Abstract
Chlamydia pneumoniae, a bacterial respiratory tract pathogen, has been associated with atherosclerosis in humans. C. pneumoniae infection of the respiratory tracts of rabbits fed a noncholesterol diet induced changes of atherosclerosis of the aorta in 6 (26.1%) of 23 animals after a single inoculum at 3 months. Multiple inocula given three times within 6 weeks resulted in grade III atherosclerosis in 8 (34.8%) of 23 rabbits, with an additional 5 (21.7%) showing increased myxoid changes in the intima-media junction and exhibiting 8 (34.8%) focal periaortitis. Control animals inoculated with carrier broth (n = 24), HEp-2 cells (n = 12), or another respiratory pathogen, Mycoplasma pneumoniae (n = 32), produced no changes of atherosclerosis after 3 months. The histological changes were dissimilar (fewer foam cells) from those of rabbits fed a 0.5% cholesterol diet but were highly similar to or indistinguishable from changes in rabbits fed a 0.15% cholesterol diet (similar to that of humans). Proinflammatory cytokines and tissue growth factors were more consistently detected in cholesterol-induced aortic lesions than those induced by C. pneumoniae. These data are compatible with de novo induction of atherogenesis by C. pneumoniae in rabbits and suggest that C. pneumoniae may be important in the pathogenesis of atherosclerosis in humans.
Human atherogenesis appears to be multifactorial in nature, as no single event can fully explain the pathogenesis of human blood vessel arteriopathy. The current concept of the pathogenesis of atherosclerosis as a response to injury (38) could be compatible with an infectious organism as an inducing agent.
Chlamydia pneumoniae is a common human bacterial pathogen that causes community-acquired pneumonia, bronchitis, and sinusitis (10, 11, 28). C. pneumoniae is distinct from but related to other members of the genus Chlamydia, which include Chlamydia trachomatis (a sexually acquired infection causing cervicitis, urethritis, pelvic inflammatory disease, tubal infertility, and ectopic pregnancy; the cause of nonvenereal transmitted conjunctivitis and trachoma in areas of the world where the diseases are endemic), Chlamydia psittacii (a zoonosis of birds that causes pneumonia and endocarditis in humans), and Chlamydia pecorium, which is not established as a human pathogen.
Seroepidemiological studies have shown an increasing prevalence of antichlamydial antibody with age, indicating the presence of acute C. pneumoniae infection beginning in childhood and extending to adulthood (12). The prevalence increases from ages 5 through 14 years, and by age 20 years, approximately 50% of persons have serum antibodies to C. pneumoniae. The seroprevalence continues to increase, reaching 75% in the elderly. The data suggest that most people are infected and reinfected throughout life.
Recently, C. pneumoniae has been associated with coronary artery disease and myocardial infarction in several seroprevalence epidemiological studies (24, 30, 35, 45, 46) and one prospective, cohort study (39). One seroprevalence study (29) also found an association between carotid artery disease and antibodies to C. pneumoniae.
More convincing is the fact that C. pneumoniae has been identified histopathologically in atherosclerotic plaques of the aorta, coronary, and carotid arteries by immunohistochemical stain, PCR, and electron microscopy (3, 21, 22, 42). Furthermore, viable C. pneumoniae has been recovered from human atheromas of the coronary artery and carotid endarterectomy specimen (14, 26, 36). These data suggest that C. pneumoniae may play a role in the pathogenesis of atherosclerosis; alternatively, it could represent nonspecific entrapment of bacteria as an innocent bystander in the diseased vessels. Recent animal studies in the rabbit and mouse models (9, 23, 32, 33) also suggest the potential for inducing intimal vascular lesions and localization of the organism in the aorta and thus may play a causal role in atherogenesis. This study was designed to assess the pathogenic role of C. pneumoniae in an animal model.
MATERIALS AND METHODS
This study was approved by the Animal Care Committee of St. Michael's Hospital, and their care was in accordance with institutional guidelines.
Animals.
One-month-old male pathogen-free New Zealand White (NZW) rabbits were fed cholesterol-free, standard chow diets (groups I to V), and two groups (VI and VII) were fed 0.5 and 0.15% (by weight) cholesterol-supplemented chow. The animals were studied in groups, and between study groups the animal care room was thoroughly cleansed aseptically and sprayed with a germicidal detergent (Quadricide PU).
Five groups of rabbits fed the cholesterol-free diet were studied: (i) 24 rabbits were inoculated once via the posterior nasopharynx with C. pneumoniae and sacrificed after 3 months; (ii) 24 rabbits were inoculated three times within 6 weeks with two separate strains of C. pneumoniae and sacrificed at 12 weeks after the first inoculation; (iii) 24 rabbits (controls) were inoculated once with carrier broth (sterile) via the nasopharynx and sacrificed at 3 months; (iv) 12 control rabbits were inoculated three times with HEp-2 cells in sucrose-phosphate-glutamic acid (SPG) buffer 2 weeks apart and sacrificed at 12 weeks after the first inoculation; (v) 32 rabbits (controls) were inoculated with another human respiratory pathogen (Mycoplasma pneumoniae) once via the nasopharynx, and then 12 were sacrificed at 10 days and the remaining 20 were sacrificed at 3 months.
Ten NZW rabbits were fed the 0.5% cholesterol (by weight) diet, were not inoculated with any microorganisms, and were sacrificed at 3 months; another 10 NZW rabbits were fed 0.15% cholesterol-supplemented chow without infection and sacrificed at 3 months.
C. pneumoniae strain and inoculum.
Two separate strains of C. pneumoniae were used in the experiments: TWAR ATCC strain VR 1310 (American Type Culture Collection, Rockville, Md.) and TWAR strain AR-39 (Washington Research Foundation, Seattle). Both strains were originally isolated from patients with respiratory infection. Viable organisms were harvested from infected cultures of HEp-2 cells (37) by disrupting infected cells with glass beads and sonification after 72 h. Organisms were partially purified by one cycle of low- and high-speed centrifugation each, resuspended in SPG buffer, and frozen in 1.0-ml aliquots at −70°C. Inoculum preparations were adjusted to contain 1.0 × 107 to 2.6 × 107 inclusion-forming units of C. pneumoniae per ml. Contamination by C. trachomatis, C. psittacii, and Mycoplasma species was excluded by analysis with PCR using genus- and species-specific primers (27). Rabbits in groups I and II received 1 ml of the prepared inocula via the nasopharynx with a catheter; the control group III animals were inoculated with uninfected SPG buffer, while control group IV animals were inoculated with HEp-2 cells and buffer.
