Abstract
Enterococcus faecalis aggregation substance (AS) mediates efficient bacterium-bacterium contact to facilitate plasmid exchange as part of a bacterial sex pheromone system. We have previously determined that AS promotes direct, opsonin-independent binding of E. faecalis to human neutrophils (PMNs) via complement receptor type 3 and other receptors on the PMN surface. We have now examined the functional consequences of this bacterium-host cell interaction. AS-bearing E. faecalis was phagocytosed and internalized by PMNs, as determined by deconvolution fluorescence microscopy. However, these bacteria were not killed by PMNs, and internalized bacteria excluded propidium iodide, indicating intact bacterial membranes. Resistance to killing occurred despite activation of PMNs, as indicated by an increase in both functional and total surface Mac-1 expression, shedding of l-selectin, and an increase in PMN extracellular superoxide and phagosomal oxidant production. Deconvolution fluorescence microscopy also revealed that phagosomes containing AS-bearing bacteria were markedly larger than phagosomes containing opsonized E. faecalis, suggesting that some modification of phagosomal maturation may be involved in AS-induced resistance to killing. PMN phagosomal pH was significantly higher after ingestion of nonopsonized AS-bearing E. faecalis than after that of opsonized bacteria. The novel ability of AS to promote intracellular survival of E. faecalis inside PMNs suggests that AS may be a virulence factor used by strains of E. faecalis.
Enterococci are important, often multidrug-resistant pathogens (36) that are the third leading cause of endocarditis (48) and nosocomial bacteremia (47). Of the two species, Enterococcus faecalis and Enterococcus faecium, responsible for almost all clinical infections, E. faecalis predominates over E. faecium by 4:1 (25). The virulence mechanisms used by E. faecalis are not well understood, but a few postulated virulence factors have been described (28). One of these is aggregation substance (AS), a plasmid-encoded adhesin which mediates efficient cell-cell contact to facilitate plasmid exchange between donor and recipient strains (11, 61). In addition to mediating bacterial binding to other enterococci, AS also plays a role in binding of E. faecalis to eukaryotic cells, including pig renal tubular cells (33) and human intestinal epithelial cells (39).
Neutrophils (PMNs) are a critical component of the human host response against bacterial infections. Invading bacteria may be opsonized by complement proteins or antibodies and subsequently phagocytosed and killed by PMNs. A key mechanism involved in innate immunity includes the interaction of bacteria opsonized with fragments of the third component of complement (iC3b) with complement receptor type 3 (CR3) on the PMN surface (64). This interaction stimulates a pathway that leads to ingestion of bacteria, activation of PMNs with migration of lysosomal granules to the developing phagosome, a respiratory burst, and eventual killing and degradation of the ingested bacteria.
In contrast to this complement-mediated pathway, we have previously determined that AS promotes direct, opsonin-independent binding of E. faecalis to PMNs (58). This interaction also involves CR3, and possibly related receptors, on the PMN surface. AS-bearing E. faecalis appeared to be phagocytosed by PMNs. In the present study, we examined the consequences of this opsonin-independent binding to PMNs and compared them to those induced by complement-mediated binding. We confirmed that AS-bearing E. faecalis were internalized by PMNs with the use of deconvolution fluorescence microscopy. Unlike opsonized bacteria, we found that E. faecalis internalized via an AS-mediated mechanism was resistant to killing. Lack of bacterial killing occurred despite activation of PMNs, as demonstrated by an increase in PMN extracellular superoxide and phagosomal oxidant production and an increase in surface Mac-1 expression and shedding of l-selectin (CD62L). However, PMN phagosomal pH was significantly higher after ingestion of nonopsonized AS-bearing E. faecalis than after ingestion of opsonized E. faecalis.
(A portion of this data has been reported in preliminary form in Abstracts of the 1996 Interscience Conference on Antimicrobial Agents and Chemotherapy [58a].)
MATERIALS AND METHODS
Reagents.
All reagents used in this study were obtained from Sigma Chemical Co. (St. Louis, Mo.) unless otherwise stated.
Bacterial strains and plasmids.
E. faecalis strains used in this study are listed in Table 1. Strains were maintained on brain-heart infusion agar plates with or without 15 μg of chloramphenicol/ml, grown overnight in M9-yeast extract medium (39) with or without 0.1 μg chloramphenicol/ml, diluted 1:50 or 1:100 into the same prewarmed medium, and grown to logarithmic phase for 4 h at 37°C with tumbling. Bacteria were then washed twice and resuspended to the desired concentration in Hanks' balanced salt solution (HBSS). Bacteria were briefly sonicated to disrupt aggregates immediately prior to use.
TABLE 1.
E. faecalis strains used in this study
| Strain | Plasmid | Descriptiona | Reference |
|---|---|---|---|
| OG1RF | None | Plasmid-free parent strain, chromosomal Rifr and Fusr | 10 |
| INY401 | pWM401 | E. faecalis shuttle vector, Cmr | 62 |
| INY1801 | pINY1801 | EcoRI c and e fragments of pCF10 cloned into pWM401; constitutive expression of Asc 10 and Sec 10 | 63 |
| INY3000 | None | Derived from OG1; contains four Tn916 insertions, does not express binding substance | 56 |
| INY3071 | pWM401 | INY3000 containing shuttle vector; similar to INY401 but does not express binding substance | 40 |
| INY3072 | pINY1801 | INY 3000 containing EcoRI c and e fragments of pCF10 cloned into pWM401; expresses Asc 10 and Sec 10 but does not express binding substance and fails to autoaggregate | 40 |
| TX1355 | Stool isolate from normal volunteer, very-low-level bacterial superoxide production | This study | |
| TX1355d | pINY1801 | TX1355 containing EcoRI c and e fragments of pCF10 cloned into pWM401; similar to INY1801 but does not produce large amounts of superoxide | This study |
Rifr, rifampin resistance; Fusr, fusidic acid resistance; Cmr, chloramphenicol resistance.
E. faecalis TX1355 was isolated from the stool of a normal volunteer and was confirmed to be E. faecalis by conventional methods (13). TX1355 itself produced superoxide in very low amounts (0.5 nm of superoxide/107 bacteria by the method described below), did not bind to PMNs in a significant amount (1.1 bacteria/PMN by the method described below), and did not hybridize with a probe for the gene for AS (5). pINY1801 was electroporated into TX1355 by a modification of a previously described method (16). In brief, TX1355 was grown overnight in Todd Hewitt broth, washed repeatedly with 10% glycerol with 0.25 M sucrose, resuspended in the same medium, and stored in 40 μl aliquots at −70°C. Bacteria were thawed, mixed with 1.2 μg of pINY1801 plasmid DNA purified from INY1801 (7), and pulsed in a 0.1-cm electroporation cuvette at a field strength of 18,000 V/cm in a 200-Ω resistor at a time constant of 3.8 ms. Directly after the pulse, 0.8 ml of Todd Hewitt broth with 0.25 M sucrose was added, and the mixture was incubated at 37°C for 90 min and plated on SR medium (per liter, 10 g of tryptone, 5 g of yeast extract, 200 g of sucrose, 10 g of glucose, 25 g of gelatin, 15 g of agar, 2.5 mM MgCl2, 2.5 mM CaCl2 [pH 6.8]) (6) with 10 μg of chloramphenicol/ml. One of the resulting transformants (TX1355d) was confirmed to contain pINY1801, to produce similarly low levels of superoxide as the parent TX1355, but now to bind to PMNs (21.3 bacteria/PMN).
Monoclonal antibodies.
Fluorescein-labeled monoclonal antibodies (MAb) MHM23 (anti-CD18 mouse immunoglobulin G1 [IgG1]; Dako Corp., Carpenteria, Calif.) used at 3 μg/ml (22), Leu-8 (anti-l-selectin mouse IgG2a; Becton Dickinson, San Jose, Calif.) used at 0.4 μg/ml, and MAb24-Cy3 (anti-CD18 IgG1; obtained from Nancy Hogg at The Imperial Cancer Fund, London, United Kingdom) used at 16 μg/ml (8, 9) were employed to examine PMN surface expression of Mac-1, l-selectin, and an activation epitope of Mac-1, respectively. MAb IB4 (anti-CD18 mouse IgG1; courtesy of David Chambers and Karl Arfors, Sidney Kimmel Cancer Center, San Diego, Calif.) at 10 μg/ml (65) was used to inhibit neutrophil-neutrophil aggregation induced by AS-expressing bacteria.
