Abstract
A new series of indoline-2-one derivatives was designed and synthesized based on the essential pharmacophoric features of VEGFR-2 inhibitors. Anti-proliferative activities were assessed for all derivatives against breast (MCF-7) and liver (HepG2) cancer cell lines, using sunitinib as a reference agent. The most potent anti-proliferative derivatives were evaluated for their VEGFR-2 inhibition activity. The effects of the most potent inhibitor, 17a, on cell cycle, apoptosis, and expression of apoptotic markers (caspase-3&-9, BAX, and Bcl-2) were studied. Molecular modeling studies, such as docking simulations, physicochemical properties prediction, and pharmacokinetic profiling were performed. The results revealed that derivatives 5b, 10e, 10g, 15a, and 17a exhibited potent anticancer activities with IC50 values from 0.74–4.62 µM against MCF-7 cell line (sunitinib IC50 = 4.77 µM) and from 1.13–8.81 µM against HepG2 cell line (sunitinib IC50 = 2.23 µM). Furthermore, these compounds displayed potent VEGFR-2 inhibitory activities with IC50 values of 0.160, 0.358, 0.087, 0.180, and 0.078 µM, respectively (sunitinib IC50 = 0.139 µM). Cell cycle analysis demonstrated the ability of 17a to induce a cell cycle arrest of the HepG2 cells at the S phase and increase the total apoptosis by 3.5-fold. Moreover, 17a upregulated the expression levels of apoptotic markers caspase-3 and -9 by 6.9-fold and 3.7-fold, respectively. In addition, 17a increased the expression level of BAX by 2.7-fold while decreasing the expression level of Bcl-2 by 1.9-fold. The molecular docking simulations displayed enhanced binding interactions and similar placement as sunitinib inside the active pocket of VEGFR-2. The molecular modeling calculations showed that all the test compounds were in accordance with Lipinski and Veber rules for oral bioavailability and had promising drug-likeness behavior.
Keywords: anti-proliferative, apoptosis, indolin-2-one, isatin, docking, sunitinib, VEGFR-2 inhibitors
1. Introduction
The treatment of cancerous diseases represents an exceptionally challenging battle for medicinal chemists to develop potent and safe chemotherapeutic agents [1,2,3,4]. These efforts mainly aim to identify and target the various biochemical processes involved in the progression and metastasis of tumors [5,6]. Angiogenesis, a complex process that involves the growth of new blood vessels from preexisting vasculature, is essential for normal organ growth and wound healing [7,8]. However, its imbalance is involved in the pathogenesis of different disorders, including cancer, psoriasis, multiple sclerosis, diabetic neuropathy, and rheumatoid arthritis [9,10,11,12,13].
Angiogenesis plays a critical role in tumor growth, invasion, and metastasis. Solid tumors are in dire need of new blood capillaries for an adequate supply of nutrients, the elimination of metabolic waste, and metastatic growth beyond their critical size [14].
The stimulation of various pre-angiogenic factors initiates angiogenesis through multiple steps, including basement membrane dissolution, migration, proliferation of endothelial cells, and finally, the formation of new capillary vessels [15,16].
Angiogenesis is controlled by several angiogenic regulators, such as vascular endothelial growth factor (VEGF) [17], fibroblast growth factor (FGF) [18], platelet-derived growth factor (PDGF) [19], transforming growth factor–β (TGF-β) [20], matrix metalloproteinases (MMPs) [21], epidermal growth factor (EGF) [22], angiopoietins [23], and integrins [24]. Among them, VEGF stands out as the most critical regulator of tumor angiogenesis [25,26]. Its actions are mainly mediated through a specific tyrosine protein kinase receptor called vascular endothelial growth factor receptor-2 (VEGFR-2) [27,28].
The binding of VEGF to VEGFR-2 leads to dimerization of two monomeric receptors and autophosphorylation of the tyrosine residues at the tail of the receptor’s intracellular domain, which initiates a signal transduction cascade that activates downstream signaling pathways [29]. These actions ultimately lead to angiogenesis and stimulate microvascular permeability, tumor proliferation, and tumor migration [30]. VEGFR-2 is overexpressed or hyperactivated in several kinds of malignancies, such as ovarian cancer [31], thyroid cancer [32], breast cancer [33], renal cancer [34], and hepatocellular carcinoma [35]. Therefore, blocking VEGF/VEGFR-2 signaling has been considered one of the most promising strategies for inhibition of angiogenesis and stopping tumor progression [36].
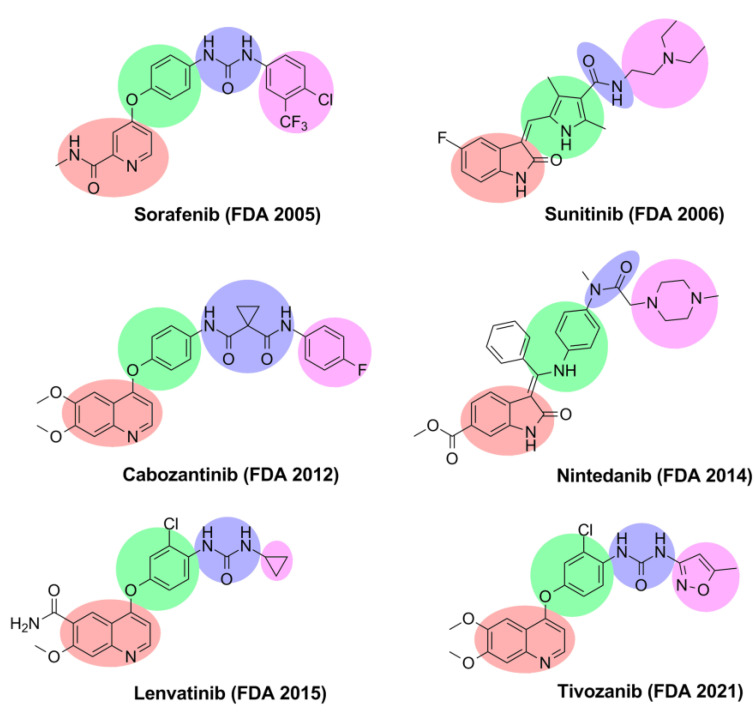
Over the past decades, many potent VEGFR-2 inhibitors have been approved by the Food and Drug Administration (FDA) to treat different types of tumors (Figure 1). According to the binding mode, VEGFR-2 inhibitors are divided into three major types [37]. Type I inhibitors are fitted in the conservative ATP active site and interact with Glu917 and Cys919 residues in the hinge region by hydrogen bonds [37]. Type I inhibitors, such as sunitinib and nintedanib, mostly bind to the active (DFG-in) conformation of VEGFR-2 [37,38]. Meanwhile, type II inhibitors target the inactive (DFG-out) conformation of VEGFR-2; these inhibitors bind to both the ATP catalytic site and the adjacent hydrophobic pocket [39]. Sorafenib, cabozantinib, lenvatinib, and tivozanib are examples of type II inhibitors [39,40]. Type III inhibitors are covalent noncompetitive inhibitors; these inhibitors bind through a covalent bond with a cysteine amino acid residue and prevent the binding of ATP at the binding site [38]. Vatalanib is considered an example of a type III inhibitor [38,41].
Figure 1.
VEGFR-2 inhibitors approved by the FDA.
Rationale and Design of the Work
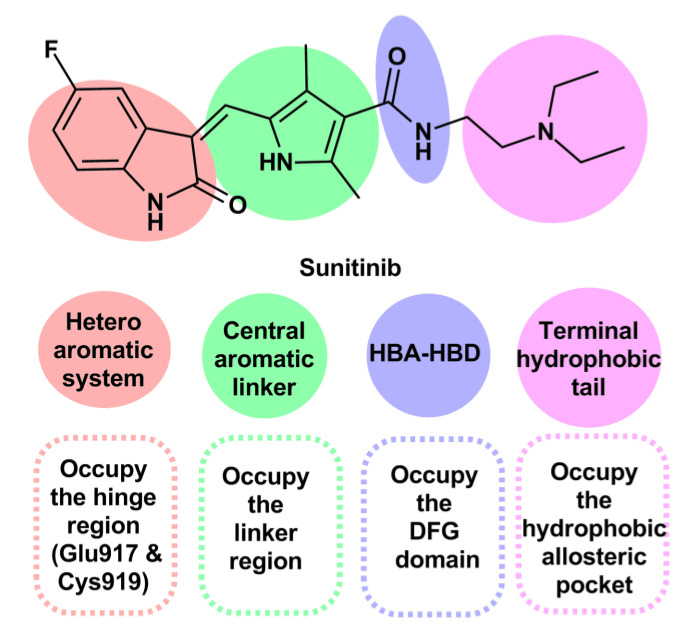
The reported virtual screening and pharmacophore modeling studies revealed that most VEGFR-2 inhibitors contain four essential pharmacophoric features [42,43,44,45]. The reported features are: (1) a flat heteroaromatic ring contains hydrogen bond acceptor center that interacts with the key Glu917 and Cys919 in the ATP binding domain (hinge region) through hydrogen bond formation [43]; (2) a central aromatic system that occupies the linker region [46]; (3) a hydrogen bond acceptor and hydrogen bond donor moiety (HBA-HBD) that fits into the DFG domain [47]; (4) a terminal hydrophobic tail placed inside the hydrophobic allosteric pocket (Figure 2) [48,49].
Figure 2.
The essential pharmacophoric features of VEGFR-2 inhibitors applied to sunitinib.
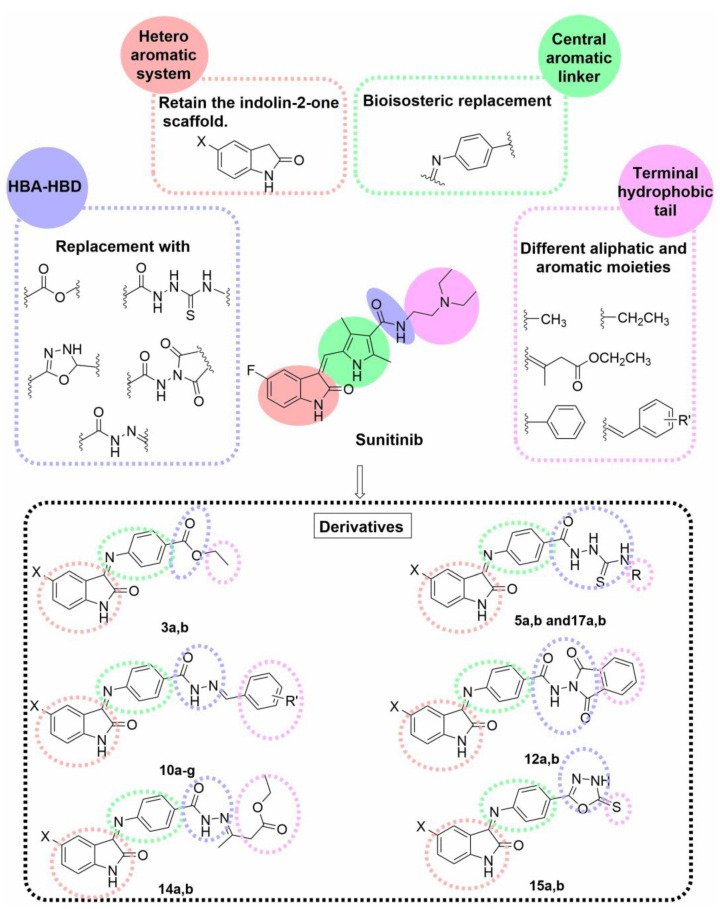
According to the previous findings and based on our previous work on anticancer agents generally and kinase inhibitors specifically [50,51,52], we reported the design of a new series of VEGFR-2 inhibitors based on the indoline-2-one scaffold [43] to attain more potent anticancer agents. Different bioisosteric modifications, including replacement, extension, ring closure, and expansion, were applied at the four essential pharmacophoric features to generate our candidate compounds, as illustrated in (Figure 3).
Figure 3.
Rationale of the designed compounds based on the pharmacophore model of VEGFR-2 inhibitors and using sunitinib as a lead compound.
The privileged indolin-2-one scaffold is regarded as one of the most promising heteroaromatic pharmacophore moieties that bind to the hinge region in the ATP active pocket of VEGFR-2 [53,54]. Therefore, the indolin-2-one nucleus was selected as the heterocyclic aromatic ring system for our target compounds. Secondly, the phenylimino moiety was chosen as the central aromatic system that occupies the linker region. Such a linker was selected to extend the available aromatic surface for interaction through ring expansion of the pyrrole ring of sunitinib to benzene and offer a possible new site for interaction through the imino nitrogen atom.
The third pharmacophoric HBA-HBD feature was realized using various moieties; ester (compounds 7a,b), thiosemicarbazide (compounds 5a,b and 17a,b), hydrazide (compounds 10a–g and 14a,b), N-(2,5-dioxopyrrolidin-1-yl)amide (compounds 12a,b), and oxadiazole ring (compounds 15a,b). Different HBA-HBD moieties were chosen to offer a wide selection of hydrogen-bond-rich groups with different geometries and variable metabolic stabilities. Lastly, the terminal hydrophobic tail was varied to be either aliphatic chains (compounds 5a,b, 7a,b, and 14a,b) or aromatic heterocyclic rings (compounds 12a,b, and 15a,b), or a substituted benzene ring with different hydrophobic, electronic, and topological groups (compounds 10a–g and 17a,b).
