Abstract
Objectives:
Radiotherapy-induced toxicity may negatively impact health-related quality of life (HRQoL). This report investigates the impact of curative-intent radiotherapy on HRQoL and toxicity in early stage and locally-advanced non-small cell lung cancer patients treated with radiotherapy or chemo-radiotherapy enrolled in the observational prospective REQUITE study.
Materials and methods:
HRQoL was assessed using the European Organisation for Research and Treatment of Cancer QLQ-C30 questionnaire up to 2 years post radiotherapy. Eleven toxicities were scored by clinicians using the Common Terminology Criteria for Adverse Events (CTCAE) version 4. Toxicity scores were calculated by subtracting baseline values. Mixed model analyses were applied to determine statistical significance (p ≤ 0.01). Meaningful clinical important differences (MCID) were determined for changes in HRQoL. Analysis was performed on the overall data, different radiotherapy techniques, multimodality treatments and disease stages.
Results:
Data of 510 patients were analysed. There was no significant change in HRQoL or its domains, except for deterioration in cognitive functioning (p = 0.01). Radiotherapy technique had no significant impact on HRQoL. The addition of chemotherapy was significantly associated with HRQoL over time (p <.001). Overall toxicity did not significantly change over time. Acute toxicities of radiation-dermatitis (p =.003), dysphagia (p =.002) and esophagitis (p <.001) peaked at 3 months and decreased thereafter. Pneumonitis initially deteriorated but improved significantly after 12 months (p =.011). A proportion of patients experienced meaningful clinically important improvements and deteriorations in overall HRQoL and its domains. In some patients, pre-treatment symptoms improved gradually.
Conclusions:
While overall HRQoL and toxicity did not change over time, some patients improved, whereas others experienced acute radiotherapy-induced toxicities and deteriorated HRQoL, especially physical and cognitive functioning. Patient characteristics, more so than radiotherapy technique and treatment modality, impact post-radiotherapy toxicity and HRQoL outcomes. This stresses the importance of considering the potential impact of radiotherapy on individuals’ HRQoL, symptoms and toxicity in treatment decision-making.
1. Introduction
Lung cancer is the leading cause of death world-wide [1], and non-small cell lung cancer (NSCLC) accounts for approximately 80% of all cases. Radiotherapy is an important treatment modality in lung cancer that aims to improve loco-regional control and survival [2]. Surgery remains the standard of care for fit early-stage (ES-)NSCLC patients, but for those who are medically unfit or unwilling to undergo surgery, stereotactic body radiotherapy (SBRT) is the therapy of choice [3]. Standard treatment for locally advanced (LA-)NSCLC in fit patients is concurrent chemotherapy and radiotherapy (cCRT) with consolidative immunotherapy [4]. However, most patients are not suitable for this treatment [5] and may therefore receive sequential chemo-radiotherapy or radiotherapy only. Three-dimensional conformal radiotherapy (3D-CRT) remains frequently used in LA-NSCLC patients, but IMRT is more effective than 3D-CRT in allowing increased dose and reducing toxicity to normal tissue.
Radiotherapy also causes toxicity. Differences in the severity can depend on various factors such as the volume of normal tissues irradiated and intrinsic differences in radiosensitivity of the normal tissues between individuals [6]. Side effects can be described as acute or late [7]. Acute adverse events occur up to 90 days post-radiotherapy. They generally resolve completely, but may affect quality of life significantly and may even cause death [8]. Acute side effects that do not heal can lead to late tissue damage, the so-called “consequential late damage” [9]. Late adverse events are mainly irreversible and progressive and have therefore a more prolonged and significant impact on patients’ daily life.
Radiation-induced toxicity may negatively impact short and long-term health-related quality of life (HRQoL), as demonstrated in head- and-neck and breast cancer patients [10–12]. HRQoL refers to the impact of treatment and disease on a patients’ daily wellbeing. It represents a subjective evaluation of the individuals’ physical, role, social, cognitive, emotional, sexual and spiritual functioning [13–15]. Assessment of HRQoL in clinical trials and in daily practice can complement traditional outcomes [15–17]. For example, HRQoL is a more accurate prognostic indicator of survival than other clinical prognosticators, such as performance status (PS); and it is particularly useful to evaluate the benefits and toxicity of therapy [18]. Not surprisingly, improving and maintaining HRQoL in oncology patients has become a key aspect of personalized medicine. Treatment-decision making should therefore balance between clinical evidence and patient preferences, which is influenced by their current and anticipated future HRQoL.
This is particularly important in frail patient populations with poor general health and prognosis, such as the lung cancer population. Unfortunately, patients with poor PS and the elderly are often excluded from clinical trials, which emphasizes the need for large observational studies to gather data on the entire patient population [19].
Such a study is REQUITE (validating pREdictive models and biomarkers of radiotherapy toxicity to reduce side effects and improve QUalITy of lifE in cancer survivors) [20], an international prospective study aimed at developing a unique resource to validate models and biomarkers that predict the risk of toxicity following radiotherapy. This current analysis provides a summary of HRQoL and toxicity in ES- and LA-NSCLC patients enrolled in the REQUITE study.
2. Materials and methods
The REQUITE (validating pREdictive models and biomarkers of radiotherapy toxicity to reduce side effects and improve QUalITy of lifE in cancer survivors) study is a multi-center, longitudinal, observational study. The objective is to validate existing models of radiotherapy-induced morbidity and incorporate biomarkers to determine patients at risk. The ultimate aim is to reduce toxicity and improve HRQoL in patients receiving radiotherapy. Patients were recruited between April 2014 and March 2017.The study collected standardized data by CRFs (a. o. demographics, comorbidities, acute and late toxicity) and tissue samples from breast, prostate and lung cancer patients. Details of the study have been published previously [20]. For lung cancer patients, the eligibility criteria were: a confirmed diagnosis of lung cancer (this manuscript only included NSCLC patients), suitable for radical radiotherapy, SBRT, sequential or concurrent chemo-radiotherapy, 18 years or older, absence of distant metastases and without malignancies in the last 5 years. Lung cancer patients were withdrawn from the study if a recurrence or a second malignancy in the thorax occurred. Participants gave written informed consent. The study was approved by local ethics committees and was registered with the Current Controlled Trials (ISRCTN98496463).
2.1. Data set
HRQoL was measured using the EORTC QLQ-C30, a questionnaire which focuses on the impact of disease and therapy on HRQoL, including the physical, role, cognitive, emotional and social functioning, symptoms, global quality of life and health status.
Toxicity was scored using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. The toxicities measured were: cough, dyspnea, chest wall pain, pneumonitis, pulmonary fibrosis, bronchial stricture, esophagitis, dysphagia, myocardial infarction, pericarditis and radiation dermatitis. Data was collected before or within the first 5 days of radiotherapy and 3, 6, 12 and 24 months post-radiotherapy with a flexibility of 4 weeks around each time point. Important to note is that baseline data were collected before radiotherapy. However, baseline data might be influenced by previous surgery or chemotherapy, particularly in those receiving sequential chemo-radiotherapy.
2.2. Data analyses
Scoring of the EORTC QLQ-C30 followed the EORTC guidelines [21]. Data imputation was done for HRQoL in case at least half of the items of a certain domain or symptom was scored [22]. No data imputation was performed for missing toxicity data.
Linear transformation was applied for each domain and item to standardize the raw score ranging from 0 to 100. Higher scores on functional scales and global health status/quality of life represent a high/healthy level of functioning whereas high scores on symptoms indicates stronger symptoms [15]. A 10-point change in any item or domain within a patient over time, was considered a threshold for a minimal clinically important difference (MCID) [15]. Meaningful improvement is defined as a 10-point increase and meaningful deterioration is defined as a 10-point decrease between two time points.
Toxicity was scored with CTCAE, with toxicity at follow-up time points being calculated by subtracting the baseline toxicity score from the subsequent score. While improvements, as well as deteriorations, were reported, only worsening toxicity scores were taken into account in statistical analyses to capture the impact of therapy. The total toxicity score was calculated with the Standardized Total Average Toxicity (STAT) score [23]. The STAT score is a scale-independent measure of toxicity that summarizes different toxicity scores into a single measurement for a patient.
All patients with either toxicity or HRQoL data were included in the analyses. Treatment was categorized into the following treatment modalities: 3D-CRT, IMRT (including rotational IMRT: tomotherapy and volumetric arc therapy (VMAT)) and SBRT, as well as multimodality approaches including concurrent or sequential chemo-radiotherapy versus radiotherapy alone. Comparisons between the latter excluded stage I patients, but included 14 stage II/III patients receiving SBRT as radiotherapy-only treatment strategy.
A linear mixed-effects model was applied to analyze the data. This model corrects for the relatedness structure of the data and missing data [13]. The following covariates were included in the full model: stage (stage I vs stage II vs stage IIIA vs stage IIIB), time point, country and radiotherapy technique (3D-CRT vs IMRT vs SBRT).
The level of statistical significance was set at p = 0.01 to correct for multiple comparisons and to adjust for a level I error. Statistical significance analyses provide data on the overall outcomes of the entire population, rather than quantifying the individual evolution over time. Therefore, this study focuses additionally on MCID and the percentages of patients improving and deteriorating over time are presented.
3. Results
3.1. Patient characteristics
In total, 561 lung cancer patients were enrolled in the REQUITE study of which 510 were eligible for analyses based on inclusion criteria and minimal data availability. Patient characteristics are shown in Table 1. See Appendix 1. for a summary of availability of HRQoL and toxicity data per time point.
Table 1.
