Abstract
While laccase of Cryptococcus neoformans is implicated in the virulence of the organism, our recent studies showing absence of melanin in the infected mouse brain has led us to a search for alternative roles for laccase in cryptococcosis. We investigated the role of laccase in protection of C. neoformans against murine alveolar macrophage (AM)-mediated antifungal activity by using a pair of congenic laccase-positive (2E-TUC) and laccase-deficient (2E-TU) strains. The laccase-positive cells with laccase derepression were more resistant to the antifungal activity of AM than a laccase-deficient strain ([28.9 ± 1.2]% versus [40.2 ± 2.6]% killing). Addition of l-dopa to Cryptococcus to produce melanin in a laccase-positive strain resulted in a slight increase in protection of C. neoformans from the antifungal activity of macrophages ([25.4 ± 3.4]% versus [28.9 ± 1.2]% killing). Recombinant cryptococcal laccase exhibited iron oxidase activity in converting Fe(II) to Fe(III). Moreover, recombinant laccase inhibited killing of C. neoformans by hydroxyl radicals catalyzed by iron in a cell-free system. Addition of the hydroxyl radical scavenger mannitol or dimethyl sulfoxide to AMs prior to the introduction of cryptococcal cells decreased killing of both strains and reduced the difference in susceptibility between the laccase-positive and laccase-deficient strains. Furthermore, laccase-mediated protection from AM killing was inhibited by the addition of Fe(II), presumably by overcoming the effects of the iron oxidase activity of cryptococcal laccase. These results suggest that the iron oxidase activity of laccase may protect C. neoformans from macrophages by oxidation of phagosomal iron to Fe(III) with a resultant decrease in hydroxyl radical formation.
Cryptococcus neoformans is an opportunistic pathogenic fungus infecting 6 to 10% of patients with AIDS as well as other immune-suppressed individuals (33). The abilities to form a capsule (7) and to grow at 37°C (23) are two well-recognized virulence factors of C. neoformans. CNLAC1, the structural gene of laccase of C. neoformans, has also been associated with the virulence of the organism in a mouse model (36, 43). Laccase oxidizes exogenous catecholamine substrates to produce melanin in vitro, which has been proposed to protect the fungus by virtue of its antioxidant properties (41, 42). However, we have recently observed that laccase-derived catecholamine oxidation products were produced in mouse brains infected with C. neoformans without the production of melanin products (27). This suggests that laccase-derived products, or even laccase itself, may have an important role in the pathogenesis of C. neoformans infection.
Reactive oxygen and nitrogen intermediates are important mediators of the activity of macrophages and neutrophils against a variety of microorganisms, including C. neoformans (2, 15, 16, 28, 30, 40). Iron catalyzes the generation of hydroxyl radicals from oxidant precursors, such as superoxide anion (O2·−) and hydrogen peroxide (H2O2) (35). This property has been shown to be an important bactericidal factor against Listeria monocytogenes, Staphylococcus aureus, and Pseudomonas aeruginosa (1, 4, 6, 18). Iron-loaded macrophages have an enhanced ability to kill or prevent replication of Brucella abortus, most likely by enhancing hydroxyl radical formation, and this effect is augmented by gamma interferon (22). The importance of hydroxyl radicals in the anticryptococcal activity of human neutrophils is supported by evidence that the susceptibility of Cryptococcus is decreased by addition of hydroxyl radical scavengers but markedly increased in the presence of iron (8). In addition, the anticryptococcal activity of nitric oxide (NO), produced by activated murine macrophages and human astrocytes (2, 15, 24, 26, 39), may also involve hydroxyl radicals. Recently, Farias-Eisner and colleagues reported that the cytotoxicity of NO to a human ovarian tumor cell line is mediated through the interaction of NO with H2O2 to produce hydroxyl radicals with iron as a catalyst by using a SIN-1 system as an NO donor (11). The toxicity of NO was increased by H2O2 but not by O2·−, and NO and H2O2 toxicity was markedly increased by catalytic quantities of Fe(III). Therefore, production of highly toxic hydroxyl radicals catalyzed by iron from both reactive oxygen and nitrogen intermediates appears to be important for antimicrobial and antitumor activities of phagocytic cells.
In the present study, we investigated the effect of laccase on mouse alveolar macrophage (AM)-mediated fungal killing by using congenic laccase-positive (2E-TUC) and laccase-deficient (2E-TU) strains. The use of congenic strains enabled us to compare the effect of laccase on cryptococcal killing by AMs in the absence of melanin. We propose that the enzyme laccase, by virtue of a newly described iron oxidase activity, may help protect C. neoformans from AM-mediated killing by maintaining macrophage iron stores in an inert, oxidized form, thereby limiting formation of hydroxyl radicals.
MATERIALS AND METHODS
Reagents.
All chemicals were from Sigma (St. Louis, Mo.) unless otherwise indicated. Diff Quick stain was from Dade International Inc., Aguada, Puerto Rico; RPMI 1640, Hanks balanced salt solution, l-glutamine, and penicillin-streptomycin were from Life Technologies, Grand Island, N.Y.; heparin was from Elkins Sinn Inc., Cherry Hill, N.Y.; defined fetal bovine serum was from HyClone, Logan, Utah; and SIN-1 chloride [1,2,3,-oxadiazolium 5-amino-3-(4-morpholinyl)-chloride] was from Cayman Chemical, Ann Arbor, Mich.
Mice.