Cells. (i) Neutrophils.
PMNs for most experiments were obtained from EDTA-anticoagulated venous blood of normal healthy volunteers by dextran sedimentation, Ficoll-Hypaque density-gradient centrifugation, and hypotonic lysis of residual erythrocytes, as previously described (45). Cells were suspended in HBSS at approximately 2 × 107 PMNs per ml. For flow cytometry experiments, PMNs were isolated from heparin-anticoagulated blood (10 U/ml) by centrifugation through a Ficoll-Hypaque gradient (Mono-Poly Resolving medium; Flow Laboratories, Inc., McLean, Va.), and the PMN band was collected, washed, suspended in phosphate-buffered saline with glucose and 1.5 mM Mg2+ at approximately 2 × 107 to 5 × 107 PMNs per ml, and kept at 4°C until use (51). Buffer with 1.5 mM Ca2+ was then added for experiments at 37°C. By each isolation method, cells were >95% PMNs by Diff Quik staining and >95% viable by trypan blue exclusion.
(ii) Monocyte-derived macrophages.
Peripheral blood mononuclear cells were isolated from EDTA-anticoagulated venous blood by dextran sedimentation and Ficoll-Hypaque density-gradient centrifugation, washed, suspended in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum; 8 × 105 cells were added to wells of 96-well microtiter plates and incubated at 37°C for 1 h in 5% CO2. Nonadherent cells were removed by washing, and the remaining cells were incubated with DMEM containing 10% fetal calf serum, 50 μg of penicillin/ml, 10 μg of streptomycin/ml, and 1,000 U of gamma interferon at 37°C in 5% CO2 for 5 to 8 days.
(iii) J774 cells.
J774A.1 cells of a murine macrophage cell line (44) (ATCC TIB 67; American Type Culture Collection, Manassas, Va.) were maintained in DMEM with 10% fetal calf serum. Cells grown in flasks were scraped off and resuspended in DMEM with 5% fetal calf serum. A total of 2 × 105 cells were added to the wells of 96-well microtiter plates and allowed to adhere for 1 h at 37°C in 5% CO2.
Bacterial internalization by PMNs.
Internalization of bacteria was examined by deconvolution fluorescence microscopy. The lipophilic carbocyanine dye DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; Molecular Probes, Eugene, Oreg.) is taken up in the PMN plasma membrane, uniformly stains the cell surface, remains as a membrane marker (41), and reportedly does not influence cell function. As bacteria are ingested by PMNs, the phagosomal membrane is formed by invaginating plasma membrane and is also highlighted by the dye. Log-phase bacteria were labeled with 0.1% fluorescein isothiocyanate (FITC) in 50 mM carbonate buffer (pH 9.5) for 30 min at 37°C protected from light, washed twice, and suspended in HBSS (2). For some experiments, bacteria were preincubated with 10% autologous serum (from the same patient from whom PMNs were isolated) for 20 min at 37°C, washed, and resuspended in HBSS. PMNs were incubated with 3 μM DiI for 5 min at room temperature and then washed three times. Bacteria were briefly sonicated to disrupt aggregates, and 200 μl of labeled bacteria (1 × 109 to 2 × 109 CFU/ml) was mixed with 200 μl of labeled PMNs (bacterium:PMN ratio of 100:1) plus 1 mM Ca2+ and 1 mM Mg2+ and incubated at 37°C with tumbling. After 10 min of incubation, cells were pelleted by brief pulsing in a tabletop microcentrifuge, washed twice to remove unattached bacteria, and resuspended in HBSS. Cells were fixed with 3.7% formaldehyde (TEM grade; Tousimis Research Corp., Rockville, Md.) for 5 min, cytocentrifuged (100 × g for 10 min) onto coverslips (Cytospin 2; Shandon, Pittsburgh, Pa.), briefly stained with 2 ng of 4′,6-diamidino-2-phenylindole (DAPI) (which stains DNA)/ml to highlight the nucleus, mounted with Elvanol (DuPont Chemical Co., Wilmington, Del.) (21), and then examined with an Olympus IX70 fluorescence microscope. Images were acquired with filters for DAPI (360-nm excitation/457-nm emission), fluorescein (490-nm excitation/528-nm emission), and Texas red (555-nm excitation/617-nm emission) with multiple optical sections through cells of interest. The images were deconvolved with the use of DeltaVision software (Applied Precision, Issaquah, Wash.). This method allows examination of serial optical sections, with subsequent data analysis enabling presentation of a three-dimensional image. Thus, an accurate assessment of the location of bacteria (e.g., intracellular versus extracellular) can be done.
Neutrophil activation. (i) Superoxide production.
Superoxide production was measured by the superoxide dismutase-inhibitable reduction of ferricytochrome c (42). PMNs (2 × 105) were added to the wells of 96-well microtiter plates and incubated at 37°C for 1 h. The supernatant and unbound PMNs were removed by tapping, and attached PMNs were incubated with approximately 2 × 106 bacteria/well in the presence of 0.19 mg of ferricytochrome c alone or with 20 μg of superoxide dismutase/ml for 60 min at 37°C prior to determination of A550 with a Bio-Tek (Winooski, Vt.) EL310 microplate reader. Controls contained PMNs alone or bacteria alone.
(ii) Phagosomal oxidant production.
The method described above determines primarily extracellular superoxide, as the 12-kDa ferricytochrome c molecule is likely excluded from the cell's interior due to its size (24). To examine oxidant production inside the phagosome, we labeled enterococci with the oxidant-sensitive fluorescent dye OxyBURST Green H2HFFDA (2′,4,5,6,7,7′-hexafluorofluorescein diacetate; Molecular Probes). OxyBURST Green H2HFFDA succinimidyl ester (5 mg) was dissolved in 0.5 ml of dimethyl sulfoxide, and 50 μl was added to 1 ml of enterococci (either TX1355 or TX1355d) at approximately 109 CFU/ml in 0.1 M sodium bicarbonate (pH 8.3) and rotated for 1 h at room temperature. Hydroxylamine (0.1 ml, 1.5 M, pH 8.5) was added, and the mixture was rotated for 1 h at room temperature and then incubated without agitation overnight at 4°C. Labeled bacteria were washed twice and resuspended with HBSS. This labeling procedure had no effect on bacterial viability. Labeled TX1355 was opsonized with 10% normal human serum on a rotator for 20 min at 37°C, washed twice, and resuspended with HBSS. PMNs labeled with DiI, as described above, were mixed with OxyBURST Green H2HFFDA-labeled bacteria in a bacterium:PMN ratio of about 100:1 in HBSS plus 1 mM Ca2+ and 1 mM Mg2+ and incubated at 37°C with tumbling. After 10 min, PMNs containing ingested bacteria were washed to remove unattached bacteria, resuspended in HBSS, fixed with formaldehyde, cytocentrifuged onto coverslips, mounted with Elvanol, and examined with an Olympus IX70 microscope, all as described above. Twenty-five PMNs containing internalized bacteria were examined for each bacterial strain, and internalized fluorescent bacteria were identified. The maximal level of fluorescence emission at 528 nm for each PMN due to internalized bacteria was quantified with DeltaVision software for individual areas of 0.066 by 0.066 μm, and the average for 25 PMNs was determined. During the entire process, tubes were flooded with nitrogen to minimize air-induced oxidation of the fluorescent dye. As a control for air- and light-induced oxidation and oxidation potentially derived from the bacteria themselves, labeled bacteria alone were fixed, processed similarly, and examined to determine the amount of fluorescence.
(iii) Expression of PMN surface proteins.
