Abstract
Echinococcus granulosus sensu lato is the causative agent of cystic echinococcosis (CE), which is a neglected zoonotic disease with an important role in human morbidity. In this study, we aimed to investigate the haplotype diversity, genetic variation, population structure and phylogeny of human E. granulosus sensu stricto (s.s.) (G1 genotype) isolates submitted to GenBank from different parts of the world by sequencing the mitochondrial CO1 and ND1 genes. The sequences of the mt-CO1 (401 bp; n = 133) and mt-ND1 (407 bp; n = 140) genes were used to analyze the haplotype, polymorphism and phylogenetic of 273 E. granulosus s.s. (G1 genotype) isolates. Mutations were observed at 31 different points in the mt-CO1 gene sequences and at 100 different points in the mt-ND1 gene sequences. Furthermore, 34 haplotypes of the mt-CO1 sequences and 37 haplotypes of the mt-ND1 sequences were identified. Tajima’s D, Fu’s Fs, and Fu’s LD values showed high negative values in both mt-CO1 and mt-ND1 gene fragments. The haplotype diversities in the sequences retrieved from GenBank in this study indicate that the genetic variation in human isolates of E. granulosus s.s. in western countries is higher than in eastern countries. This may be due to demographic expansions due to animal trades and natural selections.
Keywords: Echinococcus granulosus s.s., G1 genotype, CO1, ND1, genetic variability
1. Introduction
Cystic echinococcosis (CE) is a neglected parasitic zoonotic disease caused by Echinococcus granulosus sensu lato and has an important role in human morbidity. CE is distributed worldwide, especially in Asia, Africa, Europe, South America, Canada and Australia [1,2,3]. In 2014, CE was ranked as the third most important foodborne parasitic disease globally by the Food and Agriculture Organization (FAO) and World Health Organization (WHO), and E. granulosus s.l. infections are responsible for billions of dollars of economic loss per year [4,5,6].
In recent years, according to the mitochondrial cytochrome c oxidase subunit 1 (mt-CO1) and NADH dehydrogenase subunit 1 (mt-ND1) DNA sequences, the genetic diversity of E. granulosus s.l. was reported. These were designated E. granulosus sensu stricto (s.s.) (G1 and G3), E. equinus (G4), E. ortleppi (G5) and E. canadensis (G6/7, G8-10), and E. felidis [7,8,9]. However, E. granulosus s.s. is the most common of these, constituting the majority of human CE infections (approximately 89%), and also has the most zoonotic features [10,11].
Two interrelated hosts play an important role in the life cycle of E. granulosus s.l. Carnivores, mostly dogs, are final hosts, and many mammals, including humans, are intermediate hosts. Accidental ingestion of eggs from the feces of infected host species leads to the infection of different internal organs of the intermediate host, but mainly the liver and lungs [12,13,14].
Although CE is a benign disease, it can progress with high morbidity and mortality as a result of unexpected and serious complications [15]. However, the clinical symptoms vary based on the size, location and condition of the cyst. After ingestion, the embryos are released from the eggs into the small intestine, where they penetrate the mucous membranes, mix with the blood and reach many organs. Although a single cyst is prevalent in the majority of infected organisms, multiple cysts or cyst formation in multiple organs can be observed in 20–40% of individuals. Most cysts occur in the liver (>65%), followed by the lung (25%), while they are less common in the spleen, kidney, bone, heart and central nervous system [16,17].
Human CE is widely distributed throughout the world. The prevalence of surgically managed human CE per 100,000 has been reported at 32 in Central and Southern Peru [18], 6–20 in Southwest Chile in 2005 [19], 30 in Argentina Rio Negro [20], 1.5 in Northern Israel [21], 0.68 in Southern Israel [21], 80 in China-Xinjiang [22], 4.2 in Eastern Libya [23], 1.3–2.6 in Egypt [24], 15 in Tunisia [25], 3.6–4.6 in Algeria [26], 10.8 in Spain-Salamanca between 1980–2000 [27], 10 in France-Corsica [28], 1.3 in Italy [29] and 4.55 in Morocco in 2006 [30]. Its prevalence was also reported at between 3.5% and 6% in urban and rural areas of Brazil [31].
Communities in which sheep breeding is widespread contribute greatly to this distribution, with E. granulosus s.s. (G1 genotype) playing an important role in transmission in humans [32,33,34].
Due to the maternal inheritance and high mutation rates of mitochondrial (mt) DNA sequences, these sequences are commonly analyzed to determine the genetic structure of the population and the degree of close kinship [35]. In many studies, partial sequences of mt-CO1 and mt-ND1 genes have been used successfully to distinguish genetic variants among Echinococcus species and between E. granulosus strains [36,37,38].
Global evaluation of genetic variation among human isolates of E. granulosus s.s. (G1 genotype) is important to reveal the population dynamics of the parasite. In the current study, we evaluated the haplotype diversity, genetic variation, population structure and phylogeny of human E. granulosus s.s. (G1 genotype) submitted to GenBank from different parts of the world by analyzing the mt-CO1 and mt-ND1 gene sequences.
2. Materials and Methods
2.1. Data Collection
After filtering the mt-CO1 (n = 382) and mt-ND1 (n = 199) gene sequences containing human (Homo sapiens) isolates of the E. granulosus s.s. (G1 genotype) submitted to the National Center for Biotechnology Information, USA, (NCBI) (www.ncbi.nlm.nih.gov) database until 6 April 2022, a total of 581 gene sequences were obtained and a dataset was created.
2.2. Alignment and Phylogenetic Analysis
All the gene sequences were loaded into the CLC Sequence Viewer 8 [39] in FASTA format. All sequences were trimmed from both ends and were then aligned using the mt-CO1 (accession no. MG672129) and mt-ND1 (accession no. KU925413) reference sequences. After the removal of short gene sequences, the remaining 273 gene sequences [401 bp mt-CO1 (n = 133) and 407 bp mt-ND1 (n = 140)] were used for bioinformatic analysis. Individual phylogenetic trees were created from the sequences of both gene regions using the neighbor-joining (NJ) model and the Jukes-Cantor nucleotide distance measure. Statistical support for the specificity of the branches was obtained using 1000 bootstrap replicates. Taenia saginata and T. solium sequences were added as outgroups to show the degree of relations.
