Abstract
Alzheimer’s disease (AD) is a global concern and has become a major public health event affecting human health. Insulin is a metabolic hormone secreted mainly by the peripheral tissue pancreas. In recent years, more and more evidence has proved that insulin regulates various functions of the brain. The hippocampus, one of the earliest brain regions affected by AD, is widely distributed with insulin receptors. Studies have shown that type 2 diabetes mellitus, characterized by insulin resistance, is closely related to AD, which has drawn extensive attention to the relationship between hippocampal insulin signaling and AD. Therefore, we provide an overview of intranasal insulin administration on memory and its underlying mechanism. We also highlight the molecular link between hippocampal insulin resistance and AD and provide a theoretical basis for finding new therapeutic targets for AD in clinical practice.
Keywords: hippocampus, insulin resistance, memory impairment, type 2 diabetes mellitus
1. Introduction
Since insulin was first demonstrated to have hypoglycemic effects in 1916, followed by the identification of insulin receptors (IRs), a major role of IRs in the regulation of glucose metabolism in peripheral tissues has been established [1,2]. In the past few decades, insulin receptor (IR) function was thought to be restricted to the periphery, and the brain was traditionally considered an insulin-insensitive organ, largely based on the fact that whole-brain glucose uptake is not affected by circulating insulin levels [3]. Over the past two decades, however, studies in the field have identified a unique role for insulin in the brain. There is increasing evidence that insulin enters the brain and regulates central nervous system (CNS) functions such as eating, depression, and cognitive behavior [4,5,6]. The effects on feeding behavior and metabolism appear to be primarily mediated by the hypothalamic actions of insulin, while cognitive function and memory changes are attributed to its actions in the cortex and hippocampus.
The hippocampus is the center of learning and memory, and its dysfunction contributes to neurodegenerative diseases including Alzheimer’s disease (AD) [7,8]. Studies have shown that IRs are widely distributed in the hippocampus [9]. Whether insulin acts on the hippocampus to affect memory has been of interest. Intranasal delivery routes can effectively deliver insulin to CNS targets in a biologically active form. Although the mechanism has not been clarified, many studies in recent years have shown the ameliorative effect of intranasal insulin on memory impairment in animal models and clinical studies, respectively. Currently, type 2 diabetes mellitus (T2DM) is considered to be very prevalent due to the prevalence of obesity and population aging [10]. Notably, studies have shown that people with T2DM were twice as likely to have cognitive dysfunction [11]. Many clinical and animal models have demonstrated a close link between AD and T2DM pathology, and one of the most important links is insulin resistance [12]. In this review, we use insulin and AD as an entry point, summarize the underlying mechanisms by which insulin affects memory, and discuss the potential molecular link between insulin resistance and AD, which may help the future development of novel targets and new treatment options.
2. Insulin and Hippocampus: Memory as a Key Link
The hippocampus highly expresses IRs, so changes in insulin signaling in the brain may have significant effects on the hippocampus. Given the crucial role of the hippocampus in memory processing, there has been much interest in whether insulin regulates memory. Here, we elucidated the effects of altered insulin signaling on memory from animal and human studies and summarized the underlying mechanisms.
2.1. Evidence in Animal Studies
Changes in brain insulin levels and IR density, as well as reducing the sensitivity of IRs (i.e., insulin resistance), can lead to changes in insulin signaling. The effect of altered insulin signaling on memory function has been discussed in many animal studies. The blood-brain barrier (BBB) limits the ability to deliver drugs and peptides to the brain, and intranasal delivery provides another solution for insulin to enter the brain [13]. A study showed that intranasal insulin can be detected within 5 min in young CD-1 mice and was still present 60 min after injection [14]. In recent years, many studies have shown that intranasal insulin significantly ameliorates memory impairment in animals in various disease models (Table 1). In these disease models, 0.1–2 IU insulin showed different degrees of protective effect. Taken together, these studies suggested that intranasal insulin had a beneficial effect on memory impairment.
Table 1.
Evidence in animal studies.
| Dose of Insulin | Time of Intranasal Administration | Animal Models | Memory Detection Method | Effects on Memory | References |
|---|---|---|---|---|---|
| Low level (0.0715 IU) |
once a day, 5 days a week, 12 weeks | 18-month-old male F344 rats | Morris water maze test | No obvious effects | [15] |
| Low level (0.24 IU) |
once a day, 4 consecutive weeks | male C57BL6 mice | Radial arm water maze test | Improvement | [16] |
| Low level (0.1 IU and 0.5 IU) |
once a day, 4 consecutive weeks | kainic acid-induced chronic epileptic mice | Morris water maze test | Improvement | [17] |
| Low level (0.5 IU) |
once a day, 7 consecutive days | Wistar with methamphetamine for 10 days | Y-maze test, Novel object recognition test | Improvement | [18] |
| High level (1 IU) |
twice a day, 14 consecutive days | C57BL/6J mice treated with an I.C.V. injection of STZ | Morris water maze test | Improvement | [19] |
| High level (1.75 IU) |
once a day, 3 consecutive days | 3xTg-AD mice anesthetized with propofol/sevoflurane for 3 h | Morris water maze test, novel object recognition test | Improvement | [20] |
| High level (2 IU) |
once a day, 14 consecutive days | rats were injected with STZ (3 mg/kg, ICV) bilaterally twice, on days 1 and 3 | Morris water maze test | Improvement | [21] |
| High level (2 IU) |
once a day, 6consecutive weeks | Wistar rats were injected with 6-OHDA (12 μg/4 μL) into the unilateral medial forebrain bundle | T-maze rewarded alternation test | Improvement | [22] |
The ameliorative effect of intranasal insulin on memory depends on the normal density and function of IRs. Sufficient evidence has proved that the hippocampus IRs are closely related to learning and memory. Animal models showed that the gene expression of IRs in the hippocampus was upregulated after spatial learning [23], and the ameliorative effect of intranasal insulin on memory impairment was also affected by the levels of IRs [14]. Similarly, another study reported that the specific loss of hippocampal IRs resulted in impaired recognition and spatial memory in mice [6]. In addition, the sensitivity of IRs in regulating memory also plays an important role. Many studies have confirmed that hippocampal insulin resistance led to cognitive dysfunction [24,25]. In addition, insulin resistance was characteristic of T2DM, and type 2 diabetic mice or rats were often accompanied with cognitive dysfunction [26,27]. In conclusion, although many mechanisms remain unclear, changes in hippocampal insulin signaling are shown to regulate memory function.
2.2. Evidence in Human Studies
Insulin has been widely used as a drug to treat diabetes in clinics since it was discovered. Studies in humans have shown that intranasal insulin can bypass the BBB and reach the CNS within 1 h of administration [28]. The beneficial cognitive effects of insulin delivery to the CNS via the intranasal route have been demonstrated in a series of studies in healthy people [29,30,31,32]. In vivo animal experiments as well as in vitro studies have enabled an understanding of the ameliorative effects of intranasal insulin on cognitive dysfunction in pathological states [15,16,17,18,19,20]. In recent years, there has been increasing interest in the role of brain insulin signaling in the development of AD pathology and the prevention of cognitive impairment with intranasal insulin administration. Several studies have also explored the effect of intranasal insulin on improving memory deficits in patients with AD or MCI (mild cognitive impairment) clinically in humans (Table 2), and these clinical data consistently indicate the positive effects of intranasal insulin on cognitive function in patients. However, these studies still have their limitations. On one hand, to date, intranasal insulin is a novel treatment for patients with AD or MCI that has only been tested in a few clinical trials. On the other hand, the age and sex of the patient; the methods and criteria used to assess cognitive function; and the type, dose, and duration of insulin administration were all factors used to assess the effect of intranasal insulin on memory [33,34]. Notably, the effect of intranasal insulin on cognitive function was also influenced by apoe4 gene-carrier status. There has been evidence that ApoE ε4 negative individuals are more sensitive to the cognitive consequences of insulin resistance [33]. Patients with Apoe4 (−) showed more consistent cognitive gains compared to patients with Apoe4 (+) [35]. At present, there is sufficient evidence to show that there are few serious adverse effects observed after clinical intranasal insulin administration [35,36,37]. In conclusion, intranasal insulin has emerged as a potential treatment for neurodegenerative diseases, but further studies are needed to determine its effects on cognitive function.
Table 2.
