates/patients in cluster, n (%) | 143 (29.2%) | 113 (41.7%) | 16 (28.1%) |
| Range of isolates/patients per cluster | 2–15 | 2–37 | 2–4 |
| Intra-hospital cluster, n (%) | 14 (26.4%) | 6 (25.0%) | 0 (0.0%) |
| Widespread cluster, n (%) * | 39 (73.6%) | 18 (75.0%) | 6 (100%) |
* A total of 14/63 widespread genotypes were also intra-hospital genotypes (C. albicans [n = 6], C. parapsilosis [n = 7], and C. tropicalis [n = 1]).
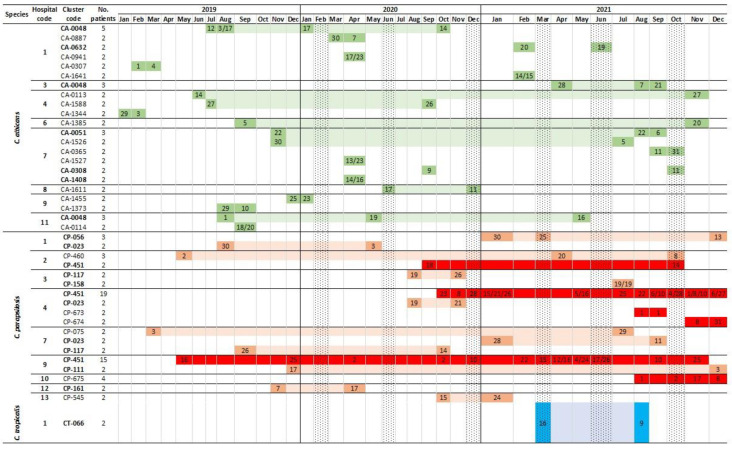
The 83 clusters involved 272 (33.3%) out of the total number of blood culture isolates (817). The percentage and range of isolates involved in clusters per species were C. albicans: 29.2%, 2–15 isolates; C. parapsilosis: 41.7%, 2–37 isolates; and C. tropicalis: 28.1%, 2–4 isolates. C. parapsilosis presented a higher proportion of isolates in clusters (p < 0.05; Table 2). The cluster analysis showed that a high proportion was widespread (75.9% widespread versus 24.1% intra-hospital clusters; p < 0.05); a number of clusters were both widespread and intra-hospital (Table 2). Figure 1 depicts a timeline for C. albicans (n = 22), C. parapsilosis (n = 18), and C. tropicalis (n = 1) intra-hospital clusters. All clusters may involve patients admitted to the hospital within a limited time period or at distant points in time. Several clusters were present before the onset of the COVID-19 pandemic; however, the number of C. parapsilosis clusters (and patients involved) was boosted during the pandemic, especially as of the second wave (Figure 1 and Figure 2).
Figure 1.
Timeline of C. albicans (green shades), C. parapsilosis (red shades), and C. tropicalis (blue shades) intra-hospital clusters found in blood cultures during the study period (2019–2021). Genotype codes in bold indicate those that were also widespread clusters. Cells in dark colours with numbers indicate the date the genotype was detected in a given patient within each cluster; cells in light shades indicate the latency period (time period spanning two time points in which isolates in a cluster were detected) of the cluster in the hospital. Cells in dark red indicate fluconazole-resistant C. parapsilosis genotypes. Dotted cell columns represent the onset of each COVID-19 wave.
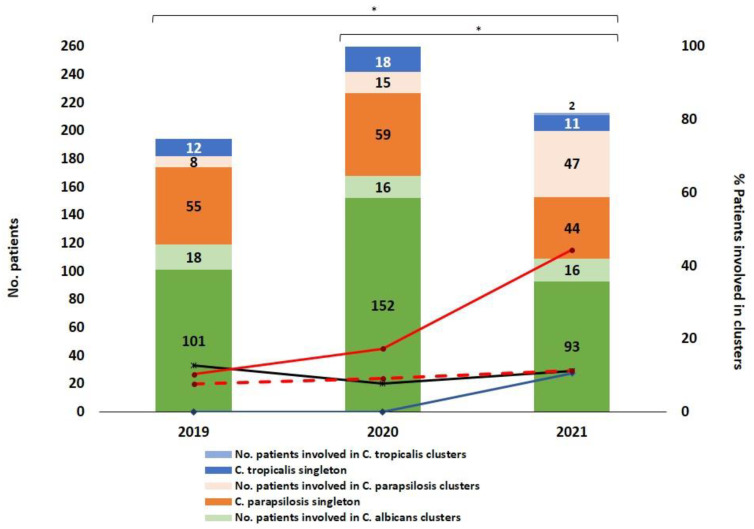
Figure 2.
Numbers (and percentage) of patients involved in singleton and intra-hospital clusters per species over the study period. * Differences reaching statistical significance (p < 0.05).
In fact, the percentage of patients involved in intra-hospital clusters of C. albicans (12.8%/7.8%/11.2%), C. parapsilosis (10.3%/17.2%/44.3%), and C. tropicalis (0%/0%/10.5%) detected in the years 2019/2020/2021, respectively, increased over time; these differences only reached statistical significance for C. parapsilosis (2019 versus 2021, and 2020 versus 2021 p < 0.05; Figure 2).
