Abstract
Methods for the synthesis of two types of isomeric dispirocompounds based on imidazothiazolotriazine and pyrrolidineoxindole, differing in the structure of imidazothiazolotriazine fragment, namely, linear dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine- 4′,3″-indolines] and angular dispiro[imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine-7,3′-pyrrolidine-4′,3″-indolines] were proposed. The first method relies on a 1,3-dipolar cycloaddition of azomethine ylides generated in situ from paraformaldehyde and N-alkylglycine derivatives to the corresponding oxindolylidene derivatives of imidazothiazolotriazine. The cycloaddition leads to a mixture of two diastereomers resulted from anti- and syn-approaches of azomethine ylide in approximately a 1:1 ratio, which were separated by column chromatography. Another method consists in rearrangement of linear dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-4′,3″-indolines] into hitherto unavailable angular dispiro[imidazo[4,5-e]thiazolo[2,3-c]-[1,2,4]triazine-7,3′-pyrrolidine-4′,3″-indolines] upon treatment with KOH. It was found that the anti-diastereomer of linear type underwent rearrangement into the isomeric angular syn-diastereomer, while the rearrangement of the linear syn-diastereomer gave the angular anti-diastereomer.
Keywords: dispirooxindoles; 1,3-dipolar cycloaddition; oxindolylidene imidazothiazolotriazines; azomethine ylides; rearrangement
1. Introduction
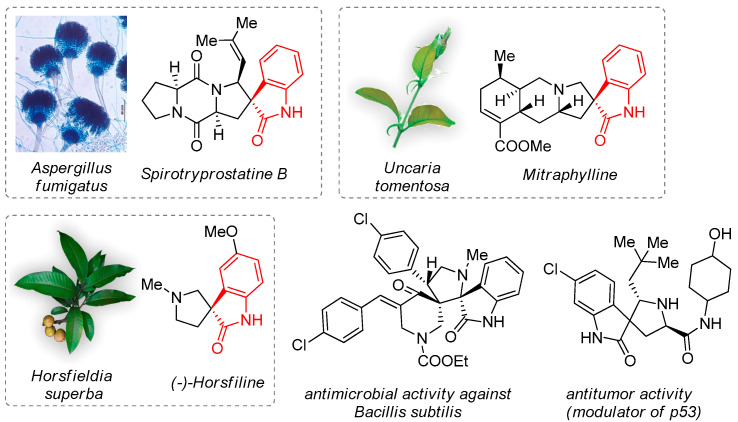
Spiro cyclic compounds have found great application in drug discovery due to the interesting conformational features and presence in natural products [1,2,3]. A conformational rigidity of the molecule allows spirocompounds to interact effectively with various active sites on many biological targets [3,4]. Therefore, the introduction of a spiro-junction in the structure affects the key properties of molecules, such as bioavailability, metabolic stability, binding ability, and target selectivity [5]. Synthetic and natural products containing a spiropyrrolidineoxindole fragment exhibit a wide scope of pharmacological activity, including antimicrobial, antitumor, antidiabetic, anticonvulsant, and antiviral effects (Figure 1) [6,7,8,9,10]. Promising biological properties encourage the researchers to develop efficient synthetic approaches to structures bearing such framework. There are numerous reports on the synthesis of various spiropyrrolidineoxindoles and dispiro-fused pyrrolidineoxindoles, additionally involving spiro-linked pharmacophore heterocycles, such as thiazolidine, imidazolidine, etc. [11,12,13,14,15]. Such hybridization resulted in a preparation of new compounds with cytotoxic, prooxidant and antidiabetic properties [15,16,17].
Figure 1.
Biologically active natural and synthetic spiropyrrolidineoxindoles.
Over the last decade, our attention was focused on thiazolo-1,2,4-triazines [18,19,20,21,22,23] because these compounds showed antiproliferative and antibacterial activities (Figure 2) [20,21,22,23,24,25].
Figure 2.
Biologically active thiazolo-1,2,4-triazines.
We previously published the synthesis of hybrids of imidazothiazolotriazine and 2,3′-spiropyrrolidinyloxindole by a 1,3-dipolar cycloaddition of an azomethine ylide generated in situ from isatins and sarcosine to arylmethylidene derivatives of imidazothiazolotriazine [26,27]. The reaction proceeded with high regio- and diastereoselectivity to give only one diastereomer. Here, we report the synthesis, structure and stereochemistry of dispirooxindoles based on imidazothiazolotriazine and 3,3′-spiropyrrolidinyloxindole.
2. Results and Discussion
2.1. Synthesis and Stereochemistry of Dispirooxindoles Based on Imidazothiazolotriazine and Pyrrolidineoxindole
To synthesize target dispirooxindoles we used previously prepared racemic oxindolylideneimidazothiazolotriazines 1a–d [20,28]. The cycloaddition of azomethine ylide generated from paraformaldehyde and sarcosine 2a to dipolarophiles 1a–d was carried out in acetonitrile under reflux for 10–14 h. According to the NMR spectroscopy data, the reaction proceeded unselectively to afford the mixture of two anti- and syn-diastereomers 3 (3aR*,4’R*,6S*,9aS*-3a,b, 3aR*,4’S*,6R*,9aR*-3c,d) and 4 (3aR*,4’S*,6R*,9aS*-4a,b,3aR*,4’R*,6S*,9aR*-4c,d) in 1:1 ratio (Scheme 1). The mixtures were separated by column chromatography (iPrOH as eluent), and each of isomers 3a–d and 4a–d was isolated individually in the yields of 31–43 and 30–42%, respectively.