Evidence in human studies.
| Objective (MCI or Mild to Moderate AD) |
Dose and Duration of Intranasal Insulin Administration | Memory Detection Method | Effects on Memory | References |
|---|---|---|---|---|
| 289 adults | 40 IU/day, 12 months |
adas-cog-12 score | No benefits | [13] |
| 60 adults | 40 IU/day, 21 days |
verbal working memory, visuospatial working memory | Improvement | [38] |
| 104 adults | 40 IU/day, 4 months |
delayed story recall, the dementia severity rating scale | Improvement | [33] |
| 49 adults | 20 IU/day, 12 months |
Alzheimer’s disease assessment scale-cognition, Alzheimer’s disease cooperative study-activities of daily living scale, a memory composite | Improvement | [39] |
| 36 adults | 40 IU/day, 4 months |
global cognition (Alzheimer’s disease assessment scale-cognition) | Improvement | [40] |
2.3. Mechanisms by Which Insulin Affects Memory
Synaptic plasticity in the hippocampus is thought to underlie learning and memory processes [41]. IRs are enriched at hippocampal synapses, where they have been proposed to modulate synaptic plasticity through interactions with the glutamatergic system [24]. AMPA and NMDA receptors are the two most important ionotropic channels gated by glutamate binding. Insulin has a strong effect on glutamate receptor signaling [24]. Studies have shown that insulin-stimulated phosphorylation of GluN2A and GluN2B subunits in the hippocampus enhanced NMDAR-mediated synaptic transmission [42,43]. In addition, insulin exhibits a strong transcriptional regulatory effect on NMDA receptors and may in turn affect synaptic function by altering the composition and kinetic properties of NMDA receptors. A recent study provided additional evidence for a functional interaction between the insulin and glutamate systems [6]. Deletion of IRs specifically downregulated the expression of the GluA1 subunit of AMPA receptors in the hippocampus. Indeed, it was shown most of the AMPA receptors containing the GluA1 subunit are near the postsynaptic membrane in recycling endosomes and can be rapidly recruited under the stimulation of calcium influx mediated by insulin or NMDA receptors. This was a key molecular mechanism for long-term enhancement (LTP), which was important for learning and memory [44,45,46]. Furthermore, insulin activates mTOR and its downstream translational regulators, 4E-BP1 and p70S6K, to stimulate translation of the dendritic spine protein, PSD95, an important postsynaptic compact protein that is responsible for excitatory synaptogenesis and function maintenance [47]. Insulin also modulates the concentration of several neurotransmitters such as acetylcholine and nitric oxide, and controls the release and uptake of GABA and norepinephrine, which in turn affects synaptic function [48,49,50]. In summary, the expression of glutamate receptors at the postsynaptic membrane, the expression of postsynaptic proteins, and the release of neurotransmitters may all influence synaptic function. Glutamate signaling may be a molecular link between brain insulin and hippocampal synaptic function, and these data underscore the critical role of insulin signaling for memory function.
3. The Source of Insulin in the Brain
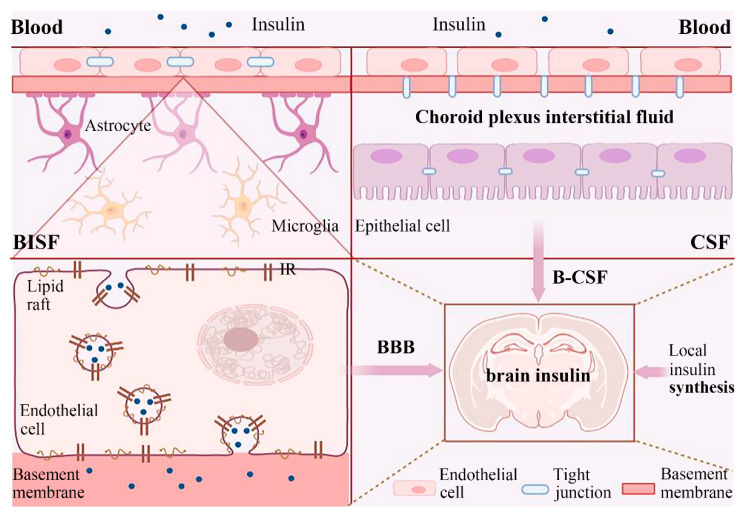
In recent years, the idea that normal brain function is not insulin-independent has also been revisited. As mentioned above, it has been confirmed in many studies that hippocampal function is affected by insulin. However, there is no doubt that peripheral insulin is produced by the pancreas, so where does insulin come from in the brain? (Figure 1).
Figure 1.
Schematic diagram showing the possible sources of brain insulin. First, the BBB is composed of a capillary basement membrane, pericytes, astrocytes, and specialized capillary endothelial cells that are interconnected with tight junctions. Peripheral insulin can cross the BBB intact through IR-specific vesicle-mediated transport in endothelial cells. Second, the B-CSF barrier has fenestrated capillaries in the choroid plexus that lack tight junctions and allow para- and trans-cellular transport across the endothelium. The B-CSF barrier is another possible route for insulin to enter the CNS. Third, there is some limited evidence suggesting the possibility of de novo insulin synthesis in the brain. BBB: blood-brain barrier, BISF: brain interstitial fluid, CSF: cerebrospinal fluid; IR: insulin receptor.
3.1. External Insulin Reaches the Brain
There was evidence that most IR isoforms in the human and mouse brain were predominantly localized in microvessels [51]. At present, peripheral insulin enters the brain through the BBB and the blood-cerebrospinal fluid (B-CSF) barrier, which are the two most concerning pathways. There is enough evidence that insulin can pass through the BBB into the brain [52]. As a special protective structure, the BBB is composed of a capillary basement membrane, pericytes, astrocytes, and specialized capillary endothelial cells that are interconnected with tight junctions [53]. A new study showed that pancreas-produced insulin interacted primarily with the IR on the luminal side of the brain vasculature [51]. IRs expressed on BBB endothelial cells play a major role in the transport of insulin to the CNS [54,55]. Recent studies have added to this view: in addition to IRs, endothelial cell-mediated insulin transport also requires lipid raft function [55]. Insulin crosses the BBB intact through IR-specific vesicle-mediated transport in endothelial cells. In addition, IRs in astrocytes also mediate insulin transport [56]. It should also not be overlooked that in vivo studies have shown that insulin across the BBB can occur independently of insulin [54], and a similar finding was obtained in another in vitro experiment [57], suggesting that the IRs may not be the only protein-mediated insulin transport in the BBB. These findings greatly increased our understanding of the pathways involved in brain insulin transport. Notably, various other events such as obesity, diabetes, and LPS-induced inflammation alter the permeability of the BBB to insulin, which may lead to changes in insulin signaling and related functions in the brain [58,59,60].
The B-CSF barrier is another possible route for insulin to enter the CNS. The B-CSF barrier has fenestrated capillaries in the choroid plexus that lack tight junctions and allow para- and trans-cellular transport across the endothelium [61]. Earlier findings supported the hypothesis that the choroid plexus has a high density of IRs and suggested that the choroid plexus may be the site of brain insulin transport to the CSF [62]. However, evidence for direct insulin receptor-mediated insulin transport across the choroid plexus is still lacking.
3.2. Local Insulin Synthesis in the Brain
The question of whether the CNS secretes insulin has been debated for a long time. Although the evidence was insufficient, previous studies have indicated that partial insulin may also be secreted by the CNS. For example, in the study of Dorn et al., radioimmunoassay analysis revealed much higher concentrations of insulin and C-peptide in the human brain than in the blood, with the highest in the hypothalamus, and immunostaining was mainly restricted to the cell soma and proximal dendrites. They observed immune response products to the two peptides in most nerve cells in all regions of the brain examined [63]. Schechter et al. further proved the presence of insulin in the CNS via rabbit neurons isolated in vitro and indicated that the neurons may be one of the synthesis sites of insulin in the brain [64]. These early studies supported that insulin was at least partly produced in the CNS. However, there are also studies showing that the brain produces little or no insulin [65]. This question has been controversial for many years. It was reported that Ins2 mRNA was strongly expressed in GABAergic glial cells in the rat cerebral cortex [66]. Nemoto et al. reported that synthesized insulin was secreted from rat hippocampal and cortical neurons’ dense-core vesicles [67]. Moreover, recent studies have reported that astrocytes isolated from the cerebral cortex of rat embryos express Ins2 mRNA and secrete insulin, which confers strong protection against AβO synaptic toxicity [68,69]. Notably, Aβ, a molecule characteristic of AD, has been reported to reduce insulin synthesis and secretion in cultured neurons and astrocytes and may cause impaired insulin signaling, which also provided new insights into the link between insulin signaling in the brain and AD [67,69]. These studies provide some evidence for the local production of insulin in the brain, and the possibility of the brain synthesizing insulin.
4. Insulin Signaling and Hippocampal Disease: AD Is a Key Point
AD is the most common form of dementia, and its most important feature is the persistent and progressive impairment of cognitive function, especially the severe decline in memory. Some human clinical data suggested that people with T2DM, which was characterized by insulin resistance, had a significantly increased risk of developing AD. In recent years, several animal studies have explored the mechanistic effects of insulin resistance on AD. Here, we summarized the molecular link between hippocampal insulin resistance and AD.