The amounts of Mac-1 and l-selectin present on the surface of PMNs before and after incubation with bacteria were assessed by flow cytometry. E. faecalis INY1801 or INY401 (107 organisms) was mixed with 106 PMNs and incubated at 37°C with agitation for 8 to 10 min. Fluorescein-labeled MAb MHM23 (to detect CD18), Leu-8 (to detect l-selectin), or MAb24-Cy3 (to detect the activation epitope of CD18) was added, and fluorescence was measured after binding for 10 min at room temperature with a FACScan (Becton-Dickinson) on the FL1 channel with linear detector settings. Data analysis was performed with FACScan Analysis Software (Becton-Dickinson), and the mean channel fluorescence was determined.
Microbicidal systems. (i) PMNs.
E. faecalis (approximately 2 × 108 CFU/ml) was incubated with PMNs (107 PMNs/ml) in HBSS plus 1 mM Ca2+ and 1 mM Mg2+ in final volumes of 200 μl in microtiter plate wells and agitated at 37°C. At intervals, 20-μl aliquots were diluted 1:10 in water for 10 min to lyse PMNs, bacteria were serially diluted, and viability was determined by the pour plate method with brain heart infusion agar (3). To examine the effect of serum opsonization on bacterial killing by PMNs, bacteria were preincubated with 10% autologous serum for 20 min at 37°C, washed, and exposed to PMNs or to HBSS alone as a control.
(ii) Monocyte-derived macrophages and J774 cells.
Cells were washed twice with HBSS and incubated with 2 × 107 bacteria/well in HBSS plus 1 mM Ca2+ and 1 mM Mg2+ at 37°C in 5% CO2 for 1 h to allow bacterial internalization. The supernatant was removed; the cells washed twice with HBSS; DMEM containing 5% fetal calf serum, 50 μg of penicillin/ml, and 10 μg of gentamicin/ml was added; and the cells were incubated at 37°C in 5% CO2. Initially and at intervals the supernatant was removed, the cells were washed twice with HBSS and lysed with 1% Triton X-100 for 5 min, bacteria were serially diluted, and viability was determined by the pour plate method, as described above. Exposure to 1% Triton X-100 for 5 min did not affect enterococcal viability.
(iii) MPO.
Human myeloperoxidase (MPO) was purified and assayed as previously described (43). Bacteria (about 109 CFU/ml) were incubated with 0.43 U of MPO/ml, 0.19 U of glucose oxidase/ml, 0.1 M NaCl, 0.04 M sodium acetate (pH 5.0), and 0.01 M glucose. Initially and at intervals, samples were removed, the reaction was stopped by the addition of sodium azide to a final concentration of 1 mM, bacteria were serially diluted, and viability was determined by the pour plate method.
Fluorescent dye exclusion.
Intracellular bacterial viability was also assessed by examining exclusion of a fluorescent dye by using the LIVE/DEAD BacLight Viability Kit (Molecular Probes). Bacteria (2 × 108/ml) either opsonized with 10% serum as described above or not were incubated with 107 PMNs/ml in HBSS plus 1 mM Ca2+ and 1 mM Mg2+ in final volumes of 200 μl in 1.2-ml tubes at 37°C for 1 h with tumbling. A fluorescent dye mixture was added to yield final concentrations of 10 μM SYTO 9 and 60 μM propidium iodide, and the bacteria were incubated for 15 min at room temperature. Then, 5 μl of the mixture was placed on a glass slide, overlaid with a coverslip, and examined within 30 min with a Nikon Optiphot fluorescence microscope. Bacteria with intact membranes appeared green from staining with SYTO 9, while bacteria with damaged membranes stained orange-red from propidium iodide. Twenty-five consecutive individual PMNs per sample that contained bacteria and that had an orange-staining nucleus (thus indicating the ability of both dyes to reach the intracellular environment; the vast majority of PMNs that contained bacteria had orange-staining nuclei) were examined, the number of apparent intracellular bacteria that stained green or orange were counted, and the percentage of bacteria that could exclude propidium iodide was determined. Bacteria alone (opsonized or unopsonized) were also incubated with SYTO 9 and propidium iodide for 15 min at room temperature to determine the percentage of bacteria in these preparations that could exclude propidium iodide.
Phagosomal pH.
Intraphagosomal pH was determined by using dual-labeled bacteria (38) and fluorescence microscopy. As the fluorescence emission intensity of fluorescein is pH dependent, while that of rhodamine is not, a ratio of fluorescein:rhodamine emission can be used to determine pH. Log-phase bacteria were labeled with 0.1% FITC in 50 mM carbonate buffer (pH 8.5) for 15 min at 37°C protected from light, 0.005% tetramethylrhodamine isothiocyanate (TRITC) was added, and incubation was done for 20 min at 37°C. Labeled bacteria were washed three times and suspended in HBSS. Labeling with both FITC and TRITC did not affect bacterial interactions with PMNs. Some bacteria were then incubated with 10% autologous serum (from the same patient from whom PMNs were isolated) for 20 min at 37°C, washed, and resuspended in HBSS. Two hundred microliters of labeled bacteria (1 × 109 to 2 × 109 CFU/ml) was mixed with 200 μl of labeled PMNs (bacteria:PMN ratio of 100:1) plus 1 mM Ca2+ and 1 mM Mg2+ and incubated at 37°C with tumbling. After 30 min of incubation, cells were pelleted by brief pulsing in a tabletop microcentrifuge, washed twice to remove unattached bacteria, and resuspended in HBSS. Ten-microliter samples were placed on a slide, covered with a coverslip which was then tacked down at the edges with nail polish, and examined with an Olympus IX70 fluorescence microscope. Intracellular bacteria were identified, and the levels of fluorescence emission at 528 nm (for fluorescein) and 617 nm (for rhodamine) (with excitation wavelengths of 490 and 555 nm, respectively) were quantified with the use of DeltaVision software for individual areas of 0.066 by 0.066 μm, with values obtained for 15 to 30 bacteria inside at least five different PMNs for each bacterial strain. The fluorescein:rhodamine emission ratio was calculated for each bacterium examined, and the mean was determined for each strain on a given day.
A standard curve was constructed daily for each strain of bacteria (opsonized INY401 and nonopsonized INY1801) by using labeled bacteria alone that were suspended in HBSS containing an additional 7 mM phosphate buffered at different pH values. The fluorescein:rhodamine emission ratios were determined for 15 to 25 bacteria at each pH, and a plot of the mean ratio versus pH was constructed. While the standard curves varied from strain to strain and from day to day, linear curve fitting by using KaleidaGraph software (version 2.1.3; Abelbeck Software) revealed that data for each standard curve approached linearity for the range of pH 4.7 to 7.3, with r values between 0.8 and 0.99. The daily mean intracellular bacterial fluorescein:rhodamine emission ratio was then converted to a value for phagosomal pH by using the corresponding standard curve for each bacterial strain.
Statistical methods.
Statistical significance was assessed by a paired 2-tailed t test, and differences were considered significant for P of < 0.05.
RESULTS
Bacterial internalization by PMNs.
Previous work using fluorescence and electron microscopy had demonstrated that E. faecalis INY401 containing the vector alone and lacking AS bound only minimally to PMNs in the absence of opsonins, while E. faecalis INY1801 expressing AS bound to PMNs in large numbers, with the majority of the bacteria appearing to be internalized (58). To confirm these latter findings, we used deconvolution fluorescence microscopy, employing the fluorescent dye DiI to label PMN plasma and phagosomal membranes. As seen in Fig. 1, the majority of PMN-bound AS-expressing INY1801 were internalized by PMNs. Multiple sections at different levels through individual PMNs by using the deconvolution fluorescence microscopy system confirmed that bacteria were actually inside PMNs and not simply attached to the exterior of cells that had extended pseudopods or membrane ruffles around externally attached bacteria. Opsonization of INY401 with normal human serum also promoted bacterial internalization by PMNs (Fig. 1). Internalization of AS-expressing bacteria was not due to autoaggregation of bacteria, as shown previously by fluorescence microscopy (58) and confirmed in this study by deconvolution fluorescence microscopy, as enterococci expressing AS in the INY3000 background (INY3072), which lack binding substance and do not autoaggregate, were also internalized in large numbers by PMNs (data not shown).