Female mice (NIH-Swiss) 7 to 8 weeks old were obtained from Harlan Industries, Indianapolis, Indiana. The animals were housed in microisolator cages and kept at the Biological Resources Laboratory of the University of Illinois at Chicago.
Preparation of C. neoformans.
A pair of congenic strains, 2E-TUC (laccase-positive) and 2E-TU (laccase-deficient), were grown on Sabouraud dextrose agar for 48 h at 30°C. Laccase expression was derepressed by growing fungal cells on asparagine agar (1 g of asparagine, 0.25 g of MgSO4, 3 g of sodium phosphate, 10 g of agar per liter, pH 6.5) without glucose for 24 h. These conditions have been used previously to detect laccase activity by whole-cell assay (44). l-Dopa (100 mg/liter) was added to the asparagine agar to confirm laccase expression or when melanin production was desired in the laccase-positive strain. Laccase-deficient cells were grown under identical conditions when they were used in the study.
Preparation of mouse AMs.
AMs were obtained as previously described (25). Briefly, after incising the skin, the trachea was isolated and a 20-gauge catheter was inserted. One milliliter of warm Hanks balanced salt solution with 10 U of heparin/ml and 50 U of penicillin-streptomycin/ml was injected into the lung through the catheter, and macrophages were collected. The lavage was repeated five times, which yielded 2 × 105 to 5 × 105 cells from each mouse. The cells were cultured in RPMI 1640 with 10% fetal calf serum, 1% l-glutamine, and 100 U of penicillin-streptomycin/ml at 37°C, 5% CO2, and 95% O2 for 2 h. Nonadherent cells were removed by washing the culture with warm medium.
AM killing of C. neoformans.
Fungicidal assays of AMs were performed in 96-half-well microtiter plates (Corning, Corning, N.Y.) by a modified method (25). Briefly, 104 opsonized C. neoformans cells (with 10% pooled mouse serum) grown on asparagine agar with or without l-dopa were added to adherent AMs at an effector-to-target ratio of 20:1 for 24 h. The AMs were subsequently lysed by 0.1% Triton X-100, and the C. neoformans cells were plated on Sabouraud agar by serial dilution. CFU were counted after incubation at 30°C for 48 h. Equivalent amounts of Cryptococcus cells grown in the culture medium without macrophages for 24 h were treated identically with Triton X-100, and CFU were recorded as a control. Mannitol (50 mM), dimethyl sulfoxide (DMSO; 200 mM), superoxide dismutase (SOD; 15 and 100 μg/ml as indicated below), or 100 μM Fe(II) was added to macrophage cultures 2 h before addition of C. neoformans. The results were expressed as the percentage of C. neoformans killed [1 − (CFU obtained in the presence of macrophages/CFU of the same strain in the absence of macrophages) × 100]. The growth rates of the two cryptococcal strains in tissue culture medium with or without oxidant inhibitors after incubation under laccase-derepressing conditions were equivalent (ratios of CFU at 24 h/CFU of initial inoculum [0 h], 1.50 ± 0.21 [2E-TUC] and 1.47 ± 0.21 [2E-TU]), and the number of CFU obtained was not affected by Triton exposure. The laccase activities of Cryptococcus cells before and after incubation in macrophage culture medium after growth under laccase-derepressing conditions were measured by a previously described method (45). The laccase activities of 2E-TUC remained similar before and after incubation in macrophage culture medium for 24 h at 37°C and 5% CO2 (A415 = 0.823/5 × 107 cells and 0.817/5 × 107 cells, respectively). In separate experiments, incubation of macrophages with 100 μM ferrous ammonium sulfate had no impact on viability as determined by trypan blue staining.
Phagocytosis of C. neoformans.
Macrophage-pathogen association was determined as described previously (25). Briefly, 4 × 105 AMs were grown in 9-mm-diameter chamber slides at 37°C, 5% CO2, and 95% O2 for 2 h. Opsonized C. neoformans cells grown with or without l-dopa substrate for melanin biosynthesis were then added to the AMs at an effector-to-target ratio of 1:20. After 2 h of incubation at 37°C and 5% CO2, nonattached C. neoformans cells were removed by washing the cell culture with phosphate-buffered saline. The slides were stained with Diff Quick stain, and phagocytosis was assessed by counting 100 macrophages. The number of AMs attached to or ingesting C. neoformans cells was recorded. Five randomly selected fields were counted. The phagocytosis index was expressed as the mean percentage of AMs attaching to or ingesting one or more organisms per 100 AMs ± standard error (SE) as defined previously (25, 42).
Iron oxidase activity of recombinant laccase.
Recombinant laccase was expressed in Pichia pastoris and purified by DEAE-Sephacel chromatography to a single band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described previously (44). Enzyme activity was measured by using epinephrine substrate as described previously (43). Laccase-catalyzed Fe(II) oxidation was measured by addition of purified recombinant laccase (10,000 U) to 200 μl of buffer containing 0.1 M sodium acetate, pH 5, and 100 μM sodium citrate containing 100 μM ferrous ammonium sulfate (37). The reaction was quenched by the addition of 50 μl of ferrozine (15 mM) at 3-min intervals. The Fe(II) remaining in the solution was quantified by measuring absorbance at 570 nm. In order to control for possible minor contaminants not observed on SDS-PAGE, equivalent amounts of purified supernatant of untransformed P. pastoris (GS115) was used as a negative control.
SIN-1-mediated killing of C. neoformans.