Patients involved in C. albicans intra-hospital clusters were cared for in intensive care units (ICU) (32%), medical (36%), surgical (12%), oncology–haematology (10%), and neonatology (10%) wards. In contrast, patients with C. parapsilosis intra-hospital clusters were more frequently cared for in the ICU (62.9%) than other wards (medical 18.6%; surgical 8.6%; oncology–haematology 8.6%; and others 1.4%). Percentage differences in the patients involved in C. albicans and C. parapsilosis intra-hospital clusters admitted to ICU wards reached statistical significance (p < 0.05). Table 3 indicates the frequencies of widespread clusters.
Table 3.
Distribution of widespread clusters of C. albicans (green shades) and C. parapsilosis (pink shades) among the participant hospitals.
| Hospital Code | Frequencies of Combinations of Widespread Genotypes | Numbers of Widespread Genotypes | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | C. albicans | C. parapsilosis | Total | |
| 1 | 3 | 2 | 6 | 2 | 0 | 7 | 1 | 4 | 0 | 3 | 2 | 1 | 0 | 0 | 1 | 15 | 5 | 20 | |
| 2 | 2 | 0 | 3 | 1 | 1 | 3 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 2 | 8 | 4 | 12 | |
| 3 | 1 | 0 | 2 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 4 | 6 | 10 | |
| 4 | 3 | 3 | 1 | 4 | 1 | 4 | 3 | 3 | 0 | 5 | 0 | 0 | 1 | 0 | 1 | 17 | 7 | 24 | |
| 5 | 3 | 2 | 1 | 3 | 1 | 1 | 1 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 7 | 7 | 14 | |
| 6 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 4 | |
| 7 | 1 | 1 | 2 | 2 | 2 | 1 | 0 | 1 | 1 | 3 | 1 | 1 | 1 | 0 | 1 | 12 | 6 | 18 | |
| 8 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 5 | |
| 9 | 3 | 3 | 3 | 3 | 4 | 1 | 3 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 6 | 8 | 14 | |
| 10 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 4 | 3 | 7 | |
| 11 | 2 | 1 | 0 | 1 | 2 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 8 | 3 | 11 | |
| 12 | 1 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | 3 | 6 | |
| 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | |
| 14 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 4 | |
| 15 | 1 | 2 | 0 | 2 | 1 | 1 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 3 | |
| 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 4 | |
Overall, hospitals 1 to 5, 7, and 9 contributed the highest numbers of widespread clusters, probably as a consequence of the size of the hospital, given that those hospitals showed high rates of candidaemia incidence and number of admissions. Table 4 shows that candidaemia incidence rates varied among hospitals, species, and over time, and the highest incidence rates were observed in 2020 and 2021 (in the hospitals for which admissions and incidence data were available). C. albicans incidence increased between the years 2019 and 2020 in most hospitals (11/16), and the highest rates were usually found in 2020 (10/16 hospitals), especially in hospitals 7 and 9. C. parapsilosis incidence increased between the years 2019 and 2020 in 7/16 hospitals. In contrast, overall candidaemia incidence gradually increased over the years in six hospitals and was mainly driven by hospitals 4, 9, and 10, the ones in which fluconazole-resistant C. parapsilosis clones spread [18]. No statistically significant differences between years were observed for C. tropicalis incidence.
Table 4.
Number of admissions and incidence of candidaemia, overall and per species, per participant hospitals, and overall, during 2019, 2020, and 2021.
| Hospital Code |
Candidaemia Incidence (Cases per 100,000 Hospital Admissions) | |||||
|---|---|---|---|---|---|---|
