Abstract
There is a large heterogeneity among patients presenting with cardiogenic shock (CS). It is crucial to better apprehend this heterogeneity in order to adapt treatments and improve prognoses in these severe patients. Notably, the presence (or absence) of a pre-existing history of chronic heart failure (CHF) at time of CS onset may be a significant part of this heterogeneity, and data focusing on this aspect are lacking. We aimed to compare CS patients with new-onset HF to those with worsening CHF in the multicenter FRENSHOCK registry. Altogether, 772 CS patients were prospectively included: 433 with a previous history of CHF and 339 without. Worsening CHF patients were older (68 +/− 13.4 vs. 62.7 +/− 16.2, p < 0.001) and had a greater burden of extra-cardiac comorbidities. At admission, acute myocardial infarction was predominantly observed in the new-onset HF group (49.9% vs. 25.6%, p < 0.001). When focusing on hemodynamic parameters, worsening CHF patients showed more congestion and higher ventricular filling pressures. Worsening CHF patients experienced higher in-hospital all-cause mortality (31.3% vs. 24.2%, p = 0.029). Our results emphasize the great heterogeneity of the patients presenting with CS. Worsening CHF patients had higher risk profiles, and this translated to a 30% increase in in-hospital all-cause mortality. The heterogeneity of this population prompts us to better determine the phenotype of CS patients to adapt their management.
Keywords: cardiogenic shock, heart failure, myocardial infarction, mechanical circulatory support
1. Introduction
Acute heart failure (AHF) remains a major cause of hospitalization and is burdened by a consequent high morbidity, high mortality, and a high rate of re-hospitalizations [1]. Cardiogenic shock (CS) represents the most severe form of AHF and is still associated with a very high rate of mortality (up to 60–70% at 1 year) [2,3,4]. It accounts for 2 to 5% of AHF patients [5,6], and its prevalence in intensive care units (ICU) can reach 14–16% of the total admissions [5,7].
Notably, there is a large diversity among patients presenting with CS, which may influence the hemodynamic profile, patient management, and prognosis. The different causes (underlying cardiopathy) that lead to CS is a significant part of this heterogeneity. Historically, the predominant cause of CS was ischemic (up to 80%), and between 5 and 10% of myocardial infarctions (MIs) were complicated by CS [8,9,10,11,12]. However, there is now a trend towards a decrease in ischemic etiologies [13,14]. For example, non-ischemic causes were recently identified as predominant causes of CS in the FRENSHOCK registry [13]. Furthermore, beyond the manifold etiologies, the presence or absence of a pre-existing documented cardiopathy and history of heart failure (HF) at the time of CS occurrence may further accentuate the heterogeneity of these patients and therefore their optimal management and prognosis. Indeed, CS may be the initial presentation of HF, occurring on a previously “healthy” heart or revealing an unknown cardiopathy. Alternatively, it may be the umpteenth destabilization of a well-documented chronic cardiopathy that is a fortiori treated and followed.
So far, there are very few data about the potential differences in patient profiles, shock management, and prognosis among these two distinct populations. In this post hoc analysis of the largest European registry of CS (the FRENSHOCK multicenter registry), we thus attempted to compare CS patients according to the presence or absence of a pre-existing history of HF at the time of admission.
2. Methods
2.1. Patient Population
The design of the FRENSCHOCK registry has already been published in detail [13,15]. Briefly, FRENSHOCK is a prospective multicenter registry conducted over a 6-month period in France between April and October 2016 (NCT02703038).
All patients (n = 772) presenting with CS were included from 52 recruiting centers if they met at least one criterion of each of the following: (i) hemodynamic criteria, defined as a low systolic blood pressure (SBP) < 90 mmHg and/or the need for maintenance with vasopressors/inotropes and/or a low cardiac index < 2.2 L/min/m2; (ii) left and/or right heart pressure elevation, defined by clinical signs, radiology, blood tests, echocardiography, or signs of invasive hemodynamic overload; and (iii) signs of organ malperfusion, which could be either clinical and/or biological. Patients could be included regardless of CS etiology and whether CS was present at admission or developed during their in-hospital course. Exclusion criteria were refusal to participate, shock from a non-cardiac origin, and post-cardiotomy CS.
The study was conducted in accordance with the guidelines for good clinical practice and French law. Written consent was obtained from all the patients. The data recorded and their handling and storage were reviewed and approved by the CCTIRS (French Health Research Data Processing Advisory Committee) (n° 15.897) and the CNIL (French Data Protection Agency) (n° DR-2016-109).
2.2. Objectives and Outcomes
The main objective of the present post hoc analysis was to assess the impact of a pre-existing history of HF on CS management and clinical outcomes. We thus compared patients with a previous diagnosis of HF (n = 433) to those without (n = 339). New-onset HF was defined as the patient having no previous history of HF after an interrogation of the patient, the patient’s family, and/or the usual practitioner. Patients with a history of HF were classified as having worsening chronic HF (CHF).
The primary outcome was in-hospital all-cause mortality.
2.3. Funding and Data Property
This registry emanated from the French Society of Cardiology and was endorsed by its Emergency and Acute Cardiovascular Care Working Group. The study was sponsored by the “Fédération Francaise de Cardiologie” and was funded by unrestricted grants from Daiichi-Sankyo and Maquet SAS.
2.4. Statistical Analysis
Continuous variables are reported as means +/− standard deviation (SD) or medians and interquartile ranges (IQR) as appropriate. Discrete variables are described as absolute numbers and percentages.
Groups (pre-existing history of CHF or not) were compared using Student’s t test or ANOVA for continuous variables and χ2 or Fisher’s exact test for discrete variables as appropriate.
To determine independent predictors of in-hospital all-cause mortality, a multivariate logistic regression analysis was used. Variables included in the final models were selected ad hoc based on their physiological relevance and potential to be associated with outcomes. Included variables were: group (pre-existing history of CHF or not), age, gender, systolic blood pressure (SBP) at admission, heart rate at admission, left ventricle ejection fraction (LVEF) at admission, presentation as MI, creatinine level at admission, arterial lactate level at admission, hemoglobin level at admission, and prothrombin time (PT) level at admission. Odds ratios (oRs) are presented with 95% confidence intervals (cIs).