The fungicidal activity of oxidants generated by SIN-1 chloride was determined by a previously described method (11). Cryptococcus cells (106) resuspended in iron-free saline were incubated with 1 ml of 5 mM SIN-1 chloride in the presence of SOD (200 U/ml) and/or catalase (400 U/ml) for 24 h at 30°C. Ferric chloride (600 μM) was added as indicated below to catalyze SIN-1-mediated hydroxyl radical production. The cells were subsequently diluted and plated on Sabouraud agar plates. The CFU were counted after incubation for 48 h at 30°C. To study the effect of laccase on SIN-1-mediated Cryptococcus killing, 10,000 U of purified recombinant laccase was added. In a separate experiment, Cryptococcus cells were incubated with saline or 600 μM ferric chloride for 24 h at 30°C. Iron had no effect on the growth of Cryptococcus cells, as shown by a comparison of the number of CFU to that in the presence of saline.
Statistics.
All experiments were repeated at least twice, and each value was measured in duplicate. The results are expressed as means ± SEs. Student's t test was used to determine significance (P < 0.05) by comparing values from two different groups.
RESULTS
Protection by laccase from AM-mediated killing.
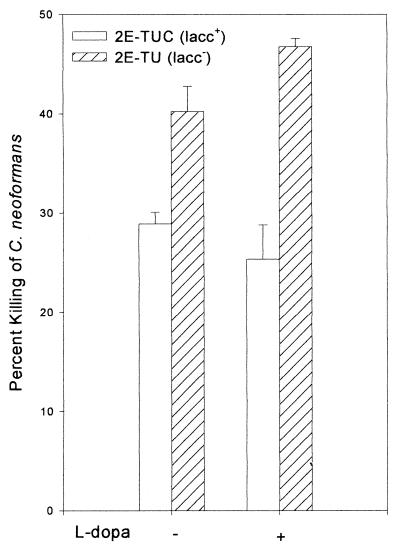
To determine the significance of laccase in cryptococcosis, we studied the effect of the enzyme on fungicidal activity of mouse AMs. Laccase expression in C. neoformans was derepressed after the organism was cultured on asparagine salt agar without glucose at 30°C for 24 h. laccase-positive cells (2E-TUC) grown under conditions of laccase derepression but without l-dopa substrate were more resistant to the antifungal activity of alveolar macrophages than those of the laccase-deficient 2E-TU strain ([28.9 ± 1.2]% killed versus [40.2 ± 2.6]%; P = 0.0037 [Fig. 1]). The lack of suitable catecholamine substrates in the macrophage culture medium and the lack of visible pigmentation during the incubation time make it highly unlikely that significant melanin could have been formed. Addition of l-dopa to the induction medium to form melanin-like pigment on cryptococcal cell walls resulted in an increase in the resistance of the laccase-positive strain ([25.4 ± 3.4]% killed) which was not significantly different (P > 0.05) from macrophage-mediated killing of C. neoformans without l-dopa. Thus, laccase contributes significant protection against macrophage-mediated killing of C. neoformans even in the absence of l-dopa substrate for melanin biosynthesis during the laccase derepression period.
FIG. 1.
Effect of laccase on antifungal activity of AMs. A macrophage-mediated fungicidal-activity assay was performed as described in Materials and Methods. The congenic laccase-positive (lacc+; 2E-TUC) and laccase-deficient (lacc−; 2E-TU) strains were grown in the presence (+) or absence (−) of l-dopa as described. Each bar represents the mean value ± SE of five separate experiments.
Effect of laccase on phagocytosis of C. neoformans by AMs.
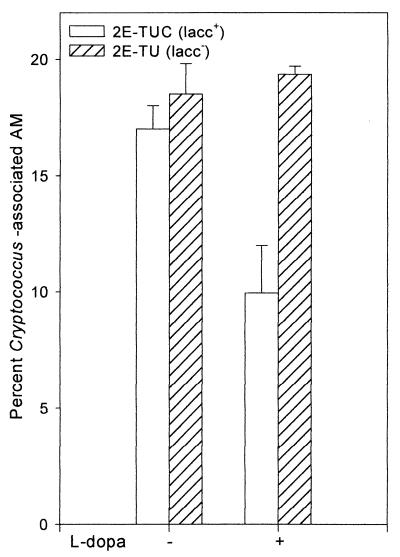
In view of the significant protection of C. neoformans by laccase from the fungicidal activity of lung macrophages, we examined whether laccase had an effect on cryptococcus-macrophage interactions. While melanin produced by C. neoformans in the presence of l-dopa inhibited phagocytosis by AMs ([10.0 ± 2.0]% with the laccase-positive strain versus [19.4 ± 0.4]% with the laccase-deficient strain), the phagocytosis indices of the two strains were similar in the absence of l-dopa substrate ([17.0 ± 1.0]% versus [18.2 ± 1.3]%) (Fig. 2). This is an expected result, since the presence of a small amount of cell wall laccase within an encapsulated strain would not be expected to alter surface properties, in contrast to the charge alterations described for melanin (42).
FIG. 2.
Effect of laccase on phagocytosis of C. neoformans by AMs. Macrophages (4 × 105) were incubated with 8 × 106 opsonized 2E-TUC or 2E-TU cells grown with (+) or without (−) l-dopa substrate for melanin biosynthesis for 2 h at 37°C and 5% CO2. Phagocytosis index = {[number of AMs attaching to or ingesting one or more organisms/100 AMs] × 100} ± SE (n = 2).
Iron oxidase activity of recombinant laccase.