Patient, disease and treatment characteristics and baseline HRQoL.
| Patient characteristics | |
| Average age, years (range), SD | 69 (39–91), 10.0 |
| Gender, n (%) | |
| Female | 149 (29.2) |
| Male | 361 (70.8) |
| Smoking status, n (%) | |
| Never smoker | 23 (4.5) |
| Ex-smoker before cancer diagnosis | 281 (55.1) |
| Ex-smoker, since cancer diagnosis | 88 (17.3) |
| Current | 115 (22.5) |
| Unknown | 3 (0.6) |
| Comorbidity, n (%) | |
| Cardio-vascular disease | 305 (59.8) |
| History of heart disease | 158 (31.0) |
| Hypertension | 251 (49.2) |
| COPD | 210 (41.2) |
| Depression | 61 (12.0) |
| Highest education, n (%) | |
| Primary school | 135 (26.5) |
| Secondary school | 84 (16.5) |
| Professional education | 46 (9.0) |
| University or equivalent | 52 (10.2) |
| Unknown | 193 (37.8) |
| Disease characteristics | |
| Disease stage, n (%) | |
| I | 169 (33.1) |
| II | 61 (12.0) |
| IIIA | 172 (33.7) |
| IIIB | 97 (19.0) |
| Unknown | 11 (2.2) |
| Histology, n (%) | |
| Adenocarcinoma | 194 (38.0) |
| Squamous cell carcinoma | 176 (34.5) |
| Large cell NOS and other types of NSCLC | 27 (5.3) |
| Unknown | 113 (22.2) |
| Treatment characteristics | |
| Radiation technique, n (%) | |
| 3D-CRT | 155 (30.4) |
| IMRT | 208 (40.8) |
| Rotational IMRT (including VMAT and tomotherapy) | 76 (14.9) |
| SBRT | 147 (28.8) |
| Combined treatment modality, n (%) | |
| Concurrent chemo-radiotherapy | 192 (37.6) |
| Sequential chemo-radiotherapy | 61 (12.0) |
| Radiotherapy alone | 257 (50.4) |
| Surgery, n (%) | 49 (9.6) |
| HRQoL baseline scores | |
| Physical functioning | 72.1 |
| Role functioning | 70.7 |
| Emotional functioning | 75.1 |
| Cognitive functioning | 83.5 |
| Social functioning | 79.7 |
| Fatigue | 34 |
| Nausea and vomiting | 7.8 |
| Pain | 16.7 |
| Dyspnoea | 35 |
| Insomina | 27.2 |
| Appetite loss | 20.6 |
| Constipation | 17.9 |
| Diarrhoea | 7 |
| Financial difficulties | 10.7 |
| Global health status/QoL | 52.7 |
| Overall HRQoL | 78.1 |
| Baseline overall HRQoL and domains per stage | |
| Stage I | |
| Physical functioning | 67.1 |
| Role functioning | 67.2 |
| Emotional functioning | 77 |
| Cognitive functioning | 82.7 |
| Social functioning | 81 |
| Overall HRQoL | 78.8 |
| Stage II | |
| Physical functioning | 69.1 |
| Role functioning | 72.2 |
| Emotional functioning | 74.9 |
| Cognitive functioning | 79.7 |
| Social functioning | 76.8 |
| Overall HRQoL | 77.4 |
| Stage IIIA | |
| Physical functioning | 75.7 |
| Role functioning | 73.7 |
| Emotional functioning | 76.9 |
| Cognitive functioning | 86.5 |
| Social functioning | 81.7 |
| Overall HRQoL | 79.8 |
| Stage IIIB | |
| Physical functioning | 75.3 |
| Role functioning | 71.7 |
| Emotional functioning | 71.6 |
| Cognitive functioning | 82.9 |
| Social functioning | 77.1 |
| Overall HRQoL | 76.5 |
Abbreviations: 3D-CRT, three-dimensional conformal radiation therapy; COPD, chronic obstructive pulmonary disease; HRQoL, health-related quality of life; IMRT, Intensity-Modulated Radiation Therapy; n, number; NOS, not otherwise specified; NSCLC, non-small cell lung cancer; SBRT, Stereotactic body radiation therapy; SD, standard deviation; QoL, quality of life; VMAT, Volumetric- Modulated Arc Therapy.
3.2. HRQoL at baseline
Baseline physical and social functioning scores varied substantially between different patient groups, whilst no meaningful differences between overall (Global health status/QoL) and other domains of HRQoL were found. An overview of HRQoL and its domains per disease stage can be found in Table 1. Similar summaries for different radiotherapy techniques and treatment modalities can be found in Appendix 2. For physical functioning, stage I (average of 67.1) and stage II (69.1) NSCLC patients reported lower scores than those with stage IIIA (75.7) and IIIB (75.3) NSCLC. In line with this, SBRT patients had lower scores (66.8) than those undergoing IMRT (71.1) or 3D-CRT (77.8), the latter having the highest baseline physical functioning score. Patients receiving concurrent chemo-radiotherapy had a higher average physical functioning score of 79.4 compared to patients who received sequential chemo-radiotherapy or radiotherapy alone, with scores of 72.9 and 65.5 respectively. Conversely, social functioning of patients receiving radiotherapy alone scored considerably higher than those receiving concurrent and sequential chemo-radiotherapy in particular (83.1 vs 80.5 vs 73.3 respectively).
3.3. HRQoL after radiotherapy
In the entire population, overall HRQoL did not significantly change over time (p =.249). The same applied to most of the domains: physical (p =.580), role (p =.232) emotional (p =.226) and social functioning (p =.086). Cognitive functioning was the only domain that significantly deteriorated over time (p = 0.01).
In terms of MCIDs compared to baseline (see Fig. 1), most patients remained stable in overall HRQoL and its domains. The most striking changes were observed at the later time points, with deterioration in physical functioning at 12 and 24 months, but the only clinically important differences were seen in an improved overall HRQoL, together with better role functioning, at 24 months.
Fig. 1.
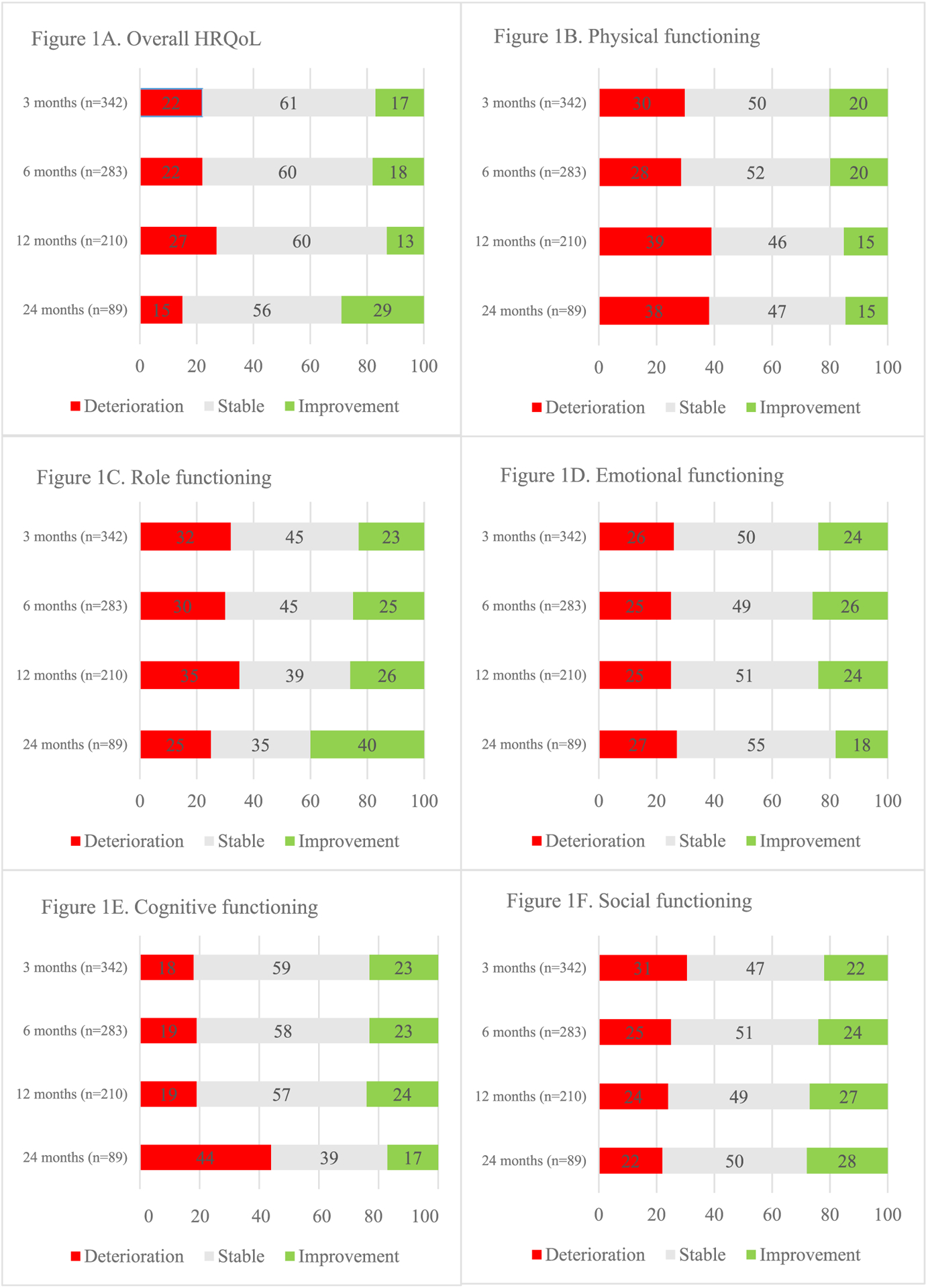
Overview of meaningful clinical important differences (deterioration or improvement) in overall HRQoL and its domains over time.
3.3.1. HRQoL for different radiotherapy techniques
On average, radiotherapy technique had no statistically significant impact on overall HRQoL (p =.349), nor its separate domains (p =.177, p =.082, p =.396, p =.358, p =.790) over time.
In terms of MCIDs, those receiving 3D-CRT deteriorated progressively over time in overall HRQoL; in physical functioning, with the most striking decline was at 12 months in 39% of the patients; and in cognitive and emotional functioning, most specifically at 24 months. Conversely, improvements were gradually seen in emotional and role functioning. In patients who received IMRT, overall HRQoL, physical and role functioning deteriorated more than social functioning over time. Among those receiving SBRT, the greatest deterioration was seen in overall HRQoL at 24 months and more patients progressively deteriorated in physical, role, cognitive and social functioning at this time point. See Fig. 2 for an overview of MCIDs in HRQoL and its domains for different radiotherapy techniques.
Fig. 2.
