Over the past 40 years, the central goal of cognitive neuroscience has been to interpret neural signals. To do so, it focuses on depicting commonalities between individuals at the population level (Raizada and Connolly, 2012). However, everyone's perception of the world is shaped differently by individual experiences and preferences (Charest et al., 2014; Lee and Geng, 2017); averaging across participants may hide such peculiarities of specific individuals' brains. Indeed, the human brain shows a considerable degree of anatomic and functional variability across individuals. This observation has often frustrated researchers that were looking for homogeneity and uniformity across individuals' brains.
Recently, however, there has been a shift in perspective: researchers are now intrigued by the presence of such variability. This interest has led to several attempts to find links between individual differences in brain organization and variability in behavior and cognition across healthy and neuroatypical individuals (Laumann et al., 2015). The transition from the group level to the individual observation has not been present equally across the methodological approaches within cognitive neuroscience. It has been most fruitful for structural MRI studies that have clear clinical utility because they report the physical structure of individual brains. In contrast, fMRI research has mostly overlooked the individual representations (Gordon et al., 2017).
An exception to this trend is represented by the recent paper by Sen et al. (2022). The authors delve into the origins of neural variability and investigate the role of heritability versus (sensory) experience in driving and shaping interindividual cortical variability in a large group of congenitally blind people. To understand the effects of sensory experience on variability, they investigated the resting-state functional connectivity (RSFC) of primary visual cortex (V1) of people born completely blind and compared it with a sighted control group.
RSFC assumes that the BOLD MRI signal is correlated between functionally related brain regions (Biswal, 2012), even if those areas are spatially segregated. RSFC has been extensively used as a tool to increase our understanding of brain function and organization at multiple scales in groups of subjects. However, these group-level analyses potentially shadow the possible individual differences in cortical organization.
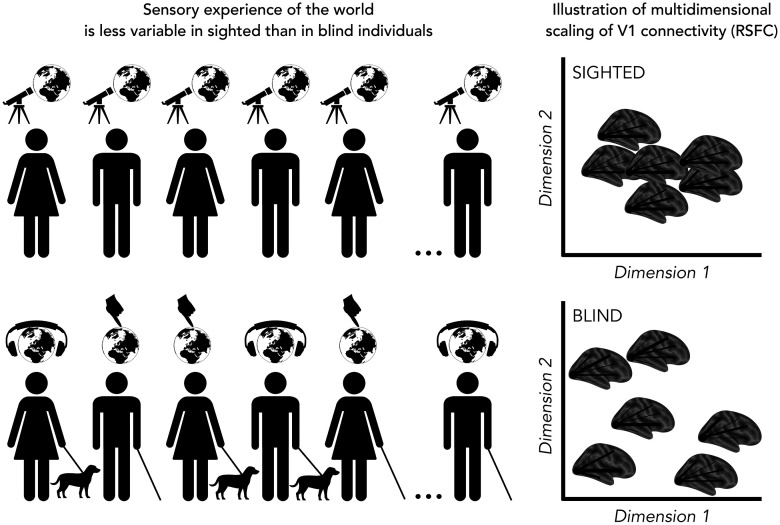
Notably, Sen et al. (2022) took an original perspective of looking at the degree of variability rather than homogeneity within groups, asking how the extreme experience of deprivation affects the degree of variability of the V1 connectivity (i.e., RSFC) profile. They found that V1 RSFC patterns were more variable across blind individuals than across sighted controls. Furthermore, individual variability was correlated with the degree of plasticity: regions showing a difference between blind and sighted individuals were also characterized by increased variability among the blind individuals (Fig. 1).
Figure 1.
Left, Illustration of the main way used to explore the environment in sighted and in blind individuals. Sighted people mostly use vision to explore the world, while people born blind use both audition and touch with some variability in selecting their preferred sense. Right, Illustration of a possible multidimensional scaling of the RSFC from V1 to the rest of the brain in sighted (top) and blind (bottom) individuals. In the sighted, the variability across subjects is lower compared with the variability across blind individuals. (This figure is for illustrative purposes, and it is not based on real data).
What could be the source of this variability? The hierarchical clustering of the RSFC profiles of blind individuals showed that spatial profiles of connectivity did not cluster based on blindness etiology, suggesting a central role for postnatal experiences rather than genetic factors linked to the causes of blindness. The authors suggested two nonmutually exclusive sources of the increased variability.
The first possible source of variability in blind people is the absence of highly consistent visual input, which is characterized by specific and similar statistical properties. A lack of the shared (visual) experience to constrain connectivity may lead to increased variability in the blind population. Indeed, individual variability in brain connectivity in newborns is greater than in adults (Molloy and Saygin, 2021). Therefore, the lack of a common visual experience could play a crucial role in maintaining and even increasing the amount of variability in the connectivity profile of V1 among blind individuals.