Statistical analyses were performed using IBM SPSS 23.0 (IBM SPSS Inc., Chicago, IL, USA). For all analyses, two-sided p values < 0.05 were considered significant.
3. Results
3.1. Baseline Characteristics
Baseline characteristics of the 772 included patients are summarized in Table 1. The mean age was 65.7 (+/− 14.9) and 71.5% of the patients were men. Histories of diabetes mellitus were present in 28% of the patients. History of renal failure and history of chronic obstructive pulmonary disease (COPD) or chronic respiratory failure were present in 29.9% and 7% of the patients, respectively.
Table 1.
Baseline characteristics.
| Overall Population |
New-Onset HF |
Worsening CHF |
p Value | |
|---|---|---|---|---|
| n = 772 | n = 339 | n = 433 | ||
| Sex male | 552 (71.5) | 222 (65.5) | 330 (76.2) | 0.001 |
| Age (years) | 65.7 (14.9) | 62.7 (16.2) | 68 (13.4) | <0.001 |
| Diabetes | 217 (28) | 73 (21.5) | 144 (33.3) | <0.001 |
| Hypertension | 364 (47.2) | 136 (40.1) | 228 (52.7) | 0.001 |
| Active smokers | 206 (27.8) | 112 (34.8) | 94 (22.4) | <0.001 |
| Hypercholesterolemia | 277 (35.9) | 76 (22.4) | 201 (46.4) | <0.001 |
| BMI (Kg/m2) | 25.8 (5.5) | 25.8 (5.3) | 25.8 (25.7) | 0.995 |
| COPD or chronic respiratory failure | 54 (7) | 16 (4.7) | 38 (8.8) | 0.029 |
| Renal failure (Cl < 60 mL/min) | 164 (21.2) | 24 (7.1) | 140 (32.3) | <0.001 |
| Peripheral artery disease | 114 (14.8) | 24 (7.1) | 90 (20.7) | <0.001 |
| Treatment before admission | ||||
| Aspirin | 288 (37.4) | 88 (26.1) | 200 (46.2) | <0.001 |
| P2Y12 inhibitors | 126 (16.3) | 53 (15.7) | 73 (16.8) | 0.671 |
| Anticoagulants | 221 (28.7) | 34 (10.1) | 187 (43.2) | <0.001 |
| VKA | 165 (21.4) | 24 (7.1) | 141 (32.6) | <0.001 |
| DOA | 56 (7.2) | 10 (3) | 46 (10.6) | <0.001 |
| Betablockers | 316 (41) | 67 (19.9) | 249 (57.5) | <0.001 |
| ACEI or ARB | 292 (37.9) | 70 (20.8) | 222 (51.3) | <0.001 |
| Sacubitril/Valsartan | 18 (2.3) | 0 | 18 (4.4) | 0.001 |
| Aldosterone antagonists | 108 (14) | 9 (2.7) | 99 (22.9) | <0.001 |
| Loop diuretics | 376 (48.7) | 65 (19.3) | 311 (71.8) | <0.001 |
| Statins | 286 (37) | 66 (19.6) | 220 (50.8) | <0.001 |
In this table, continuous variables are expressed as mean +/− SD. HF = heart failure, CHF = chronic heart failure, BMI = body mass index, SD = standard deviation, COPD = chronic obstructive pulmonary disease, VKA = vitamin K antagonist, DOA = direct oral anticoagulant, ACEI or ARB = angiotensin-converting enzyme inhibitor and an angiotensin receptor blocker.
Among the 433 patients admitted with worsening CHF (56.1%), ischemic etiology accounted for 231 patients (29.9%). Others’ causes were represented by idiopathic dilated cardiomyopathy (n = 89), left-sided valvular stenosis or incompetence (n = 81), hypertrophic cardiomyopathy (n = 13), toxic causes (n = 35), and others (n = 69). Altogether, 85 (11%) patients had mixed causes.
Patients with worsening CHF were older than those with new-onset HF (68 +/− 13.4 vs. 62.7 +/− 16.2, p < 0.001), and there were more men (76.2% vs. 65.5%, p = 0.001). In addition, more of these patients had a history of diabetes (33.3% vs. 21.5%, p < 0.001), renal failure (32.3% vs. 7.1%, p < 0.001), and respiratory failure (8.8% vs. 4.7%, p = 0.029).
3.2. Initial Presentation
These data are depicted in Table 2. In the overall population, the mean heart rate was 96 +/− 30 beats per minute and the mean SBP was 101 +/− 25 mmHg. Only 51.9% of the patient presented with sinus rhythm. The mean LVEF was 26.3% +/− 13.4, and 72.2% of the patients had a LVEF below 30%. Acute MI was identified as the precipitating factor of CS occurrence in 36.3% of the cases and severe sepsis in 11.9%.
Table 2.
Clinical presentation at admission.
| Overall Population |
New-Onset HF |
Worsening CHF |
p Value | |
|---|---|---|---|---|
| n = 772 | n = 339 | n = 433 | ||
| Heart Rate (bpm) | 95.6 (29.6) | 100 (31.1) | 91 (27.7) | <0.001 |
| Systolic blood pressure (mmHg) | 101 (25.2) | 102 (25.6) | 100 (24.8) | 0.301 |
| Diastolic blood pressure (mmHg) | 63.2 (17.4) | 65.2 (18.2) | 61 (16.6) | 0.005 |
| Mean blood pressure (mmHg) | 74.9 (18.3) | 76.6 (18.8) | 73.6 (17.9) | 0.023 |
| Sinus rhythm | 399 (51.9) | 199 (59.4) | 200 (46.2) | <0.001 |
| LVEF (%) | 26.3 (13.4) | 27.9 (14.5) | 25 (12.2) | 0.003 |
| LVEF ≤ 30% | 551 (72.2) | 228 (68.1) | 323 (75.5) | 0.023 |
| Precipitating factor None |
115 (14.9) |
31 (9.1) |
84 (19.4) |
<0.001 |
| Sepsis | 92 (11.9) | 33 (9.7) | 59 (13.6) | 0.024 |
| Myocardial infarction | 280 (36.3) | 169 (49.9) | 111 (25.6) | <0.001 |
In this table, continuous variables are expressed as mean +/− SD. HF = heart failure, CHF = chronic heart failure, BMI = body mass index, SD = standard deviation, LVEF = left ventricle ejection fraction.