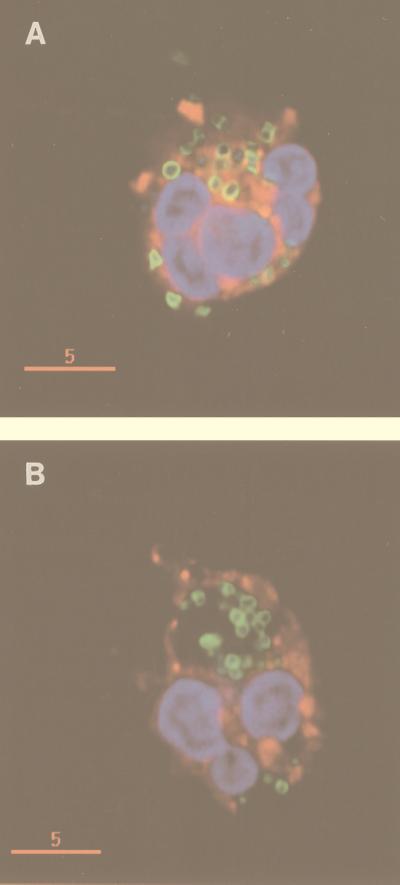
FIG. 1.
Internalization of E. faecalis INY401 (lacking AS) (A) and INY1801 (expressing AS) (B) by PMNs. FITC-labeled bacteria were opsonized with 10% serum (for INY401) or not opsonized (for INY1801) and incubated with DiI-labeled PMNs followed by fixation with formaldehyde and labeling of nucleic acid with DAPI. Cells were examined by deconvolution fluorescence microscopy, as described in Materials and Methods. The rims of the bacteria appear green from the fluorescein, PMN nuclei and bacterial nucleoids appear blue from the DAPI, and PMN plasma membrane and phagosomal membrane appear red from the DiI. Opsonized INY401 organisms are internalized by PMNs in small phagosomes, each containing one bacterium or set of diplococci, while nonopsonized INY1801 organisms are internalized in large phagosomes. The yellow rims of internalized INY401, due to overlap of the green and red fluorescent dyes, suggest close apposition of the phagosomal membrane and bacteria. Bars, 5 μm.
Bacterial resistance to killing.
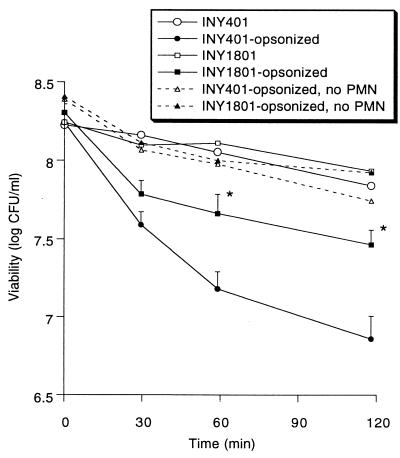
Binding and phagocytosis of bacteria by PMNs typically result in bacterial killing. Despite phagocytosis of AS-bearing E. faecalis by PMNs, INY1801 was not significantly killed during a 2-h incubation (Fig. 2). Enterococci were resistant to direct killing by normal serum alone, and serum opsonization increased bacterial killing by PMNs, as expected. However, the presence of AS resulted in relative protection against PMN-mediated killing when bacteria were opsonized, with a 0.8 ± 0.1 log decline in viability after 2 h for opsonized INY1801 versus a 1.4 ± 0.1 log decline for opsonized INY401 (P < 0.005).
FIG. 2.
Bacterial killing by PMNs. E. faecalis INY1801 or INY401 was opsonized with normal serum or not opsonized and then exposed to PMNs or HBSS alone (no PMN); microbial viability was determined. Data are the means (+ standard errors of the means for opsonized enterococci) for 5 to 12 experiments. ∗, P < 0.001 versus opsonized strain INY401.
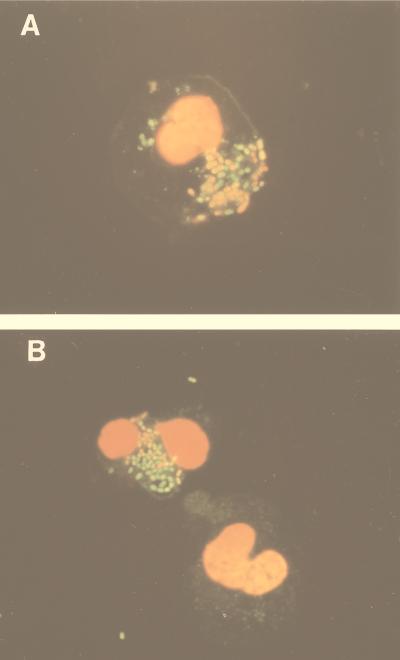
To confirm this resistance to PMN-mediated killing, we examined the integrity of bacterial membranes inside PMNs using fluorescent dyes, as this has been shown to correlate with bacterial viability (34). Internalized, nonopsonized AS-expressing bacteria maintained their ability to exclude propidium iodide to a significantly greater degree than did internalized opsonized AS-lacking bacteria (Fig. 3); after 1 h of incubation, 87% ± 4% of internalized nonopsonized INY1801 bacteria did not stain with propidium iodide, while only 60% ± 3% of opsonized INY401 bacteria excluded the dye (P < 0.01, n = 4) (Table 2). This difference was not due to differential staining characteristics of the bacteria alone, as all bacteria (opsonized or nonopsonized INY1801 or INY401) by themselves appeared similar when they were incubated with SYTO 9 and propidium iodide, with approximately 95% able to exclude propidium iodide. The ability of nonopsonized AS-expressing enterococci to maintain an intact plasma membrane inside PMNs is consistent with their resistance to killing in the viability assay.
FIG. 3.
Exclusion of propidium iodide by intracellular bacteria. Bacteria were opsonized with normal serum or not opsonized, exposed to PMNs for 60 min, and stained with SYTO 9 and propidium iodide. Representative fluorescence micrographs of opsonized E. faecalis INY401 demonstrating that the majority of intracellular bacteria stained orange-red from the propidium iodide (A) and nonopsonized INY1801 demonstrating that most of the intracellular bacteria were able to exclude the propidium iodide and stained green (B). Original magnification, ×1,000.
TABLE 2.
Resistance of E. faecalis INY1801 to intracellular killing by PMNs by fluorescent dye exclusion
| E. faecalis strain | % of internalized bacteria after incubation time (min) ofa:
|
|||
|---|---|---|---|---|
| 0 | 30 | 60 | 120 | |
| INY1801 | 100.0 ± 0.0 | 91.2 ± 3.7 | 87.2 ± 4.1 | 87.4 ± 2.3 |
| Opsonized INY401 | 90.4 ± 4.4 | 70.2 ± 6.6b | 60.2 ± 3.1b | 62.5 ± 8.3 |
Percentage of internalized bacteria able to exclude propidium iodide. Results are the means ± standard errors of the means for four experiments.
P < 0.05 versus INY1801.
AS-bearing E. faecalis was relatively resistant to killing by two other cell types as well. In the presence of human monocyte-derived macrophages, viability of AS-bearing INY1801 declined by only 0.3 ± 0.1 log after 2 h compared to a 0.9 ± 0.2 log decline for opsonized INY401 (P < 0.001, n = 6). Likewise, after 24 h of exposure to the murine macrophage cell line J774.A1, INY1801 viability was not changed (0 ± 0.2 log change), while viability of opsonized INY401 declined by 0.7 ± 0.2 log (P < 0.01, n = 5).