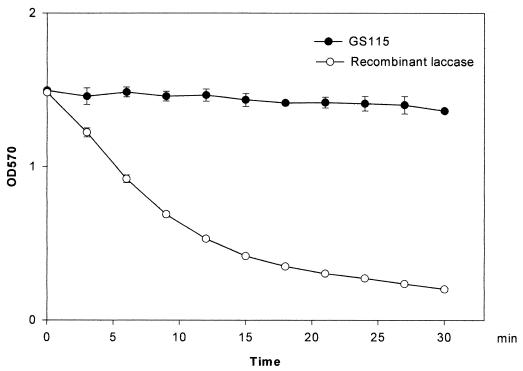
In order to investigate mechanisms for laccase protection in the absence of melanin, alternative enzyme activities were examined. We recently observed that laccase exhibits homology in its copper-binding regions to another copper enzyme, the iron transporter Fet3p, which has been shown to have iron oxidase activity (37). We tested recombinant cryptococcal laccase expressed in P. pastoris purified to homogeneity (44) and found that recombinant laccase possessed potent iron oxidase activity as well. Laccase-catalyzed Fe(II) oxidation was measured by the addition of purified recombinant laccase to a reaction mixture containing ferrous ammonium sulfate in metal-free buffer. To control for the presence of possible contaminating enzyme activity not detectable by SDS-PAGE, a supernatant of untransformed P. pastoris (GS115) purified in an identical fashion was used as a negative control. As shown in Fig. 3, the content of Fe(II) remained constant during 30 min of incubation with GS115, whereas the Fe(II) concentration decreased dramatically with 10,000 U of recombinant laccase.
FIG. 3.
Iron oxidase activity of recombinant laccase. Iron oxidase activity was measured as described in Materials and Methods. A purified supernatant from untransformed P. pastoris (GS115) was used as a negative control. The error bars indicate SE. OD570, optical density at 570 nm.
Effect of iron and recombinant laccase on killing of laccase-deficient strain by SIN-1 oxidants.
Since redox-active iron catalyzes the generation of hydroxyl radicals, we investigated the effect of hydroxyl radicals on killing mediated by cell-free oxidants generated from SIN-1 chloride. As shown in scheme 1 below, SIN-1 decomposes in a predictable manner to produce NO and O2·− in equimolar quantities. Addition of SOD converts O2·− to H2O2 plus O2. NO reduces Fe(III) to Fe(II), which reacts with H2O2 to produce highly cytotoxic hydroxyl radicals. The formation of hydroxyl radicals is accompanied by the oxidation of Fe(II) back to Fe(III). The addition of catalase instead of iron reduces H2O2 to nontoxic H2O. NO in the overall reaction is converted to NO2− and NO3−.
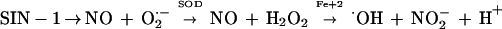 |
1 |
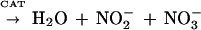 |
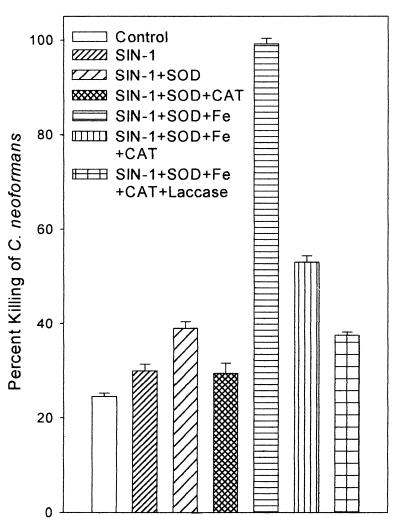
As shown in Fig. 4, 5 mM SIN-1 chloride was more toxic to 2E-TU than iron-free saline. The addition of SOD enhanced SIN-1 fungicidal activity from (30 ± 1.4)% to (39 ± 1.4)% (P = 0.02). The addition of ferric chloride, which catalyzes the formation of hydroxyl radicals, dramatically increased killing, to nearly 100%. This in vitro experiment shows that hydroxyl radicals are highly toxic to C. neoformans. The addition of catalase reduced SIN-1-mediated killing with or without Fe augmentation by reducing the availability of hydrogen peroxide substrate (P = 0.01 with SIN plus SOD plus Fe plus catalase and P = 0.03 with SIN plus SOD plus catalase compared to killing in the presence of SIN plus SOD). Killing was further reduced by 10,000 U of recombinant laccase (P = 0.005 compared to killing in the presence of SIN plus SOD plus Fe plus catalase).
FIG. 4.
Effect of SOD, catalase (CAT), iron, and recombinant laccase on SIN-1-mediated killing of 2E-TU. Each bar represents the mean percentage killed of 106 C. neoformans cells incubated at 30°C for 24 h with 5 mM SIN-1 under the conditions specified. All values represent the mean ± SE of at least two experiments, each performed in duplicate.
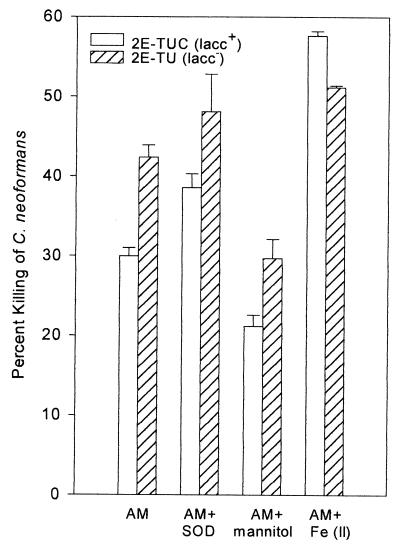
FIG. 5.