Therefore, in the current study and guided by preliminary docking studies, a new series of VEGFR-2 inhibitors based on the indolin-2-one scaffold was designed and synthesized in an endeavor to obtain potent anticancer agents. The growth inhibition activities for all the prepared compounds against the MCF-7 and HepG2 cancer cell lines were evaluated using sunitinib as the reference agent. The most potent anti-proliferative derivatives were tested for their VEGFR-2 inhibition activity. The molecular docking simulations were accomplished to predict the affinity and binding properties with VEGFR-2. Further biological investigations for the most potent inhibitor, 17a, on cell cycle, apoptosis, and expression of caspase-3&-9, BAX, and Bcl-2, were assessed to gain a better understanding of its apoptotic activity. Finally, in silico physicochemical properties and pharmacokinetic profiling were calculated to ensure the drug-likeness ability of the designed compounds.
2. Results and Discussion
2.1. Chemistry
The preparation methodologies adopted to synthesize the target compounds 3a,b–17a,b are outlined in Scheme 1, Scheme 2 and Scheme 3. The structures of the final compounds were supported by various spectral and elemental analyses. The final compounds were obtained as a mixture of E and Z isomers, and the spectral data were reported for the major isomer.
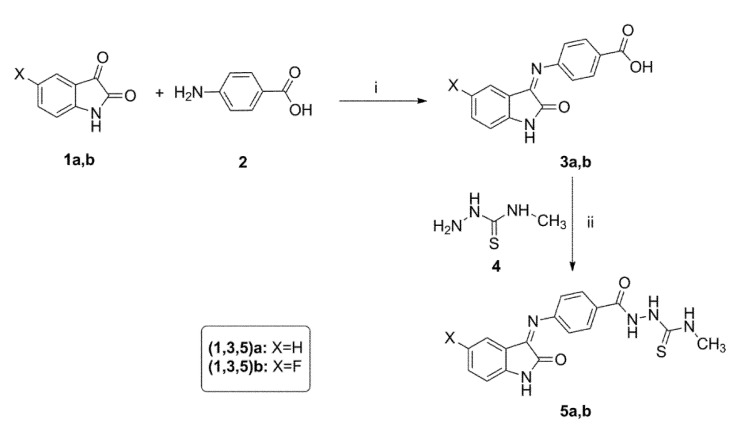
Scheme 1.
Synthesis of the target compounds 3a,b–5a,b. Reagent and conditions: (i) EtOH, AcOH, Reflux, 4 h; (ii) EDC, Et3N, HOBT, DMF, 0 °C to rt, overnight.
Scheme 2.
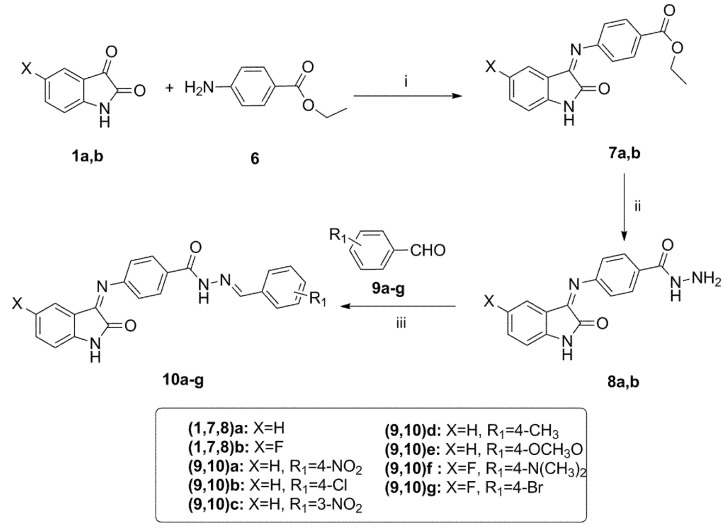
Synthesis of the target compounds 7a,b–10a–g. Reagent and conditions: (i) EtOH, AcOH, Reflux, 6 h; (ii) NH2NH2, EtOH, Reflux, 2 h; (iii) EtOH, AcOH, Reflux, 6 h.
Scheme 3.
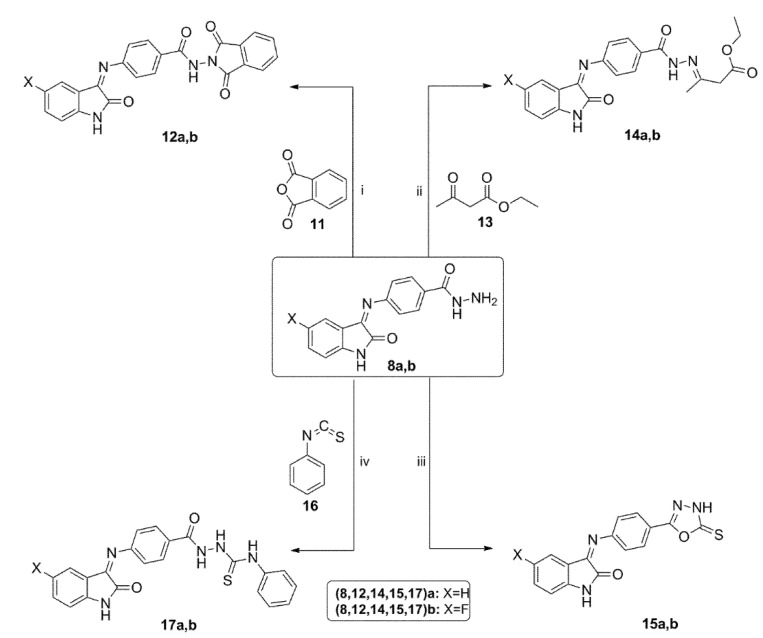
Synthesis of the target compounds 12a,b–17a,b. Reagent and conditions: (i) AcOH, sonication, 50 °C, 4 h; (ii) EtOH, AcOH, Reflux, 4 h; (iii) (a) CS2, KOH, EtOH, Reflux, 12 h, (b) 10% HCl; (iv) EtOH, Reflux, 8 h.
The synthesis of the designed N-methylthiosemicarbazides 5a,b is outlined in Scheme 1. First, benzoic acid derivatives 3a,b were obtained by condensing isatins 1a,b with 4-aminobenzoic acid 2 in refluxing ethanol and catalytic glacial acetic acid [55]. Next, acids 3a,b were subjected to an EDC/HOBt assisted coupling with 4-methylthiosemicarbazide 4 to afford the desired N-methylthiosemicarbazides 5a,b in 78–82 % yield [56].
The preparation of the target benzhydrazidehydrazones 10a–g is described in Scheme 2. The intermediate Schiff base esters 7a,b were prepared by condensation of isatins 1a,b with benzocaine 6 in ethanol under acetic acid catalysis [57]. The hydrazinolysis of esters 7a,b with hydrazine afforded the benzohydrazide derivatives 8a,b in excellent 92–93% yield. The target hydrazones 10a–g were prepared by condensation of hydrazides 8a,b with different benzaldehyde derivatives 9a–g using catalytic glacial acetic acid [58].
The synthetic procedures followed for preparation of the target compounds 12a,b–17a,b are shown in Scheme 3. The cyclic imides 12a,b were synthesized by cyclodehydration of hydrazides 8a,b with phthalic anhydride 11 in glacial acetic acid under sonication at 50 °C [59]. Treatment of hydrazides 8a,b with ethyl acetoacetate 13 under the acetic acid catalysis afforded esters 14a,b in excellent 86–88% yield [60].
Next, hydrazides 8a,b were subjected to cyclization conditions with carbon disulfide and potassium hydroxide followed by acidification to furnish the oxadiazole derivatives 15a,b in 82–86% yield [61]. The N-phenylthiosemicarbazides 17a,b were prepared by reaction of benzhydrazides 8a,b with phenyl isothiocyanate 16 in refluxing ethanol for eight hours.
2.2. Biological Investigation
2.2.1. In Vitro Cytotoxic Activity Assay
The cytotoxic activities against breast MCF-7 and liver HepG2 cancer cell lines for all the synthesized compounds 3a,b–17a,b were evaluated by MTT-based cytotoxicity assay [62]. Sunitinib was selected as a reference standard in this study, and the results are presented as IC50 in Table 1.
Table 1.
Anti-proliferative activities of compounds 3a,b–17a,b against the MCF-7 and HepG2 cell lines.
| Code | Structure | Cytotoxicity IC50 (μM) | |||
|---|---|---|---|---|---|
| X | HBA-HBD | Hydrophobic Tail | MCF-7 | HepG2 | |
| 3a | H | -COOH | --------- | 51.60 ± 2.50 | 62.41 ± 2.61 |
| 3b | F | -COOH | --------- | 43.90 ± 2.10 | 51.22 ± 2.50 |
| 5a | H | -CO-NH-NH-CS-NH- | -CH3 | 6.42 ± 0.55 | 5.48 ± 0.27 |
| 5b | F | -CO-NH-NH-CS-NH- | -CH3 | 0.99 ± 0.04 | 2.62 ± 0.13 |
| 7a | H | -CO-O- | -CH2-CH3 | 14.90 ± 0.70 | 10.94 ± 0.53 |
| 7b | F | -CO-O- | -CH2-CH3 | 3.81 ± 0.20 | 2.23 ± 0.11 |
| 8a | H | -CO-NH-NH2 | --------- | 27.20 ± 1.30 | 24.85 ± 1.21 |
| 8b | F | -CO-NH-NH2 | --------- | 40.70 ± 2.80 | 53.89 ± 2.63 |
| 10a | H | -CO-NH-N= | 4-NO2-C6H4-CH= | 40.00 ± 2.00 | 8.24 ± 0.40 |
| 10b | H | -CO-NH-N= | 4-Cl-C6H4-CH= | 23.80 ± 1.21 | 33.83 ± 1.65 |
| 10c | H | -CO-NH-N= | 3-NO2-C6H4-CH= | 14.60 ± 0.71 | 20.96 ± 1.02 |
| 10d | H | -CO-NH-N= | 4-CH3-C6H4-CH= | 13.70 ± 0.40 | 21.41 ± 1.05 |
| 10e | H | -CO-NH-N= | 4-CH3O-C6H4-CH= | 4.62 ± 0.20 | 8.81 ± 0.43 |
| 10f | F | -CO-NH-N= | 4-(CH3)2N-C6H4-CH= | 11.00 ± 0.59 | 19.16 ± 0.94 |
| 10g | F | -CO-NH-N= | 4-Br-C6H4-CH= | 0.74 ± 0.03 | 2.79 ± 0.14 |
| 12a | H | -CO-NH-N-(CO)2- | -C6H4- | 78.40 ± 3.81 | 130.20 ± 6.37 |
| 12b | F | -CO-NH-N-(CO)2- | -C6H4- | 33.80 ± 1.70 | 23.46 ± 1.15 |
| 14a | H | -CO-NH-N= | -CH2-CH3 | 7.37 ± 0.40 | 10.28 ± 0.81 |
| 14b | F | -CO-NH-N= | -CH2-CH3 | 32.90 ± 1.61 | 13.75 ± 0.67 |
| 15a | H | Oxadiazole | -CS- | 2.77 ± 0.10 | 2.30 ± 0.18 |
| 15b | F | Oxadiazole | -CS- | 19.60 ± 1.80 | 12.69 ± 0.62 |
| 17a | H | -CO-NH-NH-CS-NH- | -C6H5 | 1.44 ± 0.11 | 1.13 ± 0.06 |
| 17b | F | -CO-NH-NH-CS-NH- | -C6H5 | 25.80 ± 1.30 | 9.81 ± 0.48 |
| Sunitinib | -- | --------- | --------- | 4.77 ± 0.29 | 2.23 ± 0.11 |
All IC50 values are the mean ± SD of three different experiments.
The obtained data revealed that the tested derivatives, 3a,b–17a,b, exhibited moderate to potent cytotoxicity against the MCF-7 (IC50 = 0.74–78.40 μM) and HepG2 (IC50 = 1.13–130.20 μM) cell lines compared to the reference sunitinib. Compound 10g (IC50 = 0.74 ± 0.03 μM) displayed the most potent anti-proliferative activity against the MCF-7 cell line that was 6-folds more potent than sunitinib. Compound 5b also inhibited the growth of the breast MCF-7 cancer cell line with (IC50 = 0.99±0.04 μM), which was 5-folds more potent than sunitinib. In addition, 10g and 5b displayed a comparable cytotoxic activity to sunitinib against the hepatic HepG2 cell line. Compound 17a, compared with sunitinib, exhibited three-fold more potent growth inhibition activity against the MCF-7 cells and two-fold more potent cytotoxicity against the HepG2 cells (IC50 values of 1.44 ± 0.11 and 1.133 ± 0.06 μM), respectively. Moreover, compound 15a successfully inhibited the growth of the MCF-7 cells (IC50 = 2.77 ± 0.10, 1.7-fold more potent than sunitinib) and the HepG2 cell line (IC50 = 2.303 ± 0.18 μM). Furthermore, hydrazone 10e showed potent growth inhibition for the MCF-7 and HepG2 cell lines, with the MCF-7 cell line two times more sensitive than the HepG2 cell line.
Generally, the fluorinated derivatives showed better cytotoxic activity than their unsubstituted counterparts, except for 8b, 14b, 15b, and 17b derivatives. Remarkably, it was observed that the lack of hydrophobic tail pharmacophoric feature in compounds 3a,b and 8a,b resulted in a significant drop in the cytotoxic activity, which clearly indicates the importance of this feature for efficient binding with the allosteric hydrophobic pocket within VEGFR-2. Additionally, among the series of benzohydrazidehydrazones 10a–g, compound 10g, which has the highest iLOG P (2.93), emerged as the most potent derivative against the MCF-7, which demonstrates the importance of hydrophobic interactions and the efficacy of the hydrazide group as the HBA-HBD moiety for VEGFR-2 inhibitory activity. The use of bulky cyclic phthalimide moiety as the hydrophobic tail in compounds 12a,b decreased the cytotoxic activity, which may be attributed to steric bulkiness and inability of the HBA-HBD group to freely rotate for efficient binding inside VEGFR-2. The cytotoxicity test results were promising to evaluate the most potent derivatives for in vitro VEGFR-2 inhibition.