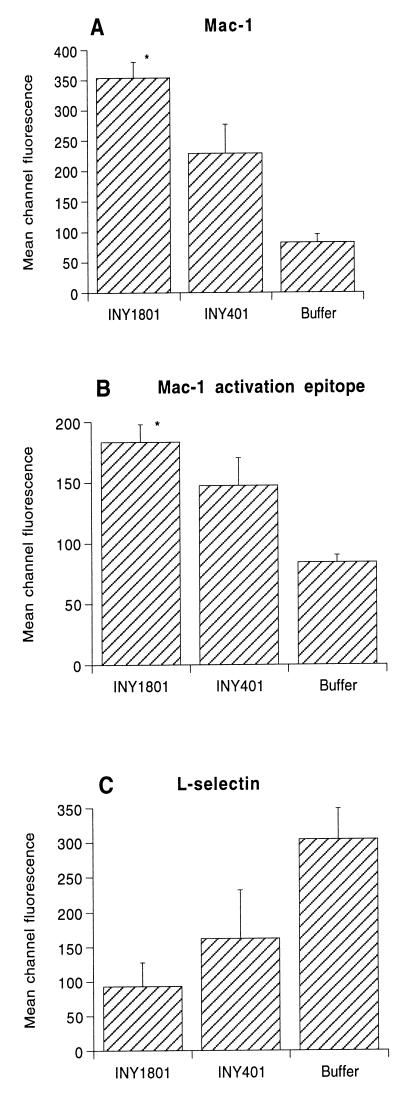
Neutrophil surface marker expression.
As bacteria undergoing phagocytosis by PMNs commonly trigger PMN activation leading to subsequent bacterial killing, we used several methods to determine if a lack of PMN activation could be responsible for the failure of PMNs to kill AS-bearing E. faecalis. First, we examined surface expression of Mac-1, l-selectin, and an activation epitope of β2-integrin defined by MAb24-Cy3 (8, 9). While both INY1801 and INY401 stimulated PMNs to increase the surface expression of Mac-1 and the MAb24-recognized activation epitope and shed l-selectin, INY1801 did so to a significantly greater extent (Fig. 4). In the presence of AS-bearing E. faecalis, we also observed a rapid neutrophil-neutrophil aggregation response (data not shown), which is a consequence of β2-integrin activation and binding (51). Minimal PMN aggregation was observed in the presence of bacteria lacking AS. As with aggregation stimulated by chemotactic factors (50), AS-mediated PMN aggregation was inhibited by anti-CD18 MAbs (data not shown).
FIG. 4.
PMN surface protein expression after incubation with bacteria. PMNs were incubated with E. faecalis INY1801 or INY401 or phosphate-buffered saline buffer control and surface expression of Mac-1, an activation epitope of Mac-1, and l-selectin were determined by flow cytometry with MAb MHM23, MAb24-Cy3, and Leu-8, respectively. Data are expressed as the mean channel fluorescence for five or six experiments; error bars represent standard errors of the means. ∗, P < 0.05 versus INY401.
Neutrophil superoxide production.
Production of highly reactive oxidant species via a respiratory burst is an important part of the PMN's antimicrobial armamentarium and is stimulated upon neutrophil activation. As the lack of respiratory burst activity could explain the ability of AS-expressing bacteria to survive inside PMNs, we examined PMN-mediated superoxide production. However, many E. faecalis organisms themselves produce an abundance of superoxide (24), including OG1RF and its derivatives, such as INY1801 and INY401 (data not shown). Thus, we identified an isolate of E. faecalis that produced only low levels of superoxide itself (TX1355) and did not carry the gene for AS or bind to PMNs (1.1 bacteria/PMN). We inserted pINY1801 by electroporation and confirmed that the resultant transformant still produced only low levels of superoxide itself but now bound to PMNs in much larger amounts (21.3 bacteria/PMN). This strain (TX1355d) was used as a probe for PMN superoxide production. TX1355d expressing AS stimulated twice as much PMN superoxide as did the parent strain TX1355 (5.5 ± 1.1 nm of superoxide/106 PMNs with TX1355d versus 2.7 ± 1.1 nm with TX1355; P < 0.05; n = 4).
Neutrophil phagosomal oxidant production.
The method described above for measuring superoxide production determines primarily extracellular superoxide, as the ferricytochrome c molecule is likely too large to enter intact cells (24). However, a distinct difference in intracellular versus extracellular PMN oxidant production has been described after stimulation with other bacteria, such as nonopsonized Neisseria gonorrhoeae (37); in that situation the vast majority of oxidants were produced intracellularly. In the present study, it was possible that while extracellular superoxide production was stimulated appropriately by AS-expressing enterococci, the bacteria could have been surviving intracellularly because of a lack of oxidant production at the critical site for antimicrobial activity, namely, the phagosome. To examine this, we labeled enterococci with the oxidant-sensitive fluorescent dye OxyBURST Green H2HFFDA, which has increased fluorescence emission upon exposure to oxidants. Bacterial labeling did not affect AS-mediated bacterial binding to PMNs and phagocytosis. Both nonopsonized AS-expressing TX1355d and opsonized AS-lacking TX1355 stimulated significant phagosomal oxidant production, with even greater oxidant production in response to TX1355d than in response to opsonized TX1355 (275 ± 13 relative fluorescence units for internalized TX1355d versus 155 ± 9 for internalized TX1355; P < 0.01, n = 3). In comparison, enterococci alone in the absence of PMNs (either TX1355d or TX1355) yielded the low values of 60 ± 3 and 61 ± 1 relative fluorescence units, and there was <25% increase in fluorescence of bacteria alone over a 1-h span, indicating a very minor amount of oxidation due to air, light, or bacterial oxidant production and confirming the validity of this tool as a measure of phagosomal oxidant production. Therefore, the presence of AS on E. faecalis stimulated both extracellular and phagosomal oxidant production, and all tested measures of PMN activation suggested that the AS-mediated resistance to PMN killing of internalized bacteria was not due to a lack of PMN activation or reactive oxidant production.
MPO-mediated killing.
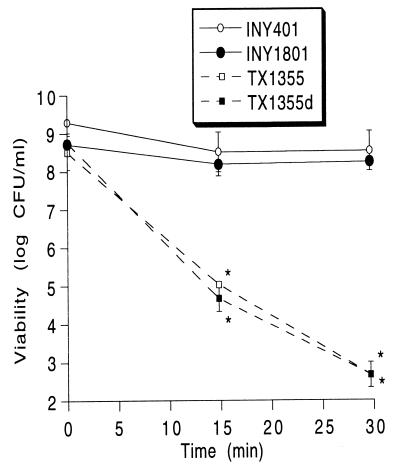
One of the major antimicrobial systems used by PMNs involves myeloperoxidase, which, together with hydrogen peroxide from the respiratory burst and chloride, generates hypochlorous acid (20) and other oxidants (54). The presence of AS itself did not affect E. faecalis susceptibility to killing by MPO-derived oxidants in a cell-free system (Fig. 5), as seen by a comparison of the viability of INY401 (lacking AS) versus that of INY1801 (expressing AS) or by TX1355 (lacking AS) versus TX1355d (expressing AS). However, INY401 and INY1801 (which produce extracellular superoxide themselves) were dramatically more resistant to MPO-mediated killing than TX1355 and TX1355d (which produce only minimal amounts of extracellular superoxide themselves). Substitution of 0.067 M sodium sulfate for 0.1 M NaCl in the MPO system yielded no bacterial killing (data not shown), confirming that the microbicidal effect in the active MPO system was due to MPO-generated oxidants.
FIG. 5.
MPO-mediated killing of enterococci. Bacteria were exposed to an MPO cell-free system, as described in Materials and Methods, and viability was determined. Data are the means for three experiments; error bars represent standard errors of the means. ∗, P < 0.05 versus INY401.
Phagosome size.
A review of the images from the deconvolution fluorescence microscopy experiments (Fig. 1) revealed that opsonized INY401 lacking AS was seen in smaller phagosomes, with each phagosome appearing to contain either one bacterium or one set of diplococci, as shown by the presence of red phagosomal membrane surrounding each ingested bacterium. The close apposition of the phagosomal membrane with each bacterium is also suggested by the altered color of the rims of internalized bacteria due to overlap of the green and red fluorescent dyes. In contrast, nonopsonized AS-expressing INY1801 was often taken up in much larger phagosomes, without close contact between bacteria and PMN membranes.
Phagosomal pH.