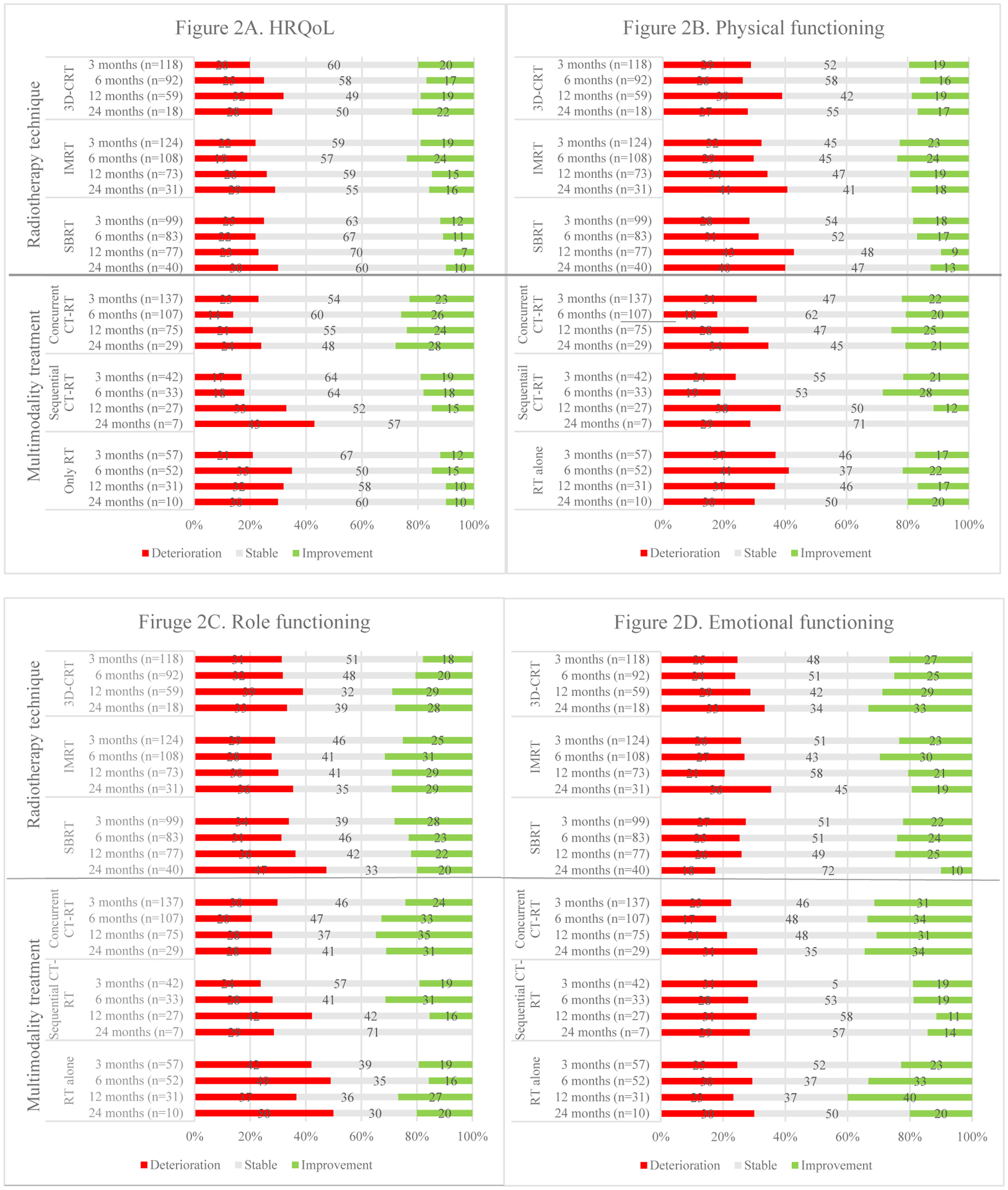
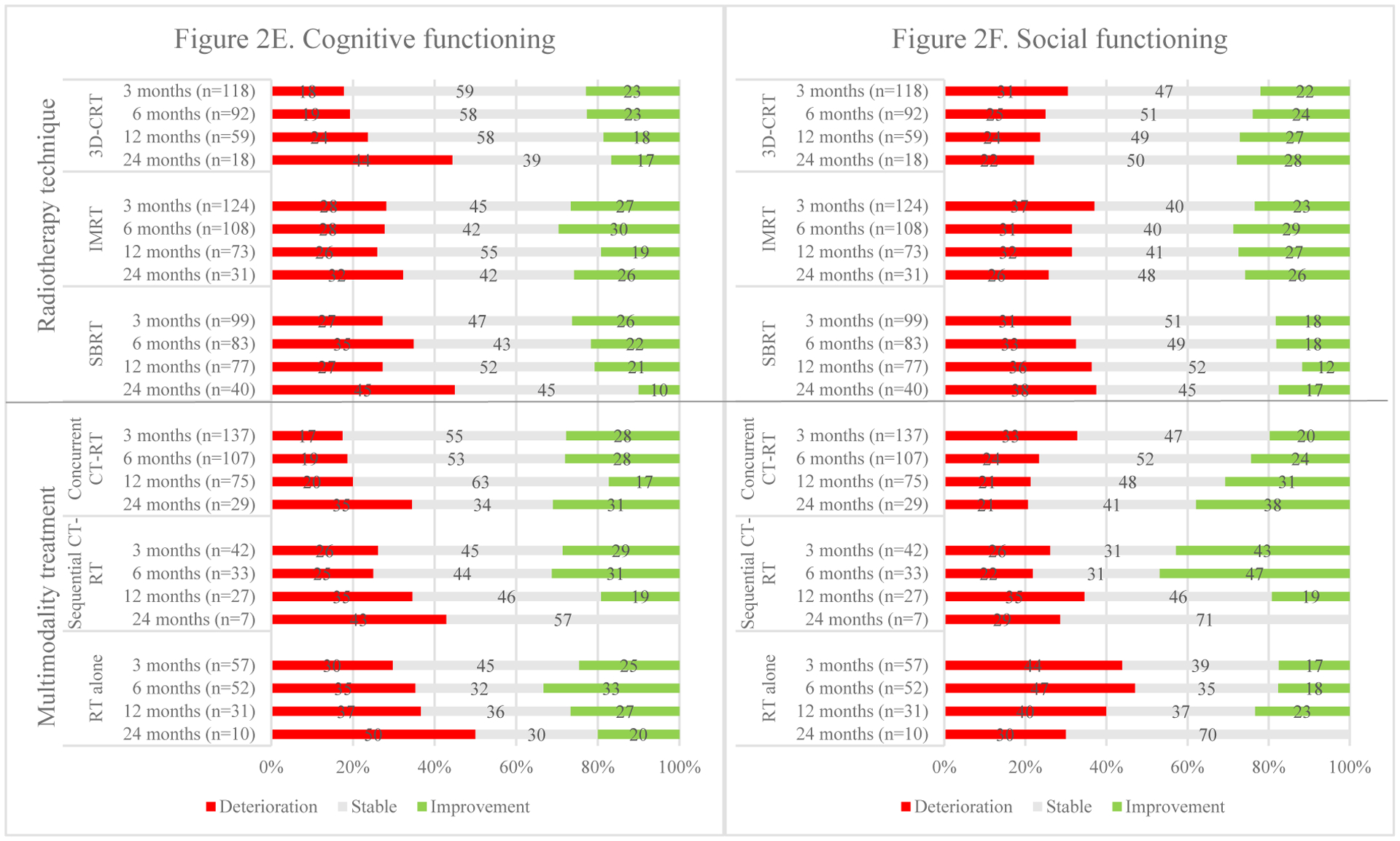
Overviews of meaningful clinical important differences (deteriorations and improvements) over time in overall HRQoL and its domains per treatment approach.
3.3.2. HRQoL for different treatment modalities
Treatment with chemotherapy was significantly associated with overall HRQoL (p <.001): those who received concurrent or sequential chemo-radiotherapy had a significantly higher post-treatment overall HRQoL (p <.001 and p =.010 respectively) than those who received radiotherapy alone at all follow-up points. No statistical significant differences in HRQoL were found post-treatment between sequential and concurrent chemo-radiotherapy.
MCIDs were observed in patients receiving concurrent chemo-radiotherapy, the most notable outcome was the substantial improvement in long-term social functioning and deterioration of cognitive functioning. Among those receiving sequential chemo-radiotherapy, more patients gradually worsened in overall HRQoL. Physical, role and cognitive functioning seemed to improve initially, but deteriorated thereafter. For stage II/III patients receiving radiotherapy without chemotherapy, considerable improvements were initially seen in emotional functioning culminating at 12 months, despite deteriorations in physical, role and social functioning in the same time frame. By 24 months, deterioration was seen in all domains as well as overall HRQoL with the greatest impact on role, emotional and especially cognitive functioning.
3.3.3. HRQoL for different disease stages
Disease stage, conversely, was associated with overall HRQoL over time (p =.008): those with stage IIIA and stage I had the best and worst overall HRQoL, respectively. Appendix 3 provides an overview of MCIDs of HRQoL per disease stage.
3.4. Symptoms at baseline
At baseline, symptoms of cough (53.6%), dyspnea (63.3%) and chest wall pain (19.2%) were not only reported more frequently, but also showed pre-radiotherapy differences between patient groups. The baseline data in Fig. 3 shows symptoms present before start of therapy. Dyspnea was most frequently reported in stage I (70.8%) and II (67.2%) patients, less so in those with stage IIIA (59.6%) and IIIB (54.6%). Pain was most commonly reported in stage II (28.3%) and IIIA (22.4%) patients but less in those with stage I (11.8%) and IIIB (18.5%). In line with this are the findings for the radiotherapy techniques: patients receiving SBRT were most likely to report dyspnea (70.5%) and least likely to report pain (10.9%) whereas those receiving 3D-CRT were least likely to experience dyspnea (57.4%) and most likely to experience pain (34.0%). Patients receiving sequential chemo-radiotherapy reported cough less often (36.5%) than those undergoing concurrent chemo-radiotherapy (56.7%) and radiotherapy alone (60.0%). The same holds for pain (9.5% vs 24.3% and 26.7%). On the other hand, those receiving radiotherapy alone were most likely to report dyspnea (70%) compared to those receiving sequential (65.1%) or concurrent chemo-radiotherapy (52.4%).
Fig. 3.