Effect of oxidant inhibitors and iron on macrophage-mediated cryptococcal killing. A macrophage-killing assay was performed in the presence of 15 μg of SOD/ml, 50 mM mannitol, or 100 μM Fe(II). Each bar represents the mean value ± SE of two experiments, each performed in duplicate.
Roles of iron and hydroxyl radical formation in AM-mediated killing.
To determine the significance of hydroxyl radicals in macrophage-mediated cryptococcal killing, the susceptibilities of the laccase-positive 2E-TUC and the laccase-deficient 2E-TU strains to AMs were compared in the presence or absence of hydroxyl radical scavengers. Macrophage killing of both strains was inhibited by the hydroxyl radical scavengers mannitol and DMSO. The AM-mediated activity against 2E-TUC was decreased from (29.9 ± 0.9)% to (22.2 ± 3.5)%, and that against 2E-TU was decreased from (42.2 ± 2.5)% to (29.6 ± 4.5)% in the presence of mannitol (Fig. 5). In a separate experiment, the hydroxyl radical scavenger DMSO was tested. The results showed that DMSO reduced killing by (7.8 ± 0.4)% for 2E-TUC and by (17.7 ± 1)% for 2E-TU. In both cases, not only did the hydroxyl radical scavengers decrease killing by AMs, but the apparent protective effect of laccase in the 2E-TUC strain was reduced (P = 0.1 in the presence of mannitol; P = 0.5 in the presence of DMSO). This suggests that hydroxyl radicals are important in AM-mediated killing of C. neoformans and that laccase, mannitol, and DMSO have a common target—reduction of hydroxyl radical concentration. Low concentrations of SOD (15 μg/ml), which catalyzes production of H2O2 from O2·−, did not significantly increase AM killing of either strain (2E-TUC, P > 0.5; 2E-TU, P > 0.1) (Fig. 5). However, at higher concentrations (100 μg/ml) in a separate experiment (not shown), SOD increased killing of 2E-TUC from 11% to 25.3% and that of 2E-TU from 18% to 38.4%. Notable was the apparent continued protection of laccase in 2E-TUC relative to that of 2E-TU in the presence of high concentrations of SOD. The increase in killing by SOD at first appears unexpected, as hydrogen peroxide has been shown to be poorly fungicidal (9, 19). However, if hydroxyl radical formation potentiates AM-mediated killing, conversion of superoxide to hydrogen peroxide by SOD would be expected to increase killing, since hydrogen peroxide is a more potent reagent than superoxide in Fe-mediated hydroxyl radical formation (35).
To further confirm the role of laccase in AM-derived hydroxyl radical production, killing assays were performed in the presence of 100 μM Fe(NH4)2(SO4)2 to determine if exogenous Fe(II) overwhelms the protective effect of laccase against AM-mediated killing. As shown in Fig. 5, exogenous Fe(II) increased AM killing of both laccase-positive and laccase-deficient strains. In addition, the enhancement was more pronounced in the laccase-positive strain (an increase from [29.9 ± 0.9]% to [57.6 ± 0.6]%; P = 0.002) than in the laccase-deficient strain (from [42.2 ± 2.5]% to [51.2 ± 0.3]%; P = 0.03). These results indicate that Fe(II) potentiates killing of Cryptococcus and that Fe(II) abolishes the protective effect of laccase, presumably by overcoming the oxidation of Fe(II) to Fe(III) by cryptococcal laccase, thus restoring the ability of macrophages to produce cytotoxic hydroxyl radicals.
DISCUSSION
Melanin formation in vitro by C. neoformans has been associated with the virulence of the organism since its description by Staib in 1962 (38). Furthermore, products derived from brain catecholamines such as melanin have been implicated in explaining the neurotrophism of this organism (34) and have prompted extensive studies on the immunobiology of cryptococcal melanin (43). For example, Wang and colleagues suggested from their in vitro work that melanin inhibits both phagocytosis and fungistasis of neutrophils and macrophages by altering the cell surface charge and scavenging oxidant radicals (41, 42). Recently, however, it has been found that while laccase-derived catecholamine oxidation products were produced in mouse brains infected with laccase-positive strains of C. neoformans, there was no evidence of polymerized melanin (27). These catecholamine oxidation products have also been detected by phage-displayed peptides that cross-react with melanin pigments (31). Since genetic knockout experiments clearly implicate laccase in virulence (36), we sought to discover if the laccase enzyme activity also contributed to the virulence of the pathogen.
We investigated the role of laccase in the antifungal activity of murine AMs by using two congenic cryptococcal strains, 2E-TUC (laccase-positive) and 2E-TU (laccase-deficient), that had been shown previously to exhibit laccase-dependent differences in virulence (36). The presence of a stable mating type in C. neoformans allows backcrossing of genetically engineered strains to produce congenic sets such as 2E-TUC and 2E-TU. Backcrossing removes extraneous genetic defects introduced during molecular biological manipulations, thus making the strains genetically and phenotypically identical except for the gene of interest. Using these strains, we found that laccase protected C. neoformans from macrophage-mediated killing. Addition of exogenous catecholamines to form melanin in these strains resulted in a small amount of additional protection that was not statistically significant. These results do not conflict with previous immunologic evaluations of cryptococcal melanin because those studies which implicated melanin alone in laccase-dependent protection did not assess macrophage-mediated killing of C. neoformans in the presence of laccase but without melanin (41, 42).