Incorporating the pharmacophoric HBA-HBD moiety within an oxadiazole ring resulted in derivatives 15a,b, which showed promising cytotoxic activities. Oxadiazole 15a was two times more potent than sunitinib against the MCF-7 cell line and exhibited close potency against the HepG2 cell line. Additionally, utilizing the thiosemicarbazide group as the HBA-HBD moiety proved to be very efficient for cytotoxic activity. The N-methyl derivative 5b was the second most potent against the MCF-7 cell line, and the N-phenyl derivative 17a was the most potent against the HepG2 cell line.
2.2.2. In Vitro VEGFR-2 Kinase Inhibitory Assay
The most potent antitumor derivatives, 5b, 10e, 10g, 15a, and 17a, were tested for VEGFR-2 kinase inhibition using sunitinib as the reference compound; the results are reported as IC50 in Table 2. [63].
Table 2.
In vitro VEGFR-2 kinase inhibitory activities of compounds 5b,10e, 10g, 15a and 17a.
| Code | IC50 (μM) a |
|---|---|
| 5b | 0.160 ± 0.008 |
| 10e | 0.358 ± 0.019 |
| 10g | 0.087 ± 0.004 |
| 15a | 0.180 ± 0.009 |
| 17a | 0.078 ± 0.003 |
| Sunitinib | 0.139 ± 0.007 |
a All IC50 values are calculated as the mean ± SD of three different experiments.
The outcomes showed that the tested compounds displayed potent inhibitory activities with IC50 from 0.078–0.358 μM. Compound 17a demonstrated the most potent VEGFR-2 inhibition activity that was 1.78-fold more potent than sunitinib (IC50 = 0.078 and 0.139 μM, respectively). Moreover, the inhibitory activity of compound 10g was 1.6-fold more than sunitinib (IC50 = 0.087 ± 0.004 μM). Derivatives 5b, 10e, and 15a demonstrated promising VEGFR-2 inhibition with IC50 values that were 0.160 ± 0.008, 0.358 ± 0.019, and 0.180 ± 0.009, respectively.
2.2.3. Cell Cycle Analysis
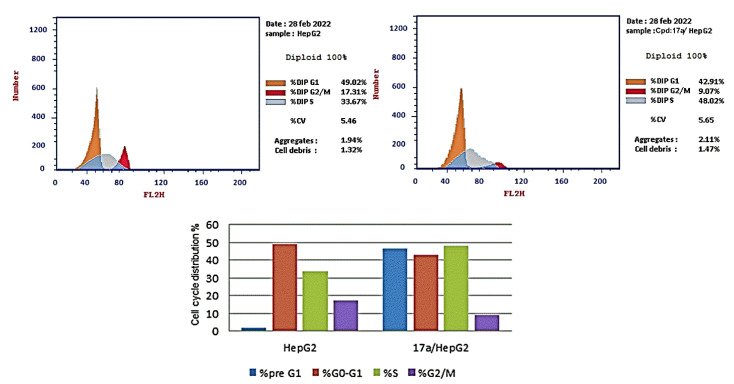
Compound 17a showed potent cytotoxic and VEGFR-2 inhibition activities, and was selected to explore its activity on the cell cycle distribution and cell proliferation of HepG2 cells. The HepG2 cells were exposed to 17a (1.13 µM equal to its anti-proliferative IC50) for 24 h, and cell cycle progression was monitored by flow cytometry; the results are reported in Table 3 [64].
Table 3.
Effect of compound 17a on cell cycle distribution in HepG2.
| Sample | Cell Cycle Distribution (%) a | |||
|---|---|---|---|---|
| %pre G1 | %G0-G1 | %S | %G2/M | |
| HepG2 | 1.91 | 49.02 | 33.67 | 17.31 |
| 17a/HepG2 | 46.38 | 42.91 | 48.02 | 9.07 |
a All percentages are expressed as the mean of three different experiments.
The obtained data revealed that compound 17a decreased the distribution at the G0-G1 phase (42.91%) and the G2/M phase (9.07%) compared with the control (49.02 and 17.31%, respectively). In addition, the percentage of cell population increased at the S phase by 1.43-fold more than the control. These findings revealed that compound 17a induced arrest of the cell cycle of the HepG2 cells at the S phase (Figure 4). Sorafenib was reported to induce a cell cycle arrest at the S phase and G2/M phase in HepG2 liver cancer cells [65].
Figure 4.
Cell cycle analysis of the HepG2 cells treated with compound 17a for 24 h using flow cytometry.
2.2.4. Apoptosis Analysis
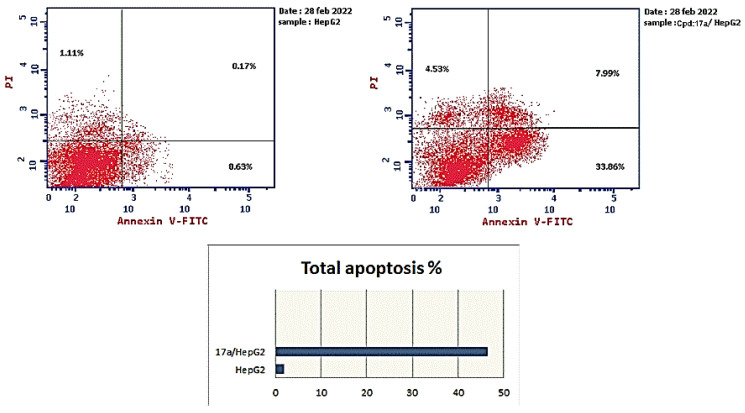
The HepG2 cells were treated with 17a (1.13 µM for 24 h), and the apoptotic effect was determined using Annexin V-FITC/PI assay. The results (Table 4) demonstrated that 17a enhanced total apoptosis by 24-fold compared to the control (46.38% and 1.91%, respectively). Additionally, 17a increased the percentage of early apoptosis compared with the control HepG2 cells (33.86% and 0.63%, respectively). Moreover, it increased the percentage of late apoptotic cells by 74-fold more than the control cells (from 0.17% to 7.99%). In addition, 17a enhanced the necrosis percentage 4-fold more than the control. These details suggested that compound 17a could induce the apoptotic mechanism of programed cell death in the HepG2 cell line (Figure 5).
Table 4.
Effect of compound 17a on apoptosis in the HepG2 cells.
| Sample | Apoptosis a | Necrosis a | ||
|---|---|---|---|---|
| Total | Early | Late | ||
| HepG2 | 1.91 | 0.63 | 0.17 | 1.11 |
| 17a/HepG2 | 46.38 | 33.86 | 7.99 | 4.53 |
a All percentages are expressed as the mean of three different experiments.
Figure 5.
Apoptosis analysis of 17a using Annexin V-FITC/PI dual staining in HepG2 cells.
2.2.5. Caspase-3 and -9 Expression Assay
The expression of the apoptotic markers (caspase-3&-9) in the HepG2 cells treated with 17a was studied to investigate the signal transduction pathway for its apoptotic activity.
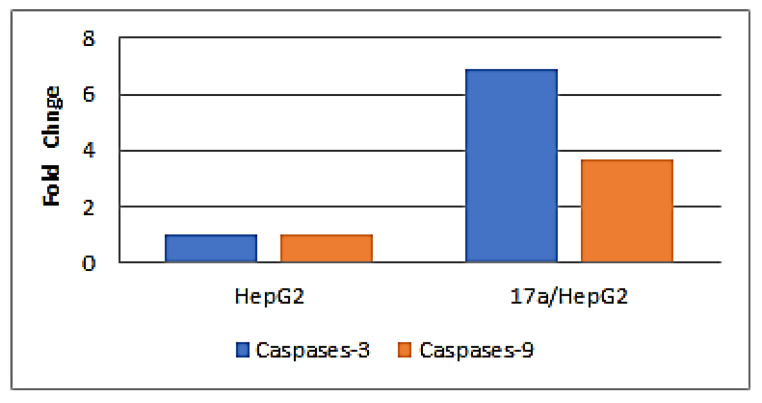
The gene expression fold change of caspase-3 and -9 in the HepG2 cells treated with 1.13 µM of compound 17a for 24 h was determined using quantitative real-time PCR analysis. The obtained results (Table 5) revealed that 17a elevated the gene expression of caspase-3 by 6.9-fold and caspase-9 by 3.7-fold more than the control HepG2 cells. The obtained data suggested that the caspase transduction pathway is involved in the apoptotic effect of compound 17a (Figure 6).
Table 5.
The gene expression fold change of caspases-3 and -9 in the HepG2 cells treated with 17a.
| Sample | Gene Expression Fold Change (Normalized to β-Actin) a | |
|---|---|---|
| Caspases-3 | Caspases-9 | |
| Cont. HepG2 | 1 | 1 |
| 17a/HepG2 | 6.889 | 3.703 |
a All results are expressed as mean of three different experiments.
Figure 6.
The gene expression fold change of caspases-3 and -9 in HepG2 cells treated with compound 17a.
2.2.6. BAX and Bcl-2 Expression Assay
The apoptotic BAX and anti-apoptotic Bcl-2 proteins play critical roles in caspase-independent apoptosis [66]. The ratio of the two proteins indicates the liability of a cell to be subjected to mitochondrial apoptosis [67].
The expression of BAX and Bcl-2 were evaluated in the HepG2 cells after treatment with 1.13 µM of compound 17a for 24 h. The Western blotting technique was utilized to determine the levels of Bax and Bcl-2 proteins and estimate the Bax/Bcl-2 ratio.
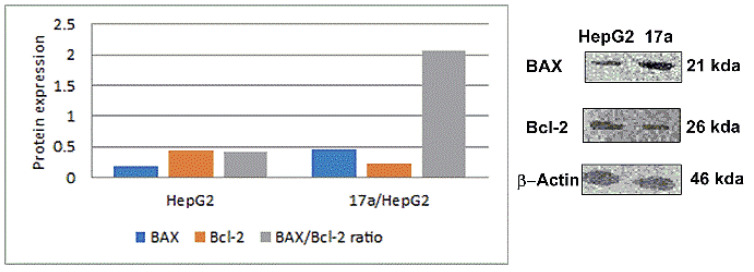
The results (Table 6) showed that 17a increased the expression of BAX by 2.7-fold more than the control cells. Moreover, it exhibited a pronounced decline in the expression level of Bcl-2 by 1.9-fold in comparison with the control. Additionally, compound 17a enhanced the BAX/Bcl-2 ratio by 5-fold. These outcomes signified that compound 17a induced mitochondrial apoptosis in the HepG2 cells (Figure 7).
Table 6.
The expression of BAX and Bcl-2 proteins in HepG2 cells after treatment with compound 17a.
| Sample | Protein Expression (Normalized to β-Actin) a | ||
|---|---|---|---|
| BAX | Bcl-2 | BAX/Bcl-2 Ratio | |
| HepG2 | 0.175 | 0.432 | 0.41 |
| 17a/HepG2 | 0.472 | 0.229 | 2.06 |
a All results are expressed as mean of three different experiments.
Figure 7.
Effect of compound 17a on the immunoblotting of BAX and Bcl-2 proteins (normalized to β-actin).
2.3. Molecular Modeling Studies
2.3.1. Docking Study
Molecular docking is the most utilized virtual drug design technique when the 3D structure of the target protein is available [68]. Simulations were carried out to predict the affinity and investigate the potential binding patterns of derivatives 3a,b–17a,b within the active pocket of VEGFR-2. A docking study was performed on VEGFR-2 tyrosine kinase co-crystallized with sunitinib (PDB: 4AGD) [69] using MOE 2020.09 computational software [70]. First, validation of the molecular docking protocol was established by re-docking sunitinib in the ATP binding domain of the VEGFR-2 active pocket. Reproduction of the same binding interactions and orientation inside the active site as the co-crystallized ligand demonstrated that the applied docking setup was appropriate for the study. This was also confirmed by the small RMSD obtained (0.6797 Å) between the native ligand and the re-docked one.
Sunitinib achieved a docking score of −16.5974 kcal/mol and was interacted by the NH and C=O groups of its indolin-2-one with the Glu917 and Cys919 of hinge residues, respectively. Additionally, it showed multiple hydrophobic interactions with Leu840, Ala866, Val916, Phe918, and Leu1035 (Figure 8).
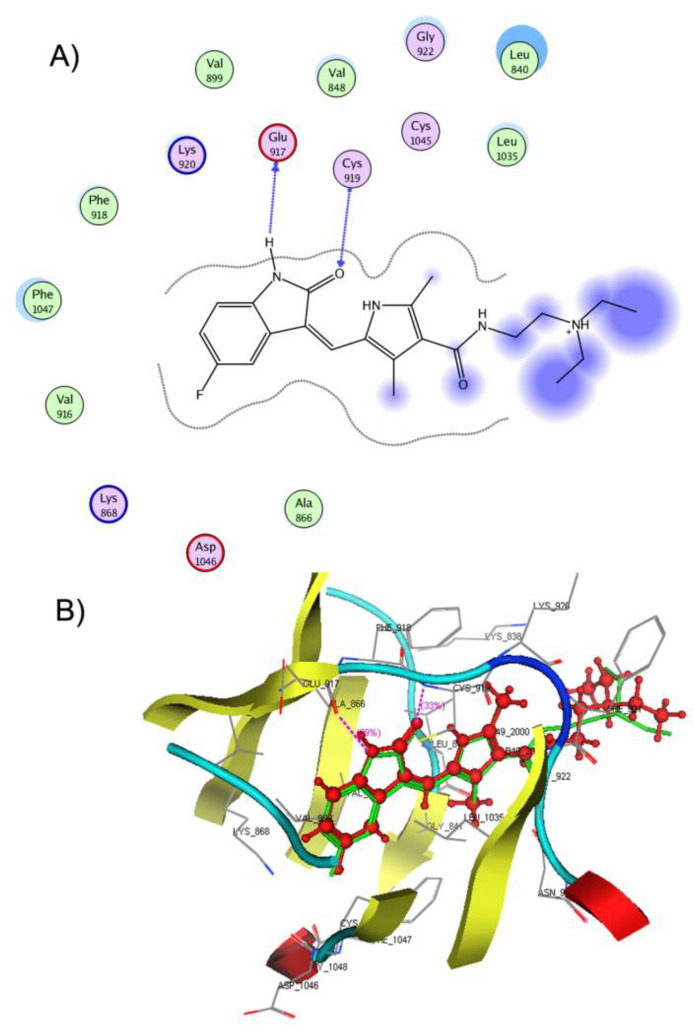
Figure 8.