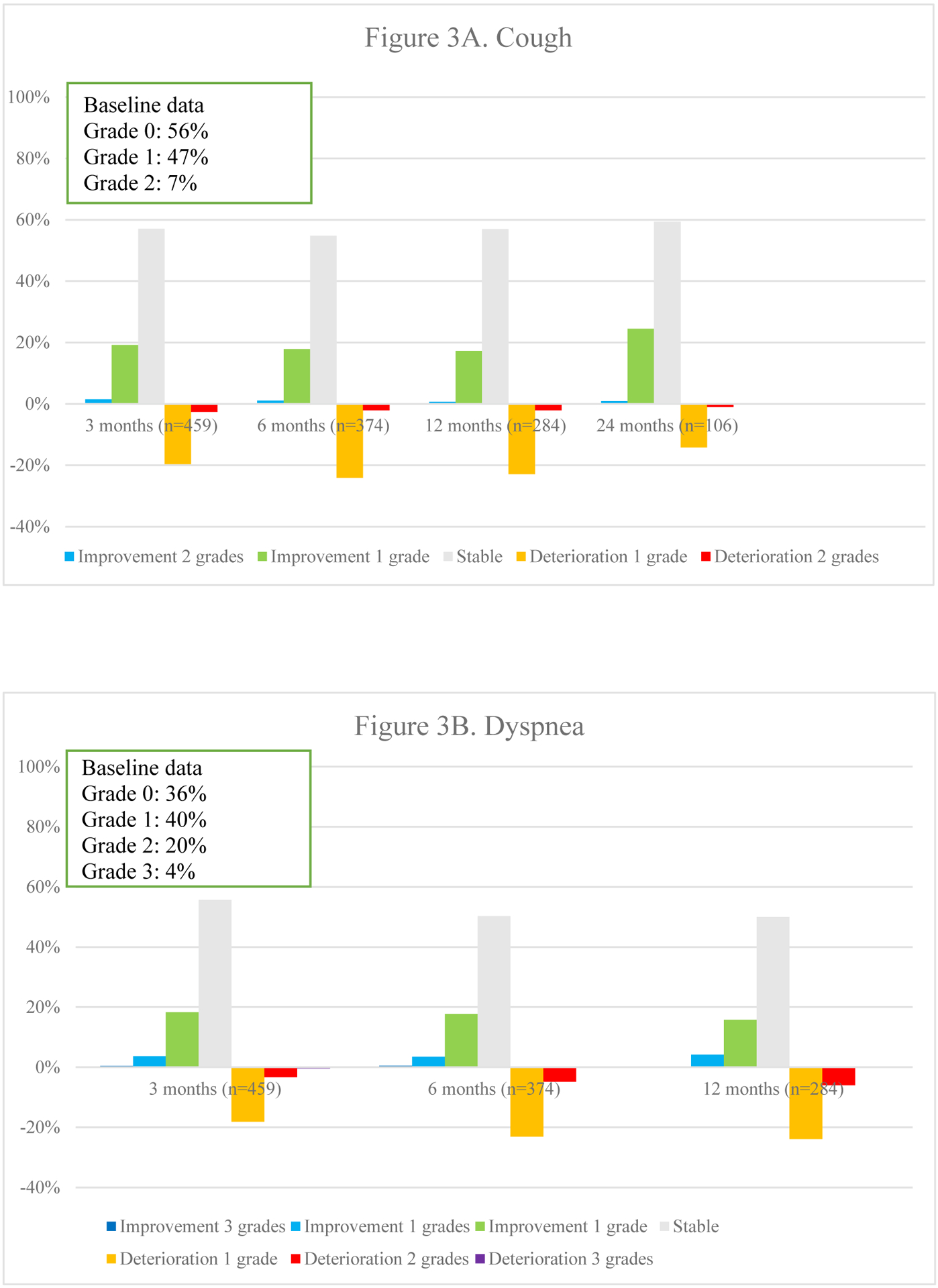
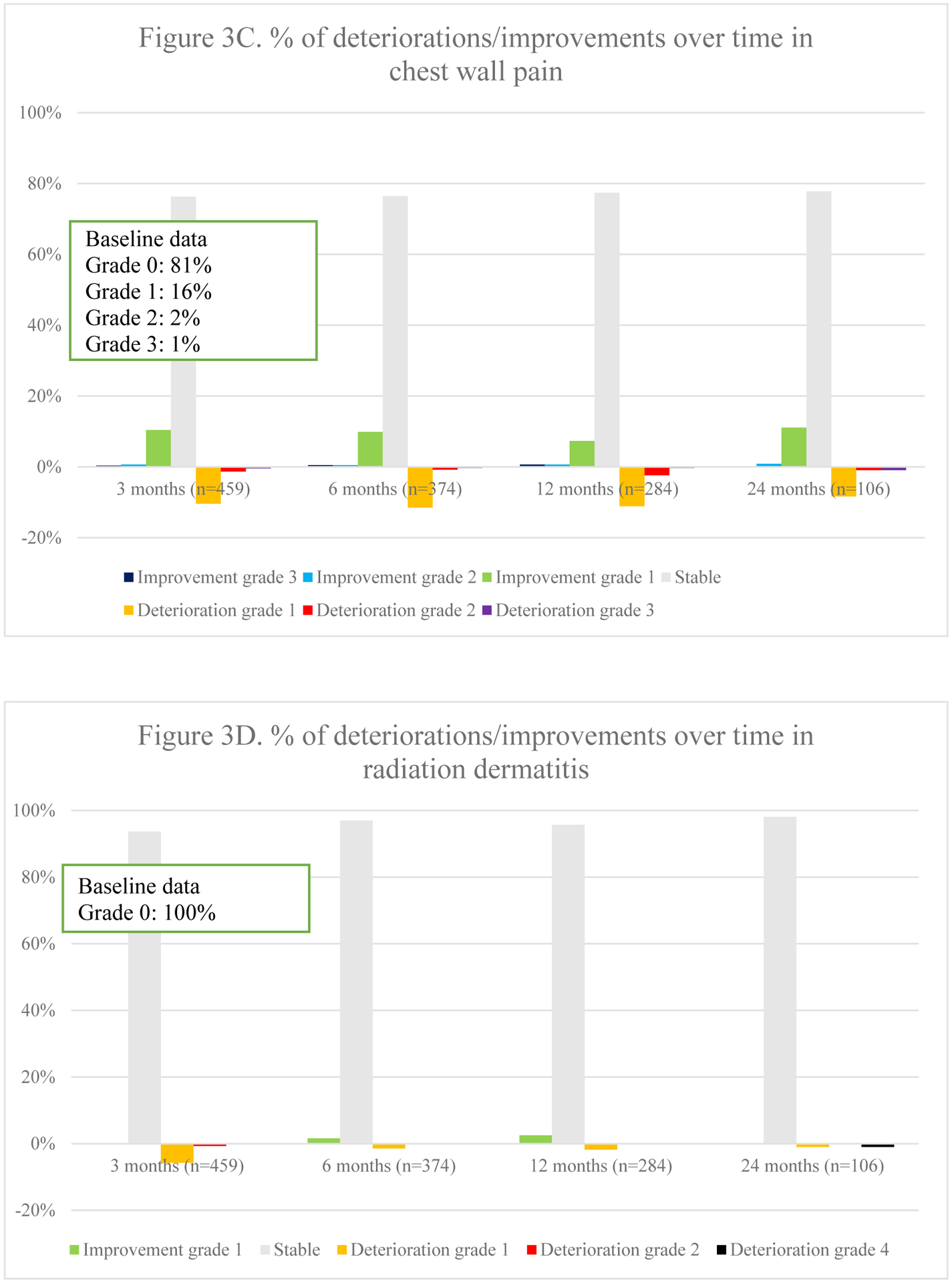
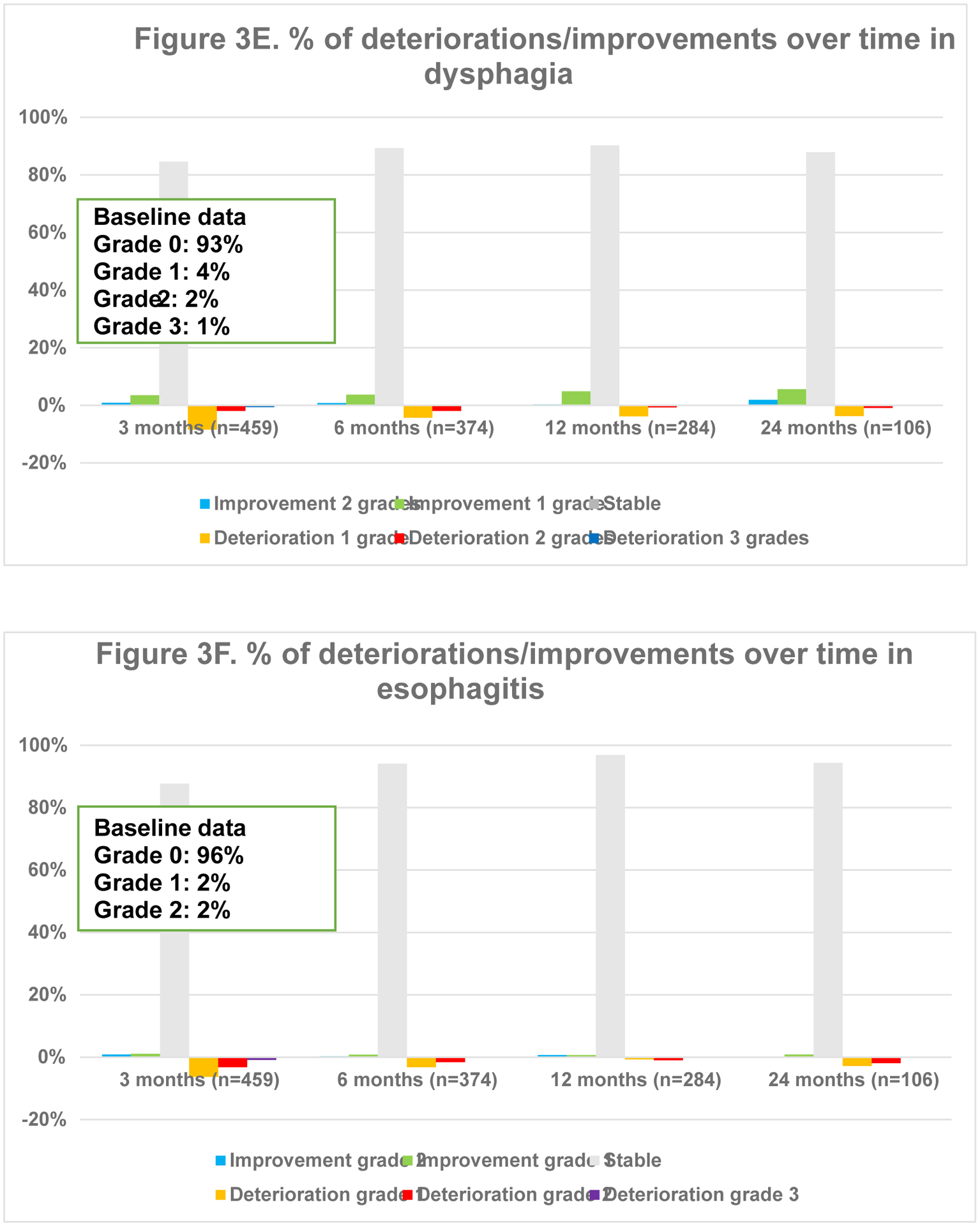
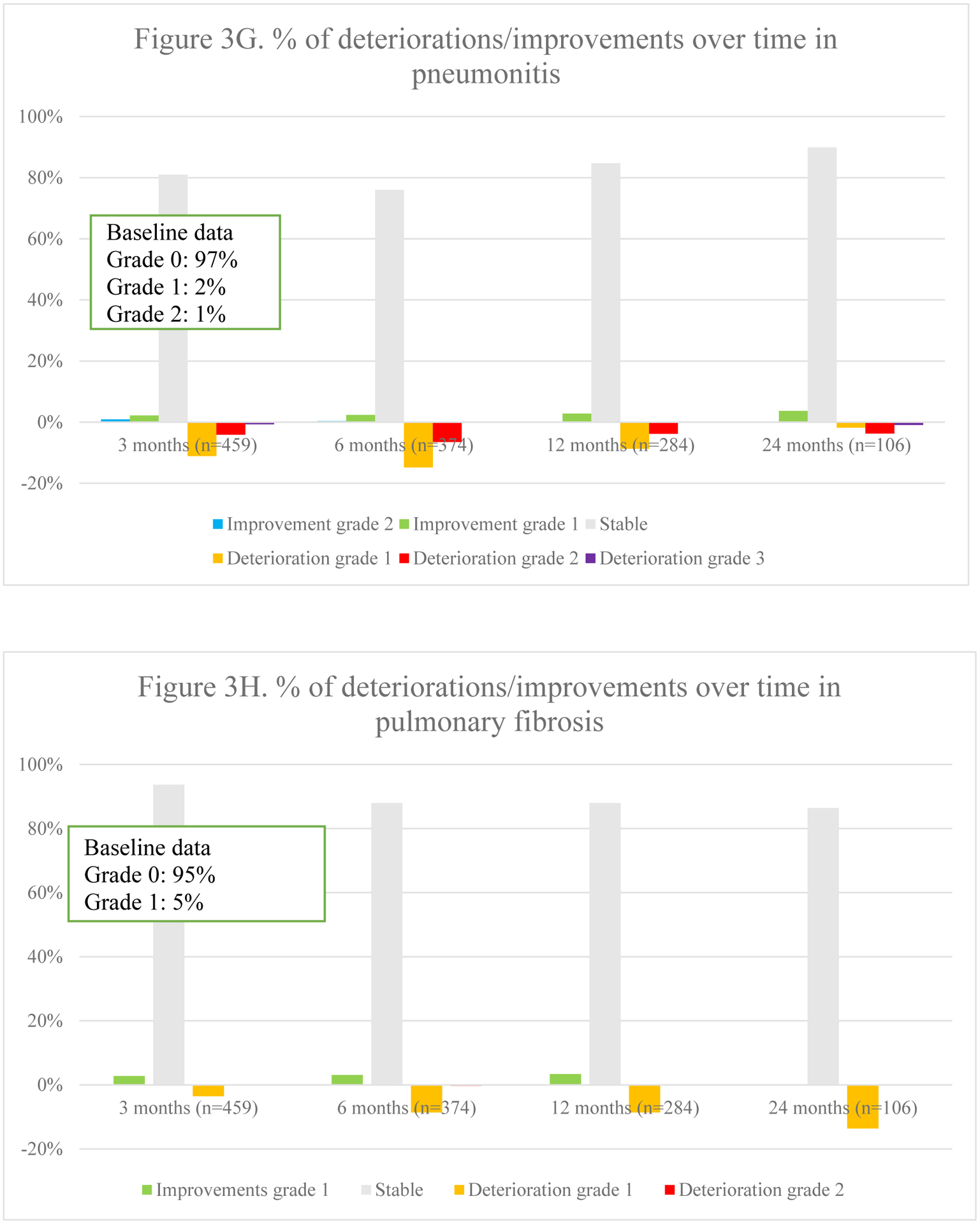
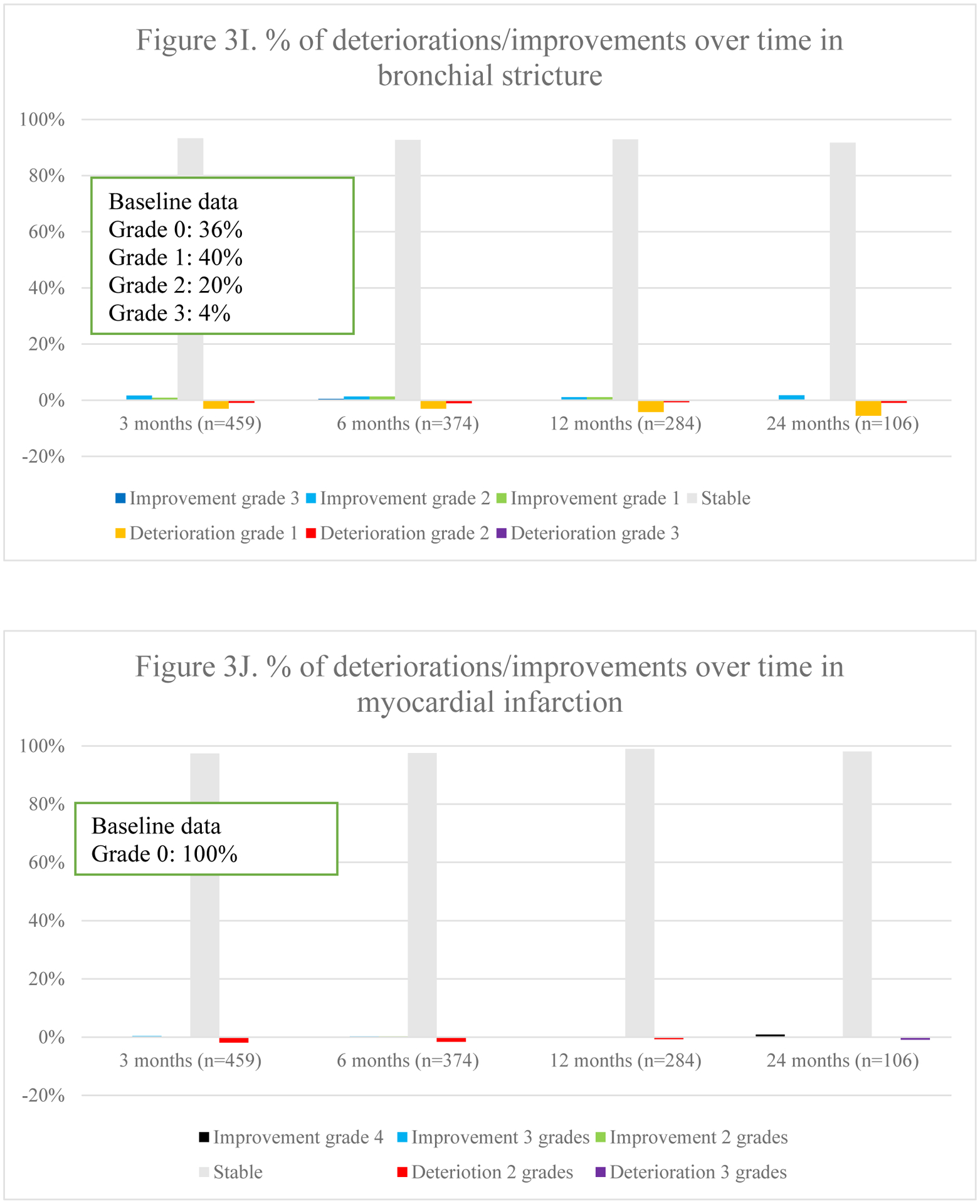
Overview of percentages of deteriorations and improvements of different toxicities over time
3.5. Toxicity after radiotherapy
Overall toxicity did not significantly differ over time (p =.544). Fig. 3 represents the course of toxicities over time. Dyspnea and cough were both positively and negatively impacted, although no statistical significance was found on average for the entire population. The typical acute radiotherapy-related toxicities of radiation-dermatitis (p =.003), dysphagia (p =.002) and esophagitis (p <.001) peaked at 3 months and decreased thereafter. Pneumonitis deteriorated initially but improved significantly at 12 and 24 months (p =.011). Finally, a trend towards increasing fibrosis (p =.045), bronchial stricture (p =.021) and pericarditis (p =.019) was observed, although the latter was extremely rare.
3.5.1. Toxicity per radiotherapy technique
In 3D-CRT, an increase in dyspnea was seen over time (Appendix 4.). These patients also experienced less improvement in chest wall pain over time. Radiation dermatitis and esophagitis were short-term toxicities. Dysphagia was either short-term or improved over time when present at baseline. Pneumonitis mainly deteriorated within the first year. In those receiving