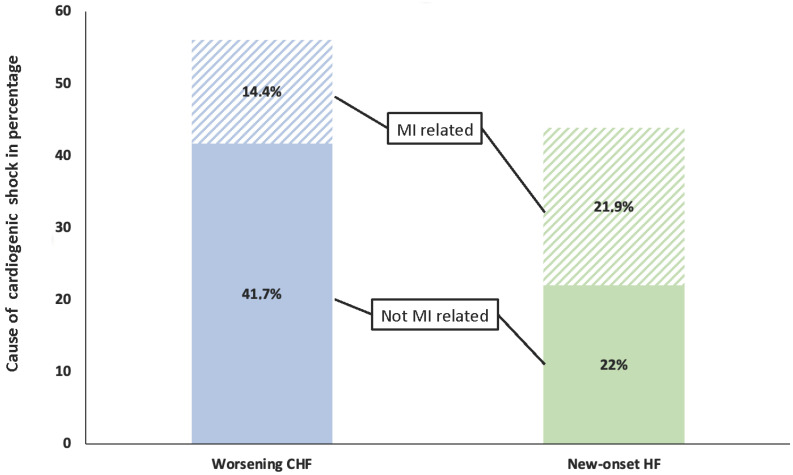
Patients with worsening CHF had a lower heart rate than those with new-onset HF (91 +/− 28 vs. 100 +/− 31, p < 0.001) and presented with sinus rhythm less often (46.2% vs. 59.4%, p < 0.001). They also had lower LVEFs (25% +/− 12.2 vs. 28% +/− 14.5, p = 0.003). Acute MI was less frequently the precipitating factor in patients with worsening CHF (25.6% vs. 49.9%, p < 0.001) (Figure 1).
Figure 1.
Graphic representation of the cardiogenic shock population based on the presence or absence of previous history of heart failure and the presence or absence of an acute myocardial infarction at admission. CS = cardiogenic shock, MI = myocardial infarction, HF = heart failure, CHF = chronic heart failure.
3.3. Biological Parameters
Biology parameters are shown in Table 3. In the overall study population, the median estimated glomerular filtration rate (eGFR) was 46 (28–67) mL/min. The median hemoglobin was 12.6 (11–14) g/dL. The median arterial lactate level was 3 (2–4.75) mmol/L, and 61.7% of the patients had a lactate level above the normal value. The median bilirubin level was 16 (9–29) mg/L and the median PT was 59% (37–77). The median BNP and NT-pro-BNP were 1150 (476–2778) and 9277 (4045–23,810) pg/mL, respectively. The median CRP level was 28 (9–69) mg/L.
Table 3.
Biology parameters at admission.
| Overall Population |
New-Onset HF |
Worsening CHF |
p Value | |
|---|---|---|---|---|
| n = 772 | n = 339 | n = 433 | ||
| eGFR (mL/min) (n = 761) |
46 [28–67] | 56 [39–77] | 40 [25–58] | <0.001 |
| Creatinine (mmol/L) (n = 751) |
133 [95–190] | 110 [84–149] | 145 [112–210] | <0.001 |
| Hemoglobin (g/dL) (n = 754) |
12.6 [11–14] | 13 [11.2–14.6] | 12 [10.6–14] | <0.001 |
| Arterial blood lactate (mmol/L) (n = 684) | 3 [2–4.75] | 3 [2–5] | 2.9 [2–4] | 0.121 |
| Arterial blood lactate > 2.2 (mmol/L) (n = 684) |
422 (61.7) | 200 (65.8) | 222 (58.4) | 0.049 |
| pH (n = 668) |
7.39 [7.28–7.46] | 7.37 [7.26–7.44] | 7.40 [7.30–7.46] | 0.007 |
| PT (%) (n = 731) |
59 [37–77] | 70 [52–85] | 48 [29–68] | <0.001 |
| SGOT (IU/L) (n = 547) |
90 [39–301] | 125 [51–377] | 69 [35–220] | 0.521 |
| SGPT (IU/L) (n = 559) |
59 [27–183] | 71 [33–194] | 48 [24–177] | 0.815 |
| Bilirubin (mmol/L) (n = 544) |
16 [9–29] | 13 [8–23] | 20 [11–34] | <0.001 |
| BNP (ng/L) (n = 264) |
1150 [476–2778] | 835 [277–2051] | 1511 [687–3157] | 0.182 |
| NT-pro-BNP (ng/L) (n = 224) |
9277 [4045–23,810] |
6306 [2063–11,730] |
12,652 [5360–30,000] |
0.006 |
| BNP or NT-pro-BNP by deciles (n = 480) | ||||
| 1 | 47 (9.8) | 36 (18.8) | 11 (3.8) | |
| 2 | 49 (10.2) | 27 (14) | 22 (7.6) | |
| 3 | 51 (10.6) | 24 (12.5) | 27 (9.4) | |
| 4 | 45 (9.3) | 18 (9.4) | 27 (9.4) | |
| 5 | 47 (9.8) | 21 (10.9) | 26 (9) | <0.001 |
| 6 | 52 (10.8) | 14 (7.2) | 38 (13.2) | |
| 7 | 46 (9.5) | 13 (6.8) | 33 (11.5) | |
| 8 | 50 (10.4) | 14 (7.2) | 36 (12.5) | |
| 9 | 41 (8.5) | 9 (4.7) | 32 (11.1) | |
| 10 | 52 (10.8) | 16 (8.3) | 36 (12.5) | |
| CRP (mg/L) (n = 406) |
28 [9–69] | 34 [8–98] | 26 [10–58] | 0.006 |
In this table, continuous variables are expressed as median [IQR]. HF = heart failure, CHF = chronic heart failure, eGFR = estimated glomerular filtration rate, IQR = interquartile range, PT = prothrombin time, SGOT = Serum Glutamooxaloacetate Transferase, SGPT = Serum Glutamopyruvate Transferase, CRP = C-reactive protein, BNP = Brain natriuretic peptide.