| Overall (No. of Admissions) * | C. albicans (2019/2020/2021) |
C. parapsilosis (2019/2020/2021) |
C. tropicalis (2019/2020/2021) |
|||
| 2019 | 2020 | 2021 | ||||
| 1 α | 114.8 (47,048) | 155.5 (42,444) | 135.7 (35,377) | 53.1/69.6/70.7α | 31.9/16.5/28.3 | 10.6/14.1/14.1 |
| 2 | 50.7 (45,357) | 63.0 (39,669) | 78.3 (39,607) | 22.0/35.3/25.2 | 15.4/7.6/25.2 | 4.4/2.5/5.0 |
| 3 β | 83.2 (32,442) | 51.4 (60,348) | 83.3 (40,832) | 40.1/24.9/34.3 | 21.6/13.3/17.1 | 0.0/0.0/4.9 |
| 4 γ | 152.9 (31,399) | 201.1 (27,841) | 231.6 (28,067) | 86.0/89.9/81.9 | 38.2/53.9/106.9β,γ | 12.7/3.6/3.6 |
| 5 | 130.7 (16,064) | 208.8 (13,886) | 137.2 (13,848) | 49.8/108.0/50.5 | 37.4/43.2/7.2 | 0.0/7.2/14.4 |
| 6 α | 53.6 (18,671) | 125.3 (16,755) | 77.4 (16,786) | 16.1/53.7/53.6 | 10.7/11.9/0.0 | 0.0/11.9/6.0 |
| 7 α,γ | 65.6 (48,757) | 131.2 (44,196) | 109.0 (44,949) | 20.5/58.8/46.7α,γ | 16.4/45.3/17.8α, β | 4.1/2.3/11.1 |
| 8 β | 104.8 (15,268) | 129.1 (13,940) | 55.6 (14,388) | 58.9/71.7/41.7 | 13.1/14.3/7.0 | 0.0/7.2/7.0 |
| 9 | 117.7 (26,348) | 171.8 (24,454) | 130.2 (24,577) | 45.5/102.2/16.3α, β | 26.6/40.9/73.2γ | 11.4/4.1/0.0 |
| 10 α,γ | 61.3 (13,049) | 150.6 (11,288) | 124.1 (11,283) | 30.7/88.6/53.2 | 7.7/17.7/44.3 | 15.3/0.0/0.0 |
| 11 | 87.4 (16,012) | 94.3 (15,902) | ND | 50.0/44.0/ND | 25.0/6.3/ND | 6.2/6.3/ND |
| 12 | 44.2 (15,826) | 86.8 (14,969) | 51.6 (15,501) | 19.0/33.4/19.4 | 19.0/33.4/6.5 | 0.0/13.4/0.0 |
| 13 α | 11.1 (9045) | 80.9 (8656) | ND | 0.0/23.1/ND | 11.1/34.7/ND | 0.0/11.6/ND |
| 14 | 34.9 (11,471) | 83.2 (9620) | ND | 26.2/10.4/ND | 8.7/31.2/ND | 0.0/10.4/ND |
| 15 | 86.2 (8116) | 14.4 (6946) | ND | 37.0/14.4/ND | 24.6/0.0/ND | 0.0/0.0/ND |
| 16 | 88.8 (4504) | 0.0 (4146) | ND | 66.6/0.0/ND | 0.0/0.0/ND | 0.0/0.0/ND |
| Overall α,γ | 85.4 (359,377) | 114.6 (355,060) | 112.9 (285,215) ** | 39.2/58.0/44.9α, β | 21.7/24.5/31.9γ | 5.3/5.4/6.7 |
ND, not done due to unavailable data about hospital admissions. * Overall incidence was calculated considering all blood culture Candida spp, species details reported elsewhere (15, 16). ** Data were calculated removing isolates from hospitals 11 to 16. α Comparisons of incidence rates between 2019 and 2020 reaching statistical significance (p < 0.05). β Comparisons of incidence rates between 2020 and 2021 reaching statistical significance (p < 0.05). γ Comparisons of incidence rates between 2019 and 2021 reaching statistical significance (p < 0.05). Colours indicate the lowest (green), intermediate (pale yellow), and highest incidence rates (red).
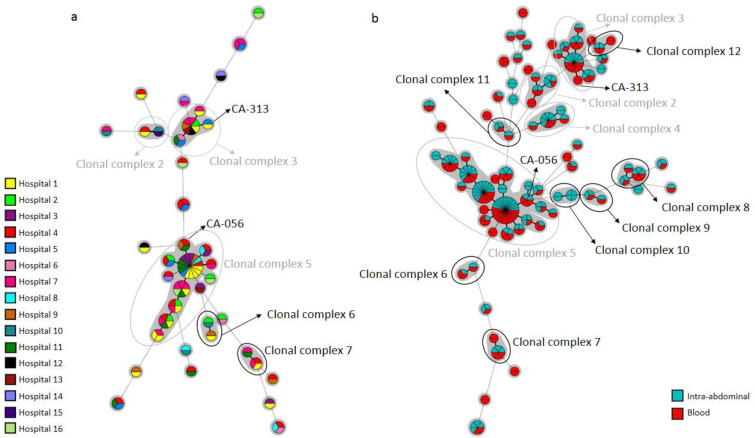
Widespread C. albicans clusters involved 2 to 7 (the latter concerning the CA-048 cluster) hospitals each; 12 clonal complexes were found (nos. 2, 3, 4, and 5 were previously detected (6), and nos. 6–12 were newly reported herein). Clonal complex no. 5 involved a large number of clusters, isolates, and hospitals (Figure 3).
Figure 3.
Minimum spanning tree showing widespread C. albicans clusters found in blood cultures shown per hospital of source (a) or combined with intra-abdominal samples (b). Circles represent different genotypes and circle size the number of isolates belonging to the same genotype. Connecting lines between circles show profile similarities. The solid bold line indicates differences in only 1 marker, solid line indicates differences in 2 markers, dashed line indicates differences in 3 markers, and dotted line indicates differences in 4 or more markers. CA-056 and CA-313 were resistant genotypes. Grey circles depict previously reported clonal complexes 2 to 5. Black circles depict clonal complex 6–12, newly reported herein.