IMRT, the most notable change was an improvement in cough at 24 months. Dyspnea worsened over time. Dysphagia and esophagitis were short-term toxicities as in 3D-CRT. Pneumonitis was mostly seen within the first 12 months. In patients receiving SBRT, dyspnea gradually deteriorated within the first 12 months. Pneumonitis, pulmonary fibrosis and bronchial stricture were most frequently reported at 24 months.
3.5.2. Treatment modalities.
In those receiving concurrent chemo-radiotherapy, cough particularly improved at 24 months, whereas dyspnea deteriorated gradually (Appendix 4.). Chest wall pain mainly deteriorated within the first 12 months. Radiation-dermatitis and esophagitis were mostly acute. Dysphagia was mainly short-term, but for those with pre-treatment dysphagia, it improved increasingly. Pneumonitis was mostly reported at 6 months. Among those receiving sequential chemo-radiotherapy, dyspnea improved over time, except for at 24 months. Pneumonitis was particularly seen at 6 months as is seen in those receiving concurrent treatment. Among stage II/III patients receiving radiotherapy alone, cough deteriorated gradually, except for at 24 months. Chest wall pain and dyspnea improved less over time, with progressively more deterioration in chest wall pain. Whereas more fibrosis was reported, less bronchial stricture and myocardial infarction was seen over time.
Similar figures per disease stage can be found in Appendix 5.