Worsening CHF patients had significantly lower eGFRs (40 (25–59) vs. 56 (39–77), p < 0.001) and hemoglobin levels (12 (10.6–14) vs. 13 (11.2–14.6), p < 0.001) than those with new-onset HF. They also had higher bilirubin (20 (11–34) vs. 13 (8–23), p < 0.001) and lower PT (48 (29–68) vs. 70 (52–85), p < 0.001). When assessed by deciles, BNP/NT-pro-BNP was significantly higher in patients with worsening CHF (p < 0.001).
3.4. Right Heart Catheterization Parameters
In our population, 83 patients had right heart catheterization: 36 in the new-onset HF group and 47 in the worsening CHF group (Table 4). The median right atrial pressure (RAP) was 10 (6–14) mmHg, the median mean pulmonary artery pressure (mPAP) was 29 (24–35) mmHg, and the median pulmonary capillary wedge pressure (PCWP) was 19 (14–25). The median cardiac index (CI) was 2.1 (1.9–3) L/min/m2.
Table 4.
Right heart catheterization parameters.
| Overall Population |
New-Onset HF |
Worsening CHF |
p Value | |
|---|---|---|---|---|
| n = 83 | n = 36 | n = 47 | ||
| Right atrial pressure (mmHg) | 10 [6–14] | 8 [5–11] | 12 [6–16] | 0.028 |
| Mean pulmonary arterial pressure (mmHg) | 29 [24–35] | 28 [21–33] | 32 [26–38] | 0.157 |
| Pulmonary capillary wedge pressure (mmHg) | 19 [14–25] | 16 [13–23] | 22 [15–29] | 0.062 |
| Cardiac index (L/min/m2) | 2.1 [1.9–3] | 2.1 [1.9–3] | 2 [1.8–2.8] | 0.391 |
In this table, continuous variables are expressed as median [IQR]. HF = heart failure, CHF = chronic heart failure, IQR = interquartile range.
Patients of the worsening CHF group had higher RAP (12 (6–16) vs. 8 (5–11), p = 0.028) and a trend for higher PCWP (22 (15–29) vs. 16 (13–23), p = 0.062).
3.5. Shock Management
In-hospital management is shown in Table 5. In the overall population, vasoactive drugs were prescribed as follows: dobutamine in 82.2% of the patients for a median time of 5 (2–8) days, noradrenaline in 53.3% of the patients for a median time of 3 (2–6) days, adrenaline in 12.3% of the patients for a median time of 1 (1,2) day, and levosimendan in only 7.4% of the cases. Intravenous diuretics were used in 82% of the cases and volume expansion in 41.6%. Organ replacement therapies were used as follows: mechanical ventilation (MV) in 291 patients (37.9%), renal replacement therapy (RRT) in 122 patients (15.8%), and mechanical circulatory support (MCS) in 143 patients (18.6%), including 48 patients with an intra-aortic balloon pump (IABP) for a median time of 2 (1–5) days, 26 patients with an Impella® device for a median of 6 (3–9) days, and 85 patients with extracorporeal life support (ECLS) for a median time of 5 (3–8) days. Coronary angiographies were performed on 399 patients (51.7%) and culprit lesions were treated in 256 patients (64.2% of those who had undergone a coronary angiography).
Table 5.
Shock management.
| Overall Population |
New-Onset HF |
Worsening CHF |
p Value | |
|---|---|---|---|---|
| n = 772 | n = 339 | n = 433 | ||
| ICU length of stay (days) | 11 [7–21] | 10 [6–20] | 12 [8–21] | 0.095 |
| In-hospital length of stay (days) | 16 [10–26] | 14 [9–24] | 16 [10–27] | 0.056 |
| Intravenous diuretics | 633 (82) | 259 (76.9) | 374 (86.8) | <0.001 |
| Volume expansion | 321 (41.6) | 168 (49.9) | 153 (35.6) | <0.001 |
| Dobutamine | 632 (82.2) | 274 (81.3) | 358 (83) | 0.534 |
| Duration (days) | 5 [2–8] | 4 [2–6] | 5 [3–9] | 0.027 |
| Max dose < 10 gamma/Kg/min | 405 (68.9) | 179 (70) | 226 (68.1) | |
| Max dose 10–15 gamma/Kg/min | 136 (23.1) | 62 (24.2) | 74 (22.3) | 0.241 |
| Max dose > 15 gamma/Kg/min | 47 (7.9) | 15 (5.9) | 32 (9.6) | |
| Noradrenaline | 410 (53.3) | 195 (57.9) | 215 (49.9) | 0.037 |
| Duration (days) | 3 [2–6] | 3 [2–5] | 3 [2–6] | 0.134 |
| Max dose < 1 mg/h | 86 (22.8) | 42 (23.1) | 44 (22.6) | |
| Max dose 1–5 mg/h | 215 (57) | 98 (53.8) | 117 (60.3) | 0.309 |
| Max dose > 5 mg/h | 75 (19.9) | 42 (23.1) | 33 (17) | |
| Adrenaline | 95 (12.3) | 51 (15.1) | 44 (10.2) | 0.042 |
| Duration (days) | 1 [1–2] | 1 [1–2] | 1 [1–4] | 0.407 |
| Max dose < 1 mg/h | 34 (38.6) | 16 (34) | 18 (43.9) | |
| Max dose 1–5 mg/h | 40 (45.4) | 22 (46) | 18 (43.9) | 0.533 |
| Max dose > 5 mg/h | 14 (15.9) | 9 (19.1) | 5 (12.2) | |
| Levosimendan | 57 (7.4) | 19 (5.6) | 38 (8.8) | 0.092 |
| Renal replacement therapy | 122 (15.8) | 52 (15.3) | 70 (16.2) | 0.745 |
| Mechanical ventilation | 291 (37.9) | 175 (51.8) | 116 (26.9) | <0.001 |
| Mechanical circulatory support | 143 (18.6) | 87 (25.6) | 56 (13) | <0.001 |
| IABP | 48 (34.5) | 31 (36.5) | 17 (31.5) | 0.552 |
| Duration (days) | 2 [1–5] | 2 [1–3] | 4 [2–5] | 0.942 |
| ECLS | 85 (60.7) | 55 (64) | 30 (55.6) | 0.326 |
| Duration (days) | 5 [3–8] | 5 [2–9] | 7 [3–8] | 0.665 |
| Impella | 26 (18.7) | 19 (22.4) | 7 (12.9) | 0.178 |
| Duration (days) | 6 [3–9] | 8 [4–10] | 5 [1–7] | 0.213 |
| Coronary angiography | 399 (51.7) | 233 (68.7) | 166 (38.3) | <0.001 |
| At least one vessel disease | 321 (41.6) | 185 (54.5) | 136 (31.4) | 0.007 |
| PCI of culprit lesion | 256 (33.2) | 171 (50.4) | 85 (19.6) | <0.001 |
In this table, continuous variables are expressed as median [IQR]. HF = heart failure, CHF = chronic heart failure, ICU = intensive care unit, IQR = interquartile range, IABP = intra-aortic balloon pump, ECLS = extracorporeal life support, PCI = percutaneous coronary intervention.