2.3. Haplotype Analysis and Networking
The haplotype analysis was carried out using the DnaSP 6 program in which the sequences were investigated in FASTA format [40]. The haplotype and nucleotide change values, nucleotide and haplotype numbers and neutrality indexes were calculated to determine the genetic structure of both gene regions. The sequences were converted to Nexus format [41] and a haplotype network was generated by using the PopArt (Population Analysis with Reticulate Trees) program [42] for a visual representation of the relationships between haplotypes.
3. Results
In this study, we analyzed a total of 273 gene sequences of E. granulosus s.s. (G1 genotype) isolates obtained from the NCBI database, consisting of 133 mt-CO1 sequences from 15 countries and 140 mt-ND1 sequences from 16 countries (Table 1).
Table 1.
Accession numbers of mt-CO1 and mt-ND1 gene fragments of E. granulosus s.s. (G1 genotype) isolates used in the study.
| mt-CO1 | mt-ND1 | ||||
|---|---|---|---|---|---|
| Origin | No. of Isolates | Accession Numbers | Origin | No. of Isolates | Accession Numbers |
| China | 43 | DQ356874-75-76-77-78-79-80/83, KJ628328-29-30-31-32-33-34-35, AB688602-03-04-05-06-07-08-09-10-11/13-14-15-16-17-18-19, MH050608-09-10-11-12-13-14-15-16-17 | Uzbekistan | 43 | MN696570/72/76-77-78-79-80-81-82-83-84-85-86-87-88-89-90-91-92-93-94-95-96-98-99, MN696600-01-02-03-04/06-07-08-09-10-11-12-13/15/19-20-21-22 |
| Tunisia | 13 | MG672264-65-66-67-68-69-70-71-72-73-74-75-76 | Algeria | 20 | MG672128, KT316342, KR349038-39-40-41-42-43-44, MG672282/84-85-86-87-88-89-90-91-92-93 |
| Pakistan | 12 | MK229295-96-97/99, MK229301-02/04/13/15/17-18-19 | China | 17 | AY572548, KJ556993-94, EU072111-12-13-14, MH050620-21-22-23-24-25-26-27-28-29 |
| Mongolia | 12 | MG672254-55, AB893242-43-44-45-46-47-48-49-50-51 | Peru | 14 | JF946597-98-99, JF946600-01-02-03-04-05-06-07-08-09/24, |
| Algeria | 12 | MG672128, MG672283-84-85-86-87-88-89-90-91-92-93 | Tunisia | 13 | MG672264-65-66-67-68-69-70-71-72-73-74-75-76 |
| Turkey | 10 | EU006783, GU951512-13, MG886833-34-35-36-37-38-39 | Iran | 9 | KT284349, MG672245, JF836800-01-02-03, JF836797-98-99 |
| Iran | 9 | KR337817, MW350099, MT073987, MG672245, MH025946-47, JQ250810/12/15 | Slovenia | 6 | MT239133-34-35/38/40/42 |
| Russia | 9 | AB777904/07-08, AB688136-37-38-39-40-41 | Spain | 4 | KU925413-14, MG672129/37 |
| Spain | 4 | MG672129/37, KU925413-14, | Iraq | 3 | FJ226756, MN231833-34 |
| Finland | 3 | MG672132, KY766884, KU925429 | Finland | 3 | MG672132, KY766884, KU925429 |
| India | 2 | JX854029-30 | Mongolia | 2 | MG672254-55 |
| Morocco | 1 | EF367266 | Poland | 2 | KT780298- KT780300 |
| Romania | 1 | MG672138 | Romania | 1 | MG672138 |
| Kazakhstan | 1 | MG672257 | Kazakhstan | 1 | MG672257 |
| Italy | 1 | MG672135 | Italy | 1 | MG672135 |
| Morocco | 1 | EF367298 | |||
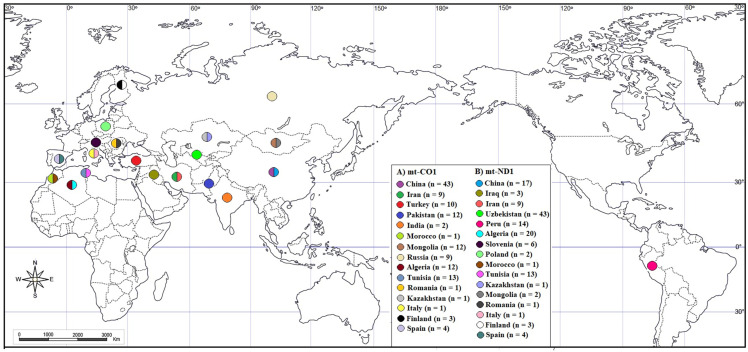
The distribution of the collected mt-CO1 and mt-ND1 sequences over the world is shown in Figure 1.
Figure 1.
Distribution map of mt-CO1 and mt-ND1 sequences of E. granulosus s.s. (G1 genotype) by geographical regions.
3.1. Polymorphism and Haplotype Analysis
Mutations were observed at 31 different points within the mt-CO1 gene sequences, with the longest conserved areas detected between 116 bp and 167 bp. Within the mt-ND1 sequences, mutations were observed at 100 different points, with conserved areas detected between 361 bp and 407 bp. No protein-coding domain was found in either of the datasets. Analysis of 133 mt-CO1 gene sequences revealed 34 different haplotypes (Table 2). Among these, Hap03 constituted the main haplotype with 79 gene sequences, of which 23 existed as a single haplotype. Analysis of 140 mt-ND1 gene sequences revealed 37 haplotypes (Table 3). Among these, Hap01 constituted the main haplotype, with 83 gene sequences, constitutes of which 28 existed as a single haplotype.
Table 2.