M. pneumoniae strain and inoculum.
An ATCC strain of M. pneumoniae (Eaton strain 15531) was cultured in Hayflick broth supplemented with glucose with an indicator dye (0.2% phenol red) to attain logarithmic-phase growth by incubation at 35°C in 5% CO2 for 4 days. The inoculum was diluted to achieve 1.0 × 107 to 1.5 × 107 CFU/ml (confirmed by colony counts on Hayflick agar) after freezing at −70°C. Group V rabbits were inoculated via the nasopharynx as in the other groups.
Serology.
Antibodies (immunoglobulin G [IgG]) to C. pneumoniae were measured by the microimmunofluorescence test (MRL Diagnostics, Cypress, Calif.). The IgG serum antibody fractions were measured by using fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (Sigma Chemical Co., St. Louis, Mo.). Sera were screened at dilutions of 1:2, and those positive were further diluted. Blood was obtained from the ear lobe marginal veins at baseline before inoculation and on the day of sacrifice. IgM and IgA antibodies were not tested because anti-rabbit IgM and IgA conjugates were not available commercially. C. pneumoniae antibodies were tested only in groups I and III.
M. pneumoniae antibodies were measured in the rabbits of group V, before inoculation and at sacrifice by the serum complement fixation test, using the chloroform-methanol extraction lipid antigen (17) Mycoplasma CF (Microbix Biosystems Inc., Toronto, Ontario, Canada). A control antigen produced from uninfected broth media from which the mycoplasma was grown was used for excluding anticomplementary activity.
Serum cholesterol.
Serum total cholesterol levels were determined from all groups of rabbits just before sacrifice, using the standard cholesterol oxidase method performed on Johnson and Johnson Ektachem 750 (1).
Pathological investigations.
The aortae were removed from the animals and cleared of any adhering fat; the vessels were divided into the arch, the proximal descending thoracic aorta, and the abdominal aorta. Before division into sections, the aorta was split longitudinally, splayed, and pinned for gross photomicrography. Two transverse sections including areas with and without evidence of atherosclerosis macroscopically were taken from each segment. Each aortic segment was examined for the presence of C. pneumoniae by staining with special immunohistochemical stains. Studies to detect C. pneumoniae particles were performed on areas with and without atherosclerotic changes.
Fresh tissues were taken for C. pneumoniae cultures. We were unable to perfusion fix the aorta and to quantitate the aortic atherosclerotic lesions or to perform staining with vital stains such as oil-red O for lesional fat content.
The lungs, liver, and spleen were also removed and examined from rabbits in groups II, IV, and V.
Light microscopy and lesion classification.
All histopathological specimens were fixed in 10% buffered formalin, processed, and paraffin embedded. Transverse and longitudinal sections of each segment were submitted on one cassette. Hematoxylin-eosin (H&E) and Movat's pentachrome stains were used on sections for histological examination. The atherosclerotic lesions were graded histologically, using a modification of the classification of Daley et al. (6): grade I (early fatty streaks) is defined as consisting of foamy cells (identified as macrophages by immunohistochemical stain) in the intima (these lesions are similar to Stary's type I and II lesions [43]); grade II (advanced fatty streaks) is defined as consisting of approximately equal numbers of foam cells and spindle-shaped cells (fibro-fatty lesions similar to Stary's type III lesion); grade III (spindle cell lesion) is defined as consisting of increased spindle-shaped (smooth muscle) cells with enlarged nuclei and their products (this lesion is unlike any lesion in Stary's classification); grade IV (advanced atheromatous lesion) is defined as the presence of core-containing pools of extracellular lipid and/or necrotic debris and fibrous cap (similar to Stary's type IV lesion). Advanced spindle cell lesion with calcification (similar histologically to Stary's type VII lesion but grossly does not impinge on or narrow the lumen as is typically seen with Stary's type VII lesion) was also considered grade IV.
Immunohistochemical study.
Immunohistochemical staining was performed on paraffin-embedded sections by the modified streptavidin-biotin-peroxidase method (4). For detection of chlamydia antigens, tissue sections were reacted with Chlamydia genus-specific mouse monoclonal antibody, directed against chlamydial lipopolysaccharide (CF2; Washington Research Foundation), using CF2-conjugated to biotin at 1:600. Two C. pneumoniae species-specific monoclonal IgG antibodies, TWAR 402 (Washington Research Foundation) at a dilution of 1:300, and a second antibody, Chlamydia Cel Pn (Cellab, Bellvue, Queensland, Australia) at 1:150 dilution, were also used. Tissue sections were reacted with normal mouse ascitic fluid (similar dilution as the antibody) as negative controls; for positive controls, C. pneumoniae infected HEp-2 cell pellets were formalin fixed, embedded in paraffin, and sectioned. Known positive lung and spleen samples from previous experiments were also used.
Tissue sections from rabbits of group V were also stained for M. pneumoniae antigen by the immunoperoxidase method using M. pneumoniae species-specific rabbit antisera (44) in a dilution of 1:1,200 (kindly provided by P. A. Quinn, Hospital for Sick Children, Toronto, Ontario, Canada). Positive controls were prepared with M. pneumoniae-infected mouse fibroblasts (3T6 cells) in minimum essential medium; cell pellets were formalin fixed and paraffin embedded. Negative controls were prepared from uninfected 3T6 cells and also with uninfected control rabbit lung tissues.
Aortic tissues with and without changes of atherosclerosis from rabbits infected with C. pneumoniae (groups I and II), from the control uninfected animals (group III), and from the cholesterol-fed group VI were stained by the immunoperoxidase method with antisera (anti-human) against von Willebrand factor for endothelial cells (IgG, 1:500 dilution; DAKO, Capinteria, Calif.), smooth muscle actin for smooth muscle cells (anti-human IgG, 1:150 dilution; DAKO) (both antibodies cross-reacted with rabbit antigens in a preliminary study in our laboratory), and MAC 387 IgG anti-human antibody (undiluted; Serotec, Mississauga, Ontario, Canada), which reacts with rabbit monocytes and macrophages; anti-rabbit antibodies to T-cell (MCA 805) and B-cell (MCA 812) markers (Serotec) were also used. Antibodies to the following cytokines and growth factors were used in immunohistochemical studies: anti-human transforming growth factor beta (anti-TGF-β) (Serotec), purified polyclonal anti-rabbit tumor necrosis factor alpha (anti-TNF-α), anti-human interleukin-1 (anti-IL-1α), and anti-mouse IL-6 (monoclonal antibodies, 1:25 dilution), anti-human platelet-derived growth factor beta (anti-PDGF-β; 1:25 dilution; Pharmingen, San Diego, Calif.) (the cytokine antibodies against IL-1α, IL-6, PDGF, and TGF-β were shown to cross-react in rabbit tissue by a pilot study conducted in our laboratory), and monoclonal antibodies to the metabolite of oxidized low-density lipoprotein (LDL) (34) and malondialdehyde-lysine (MDA-2; diluted 1:1,400), kindly provided by W. Palinski (University of California, San Diego). Negative controls consisted of phosphate-buffered saline instead of the primary antibodies performed in each case; positive controls consisted of human carotid atherosclerotic tissues for MDA-2 antibodies and human breast tumor tissue and carotid artery tissues, liver, and spleen from previous C. pneumoniae-infected rabbits for cytokine antibodies.