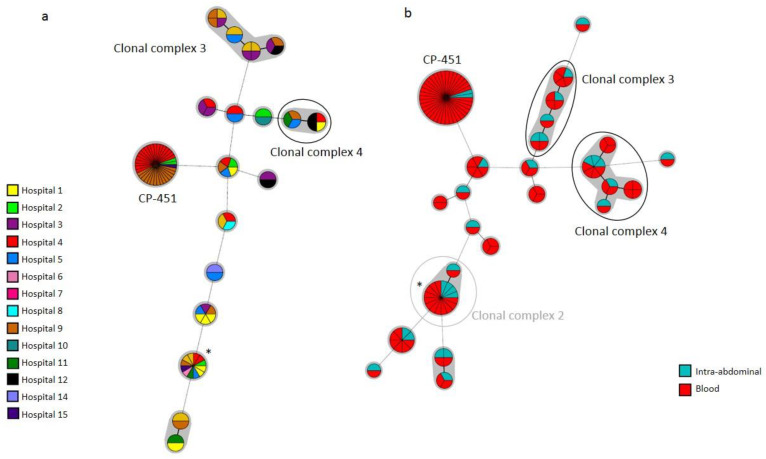
Likewise, widespread C. parapsilosis clusters involved two to nine (the latter concerning the CP-023 cluster) hospitals; four clonal complexes were found (no. 2 was previously detected [6], and nos. 3 and 4, and were newly reported herein) (Figure 4).
Figure 4.
Minimum spanning tree showing C. parapsilosis widespread clusters found in blood cultures shown per hospital of source (a) or combined with intra-abdominal samples (b). Circles represent different genotypes and circle size the number of isolates belonging to the same genotype. Connecting lines between circles show profile similarities. The solid bold line indicates differences in only 1 marker, solid line indicates differences in 2 markers, dashed line indicates differences in 3 markers, and dotted line indicates differences in 4 or more markers. CP-451 is a resistant genotype. The grey circle depicts the previously reported clonal complex 2. Black circles depict clonal complexes 3 and 4, newly reported herein. * Genotype CP-023.
Cluster CP-451 was found in four hospitals and involved a high number of isolates. Finally, widespread C. tropicalis clusters involved two to four (the latter concerning the CT-226 cluster) hospitals and were unrelated; clonal complexes were not detected.
We simultaneously collected isolates from blood cultures and intra-abdominal samples in 32 patients. Of these, identical genotypes were found in the following patients: C. albicans: 14/19; C. parapsilosis: 4/8; and C. tropicalis: 4/5. We found 579 genotypes in intra-abdominal samples, and the 100 genotypes also found in blood cultures mainly involved different patients: C. albicans (n = 59/66), C. parapsilosis (n = 21/24), and C. tropicalis (n = 8/10). Interestingly, the proportion of widespread clusters was significantly higher in genotypes found in both compartments than in those exclusively found in either blood cultures or intra-abdominal samples: C. albicans (77.3%/5.4%/2.9%), C. parapsilosis (83.3%/3.2%/0.0%), and C. tropicalis (60.0%/5.4%/4.2%), respectively, (p < 0.05) (Figure 3b and 4b).
3.2. Genotypes Involving Antifungal-Resistant Isolates
Table 5 summarizes the isolates presenting antifungal resistance to fluconazole or echinocandins.
Table 5.
Resistant genotypes found in blood cultures and intra-abdominal samples, hospitals, patients involved, and molecular resistance mechanisms.
| Species | Genotype | Hospitals Involved |
Patients Involved |
No. of Isolates and Source | Phenotypic Resistance | FKSp Substitution | ERG11p Substitution | |
|---|---|---|---|---|---|---|---|---|
| Blood | Intra -Abdominal |
|||||||
| C. albicans | CA-0056 * | H9 | 1 | 0 | 1 | Echinocandin | F641L FKS1 HS1 | ND |
| CA-0511 | H6 | 2 | 1 | 1 | Echinocandin | S645P FKS1 HS1 | ND | |
| CA-1341 | H6 | 1 | 0 | 1 | Echinocandin | S645P FKS1 HS1 | ND | |
| CA-1872 | H2 | 1 | 1 | 0 | Echinocandin | R1361H FKS1 HS2 | ND | |
| CA-0313 ** | H1 | 1 | 1 | 0 | Fluconazole | ND | None | |
| CA-1283 | H7 | 1 | 1 | 0 | Fluconazole | ND | D115E, K128T, F145L, I471L/I | |
| CA-1505 | H2 | 1 | 0 | 1 | Fluconazole | ND | A114S, Y257H | |
| CA-1674 | H1 | 1 | 0 | 1 | Fluconazole | ND | None | |
| CA-1762 | H9 | 1 | 1 | 0 | Fluconazole | ND | D116E/D, D153E/D | |
| C. parapsilosis | CP-540 | H9 | 1 | 0 | 1 | Echinocandin | None | ND |
| CP-451 | H2, H4, H9, H15 | 39 | 37 | 2 | Fluconazole | ND | Y132F, R398I | |
| CP-673 | H4 | 2 | 2 | 0 | Fluconazole | ND | Y132F, R398I | |
| CP-674 | H4 | 2 | 2 | 0 | Fluconazole | ND | Y132F, R398I | |
| CP-675 | H10 | 5 | 4 | 1 | Fluconazole | ND | G458S | |
| C. tropicalis | CT-031 *** | H10 | 1 | 0 | 1 | Echinocandin | S654P/S FKS1 HS1 + V1352I/V, V1404I/V FKS1 | ND |
| CT-081 | H6 | 1 | 0 | 1 | Fluconazole | ND | None | |
| CT-313 | H7 | 1 | 1 | 0 | Fluconazole | ND | F449V | |
* Genotype also involving susceptible isolates found in hospitals H4, H9, H11, and H15; ** Genotype also involving a susceptible isolate found in hospital H5; *** Genotype also involving susceptible isolates found in hospitals H1 and H10. ND, Not done.