Haplotypes of mt-CO1 sequences of E. granulosus s.s. (G1 genotype) and accession numbers of isolates forming groups.
| Haplotype Name | No. of Isolate | Accession Numbers |
|---|---|---|
| Hap01 | 2 | KJ628335-China, KJ628331-China |
| Hap02 | 1 | KR337817-Iran |
| Hap03 | 79 | GU951513-Turkey, GU951512-Turkey, JX854029-India, MG886839-Turkey, MG886838-Turkey, MG886837-Turkey, MG886836-Turkey, MG886835-Turkey, MG886834-Turkey, MG886833-Turkey, DQ356883-China, MW350099-Iran, MT073987-Iran, MK229301-Pakistan, MK229296-Pakistan, MH050617-China, MH050615-China, MH050614-China, MH050612-China, MH050609-China, MH050608-China, MH025946-Iran, KJ628334-China, KJ628333-China, KJ628332-China, KJ628330-China, KJ628329-China, AB688617-China, AB688616-China, AB688614-China, AB688611-China, AB688610-China, AB688609-China, AB688608-China, AB688607-China, AB688603-China, AB688602-China, JQ250815-Iran, AB893250-Mongolia, AB893249-Mongolia, AB893248-Mongolia, AB893247-Mongolia, AB893245-Mongolia, AB893244-Mongolia, AB893243-Mongolia, AB777908-Russia, AB777907-Russia, AB777904-Russia, AB688141-Russia, AB688136-Russia, MG672293-Algeria, MG672292-Algeria, MG672291-Algeria, MG672289-Algeria, MG672288-Algeria, MG672287-Algeria, MG672285-Algeria, MG672284-Algeria, MG672283-Algeria, MG672276-Tunisia, MG672275-Tunisia, MG672274-Tunisia, MG672272-Tunisia, MG672270-Tunisia, MG672269-Tunisia, MG672268-Tunisia, MG672266-Tunisia, MG672265-Tunisia, MG672264-Tunisia, MG672257-Kazakhstan, MG672254-Mongolia, MG672245-Iran, MG672135-Italy, MG672132-Finland, MG672129-Spain, MG672128-Algeria, KY766884-Finland, KU925429-Finland, KU925413-Spain |
| Hap04 | 1 | EU006783-Turkey |
| Hap05 | 2 | JX854030-India, MH050610-China |
| Hap06 | 2 | MK229304-Pakistan, MK229299-Pakistan |
| Hap07 | 1 | DQ356880-China |
| Hap08 | 1 | DQ356879-China |
| Hap09 | 2 | DQ356878-China, AB688604-China |
| Hap10 | 12 | DQ356877-China, MK229302-Pakistan, MK229297-Pakistan, MH050611-China, JQ250812-Iran, JQ250810-Iran, AB893246-Mongolia, AB688140-Russia, AB688139-Russia, MG672290-Algeria, MG672267-Tunisia, MG672255-Mongolia |
| Hap11 | 2 | DQ356876-China, DQ356875-China |
| Hap12 | 1 | DQ356874-China |
| Hap13 | 2 | MK229319-Pakistan, MK229313-Pakistan |
| Hap14 | 3 | MK229318-Pakistan, AB688618-China, MG672286-Algeria |
| Hap15 | 1 | MK229317-Pakistan |
| Hap16 | 1 | MK229315-Pakistan |
| Hap17 | 1 | MK229295-Pakistan |
| Hap18 | 2 | MH050616-China, AB688606-China |
| Hap19 | 1 | MH050613-China |
| Hap20 | 1 | EF367266-Morocco |
| Hap21 | 1 | MH025947-Iran |
| Hap22 | 1 | KJ628328-China |
| Hap23 | 1 | AB688619-China |
| Hap24 | 1 | AB688615-China |
| Hap25 | 1 | AB688613-China |
| Hap26 | 1 | AB688605-China |
| Hap27 | 1 | AB893251-Mongolia |
| Hap28 | 1 | AB893242-Mongolia |
| Hap29 | 1 | AB688138-Russia |
| Hap30 | 1 | AB688137-Russia |
| Hap31 | 1 | MG672273-Tunisia |
| Hap32 | 1 | MG672271-Tunisia |
| Hap33 | 1 | MG672138-Romania |
| Hap34 | 2 | MG672137-Spain, KU925414-Spain |
Table 3.
Haplotype of mt-ND1 sequences of E. granulosus s.s. (G1 genotype) and accession numbers of isolates forming groups.