C. pneumoniae cultures.
Aortic tissues from rabbits of groups I to III were homogenized with a sterile mortar and pestle in 1.0 to 2.0 ml of SPG medium to make a 10% (wt/vol) suspension and then kept frozen at −70°C until ready for culture. Tissue homogenates were thawed and centrifuged at 500 × g for 5 min at 4°C to sediment tissue debris, and the supernatant was aspirated for inoculation of cultures. HEp-2 cell monolayers were inoculated with 100 μl of 10% tissue homogenate. Inoculated cells were incubated at 35°C in a 5% CO2 incubator 4 days and blindly passaged once for another 4 days. Monolayers were fixed with methanol and stained with a Chlamydia genus-specific monoclonal antibody (Pathfinder Culture Confirmation reagent; Kallestad, Chaska, Minn.) conjugated to fluorescein isothiocyanate (20).
Blinding.
Investigators and technologists assessing histopathology, immunohistochemistry, serology, and cultures were blinded to the groups of animal tissues or serum being assessed to prevent bias.
RESULTS
C. pneumoniae-infected rabbits.
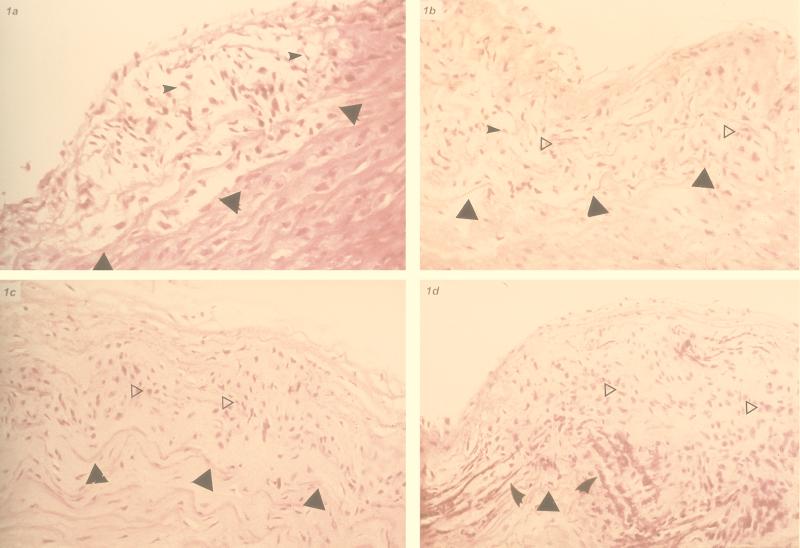
One rabbit of the single-inoculation group I had to be sacrificed prematurely soon after inoculation because of accidental injury, leaving 23 rabbits for analysis in this group. Six animals (26.1%) showed microscopic changes of atherosclerosis of the aorta (two with grade I-II changes and 4 with grade III changes) (Fig. 1). These lesions were grossly visible as discrete foci of slightly raised, pale patches. Eight rabbits also demonstrated focal increased accumulation of myxoid connective tissue ground substance in the media, three of which had changes of grade III atherosclerosis. Thus, five other rabbits (21.7%) had isolated myxoid changes. Signs of focal lymphocytic infiltration of the adventitia of the abdominal aorta (periaortitis) were also present in six rabbits (two with changes of atherosclerosis) (Fig. 1E).
FIG. 1.
C. pneumoniae-inoculated rabbit aorta. (a) Grade I lesion (fatty streak); foamy macrophages (small arrowheads) in the intima. The triangle indicates internal elastic lamina. H&E; original magnification, ×250. (b) Grade II lesion (advanced fatty streak); mixed foamy macrophages (arrowheads) and spindle smooth muscle cells (empty triangle, horizontally to right). Solid triangles indicate internal elastic lamina. H&E; original magnification, ×250. (c) Grade III lesion (fibromuscular); smooth muscle cells (empty triangle, horizontally to right) in the intima. Solid triangles indicate the internal elastic lamina. H&E; original magnification, ×250. (d) Grade IV lesion; advanced fibromuscular lesion with smooth muscle cell (empty triangle, horizontally to right) proliferation and calcification (indicated by curved arrowheads). This lesion lacks foam cells, lipid core, and fibrous cap as typical for Stary's type IV lesion or seen with 0.5% cholesterol. H&E; original magnification, ×250. (E) Periaortitis; lymphocytes and macrophages infiltrating aortic adventitia encroaching the media (star). The intima and media layer are unremarkable (arrowheads indicate the endothelial surface). H&E; original magnification, ×250.
One rabbit in the multiple-inoculation group II had to be sacrificed early due to an injury; 8 of 23 (34.8%) showed grade III-IV atherosclerosis (Fig. 1c and d), and 5 other animals (21.7%) demonstrated increased myxoid connective tissue ground substance in the intima-media junction, separating the elastic fibers (showing some fragmentation) of the aorta, with a slight increase of spindly smooth muscle cells. These latter changes are also seen in atherosclerosis. Eight (34.8%) of the rabbits also had focal periaortitis (four also had atherosclerosis). Macroscopically the visible lesions were still small discrete patches.
Controls.
None of the 36 uninfected control rabbits (groups III and IV) showed changes of atherosclerosis or periaortitis compared to the C. pneumoniae-infected groups (P = 0.009 and P = 0.03 for the single-inoculation and multiple-inoculation groups, respectively). In the M. pneumoniae-infected animals (group V), 10 of 12 (83.3%) rabbits sacrificed at day 10 showed changes of patchy, bronchopneumonia, bronchiolitis, interstitial infiltrates, and pulmonary congestion. Six (50%) of these rabbits showed lymphoid hyperplasia in the spleen and portal triaditis of the liver; one (8%) had focal periaortitis. At 3 months, 6 of 20 (30%) rabbits demonstrated mild patchy interstitial infiltrate and lymphoid hyperplasia around the airways of the lungs. Two (10%) of the rabbits also had focal periaortitis of the abdominal aorta. Immunohistochemical stain for M. pneumoniae antigen was positive in all of the animals sacrificed at day 10 in the bronchial epithelial cells and within macrophages of lymphoid infiltrate of the lung, with evidence of dissemination to the spleen (100%), liver (10%), and lymphoid infiltrate in the periaortic area. In the animals sacrificed at 3 months, M. pneumoniae antigen persisted in the lymphoid tissue around the bronchioles and in the spleens of five (25%) of the rabbits but was not detected in the liver and areas of periaortitis. None of the 32 mycoplasma-infected rabbits showed signs of atherosclerosis of the aorta compared to the C. pneumoniae-infected groups (P < 0.008). The abnormalities of the aorta, including atherosclerosis, for the various groups are summarized in Table 1.