The frequencies of resistant genotypes exclusively found in blood cultures/intra-abdominal samples/both compartments were C. albicans: 4/4/1; C. parapsilosis: 2/1/2; and C. tropicalis: 1/2/0, respectively. Most C. albicans- and C. tropicalis-resistant genotypes were present exclusively in one compartment and were, with a few exceptions, singletons. The echinocandin-resistant and intra-hospital CA-0511 cluster was found in blood cultures and intra-abdominal samples from two patients admitted to the ICU of Hospital 6; one of the patients also harboured the clonally related CA-1341 echinocandin-resistant genotype. In addition, we found three widespread clusters (CA-056, CA-313, and CT-031) that involved a resistant isolate and some susceptible isolates each. Cluster CA-056 was found in four hospitals (in blood cultures and intra-abdominal samples); clusters CA-313 (only in blood cultures) and CT-031 (only intra-abdominal samples) were found in two hospitals each (Table 5 and Figure 3).
Data concerning fluconazole-resistant C. parapsilosis isolates were reported elsewhere (15). All isolates belonged to either a clonal complex with genotypes harbouring the Y132F ERG11p substitution (CP-451, CP-673, and CP-674) or an unrelated cluster with the G458S ERG11p substitution (CP-675). All were exclusively intra-hospital clusters except for the CP-451 cluster, which was widespread and found in four hospitals (Table 5, Figure 1 and Figure 4). Noteworthy, although genotypes CP-451 and CP-675 were found in both compartments, the vast majority of isolates came from blood cultures. The CP-451 genotype was first detected in intra-abdominal samples before the COVID-19 pandemic onset in Hospital 9, and its spread in blood cultures gained traction during the COVID-19 pandemic. Moreover, the CP-451, CP-673, and CP-674 clusters were mainly responsible for the spread of C. parapsilosis clusters during the COVID-19 pandemic, and these genotypes accounted for 63% (n = 44/70) of the total number of patients involved in C. parapsilosis clusters. In fact, when resistant genotypes were excluded from the analysis, the percentage difference of patients involved in clusters in 2019/2020/2021 did not reach statistical significance (10.3%/17.2%/44.3% including fluconazole-resistant C. parapsilosis genotypes versus 7.7%/9.2%/11.3% excluding fluconazole-resistant C. parapsilosis genotypes) (Figure 2).
4. Discussion
By genotyping C. albicans, C. parapsilosis, and C. tropicalis isolates collected in 16 hospitals located in the Madrid metropolitan area, we demonstrated that the number of clusters—and patients involved—increased during the COVID-19 pandemic and this increase was mainly driven by fluconazole-resistant C. parapsilosis genotypes. Highly spread genotypes could be found not only in blood cultures but also in intra-abdominal samples, but antifungal-resistant genotypes were mainly present in blood cultures.
Surveys to study Candida spp. isolates from blood cultures and intra-abdominal samples are helpful to monitor species epidemiology and antifungal resistance, especially when genotyping of isolates is performed, and may help track antifungal-resistant clone spreading. Whereas the genotypes found in intra-abdominal samples may cause endogenous infection, those found in blood are susceptible to patient-to-patient transmission via the exogenous route [3]. Previous studies from our group demonstrated the usefulness of Candida genotyping to unravel the dynamic of isolate transmission within the hospital, as well as distinguish the presence of clusters that may suggest a common source of infection or patient-to-patient transmission, and cause infections in the form of outbreaks. We studied isolates causing candidaemia in hospital-admitted neonates and proved that some clusters were responsible for candidaemia outbreaks in that setting and that they may involve a large number of patients as far as C. parapsilosis clusters were concerned [4]. In another study, we demonstrated that the implementation of measures to decrease catheter-related infections correlated with a decrease in the number of clusters and patients with candidaemia involved, and therefore, genotyping proved the benefits of the implemented measures [7].
The CANDIMAD study is a first-in-its-class study conducted in Madrid and was devoted to assessing the burden of antifungal resistance in the city’s metropolitan area [15,16]. It allowed us to demonstrate that the rate of resistance to echinocandins was not a threat, whereas the rate of fluconazole resistance emerged in C. parapsilosis because of a genotype spreading across the region. In the present report, we complemented such observations by adding genotyping data on C. albicans, C. parapsilosis, and C. tropicalis blood cultures and intra-abdominal isolates collected in the CANDIMAD study. We detected 83 clusters in blood cultures. Intra-ward clusters (involving patients admitted to the same hospital ward) are likely indicative of hospital patient-to-patient transmission; however, we were unable to track intra-ward clusters in the present study due to the restructuring of hospitals during the COVID-19 pandemic. Therefore, potential patient-to-patient transmission was measured by the presence of intra-hospital clusters, which represented 34/83 of all clusters. Intra-hospital C. parapsilosis clusters involved a higher number of patients than those of C. albicans, as previously reported [4]. Of note, many of the clusters were detected in patients before the onset of the COVID-19 pandemic, but the number of patients involved in clusters was boosted (particularly for C. parapsilosis) during the pandemic, probably because of worsened catheter care.