4.1. The Role of the Hippocampus in AD
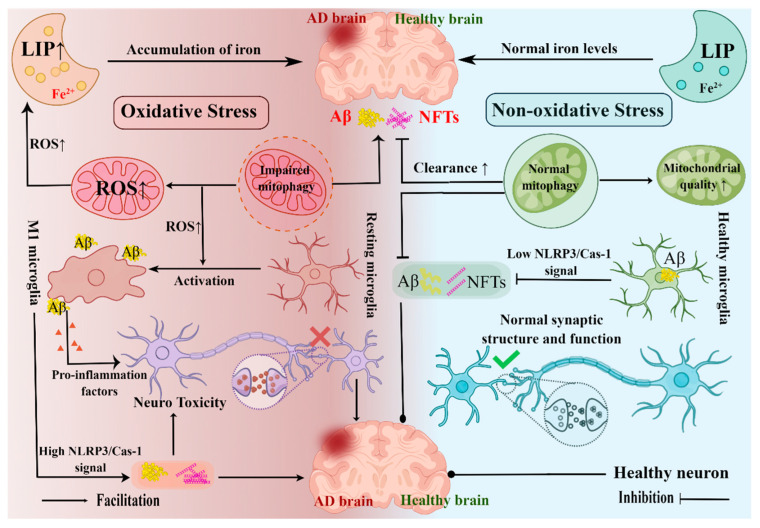
AD is usually associated with the extracellular deposition of the Aβ peptide and accumulation of hyperphosphorylated tau in neurons. Neuronal degeneration and synaptic changes caused by these pathologies are considered to constitute the main neurobiological basis of cognitive dysfunction in AD [70]. The hippocampus is one of the earliest brain regions affected by AD and reduced hippocampal volume and elevated rates of atrophy have been found in patients with early AD in many structural and functional imaging studies [7,8,71]. Therefore, alterations in hippocampal structure and function may be good candidates for predicting AD development. Here, we summarized the association of hippocampal pathology with the development of AD (primarily Aβ accumulation and tau hyperphosphorylation) (Figure 2).
Figure 2.
Association between hippocampal pathology and AD disease. AD is characterized by the accumulation of Aβ and hyperphosphorylation of tau protein which lead to neuronal degeneration and changes in synaptic structure and function, leading to neurotoxicity. The damage of mitophagy can lead to the reduction in mitochondrial quality and abnormal mitochondrial function. On the one hand, it promotes the progression of AD pathology. On the other hand, the abnormal mitochondrial function also leads to increased ROS release, which may further lead to hippocampal iron accumulation and neuroinflammation, and then lead to Aβ accumulation and hyperphosphorylation, which may eventually lead to neurotoxicity and AD. AD: Alzheimer’s disease, LIP: Labile iron pool, Aβ: Amyloid beta, NFTs: Neurofibrillary tangles, √: Protective effect, ×: Damaging effect (Drawn by Figdraw).
4.1.1. Hippocampal Neuroinflammation and AD
Neuroinflammation due to microglial activation is thought to play a key role in the ongoing neurodegeneration of AD. Activated microglia secrete a variety of proinflammatory cytokines and toxic products, leading to neuronal dysfunction and apoptosis. The transcription factor NF-κB is known to be a master regulator of inflammatory responses. Studies have shown that activation of NF-κB promoted amyloid precursor protein (APP) cleavage and Aβ production by enhancing BACE1 expression [72]. In AD, reactive microglia adjacent to Aβ plaques have been repeatedly observed in the hippocampus in both clinical data and animal experiments [73,74,75]. Not only that, but the latest research also suggested that microglia carrying being swallowed Aβ would be disseminated to other health areas of the brain, causing the formation of new Aβ [76], and Aβ deposition would continue to cause chronic activation of microglia, leading to excessive production of cytokines and chemokines, thereby deepening the microglia activation and inflammatory response. In addition to affecting Aβ production [77], studies have shown that Aβ activated the NLRP3 inflammasome in microglia to promote tau pathology and neurodegeneration [78,79]. Of note, previous in vivo and in vitro experiments have consistently shown that microglial activation drove the spread of tau tangles [78,80]. A recent study also demonstrated, for the first time from the brains of living patients, that the diffusion path of tau depends on microglial activation [81]. In conclusion, neuroinflammation is an indispensable and a key link in the upstream pathogenesis of AD. Microglia activation is not only a symptom of inflammation, but also very likely to have some association with Aβ pathology and tau pathology, and is a key role in promoting the progression of AD.
4.1.2. Hippocampal Ferroptosis and AD
In recent years, the role of ferroptosis in neurodegenerative diseases has received much attention. Iron accumulation has been observed in the brains of AD patients and AD transgenic mouse models, with excess iron accumulation in insoluble Aβ plaques and neurofibrillary tangles [82,83]. Sufficient evidence has shown a clear link between age-related elevated iron load and AD symptoms [84]. Downregulation of Ferroportin (FPN), the only known iron exporter, may be a key link between iron accumulation and AD [85,86]. Recent studies have shown decreased hippocampal FPN expression and abnormal iron deposition in the brains of AD mouse models and AD patients [84], and that increased brain iron levels may accelerate Aβ formation [87]. Similarly, the administration of specific inhibitors of ferroptosis effectively reduced neuronal death and memory impairment induced by Aβ aggregation in vitro and in vivo [84]. GPX4 is also a central regulator of ferroptosis. It has been reported that the knockdown of GPX4 in mice directly leads to age-dependent neurodegenerative changes and significant neuronal loss [88]. Iron accumulation occurs not only in neurons but also in microglia. On the one hand, iron accumulation in microglia can reduce the phagocytic ability of microglia to Aβ, leading to excessive deposition of Aβ [89,90]. On the other hand, iron accumulation can drive microglia to polarize into the proinflammatory M1 type, thereby inducing neuroinflammation [91]. In general, ferroptosis is a novel form of cell death characterized by intracellular iron overload. Excessive iron accumulation aggravates Aβ accumulation and tau hyperphosphorylation, which provides new insights into the molecular pathophysiology of AD.
4.1.3. Hippocampal Mitophagy and AD
Mitophagy is a form of cellular autophagy that selectively removes defective mitochondria. Corresponding with the age-related increase in AD incidence, there is also an age-dependent accumulation of dysfunctional mitochondria and impaired mitophagy [92]. In biopsies from human AD cases and transgenic animal models of AD, electron microscopic studies have identified the accumulation of damaged mitochondria, such as the appearance of swelling with sclerosis and distortion [93], while basal levels of mitophagy in the hippocampus of postmortem AD patients are 30–50% lower than normal [94]. These studies indicated that mitophagy was dysfunctional in the hippocampus of AD patients [95]. The mechanism of hippocampal mitophagy in AD remains largely unexplored. A recent study found that induction of mitophagy improved AD pathology and reversed memory impairment in transgenic nematodes, IPSC-derived neurons, and mouse models of AD [94]. In APP/PS1 mouse model, mitophagy reduced insoluble Aβ1-42 and Aβ1-40, and inhibited neuroinflammation and cognitive impairment through phagocytosis of Aβ plaques by microglia, suggesting that abnormal mitophagy may be one of the causes of AD. However, other studies have shown that Aβ peptide accumulation in the hippocampus of APP/PS1 mice decreased hippocampal mitochondrial mass and increased mitophagy [96]. A previous in vitro study had consistent results. The accumulation of mAPP and Aβ led to abnormal mitophagy function in hippocampal neurons [97]. These data suggested that abnormal mitophagy may be the initiator of Aβ aggregation and tau hyperphosphorylation, which can further aggravate mitochondrial dysfunction, thus forming a vicious circle in AD pathology.
4.1.4. Hippocampal Oxidative Stress and AD
Oxidative stress, a severe imbalance between the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) and antioxidant defenses, has been shown to promote the pathological progression of AD in a wide range of studies. In a recent study, the results of single-cell whole-genome sequencing data indicated higher than normal levels of single nucleotide changes associated with oxidative stress and associated DNA damage in the hippocampus and cortex of AD patients [98]. GSH, an enzyme that fights oxidative stress, was significantly depleted in the hippocampal region of patients with MCI and AD compared with healthy elderly subjects [99]. In neurons, accumulated ROS can oxidize polyunsaturated neuronal lipid products to produce active lipid byproducts, such as 4-hydroxy-2, 3-nonenal (HNE), malondialdehyde, and F2-isoprostane or glycosylated proteins to produce advanced glycosylation end products (AGEs). HNE and AGES can also overaccelerate Aβ production and tau phosphorylation. Moreover, recent studies have shown that ROS caused the overexpression of β-site APP cleavage enzyme 1 (BACE1) and increased Aβ production [100], which in turn exacerbated mitochondrial dysfunction and ROS production [101], leading to a vicious cycle. Of note, oxidative stress appears to be at the intersection of many pathological changes, such as neuroinflammation, ferroptosis, and mitochondrial dysfunction.
4.2. T2DM and AD
T2DM is a chronic endocrine disease that affects approximately 6% of the global population. The occurrence of T2DM can cause many complications in the body. Currently, the most observed neurological effects of T2DM are impaired learning and memory. Many studies have shown that humans with T2DM exhibited cognitive deficits, characterized by smaller hippocampal size and hippocampal atrophy, and poor memory in T2DM patients [102,103,104]. Similar to these results, in animal studies, T2DM mice/rats performed poorly in many behavioral tests, such as the delayed alternation T-maze task [26], the Y-maze test, the Morris maze water test [105,106], the nest building test, and the novel object recognition test. This evidence supported a strong relationship between T2DM and cognitive function. In addition, clinical and epidemiological studies have demonstrated that the risk of developing AD is twice as high in patients with T2DM compared to those without diabetes [107,108].