Acidification of PMN phagosomes normally occurs after ingestion of particles, with phagosomal pH dropping as low as 4.5 to 5.0 (27). Since phagosomal size was quite different for nonopsonized AS-expressing bacteria and opsonized bacteria, it was possible that phagosomal pH was also different in response to these two mechanisms of entry. Phagosomal pH was determined for bacteria labeled with both fluorescein and rhodamine by fluorescence microscopy. Phagosomes containing nonopsonized AS-expressing bacteria had a significantly higher pH (5.9 ± 0.5) than those containing opsonized INY401 lacking AS (4.5 ± 0.4) (P < 0.05, n = 3).
DISCUSSION
In this study, we have demonstrated that E. faecalis AS mediates opsonin-independent bacterial phagocytosis by human PMNs. Remarkably, internalized bacteria were resistant to killing. This was demonstrated in two ways, both by measurement of bacterial viability and by the ability of bacteria to maintain their membranes' integrity and exclude the fluorescent dye propidium iodide within the phagosomal compartment. While the absolute numbers are different for these two techniques, both clearly demonstrate the relative resistance to killing of AS-bearing E. faecalis compared to that of opsonized enterococci. This finding is quite unusual, in that most bacteria are readily killed by PMNs once they have been phagocytosed, even those that are typically described as intracellular pathogens because of their survival in macrophages, such as Shigella flexneri (35) or Mycobacterium tuberculosis (30). However, both S. flexneri and M. tuberculosis required opsonization for phagocytosis by PMNs, unlike AS-bearing E. faecalis, and the opsonin-independent mechanism of AS-induced entry into PMNs may be critical for intracellular survival. AS-mediated resistance to killing was not limited to PMNs but was also found with other phagocytes, such as monocyte-derived macrophages, and cells from the murine macrophage cell line J774.
The PMN response to bacteria is triggered by bacterial binding with subsequent activation of PMNs, and we examined this with several established assays of PMN activation in suspension by flow cytometry (51, 57). Activation of PMNs is associated with rapid alterations in the array of surface proteins, typically including an increase in surface expression and functional capacity of Mac-1 and concomitant shedding of l-selectin (31, 50). Within minutes of the addition of AS-expressing enterococci, upregulation of Mac-1 and the activation epitope of Mac-1 recognized by MAb24 (8, 9) were elicited. One functional consequence was the formation of neutrophil-neutrophil aggregates under sheared conditions. We also observed shedding of l-selectin upon exposure to AS-bearing bacteria. Taken together, these findings suggest that adhesion of AS-expressing E. faecalis induces rapid activation of PMNs. The presence of AS was not the only stimulus for PMN activation, as non-AS-containing E. faecalis was also found to activate PMNs, although to a significantly lesser extent. The mechanism of AS-independent activation is yet to be defined but may be related to the low-level bacterial binding to PMNs seen with strains lacking AS.
Oxygen-dependent PMN antimicrobial systems, involving a respiratory burst that yields several highly reactive forms of reduced oxygen species (32), are critical for optimal microbicidal activity. A lack of respiratory burst activity could have explained the inability of PMNs to kill internalized AS-bearing E. faecalis. However, we found that AS-bearing bacteria, in the absence of opsonins, stimulated increased PMN-mediated production of extracellular superoxide and phagosomal oxidants, indicating an active respiratory burst. Hydrogen peroxide produced by this respiratory burst may be converted by MPO into hypochlorous acid and other oxidants, which play a significant role in PMN antimicrobial activity. The presence of AS itself did not affect the resistance of E. faecalis to MPO-derived oxidants. However, enterococci are one of only two types of bacteria (along with lactococci) that have been found to produce extracellular superoxide themselves (24), and E. faecalis strains that produced superoxide themselves were markedly more resistant to MPO-mediated killing than those that did not. It is tempting to speculate that bacterial systems involved in superoxide production also may be involved in the resistance of E. faecalis to killing by MPO and PMNs. Nevertheless, TX1355 and OG1RF are completely unrelated strains, and in this study we did not examine E. faecalis strains that were isogenic except for the ability to produce superoxide, so firm conclusions cannot be drawn regarding a specific mechanism of resistance to MPO-derived oxidants.
We have previously shown that PMN CR3 is a critical surface receptor involved in AS-mediated binding to PMNs (58). The outcome of bacterial adhesion to CR3 may be dependent on multiple factors, including the activation state of CR3, the precise epitope involved in binding, and interactions with other receptors that may bind AS and initiate signal transduction (23, 55). It is thought that internalization and killing are dependent on bacterial polysaccharide binding to a lectin-like domain, in addition to recognition of particle-bound iC3b via the I domain of CR3 (59). Support for this hypothesis is the observation in this study that serum opsonization increased PMN-mediated killing of enterococci, a situation in which there likely would be binding to both the I domain and the lectin domain of CR3. It is possible that AS-mediated binding to CR3 in the absence of iC3b could result in a pathway of PMN activation that is not optimal for microbial killing. This possibility is consistent with our finding that phagosomes containing E. faecalis that had entered via an opsonin-independent AS-mediated mechanism were much larger than those containing bacteria that had entered via an opsonin-mediated mechanism. This result suggests that there were significant differences in phagosomal maturation induced by these two mechanisms of binding to CR3, which may have contributed to the intracellular survival of AS-bearing E. faecalis.
One aspect of normal PMN phagosomal maturation is acidification of phagosomes (27). Phagosomal pH 4 to 5 is thought to promote activity of a variety of PMN granule enzymes, including MPO, thus increasing their antimicrobial activity. We found a significantly higher phagosomal pH after ingestion of nonopsonized AS-expressing E. faecalis than after ingestion of opsonized AS-lacking E. faecalis. Other microorganisms, such as M. tuberculosis (52) and Legionella pneumophila (60), prevent phagosomal acidification in macrophages, and this property is considered to play a critical role in their ability to survive intracellularly. Thus, our findings of reduced phagosomal acidification induced by AS-expressing bacteria suggest that this could be a key mechanism involved in the ability of AS-bearing E. faecalis to resist PMN killing.
Other bacteria bind to phagocytes directly via CR3 in the absence of opsonins (23), even though almost none have been studied using neutrophils. Type 1-fimbriated Escherichia coli, which can bind nonopsonically to PMNs via CD66 (46) in addition to CR3 (17), can stimulate PMN activation (19, 53). However, while some have reported that type 1-fimbriated Escherichia coli was resistant to PMN-mediated killing despite internalization and activation of the respiratory burst (18, 19), we have actually found it to be more susceptible to PMN-mediated killing (29). Both N. gonorrhoeae (15) and Neisseria meningitidis (12) can undergo nonopsonic phagocytosis by PMNs, but for both of these bacteria this results in PMN-mediated killing, and at least for N. gonorrhoeae, CR3 is not involved (14). Recently, type III group B streptococci also have been shown to bind to PMNs in an opsonin-independent manner (1). However, these bacteria were not internalized by PMNs, and the interaction clearly did not involve CR3. Thus, we believe that the results presented herein are the first to describe a bacterial pathogen that can use a CR3-mediated pathway in the absence of opsonins to invade PMNs and survive intracellularly.
The importance of AS as a virulence factor has been demonstrated in animal models of endocarditis (4, 49), and AS-containing E. faecalis has frequently been found among clinical isolates (26) and has been less frequently seen in isolates from stools of healthy volunteers (5), again suggesting the importance of AS as a virulence determinant of E. faecalis. AS may serve as a virulence determinant of E. faecalis in at least two distinct ways. First, AS may facilitate the attachment of enterococci to renal (33) and intestinal (39) epithelial cells and colonization of these surfaces. Second, it may protect against PMN-mediated killing by promoting phagocytosis of bacteria by PMNs in a manner that activates PMNs but does not result in microbial killing, perhaps by inhibition of phagosomal acidification. This sequence of events may constitute a novel mechanism used by these bacteria to resist the human host defense system.
ACKNOWLEDGMENTS
We thank Robert Jordon for use of the fluorescence microscope and Nancy Hogg, David Chambers, and Karl Arfors for providing monoclonal antibodies.
Financial support was provided by grants-in-aid from the American Heart Association, Texas affiliate and national association, (to R.M.R.) and grants from the National Institutes of Health (AI31652 to S.I.S., AI19031 to M.M.M., and HL51987 to G.M.D.). S.I.S. is an Established Investigator of the American Heart Association.