The presence of genotypes involving epidemiologically unrelated patients (not admitted to the same hospital) is not infrequent, and its interpretation may be controversial [6]. Widespread clusters involve patients admitted to different hospitals and might indicate genotypes actively transferred among hospitals rather than active patient-to-patient hospital transmission. Alternatively, widespread clusters may comprise genotypes prone to cause invasive candidiasis because they are frequently present in the microbiota of healthy subjects or the environment. A number of the clusters detected in Madrid were widespread (63/83), and, as expected, they were found in the largest hospitals. The transfer of patients from smaller to large hospitals may also explain the presence of widespread clusters. Furthermore, clonal complexes are groups of genetically related widespread clusters [6], and their presence strengthens the hypothesis of frequently found genotypes prone to cause invasive candidiasis. In fact, when intra-abdominal genotypes were taken into account, the number of clonal complexes soared. It is worth noting that some of the clonal complexes found were previously detected in Madrid and outside Spain [6].
We detected an increase in the incidence of candidaemia during 2020 and 2021 in some participating hospitals, which is in line with previous reports conducted during the COVID-19 pandemic [9,10,11,12,13,14]. Such an increase could have been due to either an increase in the number of patients prone to develop candidaemia or higher patient-to-patient transmission. Our study included a large number of isolates collected before and during the COVID-19 pandemic and demonstrated that the main driver of the increase in patients involved in clusters during the COVID-19 pandemic in the Madrid region was the emergence of the widespread CP-451 fluconazole-resistant C. parapsilosis cluster harbouring the Y132F ERG11p substitution. This genotype was detected for the first time before the onset of the COVID-19 pandemic in an intra-abdominal sample in Hospital 9, where it caused an outbreak later on; it then became widespread across other hospitals, and its spread was reinforced in 2021. The transfer of patients and/or hospital material during the COVID-19 pandemic across the region may explain such spreading. Other C. parapsilosis and C. albicans clusters were also widespread but involved isolates to a lesser extent, suggesting a high potential for the dissemination of the CP-451 genotype. On the contrary, C. albicans antifungal-resistant genotypes each involved single isolates. Other studies conducted in Greece and Brazil also showed that fluconazole resistance in C. parapsilosis increased during the pandemic, probably also as a consequence of clonal spreading that could have taken place during the COVID-19 pandemic [11,14].
Our study was subject to some limitations. We were unable to study environmental isolates collected near patients and from healthcare workers, and therefore, we cannot rule out the environmental niches of the isolates or patient-to-patient transmission. Due to the lack of information regarding the candidaemia source, we were unable to study the potential correlation between catheter-related infection and intra-hospital clusters. Moreover, widespread cluster tracking would have been carried out if information regarding patient or medical material transfers across hospitals had been available. Finally, we could not rule out that some clusters were a consequence of the lack of discrimination in the typing procedure, although previous studies suggested that the microsatellite markers used herein were highly discriminative [3,4].
5. Conclusions
In conclusion, the number of clusters—and patients involved—increased during the COVID-19 pandemic mainly due to the emergence of fluconazole-resistant C. parapsilosis genotypes, predominantly in blood cultures. Our study sets an example of the value of conducting surveys of antifungal resistance in Candida spp. and the role of genotyping to track down genotype spreading across hospitals and warrants the continuation of such initiatives in the region.
Acknowledgments
We are grateful to Helena Kruyer for editing assistance. CANDIMAD study group: Judith Díaz García, Aina Mesquida, Ana Gómez, Elena Reigadas, Luis Alcalá, Carlos Sánchez Carrillo, Patricia Muñoz, Pilar Escribano, Jesús Guinea (Hospital General Universitario Gregorio Marañón); Ana Pérez de Ayala, Rosaura Pérez Muñoz, María del Carmen Vera González (Hospital Universitario 12 de Octubre); Elia Gómez García De La Pedrosa (Hospital Universitario Ramón y Cajal); Fernando González Romo, Paloma Merino Amador (Hospital Clínico San Carlos); María Soledad Cuétara, Oscar Manuel Muñoz Clemente, Víctor Antón Berenguer (Hospital Universitario Severo Ochoa); Aída Sánchez García (Hospital Universitario Infanta Sofía); Coral García Esteban, Oscar Cuevas Lobato, Guadalupe Bernal (Hospital Universitario de Getafe); Nelly Zurita, Ainhoa Gutiérrez Cobos (Hospital Universitario de La Princesa); María Muñoz Algarra, Isabel Sánchez Romero (Hospital Universitario Puerta de Hierro); Inmaculada Quiles Melero, Florinda San Juan Delgado (Hospital Universitario La Paz); María Teresa Durán Valle, Yolanda Gil Romero, Arturo Manuel Fraile Torres (Hospital Universitario de Móstoles).