Since the laccase enzyme was shown to confer protection against AM-mediated killing without the addition of exogenous substrates of melanin, alternative enzyme activities were investigated. Fungal laccases, such as that from C. neoformans, belong to the family of blue multicopper oxidase proteins that includes ascorbate oxidase and ceruloplasmin (29). Recently, the iron transporter Fet3 from Saccharomyces cerevisiae has been shown to be a member of this family by sequence homology and to exhibit a potent iron oxidase activity previously described for ceruloplasmin (37). The Fet3 protein oxidizes iron from Fe(II) to Fe(III) during transport across the plasma membrane. In the present study we found that cryptococcal laccase shares iron oxidase activity with the Fet3 protein and ceruloplasmin. It is the first report of iron oxidase activity in a laccase from any source. The presence of an iron oxidase in C. neoformans may at first seem unexpected, since it is the reduced form of iron that is taken up by the organism during growth (20). Indeed, iron reductase activity has been found in C. neoformans during log-phase growth (32), presumably required to convert environmental Fe(III) to the reduced Fe(II) form prior to transport by a Fet3-like protein. During this time of increased iron requirement, laccase expression is strongly inhibited by both glucose (43) and a cell-cycle E2F-like transcription regulator (45). However, laccase is transcribed in vitro during stationary phase (43), presumably when iron requirements are reduced. During infection in rabbit brains laccase is also transcribed, emphasizing the importance of laccase activity in virulence, despite the possible negative effects on fungal iron uptake (36). The finding of the iron oxidase activity of laccase suggests a protective mechanism for this enzyme which may be independent of host catecholamines that have been classically associated with laccase-dependent virulence (34). A role for laccase iron oxidase activity may be especially important in tissues such as the lung, where the concentration of catecholamine substrates is approximately 10-fold lower than that within dopaminergic pathways of the brain (21).
In the present studies, the chemical species SIN-1 was used as a generator of the cytotoxic metabolites NO, O2−, and H2O2 in order to assess the laccase protection of C. neoformans by its iron oxidase activity. SIN-1 demonstrated a moderate amount of killing of C. neoformans, as described previously for a human ovarian cell line (11). The importance of hydroxyl radicals in cryptococcal killing was demonstrated by a dramatic increase in killing after the addition of catalytic amounts of Fe(III) to SIN-1 and SOD. These data corroborate a previous report by Chaturvedi et al. (8), which implicated hydroxyl radicals in neutrophil-mediated killing of this organism. Protection against hydroxyl radical-mediated killing was demonstrated in this study by catalase, which is also produced by C. neoformans (12). The protective effect of catalase was augmented by further addition of recombinant laccase. Hydroxyl radicals may be produced either by the interaction of O2·− with H2O2 according to the Haber-Weiss reaction or through the interaction of NO and H2O2 (11), both of which are catalyzed by iron. Reduction of H2O2 by catalase thus decreases hydroxyl radical production. In addition, since Fe(II) is required to catalyze the formation of hydroxyl radicals (scheme 1), the iron oxidase activity of laccase may compete with reductants such as NO and subsequently limit hydroxyl radical formation.
Hydroxyl radicals appear to be important in macrophage-mediated killing of C. neoformans, since the hydroxyl radical scavengers DMSO and mannitol reduce killing by lung macrophages and the addition of exogenous iron increases it. These data are similar to those showing hydroxyl radical-dependent killing of C. neoformans by neutrophils (8) and are the first to show the importance of this species in macrophage cryptococcal killing. Reduced quantities of hydroxyl radicals produced by macrophages generally preclude direct measurement of this species by techniques such as those involving electron paramagnetic resonance (EPR)-active spin labels used for quantifying oxygen radicals in neutrophils (5, 6). In addition, since EPR-active spin labels require hydroxyl radical scavengers such as DMSO to identify and confirm radical-dependent signals, the use of these compounds may not strengthen the present data, which include the reduction of killing effect by the same hydroxyl radical scavengers (35). In an earlier study, Levitz and Dibenedetto (25) did not find a significant oxidative burst in cryptococcal killing by AMs, but it is important to recognize that they used a shorter incubation time (2 h) than the 24 h used in our studies. Oxidative mechanisms of cryptococcal killing by macrophages may thus be a relatively late phenomenon, with nonoxidative mechanisms predominating early. Nonoxidative mechanisms may involve fungicidal compounds, such as microbicidal proteins (17), defensins (14), or lysosomal enzymes (13).
Taken together, the data support a proposed mechanism for cryptococcal laccase protection against the antifungal activity of AMs which is mediated by the enzyme's iron oxidase activity. According to this scheme, Fe(III) is reduced to Fe(II) in the phagosome by reductants, which may include NO in murine macrophages (2, 15), microglia cells (3), human astrocytes (24), and macrophages within cryptococcal granulomas (10) or other reductants, such as ascorbate or reduced glutathione, in other human macrophages (26). Fe(II) then reduces H2O2 to hydroxyl radicals with conversion of iron back to Fe(III). Cryptococcal laccase-iron oxidase activity may thus protect the fungus in concert with laccase products and other identified virulence factors by maintaining Fe in an oxidized form, thereby decreasing production of antifungal hydroxyl radicals.
ACKNOWLEDGMENTS
This work was funded in part by NIH grant AI38258 and a Young Investigator Award from the Infectious Diseases Society of America and Ortho-McNeil Pharmaceuticals.