4. Discussion
This study explores HRQoL and treatment-related toxicity in lung cancer patients undergoing radiation therapy with curative intent. Research has been done previously on HRQoL and side effects in this patient population, but primarily in the context of randomized controlled trials and experimental studies [24–27]. The strength of the REQUITE study is the prospective collection of a large set of data in daily clinical practice. This multinational database constitutes patient-reported HRQoL and clinician-reported toxicity data of ES- and LA-NSCLC patients with different disease stages receiving different radiotherapy techniques from academic centers throughout Europe and the United States.
HRQoL data in this study were collected using PROMs (EORTC QLQ-C30). PROMs are standardized, validated tools that capture data from the patients’ perspective on their wellbeing and functioning [28–29]. The benefits of PROMs have been extensively described: amongst others, PROMs can aid in early detection of relapse and health deterioration and promote communication between patients and healthcare practitioners [28–32]. As such, PROMs are expected to be sensitive enough to grasp the long-term impact of radiotherapy [33].
Several studies have shown that following symptoms, toxicity and HRQoL through web-based PROM collection can result in substantial clinical benefits in individual cancer patients, such as improved survival and HRQoL [34–37]. However, only collecting electronic PROM data with symptom monitoring and providing feedback is not enough to achieve these benefits. To improve patient-centered care [38], support services are needed, including patient engagement and encouragement to participate in the intervention, an email alert system, tele-care services as well as dedicated symptom management care. One such project is the PROMPT-Care (Patient Reported Outcome Measures for Personalized Treatment and Care) intervention [39]. Patients are followed through a web-based assessment tool evaluating physical and psycho-social wellbeing. Based on algorithms, alerts inform the health care team to undertake action. Furthermore, personalized feedback and self-care advice is provided to the individual patients.
The results of this study show substantial pre-radiotherapy differences within the patient population in both functioning and symptom burden. Whereas those with ES-NSCLC and receiving SBRT reported the lowest physical functioning, most dyspnea and least pain, those receiving 3D-CRT for LA-NSCLC had the highest physical functioning, least dyspnea, but the most pain. This could be explained by the fact that ES-NSCLC patients, ineligible for surgery and therefore receiving SBRT, tend to be older, have more comorbidities, are less fit with lower baseline HRQoL than those suitable for surgical interventions [40]. LA-NSCLC patients receiving sequential chemo-radiotherapy experienced less cough and pain than those receiving either concurrent treatment or radiotherapy alone. It should be mentioned that baseline data of patients were only collected before radiotherapy, thus neglecting the potential impact of previous surgery and/or chemotherapy. Yet, the SOCCAR study, where data was collected before chemotherapy, showed no pre-treatment differences in HRQoL between patients receiving sequential and concurrent chemo-radiotherapy [41]. This may suggest that chemotherapy given prior to radiotherapy already reduces symptom burden.
Additionally, in this study LA-NSCLC patients receiving radiotherapy without chemotherapy reported the highest social functioning, but aside from this, there seems to be no consistency as to which patient group scores best or worst in overall wellbeing and functional HRQoL domains pre-radiotherapy.
In this study, both statistical significance and MCIDs were reported. Applying MCIDs, patient-derived scores that reflect changes in outcomes that are important to the patient, is common practice in HRQoL research [42–45]. Certain patients may still experience clinical improvement or deterioration even if no statistical significance is found for the entire population. Moreover, statistical significance is no guarantee that patients perceive clinical impact. Therefore, to promote patient-centered care it is important to include individual patient experiences. MCID scores allow us to identify the proportion of patients experiencing meaningful changes in treatment outcome. In this study, for example, no statistically significant difference was found in physical functioning (p =.580) over time. However, respectively 39% and 38% of patients reported a meaningful clinically important deterioration in physical functioning at 12 and 24 months after the start of radiotherapy, while 15% and 15% improved. In line with this, toxicity was calculated by subtracting baseline scores to distinguish between treatment-induced toxicity and improvement of pre-radiotherapy symptoms. While this is less common practice, this approach has previously been used in research on the evaluation of dyspnea following high-dose radiotherapy [46].
Our results show no significant impact of radiotherapy on overall HRQoL and its domains over time for the entire population, except for cognitive functioning, which significantly deteriorates with time. Previous research in ES-NSCLC already showed that radiotherapy in general has no impact on HRQoL, apart from an improved emotional functioning in those receiving SBRT [24,47]. However, a more pronounced decline in physical functioning and increase in dyspnea was reported in patients receiving 3D-CRT compared to SBRT for ES-NSCLC, emphasizing the importance of advanced radiotherapy techniques in that study [47].
In contrast to the lack of impact on the entire population, looking at the individual patient level using MCIDs, it becomes obvious that a large percentage of patients experiences a meaningful decline in HRQoL over time. In particular, physical and cognitive functioning are negatively affected. Apart from a deterioration related to the evolution of the disease itself, this suggests that radiotherapy may have long-term negative effects on HRQoL in at least a proportion of the population. This is in line with the results of the RTOG 0617 dose-escalation study. The study showed that 46.4% and 21.1% of patients receiving 3D-CRT and IMRT respectively had a clinically meaningful decline in HRQoL at 12 months post-treatment, while the clinician-reported toxicity profiles showed only minor differences between the two radiotherapy techniques (less severe pneumonitis and lower cardiac doses with IMRT) [26]. Since IMRT led to less of a decline in HRQoL compared to 3D-CRT, routine use of IMRT in LA-NSCLC was recommended. In our study, smaller meaningful declines in overall HRQoL were observed between IMRT and 3D-CRT (26% versus 32% respectively), also favouring IMRT.
Similarly, our results show that although overall toxicity does not significantly differ over time, several radiotherapy-related toxicities gradually decrease, whereas others increase. The typical acute toxicities, such as radiation-dermatitis, dysphagia and esophagitis, appear around 3 months after initiation of radiotherapy and decrease over time, mostly disappearing completely; whereas late toxicities, including radiation pneumonitis, fibrosis, bronchial stricture and pericarditis may persist for years post-radiotherapy. This is in line with the nature of radiotherapy side effects, with acute side effects occurring during or shortly after radiotherapy, while late toxicities gradually emerge in the months after therapy and may evolve till years after treatment.
Lung cancer is characterized by a high symptom burden and presence of co-morbidities. More than half of the patients reported cough (54%) and dyspnea (63%) at baseline. Radiotherapy aims to alleviate tumor-related symptoms, which was observed in 20% and 23% of patients, in which cough and dyspnea respectively improved at three months.
As it is not always easy to differentiate between pre-existing symptoms and side effects, it may also be hard to disentangle the impact of tumor stage and treatment-related approach. As such, even if patients receiving concurrent chemo-radiotherapy report toxicity, they seem to experience better HRQoL than those receiving radiotherapy alone. This could be explained by the fact that patients eligible for concurrent chemo-radiotherapy are generally in better physical health, and have higher baseline physical and social functioning, as shown by our data. Early-stage patients, ineligible for surgery due to comorbidities and poor PS, conversely, have lower baseline physical functioning than LA-NSCLC receiving radiotherapy with(out) chemotherapy, predetermining them for a larger impact of toxicity on their HRQoL.
Current radiotherapy research focuses on limiting dose to organs at risk (such as parotid gland in head and neck cancers and heart in lung cancer) thus reducing side effects and negative impact on HRQoL. As such, dose-volume based prognostic models are developed, predicting the potential clinical benefit or toxicity of a given radiotherapy intervention for a specific patient, thus individualising treatment strategies. This model-based approach has been accepted as the method to generate evidence for proton therapy in the Netherlands [48–50]. To date, prognostic models have been developed for head-and-neck, breast and lung cancer. Providing more insight into and a repository of side effects and HRQoL in different cancer populations, in view of validating models and biomarkers that predict the risk of toxicity following radiotherapy, was the primary objective of the REQUITE study.
A limitation of this study is the use of the EORTC QLQ-C30 questionnaire without the specific lung cancer module (QLQ-LC13) [51]. The EORTC QLQ-C30 is the core questionnaire covering general aspects of HRQoL, whereas the lung cancer module measures additional disease- and treatment-related symptoms. After the launch of the REQUITE study, the QLQ-LC13 was updated resulting in an elaborated module (QLQ-LC29), including toxicities and symptoms related to novel treatments and diagnostic methods [52]. The EORTC recommends using both the core and disease-specific questionnaires [53]. However, an alternative PROM was used to assess lung cancer symptoms [54]. In addition, HRQoL data were obtained directly from the patients, whereas toxicities were scored by physicians. This may have generated some inconsistencies [55]. Clinicians tend to underreport symptoms, particularly more subjective symptoms [56]. Therefore, it is recommended to collect both HRQoL and toxicity with PROMs.
Another limitation of this study, was the patient-drop out, particularly because of death and health deterioration. Missing data is a frequently encountered problem in longitudinal thoracic oncology trials [57,13]. Particularly, patients with poor baseline health and HRQoL are more likely to drop-out. This may have caused bias and limited the generalizability [14]. Therefore, it could be that the data of this article are mainly applicable to lung cancer patients with better prognosis and HRQoL and less symptomatology. However, being a real-world study, it was expected that those with poorer baseline data would be more likely to discontinue the study. To compensate for this, the mixed model method was applied in the analysis, as this approach is capable of dealing with missing data, to optimize internal validity and generalizability [58].
In conclusion, the results of this analysis suggest that radiotherapy can cause acute and late toxicity and may negatively impact HRQoL. In contrast, baseline HRQoL and tumor-related symptoms may also improve in other patients. This further highlights the importance of personalized treatment approaches and to consider both therapy side effects and impact on HRQoL to improve patient-centered decision-making.
Supplementary Material
Acknowledgement
This research was supported by the REQUITE project (www.requite.eu) sharing data. REQUITE received funding from the European Union’s Seventh Framework Programme for research, technological development, and demonstration under grant agreement no. 601826.
We thank all patients who participated in the study and the staff members at participating hospitals. DKFZ thanks Anusha Müller, Kerstin Pieper and Theodor Hanck for valuable data management.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.lungcan.2022.03.010.