Author Contributions
Conceptualization, J.G.; methodology, J.G. and P.E.; software, J.D.-G.; validation, J.G., P.E. and J.D.-G.; formal analysis, J.D.-G.; investigation, J.G. and P.E.; resources, J.G. and P.E.; data curation, J.D.-G., A.G., M.M., L.A., E.R., C.S.-C., A.P.-A., E.G.-G.d.l.P., F.G.-R., M.S.C., C.G.-E., I.Q.-M., N.D.Z., M.M.A., M.T.D.-V., A.S.-G. and P.M.; writing—original draft preparation, J.D.-G.; writing—review and editing, J.G. and P.E.; visualization, J.G. and P.E.; supervision, J.G. and P.E.; project administration, J.G. and P.E.; funding acquisition, J.G. and P.E. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the Ethics Committee of the Gregorio Marañón Hospital (CEim; study no. MICRO.HGUGM.2019-001).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
J.G. has received funds for participating in educational activities organized on behalf of Gilead, Pfizer, Mundipharma, and MSD; he has also received research funds from FIS, Gilead, Scynexis, F2G, Mundipharma, and Cidara, outside the submitted work.
Funding Statement
This study was supported by grants PI18/01155 and PI19/00074 from the Fondo de Investigación Sanitaria (FIS. Instituto de Salud Carlos III; Plan Nacional de I+D+I 2017–2020) and by grants from Instituto de Investigación Sanitaria Gregorio Marañón (II-PI-JGO-2018). The study was co-funded by the European Regional Development Fund (FEDER), ‘A way of making Europe.’ PE (CPII20/00015) is a recipient of a Miguel Servet contract supported by FIS. JG is a permanent researcher employed by the Fundación para Investigación Sanitaria del Hospital Gregorio Marañón. JD (FI19/00021) holds a predoctoral FIS grant.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pappas P.G., Lionakis M.S., Arendrup M.C., Ostrosky-Zeichner L., Kullberg B.J. Invasive candidiasis. Nat. Rev. Dis. Primers. 2018;4:18026. doi: 10.1038/nrdp.2018.26. [DOI] [PubMed] [Google Scholar]
- 2.Bassetti M., Righi E., Ansaldi F., Merelli M., Scarparo C., Antonelli M., Garnacho-Montero J., Diaz-Martin A., Palacios-Garcia I., Luzzati R., et al. A multicenter multinational study of abdominal candidiasis: Epidemiology, outcomes and predictors of mortality. Intensive Care Med. 2015;41:1601–1610. doi: 10.1007/s00134-015-3866-2. [DOI] [PubMed] [Google Scholar]
- 3.Escribano P., Guinea J., Marcos-Zambrano L.J., Martín-Rabadán P., Fernández-Cruz A., Sánchez-Carrillo C., Muñoz P., Bouza E. Is catheter-related candidemia a polyclonal infection? Med. Mycol. 2014;52:411–416. doi: 10.1093/mmy/myt018. [DOI] [PubMed] [Google Scholar]
- 4.Guinea J., Mezquita S., Gómez A., Padilla B., Zamora E., Sánchez-Luna M., Muñoz P., Escribano P. Whole genome sequencing confirms Candida albicans and Candida parapsilosis microsatellite sporadic and persistent clones causing outbreaks of candidemia in neonates. Med. Mycol. 2021;60:myab068. doi: 10.1093/mmy/myab068. [DOI] [PubMed] [Google Scholar]
- 5.Escribano P., Rodriguez-Creixems M., Sanchez-Carrillo C., Munoz P., Bouza E., Guinea J. Endemic genotypes of Candida albicans causing fungemia are frequent in the hospital. J. Clin. Microbiol. 2013;51:2118–2123. doi: 10.1128/JCM.00516-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guinea J., Arendrup M.C., Cantón R., Cantón E., García-Rodríguez J., Gómez A., Gómez-García-De La Pedrosa E., Hare R.K., Orden B., Sanguinetti M., et al. Genotyping Reveals High Clonal Diversity and Widespread Genotypes of Candida Causing Candidemia at Distant Geographical Areas. Front. Cell. Infect. Microbiol. 2020;10:166. doi: 10.3389/fcimb.2020.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Escribano P., Sanchez-Carrillo C., Munoz P., Bouza E., Guinea J. Reduction in Percentage of Clusters of Candida albicans and Candida parapsilosis Causing Candidemia in a General Hospital in Madrid, Spain. J. Clin. Microbiol. 2018;56:e00574–18. doi: 10.1128/JCM.00574-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcos-Zambrano L., Escribano P., Sanguinetti M., Gómez-García-De La Pedrosa E., De Carolis E., Vella A., Cantón R., Bouza E., Guinea J. Clusters of patients with candidaemia due to genotypes of Candida albicans and Candida parapsilosis: Differences in frequency between hospitals. Clin. Microbiol. Infect. 2015;21:677–683. doi: 10.1016/j.cmi.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Machado M., Estévez A., Sánchez-Carrillo C., Guinea J., Escribano P., Alonso R., Valerio M., Padilla B., Bouza E., Muñoz P. Incidence of Candidemia Is Higher in COVID-19 versus Non-COVID-19 Patients, but Not Driven by Intrahospital Transmission. J. Fungi. 2022;8:305. doi: 10.3390/jof8030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramos-Martínez A., Pintos-Pascual I., Guinea J., Gutiérrez-Villanueva A., Gutiérrez-Abreu E., Díaz-García J., Asensio Á., Iranzo R., Sánchez-Romero I., Muñoz-Algarra M., et al. Impact of the COVID-19 Pandemic on the Clinical Profile of Candidemia and the Incidence of Fungemia Due to Fluconazole-Resistant Candida parapsilosis. J. Fungi. 