The central feature of T2DM is insulin resistance [109]. Subsequent studies have shown that insulin resistance caused hippocampal neuroplasticity deficits [110], leading to decreased performance on hippocampal-dependent learning and memory tasks [111]. Sufficient evidence has been obtained to demonstrate the development of hippocampal insulin resistance in AD patients or AD animal models [112,113]. Collectively, these studies supported the hypothesis that hippocampal insulin resistance was a common pathological feature of T2DM and AD, with some studies also calling AD “type 3 diabetes”.
4.3. Molecular Link between Hippocampal Insulin Resistance and AD
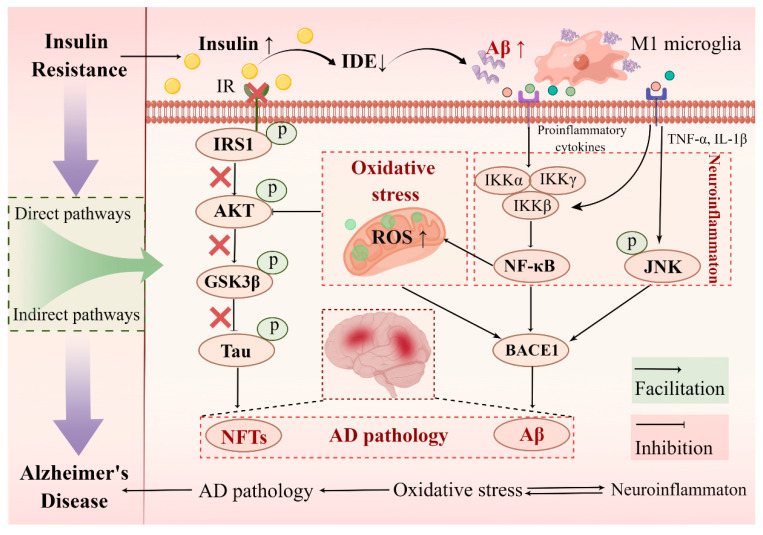
Hippocampal insulin resistance is characterized by the insensitivity of hippocampal IRs and decreased phosphorylation of insulin downstream signaling molecules. Studies have shown a strong association between hippocampal insulin resistance and AD pathology, including Aβ aggregation and tau hyperphosphorylation [25,114]. Here, we summarized recent findings on the possible mechanisms by which hippocampal insulin resistance induced AD pathology (Figure 3).
Figure 3.
A molecular link between insulin resistance and AD. Insulin resistance contributes to the development of AD pathology through both direct and indirect pathways. Brain insulin resistance reduces IDE levels and insulin signaling directly induces Aβ deposition and tau hyperphosphorylation. In addition, insulin resistance-induced neuroinflammation and oxidative stress are also involved in the regulation of AD pathological progression. IR: insulin receptor, IDE: insulin-degrading enzyme, Aβ: amyloid beta, NFTs: neurofibrillary tangles, ×: inhibiting effect (Drawn by Figdraw).
4.3.1. Direct Pathways of Hippocampal Insulin Resistance Induced AD Pathology: Aβ Aggregation and Tau Hyperphosphorylation
Aβ peptides are produced by the hydrolysis of APP. The accumulation of Aβ proteins into plaques between cells is considered to be a typical pathological feature of AD [115]. Insulin-degrading enzyme (IDE) is a widely expressed zinc-dependent metalloproteinase that contributes to the proteolytic inactivation of insulin [116]. It is worth noting that Aβ protein is also the substrate of IDE. Studies have shown that IDE plays a crucial role in the clearance of Aβ in AD [117]. Thus, IDE is also considered a link between insulin resistance and AD. In mice, increased γ-secretase activity and decreased IDE activity due to insulin resistance or hyperinsulinemia have been shown to lead to increased Aβ in the brain [118]. In addition, hyperphosphorylation of tau protein is another pathology of AD. Sufficient evidence has shown that tau phosphorylation is regulated by GSK-3β, which is regulated by insulin signaling. Studies have shown that when insulin resistance occurred in the hippocampus, the activity of PI3K/AKT, the main signaling molecule of the insulin signaling pathway, was decreased, which promoted the activation of GSK3β and phosphorylation of tau and promoted the pathological progression of AD [119].
4.3.2. Indirect Pathways of Hippocampal Insulin Resistance Induced AD Pathology: Neuroinflammation and Oxidative Stress
It has been shown that hippocampal insulin resistance led to microglia activation [120], and activated microglia released proinflammatory-related factors (IL6, TNF-a, IL-1β) and induced neuroinflammation. Current studies have shown that these proinflammatory factors can promote Aβ accumulation through three pathways. Firstly, the increase in proinflammatory factors inhibited the phagocytosis of Aβ protein by microglia and then induced the accumulation of Aβ. Second, TNF-α and IL-1β are potent stimulators of γ-secretase, leading to increased Aβ production through pathways involving the c-Jun N-terminal kinase (JNK)-dependent MAPK pathway [121]. Third, activation of the NF-κB pathway has been shown to induce ROS production and accumulation [122]. In addition, ROS may be directly affected by insulin resistance [123]. On the one hand, the increase in ROS induces the increase in BACE1 activity, thereby causing the accumulation of Aβ [124], on the other hand, it induces the generation of oxidative stress. Studies have shown that oxidative stress inactivates the AKT pathway [125], followed by increased cerebral insulin resistance, activation of GSK3β, and phosphorylation of tau. Overall, oxidative stress and neuroinflammation appear to be an important link between hippocampal insulin resistance and AD development.
5. Conclusions
It is now widely accepted that insulin in the brain plays an important role in regulating many functions of the CNS. IRs are highly expressed in many brain regions, including the hippocampus. Although mechanistic studies have been insufficiently conducted, adequate animal studies have demonstrated a significant improvement in memory impairment with insulin; however, this improvement has not been as evident in clinical studies. Some studies have shown that clinical trial delivery devices affected the effectiveness of insulin delivery in the CNS, which may be one of the possible reasons for the deviation of results between clinical trials and animal studies [13]. Previous studies have shown that the interaction between insulin and glutamatergic receptors can change hippocampal synaptic plasticity, which may be one of the key mechanisms by which insulin improves memory. As a common link between T2DM and AD, in recent years, insulin resistance has been shown to contribute directly or indirectly to the progression of AD. To date, there is no clinical treatment for AD associated with T2DM. Comparative studies that identify the various pathways involved in insulin signaling may help illustrate the relationship between AD and T2DM or their relative treatment, which may prove potential future research areas.