We thank David Pitrak for technical assistance and helpful discussions.
REFERENCES
- 1.Alford C E, King T E, Jr, Campbell P A. Role of transferrin, transferrin receptors, and iron in macrophage listericidal activity. J Exp Med. 1991;174:459–466. doi: 10.1084/jem.174.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alspaugh J A, Granger D L. Inhibition of Cryptococcus neoformans replication by nitrogen oxides supports the role of these molecules as effectors of macrophage-mediated cytostasis. Infect Immun. 1991;59:2291–2296. doi: 10.1128/iai.59.7.2291-2296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blasi E, Barluzzi R, Mazzola R, Tancini B, Saleppico S, Puliti M, Pitzurra L, Bistoni F. Role of nitric oxide and melanogenesis in the accomplishment of anticryptococcal activity by the BV-2 microglia cell line. J Neuroimmunol. 1995;58:111–116. doi: 10.1016/0165-5728(95)00016-u. [DOI] [PubMed] [Google Scholar]
- 4.Britigan B E, Edeker B L. Pseudomonas and neutrophil products modify transferrin and lactoferrin to create conditions that favor hydroxyl radical formation. J Clin Investig. 1991;88:1092–1102. doi: 10.1172/JCI115408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britigan B E, Rosen G M, Chai Y, Cohen M S. Do human neutrophils make hydroxyl radical? J Biol Chem. 1986;261:4426–4431. [PubMed] [Google Scholar]
- 6.Britigan B E, Ratcliffe H R, Buettner G R, Rosen G M. Binding of myeloperoxidase to bacteria: effect on hydroxyl radical formation and susceptibility to oxidant-mediated killing. Biochim Biophys Acta. 1996;1290:231–240. doi: 10.1016/0304-4165(96)00014-1. [DOI] [PubMed] [Google Scholar]
- 7.Chang Y C, Kwon-Chung K J. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol Cell Biol. 1994;14:4912–4919. doi: 10.1128/mcb.14.7.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaturvedi V, Wong B, Newman S L. Oxidative killing of Cryptococcus neoformans by human neutrophils. Evidence that fungal mannitol protects by scavenging reactive oxygen intermediates. J Immunol. 1996;156:3836–3840. [PubMed] [Google Scholar]
- 9.Diamond R D, Root R K, Bennett J E. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis. 1972;125:367–376. doi: 10.1093/infdis/125.4.367. [DOI] [PubMed] [Google Scholar]
- 10.Facchetti F, Vermi W, Fiorentini S, Chilosi M, Caruso A, Duse M, Notarangelo L D, Badolato R. Expression of inducible nitric oxide synthase in human granulomas and histiocytic reactions. Am J Pathol. 1999;154:145–152. doi: 10.1016/S0002-9440(10)65261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farias-Eisner R, Chaudhuri G, Aeberhard E, Fukuto J M. The chemistry and tumoricidal activity of nitric oxide/hydrogen peroxide and the implications to cell resistance/susceptibility. J Biol Chem. 1996;271:6144–6151. doi: 10.1074/jbc.271.11.6144. [DOI] [PubMed] [Google Scholar]
- 12.Fiskin A M, Zalles M C, Garrison R G. Electron cytochemical studies of Cryptococcus neoformans grown on uric acid and related sources of nitrogen. J Med Vet Mycol. 1990;28:197–207. [PubMed] [Google Scholar]
- 13.Gadebusch H H. On the mechanism of cytotoxicity by cationic tissue proteins for Cryptococcus neoformans. Z Naturforsch Sect B. 1966;21:1048–1051. [Google Scholar]
- 14.Ganz T, Selsted M E, Szklarek D, Harwig S S L, Daher K, Bainton D F, Lehrer R I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Investig. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granger D L, Hibbs J B, Jr, Perfect J R, Durack D T. Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J Clin Investig. 1988;81:1129–1136. doi: 10.1172/JCI113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hibbs J B, Jr, Taintor R R, Vavrin Z. Macrophage cytotoxicity: role for L-arginine deaminase and amino nitrogen oxidation to nitrite. Science. 1987;197:473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- 17.Hiemstra P S, Eisenhauer P B, Harwing L S, van den Barselaar M T, van Furth R, Lehrer R I. Antimicrobial proteins of murine macrophages. Infect Immun. 1993;61:3038–3046. doi: 10.1128/iai.61.7.3038-3046.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoepelman I M, Bezemer W A, Vandenbroucke-Grauls C M J E, Marx J J M, Verhoef J. Bacterial iron enhances oxygen radical-mediated killing of Staphylococcus aureus by phagocytes. Infect Immun. 1990;58:26–31. doi: 10.1128/iai.58.1.26-31.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs D H, Tinnell S B. Antioxidant function of fungal melanin. J Bacteriol. 1993;175:7102–7104. doi: 10.1128/jb.175.21.7102-7104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobson E S, Vartivarian S E. Iron assimilation in Cryptococcus neoformans. J Med Vet Mycol. 1992;30:443–450. [PubMed] [Google Scholar]
- 21.Jelinek J, Jensen A. Catecolamine concentrations in plasma and organs of the fetal guinea pig during normoxemia, hypoxemia, and asphyxia. J Dev Physiol. 1991;15:145–152. [PubMed] [Google Scholar]
- 22.Jiang X, Baldwin C L. Iron augments macrophage-mediated killing of Brucella abortus alone and in conjunction with interferon-γ. Cell Immunol. 1993;148:397–407. doi: 10.1006/cimm.1993.1121. [DOI] [PubMed] [Google Scholar]