References
- [1].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, CA Cancer J. Clin 68 (6) (2018) 394–424, 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- [2].Hanna TP, Shafiq J, Delaney GP, Vinod SK, Thompson SR, Barton MB, The population benefit of evidence-based radiotherapy: 5-Year local control and overall survival benefits, Radiother. Oncol 126 (2) (2018) 191–197, 10.1016/j.radonc.2017.11.004. [DOI] [PubMed] [Google Scholar]
- [3].von Reibnitz D, Shaikh F, Wu AJ, Treharne GC, Dick-Godfrey R, Foster A, Woo KM, Shi W, Zhang Z, Din SU, Gelblum DY, Yorke ED, Rosenzweig KE, Rimner A, Stereotactic body radiation therapy (SBRT) improves local control and overall survival compared to conventionally fractionated radiation for stage I non-small cell lung cancer (NSCLC), Acta Oncol. 57 (11) (2018) 1567–1573, 10.1080/0284186X.2018.1481292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, Cho BC, Planchard D, Paz-Ares L, Faivre-Finn C, Vansteenkiste JF, Spigel DR, Wadsworth C, Taboada M, Dennis PA, Özgüroǧlu M, Antonia SJ, Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC—Update from PACIFIC, J. Thoracic Oncol 15 (2) (2020) 288–293, 10.1016/j.jtho.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].De Ruysscher D, Botterweck A, Dirx M, Pijls-Johannesma M, Wanders R, Hochstenbag M, Dingemans A-MC, Bootsma G, Geraedts W, Simons J, Pitz C, Lambin P, Eligibility for concurrent chemotherapy and radiotherapy of locally advanced lung cancer patients: a prospective, population-based study, Ann. Oncol 20 (1) (2009) 98–102, 10.1093/annonc/mdn559. [DOI] [PubMed] [Google Scholar]
- [6].Scaife JE, Barnett GC, Noble DJ, Jena R, Thomas SJ, West CML, Burnet NG, Exploiting biological and physical determinants of radiotherapy toxicity to individualize treatment, Br. J. Radiol 88 (1051) (2015) 20150172, 10.1259/bjr.20150172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].van Diessen J, De Ruysscher D, Sonke J-J, Damen E, Sikorska K, Reymen B, van Elmpt W, Westman G, Fredberg Persson G, Dieleman E, Bjorkestrand H, Faivre-Finn C, Belderbos J, The acute and late toxicity results of a randomized phase II dose-escalation trial in non-small cell lung cancer (PET-boost trial), Radiother. Oncol 131 (2019) 166–173, 10.1016/j.radonc.2018.09.019. [DOI] [PubMed] [Google Scholar]
- [8].Pijls-Johannesma M, Houben R, Boersma L, Grutters J, Seghers K, Lambin P, Wanders R, Ruysscher DD, High-dose radiotherapy or concurrent chemo-radiation in lung cancer patients only induces a temporary, reversible decline in QoL, Radiother. Oncol 91 (3) (2009) 443–448, 10.1016/j.radonc.2009.02.010. [DOI] [PubMed] [Google Scholar]
- [9].De Ruysscher D, Niedermann G, N.G.B, Siva S, Lee AWM, Hegi-Johnson F, Radiotherapy toxicity, Nat. Rev. Disease Primers 5 (1) (2019) 14, 10.1038/s41572-019-0068-1. [DOI] [PubMed] [Google Scholar]
- [10].Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ, Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy, J. Clin. Oncol 26 (22) (2008) 3770–3776, 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- [11].Ramaekers BLT, Joore MA, Grutters JPC, van den Ende P, Jong J.d., Houben R, Lambin P, Christianen M, Beetz I, Pijls-Johannesma M, Langendijk JA, The impact of late treatment-toxicity on generic health-related quality of life in head and neck cancer patients after radiotherapy, Oral Oncol. 47 (8) (2011) 768–774, 10.1016/j.oraloncology.2011.05.012. [DOI] [PubMed] [Google Scholar]
- [12].Pignol JP, Truong P, Rakovitch E, Sattler MG, Whelan TJ, Olivotto IA, Ten years results of the Canadian breast intensity modulated radiation therapy (IMRT) randomized controlled trial, Radiother. Oncol 121 (3) (2016) 414–419, 10.1016/j.radonc.2016.08.021. [DOI] [PubMed] [Google Scholar]
- [13].van der Weijst L, Surmont V, Schrauwen W, Lievens Y, Systematic literature review of health-related quality of life in locally-advanced non-small cell lung cancer: Has it yet become state-of-the-art? Critic. Rev. Oncol./Hematol 119 (2017) 40–49, 10.1016/j.critrevonc.2017.09.014. [DOI] [PubMed] [Google Scholar]
- [14].Van Der Weijst L, Lievens Y, Schrauwen W, Surmont V, Health-related quality of life in advanced non-small cell lung cancer: A methodological appraisal based on a systematic literature review, Front. Oncol 9 (August) (2019) 1–13, 10.3389/fonc.2019.00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Osoba D, Health-related quality of life and cancer clinical trials, Therap. Adv. Med. Oncol 3 (2) (2011) 57–71, 10.1177/1758834010395342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Damm K, Roeske N, Jacob C, Health-related quality of life questionnaires in lung cancer trials: a systematic literature review, Health Econ. Rev 3 (15) (2013), 10.1186/2191-1991-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bjordal K, Impact of quality of life measurement in daily clinical practice, Ann. Oncol 15 (2004) iv279–iv282, 10.1093/annonc/mdh939. [DOI] [PubMed] [Google Scholar]
- [18].Movsas B, Moughan J, Sarna L, Langer C, Werner-Wasik M, Nicolaou N, Komaki R, Machtay M, Wasserman T, Bruner DW, Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non-small-cell lung cancer: an analysis of RTOG 9801, J. Clin. Oncol 27 (34) (2009) 5816–5822, 10.1200/JCO.2009.23.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mathur A, Challenges in clinical research, Perspect Clin Res 2 (3) (2011) 84, 10.4103/2229-3485.83223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Seibold P, Webb A, Aguado-barrera ME, et al. REQUITE : A prospective multicentre cohort study of patients undergoing radiotherapy for breast, lung or prostate cancer. 2019;138:59–67. doi: 10.1016/j.radonc.2019.04.034. [DOI] [PubMed] [Google Scholar]
- [21].Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, Haes J.C.J.M.d., Kaasa S, Klee M, Osoba D, Razavi D, Rofe PB, Schraub S, Sneeuw K, Sullivan M, Takeda F, The European organization for research and treatment of cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology, J. Natl Cancer Inst 85 (5) (1993) 365–376, 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- [22].Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, et al. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. Published online 1994:1600–1608. [DOI] [PubMed] [Google Scholar]
- [23].Barnett GC, West CML, Coles CE, Pharoah PDP, Talbot CJ, Elliott RM, Tanteles GA, Symonds RP, Wilkinson JS, Dunning AM, Burnet NG, Bentzen SM, Standardized total average toxicity score: A scale- and grade-independent measure of late radiotherapy toxicity to facilitate pooling of data from different studies, Int. J. Radiat. Oncol. Biol. Phys 82 (3) (2012) 1065–1074, 10.1016/j.ijrobp.2011.03.015. [DOI] [PubMed] [Google Scholar]
- [24].van der Voort van Zyp NC, Prévost J-B, van der Holt B, Braat C, van Klaveren RJ, Pattynama PM, Levendag PC, Nuyttens JJ, Quality of life after stereotactic radiotherapy for stage I non-small-cell lung cancer, Int. J. Radiat. Oncol. Biol. Phys 77 (1) (2010) 31–37, 10.1016/j.ijrobp.2009.04.080. [DOI] [PubMed] [Google Scholar]
- [25].Grills IS, Yan D, Martinez AA, Vicini FA, Wong JW, Kestin LL, Potential for reduced toxicity and dose escalation in the treatment of inoperable non-small-cell lung cancer: A comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation, Int. J. Radiat. Oncol. Biol. Phys 57 (3) (2003) 875–890, 10.1016/S0360-3016(03)00743-0. [DOI] [PubMed] [Google Scholar]
- [26].Movsas B, Hu C, Sloan J, Bradley J, Komaki R, Masters G, Kavadi V, Narayan S, Michalski J, Johnson DW, Koprowski C, Curran WJ, Garces YI, Gaur R, Wynn RB, Schallenkamp J, Gelblum DY, MacRae RM, Paulus R, Choy H, Quality of life analysis of a radiation dose-escalation study of patients with non-small-cell lung cancer: A secondary analysis of the radiation therapy oncology group 0617 randomized clinical trial, JAMA oncology. 2 (3) (2016) 359, 10.1001/jamaoncol.2015.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chen H, Louie AV, Boldt RG, Rodrigues GB, Palma DA, Senan S, Quality of life after stereotactic ablative radiotherapy for early-stage lung cancer: A systematic review, Clin. Lung Cancer 17 (5) (2016) e141–e149, 10.1016/j.cllc.2015.12.009. [DOI] [PubMed] [Google Scholar]
- [28].Black N, Patient reported outcome measures could help transform healthcare, BMJ (Online). 346 (7896) (2013) 1–5, 10.1136/bmj.f167. [DOI] [PubMed] [Google Scholar]
- [29].Valderas JM, Kotzeva A, Espallargues M, Guyatt G, Ferrans CE, Halyard MY, Revicki DA, Symonds T, Parada A, Alonso J, The impact of measuring patient-reported outcomes in clinical practice: A systematic review of the literature, Qual. Life Res 17 (2) (2008) 179–193, 10.1007/s11136-007-9295-0. [DOI] [PubMed] [Google Scholar]
- [30].Wintner LM, Sztankay M, Aaronson N, Bottomley A, Giesinger JM, Groenvold M, Petersen MA, van de Poll-Franse L, Velikova G, Verdonck-de Leeuw I, Holzner B, The use of EORTC measures in daily clinical practice—A synopsis of a newly developed manual, Eur. J. Cancer 68 (2016) 73–81, 10.1016/j.ejca.2016.08.024. [DOI] [PubMed] [Google Scholar]
- [31].Oldenburger E, Oldenburger F, Coolbrandt A, Isebaert S, Neyens I, Sevenants A, Van Audenhove C.h., Haustermans K, The use of patient reported outcome measures (PROMs) in palliative radiotherapy: A topical review, Radiother. Oncol 149 (2020) 94–103, 10.1016/j.radonc.2020.04.045. [DOI] [PubMed] [Google Scholar]