The second possible source of variability is individual adaptations to blindness, such as the compensatory use of nonvisual senses and cognitive faculties. Indeed, clustering analysis identified different clades of connectivity profiles classified by whether the resting-state activity in V1 was positively or negatively correlated with that in the sensorimotor, auditory, and superior frontal cortices (Sen et al., 2022). Moreover, the left-lateralization of the connectivity with the infero-frontal cortex, often reported in blindness, arose only in a subclade of blind participants. These different subprofiles might, indeed, be driven by individual adaptations to blindness, such as more or less extensive use of touch versus auditory modalities (e.g., braille vs audiobooks) or by a different role of language abilities and working memory strategies. This evidence further shows that going beyond the group-level analyses could reveal how individual sensory experiences shape the brain organization.
Future research incorporating both resting-state and task-based fMRI would be necessary to test the possible sources of variability. This would also elucidate whether the degree of variability in the functional connectivity profiles of blind individuals is related to the type of cognitive task (e.g., tactile, auditory, linguistic tasks). Consistent with this possibility, recent comparison of task-dependent versus RSFC in early blind and in sighted control individuals showed that cognitive state influences the connectivity profile of the two groups in different ways (Pelland et al., 2017). Therefore, it would be valuable to compare the RSFC results from Sen et al. (2022) with a task-dependent functional connectivity measure, to see whether the differences between blind and sighted people at the group level are also present within groups.
Another important future direction is to extend the investigation to more anterior visual regions, such as the ventral and the dorsal visual streams. Indeed, these regions reach their full development later than primary visual regions (Maurer, 2017), providing a wider window for individual experience to shape their neuronal profiles. Indeed, in sighted people, an idiosyncratic ventral occipitotemporal cortex representation of bodies, faces, places, and man-made objects has been demonstrated, beyond the well-known shared categorical structure of ventral occipitotemporal cortex representation (Charest et al., 2014; Gao et al., 2022). A possible interpretation of these results is that the representational idiosyncrasies might arise from the microstructural plasticity of cortex, which is driven by individual experience (Charest et al., 2014; Weiner et al., 2017). According to this interpretation, one might expect that this unique component of categorical representation, already present in sighted healthy individuals, is much more pronounced in those with early visual deprivation where plasticity in the ventral and dorsal visual stream is significantly enhanced (Collignon et al., 2013; Battal et al., 2022; Mattioni et al., 2022).
In this regard, recent studies (Mattioni et al., 2020; Rosenke et al., 2020) showed that, during task engagement, the functional profile of the ventral stream shows higher variability between congenitally blind people than between sighted individuals, suggesting a more idiosyncratic functional organization in the blind population. This suggests that the way in which these portions of the visual system form neural representations in blind individuals might be partially achieved in a subject-specific manner (Rosenke et al., 2020). This intriguing possibility is in line with the study of Sen et al. (2022) and raises several important, yet unresolved questions. For instance, considering that the occipital cortex of blind subjects is recruited during both auditory and tactile tasks, is there a correlation in the level of auditory and tactile recruitment across blind individuals? Or are there subject-specific profiles related to the modality of reorganization (e.g., some blind individuals may recruit the occipital cortex more for tactile stimulation and others more for auditory stimulation)?
This line of research has a critical implication to tailor visual rehabilitation for visual restoration and visual substitution based on individual profiles. Moreover, similar subject-oriented research could be applied in many other clinical domains to identify personalized targets for clinical intervention (Kohoutová et al, 2022). Therefore, we should look forward to more studies of interindividual variability in the future.
Footnotes
Editor's Note: These short reviews of recent JNeurosci articles, written exclusively by students or postdoctoral fellows, summarize the important findings of the paper and provide additional insight and commentary. If the authors of the highlighted article have written a response to the Journal Club, the response can be found by viewing the Journal Club at www.jneurosci.org. For more information on the format, review process, and purpose of Journal Club articles, please see http://jneurosci.org/content/jneurosci-journal-club.
C.B. is postdoctoral researcher at Fond National de la Recherche Scientifique de Belgique. We thank Tomas Lenc for helpful comments on an earlier version of this draft.
The authors declare no competing financial interests.