Noradrenaline and adrenaline were used less often in patients presenting with worsening CHF (49.9% vs. 57.9%, p = 0.03 and 10.2% vs. 15.1%, p = 0.04, respectively). Diuretics were used more often (86.8% vs. 76.9%, p < 0.001) and volume expansion was used less often (35.6% vs. 49.9%, p < 0.001). Organ replacement therapies, i.e., MV (26.9% vs. 51.8%, p < 0.001) and MCS (13% vs. 25.6%, p < 0.001) were also used less often. Coronary angiographies were performed less frequently (38.3% vs. 68.7%, p < 0.001) and culprit lesions were less frequently identified (51.2% of those who undergone a coronary angiography vs. 73.4%, p < 0.001).
3.6. In-Hospital Outcomes
The median in-hospital length of stay was 16 (10–26) days and the median length of stay in ICU was 11 (7–21) days. The in-hospital all-cause mortality rate was 28% (n = 217 patients).
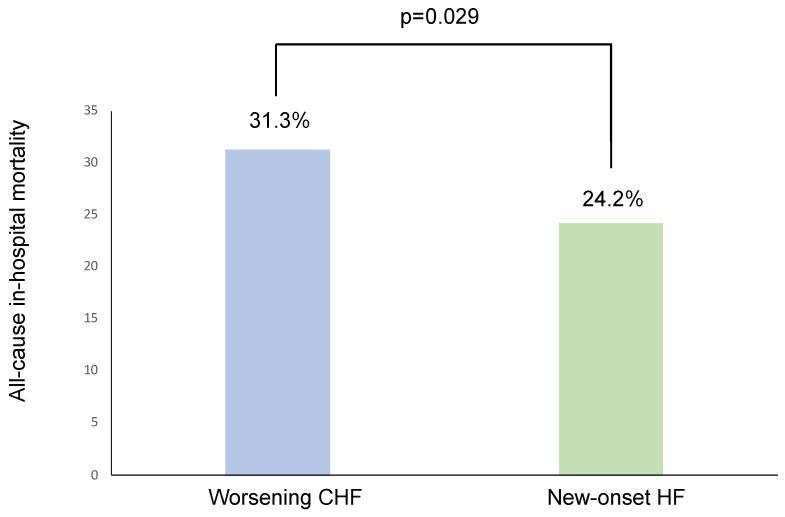
Worsening CHF patients had a non-significant trend for longer in-hospital stays (16 (10–27) days vs. 14 (9–24) days, p = 0.056). They experienced a higher all-cause mortality rate (31.3% vs. 24.2%, p = 0.029, Figure 2). After multivariate analysis, independent predictors of in-hospital all-cause mortality were age, heart rate, SBP, LVEF, hemoglobin, and arterial blood lactate levels (Table 6). Worsening CHF was not independently associated with in-hospital all-cause mortality (OR = 1.051, 95% CI = 0.680–1.624, p = 0.823).
Figure 2.
All-cause in-hospital mortality in both groups. HF = heart failure, CHF = chronic heart failure.
Table 6.
Multivariate analysis (in-hospital all-cause mortality).
| Odds Ratio | 95% Confidence Interval |
p Value | |
|---|---|---|---|
| Sex male | 1.149 | 0.737–1.794 | 0.540 |
| Age (per year) | 1.028 | 1.012–1.043 | <0.001 |
| Myocardial infarction as precipitating factor | 1.451 | 0.949–2.220 | 0.086 |
| Heart rate (per 1 bpm) | 1.008 | 1.001–1.014 | 0.019 |
| Systolic blood pressure (per 1 mmHg) | 0.987 | 0.979–0.995 | 0.002 |
| LVEF (per 1%) | 0.975 | 0.959–0.992 | 0.003 |
| Creatinin (per 1 mmol/L) | 1.002 | 0.999–1.004 | 0.147 |
| Hemoglobin (per 1 g/dL) | 0.901 | 0.822–0.987 | 0.026 |
| Arterial blood lactate (per 1 mmol/L) | 1.101 | 1.042–1.163 | 0.001 |
| PT (per 1%) | 0.992 | 0.983–1.001 | 0.070 |
| Worsening CHF | 1.051 | 0.680–1.624 | 0.823 |
LVEF = left ventricle ejection fraction, CHF = chronic heart failure, PT = prothrombin time.
4. Discussion
AHF and especially patients presenting with CS continue to experience poor in-hospital prognosis [8,11,12]. Incremental insights into risk stratification could be of paramount importance to improve patient management and therefore clinical outcomes. CS related to acute MI (AMI-CS) has been the focus of intense investigations [16,17]. However, the number of CS patients related to other etiologies (commonly designated as HF-CS) is now more prevalent in the contemporary era [7,13]. Clinical characteristics and in-hospital outcomes between HF-CS and AMI-CS were recently reported and showed better survival in HF-CS patients [18]. One could argue that AMI patients often represent the vast majority of new-onset HF etiologies among CS patients and are therefore equal to new-onset HF. This assumption was not observed in our population and is probably unfounded. Indeed, CS was the first manifestation of HF for almost half of our patients and among them, AMI was considered as the triggering factor in only 54.8% of the cases. Interestingly, AMI was also identified as a precipitating factor for 31.6% of the worsening CHF patients. The whole spectrum of CS patients is clearly difficult to apprehend and there is a large variability among patient profiles in daily practice. In our study, worsening CHF patients had more extra-cardiac comorbidities as compared to those of the new-onset HF group. They were also significantly older, had more history of diabetes, renal failure, COPD, and peripheral artery disease. This observation is in perfect accordance with the previous literature in the field [19,20].