| Haplotype Name | No. of Isolate | Accession Numbers |
|---|---|---|
| Hap01 | 83 | KU925413-Spain, EU072111-China, FJ226756-Iraq, KT284349-Iran, JF836803-Iran, JF836802-Iran, JF836801-Iran, JF836799-Iran, JF836797-Iran, MN696622-Uzbekistan, MN696621-Uzbekistan, MN696620-Uzbekistan, MN696619-Uzbekistan, MN696606-Uzbekistan, MN696596-Uzbekistan, MN696591-Uzbekistan, MN696583-Uzbekistan, MN696572-Uzbekistan, MN696570-Uzbekistan, JF946609-Peru, JF946608-Peru, JF946607-Peru, JF946606-Peru, JF946605-Peru, JF946604-Peru, JF946603-Peru, JF946602-Peru, JF946601-Peru, JF946600-Peru, JF946599-Peru, JF946598-Peru, JF946597-Peru, KR349044-Algeria, KR349042-Algeria, KR349038-Algeria, MT239138-Slovenia, MT239133-Slovenia, KT780300-Poland, KT780298-Poland, JF946624-Peru, MH050629-China, MH050628-China, MH050626-China, MH050625-China, MH050622-China, MH050621-China, MH050620-China, KJ556994-China, KJ556993-China, MN231834-Iraq, EF367298-Morocco, MG672293-Algeria, MG672292-Algeria, MG672291-Algeria, MG672289-Algeria, MG672288-Algeria, MG672287-Algeria, MG672285-Algeria, MG672284-Algeria, MG672283-Algeria, MG672276-Tunisia, MG672275-Tunisia, MG672274-Tunisia, MG672273-Tunisia, MG672272-Tunisia, MG672271-Tunisia, MG672270-Tunisia, MG672268-Tunisia, MG672267-Tunisia, MG672266-Tunisia, MG672265-Tunisia, MG672264-Tunisia, MG672257-Kazakhstan, MG672255-Mongolia, MG672254-Mongolia, MG672245-Iran, MG672138-Romania, MG672135-Italy, MG672132-Finland, MG672129-Spain, MG672128-Algeria, KY766884-Finland, KU925429-Finland |
| Hap02 | 1 | EU072114-China |
| Hap03 | 2 | EU072113-China, JF836798-Iran |
| Hap04 | 2 | EU072112-China, AY572548-China |
| Hap05 | 15 | JF836800-Iran, MN696613-Uzbekistan, MN696612-Uzbekistan, MN696610-Uzbekistan, MN696609-Uzbekistan, MN696607-Uzbekistan, MN696599-Uzbekistan, MN696598-Uzbekistan, MN696590-Uzbekistan, MN696589-Uzbekistan, MN696587-Uzbekistan, MN696585-Uzbekistan, MN696584-Uzbekistan, MN696582-Uzbekistan, MN696581-Uzbekistan |
| Hap06 | 1 | MN696615-Uzbekistan |
| Hap07 | 2 | MN696611-Uzbekistan, MN696608-Uzbekistan |
| Hap08 | 1 | MN696604-Uzbekistan |
| Hap09 | 1 | MN696603-Uzbekistan |
| Hap10 | 1 | MN696602-Uzbekistan |
| Hap11 | 1 | MN696601-Uzbekistan |
| Hap12 | 1 | MN696600-Uzbekistan |
| Hap13 | 1 | MN696595-Uzbekistan |
| Hap14 | 1 | MN696594-Uzbekistan |
| Hap15 | 1 | MN696593-Uzbekistan |
| Hap16 | 1 | MN696592-Uzbekistan |
| Hap17 | 1 | MN696588-Uzbekistan |
| Hap18 | 1 | MN696586-Uzbekistan |
| Hap19 | 1 | MN696580-Uzbekistan |
| Hap20 | 1 | MN696579-Uzbekistan |
| Hap21 | 1 | MN696578-Uzbekistan |
| Hap22 | 1 | MN696577-Uzbekistan |
| Hap23 | 1 | MN696576-Uzbekistan |
| Hap24 | 1 | KT316342-Algeria |
| Hap25 | 1 | KR349043-Algeria |
| Hap26 | 2 | KR349041-Algeria, MG672286-Algeria |
| Hap27 | 2 | KR349040-Algeria, MG672290-Algeria |
| Hap28 | 2 | KR349039-Algeria, MG672269-Tunisia |
| Hap29 | 1 | MT239142-Slovenia |
| Hap30 | 1 | MT239140-Slovenia |
| Hap31 | 1 | MT239135-Slovenia |
| Hap32 | 1 | MT239134-Slovenia |
| Hap33 | 1 | MH050627-China |
| Hap34 | 1 | MH050624-China |
| Hap35 | 1 | MH050623-China |
| Hap36 | 1 | MN231833-Iraq |
| Hap37 | 2 | MG672137-Spain KU925414-Spain |
3.2. Haplotype Network
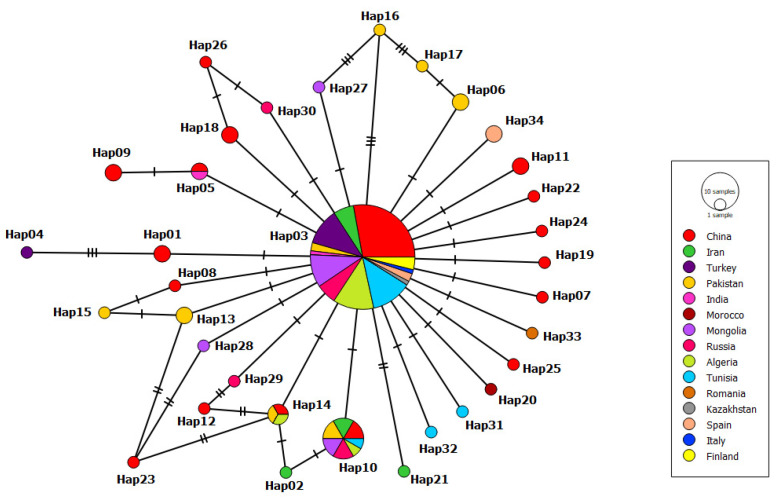
The mt-CO1 haplotype network consisted of 34 haplotypes (Figure 2). A comparison of the main haplotype with the others in this network revealed between one and seven mutations. The main haplotype was Hap03, accounting for 59.39% (79/133) of the haplotype network, followed by Hap10, accounting for 9.02%. (12/133). A unique single haplotype constituted 67.64% (23/34) of the haplotype network. Single haplotypes were from China (n = 9), Pakistan (n = 3), Iran (n = 2), Tunisia (n = 2), Russia (n = 2), Mongolia (n = 2), Turkey (n = 1), Romania (n = 1) and Morocco (n = 1).
Figure 2.
Appearance of mt-CO1 (401 bp) haplotypes E. granulosus s.s. (G1 genotype) sequences. The number of mutations that distinguish haplotypes is indicated by screening marks. The geographical distribution of haplotypes is shown in different colors. The size of the circles is related to the haplotype frequency.