2022;8:451. doi: 10.3390/jof8050451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Routsi C., Meletiadis J., Charitidou E., Gkoufa A., Kokkoris S., Karageorgiou S., Giannopoulos C., Koulenti D., Andrikogiannopoulos P., Perivolioti E., et al. Epidemiology of Candidemia and Fluconazole Resistance in an ICU before and during the COVID-19 Pandemic Era. Antibiotics. 2022;11:771. doi: 10.3390/antibiotics11060771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papadimitriou-Olivgeris M., Kolonitsiou F., Kefala S., Spiliopoulou A., Aretha D., Bartzavali C., Siapika A., Marangos M., Fligou F. Increased incidence of candidemia in critically ill patients during the Coronavirus Disease 2019 (COVID-19) pandemic. Braz. J. Infect. Dis. 2022;26:102353. doi: 10.1016/j.bjid.2022.102353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nucci M., Barreiros G., Guimarães L.F., Deriquehem V.A.S., Castiñeiras A.C., Nouér S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses. 2021;64:152–156. doi: 10.1111/myc.13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomaz D.Y., Del Negro G.M.B., Ribeiro L.B., da Silva M., Carvalho G.O.M.H., Camargo C.H., de Almeida J.N., Motta A.L., Siciliano R.F., Sejas O.N.E., et al. A Brazilian Inter-Hospital Candidemia Outbreak Caused by Fluconazole-Resistant Candida parapsilosis in the COVID-19 Era. J. Fungi. 2022;8:100. doi: 10.3390/jof8020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Díaz-García J., Gómez A., Alcalá L., Reigadas E., Sánchez-Carrillo C., Pérez-Ayala A., Pérez-Ayala A., Gómez-García de la Pedrosa E., González-Romo F., Merino-Amador P., et al. Evidence of Fluconazole-Resistant Candida parapsilosis Genotypes Spreading across Hospitals Located in Madrid, Spain and Harboring the Y132F ERG11p Substitution. Antimicrob Agents Chemother. 2022;66:e0071022. doi: 10.1128/aac.00710-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Díaz-García J., Gómez A., Machado M., Alcalá L., Reigadas E., Sánchez-Carrillo C., Pérez-Ayala A., Gómez-García-De-La-Pedrosa E., González-Romo F., Cuétara M.S., et al. Blood and intra-abdominal Candida spp. from a multicentre study conducted in Madrid using EUCAST: Emergence of fluconazole resistance in Candida parapsilosis, low echinocandin resistance and absence of Candida auris. J. Antimicrob. Chemother. 2022;77:3102–3109. doi: 10.1093/jac/dkac288. [DOI] [PubMed] [Google Scholar]
- 17.Pfaller M.A., Andes D., Diekema D.J., Espinel-Ingroff A., Sheehan D., CLSI Subcommittee for Antifungal Susceptibility Testing Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: Time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist. Updat. 2010;13:180–195. doi: 10.1016/j.drup.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Díaz-García J., Mesquida A., Sánchez-Carrillo C., Reigadas E., Muñoz P., Escribano P., Guinea J. Monitoring the Epidemiology and Antifungal Resistance of Yeasts Causing Fungemia in a Tertiary Care Hospital in Madrid, Spain: Any Relevant Changes in the Last 13 Years? Antimicrob. Agents Chemother. 2021;65:e01827-20. doi: 10.1128/AAC.01827-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botterel F., Desterke C., Costa C., Bretagne S. Analysis of microsatellite markers of Candida albicans used for rapid typing. J. Clin. Microbiol. 2001;39:4076–4081. doi: 10.1128/JCM.39.11.4076-4081.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sampaio P., Gusmão L., Correia A., Alves C., Rodrigues A.G., Pina-Vaz C., Amorim A., Pais C. New Microsatellite Multiplex PCR for Candida albicans Strain Typing Reveals Microevolutionary Changes. J. Clin. Microbiol. 2005;43:3869–3876. doi: 10.1128/JCM.43.8.3869-3876.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabino R., Sampaio P., Rosado L., Stevens D.A., Clemons K.V., Pais C. New polymorphic microsatellite markers able to distinguish among Candida parapsilosis sensu stricto isolates. J. Clin. Microbiol. 2010;48:1677–1682. doi: 10.1128/JCM.02151-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaz C., Sampaio P., Clemons K.V., Huang Y.C., Stevens D.A., Pais C. Microsatellite multilocus genotyping clarifies the relationship of Candida parapsilosis strains involved in a neonatal intensive care unit outbreak. Diagn. Microbiol. Infect. Dis. 2011;71:159–162. doi: 10.1016/j.diagmicrobio.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y., Zhou H.-J., Che J., Li W.-G., Bian F.-N., Yu S.-B., Zhang L.-J., Lu J. Multilocus microsatellite markers for molecular typing of Candida tropicalis isolates. BMC Microbiol. 2014;14:245. doi: 10.1186/s12866-014-0245-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.