Scheme 1.
Synthesis of racemic spiropyrrolidineoxindoles 3a–d, 4a–d via 1,3-dipolar cycloaddition using paraformaldehyde and sarcosine as azomethine ylide precursors.
The stereoselectivity of the cycloaddition can be explained by the possible approach of sterically unhindered “small” azomethine ylide to the plane of the double bond of dipolarophile both from the opposite side (anti-) and from the same side (syn-) to which the imidazolidine cycle deviates (Scheme 2).
Scheme 2.
Modes of approach of azomethine ylide.
To increase the selectivity of the reaction we prepared more sterically hindered amino acid, i.e., N-cyclohexylglycine. Indeed, compounds 3 and 4 were obtained in 1.5:1 ratio (Scheme 3). In addition, diastereomer 3 underwent Mannich amino alkylation at the N(9) nitrogen atom. Since compounds 3e (3aR*,4′R*,6S*,9aS*) and 4e (3aR*,4′S*,6R*,9aS*) are not diastereomers, they were easily separated by crystallization. Earlier [29], we already observed aminomethylation at the oxindole nitrogen atom during cycloaddition reaction. Thus, we switched to N-methyloxindolylidene derivatives 1 and 5.
Scheme 3.
Synthesis of racemic spiropyrrolidineoxindoles 3e and 4e via 1,3-dipolar cycloaddition using paraformaldehyde and N-cyclohexylglycine as azomethine ylide precursors.
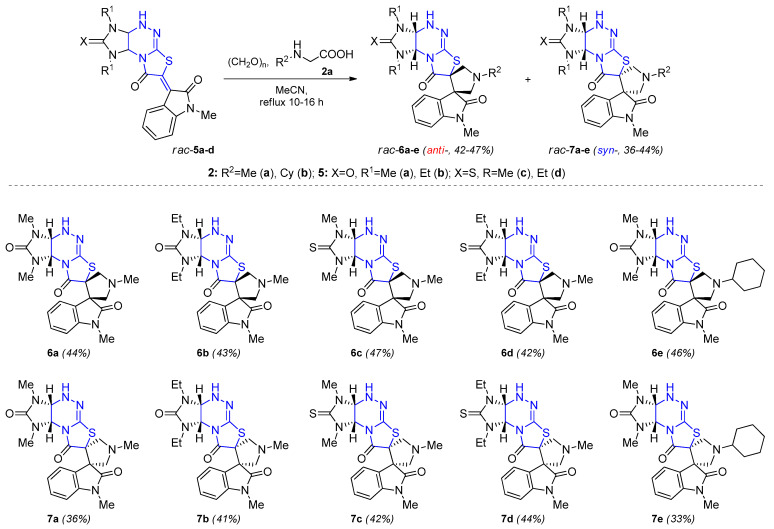
Oxindolylidene derivatives of isomeric angular structure 5a–d reacted with paraformaldehyde and amino acids 2a,b similarly to provide the formation of hitherto unavailable anti- and syn-diastereomers 6 and 7 in approximately the same ratio (Scheme 4). The yields of diastereomers 6 (3aR*,4′R*,7S*,9aS*-6a,b,e, 3aR*,4′R*,7S*,9aR*-6c,d) and 7 (3aR*,4′S*,7R*,9aS*-7a,b,e, 3aR*,4′S*,7R*,9aR*-7c,d) after separation by column chromatography were 42–47% and 33–44%, respectively.
Scheme 4.
Synthesis of isomeric racemic spiropyrrolidineoxindoles 6a–e, 7a–e via 1,3-dipolar cycloaddition using paraformaldehyde and N-alkylglycine derivatives as azomethine ylide precursors.
Another approach to the compounds 6 and 7 is the rearrangement of isomers 3 and 4 upon treatment with KOH under reflux in methanol. Taking diastereomers 3a and 4a as examples, we found that the anti-diastereomer 3a (3aR*,4′R*,6S*,9aS*) underwent rearrangement into the isomeric syn-diastereomer 7a (3aR*,4′S*,7R*,9aS*), while the rearrangement of the syn-diastereomer 4a (3aR*,4′S*,6R*,9aS*) gave rise to the isomeric anti-diastereomer 6a (3aR*,4′R*,7S*,9aS*). The yields of the rearrangement products 6a and 7a were 80 and 92%, respectively (Scheme 5).
Scheme 5.
Synthesis of isomeric racemic spiropyrrolidineoxindoles 6a and 7a via rearrangement of spiropyrrolidineoxindoles 4a and 3a.
The presumable rearrangement mechanism is depicted in Scheme 6. We assume that the rearrangement occurs as transamidation reaction upon treatment with KOH in methanol [27]. The nucleophilic attack of the methoxide anion onto the carbon atom C(7) results in the cleavage of the C(7)–N(8) bond followed by rotation of the spiropyrrolidinyloxindole moiety around the C–S bond and the recyclization involving the nitrogen atom N(4) of the triazine ring (Scheme 6).