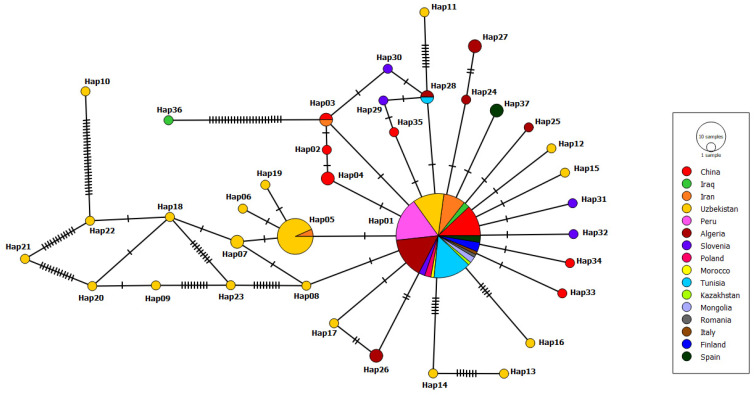
The mt-ND1 haplotype network consisted of 37 haplotypes (Figure 3). A comparison of the main haplotype with the others in this network revealed between one and 50 mutations. The main haplotype was Hap01, accounting for 59.28% (83/140) of the haplotype network, followed by Hap05, accounting for 10.71% (15/140). A unique single haplotype constituted 75.67% (28/37) of the haplotype network. Single haplotypes were from Uzbekistan (n = 17), Slovenia (n = 4), China (n = 4), Algeria (n = 2) and Iraq (n = 1).
Figure 3.
Appearance of mt-ND1 (407 bp) haplotypes of E. granulosus s.s. (G1 genotype) sequences. The number of mutations that distinguish haplotypes is indicated by screening marks. The geographical distribution of haplotypes is shown in different colors. The size of the circles is related to the haplotype frequency.
The nucleotide positions of the mt-CO1 and mt-ND1 genes among the haplotypes were presented in Supplementary Tables S1 and S2.
3.3. Phylogenetic Tree
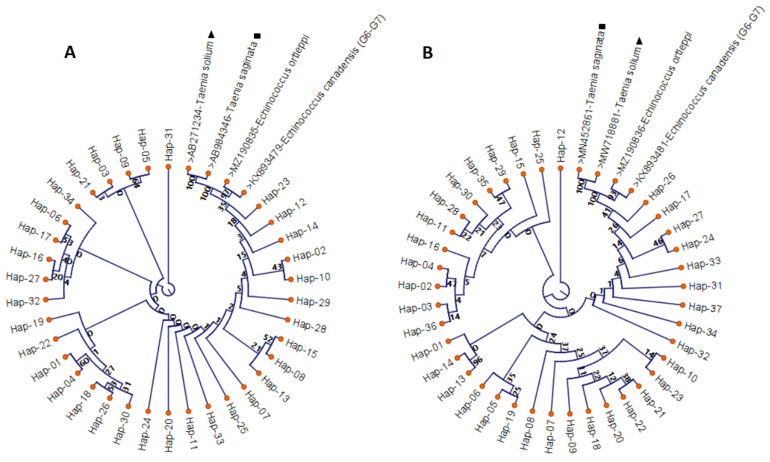
The results of the phylogenetic analysis were consistent with the haplotype network. The phylogenetic tree generated by aligning the mt-CO1 gene sequences is shown in Figure 4A. In this tree, Hap04 (EU006783), Hap12 (DQ356874), Hap16 (MK229315) and Hap23 (AB688619) were the haplotypes farthest apart, with mutations at seven points. The phylogenetic tree generated by aligning the mt-ND1 gene sequences is shown in Figure 4B. In this tree, Hap10 (MN696602) and Hap36 (MN231833) were the haplotypes farthest apart, with mutations at 50 points. Taenia saginata and T. solium were added as outgroups in both phylogenetic trees.
Figure 4.
Phylogenetic tree view of E. granulosus s.s. (G1 genotype) sequences using mt-CO1 (401 bp) (A) and mt-ND1 (407 bp) (B) gene and reference sequences. CLC Sequence Viewer 8 was used to generate a Maximum Likelihood tree based on the Neighbor Joining model. The reliability of the tree was evaluated with 1000 bootstrap iterations. ■ Taenia saginata ▲ Taenia solium.
3.4. Gene Flow, Diversity and Neutrality Analysis
The diversity and neutrality indices of the mt-CO1 and mt-ND1 groups are shown in Table 4. Tajima’s D (Tajima, 1989) and Fu’s FS (Fu, 1997) values were calculated to determine whether populations were subject to selection pressure. Tajima D, Fu’s Fs and Fu’s LD values showed high negative values in both the mt-CO1 and mt-ND1 regions, providing evidence of a large number of alleles.
Table 4.
Diversity and neutrality indices obtained using nucleotide data of the mt-CO1 (401 bp) and mt-ND1 (407 bp) genes of E. granulosus s.s. (G1 genotype).
| n | H | hd ± SD | πd ± SD | Tajima’s D | p Value | Fu’s Fs | p Value | FLD | p Value | FLF | p Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mt-CO1 | 133 | 34 | 0.640 ± 0.048 | 0.00255 ± 0.00031 | −2.47269 | *. p < 0.01 | −49.797 | 0.000 | −3.97170 | p < 0.02 | −4.03871 | p < 0.02 |
| mt-ND1 | 140 | 37 | 0.639 ± 0.047 | 0.00611 ± 0.00147 | −2.80355 | *. p < 0.001 | −31.231 | 0.000 | −8.50154 | p < 0.02 | −7.14019 | p < 0.02 |
n: Number of isolates, H: number of haplotypes; hd: haplotype diversity; πd: nucleotide diversity; SD: standard deviation; FLD: Fu and Li’s D * test statistic; FLF: Fu and Li’s F * test statistic.
4. Discussion
Genetic diversity and population structure of E. granulosus s.s. (G1 genotype) were investigated in the current study. This was carried out using sequenced data of mt-CO1 and mt-ND1 retrieved from GenBank, commonly used for the differentiation of Echinococcus species. Results obtained in this study emerged information about gene flow and population dynamics in human E. granulosus s.s. infections globally. A total of 133 mt-CO1 (401 bp) and 140 mt-ND1 (407 bp) gene sequences of E. granulosus s.s. (G1 genotype) human isolates already registered in the NCBI database were used for us in silico analyses to determine the genetic diversity and variations of the E. granulosus s.s. (G1 genotype) human isolates.