(A) 2D representation of the ligand interactions and (B) 3D representation of the re-docked sunitinib in the active site of VEGFR-2 (PDB ID: 4AGD). The native ligand is represented by green stick and the re-docked ligand is depicted as red ball and stick. Atom coloration (gray, carbon; blue, nitrogen; red, oxygen; yellow, sulfur; white, hydrogen; brown, bromine; green, fluorine).
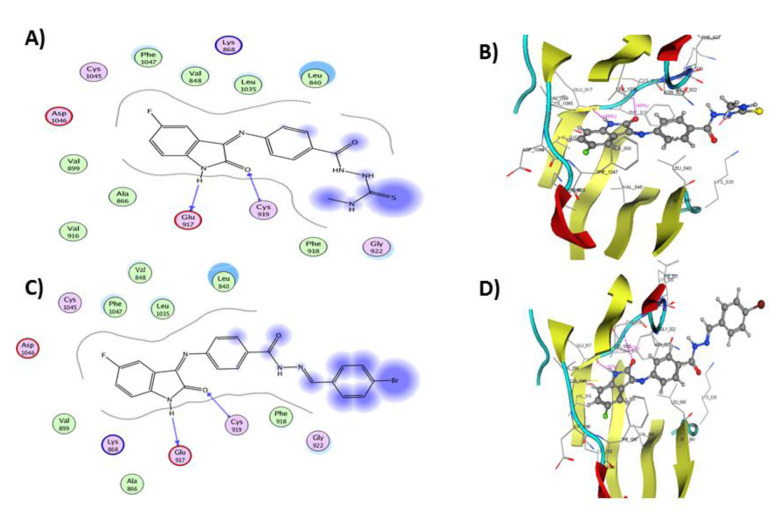
The docking simulation of 5b in the ATP binding pocket of VEGFR-2 (Figure 9) showed that 5b was fitted in the hinge region and the docking score was −19.0360 kcal/mol. Compound 5b formed two hydrogen bond interactions by the NH and CO groups of its indolin-2-one scaffold with Glu917 and Cys919 hinge residues, respectively. Additionally, several hydrophobic and Van der Waals interactions were observed with Leu840, Val848, Ala866, Val916, Phe918, Leu1035, and Phe 1047.
Figure 9.
(A) 2D representation of the ligand interactions and (B) 3D representation of the docking of 5b in the active site of VEGFR-2 (PDB ID: 4AGD), (C) 2D representation of the ligand interactions and (D) 3D representation of the docking of 10g in the active site of VEGFR-2 (PDB ID: 4AGD). Ligands 5b and 10g are depicted in ball and stick. Amino acid residues are represented in line. Atom coloration (gray, carbon; blue, nitrogen; red, oxygen; yellow, sulfur; white, hydrogen; brown, bromine; green, fluorine).
The docking pose of compound 10g into the ATP binding domain of VEGFR-2 (Figure 9) showed two hydrogen bond interactions: isatin NH of the ligand with Glu917 and isatin C=O with Cys919. Additionally, several Van der Waals and hydrophobic contacts were observed with Val848, Ala866, Val916, Phe918, Leu1035, and Phe 1047. These interactions were reflected in the docking score of 10g (−21.2368 kcal/mol) and supported the obtained in vitro activity results.
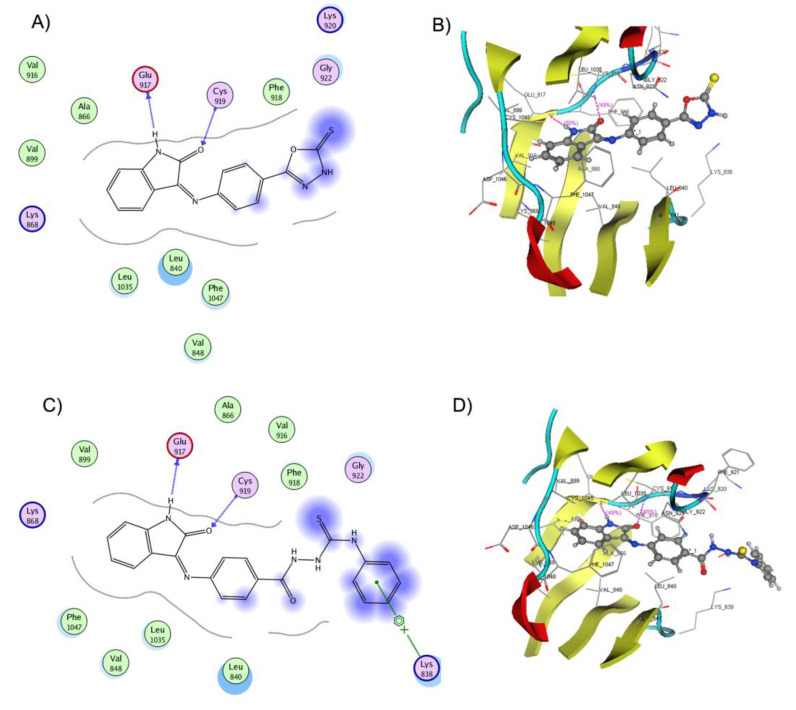
The binding mode of compound 15a (docking score −17.2599 kcal/mol) inside VEGFR-2 is shown in Figure 10. It displayed two hydrogen bond interactions by the NH and C=O groups of its isatin ring with Glu917 and Cys919 residues, respectively. Furthermore, a dipole interaction was observed between the S atom of the oxadiazole ring and Lys920 residue. Moreover, various Van der Waals and hydrophobic contacts were observed with Leu840, Val848, Ala866, Val916, Phe918, Leu1035, and Phe 1047.
Figure 10.
(A) 2D representation of the ligand interactions and (B) 3D representation of the docking of 15a in the active site of VEGFR-2 (PDB ID: 4AGD), (C) 2D representation of the ligand interactions and (D) 3D representation of the docking of 17a in the active site of VEGFR-2 (PDB ID: 4AGD). Ligands 15a and 17a are depicted in ball and stick. Amino acid residues are represented in line. Atom coloration (gray, carbon; blue, nitrogen; red, oxygen; yellow, sulfur; white, hydrogen; brown, bromine; green, fluorine).
Considering the binding mode of compound 17a into the active pocket of VEGFR-2 (docking score −20.1061 kcal/mol), it formed two hydrogen bonds by the NH and C=O groups of its indolinone nucleus with Glu917 and Cys919, respectively (Figure 10). Additionally, an arene cation interaction was noticed between the terminal benzene ring and Lys838 residue. In addition, many hydrophobic and Van der Waals interactions were regarded with Leu840, Val848, Ala866, Val899, Val916, Phe918, Leu1035, and Phe 1047. The molecular docking study revealed the ability of the tested compound to interact with the key amino acids in the ATP active site of VEGFR-2. The binding interactions and energy binding scores are in agreement with the obtained experimental in vitro anticancer and VEGFR-2 kinase inhibition activities for these compounds.
2.3.2. Physicochemical Properties and Drug-Likeness Predictions
The estimation of physicochemical parameters and drug-likeness profiles for the designed molecules is a crucial step for the development process of new drug candidates. Several active drug candidates were rejected during clinical trials because of their poor pharmacokinetics. Accordingly, in silico tools were developed to predict physicochemical parameters and drug-likeness profiles at an early stage of drug design to save time, reduce cost, and increase chances of success [71]. The oral bioavailability of the target compounds 3a,b–17a,b were evaluated based on drug-likeness parameters of the Lipinski and the Veber rules.
The Lipinski rule of five is considered one of the highly influential rules in pharmacokinetic drug design. The Lipinski rule states that orally active molecules should have three of the following properties: molecular weight (MW) is less than 500, octanol/water partition coefficient (iLOG P) is less than five, and H-bond acceptors (HAs) and donors (HDs) are not more than ten and five, respectively [72]. The Veber rule states that compounds with a total polar surface area (TPSA) less than 140 Å2 and rotatable bonds (RBs) less than 10 were found to have better oral bioavailability [73].
The physicochemical properties of VEGFR-2 inhibitors 3a,b–17a,b were calculated using the SwissADME online tool [74], and the key parameters are summarized in Table 7.
Table 7.
Lipinski and Veber Parameters of the synthesized compounds 3a,b–17a,b.
| Code | Lipinski Rule of Five | Veber Rule | ||||||
|---|---|---|---|---|---|---|---|---|
| MW (g/mol) | iLOG P | HDs | HAs | Violations | RBs | TPSA (Å2) | Violations | |
| 3a | 266.25 | 1.63 | 2 | 4 | 0 | 2 | 78.76 | 0 |
| 3b | 284.24 | 1.39 | 2 | 5 | 0 | 2 | 78.76 | 0 |
| 5a | 353.40 | 1.96 | 4 | 3 | 0 | 6 | 126.71 | 0 |
| 5b | 371.39 | 2.40 | 4 | 4 | 0 | 6 | 126.71 | 0 |
| 7a | 294.30 | 2.52 | 1 | 4 | 0 | 4 | 67.76 | 0 |
| 7b | 312.30 | 2.43 | 1 | 5 | 0 | 4 | 67.76 | 0 |
| 8a | 280.28 | 1.28 | 3 | 4 | 0 | 3 | 96.58 | 0 |
| 8b | 298.27 | 0.78 | 3 | 5 | 0 | 3 | 96.58 | 0 |
| 10a | 413.39 | 1.70 | 2 | 6 | 0 | 6 | 128.74 | 0 |
| 10b | 462.83 | 2.73 | 2 | 4 | 0 | 5 | 82.92 | 0 |
| 10c | 413.39 | 1.52 | 2 | 6 | 0 | 6 | 128.74 | 0 |
| 10d | 382.41 | 2.74 | 2 | 4 | 0 | 5 | 82.92 | 0 |
| 10e | 398.41 | 2.11 | 2 | 5 | 0 | 6 | 92.15 | 0 |
| 10f | 429.45 | 2.27 | 2 | 5 | 0 | 6 | 86.16 | 0 |
| 10g | 465.27 | 2.93 | 2 | 5 | 0 | 5 | 82.92 | 0 |
| 12a | 410.38 | 1.89 | 2 | 5 | 0 | 4 | 107.94 | 0 |
| 12b | 428.37 | 2.09 | 2 | 6 | 0 | 4 | 107.94 | 0 |
| 14a | 392.41 | 2.48 | 2 | 6 | 0 | 8 | 109.22 | 0 |
| 14b | 410.40 | 2.59 | 2 | 7 | 0 | 8 | 109.22 | 0 |
| 15a | 322.34 | 1.99 | 2 | 4 | 0 | 2 | 115.37 | 0 |
| 15b | 346.33 | 2.10 | 2 | 5 | 0 | 2 | 115.37 | 0 |
| 17a | 415.47 | 2.39 | 4 | 3 | 0 | 7 | 126.71 | 0 |
| 17b | 433.46 | 2.81 | 4 | 4 | 0 | 7 | 126.71 | 0 |
The physicochemical properties data revealed that the MWs of the designed compounds were in the range from 266.25 to 465.27 g/mol, with 3a as the lowest and 10g as the highest, respectively. The iLOG P values varied between 0.78 and 2.93, with 8b as lowest and 10g as highest, respectively. The HDs and HAs of the molecules have been found in the range from 2 to 4 and 3 to 7, respectively. The number of RBs of the tested compounds was between 2 and 8. Compounds 7a,b had the lowest TPSA with a value of 67.76 Å2, while compounds 10a and 10c had the highest TPSA with a value of 128.74 Å2. The obtained data demonstrated that all the target compounds 3a,b–17a,b were in accordance with the Lipinski and Veber rules.
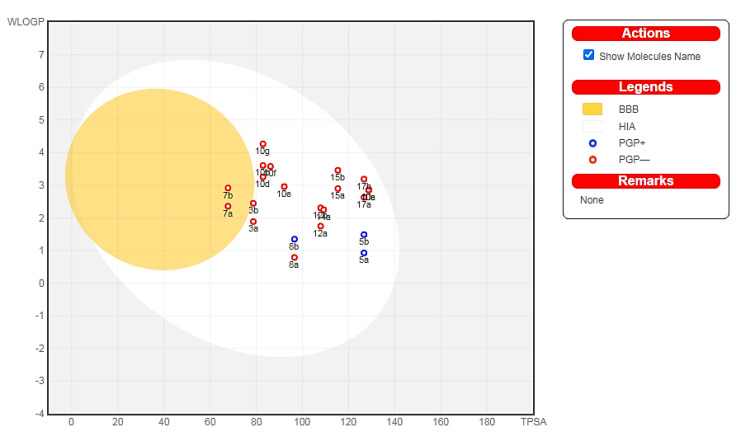
Additionally, two important pharmacokinetic parameters, passive GI absorption (HIA) and brain accessibility (BBB), of compounds 3a,b–17a,b were predicted and graphically depicted by the Brain Or IntestinaL EstimateD permeation method (BOILED-Egg). The BOILED-Egg method predicts pharmacokinetic parameters by computing the lipophilicity (WLOG P) against the TPSA, as shown in Figure 11. The molecules located in the white region have a high probability of passive gastrointestinal absorption, while the molecules located in the yellow region (yolk) have a high probability of brain access. Moreover, the blue points represent molecules predicted to be actively effluxed by the P-glycoprotein (PGP+), while the red points represent molecules that are not predicted to be actively effluxed by the P-glycoprotein (PGP−) [75].