REFERENCES
- 1.Albanyan E A, Smith C W, Edwards M S. Abstracts of the 36th Annual Meeting of the Infectious Diseases Society of America. 1998. Nonopsonic interaction of type III group B Streptococcus (III GBS) with human neutrophils (PMN), abstr. 120. Denver, Colo. [Google Scholar]
- 2.Arduino R C, Jacques-Palaz K, Murray B E, Rakita R M. Resistance of Enterococcus faecium to neutrophil-mediated phagocytosis. Infect Immun. 1994;62:5587–5594. doi: 10.1128/iai.62.12.5587-5594.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arduino R C, Murray B E, Rakita R M. Roles of antibodies and complement in phagocytic killing of enterococci. Infect Immun. 1994;62:987–993. doi: 10.1128/iai.62.3.987-993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow J W, Thal L A, Perri M B, Vazquez J A, Donabedian S M, Clewell D B, Zervos M J. Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental Enterococcal endocarditis. Antimicrob Agents Chemother. 1993;37:2474–2477. doi: 10.1128/aac.37.11.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coque T M, Patterson M E, Steckelberg J M, Murray B E. Incidence of hemolysin, gelatinase, and aggregation substance among enterococci isolated from patients with endocarditis and other infections and from feces of hospitalized and community-based persons. J Infect Dis. 1995;171:1223–1229. doi: 10.1093/infdis/171.5.1223. [DOI] [PubMed] [Google Scholar]
- 6.Cruz-Rodz A L, Gilmore M S. High efficiency introduction of plasmid DNA into glycine treated Enterococcus faecalis by electroporation. Mol Gen Genet. 1990;224:152–154. doi: 10.1007/BF00259462. [DOI] [PubMed] [Google Scholar]
- 7.Currier T C, Nester E W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976;76:431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- 8.Dransfield I, Cabanas C, Craig A, Hogg N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J Cell Biol. 1992;116:219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dransfield I, Hogg N. Regulated expression of Mg+2 binding epitope on leukocyte integrin alpha subunits. EMBO J. 1989;8:3759–3765. doi: 10.1002/j.1460-2075.1989.tb08552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunny G M, Craig R A, Carron R L, Clewell D B. Plasmid transfer in Streptococcus faecalis: production of multiple pheromones by recipients. Plasmid. 1979;2:454–465. doi: 10.1016/0147-619x(79)90029-5. [DOI] [PubMed] [Google Scholar]
- 11.Dunny G M, Leonard B A, Hedberg P J. Pheromone-inducible conjugation in Enterococcus faecalis: interbacterial and host-parasite chemical communication. J Bacteriol. 1995;177:871–876. doi: 10.1128/jb.177.4.871-876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estabrook M E, Zhou D, Apicella M A. Nonopsonic phagocytosis of group C Neisseria meningitidis by human neutrophils. Infect Immun. 1998;66:1028–1036. doi: 10.1128/iai.66.3.1028-1036.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Facklam R R, Collins M D. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989;27:731–734. doi: 10.1128/jcm.27.4.731-734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrell C F, Rest R F. Up-regulation of human neutrophil receptors for Neisseria gonorrhoeae expressing PII outer membrane protein. Infect Immun. 1990;58:2777–2784. doi: 10.1128/iai.58.9.2777-2784.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer S H, Rest R F. Gonococci possessing only certain P.II outer membrane proteins interact with human neutrophils. Infect Immun. 1988;56:1574–1579. doi: 10.1128/iai.56.6.1574-1579.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friesenegger A, Fiedler S, Devriese L A, Wirth R. Genetic transformation of various species of Enterococcus by electroporation. FEMS Microbiol Lett. 1991;79:323–328. doi: 10.1016/0378-1097(91)90106-k. [DOI] [PubMed] [Google Scholar]
- 17.Gbarah A, Gahmberg C G, Ofek I, Jacobi U, Sharon N. Identification of the leukocyte adhesion molecules CD11 and CD18 as receptors for type 1-fimbriated (mannose-specific) Escherichia coli. Infect Immun. 1991;59:4524–4530. doi: 10.1128/iai.59.12.4524-4530.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goetz M B, Kuriyama S M, Silverblatt F J. Phagolysosome formation by polymorphonuclear neutrophilic leukocytes after ingestion of Escherichia coli that express type 1 pili. J Infect Dis. 1987;156:229–233. doi: 10.1093/infdis/156.1.229. [DOI] [PubMed] [Google Scholar]
- 19.Goetz M B, Silverblatt F J. Stimulation of human polymorphonuclear leukocyte oxidative metabolism by type 1 pili from Escherichia coli. Infect Immun. 1987;55:534–540. doi: 10.1128/iai.55.3.534-540.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison J E, Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976;251:1371–1374. [PubMed] [Google Scholar]
- 21.Heimer G V, Taylor C E D. Improved mountant for immunofluorescence preparations. J Clin Pathol. 1973;27:254–256. doi: 10.1136/jcp.27.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hildreth J E K, Gotch F M, Hildreth P D K, McMichael A J. A human lymphocyte-associated antigen involved in cell-mediated lympholysis. Eur J Immunol. 1983;13:202–208. doi: 10.1002/eji.1830130305. [DOI] [PubMed] [Google Scholar]
- 23.Hoepelman A I M, Tuomanen E I. Consequences of microbial attachment: directing host cell functions with adhesins. Infect Immun. 1992;60:1729–1733. doi: 10.1128/iai.60.5.1729-1733.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huycke M M, Joyce W, Wack M F. Augmented production of extracellular superoxide by blood isolates of Enterococcus faecalis. J Infect Dis. 1996;173:743–746. doi: 10.1093/infdis/173.3.743. [DOI] [PubMed] [Google Scholar]
- 25.Huycke M M, Sahm D F, Gilmore M S. Multiple-drug resistant Enterococci: the nature of the problem and an agenda for the future. Emerg Infect Dis. 1998;4:239–249. doi: 10.3201/eid0402.980211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ike Y, Hashimoto H, Clewell D B. High incidence of hemolysin production by Enterococcus (Streptococcus) faecalis strains associated with human parenteral infections. J Clin Microbiol. 1987;25:1524–1528. doi: 10.1128/jcm.25.8.1524-1528.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacques Y V, Bainton D F. Changes in pH within phagocytic vacuoles of human neutrophils and monocytes. Lab Investig. 1978;39:179–185. [PubMed] [Google Scholar]
- 28.Jett B D, Huycke M M, Gilmore M S. Virulence of enterococci. Clin Microbiol Rev. 1994;7:462–478. doi: 10.1128/cmr.7.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson J R, Skubitz K M, Nowicki B J, Jacques-Palaz K, Rakita R M. Nonlethal adherence to human neutrophils mediated by Dr antigen-specific adhesins of Escherichia coli. Infect Immun. 1995;63:309–316. doi: 10.1128/iai.63.1.309-316.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones G S, Amirault H J, Andersen B R. Killing of Mycobacterium tuberculosis by neutrophils: a nonoxidative process. J Infect Dis. 1990;162:700–704. doi: 10.1093/infdis/162.3.700. [DOI] [PubMed] [Google Scholar]
- 31.Kishimoto T K, Jutila M A, Berg E C, Butcher E C. Neutrophil Mac-1 and Mel-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- 32.Klebanoff S J. Oxygen metabolites from phagocytes. In: Gallin J I, Goldstein I M, Snyderman R, editors. Inflammation: basic principles and clinical correlates. New York, N.Y: Raven Press; 1992. pp. 541–588. [Google Scholar]
- 33.Kreft B, Marre R, Schramm U, Wirth R. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect Immun. 1992;60:25–30. doi: 10.1128/iai.60.1.25-30.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd D, Hayes A J. Vigour, vitality and viability of microorganisms. FEMS Microbiol Lett. 1995;133:1–7. [Google Scholar]