Scheme 6.
The presumable reaction mechanism of the rearrangement of compounds 3a and 4a into isomers 7a and 6a.
2.2. Structural Determination of Compounds 3,4,6,7
The structures of the compounds obtained were elucidated by spectral methods; single crystal X-ray diffraction of 3a, 4e and 7c was also carried out.
Compounds 3a and 7c crystalize in centrosymmetric space groups P21/c and respectively, while 4e structure crystalizes in an acentric orthorhombic space group P212121 as an inversion twin. Thus, these compounds crystalize as racemic mixtures of two enantiomers. The relative configurations of chiral centers of independent part of compounds 3a (3aR*,4′R*,6S*,9aS*), 4e (3aR*,4′S*,6R*,9aS*) and 7c (3aR*,4′S*,7R*,9aR*) were unambiguously assigned by single crystal X-ray diffraction (Figure 3, Figure 4 and Figure 5).
Figure 3.
General view and fragment of crystal packing of 3a in crystal. Anisotropic displacement parameters are drawn at 50% probability level.
Figure 4.
General view and fragment of crystal packing of 4e in crystal (only one disordered component is shown). Anisotropic displacement parameters are drawn at 20% probability level.
Figure 5.
General view and fragment of crystal packing of 7c in crystal. Anisotropic displacement parameters are drawn at 50% probability level.
Bond lengths and angles in the structures are within normal ranges with some exceptions, that was confirmed by the Mogul geometry check [30]. The exceptions are deviations of bond lengths and angles of the oxo- and thioimidazole cycle from average values for similar fragments of the previously published compounds. The list of selected bond lengths and angles can be seen in Table 1.
Table 1.
Selected bond lengths (Å) and angles (°) for racemates 3a, 4e and 7c.

| |||
|---|---|---|---|
| Compound | 3a | 4e | 7c |
| Bond lengths (Å) | |||
| A | |||
| C-N | 1.3682(17)–1.4527(16) | 1.356(4)–1.452(4) | 1.3477(14)–1.4828(14) |
| C-C | 1.5367(17) | 1.520(5) | 1.5313(14) |
| B | |||
| N-N | 1.4079(14) | 1.420(4) | 1.4103(13) |
| C-N | 1.3905(16)–1.4664(16) | 1.389(4)–1.475(5) | 1.4046(13)–1.4832(12) |
| C=N | 1.2601(16) | 1.270(4) | 1.2748(13) |
| C | |||
| S-C | 1.7557(12)–1.8187(12) | 1.755(3)–1.827(3) | 1.7637(10)–1.8254(10) |
| C-N | 1.3641(15) | 1.362(4) | 1.3706(13) |
| C-C | 1.5241(17) | 1.525(5) | 1.5297(14) |
| D | |||
| C-N | 1.4649(16)–1.4717(16) | 1.452(5)–1.468(5) | 1.4660(14)–1.4720(14) |
| C-C | 1.5364(16)–1.5517(17) | 1.545(5)–1.552(5) | 1.5319(14)–1.5728(14) |
| E | |||
| C-N | 1.3637(16)–1.3993(17) | 1.355(4)–1.410(4) | 1.3632(13)–1.4097(13) |
| C-C | 1.3994(17)–1.5446(17) | 1.385(5)–1.547(5) | 1.3999(14)–1.5505(14) |
| Dihedral angles between two spiro-jointed cycles (°) | |||
| CD | 89.1(1) | 89.1(3) | 89.0(1) |
| DE | 89.5(1) | 86.3(3) | 88.3(1) |
| Torsion angles between two adjacent cycles (°) | |||
| AB | |||
| N-C-C-N | 89.7(1), −150.9(1) | 152.8(3), −87.3(3) | 149.3(1), −92.8(1) |
| BC | |||
| C-N-C-N | −173.6(1) | 179.2(3) | 157.9(1) |
| N-N-C-S (3a, 4e) C-N-C-S (7c) |
−176.4(1) | −179.5(2) | 163.2(1) |
The five-membered imidazole fragment has an envelope conformation in 3a (with atom C(2) deviated by 0.46(2) Å) and a twist conformation in 4e and 7c. The bicyclic thiazolotriazine fragment is almost planar in 3a and 4e with one carbon atom (C(2) and C(3), respectively) deviated by 0.37(3) and 0.47(3) Å from the mean plane, respectively. In 7c this fragment is significantly twisted with atoms N(3), N(4) and S(2) deviated from the mean plane of C(3)-C(2)-N(5)-C(5)-C(6) atoms by 0.78(3), 0.34(3) and 0.46(3) Å, respectively. The five-membered pyrrolidine fragment has an envelope conformation in 3a, 4e and 7c with atoms C(6), C(6) and C(9) deviated by 0.56(1), 0.57(2) and 0.60(1) Å, respectively. The bridgehead torsion angle H(2)-C(2)-C(3)-H(3) is in the range 30.03–32.86°, which additionally characterizes the distortion of the imidazothiazolotriazine fragments. The mean planes of neighboring spiro-jointed five-membered cycles are practically perpendicular to each other. The nitrogen atoms N(3) and N(6) are slightly pyramidalized with the sum of bond angles at these atoms less than 340° (Table S2).