Figure 11.
BOILED-Egg depiction of the designed molecules.
The results revealed that all the tested derivatives were suggested to have good intestinal absorption and bioavailability according to the Lipinski and Veber estimated parameters. Compounds 7a,b were predicted to display high blood-brain permeability and gastrointestinal absorption. All the tested compounds, with the exception of 5a,b and 8b, were predicted to be not subjected to active efflux by P-glycoprotein (red dot). Compounds 5b, 10e, 10g, 15a, and 17a could be potential candidates for drug discovery because of their potent cytotoxic activity and their good drug-likeness and pharmacokinetic properties.
3. Materials and Methods
3.1. Chemistry
All commercially purchased chemicals were used as received. Experiments were conducted under nitrogen or argon. Thin-layer chromatography (TLC) was performed on Merck TLC silica gel 60 F254 pre-coated aluminum sheets. Melting points were determined using Stuart electrothermal melting point apparatus and were uncorrected. Infrared spectra were recorded as KBr discs on a Thermo-912AO683 FT-IR spectrophotometer and were reported in frequency of absorption (cm−1). NMR spectra were recorded on a Bruker 400 MHz NMR spectrometer (Supplementary Figures S3–S19). Chemical shift (δ) values were reported in parts per million (ppm) relative to internal standard tetramethylsilane at δ 0.00 ppm. Coupling constants (J) were reported in Hertz. The spin multiplicity was abbreviated as: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet. Elemental analysis was performed on AnalysenSysteme GmbH-D-63452-HANAU apparatus and a Perkin Elmer 2400 CHN elemental analyzer. The values of elemental analyses were within ± 0.4% of the theoretical values.
3.1.1. Synthesis of 4-((5-Substituted-2-oxoindolin-3-ylidene)amino)benzoic acid 3a,b
Acids 3a,b were prepared according to the general procedure described in [55].
3.1.2. General Procedure for Synthesis of 2-(4-((5-Substituted-2-oxoindolin-3-ylidene)amino)benzoyl)-N-methylhydrazinecarbothioamide 5a,b
To a mixture of benzoic acid, derivatives 3a,b (3 mmol), EDC (3.3 mmol), 4-methylthiosemicarbazide 4 (3.6 mmol) and HOBt (3.9 mmol) in DMF (20 mL) were added to triethylamine (9 mmol) at 0 °C. The reaction mixture was stirred at 0 °C for one hour. Next, the ice bath was removed, and the reaction mixture was stirred at room temperature overnight. The reaction was quenched by addition of water (40 mL). The precipitated solid was filtered and purified by flash column chromatography using (hexanes/EtOAc, 3:1) as the mobile phase system to give N-methylthiosemicarbazises 5a,b.
N-Methyl-2-(4-((2-oxoindolin-3-ylidene)amino)benzoyl)hydrazinecarbothioamide 5a
Yellow powder (yield, 78 %); mp (°C) 249-251; FT-IR (KBr, cm−1): 3235 (NH), 1691(C=O), 1669 (C=O), 1587 (C=N); 1H NMR (400 MHz, DMSO) δ 12.61 (s, 2H), 11.22 (s, 1H), 9.25 (d, J = 4.5 Hz, 1H), 7.75–7.52 (m, 2H), 7.48–7.20 (m, 2H), 7.23–7.02 (m, 2H), 6.94 (d, J = 7.8 Hz, 2H), 3.10 (d, J = 4.6 Hz, 3H); 13C NMR (101 MHz, DMSO) δ 178.12, 163.10, 142.71, 132.05, 131.96, 131.65, 131.58, 122.80, 121.15, 121.04, 120.80, 120.56, 120.48, 111.54, 31.84; ESI-MS m/z: 354.3 [M+H]+; Anal. Calcd. for C17H15N5O2S: C, 57.78; H, 4.28; N, 19.82. Found: C, 57.62; H, 4.48; N, 19.95.
2-(4-((5-Fluoro-2-oxoindolin-3-ylidene)amino)benzoyl)-N-methylhydrazinecarbothioamide 5b
Orange powder (yield, 82 %); mp (°C) 252-254; FT-IR (KBr, cm−1): 3284 (NH), 1689 (C=O), 1654 (C=O), 1588 (CH=N); 1H NMR (400 MHz, DMSO) δ 12.48 (s, 1H), 11.21 (s, 2H), 9.31 (d, J = 4.5 Hz, 1H), 7.63–7.28 (m, 2H), 7.35–6.98 (m, 3H), 7.10–6.75 (m, 2H), 3.10 (d, J = 4.6 Hz, 3H); 13C NMR (101 MHz, DMSO) δ 178.09, 163.17, 159.87, 157.51, 138.94, 131.38 (d, J = 3.4 Hz), 121.95, 121.86, 117.88, 117.64, 112.62, 112.54, 108.16, 107.91, 31.78; ESI-MS m/z: 372.2 [M+H]+; Anal. Calcd. for C17H14FN5O2S: C, 54.89; H, 3.80; N, 18.86. Found: C, 55.05; H, 3.94; N, 18.97.
3.1.3. Synthesis of Ethyl 4-((5-Substituted-2-oxoindolin-3-ylidene)amino)benzoate 7a,b
Esters 7a,b were prepared according to the general procedure described in [76].
3.1.4. Synthesis of 4-((5-Substituted-2-oxoindolin-3-ylidene)amino)benzohydrazide 8a,b
Hydrazides 8a,b were prepared according to the general procedure described in [61].
3.1.5. General Procedure for Synthesis of N′-(4-Substitutedbenzylidene)-4-((5-substituted-2-oxoindolin-3-ylidene)amino)benzohydrazide 10a–g
Benzaldehyde derivatives 9a–g (1.8 mmol) were added to a solution of hydrazides 8a,b (1.8 mmol) in absolute ethanol (10 mL). Next, 0.3 mL of glacial acetic acid was added, and the reaction mixture was heated under reflux for 6 h. After cooling, the precipitate was collected, and the crude precipitate was recrystallized from methanol to give hydrazones 10a–g.
N′-(4-Nitrobenzylidene)-4-((2-oxoindolin-3-ylidene)amino)benzohydrazide 10a
Orange powder (yield, 94%, E: Z = 5.3: 1); mp (°C) 220–222; FT-IR (KBr, cm−1): 3412 (NH), 1653 (C=O), 1636 (C=O), 1588 (C=N); 1H NMR (400 MHz, DMSO) δ 11.78 (s, 1H), 10.56–9.57 (d, J = 14.2 Hz, 1H), 8.49 (s, 1H), 8.29 (d, J = 8.8 Hz, 2H), 7.95 (d, J = 8.7 Hz, 2H), 7.71 (d, J = 8.6 Hz, 2H), 7.04–6.87 (m, 2H), 6.63 (d, J = 8.6 Hz, 2H), 5.91-5.86 (m, 2H); 13C NMR (101 MHz, DMSO) δ 164.67, 163.25, 156.87, 153.08, 147.99, 141.61, 139.11, 134.62, 130.17, 128.12, 126.69, 124.53, 122.73, 121.83, 119.46, 117.93, 113.09, 110.44; ESI-MS m/z: 414.5 [M+H]+; Anal. Calcd. for C22H15N5O4: C, 63.92; H, 3.66; N, 16.94. Found: C, 63.84; H, 3.89; N, 16.79.
N′-(4-Chlorobenzylidene)-4-((2-oxoindolin-3-ylidene)amino)benzohydrazide 10b
White powder (yield, 89%, E: Z = 8.9: 1); mp (°C) 237–239; FT-IR (KBr, cm−1): 3398 (NH), 1666 (C=O), 1646 (C=O), 1596 (C=N); 1H NMR (400 MHz, DMSO) δ 11.55 (s, 1H), 8.40 (s, 1H), 7.82–7.51 (m, 3H), 7.51 (d, J = 8.5 Hz, 2H), 6.9–7.2 (m, 4H), 6.62 (d, J = 8.6 Hz, 2H), 5.90–5.72 (m, 2H); 13C NMR (101 MHz, DMSO) δ 163.35, 152.86, 144.95, 144.76, 134.52, 134.46, 134.25, 134.16, 129.93, 129.72, 129.52, 129.36, 129.20, 129.12, 128.91, 119.87, 119.80, 119.72, 113.19, 113.08; ESI-MS m/z: 403.9 [M+H]+; Anal. Calcd. for C22H15ClN4O2: C, 65.59; H, 3.75; N, 13.91. Found: C, 65.65; H, 3.97; N, 14.11.
N′-(3-Nitrobenzylidene)-4-((2-oxoindolin-3-ylidene)amino)benzohydrazide 10c
Orange powder (yield, 93%, E: Z = 8:1); mp (°C) 200–201; FT-IR (KBr, cm−1): 3312 (NH), 1689 (C=O), 1664 (C=O), 1604 (C=N); 1H NMR (400 MHz, DMSO) δ 10.94 (s, 1H), 10.77–10.49 (m, 1H), 8.82–8.67 (m, 2H), 8.51–8.36 (m, 2H), 7.98–7.26 (m, 4H), 7.15 (t, J = 7.6 Hz, 1H), 7.14–6.73 (m, 4H); 13C NMR (101 MHz, DMSO) δ 164.72, 157.36, 150.49, 148.79, 145.73, 139.10, 135.47, 134.40, 131.29, 129.20, 126.58, 124.02, 122.93, 121.82, 117.92, 116.59, 111.48, 110.43; ESI-MS m/z: 414.3 [M+H]+; Anal. Calcd. for C22H15N5O4: C, 63.92; H, 3.66; N, 16.94. Found: C, 63.96; H, 3.71; N, 17.14.
N′-(4-Methylbenzylidene)-4-((2-oxoindolin-3-ylidene)amino)benzohydrazide 10d
Reddish orange powder (yield, 84%, E: Z = 6.7:1); mp (°C) 234–236; FT-IR (KBr, cm−1): 3358 (NH), 1661 (C=O), 1636 (C=O), 1581 (C=N); 1H NMR (400 MHz, DMSO) δ 11.03 (s, 1H), 10.71–9.56 (m, 1H), 8.67 (s, 1H), 7.99–7.87 (m, 1H), 7.77 (d, J = 8.0 Hz, 2H), 7.65–7.46 (m, 1H), 7.42–7.33 (m, 3H), 7.16 (td, J = 7.7, 1.0 Hz, 2H), 7.02–6.91 (m, 3H), 2.37 (s, 3H); 13C NMR (101 MHz, DMSO) δ 165.08, 163.26, 161.73, 145.67, 141.80, 139.11, 134.88, 131.67, 130.31, 129.98, 128.79, 127.51, 126.69, 122.73, 121.83, 117.93, 116.27, 110.44, 21.63; ESI-MS m/z: 383.7 [M+H]+; Anal. Calcd. for C23H18N4O2: C, 72.24; H, 4.74; N, 14.65. Found: C, 72.33; H, 4.81; N, 14.55.
N′-(4-Methoxybenzylidene)-4-((2-oxoindolin-3-ylidene)amino)benzohydrazide 10e
Orange powder (yield, 87%, E: Z = 5.7:1); mp (°C) 239–240; FT-IR (KBr, cm−1): 3339 (NH), 1683(C=O), 1659 (C=O), 1611 (C=N); 1H NMR (400 MHz, DMSO) δ 11.02 (s, 1H), 10.86 (s, 1H), 8.65 (d, J = 3.8 Hz, 2H), 7.98 (d, J = 8.8 Hz, 1H), 7.82 (d, J = 8.7 Hz, 2H), 7.43–7.38 (m, 1H), 7.15 (d, J = 8.8 Hz, 2H), 7.05 (dd, J = 10.3, 6.9 Hz, 4H), 6.93 (t, J = 7.3 Hz, 1H), 3.84 (s, 3H); 13C NMR (101 MHz, DMSO) δ 165.25, 163.12, 162.15, 160.99, 151.23, 145.68, 134.90, 133.96, 131.53, 130.46, 129.37, 128.68, 127.04, 123.04, 117.13, 115.27, 114.87, 111.60, 55.86; ESI-MS m/z: 399.2 [M+H]+; Anal. Calcd. for C23H18N4O3: C, 69.34; H, 4.55; N, 14.06. Found: C, 69.39; H, 4.47; N, 14.31.
N′-(4-(Dimethylamino)benzylidene)-4-((5-fluoro-2-oxoindolin-3-ylidene)amino)benzohydrazide 10f
Reddish orange powder (yield, 91%, E: Z = 8:1); mp (°C) 202–204; FT-IR (KBr, cm−1): 3296 (NH), 1678 (C=O), 1643 (C=O), 1595 (C=N); 1H NMR (400 MHz, DMSO) δ 11.59 (s, 1H), 10.92 (m, 1H), 8.69 (s, 1H), 8.08–7.87 (m, 1H), 7.84 (d, J = 8.9 Hz, 1H), 7.44–7.30 (m, 1H), 7.30–7.18 (m, 1H), 7.18–7.04 (m, 1H), 7.07–6.93 (m, 1H), 6.95–6.77 (m, 4H), 6.81–6.68 (m, 1H), 3.07 (s, 3H), 2.92 (s, 3H); 13C NMR (101 MHz, DMSO) δ 167.11, 165.70, 163.55, 157.41, 153.86, 151.02, 141.13, 135.96 (d, J = 3.1 Hz), 132.01, 127.93, 124.92, 123.26, 120.61, 119.67, 114.67, 112.76, 112.36, 105.39, 40.45, 40.14; ESI-MS m/z: 430.6 [M+H]+; Anal. Calcd. for C24H20FN5O2: C, 67.12; H, 4.69; N, 16.31. Found: C, 67.37; H, 4.86; N, 16.52.