Author Contributions
Conceptualization, Y.C. and Q.L.; writing manuscript, Q.L.; visualization, Z.W., J.C. and Y.D.; supervision, Z.W.; funding acquisition, Y.C. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China: 31873000, National Natural Science Foundation of China: 32172801, and Beijing Natural Science Foundation: 6222019.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yaribeygi H., Farrokhi F.R., Butler A.E., Sahebkar A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019;234:8152–8161. doi: 10.1002/jcp.27603. [DOI] [PubMed] [Google Scholar]
- 2.Arnold S.E., Arvanitakis Z., Macauley-Rambach S.L., Koenig A.M., Wang H.Y., Ahima R.S., Craft S., Gandy S., Buettner C., Stoeckel L.E., et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018;14:168–181. doi: 10.1038/nrneurol.2017.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koepsell H. Glucose transporters in brain in health and disease. Pflugers Arch. 2020;472:1299–1343. doi: 10.1007/s00424-020-02441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muta K., Morgan D.A., Rahmouni K. The role of hypothalamic mTORC1 signaling in insulin regulation of food intake, body weight, and sympathetic nerve activity in male mice. Endocrinology. 2015;156:1398–1407. doi: 10.1210/en.2014-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasca C., Dobbin J., Bigio B., Watson K., de Angelis P., Kautz M., Cochran A., Mathe A.A., Kocsis J.H., Lee F.S., et al. Insulin receptor substrate in brain-enriched exosomes in subjects with major depression: On the path of creation of biosignatures of central insulin resistance. Mol. Psychiatry. 2021;26:5140–5149. doi: 10.1038/s41380-020-0804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soto M., Cai W., Konishi M., Kahn C.R. Insulin signaling in the hippocampus and amygdala regulates metabolism and neurobehavior. Proc. Natl. Acad. Sci. USA. 2019;116:6379–6384. doi: 10.1073/pnas.1817391116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park K.H., Noh Y., Choi E.J., Kim H., Chun S., Son Y.D. Functional Connectivity of the Hippocampus in Early- and vs. Late-Onset Alzheimer’s Disease. J. Clin. Neurol. 2017;13:387–393. doi: 10.3988/jcn.2017.13.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maruszak A., Thuret S. Why looking at the whole hippocampus is not enough-a critical role for anteroposterior axis, subfield and activation analyses to enhance predictive value of hippocampal changes for Alzheimer’s disease diagnosis. Front. Cell. Neurosci. 2014;8:95. doi: 10.3389/fncel.2014.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gralle M., Labrecque S., Salesse C., De Koninck P. Spatial dynamics of the insulin receptor in living neurons. J. Neurochem. 2021;156:88–105. doi: 10.1111/jnc.14950. [DOI] [PubMed] [Google Scholar]
- 10.Zimmet P., Shi Z., El-Osta A., Ji L. Epidemic T2DM, early development and epigenetics: Implications of the Chinese Famine. Nat. Rev. Endocrinol. 2018;14:738–746. doi: 10.1038/s41574-018-0106-1. [DOI] [PubMed] [Google Scholar]
- 11.Antal B., McMahon L.P., Sultan S.F., Lithen A., Wexler D.J., Dickerson B., Ratai E.M., Mujica-Parodi L.R. Type 2 diabetes mellitus accelerates brain aging and cognitive decline: Complementary findings from UK Biobank and meta-analyses. Elife. 2022;11:e73138. doi: 10.7554/eLife.73138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talbot K., Wang H.Y., Kazi H., Han L.Y., Bakshi K.P., Stucky A., Fuino R.L., Kawaguchi K.R., Samoyedny A.J., Wilson R.S., et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Investig. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craft S., Raman R., Chow T.W., Rafii M.S., Sun C.K., Rissman R.A., Donohue M.C., Brewer J.B., Jenkins C., Harless K., et al. Safety, Efficacy, and Feasibility of Intranasal Insulin for the Treatment of Mild Cognitive Impairment and Alzheimer Disease Dementia: A Randomized Clinical Trial. JAMA Neurol. 2020;77:1099–1109. doi: 10.1001/jamaneurol.2020.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhea E.M., Humann S.R., Nirkhe S., Farr S.A., Morley J.E., Banks W.A. Intranasal Insulin Transport is Preserved in Aged SAMP8 Mice and is Altered by Albumin and Insulin Receptor Inhibition. J. Alzheimers Dis. 2017;57:241–252. doi: 10.3233/JAD-161095. [DOI] [PubMed] [Google Scholar]
- 15.Frazier H.N., Ghoweri A.O., Sudkamp E., Johnson E.S., Anderson K.L., Fox G., Vatthanaphone K., Xia M., Lin R.L., Hargis-Staggs K.E., et al. Long-Term Intranasal Insulin Aspart: A Profile of Gene Expression, Memory, and Insulin Receptors in Aged F344 Rats. J. Gerontol. A Biol. Sci. Med. Sci. 2020;75:1021–1030. doi: 10.1093/gerona/glz105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apostolatos A., Song S., Acosta S., Peart M., Watson J.E., Bickford P., Cooper D.R., Patel N.A. Insulin promotes neuronal survival via the alternatively spliced protein kinase CdeltaII isoform. J. Biol. Chem. 2012;287:9299–9310. doi: 10.1074/jbc.M111.313080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng S., Yang J., Wang Y., Fan Y., Tang F., Hou C., Yu J., Wang X., Jiang G. Low-dose intranasal insulin improves cognitive function and suppresses the development of epilepsy. Brain Res. 2020;1726:146474. doi: 10.1016/j.brainres.2019.146474. [DOI] [PubMed] [Google Scholar]
- 18.Beirami E., Oryan S., Seyedhosseini Tamijani S.M., Ahmadiani A., Dargahi L. Intranasal insulin treatment restores cognitive deficits and insulin signaling impairment induced by repeated methamphetamine exposure. J. Cell. Biochem. 2018;119:2345–2355. doi: 10.1002/jcb.26398. [DOI] [PubMed] [Google Scholar]
- 19.Lv H., Tang L.J., Guo C.S., Jiang Y.M., Gao C., Wang Y.F., Jian C.D. Intranasal insulin administration may be highly effective in improving cognitive function in mice with cognitive dysfunction by reversing brain insulin resistance. Cogn. Neurodynamics. 2020;14:323–338. doi: 10.1007/s11571-020-09571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y., Dai C.L., Wu Z., Iqbal K., Liu F., Zhang B., Gong C.X. Intranasal Insulin Prevents Anesthesia-Induced Cognitive Impairment and Chronic Neurobehavioral Changes. Front. Aging Neurosci. 2017;9:136. doi: 10.3389/fnagi.2017.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajasekar N., Nath C., Hanif K., Shukla R. Intranasal insulin improves cerebral blood flow, Nrf-2 expression and BDNF in STZ (ICV)Q-induced memory impaired rats. Life Sci. 2017;173:1–10. doi: 10.1016/j.lfs.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Yang L.Y., Zhang X., Li S.S., Wang H.M., Zhang X.N., Liu L.J., Xie A.M. Intranasal insulin ameliorates cognitive impairment in a rat model of Parkinson’s disease through Akt/GSK3 beta signaling pathway. Life Sci. 2020;259:118159. doi: 10.1016/j.lfs.2020.118159. [DOI] [PubMed] [Google Scholar]
- 23.Dou J.T., Chen M., Dufour F., Alkon D.L., Zhao W.Q. Insulin receptor signaling in long-term memory consolidation following spatial learning. Learn. Mem. 2005;12:646–655. doi: 10.1101/lm.88005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grillo C.A., Piroli G.G., Lawrence R.C., Wrighten S.A., Green A.J., Wilson S.P., Sakai R.R., Kelly S.J., Wilson M.A., Mott D.D., et al. Hippocampal Insulin Resistance Impairs Spatial Learning and Synaptic Plasticity. Diabetes. 2015;64:3927–3936. doi: 10.2337/db15-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng L., Fang X., Xu F., Liu S., Qian Y., Gong X., Zhao X., Ma Z., Xia T., Gu X. Amelioration of Hippocampal Insulin Resistance Reduces Tau Hyperphosphorylation and Cognitive Decline Induced by Isoflurane in Mice. Front. Aging Neurosci. 2021;13:686506. doi: 10.3389/fnagi.2021.686506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang P.A., Sun Q., Li Y.C., Weng R.X., Wu R., Zhang H.H., Xu G.Y. Overexpression of Purinergic P2X4 Receptors in Hippocampus Rescues Memory Impairment in Rats with Type 2 Diabetes. Neurosci. Bull. 2020;36:719–732. doi: 10.1007/s12264-020-00478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yermakov L.M., Griggs R.B., Drouet D.E., Sugimoto C., Williams M.T., Vorhees C.V., Susuki K. Impairment of cognitive flexibility in type 2 diabetic db/db mice. Behav. Brain Res. 2019;371:111978. doi: 10.1016/j.bbr.2019.111978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Born J., Lange T., Kern W., McGregor G.P., Bickel U., Fehm H.L. Sniffing neuropeptides: A transnasal approach to the human brain. Nat. Neurosci. 2002;5:514–516. doi: 10.1038/nn0602-849. [DOI] [PubMed] [Google Scholar]
- 29.Benedict C., Hallschmid M., Hatke A., Schultes B., Fehm H.L., Born J., Kern W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Benedict C., Hallschmid M., Schultes B., Born J., Kern W. Intranasal insulin to improve memory function in humans. Neuroendocrinology. 2007;86:136–142. doi: 10.1159/000106378. [DOI] [PubMed] [Google Scholar]
- 31.Benedict C., Kern W., Schultes B., Born J., Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J. Clin. Endocrinol. Metab. 2008;93:1339–1344. doi: 10.1210/jc.2007-2606. [DOI] [PubMed] [Google Scholar]