Although the prevalence and incidence of CE have decreased significantly in recent years, it still remains an important public health concern, especially in some countries and geographical regions that cannot implement a control program due to economic difficulties [43]. In addition, it is an important problem for human health in developing countries where animal husbandry is intense, and sheep meat is consumed intensively [44]. The incidence of CE increases with age and is more common between the ages of 20 and 40 years. The incidence of the disease is higher in societies with a low socio-economic ratio [45].
The results of the current study show an extremely high global haplotype diversity within the G1 genotype. The 273 samples analyzed represented a total of 34 haplotypes for mt-CO1 and 37 for mt-ND1. High genetic diversity within E. granulosus s.s. has also been reported by Kinkar et al. [46]. They [46] analyzed 212 samples (near complete mitochondrial sequence) and found 171 haplotypes (overall haplotype diversity was 0.994). The main reason for the haplotype difference between the studies is related to the length of the gene regions analyzed. Therefore, more haplotypes can be determined by sequencing longer mitochondrial gene fragments.
Neutrality indices such as Tajima D, Fu’s Fs, and Fu’s LD were used to measure nucleotide variability and population expansion [47]. The Tajima D test evaluates the deviation of populations from the standard neutral model, with a positive Tajima D value representing heterozygosity, defined as having a selective advantage, while negative values indicate that a particular allele has a selective advantage over the other allele. A negative value also indicates a rapid increase in the population [48,49]. In our study, Tajima D values were low in both the mt-CO1 and mt-ND1 gene fragments, indicating a high probability of population increase in the future. However, the lower Tajima D value of the mt-ND1 gene sequence (−2.80355) compared with that of the mt-CO1 gene sequence (−2.47269) indicates a higher rate of population growth in the former. The negative value of the neutrality indices Tajima’s D suggests population expansion (Animal movements among the countries indicate that this expansion may continue in the coming years. Fu’s FS represents a marker of sensitivity to population growth, with a significantly negative value (p < 0.05), indicating that the populations have common growth patterns and belong to the same gene pool [50,51]. Our analysis yielded highly negative and statistically significant Fu’s Fs values in both the mt-CO1 and mt-ND1 haplotype groups, indicating that these populations are subject to expansion globally.
Nucleotide diversity was examined to determine the degree of polymorphism in the population. We determined that the mean nucleotide difference of the mt-ND1 (0.00611) gene sequence was higher than that of the mt-CO1 (0.00255) gene sequence. In addition, haplotype diversity was assessed to evaluate the uniqueness of haplotypes within the population. In our study, the values of the mt-CO1 (0.640) and mt-ND1 (0.639) gene sequences were very similar.
In total, 34 haplotypes were identified in our analysis of the mt-CO1 gene sequences. The main haplotype constituted 59.39% of the total network, and there were 23 single haplotypes. Thirty-seven different haplotypes were identified in our analysis of the mt-ND1 gene sequence. The main haplotype constituted 59.28% of the total network, and there were 28 single haplotypes. The major haplotypes represent a single ancestor.
In total, 31 different mutations were detected across the 401 bp mt-CO1 gene sequences, and 100 different mutations were detected within the 407 bp mt-ND1 sequences. The higher mutation rates may reflect the long and complex evolutionary history of E. granulosus. The genetic diversity within E. granulosus s.s. (G1 genotype) is very high worldwide, and the observed complex phylogeographic patterns emerging from the phylogenetic and geographic analyses suggest that the current distribution of E. granulosus s.s. (G1 genotype) has been shaped by the intensive animal trade [46]. The high number of haplotypes detected in some Asian and Middle Eastern countries (China, Mongolia, Pakistan, Iran, etc.) in this study may indicate that E. granulosus s.s. (G1 genotype) has existed in these countries for many years compared to some western countries (Finland and Spain).
5. Conclusions
E. granulosus s.s. (G1 genotype) poses an important problem in communities where sheep breeding is common. Although different molecular studies have been conducted to date, this study is the first bioinformatics study to evaluate the genetic structure and gene flow of human isolates of the E. granulosus s.s. (G1 genotype) collected worldwide. In this study, all the sequence data reported from humans related to E. granulosus s.s. (G1 genotype), the most common species in humans were screened, and aggregated data were given comparatively. We think that this study can fill the knowledge gaps on the subject. Our findings also represent an important step in future epidemiological, bioecological, vaccine and diagnostic studies that could yield efficient treatments for species/strains.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens11111346/s1, Table S1: Nucleotide variation positions of the mt-CO1 (401 bp) gene among 34 haplotypes analyzed; Table S2: Nucleotide variation positions of the mt-ND1 (407 bp) gene among 37 haplotypes analyzed.