TABLE 1.
Summary of atherosclerotic and other findings in the aortae of infected and uninfected rabbits
| Finding | No. (%)
|
|||||
|---|---|---|---|---|---|---|
| Controls
|
C. pneumoniae infected
|
|||||
| Uninfected (n = 36) | Mycoplasma infected (n = 32) | Cholesterol fed
|
||||
| 0.5% (n = 10) | 0.15% (n = 10) | Single inoculum (n = 23) | Multiple inocula (n = 23) | |||
| Atherosclerosis | 0 | 0 | 10 (100) | 2 (20) | 6 (26.1) | 8 (34.8) |
| Myxoid changesa | 0 | 0 | 0 | 0 | 5 (21.7) | 5 (21.7) |
| Periaortitis | 0 | 3 (9.4) | 0 | 0 | 6 (26.1) | 8 (34.8) |
Accumulation of matrix ground substance within the intima-media, separating elastic fibers and with slight increase of smooth muscle cells but no other changes of atherosclerosis as defined.
Cholesterol-fed rabbits (group VI).
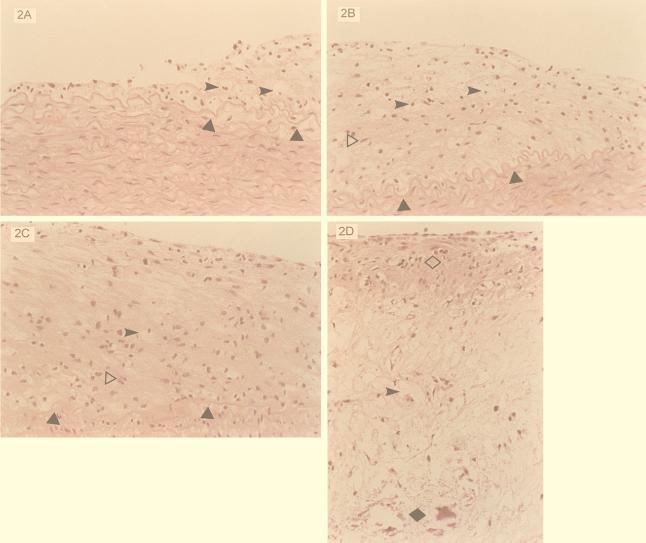
The 10 uninfected rabbits fed the 0.5% cholesterol diet all had diffuse gross changes of atherosclerosis at 3 months. All 10 had grade I-IV changes, but 6 (60%) had predominantly grade III-IV changes, and 4 had mostly grade II atherosclerosis (Fig. 2). Histologically the lesions were more diffuse and advanced than those seen in the infected animals. Moreover, foam cells were more prominent and widespread and present in all grades (Fig. 2), compared to predominantly spindle muscle cells and sparsely detected foam cells in the aortic lesions of the C. pneumoniae-induced lesions. Whereas the 0.5% cholesterol-fed rabbit type IV lesions closely resemble Stary's type IV lesion, with a lipid core and fibrous cap (Fig. 2D), the infected-rabbit advanced lesions were more fibromuscular, with calcification without a lipid core (Fig. 1d).
FIG. 2.
Aorta of rabbit fed 0.5% cholesterol diet. (A) Grade I lesion. The fatty streak lesion is seen adjacent to unremarkable intima-media. Triangles indicate internal elastic lamina, and arrowheads indicate foamy macrophage. H&E; original magnification, ×250. (B) Grade II lesions; moderate expansion of the intima with mixed foamy macrophages (arrowheads) and spindle smooth muscle cells (empty triangle, horizontally to right). Solid triangles indicate internal elastic lamina. H&E; original magnification, ×250. (C) Grade III lesion; moderate to marked expansion of the intima with predominantly spindle smooth muscle cells (empty triangle, horizontally to right) occupying the entire intima. Solid triangles indicate internal elastic lamina, and the arrowhead indicates a foamy macrophage (which is absent in the infected type III lesion). H&E; original magnification, ×250. (D) Grade IV lesion (advanced atheromatous lesion); a fibromuscular cap (empty diamond) with mainly foamy macrophages (arrowhead) and cholesterol clefts and calcification (solid diamond) at the base of the atheromatous plaque. Note difference from infected grade IV lesion (Fig. 1d). H&E; original magnification, ×100.
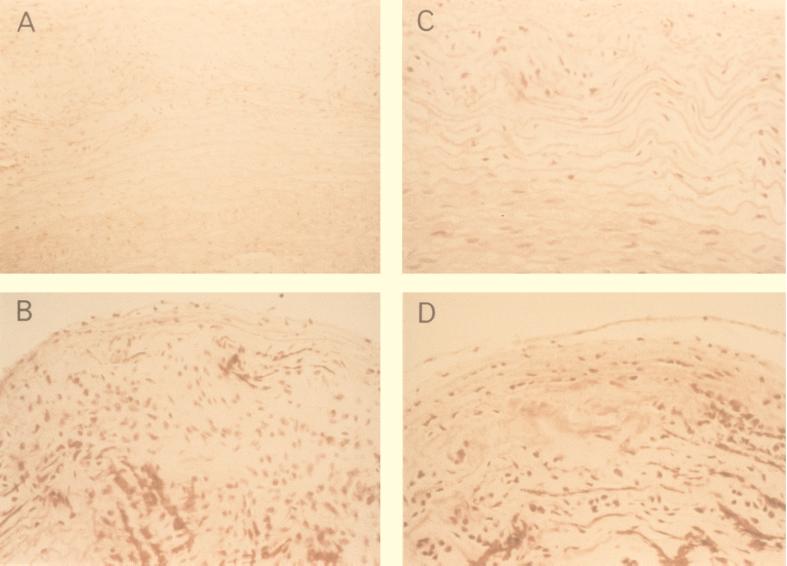
The mean serum cholesterol concentration was significantly elevated in the 0.5% cholesterol-fed group (53.2 ± 13.6 mmol/liter) compared to the uninfected controls fed standard chow (0.8 ± 0.3 mmol/liter) or the C. pneumoniae-infected groups (0.8 ± 0.3 and 0.9 ± 0.2 mmol/liter), but there was no significant difference in cholesterol levels between the C. pneumoniae-infected rabbits and the uninfected controls fed cholesterol-free chow. In rabbits fed the 0.15% cholesterol diet, grossly visible lesions were not seen in the aorta. However, microscopically two rabbits (20%) showed changes of early atherosclerosis (grade III-IV) which closely resemble the lesions in the infected rabbits histologically (Fig. 3). The mean serum cholesterol concentration of this group (4.1 ± 2 mmol/liter) is similar to that seen in humans.
FIG. 3.