- 35.Mandic-Mulec I, Weiss J, Zychlinsky A. Shigella flexneri is trapped in polymorphonuclear leukocyte vacuoles and efficiently killed. Infect Immun. 1997;65:110–115. doi: 10.1128/iai.65.1.110-115.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray B E. The life and times of the enterococcus. Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naids F L, Rest R F. Stimulation of human neutrophil oxidative metabolism by nonopsonized Neisseria gonorrhoeae. Infect Immun. 1991;59:4383–4390. doi: 10.1128/iai.59.12.4383-4390.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh Y-K, Straubinger R M. Intracellular fate of Mycobacterium avium: use of dual-label spectrofluorometry to investigate the influence of bacterial viability and opsonization of phagosomal pH and phagosome-lysosome interaction. Infect Immun. 1996;64:319–325. doi: 10.1128/iai.64.1.319-325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olmsted S B, Dunny G M, Erlandsen S L, Wells C L. A plasmid-encoded surface protein on Enterococcus faecalis augments its internalization by cultured intestinal epithelial cells. J Infect Dis. 1994;170:1549–1556. doi: 10.1093/infdis/170.6.1549. [DOI] [PubMed] [Google Scholar]
- 40.Olmsted S B, Kao S-M, van Putte L J, Gallo J C, Dunny G M. Role of the pheromone-inducible surface protein Asc10 in mating aggregate formation and conjugal transfer of the Enterococcus faecalis plasmid pCF10. J Bacteriol. 1991;173:7665–7672. doi: 10.1128/jb.173.23.7665-7672.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petty H R, Francis J W. Polymorphonuclear leukocyte histamine receptors: occurrence in cell surface clusters and their redistribution during locomotion. Proc Natl Acad Sci USA. 1986;83:4332–4335. doi: 10.1073/pnas.83.12.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pick E. Microassays for superoxide and hydrogen peroxide production and nitroblue tetrazolium reduction using an enzyme immunoassay microplate reader. Methods Enzymol. 1986;132:407–421. doi: 10.1016/s0076-6879(86)32026-3. [DOI] [PubMed] [Google Scholar]
- 43.Rakita R M, Michel B R, Rosen H. Differential inactivation of Escherichia coli membrane dehydrogenases by a myeloperoxidase-mediated antimicrobial system. Biochemistry. 1990;29:1075–1080. doi: 10.1021/bi00456a033. [DOI] [PubMed] [Google Scholar]
- 44.Ralph P, Nakoinz I. Direct toxic effects of immunopotentiators on monocytic, myelomonocytic, and histiocytic or macrophage tumor cells in culture. Cancer Res. 1977;37:546–550. [PubMed] [Google Scholar]
- 45.Rosen H, Michel B R, Chait A. Phagocytosis of opsonized oil droplets by neutrophils. J Immunol Methods. 1991;144:117–125. doi: 10.1016/0022-1759(91)90237-a. [DOI] [PubMed] [Google Scholar]
- 46.Sauter S L, Rutherfurd S M, Wagener C, Shively J E, Hefta S A. Identification of the specific oligosaccharide sites recognized by type 1 fimbriae from Escherichia coli on nonspecific cross-reacting antigen, a CD66 cluster granulocyte glycoprotein. J Biol Chem. 1993;268:15510–15516. [PubMed] [Google Scholar]
- 47.Schaberg D R, Culver D H, Gaynes R P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991;91:72S–75S. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- 48.Scheld W M, Sande M A. Endocarditis and intravascular infections. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill-Livingstone; 1995. pp. 740–782. [Google Scholar]
- 49.Schlievert P M, Gahr P J, Assimacopoulos A P, Dinges M M, Stoehr J A, Harmala J W, Hirt H, Dunny G M. Aggregation and binding substances enhance pathogenicity in rabbit models of Enterococcus faecalis endocarditis. Infect Immun. 1998;66:218–223. doi: 10.1128/iai.66.1.218-223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simon S I, Chambers D C, Butcher E, Sklar L A. Neutrophil aggregation is β2-integrin and L-selectin dependent in blood and isolated cells as measured by flow cytometry. J Immunol. 1992;149:2765–2771. [PubMed] [Google Scholar]
- 51.Simon S I, Rochon Y P, Lynam E B, Smith C W, Anderson D C, Sklar L A. β2-Integrin and L-selectin are obligatory receptors in neutrophil aggregation. Blood. 1993;82:1097–1106. [PubMed] [Google Scholar]
- 52.Sturgill-Koszycki S, Schlesinger P H, Chakraborty P, Haddix P L, Collins H L, Fok A K, Allen R D, Gluck S L, Heuser J, Russell D G. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 53.Tewari R, MacGregor J I, Ikeda T, Little J R, Hultgren S J, Abraham S N. Neutrophil activation by nascent FimH subunits of type 1 fimbriae purified from the periplasm of Escherichia coli. J Biol Chem. 1993;268:3009–3015. [PubMed] [Google Scholar]
- 54.Thomas E L. Myeloperoxidase, hydrogen peroxide, chloride antimicrobial system: nitrogen-chlorine derivatives of bacterial components in bactericidal action against Escherichia coli. Infect Immun. 1979;23:522–531. doi: 10.1128/iai.23.2.522-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Todd R F., III The continuing saga of complement receptor type 3 (CR3) J Clin Investig. 1996;98:1–2. doi: 10.1172/JCI118752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trotter K M, Dunny G M. Mutants of Enterococcus faecalis deficient as recipients in mating with donors carrying pheromone-inducible plasmids. Plasmid. 1990;24:57–67. doi: 10.1016/0147-619x(90)90025-8. [DOI] [PubMed] [Google Scholar]
- 57.Tsang Y T M, Neelamegham S, Hu Y, Berg E L, Burns A R, Smith C W, Simon S I. Synergy between L-selectin signaling and chemotactic activation during neutrophil adhesion and transmigration. J Immunol. 1997;159:4566–4577. [PubMed] [Google Scholar]
- 58.Vanek, N. N., S. I. Simon, K. Jacques-Palaz, M. M. Mariscalco, G. M. Dunny, and R. M. Rakita. Enterococcus faecalis aggregation substance promotes opsonin-independent binding to human neutrophils via a complement receptor type 3-mediated mechanism. FEMS Immunol. Med. Microbiol., in press. [DOI] [PubMed]
- 58a.Vanek N N, Simon S I, Jacques-Palaz K, Mariscalco M M, Dunny G M, Rakita R M. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Enterococcus faecalis bearing aggregation substance (AS) bind to and activate neutrophils (PMNs) but are not killed, abstr. B23; p. 25. [Google Scholar]
- 59.Vetvicka V, Thornton B P, Ross G D. Soluble β-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J Clin Investig. 1996;98:50–61. doi: 10.1172/JCI118777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiater L A, Dunn K, Maxfield F R, Shuman H A. Early events in phagosome establishment are required for intracellular survival of Legionella pneumophila. Infect Immun. 1998;66:4450–4460. doi: 10.1128/iai.66.9.4450-4460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wirth R. The sex pheromone system of Enterococcus faecalis. Eur J Biochem. 1994;222:235–246. doi: 10.1111/j.1432-1033.1994.tb18862.x. [DOI] [PubMed] [Google Scholar]
- 62.Wirth R, An F Y, Clewell D B. Highly efficient protoplast transformation system for Streptococcus faecalis and a new Escherichia coli-S. faecalis shuttle vector. J Bacteriol. 1986;165:831–836. doi: 10.1128/jb.165.3.831-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wirth R, Olmsted S B, Galli D, Dunny G M. Comparative analysis of cAD1 and cCF10 induced aggregation substances of Enterococcus faecalis. In: Dunny G M, Cleary P P, McKay L L, editors. Genetics and molecular biology of streptococci, lactococci, and enterococci. Washington, D.C: American Society for Microbiology; 1991. pp. 34–38. [Google Scholar]
- 64.Wright S D. Receptors for complement and the biology of phagocytosis. In: Gallin J I, Goldstein I M, Snyderman R, editors. Inflammation: basic principles and clinical correlates. 2nd ed. New York, N.Y: Raven Press; 1992. pp. 477–495. [Google Scholar]
- 65.Wright S D, Rao P E, Van Voorhis W C, Craigmyle L S, Iida K, Talle M A, Westberg E F, Goldstein G, Silverstein S C. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci USA. 1983;80:5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]