Notably, the CS pathophysiology of new-onset HF or decompensation of a pre-existing CHF may considerably differ. Indeed, new-onset HF is more often characterized by a sudden decrease in cardiac output without adaptative remodeling, resulting in a more pronounced hypoperfusion; in comparison, CHF is progressive by nature, with prolonged exposure to neurohormonal and hemodynamic perturbations that will durably affect all organs (especially the liver and kidneys) and lead to histological and functional changes even in the absence of severe tissular hypoperfusion and/or hypoxia. Different phenotypes of CS according to HF duration before the index event are therefore not surprising. The CS hemodynamic profile at presentation is of major importance when it comes to treatment adaptation and prognosis evaluation. Recently, Zweck et al. described three distinct phenotypes using a machine-learning approach (demographic, hemodynamic, and biological variables were used): non-congested (phenotype I), cardio-renal (phenotype II), and cardio-metabolic CS (phenotype III), which were closely linked to in-hospital deaths, the worst prognosis being observed for the phenotype III [21]. Importantly, mortality trends of these three phenotypes were not significantly different in AMI-CS and HF-CS patients (respectively, 21% vs. 10% for phenotype I, 45% vs. 32% for phenotype II, and 55% vs. 52% for phenotype III). In addition, Thayer et al. yielded important insights and showed a close association between CS mortality and patients’ congestion profiles based on the evaluation of left and right ventricular pressures [18]. They stratified patients into four categories (euvolemic, left, right, or bi-ventricular congestion). Right-sided and bi-ventricular congestion were significantly associated with the highest risks of in-hospital mortality in both AMI-CS and HF-CS subgroups. In multivariate analysis, the CS profile but not the cause of CS (AMI vs. not) was independently associated with mortality in their study. These studies have highlighted the fact that CS mortality seems to be related to patient hemo-metabolic status rather than to the underlying etiology of CS, even if both are sometimes strongly correlated. All these concerns may have a critical impact on therapeutic management.
In our analysis, worsening CHF patients showed a higher degree of congestion with higher RAP and a trend for higher PCWP, which seems consistent with initial CS management: more intravenous diuretic use and less volume expansion. Management of CS also involves hemodynamic stabilization with catecholamine therapy and sometimes organ support (MV, RRT, MCS). Despite limited available data on its true efficacy in improving prognosis, use of MCS is growing in the contemporary era. MCS is, however, associated with a high rate of complications that could subsequently influence outcomes, including major bleeding, thrombosis, hemolysis, stroke, and infection. MCS was inserted in 18.6% of the patients in our population, with a more frequent use in new-onset HF patients (25.6% vs. 13%, p = 0.001), most likely related to the fact that these patients were younger and had less extra-cardiac comorbidities. Interestingly, the duration of HF has an important impact on prognosis in patients receiving MCS. In the INTERMACS registry, despite a more severe presentation at the time of MCS implantation, acute de novo HF patients had better prognoses than CHF ones in each level of INTERMACS severity [22].
Among patients presenting with AHF, previous studies have revealed that pre-existing history of HF independently predicts mortality [23,24]. Data emanating from the ASCEND-HF trial revealed that HF duration ≥ 1 month was associated with increased mortality (>1 to 12 months, hazard ratio (HR): 1.89; 95% CI: 1.35 to 2.65; >12 to 60 months, HR: 1.82; 95% CI: 1.33 to 2.48; and >60 months, HR: 2.02; 95% CI: 1.47 to 2.77) [24]. These results were in accordance with those of a Danish nationwide cohort [23], which included 17,176 patients with a first admission for AHF. In this cohort, worsening CHF patients (n = 8316) had a higher rate of all-cause mortality or HF readmission during follow-up (HR 1.37, 95% CI 1.31–1.43). Moreover, it has been reported that a shorter duration of HF was associated with a higher probability of function recovery and a better prognosis in several clinical scenarios, such as before cardiac resynchronization therapy implantation [25,26]. However, these studies excluded patients with CS. Data on this specific subset of patients are sparse and represent an important gap in knowledge. In our large prospective cohort of CS patients, the worsening CHF subgroup was associated with a greater burden of extra-cardiac comorbidities, lower LVEF, and more pronounced organ failure. Importantly, in-hospital mortality was 30% higher in the worsening CHF group as compared to the new-onset HF group (31.3% vs. 24.2%). A pre-existing history of HF, however, was not independently associated with in-hospital mortality by multivariate analysis, highlighting again that these patients largely differ in many points such as age, extra-cardiac comorbidities, and hemodynamic profile.
5. Strengths and Limitations
Our work prospectively included 772 consecutive CS patients (the larger European cohort) from a broad spectrum of etiologies. In addition, we used a contemporary and pragmatic definition of CS that considerably strengthens our results. However, it may be challenging in clinical daily practice to determine the chronicity of HF, and some patients could have been misclassified. Initial admission in primary centers may have influenced early patients’ management and may explain the high rate of IABP use. Few patients underwent right heart catheterization, and results emanating from this subgroup should be considered as hypothesis-generating. Finally, the delay between initial HF diagnosis and CS occurrence is not available in our database and may carry relevant information.
6. Conclusions
In summary, our results emphasize the great heterogeneity among patients presenting with CS. The presence or absence of a pre-existing HF is a significant part of this heterogeneity. CS patients of the worsening CHF group were older and had lower LVEFs, more extra-cardiac comorbidities, more congestion, and more organ failure as compared to those of the new-onset HF group. Importantly, this translated in a higher risk profile and a 30% increase in in-hospital all-cause mortality, although this association disappeared after adjustment. The heterogeneity of this population prompts us to better determine the phenotypes of patients in terms of clinical, biological, and hemodynamic characteristics, but also HF duration, which may have important implications for future trials and evaluations of tailored therapies.