(A and B) Grade III and IV lesions from rabbits infected with C. pneumoniae; (C and D) grade III and IV lesions in 0.15% cholesterol-fed rabbits. Note the similarity. H&E stain; original magnification, ×250.
Immunocytochemical stains.
The results of staining with the special stains for cellular markers and C. pneumoniae antigen are summarized in Table 2.
TABLE 2.
Cytological changes associated with C. pneumoniae-induced atherosclerotic, myxoid, and periaortic lesionsa
| Cell type | Controls (aorta) | Cholesterol fedb (atherosclerosis) |
C. pneumoniae infected
|
||
|---|---|---|---|---|---|
| Atherosclerosis | Myxoidc | Periaortitis | |||
| B cell | 0 (0/25) | +–++ (6/10) | 0–+ (2/17) | 0–+ (1/21) | +–+++ (4/14) |
| T cell | 0 (0/25) | ++–+++ (7/10) | +–++ (5/17) | 0–+ (1/21) | +–+++ (6/14) |
| Macrophage | 0 (0/25) | ++–+++ (10/10) | + (6/17) | 0–+ (3/21) | +–+++ (10/14) |
| Smooth muscle | N (25) | +++ (10/10) | +–+++ (11/17) | + (9/21) | 0 (0/14) |
| C. pneumoniae (sum of all stains) | 0 (0/25) | 0 (0/10) | +–++ (7/17) | +–++ (9/21) | + (5/14) |
Grading system: 0, negative; N, positive normal amount; +, one focus with ≥5 cells positive; ++, two to five foci each with ≥5 cells positive; +++, over five foci each with ≥5 cells positive. Number of lesions positive over total lesions (or aortic sections) tested is given in parentheses.
Animals were fed 0.5% cholesterol-containing diets.
Focal area of accumulation of myxoid ground substance in the media without other changes.
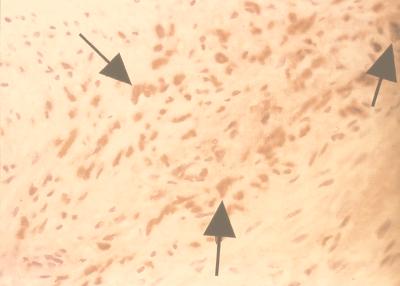
The cell markers were positive and increased to various degrees in the cholesterol-induced atherosclerotic lesions, with uniform increase of macrophages and smooth muscle cells and lower detection of T and B cells (60 to 70%). All aortae of the uninfected animals (fed standard chow) were negative for these cell markers except for the normal presence of smooth muscle cells in the media. In the C. pneumoniae-induced atherosclerotic lesions, smooth muscle cells were increased by histological appearance in all with grade III lesions, but the cell marker for smooth muscle cells was increased above normal in only 65% (indicating possible low sensitivity of the anti-human antibody in rabbits). Similarly, T cells and macrophages were found to be increased by immunocytochemistry in 29.5 to 35.3%. C. pneumoniae antigen was detected in 41% of the infection-induced (mainly grade III-IV) atherosclerotic lesions (Fig. 4), 42.9% of the myxoid lesions, and 35.7% of periaortitis foci but in none of the controls or animals with cholesterol-induced atherosclerosis.
FIG. 4.
Grade III (fibromuscular) lesion with C. pneumoniae demonstrated by immunohistochemistry; positive staining in macrophage indicated by arrowhead. Hematoxylin counterstained; original magnification, ×250.
The results of the immunocytochemistry for cytokines, growth factors, and MDA-2 are summarized in Table 3. Lesions of animals with cholesterol-induced atherosclerosis were consistently more positive for cytokines (50 to 60%), growth factors (100%), and MDA-2 (70%), whereas lesions of animals with C. pneumoniae-induced atherosclerosis were rarely positive for the cytokines or MDA-2 but were more commonly positive for tissue growth factors TGF-β and PDGF (29.4 to 41.2%).
TABLE 3.
Cytokines, HSP, and oxidized LDL (MDA-2) in atherosclerotic lesions and aortic tissuea
| Marker | Controls (aorta) | Cholesterol fed (atherosclerosis) |
C. pneumoniae infected
|
||
|---|---|---|---|---|---|
| Atherosclerosis | Myxoidb | Periaortitis | |||
| TNF-α | 0 (0/25) | +–++ (5/10) | 0–+ (2/17) | 0–+ (3/21) | 0–+ (1/16) |
| IL-1α | 0 (0/25) | +–++ (5/10) | 0–+ (1/17) | 0 (0/21) | 0–+++ (3/14) |
| IL-6 | 0 (0/25) | +–++ (6/10) | 0–+ (2/17) | 0–++ (1/21) | 0–+ (2/16) |
| TGF-β | 0 (0/25) | ++–+++ (10/10) | +–++ (7/17) | +–++ (10/21) | 0–+ (6/16) |
| PDGF | 0 (0/25) | +–+++ (6/6) | +–++ (5/17) | 0–+ (2/21) | 0–++ (3/16) |
| MDA-2 | 0 (0/25) | +–+++ (7/10) | 0–+ (1/17) | 0 (0/21) | 0 (0/16) |
Grading system: 0, no positive stains; +, one focus with ≥5 cells positive; ++, two to five foci each with ≥5 cells positive; +++, over five foci each with ≥5 cells positive. Number of lesions positive over total tested is given in parentheses.
Focal area with isolated myxoid ground substance accumulation in the media.
Serology.
C. pneumoniae antibody (IgG) response was assessed in the single-inoculation group I and uninfected control group III. All baseline sera (before inoculation) were negative for C. pneumoniae antibodies (<1:2). In group I, 20 (83.3%) demonstrated significant antibody response (seroconversion), whereas none of 24 control rabbits did so. There was no correlation with the mean antibody titer and development of atherosclerosis or periaortitis. The mean antibody titer in animals with atherosclerosis or periaortitis was 1:25 ± 19 (standard deviation) (range, 1:2 to 1:64), whereas animals without these changes had a mean titer of 1:38 ± 64 (range, <1:2 to 1:256).
In the animals infected with M. pneumoniae (group V), all baseline preinoculated sera were negative for complement-fixing antibodies (<1:4) to M. pneumoniae. In the acute infection batch with day 10 sacrifice, 10 of 12 (83.3%) showed acute seroconversion (mean titer of 1:16 ± 18 [range, <1:4 to 1:64]). All 20 (100%) of the chronic-infection animals sacrificed at 3 months demonstrated seroconversion, with peak titers occurring at 28 days postinoculation (mean titer, 1:54 ± 36 [range, 1:16 to 1:128]) and a twofold decline in antibody titer at sacrifice (1:27 ± 18).
Chlamydia cultures.