N′-(4-Bromobenzylidene)-4-((5-fluoro-2-oxoindolin-3-ylidene)amino)benzohydrazide 10g
Orange powder (yield, 90%, E: Z = 7.3:1); mp (°C) 227–229; FT-IR (KBr, cm−1): 3312 (NH), 1675(C=O), 1657 (C=O), 1586 (C=N); 1H NMR (400 MHz, DMSO) δ 10.94 (s, 1H), 10.75–10.57 (m, 1H), 8.69 (s, 1H), 7.95 (d, J = 8.2 Hz, 3H), 7.83 (d, J = 6.8 Hz, 3H), 7.64 (d, J = 8.1 Hz, 1H), 7.32 (t, J = 8.9 Hz, 1H), 7.22–6.71 (m, 3H); 13C NMR (101 MHz, DMSO) δ 164.91, 163.46, 161.20, 159.13, 156.77, 150.87, 142.01, 132.88, 131.26 (d, J = 1.2 Hz), 126.57, 120.93, 120.70, 117.19, 117.10, 115.95, 115.70, 113.47, 112.52; ESI-MS m/z: 467.3 [M+H]+; Anal. Calcd. for C22H14FBrN4O2: C, 56.79; H, 3.03; N, 12.04. Found: C, 56.84; H, 3.11; N, 12.27.
3.1.6. General Procedure for Synthesis of N-(1,3-dioxoisoindolin-2-yl)-4-((5-substituted-2-oxoindolin-3-ylidene)amino)benzamide 12a,b
A mixture of hydrazide derivatives 8a,b (2.1 mmol) and phthalic anhydride 11 (2.1 mmol) in 10 mL glacial acetic acid was stirred under sonication for 4 h at 50 °C. Next, the reaction mixture was cooled, and the reaction was quenched by addition of water (30 mL). The separated precipitate was collected and purified by flash column chromatography using (hexane/EtOAc, 4:1) as an eluent to afford cyclic imides 12a,b.
N-(1,3-Dioxoisoindolin-2-yl)-4-((2-oxoindolin-3-ylidene)amino)benzamide 12a
Orange powder (yield, 75%); mp (°C) 187–189; FT-IR (KBr, cm−1): 3365 (NH), 1696 (C=O), 1663 (C=O), 1596 (C=N); 1H NMR (400 MHz, DMSO) δ 12.84 (s, 1H), 11.25 (s, 1H), 8.43–7.85 (m, 1H), 7.86–7.61 (m, 2H), 7.70–7.46 (m, 2H), 7.47–7.25 (m, 2H), 7.25–6.77 (m, 5H); 13C NMR (101 MHz, DMSO) δ 173.14, 167.66, 162.99, 162.94, 142.74, 132.54, 131.83, 131.27, 127.49, 122.98, 121.81, 120.89, 120.31, 120.27, 117.91, 111.64, 111.54, 110.42; ESI-MS m/z: 411.2 [M+H]+; Anal. Calcd. for C23H14N4O4: C, 67.31; H, 3.44; N, 13.65. Found: C, 67.29; H, 3.70; N, 13.76.
N-(1,3-Dioxoisoindolin-2-yl)-4-((5-fluoro-2-oxoindolin-3-ylidene)amino)benzamide 12b
Orange powder (yield, 81%); mp (°C) 225–227; FT-IR (KBr, cm−1): 3342 (NH), 1689 (C=O), 1670 (C=O), 1612 (C=N); 1H NMR (400 MHz, DMSO) δ 10.73–10.65 (m, 1H), 9.81 (d, J = 14.8 Hz, 1H), 8.06–7.53 (m, 2H), 7.51–7.26 (m, 1H), 7.30–7.07 (m, 2H), 7.13–6.86 (m, 3H), 6.89–6.38 (m, 3H); 13C NMR (101 MHz, DMSO) δ 167.72, 163.47, 159.71, 157.37, 139.11, 135.23 (d, J = 1.4 Hz), 126.19, 126.16, 124.15, 124.06, 113.71, 113.47, 112.90, 111.30, 111.22, 105.08, 105.01, 104.75; ESI-MS m/z: 429.5 [M+H]+; Anal. Calcd. for C23H13FN4O4: C, 64.49; H, 3.06; N, 13.08. Found: C, 64.21; H, 2.89; N, 13.22.
3.1.7. General Procedure for Synthesis of Ethyl 3-(2-(4-((5-Substituted-2-oxoindolin-3-ylidene)amino)benzoyl)hydrazono)butanoate 14a,b
Ethyl acetoacetate 13 (2 mmol) was added to a solution of hydrazides 8a,b (2 mmol) in absolute ethanol (10 mL). After the addition of 0.2 mL of glacial acetic acid, the reaction mixture was heated under reflux for 4 h. After cooling, the formed precipitate was collected, and the crude solid was recrystallized from methanol to afford hydrazones 14a,b.
Ethyl 3-(2-(4-((2-oxoindolin-3-ylidene)amino)benzoyl)hydrazono)butanoate 14a
Yellow powder (yield, 86%); mp (°C) 206–208; FT-IR (KBr, cm−1) 3321 (NH), 1685(C=O), 1673 (C=O), 1657 (C=O), 1614 (C=N); 1H NMR (400 MHz, DMSO) δ 14.19 (s, 1H), 11.03 (s, 1H), 7.48 (d, J = 7.2 Hz, 1H), 7.43–7.24 (m, 2H), 7.16 (t, J = 7.6 Hz, 1H), 7.12–6.96 (m, 2H), 6.95–6.81 (m, 2H), 5.07 (s, 2H), 4.13 (q, J = 6.8 Hz, 2H), 2.26 (s, 3H), 1.23 (t, J = 7.0 Hz, 3H); 13C NMR (101 MHz, DMSO) δ 167.61, 163.25, 162.26, 156.36, 141.77, 139.11, 132.36, 130.51, 127.50, 122.41, 121.10, 120.09, 117.92, 111.10, 110.43, 91.95, 59.49, 18.50, 14.85; ESI-MS m/z: 393.3 [M+H]+; Anal. Calcd. for C21H20N4O4: C, 64.28; H, 5.14; N, 14.28. Found: C, 63.96; H, 4.95; N, 13.95.
Ethyl 3-(2-(4-((5-Fluoro-2-oxoindolin-3-ylidene)amino)benzoyl)hydrazono)butanoate 14b
Yellow powder (yield, 88%); mp (°C) 213–215; FT-IR (KBr, cm−1): 3354 (NH), 1698(C=O), 1675 (C=O), 1648 (C=O), 1598 (C=N); 1H NMR (400 MHz, DMSO) δ 14.20 (s, 1H), 11.04 (s, 1H), 7.37–7.13 (m, 2H), 7.16–7.13 (m, 2H), 7.11–6.73 (m, 3H), 5.11 (s, 2H), 4.13 (q, J = 7.1 Hz, 2H), 2.25 (s, 3H), 1.23 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, DMSO) δ 167.56, 162.41, 159.77, 157.41, 156.20, 137.92, 131.83, 131.80 (d, J = 3.3 Hz), 122.43, 122.34, 116.80, 116.56, 112.02, 107.16, 106.91, 92.77, 59.60, 18.38, 14.82; ESI-MS m/z: 411.2 [M+H]+; Anal. Calcd. for C21H19FN4O4: C, 61.46; H, 4.67; N, 13.65. Found: C, 61.75; H, 4.88; N, 13.82.
3.1.8. General Procedure for Synthesis of 5-Substituted-3-((4-(5-thioxo-4,5-dihydro-1,3,4-oxadiazol-2-yl)phenyl)imino)indolin-2-one 15a,b
Potassium hydroxide (2 mmol) was added to a solution of hydrazides 8a,b (2 mmol) and carbon disulfide (4 mmol) in 20 mL of absolute ethanol. The reaction mixture was refluxed for 12 h. Next, the reaction mixture was cooled, and the solvent was evaporated. The residue was dissolved in water and acidified with 10% HCl. The formed precipitate was collected and recrystallized from ethanol to generate the corresponding oxadiazole derivatives 15a,b.
3-((4-(5-Thioxo-4,5-dihydro-1,3,4-oxadiazol-2-yl)phenyl)imino)indolin-2-one 15a
Yellow powder (yield, 86%); mp (°C) 219–220; FT-IR (KBr, cm−1): 3252 (NH), 1679(C=O), 1612 (C=N); 1H NMR (400 MHz, DMSO) δ 10.71 (s, 1H), 10.54 (s, 1H), 7.37 (d, J = 7.5 Hz, 2H), 7.30–7.10 (m, 2H), 7.08–6.77 (m, 4H); 13C NMR (101 MHz, DMSO) δ 186.12, 163.25, 160.80, 139.11, 127.52, 127.36, 126.67, 122.88, 122.73, 121.94, 121.84, 118.09, 117.93, 110.44; ESI-MS m/z: 323.5 [M+H]+; Anal. Calcd. for C16H10N4O2S: C, 59.62; H, 3.13; N, 17.38. Found: C, 59.68; H, 3.28; N, 17.51.
5-Fluoro-3-((4-(5-thioxo-4,5-dihydro-1,3,4-oxadiazol-2-yl)phenyl)imino)indolin-2-one 15b
Yellow powder (yield, 82%); mp (°C) 231–233; FT-IR (KBr, cm−1): 3263 (NH), 1668 (C=O), 1607 (C=N); 1H NMR (400 MHz, DMSO) δ 10.67 (d, J = 15.0 Hz, 2H), 7.17–7.14 (m, 1H), 7.08–6.91 (m, 3H), 6.91–6.73 (m, 3H); 13C NMR (101 MHz, DMSO) δ 186.84, 159.70, 157.36, 135.20 (d, J = 1.2 Hz), 135.19, 126.19, 126.16, 124.06, 113.69, 113.46, 111.29, 111.20, 105.01, 104.76; ESI-MS m/z: 341.3 [M+H]+; Anal. Calcd. for C16H9FN4O2S: C, 56.47; H, 2.67; N, 16.46. Found: C, 56.51; H, 2.70; N, 16.53.
3.1.9. General Procedures for Synthesis of 2-(4-((5-Substituted-2-oxoindolin-3-ylidene)amino)benzoyl)-N-substitutedhydrazinecarbothioamide 17a,b
Phenyl isothiocyanate 16 (1.9 mmol) was added to a solution of hydrazides 8a,b (1.9 mmol) in absolute ethanol (10 mL). The reaction mixture was refluxed for 8 h. After cooling, the precipitated solid was collected and purified by flash column chromatography using (hexanes/EtOAc, 3:2) as an eluent to yield N-phenylthiosemicarbazides 17a,b.
2-(4-((2-Oxoindolin-3-ylidene)amino)benzoyl)-N-phenylhydrazinecarbothioamide 17a
Yellow powder (yield, 93%); mp (°C) 211–213; FT-IR (KBr, cm−1): 3342 (NH), 1686 (C=O), 1654 (C=O), 1602 (C=N); 1H NMR (400 MHz, DMSO) δ 10.72 (s, 1H), 10.64–10.48 (m, 1H), 10.05 (d, J = 17.9 Hz), 9.57 (d, J = 13.4 Hz, 1H), 7.95 (d, J = 7.5 Hz, 1H), 7.89–7.60 (m, 2H), 7.56–7.30 (m, 6H), 7.17 (t, J = 7.3 Hz, 2H), 6.99 (t, J = 7.4 Hz, 1H), 6.88 (d, J = 7.6 Hz, 1H); 13C NMR (101 MHz, DMSO) δ 179.93, 163.27, 139.68, 139.12, 129.03, 128.92, 128.45, 127.54, 126.71, 126.17, 125.18, 124.92, 124.17, 122.74, 122.50, 121.86, 117.95, 110.46; ESI-MS m/z: 415.6 [M+H]+; Anal. Calcd. for C22H17N5O2S: C, 63.60; H, 4.12; N, 16.86. Found: C, 63.52; H, 4.29; N, 16.63.
2-(4-((5-Fluoro-2-oxoindolin-3-ylidene)amino)benzoyl)-N-phenylhydrazinecarbothioamide 17b
Orange powder (yield, 92%); mp (°C) 203–205; FT-IR (KBr, cm−1): 3339 (NH), 1694 (C=O), 1663 (C=O), 1593 (C=N); 1H NMR (400 MHz, DMSO) δ 12.70 (s, 1H), 11.14 (s, 1H), 10.68 (d, J = 15.0 Hz, 1H), 9.82 (d, J = 14.9 Hz, 1H), 7.67–7.61 (m, 2H), 7.45 (t, J = 7.7 Hz, 1H), 7.38–7.29 (m, 3H), 7.25–7.13 (m, 3H), 7.02–6.92 (m, 2H), 6.87–6.81 (m, 1H); 13C NMR (101 MHz, DMSO) δ 176.78, 163.48, 159.71, 157.37, 139.23, 138.78, 135.21 (d, J = 1.2 Hz), 128.94, 126.72, 126.20, 126.16, 124.06, 113.70, 113.46, 111.29, 111.21, 105.01, 104.76; ESI-MS m/z: 435.3 [M+H]+; Anal. Calcd. for C22H16FN5O2S: C, 60.96; H, 3.72; N, 16.16. Found: C, 60.69; H, 3.56; N, 16.33.