- [32].Howell D, Molloy S, Wilkinson K, Green E, Orchard K, Wang K, Liberty J, Patient-reported outcomes in routine cancer clinical practice: a scoping review of use, impact on health outcomes, and implementation factors, Ann. Oncol 26 (9) (2015) 1846–1858, 10.1093/annonc/mdv181. [DOI] [PubMed] [Google Scholar]
- [33].Faithfull S, Lemanska A, Chen T, Patient-reported Outcome Measures in Radiotherapy: Clinical Advances and Research Opportunities in Measurement for Survivorship, Clinical Oncology. 27 (11) (2015) 679–685, 10.1016/j.clon.2015.07.007. [DOI] [PubMed] [Google Scholar]
- [34].Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, Rogak L, Bennett AV, Dueck AC, Atkinson TM, Chou JF, Dulko D, Sit L, Barz A, Novotny P, Fruscione M, Sloan JA, Schrag D, Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial, J. Clin. Oncol 34 (6) (2016) 557–565, 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Denis F, Lethrosne C, Pourel N, Molinier O, Pointreau Y, Domont J, Bourgeois H, Senellart H, Trémolières P, Lizée T, Bennouna J, Urban T, El Khouri C, Charron A, Septans A-L, Balavoine M, Landry S, Solal-Céligny P, Letellier C, Randomized trial comparing a web-mediated follow-up with routine surveillance in lung cancer patients, J. Natl Cancer Inst 109 (9) (2017), 10.1093/jnci/djx029. [DOI] [PubMed] [Google Scholar]
- [36].Denis F, Viger L, Charron A, Voog E, Letellier C, Detecting lung cancer relapse using self-evaluation forms weekly filled at home: The sentinel follow-up, Support. Care Cancer 22 (1) (2014) 79–85, 10.1007/s00520-013-1954-9. [DOI] [PubMed] [Google Scholar]
- [37].LeBlanc TW, Nickolich M, Rushing CN, Samsa GP, Locke SC, Abernethy AP, What bothers lung cancer patients the most? A prospective, longitudinal electronic patient-reported outcomes study in advanced non-small cell lung cancer, Support. Care Cancer 23 (12) (2015) 3455–3463, 10.1007/s00520-015-2699-4. [DOI] [PubMed] [Google Scholar]
- [38].Kroenke K, Cheville AL, Symptom improvement requires more than screening and feedback, JCO. 34 (27) (2016) 3351–3352, 10.1200/JCO.2016.67.7708. [DOI] [PubMed] [Google Scholar]
- [39].Girgis A, Durcinoska I, Arnold A, Descallar J, Kaadan N, Koh E-S, Miller A, Ng W, Carolan M, Della-Fiorentina SA, Avery S, Delaney GP, Web-based patient-reported outcome measures for personalized treatment and care (PROMPT-Care): multicenter pragmatic nonrandomized trial, J Med Internet Res. 22 (10) (2020) e19685, 10.2196/19685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Schwartz RM, Alpert N, Rosenzweig K, Flores R, Taioli E, Changes in quality of life after surgery or radiotherapy in early-stage lung cancer, J Thorac Dis. 11 (1) (2019) 154–161, 10.21037/jtd.2018.12.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Maguire J, Khan I, McMenemin R, O’Rourke N, McNee S, Kelly V, Peedell C, Snee M, SOCCAR: A randomised phase II trial comparing sequential versus concurrent chemotherapy and radical hypofractionated radiotherapy in patients with inoperable stage III Non-Small Cell Lung Cancer and good performance status, Eur. J. Cancer 50 (17) (2014) 2939–2949, 10.1016/j.ejca.2014.07.009. [DOI] [PubMed] [Google Scholar]
- [42].Coens C, Pe M, Dueck AC, Sloan J, Basch E, Calvert M, Campbell A, Cleeland C, Cocks K, Collette L, Devlin N, Dorme L, Flechtner H-H, Gotay C, Griebsch I, Groenvold M, King M, Kluetz PG, Koller M, Malone DC, Martinelli F, Mitchell SA, Musoro JZ, O’Connor D, Oliver K, Piault-Louis E, Piccart M, Quinten C, Reijneveld JC, Schürmann C, Smith AW, Soltys KM, Taphoorn MJB, Velikova G, Bottomley A, International standards for the analysis of quality-of-life and patient-reported outcome endpoints in cancer randomised controlled trials: recommendations of the SISAQOL Consortium, Lancet Oncol. 21 (2) (2020) e83–e96, 10.1016/S1470-2045(19)30790-9. [DOI] [PubMed] [Google Scholar]
- [43].Musoro ZJ, Hamel J-F, Ediebah DE, Cocks K, King MT, Groenvold M, Sprangers MAG, Brandberg Y, Velikova G, Maringwa J, Flechtner H-H, Bottomley A, Coens C, Establishing anchor-based minimally important differences (MID) with the EORTC quality-of-life measures: A meta-analysis protocol, BMJ Open. 8 (1) (2018) e019117, 10.1136/bmjopen-2017-019117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Osoba D, Rodrigues G, Myles J, Zee B, Pater J, Interpreting the significance of changes in health-related quality-of- life scores, J. Clin. Oncol 16 (1) (1998) 139–144, 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- [45].Cook CE, Clinimetrics Corner: The Minimal Clinically Important Change Score (MCID): A Necessary Pretense, Journal of Manual & Manipulative Therapy. 16 (4) (2008) 82E–83E, 10.1179/jmt.2008.16.4.82e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].De Ruysscher D, Dehing C, Yu S, Wanders R, Öllers M, Dingemans A-M, Bootsma G, Hochstenbag M, Geraedts W, Pitz C, Simons J, Boersma L, Borger J, Dekker A, Lambin P, Dyspnea evolution after high-dose radiotherapy in patients with non-small cell lung cancer, Radiother. Oncol 91 (3) (2009) 353–359, 10.1016/j.radonc.2008.10.006. [DOI] [PubMed] [Google Scholar]
- [47].Widder J, Postmus D, Ubbels JF, Wiegman EM, Langendijk JA, Survival and quality of life after stereotactic or 3D-Conformal radiotherapy for inoperable early-stage lung cancer, Int. J. Radiat. Oncol. Biol. Phys 81 (4) (2011) E291–E297, 10.1016/j.ijrobp.2011.03.052. [DOI] [PubMed] [Google Scholar]
- [48].Lievens Y, Access to innovative radiotherapy: how to make it happen from an economic perspective? Acta Oncol. 56 (11) (2017) 1353–1358, 10.1080/0284186X.2017.1348622. [DOI] [PubMed] [Google Scholar]
- [49].KNAW. Evaluation of new technology in health care. In need of guidance for relevant evidence. Amsterdam. Published online 2014. doi: 10.1097/00005650-198706000-00012. [DOI] [Google Scholar]
- [50].Langendijk JA, Lambin P, De Ruysscher D, Widder J, Bos M, Verheij M, Selection of patients for radiotherapy with protons aiming at reduction of side effects: The model-based approach, Radiother. Oncol 107 (3) (2013) 267–273, 10.1016/j.radonc.2013.05.007. [DOI] [PubMed] [Google Scholar]
- [51].Koller M, Warncke S, Hjermstad MJ, Arraras J, Pompili C, Harle A, Johnson CD, Chie W-C, Schulz C, Zeman F, van Meerbeeck JP, Kuliś D, Bottomley A, Use of the lung cancer-specific Quality of Life Questionnaire EORTC QLQ-LC13 in clinical trials: A systematic review of the literature 20 years after its development, Cancer 121 (24) (2015) 4300–4323, 10.1002/cncr.29682. [DOI] [PubMed] [Google Scholar]
- [52].Koller M, Shamieh O, Hjermstad MJ, Hornslien K, Young T, Chalk T, Ioannidis G, Harle A, Johnson CD, Tomaszewski KA, Serpentini S, Pinto M, van der Weijst L, Janssens A, Morag O, Chie W-C, Arraras JI, Pompili C, Jungraithmayr W, Hechtner M, Katsochi D, Müller K, Gräfenstein L, Schulz C, Bottomley A, Psychometric properties of the updated EORTC module for assessing quality of life in patients with lung cancer (QLQ-LC29): an international, observational field study, Lancet Oncol. 21 (5) (2020) 723–732, 10.1016/S1470-2045(20)30093-0. [DOI] [PubMed] [Google Scholar]
- [53].Bergman B, Aaronson NK, Ahmedzai S, Kaasa S, Sullivan M, The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life, Eur. J. Cancer 30A (5) (1994) 635–642, 10.1016/j.ejca.2010.08.021. [DOI] [PubMed] [Google Scholar]
- [54].Christodoulou M, McCloskey P, Stones N, Bayman N, Burt P, Chittalia A, Harris M, Lee L, Pemberton L, Sheikh H, Swindell R, Faivre-Finn C, Investigation of a Patient Reported Outcome tool to assess radiotherapy-related toxicity prospectively in patients with lung cancer, Radiother. Oncol 112 (2) (2014) 244–249, 10.1016/j.radonc.2014.07.008. [DOI] [PubMed] [Google Scholar]
- [55].Basch E, Jia X, Heller G, et al. , Adverse symptom event reporting by patients vs clinicians: Relationships with clinical outcomes, J. Natl Cancer Inst 101 (23) (2009) 1624–1632, 10.1093/jnci/djp386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Farr KP, Khalil AA, Grau C, Patient-reported lung symptoms and quality of life before and after radiation therapy for non-small cell lung cancer: correlation with radiation pneumonitis and functional imaging, Acta Oncol. 58 (10) (2019) 1523–1527, 10.1080/0284186X.2019.1634835. [DOI] [PubMed] [Google Scholar]
- [57].Hollen PJ, Gralla RJ, Cox C, Eberly SW, Kris MG, A dilemma in analysis: issues in the serial measurement of quality of life in patients with advanced lung cancer, Lung Cancer 18 (2) (1997) 119–136. [DOI] [PubMed] [Google Scholar]
- [58].Bottomley A, Pe M, Sloan J, Basch E, Bonnetain F, Calvert M, Campbell A, Cleeland C, Cocks K, Collette L, Dueck AC, Devlin N, Flechtner H-H, Gotay C, Greimel E, Griebsch I, Groenvold M, Hamel J-F, King M, Kluetz PG, Koller M, Malone DC, Martinelli F, Mitchell SA, Moinpour CM, Musoro J, O’Connor D, Oliver K, Piault-Louis E, Piccart M, Pimentel FL, Quinten C, Reijneveld JC, Schürmann C, Smith AW, Soltys KM, Taphoorn MJB, Velikova G, Coens C, Analysing data from patient-reported outcome and quality of life endpoints for cancer clinical trials: a start in setting international standards, Lancet Oncol. 17 (11) (2016) e510–e514, 10.1016/S1470-2045(16)30510-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