Presence of H(N) atom allows H-bonding for three solids (Figure 3, Figure 4 and Figure 5). In 3a centrosymmetric dimers are formed by two bifurcated contacts N(3)…O(2), N(3)…N(6). In 4e and 7c infinite chains are observed. For 4e a solvent methanol acts as a bridge between two molecules.
The structures of compounds 3a, 3b, 6a and 7b were also confirmed by NMR data in DMSO solutions using NOESY 2D correlation technique. Hydrogen atoms of alkyl group at the nitrogen atom N(1) and hydrogen atoms H-4″, H-5″ of oxindole fragment in compounds 3 are spatially close. The presence of cross-peaks of these protons in the NOESY spectra is characteristic of the anti-diastereomers 3a, 3b. In the NOESY spectra of anti-diastereomer 6a, correlations between protons of N(1)Me group and H-4″ of oxindole fragment, between protons of N(3)Me and N(4)H groups, as well as between protons H-7″ and N(1′)Me of oxindole fragment were observed. In the NOESY spectrum of syn-diastereomer 7b cross-peaks of NMe group and oxindole fragment protons are absent (see Figure 6 and Supplementary Information).
Figure 6.
Fragment of the NOESY spectrum of anti-diastereomer 3a with correlations of N(1)CH3-group protons with N(9)H and oxindole fragment H-4″, H-5″ protons.
In the spectra of angular structures 4 and 7, downfield shifts of the proton signal of the NH group from 6.73–7.05 to 7.43–7.66 ppm were observed in comparison with the spectra of the linear structures 3 and 6. Downfield shifts from 4.59–4.87 to 5.35–5.85 ppm were also revealed for the signal of one of the bridging protons H(3a) or H(9a) (Figure 7).
Figure 7.
1H NMR spectra of compounds 3b, 4b, 6b and 7b in DMSO-d6 in the region of 2.6–8.0 ppm.
Thus, we obtained two diastereomers of each type of dispirocompounds of linear and angular structure in individual form and proved their structures and stereochemistry by spectral methods and single crystal X-ray diffraction.
3. Materials and Methods
3.1. General Information
Melting points were determined in open glass capillaries on a Gallenkamp (Sanyo) melting point apparatus. IR spectra were recorded on a Bruker ALPHA instrument in KBr pellets. High resolution mass spectra (HRMS) were measured on a Bruker micrOTOF II instrument using electrospray ionization (ESI). The measurements were done in a positive ion mode (interface capillary voltage—4500 V) or in a negative ion mode (3200 V); mass range from m/z 50 to m/z 3000 Da; external or internal calibration was done with electrospray calibrant solution (Fluka). A syringe injection was used for solutions in acetonitrile or methanol (flow rate 3 mL min−1). Nitrogen was applied as a dry gas; the interface temperature was set at 180 °C. 1H NMR and 13C NMR spectra were recorded on a Bruker AM300 spectrometer operating at 300.13 MHz and 75.5 MHz, respectively, Bruker DRX 500 spectrometer (500.13 MHz and 125.76 MHz, respectively) and Bruker DRX 600 spectrometer (Bruker, USA; 600.13 MHz and 150.90 MHz, respectively) using DMSO-d6 as a solvent. Chemical shifts (δ) are given in ppm from TMS as an internal standard. All reactions were run in air.
Column chromatography was carried out with silica gel (Acros Silica gel, for column chromatography, 60 Angstrom pore size, 0.040–0.063 mm) and to monitor the preparative separations, analytical thin layer chromatography (TLC) was performed at room temperature on pre-coated 0.25 mm thick silica gel 60 F254 aluminum plates 20 × 20 cm Merck, Darmstadt, Germany. Propanol-2 was used as eluent without preliminary purification.
The X-ray data collection for samples 3a and 7c were performed on a Bruker APEX DUO diffractometer equipped with an Apex II CCD detector (graphite monochromator, ω-scans), and for sample 4e on a Bruker Quest D8 diffractometer equipped with a Photon-III area-detector (graphite monochromator, shutterless φ- and ω-scan technique), using Mo Kα-radiation (λ = 0.71073 Å).
The intensity data were integrated by the Bruker SAINT software package [31] and were corrected for absorption and decay using the SADABS program [32]. The structures were solved using the SHELXT program [33] and refined by the full-matrix least-squares technique against F2hkl in anisotropic approximation for non-hydrogen atoms with the SHELXL program [34]. Hydrogen atoms connected to heteroatoms were found from difference Fourier synthesis and refined isotropically. Other hydrogen atoms were placed in calculated positions and refined in the riding model with Uiso(H) = 1.5Ueq(Cm) for methyl groups and 1.2Ueq(Ci) for other carbon atoms to which corresponding H atoms are bonded. The structure 4e was refined as an inversion twin with calculated BASF parameter equal to 0.58. The cyclohexyl fragment in 4e was found to be disordered by two positions with refined relative occupancies 0.68:0.32, ISOR instruction was used for the disordered atoms. For 4e a SQUEEZE method implemented in the PLATON program [35] was applied to model the disordered methanol molecule.