3.2. Biological Investigation
3.2.1. In Vitro Cytotoxic Activity Assay
The anticancer activities of the target compounds 3a,b–17a,b were quantitatively assessed using the MTT protocol against the MCF-7 and HepG2 cell lines, as described in the Supplementary Materials.
3.2.2. In Vitro VEGFR-2 Kinase Inhibitory Assay
The most potent anti-proliferative derivatives, 5b, 10e, 10g, 15a, and 17a, were tested for their VEGFR-2 kinase inhibition using a VEGFR2 (KDR) Kinase Assay Kit (Enzyme-Linked Immunosorbent Assay), as described in the Supplementary Materials.
3.2.3. Cell Cycle Analysis
The liver HepG2 cells were treated with 1.13 μM of 17a, and the effect on the cell cycle distribution was evaluated by flow cytometric analysis, as described in the Supplementary Materials.
3.2.4. Apoptosis Analysis
The apoptotic ability of compound 17a to the HepG2 cells was assessed using Annexin V-FITC/PI dual staining by flow cytometric analysis, as shown in the Supplementary Materials.
3.2.5. Caspase-3 and -9 Expression Assay
The expression of caspase-3 and -9 in the liver HepG2 cells treated with 1.13 μM of 17a was determined using quantitative real-time PCR analysis, as presented in the Supplementary Materials.
3.2.6. BAX and Bcl-2 Expression Assay
The expression of apoptotic BAX and antiapoptotic Bcl-2 proteins in the liver HepG2 cells treated with 1.13 μM of 17a was determined using Western blot analysis, as described in the Supplementary Materials.
3.3. Molecular Modeling Studies
3.3.1. Molecular Docking Study
The molecular docking simulation studies were performed on a Dell precision T3600 workstation with Intel Xeon® CPU-1650.0 @ 3.20 GHz with Windows 7 operating system using Molecular Operating Environment software (MOE 2020.09) [70]; the docking protocol is described in the Supplementary Materials.
3.3.2. Physicochemical Properties and Drug Likeness Predictions
The physicochemical parameters of drug-likeness and the pharmacokinetic properties, such as gastrointestinal absorption, brain permeability, and P-glycoprotein efflux, were estimated using the SwissADME online tool (http://www.swissadme.ch/ access date 5 March 2022) for calculations [74].
4. Conclusions
In this investigation, a series of indoline-2-one derivatives 3a,b–17a,b were designed and synthesized based on the pharmacophore model of the reported VEGFR-2 inhibitors. The results showed that compounds 5b, 10e, 10g, 15a, and 17a exhibited the most potent anticancer activities with IC50 values from 0.74–4.62 µM against the breast MCF-7 cancer cell line (sunitinib IC50 = 4.77 µM) and from 1.13–8.80 µM against the liver HepG2 cancer cell line (sunitinib IC50 = 2.23 µM). Furthermore, these members displayed potent VEGFR-2 kinase inhibitory activities (IC50 from 0.078–0.358 µM) compared with sunitinib (IC50 = 0.139 µM). Moreover, the cell cycle of HepG2 cells was blocked by compound 17a at the S phase, and the total apoptosis was enhanced by 24-fold. In addition, compound 17a increased the expression of caspase-3, -9, and BAX by 6.88, 3.703, and 2.69-fold, and decreased the expression of Bcl-2 by 1.88 fold. The docking simulations demonstrated that the prepared compounds have similar interactions and orientation to sunitinib inside VEGFR-2. Finally, the molecular modeling studies showed that all the target compounds are not violating Lipinski and Veber rules for oral bioavailability and have promising drug-likeness behavior.
Acknowledgments
This work was funded by the Deanship of Scientific Research at Jouf University Under Grant Number (DSR2022-RG-0147).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph15111416/s1, Figure S1. Simple linear regression for the correlation between cytotoxicity against HepG2 cell line and VEGFR-2 inhibition; Figure S2. Simple linear regression for the correlation between cytotoxicity against MCF-7 cell line and VEGFR-2 inhibition; Figures S3–S19. 1H and 13 C NMR spectra of compound 5–17.
Author Contributions
Conceptualization, A.M.H., H.M.A.-R. and M.K.A.E.-G.; methodology, A.M.H., H.M.A.-R., M.A.A. and M.K.A.E.-G.; validation, A.M.H., H.M.A.-R., S.N.A.B. and M.K.A.E.-G. formal analysis, A.M.H., H.M.A.-R. and M.K.A.E.-G.; investigation, S.N.A.B., A.M. and M.E. Data curation, A.M.H. and M.K.A.E.-G. writing—original draft preparation, A.M.H., H.M.A.-R. and M.K.A.E.-G. writing—review and editing, M.A.A., S.N.A.B., A.M., M.E., project administration, H.M.A.-R. and M.A.A., S.N.A.B., A.M.H., M.E., funding acquisition, M.A.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available within article and Supplementary Materials.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding Statement
Deanship of Scientific Research at Jouf University Under Grant Number (DSR2022-RG-0147).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pors K., Goldberg F.W., Leamon C.P., Rigby A.C., Snyder S.A., Falconer R.A. The changing landscape of cancer drug discovery: A challenge to the medicinal chemist of tomorrow. Drug Discov. Today. 2009;14:1045–1050. doi: 10.1016/j.drudis.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Barreca M., Spanò V., Raimondi M.V., Bivacqua R., Giuffrida S., Montalbano A., Cavalli A., Bertoni F., Barraja P. GPCR Inhibition in Treating Lymphoma. ACS Med. Chem. Lett. 2022;13:358–364. doi: 10.1021/acsmedchemlett.1c00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duró C., Jernei T., Szekeres K.J., Láng G.G., Oláh-Szabó R., Bősze S., Szabó I., Hudecz F., Csámpai A. Synthesis and SAR Analysis of Novel 4-Hydroxytamoxifen Analogues Based on Their Cytotoxic Activity and Electron-Donor Character. Molecules. 2022;27:6758. doi: 10.3390/molecules27196758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finiuk N., Kryshchyshyn-Dylevych A., Holota S., Klyuchivska O., Kozytskiy A., Karpenko O., Manko N., Ivasechko I., Stoika R., Lesyk R. Novel hybrid pyrrolidinedione-thiazolidinones as potential anticancer agents: Synthesis and biological evaluation. Eur. J. Med. Chem. 2022;238:114422. doi: 10.1016/j.ejmech.2022.114422. [DOI] [PubMed] [Google Scholar]
- 5.Hu L., Fan M., Shi S., Song X., Wang F., He H., Qi B. Dual target inhibitors based on EGFR: Promising anticancer agents for the treatment of cancers (2017-) Eur. J. Med. Chem. 2022;227:113963. doi: 10.1016/j.ejmech.2021.113963. [DOI] [PubMed] [Google Scholar]
- 6.Luo S., Dang X., Wang J., Yuan C., Hu Y., Lei S., Zhang Y., Lu D., Jiang F., Fu L. Biological evaluation of mitochondria targeting small molecules as potent anticancer drugs. Bioorganic Chem. 2021;114:105055. doi: 10.1016/j.bioorg.2021.105055. [DOI] [PubMed] [Google Scholar]
- 7.Parmar D., Apte M. Angiopoietin inhibitors: A review on targeting tumor angiogenesis. Eur. J. Pharmacol. 2021;899:174021. doi: 10.1016/j.ejphar.2021.174021. [DOI] [PubMed] [Google Scholar]
- 8.De Palma M., Biziato D., Petrova T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer. 2017;17:457–474. doi: 10.1038/nrc.2017.51. [DOI] [PubMed] [Google Scholar]
- 9.Lugano R., Ramachandran M., Dimberg A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020;77:1745–1770. doi: 10.1007/s00018-019-03351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young H.S., Summers A., Brenchley P., Griffiths C. Mechanisms of disease: Angiogenesis, vascular endothelial growth factor (VEGF) and psoriasis1 1Disclosure not available at press time. J. Am. Acad. Dermatol. 2004;50:P146. doi: 10.1016/j.jaad.2003.10.510. [DOI] [Google Scholar]
- 11.Kirk S., Frank J.A., Karlik S. Angiogenesis in multiple sclerosis: Is it good, bad or an epiphenomenon? J. Neurol. Sci. 2004;217:125–130. doi: 10.1016/j.jns.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Dewanjee S., Das S., Das A.K., Bhattacharjee N., Dihingia A., Dua T.K., Kalita J., Manna P. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur. J. Pharmacol. 2018;833:472–523. doi: 10.1016/j.ejphar.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y., Wu H., Deng R. Angiogenesis as a potential treatment strategy for rheumatoid arthritis. Eur. J. Pharmacol. 2021;910:174500. doi: 10.1016/j.ejphar.2021.174500. [DOI] [PubMed] [Google Scholar]
- 14.Rajabi M., Mousa S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines. 2017;5:34. doi: 10.3390/biomedicines5020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuazo-Gaztelu I., Casanovas O. Unraveling the Role of Angiogenesis in Cancer Ecosystems. Front. Oncol. 2018;8:248. doi: 10.3389/fonc.2018.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoff P.M., Machado K.K. Role of angiogenesis in the pathogenesis of cancer. Cancer Treat. Rev. 2012;38:825–833. doi: 10.1016/j.ctrv.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Shibata M., Kono K., Takenoshita S. Inhibiting VEGF in cancer immunotherapy. Clin. Immunol. Commun. 2022;2:12–16. doi: 10.1016/j.clicom.2021.12.003. [DOI] [Google Scholar]
- 18.Turner N., Grose R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 19.Östman A., Heldin C.H. Advances in Cancer Research. Volume 97. Academic Press; Cambridge, MA, USA: 2007. PDGF Receptors as Targets in Tumor Treatment; pp. 247–274. [DOI] [PubMed] [Google Scholar]
- 20.Massagué J. TGFβ in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cathcart J., Pulkoski-Gross A., Cao J. Targeting matrix metalloproteinases in cancer: Bringing new life to old ideas. Genes Dis. 2015;2:26–34. doi: 10.1016/j.gendis.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yewale C., Baradia D., Vhora I., Patil S., Misra A. Epidermal growth factor receptor targeting in cancer: A review of trends and strategies. Biomaterials. 2013;34:8690–8707. doi: 10.1016/j.biomaterials.2013.07.100. [DOI] [PubMed] [Google Scholar]
- 23.Hu B., Cheng S.-Y. Angiopoietin-2: Development of inhibitors for cancer therapy. Curr. Oncol. Rep. 2009;11:111–116. doi: 10.1007/s11912-009-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamidi H., Ivaska J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer. 2018;18:533–548. doi: 10.1038/s41568-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verheul H.M.W., Pinedo H.M. The Role of Vascular Endothelial Growth Factor (VEGF) in Tumor Angiogenesis and Early Clinical Development of VEGFReceptor Kinase Inhibitors. Clin. Breast Cancer. 2000;1:S80–S84. doi: 10.3816/CBC.2000.s.015. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 27.Cébe-Suarez S., Zehnder-Fjällman A., Ballmer-Hofer K. The role of VEGF receptors in angiogenesis; complex partnerships. Cell. Mol. Life Sci. 2006;63:601. doi: 10.1007/s00018-005-5426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X.-R., Wang S., Li W.-B., Xu K.-Y., Qiao X.-P., Jing X.-L., Wang Z.-X., Yang C.-J., Chen S.-W. Design, synthesis and biological evaluation of novel 2-(4-(1H-indazol-6-yl)-1H-pyrazol-1-yl)acetamide derivatives as potent VEGFR-2 inhibitors. Eur. J. Med. Chem. 2021;213:113192. doi: 10.1016/j.ejmech.2021.113192. [DOI] [PubMed] [Google Scholar]
- 29.Fan H., Wei D., Zheng K., Qin X., Yang L., Yang Y., Duan Y., Xu Y., Hu L. Discovery of Dioxino[2,3-f]quinazoline derivative VEGFR-2 inhibitors exerting significant antipro-liferative activity in HUVECs and mice. Eur. J. Med. Chem. 2019;175:349–356. doi: 10.1016/j.ejmech.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Alsaif N.A., Taghour M.S., Alanazi M.M., Obaidullah A.J., Alanazi W.A., Alasmari A., Albassam H., Dahab M.A., Mahdy H.A. Identification of new [1,2,4]triazolo[4,3-a]quinoxalines as potent VEGFR-2 tyrosine kinase inhibitors: Design, synthesis, anticancer evaluation, and in silico studies. Bioorganic Med. Chem. 2021;46:116384. doi: 10.1016/j.bmc.2021.116384. [DOI] [PubMed] [Google Scholar]