- 32.Ritze Y., Kern W., Ebner E.M., Jahn S., Benedict C., Hallschmid M. Metabolic and Cognitive Outcomes of Subchronic Once-Daily Intranasal Insulin Administration in Healthy Men. Front. Endocrinol. (Lausanne) 2018;9:663. doi: 10.3389/fendo.2018.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Claxton A., Baker L.D., Wilkinson C.W., Trittschuh E.H., Chapman D., Watson G.S., Cholerton B., Plymate S.R., Arbuckle M., Craft S. Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer’s disease. J. Alzheimers Dis. 2013;35:789–797. doi: 10.3233/JAD-122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kullmann S., Veit R., Peter A., Pohmann R., Scheffler K., Haring H.U., Fritsche A., Preissl H., Heni M. Dose-Dependent Effects of Intranasal Insulin on Resting-State Brain Activity. J. Clin. Endocrinol. Metab. 2018;103:253–262. doi: 10.1210/jc.2017-01976. [DOI] [PubMed] [Google Scholar]
- 35.Avgerinos K.I., Kalaitzidis G., Malli A., Kalaitzoglou D., Myserlis P.G., Lioutas V.A. Intranasal insulin in Alzheimer’s dementia or mild cognitive impairment: A systematic review. J. Neurol. 2018;265:1497–1510. doi: 10.1007/s00415-018-8768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lioutas V.A., Alfaro-Martinez F., Bedoya F., Chung C.C., Pimentel D.A., Novak V. Intranasal Insulin and Insulin-Like Growth Factor 1 as Neuroprotectants in Acute Ischemic Stroke. Transl. Stroke Res. 2015;6:264–275. doi: 10.1007/s12975-015-0409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long C., Han X., Yang Y., Li T., Zhou Q., Chen Q. Efficacy of intranasal insulin in improving cognition in mild cognitive impairment or dementia: A systematic review and meta-analysis. Front. Aging Neurosci. 2022;14:963933. doi: 10.3389/fnagi.2022.963933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Claxton A., Baker L.D., Hanson A., Trittschuh E.H., Cholerton B., Morgan A., Callaghan M., Arbuckle M., Behl C., Craft S. Long-Acting Intranasal Insulin Detemir Improves Cognition for Adults with Mild Cognitive Impairment or Early-Stage Alzheimer’s Disease Dementia. J. Alzheimers Dis. 2015;44:897–906. doi: 10.3233/JAD-141791. [DOI] [PubMed] [Google Scholar]
- 39.Kellar D., Lockhart S.N., Aisen P., Raman R., Rissman R.A., Brewer J., Craft S. Intranasal Insulin Reduces White Matter Hyperintensity Progression in Association with Improvements in Cognition and CSF Biomarker Profiles in Mild Cognitive Impairment and Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2021;8:240–248. doi: 10.14283/jpad.2021.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Craft S., Claxton A., Baker L.D., Hanson A.J., Cholerton B., Trittschuh E.H., Dahl D., Caulder E., Neth B., Montine T.J., et al. Effects of Regular and Long-Acting Insulin on Cognition and Alzheimer’s Disease Biomarkers: A Pilot Clinical Trial. J. Alzheimers Dis. 2017;57:1325–1334. doi: 10.3233/JAD-161256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mendez P., Stefanelli T., Flores C.E., Muller D., Luscher C. Homeostatic Plasticity in the Hippocampus Facilitates Memory Extinction. Cell Rep. 2018;22:1451–1461. doi: 10.1016/j.celrep.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 42.Skeberdis V.A., Lan J., Zheng X., Zukin R.S., Bennett M.V. Insulin promotes rapid delivery of N-methyl-D- aspartate receptors to the cell surface by exocytosis. Proc. Natl. Acad. Sci. USA. 2001;98:3561–3566. doi: 10.1073/pnas.051634698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christie J.M., Wenthold R.J., Monaghan D.T. Insulin causes a transient tyrosine phosphorylation of NR2A and NR2B NMDA receptor subunits in rat hippocampus. J. Neurochem. 1999;72:1523–1528. doi: 10.1046/j.1471-4159.1999.721523.x. [DOI] [PubMed] [Google Scholar]
- 44.Derkach V.A., Oh M.C., Guire E.S., Soderling T.R. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat. Rev. Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 45.Passafaro M., Piech V., Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat. Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- 46.Park M., Penick E.C., Edwards J.G., Kauer J.A., Ehlers M.D. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- 47.Lee C.C., Huang C.C., Wu M.Y., Hsu K.S. Insulin stimulates postsynaptic density-95 protein translation via the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway. J. Biol. Chem. 2005;280:18543–18550. doi: 10.1074/jbc.M414112200. [DOI] [PubMed] [Google Scholar]
- 48.Yarube I., Ayo J., Magaji R., Umar I. Insulin treatment increases brain nitric oxide and oxidative stress, but does not affect memory function in mice. Physiol. Behav. 2019;211:112640. doi: 10.1016/j.physbeh.2019.112640. [DOI] [PubMed] [Google Scholar]
- 49.Watson G.S., Bernhardt T., Reger M.A., Cholerton B.A., Baker L.D., Peskind E.R., Asthana S., Plymate S.R., Frolich L., Craft S. Insulin effects on CSF norepinephrine and cognition in Alzheimer’s disease. Neurobiol. Aging. 2006;27:38–41. doi: 10.1016/j.neurobiolaging.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 50.Hammoud H., Netsyk O., Tafreshiha A.S., Korol S.V., Jin Z., Li J.P., Birnir B. Insulin differentially modulates GABA signalling in hippocampal neurons and, in an age-dependent manner, normalizes GABA-activated currents in the tg-APPSwe mouse model of Alzheimer’s disease. Acta Physiol. 2021;232:e13623. doi: 10.1111/apha.13623. [DOI] [PubMed] [Google Scholar]
- 51.Leclerc M., Bourassa P., Tremblay C., Caron V., Sugere C., Emond V., Bennett D.A., Calon F. Cerebrovascular insulin receptors are defective in Alzheimer’s disease. Brain. 2022:awac309. doi: 10.1093/brain/awac309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown C., Pemberton S., Babin A., Abdulhameed N., Noonan C., Brown M.B., Banks W.A., Rhea E.M. Insulin blood-brain barrier transport and interactions are greater following exercise in mice. J. Appl. Physiol. (1985) 2022;132:824–834. doi: 10.1152/japplphysiol.00866.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee S.H., Zabolotny J.M., Huang H., Lee H., Kim Y.B. Insulin in the nervous system and the mind: Functions in metabolism, memory, and mood. Mol. Metab. 2016;5:589–601. doi: 10.1016/j.molmet.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rhea E.M., Rask-Madsen C., Banks W.A. Insulin transport across the blood-brain barrier can occur independently of the insulin receptor. J. Physiol. 2018;596:4753–4765. doi: 10.1113/JP276149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gray S.M., Aylor K.W., Barrett E.J. Unravelling the regulation of insulin transport across the brain endothelial cell. Diabetologia. 2017;60:1512–1521. doi: 10.1007/s00125-017-4285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garcia-Caceres C., Quarta C., Varela L., Gao Y., Gruber T., Legutko B., Jastroch M., Johansson P., Ninkovic J., Yi C.X., et al. Astrocytic Insulin Signaling Couples Brain Glucose Uptake with Nutrient Availability. Cell. 2016;166:867–880. doi: 10.1016/j.cell.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hersom M., Helms H.C., Schmalz C., Pedersen T.A., Buckley S.T., Brodin B. The insulin receptor is expressed and functional in cultured blood-brain barrier endothelial cells but does not mediate insulin entry from blood to brain. Am. J. Physiol. Endocrinol. Metab. 2018;315:E531–E542. doi: 10.1152/ajpendo.00350.2016. [DOI] [PubMed] [Google Scholar]
- 58.Xaio H., Banks W.A., Niehoff M.L., Morley J.E. Effect of LPS on the permeability of the blood-brain barrier to insulin. Brain Res. 2001;896:36–42. doi: 10.1016/S0006-8993(00)03247-9. [DOI] [PubMed] [Google Scholar]
- 59.Urayama A., Banks W.A. Starvation and triglycerides reverse the obesity induced impairment of insulin transport at the blood-brain barrier. Endocrinology. 2008;149:3592–3597. doi: 10.1210/en.2008-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Banks W.A. The Blood-Brain Barrier Interface in Diabetes Mellitus: Dysfunctions, Mechanisms and Approaches to Treatment. Curr. Pharm. Des. 2020;26:1438–1447. doi: 10.2174/1381612826666200325110014. [DOI] [PubMed] [Google Scholar]
- 61.Baranowska-Bik A., Bik W. Insulin and brain aging. Prz Menopauzalny. 2017;16:44–46. doi: 10.5114/pm.2017.68590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baskin D.G., Brewitt B., Davidson D.A., Corp E., Paquette T., Figlewicz D.P., Lewellen T.K., Graham M.K., Woods S.G., Dorsa D.M. Quantitative autoradiographic evidence for insulin receptors in the choroid plexus of the rat brain. Diabetes. 1986;35:246–249. doi: 10.2337/diab.35.2.246. [DOI] [PubMed] [Google Scholar]
- 63.Dorn A., Rinne A., Bernstein H.G., Hahn H.J., Ziegler M. Insulin and C-peptide in human brain neurons (insulin/C-peptide/brain peptides/immunohistochemistry/radioimmunoassay) J. Fur Hirnforsch. 1983;24:495–499. [PubMed] [Google Scholar]
- 64.Schechter R., Holtzclaw L., Sadiq F., Kahn A., Devaskar S. Insulin synthesis by isolated rabbit neurons. Endocrinology. 1988;123:505–513. doi: 10.1210/endo-123-1-505. [DOI] [PubMed] [Google Scholar]