Author Contributions
Conceptualization and design, S.S., J.C. and H.A.; Analysis and interpretation of data, F.C., M.A.S., S.G.K. and H.K.K.; Writing—Original Draft Preparation, F.C. and M.A.S.; Statistical analysis, M.A.S.; Supervision, S.S. and J.C.; Writing—Review and Editing, H.A., N.J., S.S. and J.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by the National Natural Science Foundation of China (Nos. 81971969, 82272369, and 81772225 to J.C.) and the Three-Year Public Health Action Plan (2020-2022) of Shanghai (No. GWV-10.1-XK13 to J.C.). The funders had no role in the study design, the data collection, and analysis, the decision to publish, or the preparation of the manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McManus D.P., Thompson R.C.A. Molecular epidemiology of cystic echinococcosis. Parasitology. 2003;127:37–51. doi: 10.1017/S0031182003003524. [DOI] [PubMed] [Google Scholar]
- 2.Batsch A.J.G.K. Naturgeschichte Der Bandwurmgattung Überhaupt und Ihrer Arten Insbesondere. Bey Johann Jacob Gebauer; Halle, Germany: 1786. [Google Scholar]
- 3.Moro P., Schantz P.M. Cystic echinococcosis in the Americas. Parasitol. Int. 2006;55:181–186. doi: 10.1016/j.parint.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 4.Deplazes P., Rinaldi L., Rojas C.A., Torgerson P.R., Harandi M.F., Romig T., Antolova D., Schurer J.M., Lahmar S., Cringoli G., et al. Global distribution of alveolar and cystic echinococcosis. Adv. Parasitol. 2017;95:315–493. doi: 10.1016/bs.apar.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Multicriteria-Based Ranking for Risk Management of Food-Borne Parasites: Report of a Joint FAO. WHO; Geneva, Switzerland: 2014. [Google Scholar]
- 6.World Health Organization . Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases: Second WHO Report on Neglected Diseases. WHO; Geneva, Switzerland: 2013. [Google Scholar]
- 7.Vuitton D.A., McManus D.P., Rogan M.T., Romig T., Gottstein B., Naidich A., Tuxun T., Wen H., da Silva A.M. International consensus on terminology to be used in the field of echinococcoses. Parasite. 2020;27:41. doi: 10.1051/parasite/2020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakao M., McManus D.P., Schantz P.M., Craig P.S., Ito A. A molecular phylogeny of the genus Echinococcus inferred from complete mitochondrial genomes. Parasitology. 2006;134:713–722. doi: 10.1017/S0031182006001934. [DOI] [PubMed] [Google Scholar]
- 9.Busi M., Šnábel V., Varcasia A., Garippa G., Perrone V., De Liberato C., D’Amelio S. Genetic variation within and between G1 and G3 genotypes of Echinococcus granulosus in Italy revealed by multilocus DNA sequencing. Vet. Parasitol. 2007;150:75–83. doi: 10.1016/j.vetpar.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Rojas C.A.A., Romig T., Lightowlers M.W. Echinococcus granulosus sensu lato genotypes infecting humans–review of current knowledge. Int. J. Parasitol. 2014;44:9–18. doi: 10.1016/j.ijpara.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Lymbery A.J. Phylogenetic pattern, evolutionary processes and species delimitation in the genus Echinococcus. Adv. Parasitol. 2017;95:111–145. doi: 10.1016/bs.apar.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins D.J., Romig T., Thompson R.C.A. Emergence/re-emergence of Echinococcus spp.—A global update. Int. J. Parasitol. 2005;35:1205–1219. doi: 10.1016/j.ijpara.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Lewall D.B. Hydatid disease: Biology, pathology, imaging and classification. Clin. Radiol. 1998;53:863–874. doi: 10.1016/S0009-9260(98)80212-2. [DOI] [PubMed] [Google Scholar]
- 14.Thompson R.C.A. The taxonomy, phylogeny and transmission of Echinococcus. Exp. Parasitol. 2008;119:439–446. doi: 10.1016/j.exppara.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Patrice Bourée M.D. Hydatidosis: Dynamics of transmission. World J. Surg. 2001;25:429. doi: 10.1007/s002680020001. [DOI] [PubMed] [Google Scholar]
- 16.Kammerer W.S., Schantz P.M. Echinococcal disease. Infect. Dis. Clin. N. Am. 1993;7:605–618. doi: 10.1016/S0891-5520(20)30545-6. [DOI] [PubMed] [Google Scholar]
- 17.Moro P., Schantz P.M. Echinococcosis: A review. Int. J. Infect. Dis. 2009;13:125–133. doi: 10.1016/j.ijid.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 18.Moro P.L., Lopera L., Cabrera M., Cabrera G., Silva B., Gilman R.H., Moro M.H. Endemic focus of cystic echinococcosis in a coastal city of Peru. Am. J. Trop. Med. Hyg. 2004;71:327–329. doi: 10.4269/ajtmh.2004.71.327. [DOI] [PubMed] [Google Scholar]
- 19.Grosso G., Gruttadauria S., Biondi A., Marventano S., Mistretta A. Worldwide epidemiology of liver hydatidosis including the Mediterranean area. World J. Gastroenterol. 2012;18:1425. doi: 10.3748/wjg.v18.i13.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haag K.L., Ayala F.J., Kamenetzky L., Gutierrez A.M., Rosenzvit M. Livestock trade history, geography, and parasite strains: The mitochondrial genetic structure of Echinococcus granulosus in Argentina. J. Parasitol. 2004;90:234–239. doi: 10.1645/GE-173R. [DOI] [PubMed] [Google Scholar]
- 21.Shimshony A. Epidemiology of emerging zoonoses in Israel. Emerg. Infect. Dis. 1997;3:229. doi: 10.3201/eid0302.970221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bart J.M., Abdukader M., Zhang Y.L., Lin R.Y., Wang Y.H., Nakao M., Ito A., Craig P.S., Piarroux R., Vuitton D.A., et al. Genotyping of human cystic echinococcosis in Xinjiang, PR China. Parasitology. 2006;133:571–579. doi: 10.1017/S0031182006000734. [DOI] [PubMed] [Google Scholar]
- 23.Buishi I.E., Njoroge E.M., Bouamra O., Craig P.S. Canine echinococcosis in northwest Libya: Assessment of coproantigen ELISA, and a survey of infection with analysis of risk-factors. Vet. Parasitol. 2005;130:223–232. doi: 10.1016/j.vetpar.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed E.S., Helmy H., El Setouhy M., Craig P.S., Ramzy R.M.R. A retrospective hospital study of human cystic echinococcosis in Egypt. East. Mediterr. Health J. 2004;10:349–357. [PubMed] [Google Scholar]