C. pneumoniae was not recovered from any of the aortic tissues cultured in this study.
DISCUSSION
We have reported previously that C. pneumoniae infection in a rabbit model can result in changes of atherosclerosis in 2 of 11 rabbits within 1 month of infection (9), while uninfected rabbits fed standard cholesterol-free chow do not develop changes of atherosclerosis. In our initial study we used C. pneumoniae ATCC strain VR 1310 (single inoculation) and demonstrated dissemination of the organism from the lungs to the liver, spleen, and aorta. The results of the present study have confirmed and extended these observations. Using a different strain of C. pneumoniae, we have demonstrated that a single inoculum results in grade I-III atherosclerosis of the aorta in 6 of 23 (26.1%), versus 0 of 24 (0%) for uninfected control animals in 3 months (P = 0.009). We have further shown that repeated inoculation (three times) with two different strains can result in higher prevalence of atherosclerosis, 34.5% with grade III-IV changes and another 21.7% showing earlier changes of myxoid ground substance accumulation at the intima-media junction with fragmentation of the elastic fibers in the media of the aorta (which were not seen in the uninfected cholesterol-free fed controls or the mycoplasma-infected rabbits). Although these isolated changes of the vascular extracellular matrix are not included in the grading of atherosclerosis per se, they are recognized changes that are frequently seen in atherosclerosis in humans (41, 47). Furthermore, we have clearly demonstrated that the atherosclerosis induced by C. pneumoniae infection is not related to increases in serum cholesterol.
Macroscopically, the lesions induced by C. pneumoniae in the aorta were small, slightly raised and just visible discrete lesions (few in number), whereas the aortae in rabbits fed 0.5% cholesterol diet were diffusely and prominently involved by gross inspection. However, in animals fed the lower-cholesterol (0.15%) diet, the lesions were not usually visible to the naked eye and microscopically closely resembled lesions of the infected rabbits. It should be noted that the serum cholesterol levels in the rabbits fed 0.5% cholesterol were 50-fold higher than those in the control rabbits and 10 times the recommended level for humans, whereas the serum cholesterol concentration in the 0.15% cholesterol diet falls within the recommended level for humans.
To exclude a nonspecific effect of respiratory tract infection in the rabbit as an inducer of atherosclerosis, we have produced a model for M. pneumoniae pneumonia in the rabbit. There was no previous M. pneumoniae rabbit model reported except for arthritis by direct inoculation (5). Thus, we have established an acute and chronic mycoplasma infection model. The clinical manifestation of M. pneumoniae infection in humans is very similar to and clinically indistinguishable from that for C. pneumoniae. However, M. pneumoniae differs from C. pneumoniae in being an extracellular pathogen and lacking lipopolysaccharide (LPS) or a cell wall. We have demonstrated that the pulmonary pathology and systemic dissemination of M. pneumoniae in the rabbit are exceedingly similar to those in our rabbit model with C. pneumoniae (9). Although both C. pneumoniae and M. pneumoniae can produce focal periaortitis, none of the 32 mycoplasma-infected animals showed changes of atherosclerosis. Thus, nonspecific immune stimulation does not appear to be the mechanism responsible for induction of atherosclerosis in the rabbit model.
Although we could demonstrate C. pneumoniae antigen by immunohistochemistry in the aorta with atherosclerotic changes, we were unable to isolate the organisms in any of the tissues. Recovery of C. pneumoniae from tissues of rabbits has been difficult. In a previous study (31), C. pneumoniae was isolated only from the nasopharynx 2 to 3 days after inoculation and not from other tissues in the rabbit, although the organism was detected in these tissues by immunocytochemistry or PCR. Attempts to isolate C. pneumoniae from human atherosclerotic plaques have also resulted in limited success. This has led to the postulate that chlamydia may exist in tissues in a persistent or latent state, making isolation by standard culture techniques more difficult (2).
Previous attempts to establish a causal effect of C. pneumoniae in the pathogenesis of atherosclerosis were unsuccessful in the murine model (32). In apolipoprotein E-deficient transgenic mice, which spontaneously develop atherosclerosis, and C57BL/6J mice fed atherogenic and nonatherogenic diets, C. pneumoniae was detected in the aorta and atherosclerotic lesions, but a cause-and-effect relationship could not be established. We had initially described (9) grade I and III lesions in our rabbit model within a month after a single inoculation; in the present study we found more varied, advanced, and grossly visible lesions which may be related to multiple inoculation and longer duration of infection. Recently, Laitinen et al. (23) confirmed our initial observation in a rabbit model and found focal intimal thickening or fibrous plaques resembling atherosclerosis in six of nine rabbits inoculated twice with C. pneumoniae. In one of their rabbits, a fibrous plaque with calcification similar to our calcified type IV lesion was found. Whereas Laitinen et al. did not find foam cells in any of their lesions, we were able to detect foam cells in grade I and II lesions (Fig. 1a and b) in our infected model. Furthermore, C. pneumoniae has been shown to enhance or accelerate atherosclerosis in cholesterol-fed rabbits (33), with partial reversal by azithromycin.
The exact mechanisms by which C. pneumoniae infection is able to induce atherosclerosis in our rabbit model is unclear. It is clearly not related to increases in serum cholesterol levels. The possible mechanisms include stimulation of macrophage cytotoxic responses (oxidative burst, activation of complement, secretion of cytokines, etc.) following exposure to C. pneumoniae. C. pneumoniae may activate the inflammatory process by direct stimulation of the complement pathway by chlamydial LPS or other antigens or by immune complexes.
These processes can result in cellular injury (endothelial damage) and affect local cholesterol metabolism (8), as LPS may enhance the LDL receptor pathway in human macrophages (25). Macrophage scavenger receptors, which mediate the uptake of lipids into macrophages (19), have been shown to bind to gram-negative bacterial LPS (13) in the process of phagocytosis and clearance. The immune response to C. pneumoniae infection may also generate the production of reactive oxygen species (7) which may enhance oxidized LDL, further leading to foamy cell formation.
Recently, it has been demonstrated that C. pneumoniae can induce foam cell formation in vitro when incubated with human mononuclear cells (15), and these changes may be due to chlamydial LPS (16). Furthermore, Kol et al. (18) have demonstrated chlamydial heat shock protein 60 in human atheroma, which can regulate TNF-α and metalloproteinase expression.
The failure of our immunohistochemical studies to detect the proinflammatory cytokines in the atherosclerotic lesions of most of the infected rabbits does not preclude the above hypotheses. The low yield may be explained by transient increases in the cytokines earlier in the process of atherogenesis induced by C. pneumoniae or by limited availability of high-avidity antibodies for rabbit studies. Conversely, the more uniform presence of the cytokines and growth factors in the cholesterol-induced lesions may represent ongoing stimulation via oxidized LDL. Further studies will be required to explore and define the exact mechanisms responsible for the changes detected in the infected rabbits.