Acknowledgments
FRENSHOCK is a registry of the French Society of Cardiology, managed by its Emergency and Acute Cardiovascular Care Working Group. Our thanks go out to all the devoted personnel of Société Française de Cardiologie who actively participate in the upkeep of the registry especially N. Naccache, E. Drouet, and V.Bataille. The authors are deeply indebted to all the physicians who took care of the patients at the participating institutions.
Author Contributions
Conceptualization: G.S., C.D., M.F. and G.L. (Gilles Lemesle); methodology: C.D., F.R., E.P. and G.L. (Gilles Lemesle); software: G.S.; validation: C.D., N.L. and G.L. (Gilles Lemesle); formal analysis: G.L. (Gilles Lemesle); investigation: C.D., F.R., E.P., L.B., G.L. (Guillaume Leurent), B.V., B.L. (Bruno Levy), J.T., B.H., G.V., N.C., B.L. (Benoît Lattuca), C.B., J.B., V.L., P.H., É.B.-C., N.L. and G.L. (Gilles Lemesle); resources: C.D., F.R., E.P., L.B., G.L. (Guillaume Leurent), B.V., B.L. (Bruno Levy), J.T., B.H., G.V., N.C., B.L. (Benoît Lattuca), C.B., J.B., V.L., P.H., É.B.-C., N.L. and G.L. (Gilles Lemesle); data curation: C.D., F.R., E.P., L.B., G.L. (Guillaume Leurent), B.V., B.L. (Bruno Levy), J.T., B.H., G.V., N.C., B.L. (Benoît Lattuca), C.B., J.B., V.L., P.H., É.B.-C., N.L. and G.L. (Gilles Lemesle); writing—original draft preparation: G.S., C.D., M.F., N.L. and G.L. (Gilles Lemesle); writing—review and editing: G.S., C.D., M.F., N.L. and G.L. (Gilles Lemesle); visualization: C.D. and G.L. (Gilles Lemesle); supervision: G.L. (Gilles Lemesle); project administration: C.D. and G.L. (Gilles Lemesle); funding acquisition: C.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
Gilles Lemesle reported personal fees from Alnylam, Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, MSD, Mylan, Novartis, Novonordisk, Pfizer, Sanofi-Aventis, and Servier. Other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Funding Statement
The study was sponsored by the Fédération Française de Cardiologie and was funded by unrestricted grants from Daiichi-Sankyo and Maquet SAS. Complementary grants will be sought for dedicated research projects within the main study.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McDonagh T.A., Metra M., Adamo M., Gardner R.S., Baumbach A., Bohm M., Burri H., Butler J., Celutkiene J., Chioncel O., et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 2.Chioncel O., Parissis J., Mebazaa A., Thiele H., Desch S., Bauersachs J., Harjola V.P., Antohi E.L., Arrigo M., Ben Gal T., et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020;22:1315–1341. doi: 10.1002/ejhf.1922. [DOI] [PubMed] [Google Scholar]
- 3.Hollenberg S.M., Kavinsky C.J., Parrillo J.E. Cardiogenic shock. Ann. Intern. Med. 1999;131:47–59. doi: 10.7326/0003-4819-131-1-199907060-00010. [DOI] [PubMed] [Google Scholar]
- 4.Shah R.U., de Lemos J.A., Wang T.Y., Chen A.Y., Thomas L., Sutton N.R., Fang J.C., Scirica B.M., Henry T.D., Granger C.B. Post-Hospital Outcomes of Patients with Acute Myocardial Infarction with Cardiogenic Shock: Findings From the NCDR. J. Am. Coll. Cardiol. 2016;67:739–747. doi: 10.1016/j.jacc.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 5.Chioncel O., Mebazaa A., Harjola V.P., Coats A.J., Piepoli M.F., Crespo-Leiro M.G., Laroche C., Seferovic P.M., Anker S.D., Ferrari R., et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: The ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017;19:1242–1254. doi: 10.1002/ejhf.890. [DOI] [PubMed] [Google Scholar]
- 6.Maggioni A.P., Dahlstrom U., Filippatos G., Chioncel O., Leiro M.C., Drozdz J., Fruhwald F., Gullestad L., Logeart D., Metra M., et al. EURObservational Research Programme: The Heart Failure Pilot Survey (ESC-HF Pilot) Eur. J. Heart Fail. 2010;12:1076–1084. doi: 10.1093/eurjhf/hfq154. [DOI] [PubMed] [Google Scholar]
- 7.Berg D.D., Bohula E.A., van Diepen S., Katz J.N., Alviar C.L., Baird-Zars V.M., Barnett C.F., Barsness G.W., Burke J.A., Cremer P.C., et al. Epidemiology of Shock in Contemporary Cardiac Intensive Care Units. Circ. Cardiovasc. Qual. Outcomes. 2019;12:e005618. doi: 10.1161/CIRCOUTCOMES.119.005618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babaev A., Frederick P.D., Pasta D.J., Every N., Sichrovsky T., Hochman J.S., Investigators N. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2005;294:448–454. doi: 10.1001/jama.294.4.448. [DOI] [PubMed] [Google Scholar]
- 9.Harjola V.P., Lassus J., Sionis A., Kober L., Tarvasmaki T., Spinar J., Parissis J., Banaszewski M., Silva-Cardoso J., Carubelli V., et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur. J. Heart Fail. 2015;17:501–509. doi: 10.1002/ejhf.260. [DOI] [PubMed] [Google Scholar]
- 10.Sleeper L.A., Reynolds H.R., White H.D., Webb J.G., Dzavik V., Hochman J.S. A severity scoring system for risk assessment of patients with cardiogenic shock: A report from the SHOCK Trial and Registry. Am. Heart J. 2010;160:443–450. doi: 10.1016/j.ahj.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolte D., Khera S., Dabhadkar K.C., Agarwal S., Aronow W.S., Timmermans R., Jain D., Cooper H.A., Frishman W.H., Menon V., et al. Trends in Coronary Angiography, Revascularization, and Outcomes of Cardiogenic Shock Complicating Non-ST-Elevation Myocardial Infarction. Am. J. Cardiol. 2016;117:1–9. doi: 10.1016/j.amjcard.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Aissaoui N., Puymirat E., Delmas C., Ortuno S., Durand E., Bataille V., Drouet E., Bonello L., Bonnefoy-Cudraz E., Lesmeles G., et al. Trends in cardiogenic shock complicating acute myocardial infarction. Eur. J. Heart Fail. 2020;22:664–672. doi: 10.1002/ejhf.1750. [DOI] [PubMed] [Google Scholar]