- 25.Sadjjadi S.M. Present situation of echinococcosis in the Middle East and Arabic North Africa. Parasitol. Int. 2006;55:197–202. doi: 10.1016/j.parint.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 26.Bardonnet K., Benchikh-Elfegoun M.C., Bart J.M., Harraga S., Hannache N., Haddad S., Dumon H., Vuitton D.A., Piarroux R. Cystic echinococcosis in Algeria: Cattle act as reservoirs of a sheep strain and may contribute to human contamination. Vet. Parasitol. 2003;116:35–44. doi: 10.1016/S0304-4017(03)00255-3. [DOI] [PubMed] [Google Scholar]
- 27.Mwambete K.D., Ponce-Gordo F., Cuesta-Bandera C. Genetic identification and host range of the Spanish strains of Echinococcus granulosus. Acta Trop. 2004;91:87–93. doi: 10.1016/j.actatropica.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Eckert J., Thompson R.C. Echinococcus strains in Europe: A review. Ann. Trop. Med. Parasitol. 1998;39:1–8. [PubMed] [Google Scholar]
- 29.Garippa G. Updates on cystic echinococcosis (CE) in Italy. Parassitologia. 2006;48:57. [PubMed] [Google Scholar]
- 30.Azlaf R., Dakkak A. Epidemiological study of the cystic echinococcosis in Morocco. Vet. Parasitol. 2006;137:83–93. doi: 10.1016/j.vetpar.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 31.La Rue M.L.D., Dinkel A., Mackenstedt U., Romig T. New data on Echinococcus spp. in southern Brazil. Rev. Inst. Med. Trop. Sao Paulo. 2006;48:103–104. doi: 10.1590/S0036-46652006000200009. [DOI] [PubMed] [Google Scholar]
- 32.Thompson R.C.A., McManus D. Aetiology: Parasites and life-cyles. In: Eckert J., Gemmell M.A., Meslin F.-X., Pawlowski Z.S., editors. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. World Organisation for Animal Health; Paris, France: 2001. pp. 1–19. [Google Scholar]
- 33.Eckert J., Gemmell M.A., Meslin F.X., Pawlowski Z.S. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. World Organisation for Animal Health; Paris, France: 2001. [Google Scholar]
- 34.McManus D.P. The molecular epidemiology of Echinococcus granulosus and cystic hydatid disease. Trans. R. Soc. Trop. Med. Hyg. 2002;96:151–157. doi: 10.1016/S0035-9203(02)90068-4. [DOI] [PubMed] [Google Scholar]
- 35.Jia W., Yan H., Lou Z., Ni X., Dyachenko V., Li H., Littlewood D.T.J. Mitochondrial genes and genomes support a cryptic species of tapeworm within Taenia taeniaeformis. Acta Trop. 2012;123:154–163. doi: 10.1016/j.actatropica.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Bowles J., McManus D.P. NADH dehydrogenase 1 gene sequences compared for species and strains of the genus Echinococcus. Int. J. Parasitol. 1993;23:969–972. doi: 10.1016/0020-7519(93)90065-7. [DOI] [PubMed] [Google Scholar]
- 37.Bowles J., Blair D., McManus D.P. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992;54:165–173. doi: 10.1016/0166-6851(92)90109-W. [DOI] [PubMed] [Google Scholar]
- 38.Bowles J., Blair D., McManus D.P. Molecular genetic characterization of the cervid strain (‘northern form’) of Echinococcus granulosus. Parasitology. 1994;109:215–221. doi: 10.1017/S0031182000076332. [DOI] [PubMed] [Google Scholar]
- 39.Knudsen B., Knudsen T., Flensborg M., Sandmann H., Heltzen M., Andersen A., Sinding J.B. CLC Main Workbench, Version 5.5. CLC bio; Aarhus, Denmark: 2007. [Google Scholar]
- 40.Rozas J., Ferrer-Mata A., Sánchez-DelBarrio J.C., Guirao-Rico S., Librado P., Ramos-Onsins S.E., Sánchez-Gracia A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017;34:3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- 41.Maddison D.R., Swofford D.L., Maddison W.P. NEXUS: An extensible file format for systematic information. Syst. Biol. 1997;46:590–621. doi: 10.1093/sysbio/46.4.590. [DOI] [PubMed] [Google Scholar]
- 42.Leigh J.W., Bryant D. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015;6:1110–1116. doi: 10.1111/2041-210X.12410. [DOI] [Google Scholar]
- 43.Tünger Ö. Dünyada Kistik Ekinokokkoz Epidemiyolojisi. Turkiye Parazitol. Derg. 2013;37:47–52. doi: 10.5152/tpd.2013.12. [DOI] [PubMed] [Google Scholar]
- 44.Eckert J., Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Unat E.K., Yücel A., Atlas K., Samastı M. Unat’ın Tıp Parazitolojisi. Cerrahpasa Med. J. 1995;15:440–459. [Google Scholar]
- 46.Kinkar L., Laurimäe T., Acosta-Jamett G., Andresiuk V., Balkaya I., Casulli A., Gasser R.B., van der Giessen J., González L.M., Haag K.L., et al. Global phylogeography and genetic diversity of the zoonotic tapeworm Echinococcus granulosus sensu stricto genotype G1. Int. J. Parasitol. 2018;48:729–742. doi: 10.1016/j.ijpara.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 47.Ramos-Onsins S.E., Rozas J. Statistical properties of new neutrality tests against population growth. Mol. Biol. Evol. 2002;19:2092–2100. doi: 10.1093/oxfordjournals.molbev.a004034. [DOI] [PubMed] [Google Scholar]
- 48.Vamathevan J.J., Hasan S., Emes R.D., Amrine-Madsen H., Rajagopalan D., Topp S.D., Kumar V., Word M., Simmons M.D., Foord S.M., et al. The role of positive selection in determining the molecular cause of species differences in disease. BMC Evol. Biol. 2008;8:1–14. doi: 10.1186/1471-2148-8-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stephens J.C., Schneider J.A., Tanguay D.A., Choi J., Acharya T., Stanley S.E., Jiang R., Messer C.J., Chew A., Han J.H., et al. Haplotype variation and linkage disequilibrium in 313 human genes. Science. 2001;293:489–493. doi: 10.1126/science.1059431. [DOI] [PubMed] [Google Scholar]
- 50.Fu Y.-X. Statistical tests of neutrality of mutations against population growth, hitchhiking and back ground selection. Genetics. 1997;147:915–925. doi: 10.1093/genetics/147.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y.L., Kong X.Y., Yu Z.N., Kong J., Ma S., Chen L.M. Genetic diversity and historical demography of Chinese shrimp Feneropenaeus chinensis in Yellow Sea and Bohai Sea based on mitochondrial DNA analysis. Afr. J. Biotechnol. 2009;8:1193–1202. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.