- 65.Banks W.A., Jaspan J.B., Kastin A.J. Selective, physiological transport of insulin across the blood-brain barrier: Novel demonstration by species-specific radioimmunoassays. Peptides. 1997;18:1257–1262. doi: 10.1016/S0196-9781(97)00198-8. [DOI] [PubMed] [Google Scholar]
- 66.Molnar G., Farago N., Kocsis A.K., Rozsa M., Lovas S., Boldog E., Baldi R., Csajbok E., Gardi J., Puskas L.G., et al. GABAergic neurogliaform cells represent local sources of insulin in the cerebral cortex. J. Neurosci. 2014;34:1133–1137. doi: 10.1523/JNEUROSCI.4082-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nemoto T., Toyoshima-Aoyama F., Yanagita T., Maruta T., Fujita H., Koshida T., Yonaha T., Wada A., Sawaguchi A., Murakami M. New insights concerning insulin synthesis and its secretion in rat hippocampus and cerebral cortex: Amyloid-beta1-42-induced reduction of proinsulin level via glycogen synthase kinase-3beta. Cell. Signal. 2014;26:253–259. doi: 10.1016/j.cellsig.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 68.Pitt J., Wilcox K.C., Tortelli V., Diniz L.P., Oliveira M.S., Dobbins C., Yu X.W., Nandamuri S., Gomes F.C.A., DiNunno N., et al. Neuroprotective astrocyte-derived insulin/insulin-like growth factor 1 stimulates endocytic processing and extracellular release of neuron-bound Abeta oligomers. Mol. Biol. Cell. 2017;28:2623–2636. doi: 10.1091/mbc.e17-06-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takano K., Koarashi K., Kawabe K., Itakura M., Nakajima H., Moriyama M., Nakamura Y. Insulin expression in cultured astrocytes and the decrease by amyloid beta. Neurochem. Int. 2018;119:171–177. doi: 10.1016/j.neuint.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 70.Montero-Crespo M., Dominguez-Alvaro M., Alonso-Nanclares L., DeFelipe J., Blazquez-Llorca L. Three-dimensional analysis of synaptic organization in the hippocampal CA1 field in Alzheimer’s disease. Brain. 2021;144:553–573. doi: 10.1093/brain/awaa406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Braak H., Braak E., Bohl J. Staging of Alzheimer-related cortical destruction. Eur. Neurol. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- 72.Ly P.T.T., Wu Y.L., Zou H.Y., Wang R.T., Zhou W.H., Kinoshita A., Zhang M.M., Yang Y., Cai F., Woodgett J., et al. Inhibition of GSK3 beta-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J. Clin. Investig. 2013;123:224–235. doi: 10.1172/JCI64516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hemonnot-Girard A.L., Valverde A.J., Hua J., Delaygue C., Linck N., Maurice T., Rassendren F., Hirbec H. Analysis of CX3CR1 haplodeficiency in male and female APP(swe)/PSEN1(dE9) mice along Alzheimer disease progression. Brain Behav. Immun. 2021;91:404–417. doi: 10.1016/j.bbi.2020.10.021. [DOI] [PubMed] [Google Scholar]
- 74.Bradburn S., Murgatroyd C., Ray N. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Ageing Res. Rev. 2019;50:1–8. doi: 10.1016/j.arr.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 75.Popp J., Oikonomidi A., Tautvydaite D., Dayon L., Bacher M., Migliavacca E., Henry H., Kirkland R., Severin I., Wojcik J., et al. Markers of neuroinflammation associated with Alzheimer’s disease pathology in older adults. Brain Behav. Immun. 2017;62:203–211. doi: 10.1016/j.bbi.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 76.d’Errico P., Ziegler-Waldkirch S., Aires V., Hoffmann P., Mezo C., Erny D., Monasor L.S., Liebscher S., Ravi V.M., Joseph K., et al. Microglia contribute to the propagation of a beta into unaffected brain tissue. Nat. Neurosci. 2022;25:20–25. doi: 10.1038/s41593-021-00951-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Friker L.L., Scheiblich H., Hochheiser I.V., Brinkschulte R., Riedel D., Latz E., Geyer M., Heneka M.T. Beta-Amyloid Clustering around ASC Fibrils Boosts Its Toxicity in Microglia. Cell Rep. 2020;30:3743–3754 e6. doi: 10.1016/j.celrep.2020.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ising C., Venegas C., Zhang S., Scheiblich H., Schmidt S.V., Vieira-Saecker A., Schwartz S., Albasset S., McManus R.M., Tejera D., et al. NLRP3 inflammasome activation drives tau pathology. Nature. 2019;575:669–673. doi: 10.1038/s41586-019-1769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heneka M.T., Kummer M.P., Stutz A., Delekate A., Schwartz S., Vieira-Saecker A., Griep A., Axt D., Remus A., Tzeng T.C., et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoppe S.C., Lin Y., Oakley D., Roe A.D., DeVos S.L., Hanlon D., Hyman B.T. The role of microglia in processing and spreading of bioactive tau seeds in Alzheimer’s disease. J. Neuroinflamm. 2018;15:269. doi: 10.1186/s12974-018-1309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pascoal T.A., Benedet A.L., Ashton N.J., Kang M.S., Therriault J., Chamoun M., Savard M., Lussier F.Z., Tissot C., Karikari T.K., et al. Microglial activation and tau propagate jointly across Braak stages. Nat. Med. 2021;27:1592–1597. doi: 10.1038/s41591-021-01456-w. [DOI] [PubMed] [Google Scholar]
- 82.Antharam V., Collingwood J.F., Bullivant J.P., Davidson M.R., Chandra S., Mikhaylova A., Finnegan M.E., Batich C., Forder J.R., Dobson J. High field magnetic resonance microscopy of the human hippocampus in Alzheimer’s disease: Quantitative imaging and correlation with iron. Neuroimage. 2012;59:1249–1260. doi: 10.1016/j.neuroimage.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao L.L., Jiang Z.H., Cai Z.C., Cai M., Zhang Q., Ma Y.Y., Li G.L., Zhao F.Z., Ma Q. Brain iron deposition analysis using susceptibility weighted imaging and its association with body iron level in patients with mild cognitive impairment. Mol. Med. Rep. 2017;16:8209–8215. doi: 10.3892/mmr.2017.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bao W.D., Pang P., Zhou X.T., Hu F., Xiong W., Chen K., Wang J., Wang F., Xie D., Hu Y.Z., et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021;28:1548–1562. doi: 10.1038/s41418-020-00685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cruz-Alonso M., Fernandez B., Navarro A., Junceda S., Astudillo A., Pereiro R. Laser ablation ICP-MS for simultaneous quantitative imaging of iron and ferroportin in hippocampus of human brain tissues with Alzheimer’s disease. Talanta. 2019;197:413–421. doi: 10.1016/j.talanta.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 86.Lu L.N., Qian Z.M., Wu K.C., Yung W.H., Ke Y. Expression of Iron Transporters and Pathological Hallmarks of Parkinson’s and Alzheimer’s Diseases in the Brain of Young, Adult, and Aged Rats. Mol. Neurobiol. 2017;54:5213–5224. doi: 10.1007/s12035-016-0067-0. [DOI] [PubMed] [Google Scholar]
- 87.Leskovjan A.C., Kretlow A., Lanzirotti A., Barrea R., Vogt S., Miller L.M. Increased brain iron coincides with early plaque formation in a mouse model of Alzheimer’s disease. Neuroimage. 2011;55:32–38. doi: 10.1016/j.neuroimage.2010.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hambright W.S., Fonseca R.S., Chen L., Na R., Ran Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017;12:8–17. doi: 10.1016/j.redox.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McIntosh A., Mela V., Harty C., Minogue A.M., Costello D.A., Kerskens C., Lynch M.A. Iron accumulation in microglia triggers a cascade of events that leads to altered metabolism and compromised function in APP/PS1 mice. Brain Pathol. 2019;29:606–621. doi: 10.1111/bpa.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zeineh M.M., Chen Y., Kitzler H.H., Hammond R., Vogel H., Rutt B.K. Activated iron-containing microglia in the human hippocampus identified by magnetic resonance imaging in Alzheimer disease. Neurobiol. Aging. 2015;36:2483–2500. doi: 10.1016/j.neurobiolaging.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kroner A., Greenhalgh A.D., Zarruk J.G., dos Santos R.P., Gaestel M., David S. TNF and Increased Intracellular Iron Alter Macrophage Polarization to a Detrimental M1 Phenotype in the Injured Spinal Cord. Neuron. 2014;83:1098–1116. doi: 10.1016/j.neuron.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 92.Hirai K., Aliev G., Nunomura A., Fujioka H., Russell R.L., Atwood C.S., Johnson A.B., Kress Y., Vinters H.V., Tabaton M., et al. Mitochondrial abnormalities in Alzheimer’s disease. J. Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang W., Yin J., Ma X., Zhao F., Siedlak S.L., Wang Z., Torres S., Fujioka H., Xu Y., Perry G., et al. Inhibition of mitochondrial fragmentation protects against Alzheimer’s disease in rodent model. Hum. Mol. Genet. 2017;26:4118–4131. doi: 10.1093/hmg/ddx299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fang E.F., Hou Y., Palikaras K., Adriaanse B.A., Kerr J.S., Yang B., Lautrup S., Hasan-Olive M.M., Caponio D., Dan X., et al. Mitophagy inhibits amyloid-beta and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 2019;22:401–412. doi: 10.1038/s41593-018-0332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nixon R.A., Yang D.S. Autophagy failure in Alzheimer’s disease-locating the primary defect. Neurobiol. Dis. 2011;43:38–45. doi: 10.1016/j.nbd.