In summary, C. pneumoniae infection can induce early macroscopic and histological changes of atherosclerosis in the aortae of rabbits (without a cholesterol diet), and these changes closely resemble the lesions induced by a very low cholesterol diet (0.15%), which result in serum cholesterol concentration in the range recommended for humans.
ACKNOWLEDGMENTS
We are grateful to M. Petric and K. Leung, The Hospital for Sick Children, for assistance in performing the mycoplasma serology; P. Lynn, McMaster University Bacteriology Laboratory, and D. Noda for technical assistance; D. Bajhan for preparation of the manuscript; P. Connelly and A. Gotlieb for reviewing the manuscript; J. Li for assistance with statistical analysis; and M. Moreland for assistance with the figures for illustration.
REFERENCES
- 1.Allain C C, Poon L S, Chan C S, Richmond W, Fu P C. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 2.Beatty W L, Morrison R P, Byrne G I. Persistent chlamydia: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol Rev. 1994;58:686–699. doi: 10.1128/mr.58.4.686-699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell L A, O'Brien E R, Cappuccio A L, Kuo C C, Wang S P, Stewart D, Patton D L, Cummings P K, Grayston J T. Detection of Chlamydia pneumoniae (TWAR) in human atherectomy tissue arteries. J Infect Dis. 1995;172:585–588. doi: 10.1093/infdis/172.2.585. [DOI] [PubMed] [Google Scholar]
- 4.Cartun R W, Pedersen C A. An immunocytochemical technique offering increased sensitivity and lowered cost with a streptavidin. Horseradish peroxidase conjugate. J Histotechnol. 1989;12:273–277. [Google Scholar]
- 5.Cedillo L. Experimental arthritis induced by Mycoplasma pneumoniae in rabbits. J Rheumatol. 1992;19:344–347. [PubMed] [Google Scholar]
- 6.Daley S J, Klemp K F, Guyton J R, Rogers K A. Cholesterol-fed and casein-fed rabbit models of atherosclerosis. Part 2. Differing morphological severity of atherosclerosis despite matched plasma cholesterol levels. Arterioscler Thromb. 1994;14:105–114. doi: 10.1161/01.atv.14.1.105. [DOI] [PubMed] [Google Scholar]
- 7.Diaz M, Frei B, Vita J A, Keaney J F., Jr Antioxidants and atherosclerotic heart disease. N Engl J Med. 1997;337:408–416. doi: 10.1056/NEJM199708073370607. [DOI] [PubMed] [Google Scholar]
- 8.Fogelman, A. M., J. Seager, H. Nevab, M. E. Haberland, and P. A. Edwards. 1984. Bacterial endotoxin prevents the expression of scavenger receptors activity or macrophages derived from human monocytes. Circulation 70S.11.
- 9.Fong I W, Chiu B, Viira E, Fong M W, Jang D, Mahony J B. Rabbit model for Chlamydia pneumoniae infection. J Clin Microbiol. 1997;35:48–52. doi: 10.1128/jcm.35.1.48-52.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grayston J T, Aldous M B, Easton A, Wang S P, Kuo C C, Campbell L A, Altman J. Evidence that Chlamydia pneumoniae causes pneumonia and bronchitis. J Infect Dis. 1993;168:1231–1235. doi: 10.1093/infdis/168.5.1231. [DOI] [PubMed] [Google Scholar]
- 11.Grayston J T, Campbell L A, Kuo C C, Mordhorst C H, Saiiku P, Thom D H, Wang S P. A respiratory tract pathogen: Chlamydia pneumoniae strain TWAR. J Infect Dis. 1990;161:618–625. doi: 10.1093/infdis/161.4.618. [DOI] [PubMed] [Google Scholar]
- 12.Grayston J T. Infections caused by C. pneumoniae strain TWAR. Clin Infect Dis. 1992;15:757–761. doi: 10.1093/clind/15.5.757. [DOI] [PubMed] [Google Scholar]
- 13.Hampton R Y, Goldenbock D T, Penman M, Krieger M. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 1991;352:342–344. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- 14.Jackson L A, Campbell L A, Kuo C C, Rodriguez D I, Lee A, Grayston J T. Isolation of Chlamydia pneumoniae from a carotid endarterectomy specimen. J Infect Dis. 1997;176:292–295. doi: 10.1086/517270. [DOI] [PubMed] [Google Scholar]
- 15.Kalayoglu M V, Byrne G I. Induction of macrophage foam cell formation by Chlamydia pneumoniae. J Infect Dis. 1998;177:725–729. doi: 10.1086/514241. [DOI] [PubMed] [Google Scholar]
- 16.Kalayoglu M V, Byrne G I. A Chlamydia pneumoniae component that induces macrophage foam cell formation is chlamydial lipopolysaccharide. Infect Immun. 1998;66:5067–5072. doi: 10.1128/iai.66.11.5067-5072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenny G E. Serodiagnosis. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C: American Society for Microbiology; 1992. pp. 505–512. [Google Scholar]
- 18.Kol A, Sukhova G K, Lichtman A H, Libby P. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumour necrosis factor-α and matrix metalloproteinase expression. Circulation. 1998;98:300–307. doi: 10.1161/01.cir.98.4.300. [DOI] [PubMed] [Google Scholar]
- 19.Krieger M. Molecular flypaper and atherosclerosis: structure of the macrophage scavenger and receptor. Trends Biochem Sci. 1992;17:141–146. doi: 10.1016/0968-0004(92)90322-z. [DOI] [PubMed] [Google Scholar]
- 20.Kuo C C, Chen H H, Wang P S, Grayston J T. Identification of a new group of Chlamydia psittaci strains called TWAR. J Clin Microbiol. 1986;24:1034–1037. doi: 10.1128/jcm.24.6.1034-1037.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo C C, Gown A M, Benditt E P, Grayston J T. Detection of Chlamydia pneumoniae in aortic lesions of atherosclerosis by immunocytochemical stain. Arterioscler Thromb. 1993;13:1500–1504. doi: 10.1161/01.atv.13.10.1501. [DOI] [PubMed] [Google Scholar]
- 22.Kuo C C, Shor A, Campbell L A, Fukushi H, Patton D L, Grayston J T. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J Infect Dis. 1993;167:841–849. doi: 10.1093/infdis/167.4.841. [DOI] [PubMed] [Google Scholar]
- 23.Laitinen K, Laurila A, Pyhala L, Leinonen M, Saikku P. Chlamydia pneumoniae infection induces inflammatory changes in the aortas of rabbits. Infect Immunol. 1997;65:4832–4835. doi: 10.1128/iai.65.11.4832-4835.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linnanmaki E, Leinonen M, Mattila K, Nieminen M S, Valtonen V, Saikku P. Chlamydia pneumoniae specific circulating immune complexes in patients with chronic coronary heart disease. Circulation. 1993;87:1130–1134. doi: 10.1161/01.cir.87.4.1130. [DOI] [PubMed] [Google Scholar]