- 13.Delmas C., Roubille F., Lamblin N., Bonello L., Leurent G., Levy B., Elbaz M., Danchin N., Champion S., Lim P., et al. Baseline characteristics, management, and predictors of early mortality in cardiogenic shock: Insights from the FRENSHOCK registry. ESC Heart Fail. 2022;9:408–419. doi: 10.1002/ehf2.13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonello L., Laine M., Puymirat E., Ceccaldi V., Gaubert M., Paganelli F., Thuny P.F., Dabry T., Schurtz G., Delmas C., et al. Etiology and Prognosis of Cardiogenic Shock in a Secondary Center without Surgical Back-Up. Cardiol. Res. Pract. 2019;2019:3869603. doi: 10.1155/2019/3869603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delmas C., Puymirat E., Leurent G., Elbaz M., Manzo-Silberman S., Bonello L., Gerbaud E., Bataille V., Levy B., Lamblin N., et al. Design and preliminary results of FRENSHOCK 2016: A prospective nationwide multicentre registry on cardiogenic shock. Arch. Cardiovasc. Dis. 2019;112:343–353. doi: 10.1016/j.acvd.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Samsky M.D., Morrow D.A., Proudfoot A.G., Hochman J.S., Thiele H., Rao S.V. Cardiogenic Shock After Acute Myocardial Infarction: A Review. JAMA. 2021;326:1840–1850. doi: 10.1001/jama.2021.18323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myrda K., Gąsior M., Dudek D., Nawrotek B., Niedziela J., Wojakowski W., Gierlotka M., Grygier M., Stępińska J., Witkowski A., et al. One-Year Outcome of Glycoprotein IIb/IIIa Inhibitor Therapy in Patients with Myocardial Infarction-Related Cardiogenic Shock. J. Clin. Med. 2021;29:5059. doi: 10.3390/jcm10215059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thayer K.L., Zweck E., Ayouty M., Garan A.R., Hernandez-Montfort J., Mahr C., Morine K.J., Newman S., Jorde L., Haywood J.L., et al. Invasive Hemodynamic Assessment and Classification of In-Hospital Mortality Risk among Patients with Cardiogenic Shock. Circ. Heart Fail. 2020;13:e007099. doi: 10.1161/CIRCHEARTFAILURE.120.007099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatt A.S., Berg D.D., Bohula E.A., Alviar C.L., Baird-Zars V.M., Barnett C.F., Burke J.A., Carnicelli A.P., Chaudhry S.P., Daniels L.B., et al. De Novo vs Acute-on-Chronic Presentations of Heart Failure-Related Cardiogenic Shock: Insights from the Critical Care Cardiology Trials Network Registry. J. Card. Fail. 2021;27:1073–1081. doi: 10.1016/j.cardfail.2021.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones T.L., Tan M.C., Nguyen V., Kearney K.E., Maynard C.C., Anderson E., Mahr C., McCabe J.M. Outcome differences in acute vs. acute on chronic heart failure and cardiogenic shock. ESC Heart Fail. 2020;7:1118–1124. doi: 10.1002/ehf2.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zweck E., Thayer K.L., Helgestad O.K.L., Kanwar M., Ayouty M., Garan A.R., Hernandez-Montfort J., Mahr C., Wencker D., Sinha S.S., et al. Phenotyping Cardiogenic Shock. J. Am. Heart Assoc. 2021;10:e020085. doi: 10.1161/JAHA.120.020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loyaga-Rendon R.Y., Acharya D., Pamboukian S.V., Tallaj J.A., Cantor R., Starling R.C., Naftel D.C., Kirklin J.K. Duration of Heart Failure Is an Important Predictor of Outcomes After Mechanical Circulatory Support. Circ. Heart Fail. 2015;8:953–959. doi: 10.1161/CIRCHEARTFAILURE.115.002321. [DOI] [PubMed] [Google Scholar]
- 23.Butt J.H., Fosbol E.L., Gerds T.A., Andersson C., McMurray J.J.V., Petrie M.C., Gustafsson F., Madelaire C., Kristensen S.L., Gislason G.H., et al. Readmission and death in patients admitted with new-onset versus worsening of chronic heart failure: Insights from a nationwide cohort. Eur. J. Heart Fail. 2020;22:1777–1785. doi: 10.1002/ejhf.1800. [DOI] [PubMed] [Google Scholar]
- 24.Greene S.J., Hernandez A.F., Dunning A., Ambrosy A.P., Armstrong P.W., Butler J., Cerbin L.P., Coles A., Ezekowitz J.A., Metra M., et al. Hospitalization for Recently Diagnosed Versus Worsening Chronic Heart Failure: From the ASCEND-HF Trial. J. Am. Coll. Cardiol. 2017;69:3029–3039. doi: 10.1016/j.jacc.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein D.J., Maybaum S., MacGillivray T.E., Moore S.A., Bogaev R., Farrar D.J., Frazier O.H., HeartMate I.I.C.I. Young patients with nonischemic cardiomyopathy have higher likelihood of left ventricular recovery during left ventricular assist device support. J. Card. Fail. 2012;18:392–395. doi: 10.1016/j.cardfail.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Verbrugge F.H., Dupont M., Vercammen J., Jacobs L., Verhaert D., Vandervoort P., Tang W.H., Mullens W. Time from emerging heart failure symptoms to cardiac resynchronisation therapy: Impact on clinical response. Heart. 2013;99:314–319. doi: 10.1136/heartjnl-2012-302807. [DOI] [PubMed] [Google Scholar]