Detailed crystallographic information is given in Table S1. CCDC 2215817-2215819 contain supplementary crystallographic data for this paper. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures (accessed on 20 October 2022).
3.2. General Procedure for the Preparation of Dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-4′,3′′-indole] 3a–e and 4a–e
Dipolarophile 1a–d (0.5 mmol), paraformaldehyde (0.060 g, 2.0 mmol) and N-alkylglycine 1a,b (2.0 mmol) were charged in a flask and acetonitrile (40 mL) was added. The resulting suspension was refluxed with stirring for 10–14 h until the red color disappeared. The solvent was then removed under reduced pressure to afford a solid product. This solid was dissolved in isopropyl alcohol (10 mL) and separated via column chromatography using isopropyl alcohol as eluent. Anti-diastereomers 3a–e have a retention factor Rf = 0.20–0.39, syn-diastereomers 4a–e have a retention factor Rf = 0.43–0.60.
(3aR*,4′R*,6S*,9aS*)-1,1′,1″,3-Tetramethyl-3,3a,9,9a-tetrahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-4′,3″-indoline]-2,2″,7(1H)-trione (3a). Yield 95 mg (43%); Rf = 0.21; white solid; mp: 280–282 °C. IR (KBr): ν 3414 (NH), 3065, 3055 (ArH), 2936, 2861 (Alk), 1700, 1653, 1644, 1611 (C=O, C=N) cm−1. 1H NMR (500 MHz, DMSO-d6): δ 2.45 (s, 3H, NCH3), 2.49 (s, 3H, 1′-NCH3), 2.59 (s, 3H, NCH3), 3.11 (d, J = 10.2 Hz, 1H, CH2), 3.14 (s, 3H, 1′′-NCH3), 3.40 (d, J = 10.2 Hz, 1H, CH2), 3.48 (d, J = 9.9 Hz, 1H, CH2), 3.74 (d, J = 10.5 Hz, 1H, CH2), 4.56–4.60 (m, 2H, 3a-H, 9a-H), 6.75 (d, J = 1.8 Hz, 1H, 9-NH), 6.98–7.03 (m, 2H, 5′′-H, 7′′-H), 7.09 (d, J = 7.2 Hz, 1H, 4′′-H), 7.34 (t, J = 6.9 Hz, 1H, 6′′-H). 13C NMR (75 MHz, DMSO-d6): δ 26.07, 26.64, 27.54 (1,1′′,3-NCH3), 42.03 (1′-NCH3), 60.04, 60.20 (C-3′, C-3′′), 61.89, 63.96 (C-2′, C-5′), 64.73, 65.54 (C-3a, C-9a), 109.12 (C-7′′), 122.54, 123.14, 123.88, 129.54 (C-3a′′, C-4′′, C-5′′, C-6′′), 144.41 (C-7a′′), 148.60 (4a-C=N), 158.26 (2-C=O), 168.50 (7-C=O), 176.12 (2′′-C=O). HRMS (ESI): Calculated for C20H23N7O3S [M + H]+: 442.1647, Found: 442.1656.
(3aR*,4′R*,6S*,9aS*)-1,3-Diethyl-1′,1″-dimethyl-3,3a,9,9a-tetrahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-4′,3″-indoline]-2,2″,7(1H)-trione (3b). Yield 73 mg (31%); Rf = 0.24; white solid; mp: 252–254 °C. IR (KBr): ν 3245, 3163 (NH), 3063, 3015 (ArH), 2967, 2873, 2876 (Alk), 1756, 1676, 1682, 1635 (C=O, C=N) cm−1. 1H NMR (500 MHz, DMSO-d6): δ 0.88 (t, J = 6.9 Hz, 3H, CH3), 1.01 (t, J = 7.2 Hz, 3H, CH3), 2.48 (s, 3H, 1′-NCH3), 2.85–2.99 (m, 3H, NCH2), 3.10 (d, J = 10.2 Hz, CH2), 3.13 (s, 3H, 1′′-NCH3), 3.16–3.23 (m, 1H, NCH2), 3.41 (d, J = 10.5 Hz, 1H, CH2), 3.50 (d, J = 10.1 Hz, 1H, CH2), 3.73 (d, J = 10.4 Hz, 1H, CH2), 4.68 (d, J = 5.8 Hz, 1H, 3a-H), 4.72 (d, J = 5.6 Hz, 1H, 9a-H), 6.73 (s, 1H, 9-NH), 6.96–7.02 (m, 2H, 5′′-H, 7′′-H), 7.13 (d, J = 7.4 Hz, 1H, 4′′-H), 7.33 (t, J = 7.7 Hz, 1H, 6′′-H). 13C NMR (150 MHz, DMSO-d6): δ 13.43, 14.45 (2CH3), 27.28 (1′′-NCH3), 35.00, 35.85 (1,3-NCH2), 43.24 (1′-NCH3), 60.98, 61.56 (C-3′, C-3′′), 63.37 (C-2′, C-5′), 64.39, 65.88 (C-3a, C-9a), 110.28 (C-7′′), 123.89, 124.58, 125.58, 130.69 (C-3a′′, C-4′′, C-5′′, C-6′′), 145.62 (4a-C=N), 149.93 (C-7a′′), 158.58 (2-C=O), 169.61 (7-C=O), 177.43 (2′′-C=O). HRMS (ESI): Calculated for C22H27N7O3S [M + H]+: 470.1974, Found: 470.1969.