- 23.Kwon-Chung K J, Polacheck I, Popkin T. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J Bacteriol. 1982;150:1414–1421. doi: 10.1128/jb.150.3.1414-1421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S C, Dickson D W, Brosnan C F, Casadevall A. Human astrocytes inhibit Cryptococcus neoformans growth by a nitric oxide-mediated mechanism. J Exp Med. 1994;180:365–369. doi: 10.1084/jem.180.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levitz S M, Dibenedetto D J. Paradoxical role of capsule in murine bronchoalveolar macrophage-mediated killing of Cryptococcus neoformans. J Immunol. 1989;142:659–665. [PubMed] [Google Scholar]
- 26.Liochev S. The role of iron-sulfur clusters in in vivo hydroxyl radical production. Free Radic Res. 1996;25:369–384. doi: 10.3109/10715769609149059. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Wakamatsu K, Ito S, Williamson P R. Catecholamine oxidative products, but not melanin, are produced by Cryptococcus neoformans during neuropathogenesis in mice. Infect Immun. 1999;67:108–112. doi: 10.1128/iai.67.1.108-112.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lovchik J A, Lyons C R, Lipscomb M F. A role for gamma interferon-induced nitric oxide in pulmonary clearance of Cryptococcus neoformans. Am J Respir Cell Mol Biol. 1995;13:116–124. doi: 10.1165/ajrcmb.13.1.7598935. [DOI] [PubMed] [Google Scholar]
- 29.Messerschmidt A, Huber R. The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin. Modelling and structural relationships. Eur J Biochem. 1990;187:341–352. doi: 10.1111/j.1432-1033.1990.tb15311.x. [DOI] [PubMed] [Google Scholar]
- 30.Murray H W, Cartelli D M. Killing of intracellular Leishmania donovani by human mononuclear phagocytes: evidence for oxygen-dependent and -independent Leishmaniacidal activity. J Clin Investig. 1983;72:32–44. doi: 10.1172/JCI110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nosanchuk J D, Valadon P, Feldmesser M, Casadevall A. Melanization of Cryptococcus neoformans in murine infection. Mol Cell Biol. 1999;19:745–750. doi: 10.1128/mcb.19.1.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nyhus K J, Wilborn A T, Jacobson E S. Ferric iron reduction by Cryptococcus neoformans. Infect Immun. 1997;65:434–438. doi: 10.1128/iai.65.2.434-438.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinner R W, Hajjeh R A, Powderly W G. Prospects for preventing cryptococcosis in persons infected with human immunodeficiency virus. Clin Infect Dis. 1995;21(Suppl. 1):S103–S107. doi: 10.1093/clinids/21.supplement_1.s103. [DOI] [PubMed] [Google Scholar]
- 34.Polacheck I. The discovery of melanin production in C. neoformans and its impact on diagnosis and the study of virulence. Zentbl Bakteriol. 1991;276:120–123. doi: 10.1016/s0934-8840(11)80225-2. [DOI] [PubMed] [Google Scholar]
- 35.Rosen G M, Pou S, Ramos C L, Cohen M S, Britigan B E. Free radicals and phagocytic cells. FASEB J. 1995;9:200–209. doi: 10.1096/fasebj.9.2.7540156. [DOI] [PubMed] [Google Scholar]
- 36.Salas S D, Bennett J E, Kwon-Chung K J, Perfect J R, Williamson P R. Effect of the laccase gene, CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med. 1996;184:377–386. doi: 10.1084/jem.184.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva D, Davis-Kaplan S, Fergestad J, Kaplan J. Purification and characterization of Fet3 protein, a yeast homologue of ceruloplasmin. J Biol Chem. 1997;272:14208–14213. doi: 10.1074/jbc.272.22.14208. [DOI] [PubMed] [Google Scholar]
- 38.Staib F. Cryptococcus neoformans and Guizotia abyssinica (syn. G. oleifera D. C.) (farbreadktion fur C. neoformans) Z Hyg. 1962;148:466–475. [Google Scholar]
- 39.Tohyama M, Kawakami K, Futenma M, Saito A. Enhancing effect of oxygen radical scavengers on murine macrophage anticryptococcal activity through production of nitric oxide. Clin Exp Immunol. 1996;103:436–441. doi: 10.1111/j.1365-2249.1996.tb08299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker L, Lowries D B. Killing of Mycobacterium microti by immunologically activated macrophages. Nature. 1981;293:69–71. doi: 10.1038/293069a0. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Casadevall A. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to nitrogen- and oxygen-derived oxidants. Infect Immun. 1994;62:3004–3007. doi: 10.1128/iai.62.7.3004-3007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Aisen P, Casadevall A. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect Immun. 1995;63:3131–3136. doi: 10.1128/iai.63.8.3131-3136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williamson P R. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: identification as a laccase. J Bacteriol. 1994;176:656–664. doi: 10.1128/jb.176.3.656-664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williamson P R, Wakamatsu K, Ito S. Melanin biosynthesis in Cryptococcus neoformans. J Bacteriol. 1998;180:1570–1572. doi: 10.1128/jb.180.6.1570-1572.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang S, Varma A, Williamson P R. The yeast Cryptococcus neoformans uses “mammalian” enhancer sites in the regulation of the virulence gene, CNLAC1. Gene. 1999;227:231–240. doi: 10.1016/s0378-1119(98)00590-3. [DOI] [PubMed] [Google Scholar]