References
- Battal C, Gurtubay-Antolin A, Rezk M, Mattioni S, Bertonati G, Occelli V, Bottini R, Targher S, Maffei C, Jovicich J, Collignon O (2022) Structural and functional network-level reorganization in the coding of auditory motion directions and sound source locations in the absence of vision. J Neurosci 42:4652–4668. 10.1523/JNEUROSCI.1554-21.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB (2012) Resting state fMRI: a personal history. Neuroimage 62:938–944. 10.1016/j.neuroimage.2012.01.090 [DOI] [PubMed] [Google Scholar]
- Charest I, Kievit RA, Schmitz TW, Deca D, Kriegeskorte N (2014) Unique semantic space in the brain of each beholder predicts perceived similarity. Proc Natl Acad Sci USA 111:14565–14570. 10.1073/pnas.1402594111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collignon O, Dormal G, Albouy G, Vandewalle G, Voss P, Phillips C, Lepore F (2013) Impact of blindness onset on the functional organization and the connectivity of the occipital cortex. Brain 136:2769–2783. 10.1093/brain/awt176 [DOI] [PubMed] [Google Scholar]
- Gao X, Wen M, Sun M, Rossion B (2022) A genuine interindividual variability in number and anatomical localization of face-selective regions in the human brain. Cereb Cortex 32:4834–4856. 10.1093/cercor/bhab519 [DOI] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Gilmore AW, Newbold DJ, Greene DJ, Berg JJ, Ortega M, Hoyt-Drazen C, Gratton C, Sun H, Hampton JM, Coalson RS, Nguyen AL, McDermott KB, Shimony JS, Snyder AZ, Schlaggar BL, Petersen SE, Nelson SM, Dosenbach NU (2017) Precision functional mapping of individual human brains. Neuron 95:791–807.e7. 10.1016/j.neuron.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohoutová L, Atlas LY, Büchel C, Buhle JT, Geuter S, Jepma M, Koban L, Krishnan A, Lee DH, Lee S, Roy M, Schafer SM, Schmidt L, Wager TD, Woo CW (2022) Individual variability in brain representations of pain. Nat Neurosci 25:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann TO, Gordon EM, Adeyemo B, Snyder AZ, Joo SJ, Chen MY, Gilmore AW, McDermott KB, Nelson SM, Dosenbach NU, Schlaggar BL, Mumford JA, Poldrack RA, Petersen SE (2015) Functional system and areal organization of a highly sampled individual human brain. Neuron 87:657–670. 10.1016/j.neuron.2015.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Geng JJ (2017) Idiosyncratic patterns of representational similarity in prefrontal cortex predict attentional performance. J Neurosci 37:1257–1268. 10.1523/JNEUROSCI.1407-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioni S, Rezk M, Battal C, Bottini R, Mendoza KE, Oosterhof NN, Collignon O (2020) Categorical representation from sound and sight in the ventral occipito-temporal cortex of sighted and blind. Elife 9:e50732. 10.7554/eLife.50732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioni S, Rezk M, Battal C, Vadlamudi J, Collignon O (2022) Impact of blindness onset on the representation of sound categories in occipital and temporal cortices. Elife 11:e79370. 10.7554/eLife.79370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer D (2017) Critical periods re-examined: evidence from children treated for dense cataracts. Cogn Dev 42:27–36. 10.1016/j.cogdev.2017.02.006 [DOI] [Google Scholar]
- Molloy MF, Saygin ZM (2021) Individual variability in the innate functional organization of the human brain. bioRxiv 436788. 10.1101/2021.03.24.436788. [DOI] [Google Scholar]
- Pelland M, Orban P, Dansereau C, Lepore F, Bellec P, Collignon O (2017) State-dependent modulation of functional connectivity in early blind individuals. Neuroimage 147:532–541. 10.1016/j.neuroimage.2016.12.053 [DOI] [PubMed] [Google Scholar]
- Raizada RD, Connolly AC (2012) What makes different people's representations alike: neural similarity space solves the problem of across-subject fMRI decoding. J Cogn Neurosci 24:868–877. 10.1162/jocn_a_00189 [DOI] [PubMed] [Google Scholar]
- Rosenke M, Van den Hurk J, Margalit E, de Beeck HO, Grill-Spector K, Weiner KS (2020) Extensive individual differences of category information in ventral temporal cortex in the congenitally blind. bioRxiv 151092. 10.1101/2020.06.14.151092. [DOI] [Google Scholar]
- Sen S, Khalsa NN, Tong N, Ovadia-Caro S, Wang X, Bi Y, Striem-Amit E (2022) The role of visual experience in individual differences of brain connectivity. J Neurosci 42:5070–5084. 10.1523/JNEUROSCI.1700-21.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner KS, Barnett MA, Lorenz S, Caspers J, Stigliani A, Amunts K, Zilles K, Fischl B, Grill-Spector K (2017) The cytoarchitecture of domain-specific regions in human high-level visual cortex. Cereb Cortex 27:146–161. 10.1093/cercor/bhw361 [DOI] [PMC free article] [PubMed] [Google Scholar]