(3aR*,4′S*,6R*,9aR*)-1,1′,1″,3-Tetramethyl-2-thioxo-1,2,3,3a,9,9a-hexahydro-7H-dispro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-4′,3″-indoline]-2″,7-dione (3c). Yield 80 mg (35%); Rf = 0.20; white solid; mp: 228–230 °C. IR (KBr): ν 3513 (NH), 3024 (ArH), 2966, 2927, 2849 (Alk), 1733, 1708, 1633, 1615 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 2.47 (s, 3H, 1′-NCH3), 2.89 (s, 3H, NCH3), 2.99 (s, 3H, NCH3), 3.10–3.14 (m, 4H, 1′′-NCH3, CH2), 3.37 (d, J = 10.8 Hz, 1H, CH2), 3.46 (d, J = 9.9 Hz, 1H, CH2), 3.70 (d, J = 10.5 Hz, 1H, CH2), 4.82–4.89 (m, 2H, 3a-H, 9a-H), 7.03–7.07 (m, 3H, 5′′-H, 7′′-H, 9-NH), 7.17 (d, J = 7.5 Hz, 1H, 4′′-H), 7.37 (t, J = 7.5 Hz, 1H, 6′′-H). 13C NMR (75 MHz, DMSO-d6): δ 26.07 (1′′-NCH3), 30.80, 31.05 (1,3-NCH3), 41.92 (1′-NCH3), 60.23, 60.43 (C-3′, C-3′′), 61.60, 63.02 (C-2′, C-5′), 66.79, 67.84 (C-3a, C-9a), 108.96 (C-7′′), 122.98, 123.11, 124.27, 129.65 (C-3a′′, C-4′′, C-5′′, C-6′′), 144.53 (C-7a′′), 149.30 (4a-C=N), 167.82 (7-C=O), 175.96 (2′′-C=O), 182.69 (2-C=S). HRMS (ESI): Calculated for C20H23N7O2S2 [M + H]+: 458.1427, Found: 458.1427.
(3aR*,4′S*,6R*,9aR*)-1,3-Diethyl-1′,1″-dimethyl-2-thioxo-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-4′,3″-indoline]-2″,7-dione (3d). Yield 80 mg (33%); Rf = 0.25; white solid; mp: 155–157 °C. IR (KBr): ν 3433 (NH), 3233 (ArH), 2973, 2939, 2876 (Alk), 1707, 1641, 1613 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6) δ 0.92 (t, J = 7.0 Hz, 3H, CH3), 1.07 (t, J = 7.0 Hz, 3H, CH3), 2.49 (s, 3H, 1′-NCH3), 3.10–3.32 (m, 6H, 1′′-NCH3, CH2), 3.41–3.51 (m, 3H, CH2), 3.71–3.81 (m, 2H, CH2), 4.88 (d, J = 6.7 Hz, 1H, 3a-H), 5.03 (dd, J = 6.7, 2.4 Hz, 1H, 9a-H), 6.85 (d, J = 2.3 Hz, 1H, 9-NH), 6.95–7.02 (m, 2H, 5′′-H, 7′′-H), 7.12 (d, J = 7.5 Hz, 1H, 4′′-H), 7.33 (t, J = 7.6 Hz, 1H, 6′′-H). 13C NMR (75 MHz, DMSO-d6): δ 11.63, 12.84 (2CH3), 26.07 (1′′-NCH3), 37.63, 37.88 (1,3-NCH2), 42.02 (1′-NCH3), 59.70, 60.33 (C-3′, C-3′′), 62.06, 63.82, 64.56, 65.84 (2CH2, C-3a, C-9a), 109.09 (C-7′′), 122.74, 123.20, 124.28, 129.51 (C-3a′′, C-4′′, C-5′′, C-6′′), 144.34 (4a-C=N), 149.99 (C-7a′′), 168.24 (7-C=O), 176.16 (2′′-C=O), 180.72 (2-C=S). HRMS (ESI): Calculated for C22H27N7O2S2 [M + H]+: 486.1729, Found: 486.1740.