- 25.Lopes-Virella M, Virella G. Immunological and microbiological factors in the pathogenesis of atherosclerosis. Clin Immunol Immunopathol. 1985;37:337–386. doi: 10.1016/0090-1229(85)90107-2. [DOI] [PubMed] [Google Scholar]
- 26.Maas M, Bartels S C, Engel P M, Momat U, Sievers H H. Endovascular presence of viable Chlamydia pneumoniae is a common phenomenon in coronary artery disease. Am J Coll Cardiol. 1998;31:827–832. doi: 10.1016/s0735-1097(98)00016-3. [DOI] [PubMed] [Google Scholar]
- 27.Mahony J B, Luinstra K E, Chernesky M A. Diagnosis of Chlamydia trachomatis and Chlamydia pneumoniae respiratory tract infections by multiplex PCR. In: Ofilia J, et al., editors. Chlamydia infections. Escullapio Italy: Societe Editrice; 1994. pp. 370–373. [Google Scholar]
- 28.Marrie T M. Community-acquired pneumonia. Clin Infect Dis. 1993;18:501–515. doi: 10.1093/clinids/18.4.501. [DOI] [PubMed] [Google Scholar]
- 29.Melnick S L, Shahar E, Folsom A R, Grayston J T, Sorlie P D, Wang S P, Szklo M. Past infection by Chlamydia pneumoniae strain TWAR and asymptomatic carotid atherosclerosis. Am J Med. 1993;95:499–504. doi: 10.1016/0002-9343(93)90332-j. [DOI] [PubMed] [Google Scholar]
- 30.Mendall M A, Carrington D, Strachan D, Patel P, Molineaux N, Jevi J, Toosey T, Camm A J, Northfield T C. Chlamydia pneumoniae: risk factor for sero-positivity and association with coronary artery disease. J Infect. 1995;30:121–128. doi: 10.1016/s0163-4453(95)80006-9. [DOI] [PubMed] [Google Scholar]
- 31.Moazed T C, Kuo C C, Patton D L, Grayston J T, Campbell L A. Experimental rabbit models of Chlamydia pneumoniae infection. Am J Pathol. 1996;148:667–676. [PMC free article] [PubMed] [Google Scholar]
- 32.Moazed T C, Kuo C C, Grayston J T, Campbell L A. Murine models of Chlamydia pneumoniae infection and atherosclerosis. J Infect Dis. 1997;175:883–890. doi: 10.1086/513986. [DOI] [PubMed] [Google Scholar]
- 33.Muhlestein J B, Anderson J L, Hammond E H, Zhao L, Trehan S, Schwabe E P, Carlquist J F. Infection with Chlamydia pneumoniae accelerates the development of atherosclerosis and the treatment with azithromycin prevents it in a rabbit model. Circulation. 1998;97:633–636. doi: 10.1161/01.cir.97.7.633. [DOI] [PubMed] [Google Scholar]
- 34.Palinski W, Yia-Herttuala S, Rosenfeld M E, Butler S W, Sacher S A, Parthasarathy S, Curtiss L K, Witztum J L. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis. 1990;10:325–335. doi: 10.1161/01.atv.10.3.325. [DOI] [PubMed] [Google Scholar]
- 35.Patel P, Mendall M A, Carrington D, Strachan D P, Leatham E. Association of Helicobacter pylori and Chlamydia pneumoniae infections with coronary heart disease and cardiovascular risk factor. BMJ. 1995;311:711–714. doi: 10.1136/bmj.311.7007.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramirez J the Chlamydia pneumoniae/Atherosclerotic Study Group. Isolation of Chlamydia pneumoniae from the coronary artery of a patient with atherosclerosis. Ann Intern Med. 1996;125:979–982. doi: 10.7326/0003-4819-125-12-199612150-00008. [DOI] [PubMed] [Google Scholar]
- 37.Roblin P M, Dumornay W, Hammerschlag M R. Use of HEP-2 cells for improved isolation and passage of Chlamydia pneumoniae. J Clin Microbiol. 1992;30:1968–1971. doi: 10.1128/jcm.30.8.1968-1971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 39.Saikku P, Leinonen M, Tenakanen L, Linnanmaki E, Ekman M R, Manninen V, Manttari M, Frick M H, Huttunen J K. Chronic Chlamydia pneumoniae infection as a risk factor for coronary heart disease in the Helsinki heart study. Ann Intern Med. 1992;116:273–278. doi: 10.7326/0003-4819-116-4-273. [DOI] [PubMed] [Google Scholar]
- 40.Saikku P, Mattila K, Nieminen M S, Leinonen M, Ekman M R. Serological evidence of an association of a novel chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;ii:983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- 41.Sandberg L B, Soskel N T, Leslie J G. Elastin structure biosynthesis and relation to disease states. N Engl J Med. 1981;304:566–579. doi: 10.1056/NEJM198103053041004. [DOI] [PubMed] [Google Scholar]
- 42.Shor A, Kuo C C, Patton D L. Detection of Chlamydia pneumoniae in coronary artery fatty streaks and atheromatous plaques. S Afr Med J. 1992;82:158–161. [PubMed] [Google Scholar]
- 43.Stary H C. The histological classification of atherosclerotic lesions in human coronary arteries. In: Fuster V, Ross R, Topol E J, editors. Atherosclerosis and coronary artery disease. Philadelphia, Pa: Lippincott-Raven Publishers; 1995. pp. 463–474. [Google Scholar]
- 44.Th'ng C, Quinn P A. Preparation of antisera to serovars of Ureaplasma urealyticum, Mycoplasma hominis, and Mycoplasma pneumoniae. In: Stanek G, Cassell G H, Tulley J G, Whitcomb R F, editors. Recent advances in mycoplasmaology. Stuttgart, Germany: Gustave Fischer Verlag; 1990. pp. 795–797. [Google Scholar]
- 45.Thom D H, Grayston J T, Siscovich D S, Wang S P, Weiss N S, Daling J R. Association of prior infection with Chlamydia pneumoniae and angiographically demonstrated coronary artery disease. JAMA. 1992;268:68–72. [PubMed] [Google Scholar]
- 46.Thom D H, Wang S P, Grayston J T, Siscovick D S, Stewart D K, Kronmal R A, Weiss N S. Chlamydia pneumoniae strain TWAR antibody and angiographically demonstrated coronary artery disease. Arterioscler Thromb. 1991;11:547–551. doi: 10.1161/01.atv.11.3.547. [DOI] [PubMed] [Google Scholar]
- 47.Wight T N. The vascular extracellular matrix. In: Fuster V, Ross R, Topal E J, editors. Atherosclerosis and coronary artery disease. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 421–439. [Google Scholar]