- 31.An D., Banerjee S., Lee J.-M. Recent advancements of antiangiogenic combination therapies in ovarian cancer. Cancer Treat. Rev. 2021;98:102224. doi: 10.1016/j.ctrv.2021.102224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Capdevila J., Robinson B., Sherman S.I., Jarzab B., Lin C.C., Vaisman F., Hoff A.O., Hitre E., Bowles D.W., Sen S., et al. LBA67 Cabozantinib versus placebo in patients with radioiodine-refractory differentiated thyroid cancer who have progressed after prior VEGFR-targeted therapy: Updated results from the phase III COSMIC-311 trial and prespecified subgroup analyses by prior therapy. Ann. Oncol. 2021;32:S1343. doi: 10.1016/j.annonc.2021.08.2148. [DOI] [Google Scholar]
- 33.Han M.İ., Atalay P., Tunç C.Ü., Ünal G., Dayan S., Aydın Ö., Küçükgüzel Ş.G. Design and synthesis of novel (S)-Naproxen hydrazide-hydrazones as potent VEGFR-2 inhibitors and their evaluation in vitro/in vivo breast cancer models. Bioorganic Med. Chem. 2021;37:116097. doi: 10.1016/j.bmc.2021.116097. [DOI] [PubMed] [Google Scholar]
- 34.Mahmoud H.K., Farghaly T.A., Abdulwahab H.G., Al-Qurashi N.T., Shaaban M.R. Novel 2-indolinone thiazole hybrids as sunitinib analogues: Design, synthesis, and potent VEGFR-2 inhibition with potential anti-renal cancer activity. Eur. J. Med. Chem. 2020;208:112752. doi: 10.1016/j.ejmech.2020.112752. [DOI] [PubMed] [Google Scholar]
- 35.Fernández M., Semela D., Bruix J., Colle I., Pinzani M., Bosch J. Angiogenesis in liver disease. J. Hepatol. 2009;50:604–620. doi: 10.1016/j.jhep.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Shojaei F. Anti-angiogenesis therapy in cancer: Current challenges and future perspectives. Cancer Lett. 2012;320:130–137. doi: 10.1016/j.canlet.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y., Gray N.S. Rational design of inhibitors that bind to inactive kinase conformations. Nat. Chem. Biol. 2006;2:358–364. doi: 10.1038/nchembio799. [DOI] [PubMed] [Google Scholar]
- 38.Modi S.J., Kulkarni V.M. Vascular Endothelial Growth Factor Receptor (VEGFR-2)/KDR Inhibitors: Medicinal Chemistry Perspective. Med. Drug Discov. 2019;2:100009. doi: 10.1016/j.medidd.2019.100009. [DOI] [Google Scholar]
- 39.Huang L., Huang Z., Bai Z., Xie R., Sun L., Lin K. Development and strategies of VEGFR-2/KDR inhibitors. Future Med. Chem. 2012;4:1839–1852. doi: 10.4155/fmc.12.121. [DOI] [PubMed] [Google Scholar]
- 40.Blanc J., Geney R., Menet C. Type II Kinase Inhibitors: An Opportunity in Cancer for Rational Design. Anti-Cancer Agents Med. Chem. 2013;13:731–747. doi: 10.2174/1871520611313050008. [DOI] [PubMed] [Google Scholar]
- 41.Zhang C., Tan C., Ding H., Xin T., Jiang Y. Selective VEGFR Inhibitors for Anticancer Therapeutics in Clinical Use and Clinical Trials. Curr. Pharm. Des. 2012;18:2921–2935. doi: 10.2174/138161212800672732. [DOI] [PubMed] [Google Scholar]
- 42.Xie Q.-Q., Xie H.-Z., Ren J.-X., Li L.-L., Yang S.-Y. Pharmacophore modeling studies of type I and type II kinase inhibitors of Tie2. J. Mol. Graph. Model. 2009;27:751–758. doi: 10.1016/j.jmgm.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Lee K., Jeong K.-W., Lee Y., Song J.Y., Kim M.S., Lee G.S., Kim Y. Pharmacophore modeling and virtual screening studies for new VEGFR-2 kinase inhibitors. Eur. J. Med. Chem. 2010;45:5420–5427. doi: 10.1016/j.ejmech.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Bajusz D., Ferenczy G.G., Keseru M.G. Structure-based Virtual Screening Approaches in Kinase-directed Drug Discovery. Curr. Top. Med. Chem. 2017;17:2235–2259. doi: 10.2174/1568026617666170224121313. [DOI] [PubMed] [Google Scholar]
- 45.Sangande F., Julianti E., Tjahjono D.H. Ligand-Based Pharmacophore Modeling, Molecular Docking, and Molecular Dynamic Studies of Dual Tyrosine Kinase Inhibitor of EGFR and VEGFR2. Int. J. Mol. Sci. 2020;21:7779. doi: 10.3390/ijms21207779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Machado V.A., Peixoto D., Costa R., Froufe H.J.C., Calhelha R.C., Abreu R.M.V., Ferreira I.C.F.R., Soares R., Queiroz M.-J.R.P. Synthesis, antiangiogenesis evaluation and molecular docking studies of 1-aryl-3-[(thieno[3,2-b]pyridin-7-ylthio)phenyl]ureas: Discovery of a new substitution pattern for type II VEGFR-2 Tyr kinase inhibitors. Bioorganic Med. Chem. 2015;23:6497–6509. doi: 10.1016/j.bmc.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z., Wang N., Han S., Wang D., Mo S., Yu L., Huang H., Tsui K., Shen J., Chen J. Dietary Compound Isoliquiritigenin Inhibits Breast Cancer Neoangiogenesis via VEGF/VEGFR-2 Signaling Pathway. PLoS ONE. 2013;8:e68566. doi: 10.1371/journal.pone.0068566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeidan M.A., Mostafa A.S., Gomaa R.M., Abou-zeid L.A., El-Mesery M., El-Sayed M.A.A., Selim K.B. Design, synthesis and docking study of novel picolinamide derivatives as anticancer agents and VEGFR-2 inhibitors. Eur. J. Med. Chem. 2019;168:315–329. doi: 10.1016/j.ejmech.2019.02.050. [DOI] [PubMed] [Google Scholar]
- 49.Abdel-Mohsen H.T., Omar M.A., El Kerdawy A.M., Mahmoud A.E.E., Ali M.M., El Diwani H.I. Novel potent substituted 4-amino-2-thiopyrimidines as dual VEGFR-2 and BRAF kinase inhibitors. Eur. J. Med. Chem. 2019;179:707–722. doi: 10.1016/j.ejmech.2019.06.063. [DOI] [PubMed] [Google Scholar]
- 50.El-Sherief H.A.M., Youssif B.G.M., Abbas Bukhari S.N., Abdelazeem A.H., Abdel-Aziz M., Abdel-Rahman H.M. Synthesis, anticancer activity and molecular modeling studies of 1,2,4-triazole derivatives as EGFR inhibitors. Eur. J. Med. Chem. 2018;156:774–789. doi: 10.1016/j.ejmech.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 51.Amin N.H., Elsaadi M.T., Zaki S.S., Abdel-Rahman H.M. Design, synthesis and molecular modeling studies of 2-styrylquinazoline derivatives as EGFR inhibitors and apoptosis inducers. Bioorganic Chem. 2020;105:104358. doi: 10.1016/j.bioorg.2020.104358. [DOI] [PubMed] [Google Scholar]
- 52.Hisham M., Hassan H.A., Gomaa H.A.M., Youssif B.G.M., Hayallah A.M., Abdel-Aziz M. Structure-based design, synthesis and antiproliferative action of new quinazoline-4-one/chalcone hybrids as EGFR inhibitors. J. Mol. Struct. 2022;1254:132422. doi: 10.1016/j.molstruc.2022.132422. [DOI] [Google Scholar]
- 53.Chaudhari P., Bari S., Surana S., Shirkhedkar A., Wakode S., Shelar S., Racharla S., Ugale V., Ghodke M. Logical synthetic strategies and structure-activity relationship of indolin-2-one hybrids as small molecule anticancer agents: An overview. J. Mol. Struct. 2022;1247:131280. doi: 10.1016/j.molstruc.2021.131280. [DOI] [Google Scholar]
- 54.Yousefian M., Ghodsi R. Structure–activity relationship studies of indolin-2-one derivatives as vascular endothelial growth factor receptor inhibitors and anticancer agents. Arch. Pharm. 2020;353:2000022. doi: 10.1002/ardp.202000022. [DOI] [PubMed] [Google Scholar]
- 55.Farooq M., Al Marhoon Z.M., Taha N.A., Baabbad A.A., Al-Wadaan M.A., El-Faham A. Synthesis of Novel Class of <i>N</i>-Alkyl-isatin-3-iminobenzoic Acid Derivatives and Their Biological Activity in Zebrafish Embryos and Human Cancer Cell Lines. Biol. Pharm. Bull. 2018;41:350–359. doi: 10.1248/bpb.b17-00674. [DOI] [PubMed] [Google Scholar]
- 56.Angibaud P., Saha A.K., Bourdrez X., End D.W., Freyne E., Lezouret P., Mannens G., Mevellec L., Meyer C., Pilatte I., et al. 4-Methyl-1,2,4-triazol-3-yl heterocycle as an alternative to the 1-methylimidazol-5-yl moiety in the Farnesyltransferase inhibitor ZARNESTRA™. Bioorganic Med. Chem. Lett. 2003;13:4361–4364. doi: 10.1016/j.bmcl.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 57.Luczywo A., González L.G., Aguiar A.C.C., Oliveira de Souza J., Souza G.E., Oliva G., Aguilar L.F., Casal J.J., Guido R.V.C., Asís S.E., et al. 3-aryl-indolinones derivatives as antiplasmodial agents: Synthesis, biological activity and computational analysis. Nat. Prod. Res. 2021;36:3887–3893. doi: 10.1080/14786419.2021.1895149. [DOI] [PubMed] [Google Scholar]
- 58.Afsah E.M., Elmorsy S.S., Abdelmageed S.M., Zaki Z.E. Synthesis of some new hydrazide-hydrazones related to isatin and its Mannich and Schiff bases. Z. Für Nat. B. 2016;71:1147–1157. doi: 10.1515/znb-2016-0130. [DOI] [Google Scholar]
- 59.Milovanović V.M., Petrović Z.D., Novaković S., Bogdanović G.A., Petrović V.P., Simijonović D. Green synthesis of benzamide-dioxoisoindoline derivatives and assessment of their radical scavenging activity—Experimental and theoretical approach. Tetrahedron. 2020;76:131456. doi: 10.1016/j.tet.2020.131456. [DOI] [Google Scholar]
- 60.Chaitanya M.V.S.R.K., Dubey P.K. A simple and efficient synthesis of novel naphthyridine-1-H-pyrazole-4-carboxylic acid esters/carbaldehydes using Vilsmeier-Haack reagent. Heterocycl. Commun. 2013;19:49–55. doi: 10.1515/hc-2012-0097. [DOI] [Google Scholar]
- 61.Arief M.M.H., Aly A.A., Khalil A.A., Mohamed H.I. Utility of 4-(isatin-3-ylideneamino)benzohydrazide in the synthesis of bioactive N-heterocyclic compounds. J. Chem. Pharm. Res. 2014;6:327–335. [Google Scholar]
- 62.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 63.Sharma K., Suresh P.S., Mullangi R., Srinivas N.R. Quantitation of VEGFR2 (vascular endothelial growth factor receptor) inhibitors—Review of assay methodologies and perspectives. Biomed. Chromatogr. 2015;29:803–834. doi: 10.1002/bmc.3370. [DOI] [PubMed] [Google Scholar]
- 64.Pietenpol J.A., Stewart Z.A. Cell cycle checkpoint signaling: Cell cycle arrest versus apoptosis. Toxicology. 2002;181–182:475–481. doi: 10.1016/S0300-483X(02)00460-2. [DOI] [PubMed] [Google Scholar]
- 65.Bahman A.A., Abaza M.S.I., Khoushiash S.I., Al-Attiyah R.J. Sequence-dependent effect of sorafenib in combination with natural phenolic compounds on hepatic cancer cells and the possible mechanism of action. Int. J. Mol. Med. 2018;42:1695–1715. doi: 10.3892/ijmm.2018.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dadsena S., King L.E., García-Sáez A.J. Apoptosis regulation at the mitochondria membrane level. Biochim. Biophys. Acta (BBA) Biomembr. 2021;1863:183716. doi: 10.1016/j.bbamem.2021.183716. [DOI] [PubMed] [Google Scholar]
- 67.Perlman H., Zhang X., Chen M.W., Walsh K., Buttyan R. An elevated bax/bcl-2 ratio corresponds with the onset of prostate epithelial cell apoptosis. Cell Death Differ. 1999;6:48–54. doi: 10.1038/sj.cdd.4400453. [DOI] [PubMed] [Google Scholar]
- 68.Abdolmaleki A., Shiri F., Ghasemi J.B. Chapter 11—Use of Molecular Docking as a Decision-Making Tool in Drug Discovery. In: Coumar M.S., editor. Molecular Docking for Computer-Aided Drug Design. Academic Press; Cambridge, MA, USA: 2021. pp. 229–243. [Google Scholar]
- 69.McTigue M., Murray B.W., Chen J.H., Deng Y.-L., Solowiej J., Kania R.S. Molecular conformations, interactions, and properties associated with drug efficiency and clinical performance among VEGFR TK inhibitors. Proc. Natl. Acad. Sci. USA. 2012;109:18281–18289. doi: 10.1073/pnas.1207759109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Molecular Operating Environment (MOE) 2020.09. Chemical Computing Group ULC; Montreal, QC, Canada: 2022. [Google Scholar]
- 71.Gleeson M.P., Hersey A., Montanari D., Overington J. Probing the links between in vitro potency, ADMET and physicochemical parameters. Nat. Rev. Drug Discov. 2011;10:197–208. doi: 10.1038/nrd3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001;46:3–26. doi: 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 73.Veber D.F., Johnson S.R., Cheng H.-Y., Smith B.R., Ward K.W., Kopple K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002;45:2615–2623. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 74.Daina A., Michielin O., Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Daina A., Zoete V. A BOILED-Egg to Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem. 2016;11:1117–1121. doi: 10.1002/cmdc.201600182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aldokheily M.E., Mekky A.H., Rahem S.A. Preparation of Polymer Nanoparticles and Doping by Some Schiff Base Compounds by using Microemulsion Systems. Chem. Methodol. 2022;6:494–500. doi: 10.22034/chemm.2022.334829.1459. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available within article and Supplementary Materials.