(3aR*,4′R*,6S*,9aS*)-1′-Cyclohexyl-9-((cyclohexyl(methyl)amino)methyl)-1,1″,3-trimethyl-3,3a,9,9a-tetrahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-4′,3″-indoline]-2,2″,7(1H)-trione (3e). Yield 110 mg (35%); Rf = 0.39; white solid; mp: 179–181 °C. IR (KBr): ν 2930, 2854 (Alk), 1709, 1641, 1610 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 1.14–1.28 (m, 10H, Cy), 1.52–1.83 (m, 10H, Cy), 2.36–2.41 (m, 4H, NCH3, Cy), 2.47 (s, 3H, NCH3), 2.60–2.63 (m, 4H, NCH3, Cy), 3.15 (s, 3H, 1′′-NCH3), 3.20 (d, J = 9.9 Hz, 1H, CH2), 3.47–3.57 (m, 2H, CH2), 3.72 (d, J = 12.7 Hz, 1H, 9-NCH2), 3.79 (d, J = 10.0 Hz, 1H, CH2), 4.05 (d, J = 12.7 Hz, 1H, 9-NCH2), 4.70 (d, J = 5.8 Hz, 1H, 9a-H), 4.76 (d, J = 5.7 Hz, 1H, 3a-H), 6.99–7.05 (m, 2H, 5′′-H, 7′′-H), 7.11 (d, J = 7.4 Hz, 1H, 4′′-H), 7.35 (t, J = 7.6 Hz, 1H, 6′′-H). 13C NMR (75 MHz, DMSO-d6): δ 23.78, 23.93, 25.49, 25.59, 26.01, 26.61, 27.41, 28.26, 28.92, 30.88, 31.31, 36.23 (4NCH3, Cy), 57.40, 58.80, 59.29, 59.74, 60.17, 62.06, 63.46, 68.70, 72.42 (C-2′, C-3′, C-3′′, C-5′, C-3a, C-9a, 9-NCH2, 2Cy), 109.00 (C-7′′), 122.43, 123.33, 124.09, 129.47 (C-3a′′, C-4′′, C-5′′, C-6′′), 144.41 (C-7a′′), 147.85 (4a-C=N), 157.80 (2-C=O), 167.59 (7-C=O), 175.97 (2′′-C=O). HRMS (ESI): Exact mass calcd for C33H46N8O3S [M + H]+: 635.3486, Found: 635.3497.
(3aR*,4′S*,6R*,9aS*)-1,1′,1″,3-Tetramethyl-3,3a,9,9a-tetrahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-4′,3″-indoline]-2,2″,7(1H)-trione (4a). Yield 92 mg (42%); Rf = 0.53; white solid; mp: 164–166 °C. IR (KBr): ν 3468, 3188 (NH), 3053, 3026 (ArH), 2934, 2854 (Alk), 1716, 1700, 1644 (C=O, C=N) cm−1. 1H NMR (500 MHz, DMSO-d6): δ 2.47 (s, 3H, 1′-NCH3), 2.57 (s, 3H, NCH3), 2.64 (s, 3H, NCH3), 3.11 (d, J = 10.2 Hz, 1H, CH2), 3.14 (s, 3H, 1′′-NCH3), 3.40 (d, J = 10.7 Hz, 1H, CH2), 3.47 (d, J = 10.2 Hz, 1H, CH2), 3.71 (d, J = 10.7 Hz, 1H, CH2), 4.52 (dd, J = 5.7, 2.5 Hz, 1H, 9a-H), 4.60 (d, J = 5.8 Hz, 1H, 3a-H), 6.83 (d, J = 2.1 Hz, 1H, 9-NH), 7.05–7.06 (m, 2H, 5′′-H, 7′′-H), 7.17 (d, J = 7.4 Hz, 1H, 4′′-H), 7.37 (t, J = 7.6 Hz, 1H, 6′′-H). 13C NMR (75 MHz, DMSO-d6): δ 26.07, 26.71, 27.69 (1,1′′,3-NCH3), 41.96 (1′-NCH3), 60.42, 61.67, 63.20 (C-2′, C-3′, C-3′′, C-5′), 65.03, 65.64 (C-3a, C-9a), 108.96 (C-7′′), 123.06, 123.10, 124.29, 129.66 (C-3a′′, C-4′′, C-5′′, C-6′′), 144.58 (C-7a′′), 148.24 (4a-C=N), 158.62 (2-C=O), 167.97 (7-C=O), 176.05 (2′′-C=O). HRMS (ESI): Calculated for C20H23N7O3S [M + H]+: 442.1665, Found: 442.1656.
(3aR*,4′S*,6R*,9aS*)-1,3-Diethyl-1′,1″-dimethyl-3,3a,9,9a-tetrahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-4′,3″-indoline]-2,2″,7(1H)-trione (4b). Yield 70 mg (30%); Rf = 0.60; white solid; mp: 217–219 °C. IR (KBr): ν 3495, 3175 (NH), 3057 (ArH), 2980, 2959, 2935, 2898, 2874 (Alk), 1738, 1709, 1693, 1634, 161 (C=O, C=N) cm−1. 1H NMR (500 MHz, DMSO-d6): δ 0.95 (t, J = 7.1 Hz, 3H, CH3), 1.04 (t, J = 7.2 Hz, 3H, CH3), 2.47 (s, 3H, 1′-NCH3), 2.93–3.07 (m, 2H, NCH2), 3.10–3.17 (m, 5H, 1′′-NCH3, CH2, NCH2), 3.21–3.30 (m, 2H, CH2, NCH2), 3.49 (d, J = 10.2 Hz, 1H, CH2), 3.73 (d, J = 10.6 Hz, 1H, CH2), 4.63–4.68 (m, 2H, 3a-H, 9a-H), 6.85 (d, J = 2.2 Hz, 1H, 9-NH), 7.04–7.07 (m, 2H, 5′′-H, 7′′-H), 7.18 (d, J = 7.3 Hz, 1H, 4′′-H), 7.37 (t, J = 7.7 Hz, 1H, 6′′-H). 13C NMR (125 MHz, DMSO-d6): δ 13.22, 13.28 (2CH3), 26.15 (1′′-NCH3), 34.25, 35.29 (1,3-NCH2), 42.07 (1′-NCH3), 60.36, 60.57 (C-3′, C-3′′), 61.76, 63.00, 63.53, 64.35 (C-2′, C-3a, C-5′, C-9a), 109.05 (C-7′′), 123.11, 123.20, 124.45, 129.75 (C-3a′′, C-4′′, C-5′′, C-6′′), 144.69 (4a-C=N), 148.17 (C-7a′′), 157.84 (2-C=O), 168.00 (7-C=O), 176.14 (2′′-C=O). HRMS (ESI): Calculated for C22H27N7O3S [M + H]+: 470.1965, Found: 470.1969.