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de la Cueva M., Antequera D., Ordonez-Gutierrez L., Wandosell F., Camins A., Carro E., Bartolome F. Amyloid-beta impairs mitochondrial dynamics and autophagy in Alzheimer’s disease experimental models. Sci. Rep. 2022;12:10092. doi: 10.1038/s41598-022-13683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reddy P.H., Yin X., Manczak M., Kumar S., Pradeepkiran J.A., Vijayan M., Reddy A.P. Mutant APP and amyloid beta-induced defective autophagy, mitophagy, mitochondrial structural and functional changes and synaptic damage in hippocampal neurons from Alzheimer’s disease. Hum. Mol. Genet. 2018;27:2502–2516. doi: 10.1093/hmg/ddy154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miller M.B., Huang A.Y., Kim J., Zhou Z., Kirkham S.L., Maury E.A., Ziegenfuss J.S., Reed H.C., Neil J.E., Rento L., et al. Somatic genomic changes in single Alzheimer’s disease neurons. Nature. 2022;604:714–722. doi: 10.1038/s41586-022-04640-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mandal P.K., Shukla D., Tripathi M., Ersland L. Cognitive Improvement with Glutathione Supplement in Alzheimer’s Disease: A Way Forward. J. Alzheimer’s Dis. 2019;68:531–535. doi: 10.3233/JAD-181054. [DOI] [PubMed] [Google Scholar]
- 100.Tan J., Li Q.X., Evin G. Effects of Mild and Severe Oxidative Stress on BACE1 Expression and APP Amyloidogenic Processing. Methods Mol. Biol. 2016;1303:101–116. doi: 10.1007/978-1-4939-2627-5_4. [DOI] [PubMed] [Google Scholar]
- 101.Butterfield D.A., Sultana R. Methionine-35 of abeta(1-42): Importance for oxidative stress in Alzheimer disease. J. Amino Acids. 2011;2011:198430. doi: 10.4061/2011/198430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao Y., Xiao Y., Miao R., Zhao J., Zhang W., Huang G., Ma F. The characteristic of cognitive function in Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2015;109:299–305. doi: 10.1016/j.diabres.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Y.W., Zhang J.Q., Liu C., Wei P., Zhang X., Yuan Q.Y., Yin X.T., Wei L.Q., Cui J.G., Wang J. Memory dysfunction in type 2 diabetes mellitus correlates with reduced hippocampal CA1 and subiculum volumes. Chin. Med. J. 2015;128:465–471. doi: 10.4103/0366-6999.151082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang R.R., Jia B.H., Xie L., Ma S.H., Yin J.J., Sun Z.B., Le H.B., Xu W.C., Huang J.Z., Luo D.X. Spatial working memory impairment in primary onset middle-age type 2 diabetes mellitus: An ethology and BOLD-fMRI study. J. Magn. Reson. Imaging. 2016;43:75–87. doi: 10.1002/jmri.24967. [DOI] [PubMed] [Google Scholar]
- 105.Soares E., Prediger R.D., Nunes S., Castro A.A., Viana S.D., Lemos C., De Souza C.M., Agostinho P., Cunha R.A., Carvalho E., et al. Spatial memory impairments in a prediabetic rat model. Neuroscience. 2013;250:565–577. doi: 10.1016/j.neuroscience.2013.07.055. [DOI] [PubMed] [Google Scholar]
- 106.Singh A., Bodakhe S.H. Resveratrol attenuates behavioural impairment associated with learning and memory in rats with diabetes induced by a high-fat diet and streptozotocin. Br. J. Pharmacol. 2022;179:4673–4691. doi: 10.1111/bph.15895. [DOI] [PubMed] [Google Scholar]
- 107.Morris J.K., Vidoni E.D., Honea R.A., Burns J.M., Alzheimer’s Disease Neuroimaging I. Impaired glycemia increases disease progression in mild cognitive impairment. Neurobiol. Aging. 2014;35:585–589. doi: 10.1016/j.neurobiolaging.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Potenza M.A., Sgarra L., Desantis V., Nacci C., Montagnani M. Diabetes and Alzheimer’s Disease: Might Mitochondrial Dysfunction Help Deciphering the Common Path? Antioxidants. 2021;10:1257. doi: 10.3390/antiox10081257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wakabayashi T., Yamaguchi K., Matsui K., Sano T., Kubota T., Hashimoto T., Mano A., Yamada K., Matsuo Y., Kubota N., et al. Differential effects of diet- and genetically-induced brain insulin resistance on amyloid pathology in a mouse model of Alzheimer’s disease. Mol. Neurodegener. 2019;14:15. doi: 10.1186/s13024-019-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schmitz L., Kuglin R., Bae-Gartz I., Janoschek R., Appel S., Mesaros A., Jakovcevski I., Vohlen C., Handwerk M., Ensenauer R., et al. Hippocampal insulin resistance links maternal obesity with impaired neuronal plasticity in adult offspring. Psychoneuroendocrinology. 2018;89:46–52. doi: 10.1016/j.psyneuen.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 111.Spinelli M., Fusco S., Mainardi M., Scala F., Natale F., Lapenta R., Mattera A., Rinaudo M., Li Puma D.D., Ripoli C., et al. Brain insulin resistance impairs hippocampal synaptic plasticity and memory by increasing GluA1 palmitoylation through FoxO3a. Nat. Commun. 2017;8:2009. doi: 10.1038/s41467-017-02221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Denver P., English A., McClean P.L. Inflammation, insulin signaling and cognitive function in aged APP/PS1 mice. Brain Behav. Immun. 2018;70:423–434. doi: 10.1016/j.bbi.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 113.Willette A.A., Modanlo N., Kapogiannis D., Alzheimer’s Disease Neuroimaging Initiative Insulin resistance predicts medial temporal hypermetabolism in mild cognitive impairment conversion to Alzheimer disease. Diabetes. 2015;64:1933–1940. doi: 10.2337/db14-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cui Y., Tang T.Y., Lu C.Q., Ju S. Insulin Resistance and Cognitive Impairment: Evidence From Neuroimaging. J. Magn. Reson. Imaging. 2022;56:1621–1649. doi: 10.1002/jmri.28358. [DOI] [PubMed] [Google Scholar]
- 115.Huang Y.T., Happonen K.E., Burrola P.G., O’Connor C., Hah N., Huang L., Nimmerjahn A., Lemke G. Microglia use TAM receptors to detect and engulf amyloid beta plaques. Nat. Immunol. 2021;22:586–594. doi: 10.1038/s41590-021-00913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maianti J.P., Tan G.A., Vetere A., Welsh A.J., Wagner B.K., Seeliger M.A., Liu D.R. Substrate-selective inhibitors that reprogram the activity of insulin-degrading enzyme. Nat. Chem. Biol. 2019;15:565–574. doi: 10.1038/s41589-019-0271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sahoo B.R., Panda P.K., Liang W., Tang W.J., Ahuja R., Ramamoorthy A. Degradation of Alzheimer’s Amyloid-beta by a Catalytically Inactive Insulin-Degrading Enzyme. J. Mol. Biol. 2021;433:166993. doi: 10.1016/j.jmb.2021.166993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Starks E.J., O’Grady J.P., Hoscheidt S.M., Racine A.M., Carlsson C.M., Zetterberg H., Blennow K., Okonkwo O.C., Puglielli L., Asthana S., et al. Insulin Resistance is Associated with Higher Cerebrospinal Fluid Tau Levels in Asymptomatic APOE epsilon 4 Carriers. J. Alzheimers Dis. 2015;46:525–533. doi: 10.3233/JAD-150072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang W., Liu Y., Xu Q.Q., Xian Y.F., Lin Z.X. Sulforaphene Ameliorates Neuroinflammation and Hyperphosphorylated Tau Protein via Regulating the PI3K/Akt/GSK-3beta Pathway in Experimental Models of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2020;2020:4754195. doi: 10.1155/2020/4754195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhao W.H., Xu W.M. Glutaredoxin 2 (GRX2) deficiency exacerbates high fat diet (HFD)-induced insulin resistance, inflammation and mitochondrial dysfunction in brain injury: A mechanism involving GSK-3 beta. Biomed. Pharmacother. 2019;118:108940. doi: 10.1016/j.biopha.2019.108940. [DOI] [PubMed] [Google Scholar]
- 121.Liao Y.F., Wang B.J., Cheng H.T., Kuo L.H., Wolfe M.S. Tumor necrosis factor-alpha, interleukin-1 beta, and interferon-gamma stimulate gamma-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. J. Biol. Chem. 2004;279:49523–49532. doi: 10.1074/jbc.M402034200. [DOI] [PubMed] [Google Scholar]
- 122.Khan M.S., Muhammad T., Ikram M., Kim M.O. Dietary Supplementation of the Antioxidant Curcumin Halts Systemic LPS-Induced Neuroinflammation-Associated Neurodegeneration and Memory/Synaptic Impairment via the JNK/NF-kappa B/Akt Signaling Pathway in Adult Rats. Oxid. Med. Cell. Longev. 2019;2019:7860650. doi: 10.1155/2019/7860650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ruegsegger G.N., Vanderboom P.M., Dasari S., Klaus K.A., Kabiraj P., McCarthy C.B., Lucchinetti C.F., Nair K.S. Exercise and metformin counteract altered mitochondrial function in the insulin-resistant brain. JCI Insight. 2019;4:e130681. doi: 10.1172/jci.insight.130681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zuo L., Hemmelgarn B.T., Chuang C.C., Best T.M. The Role of Oxidative Stress-Induced Epigenetic Alterations in Amyloid-beta Production in Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2015;2015:604658. doi: 10.1155/2015/604658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rains J.L., Jain S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]