(3aR*,4′R*,6S*,9aR*)-1,1′,1″,3-Tetramethyl-2-thioxo-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-4′,3″-indoline]-2″,7-dione (4c). Yield 87 m (38%); Rf = 0.43; white solid; mp: 273–275 °C. IR (KBr): ν 3399 (NH), 3125 (ArH), 2936, 2921, 2855 (Alk), 1726, 1707, 1603, 1609 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 2.51 (s, 3H, 1′-NCH3), 2.79 (s, 3H, NCH3), 2.94 (s, 3H, NCH3), 3.12–3.16 (m, 4H, CH2, 1′′-NCH3), 3.42 (d, J = 10.5 Hz, 1H, CH2), 3.49 (d, J = 10.2 Hz, 1H, CH2), 3.75 (d, J = 10.5 Hz, 1H, CH2), 4.85 (d, J = 6.3 Hz, 1H, 3a-H), 4.92 (d, J = 6.3 Hz, 1H, 9a-H), 6.95 (s, 1H, 9-NH), 7.02–7.11 (m, 3H, 4′′-H, 5′′-H, 7′′-H), 7.35 (t, J = 7.5 Hz, 1H, 6′′-H). 13C NMR (75 MHz, DMSO-d6): δ 26.06 (1′′-NCH3), 30.62, 30.95 (1,3-NCH3), 41.98 (1′-NCH3), 59.98, 60.15 (C-3′, C-3′′), 61.80, 63.88 (C-2′, C-5′), 66.41, 67.88 (C-3a, C-9a), 109.11 (C-7′′), 122.62, 123.01, 123.72, 129.54 (C-3a′′, C-4′′, C-5′′, C-6′′), 144.34 (C-7a′′), 149.80 (4a-C=N), 168.35 (7-C=O), 176.05 (2′′-C=O), 182.36 (2-C=S). HRMS (ESI): Calculated for C20H23N7O2S2 [M + H]+: 458.1426, Found: 458.1427.
(3aR*,4′R*,6S*,9aR*)-1,3-Diethyl-1′,1″-dimethyl-2-thioxo-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-4′,3″-indoline]-2″,7-dione (4d). Yield 73 mg (30%); Rf = 0.54; white solid; mp: 224–226 °C. IR (KBr): ν 3433 (NH), 3261, 3210 (ArH), 2969, 2932, 2858 (Alk), 1728, 1701, 1641, 1612 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.99 (t, J = 6.9 Hz, 3H, CH3), 1.11 (t, J = 7.1 Hz, 3H, CH3), 2.46 (s, 3H, 1′-NCH3), 3.10–3.14 (m, 4H, 1′′-NCH3, CH2), 3.19–3.34 (m, 2H, NCH2, CH2), 3.41–3.56 (m, 3H, NCH2, CH2), 3.71–3.83 (m, 2H, NCH2, CH2), 4.88 (d, J = 6.6 Hz, 1H, 3a-H), 4.96 (dd, J = 6.7, 2.7 Hz, 1H, 9a-H), 7.03–7.07 (m, 3H, 5′′-H, 7′′-H, 9-NH), 7.18 (d, J = 7.6 Hz, 1H, 4′′-H), 7.37 (t, J = 7.7 Hz, 1H, 6′′-H). 13C NMR (75 MHz, DMSO-d6): δ 12.30, 12.92 (2CH3), 26.14 (1′′-NCH3), 38.24, 38.34 (1,3-NCH2), 42.04 (1′-NCH3), 60.36 (C-3′, C-3′′), 61.67, 63.30 (C-2′, C-5′), 64.73, 66.71 (C-3a, C-9a), 109.06 (C-7′′), 123.00, 123.19, 124.43, 129.79 (C-3a′′, C-4′′, C-5′′, C-6′′), 144.62 (4a-C=N), 149.24 (C-7a′′), 167.87 (7-C=O), 176.06 (2′′-C=O), 181.23 (2-C=S). HRMS (ESI): Calculated for C22H27N7O2S2 [M + H]+: 486.1749, Found: 486.1740.
(3aR*,4′S*,6R*,9aS*)-1′-Cyclohexyl-1,1″,3-trimethyl-3,3a,9,9a-tetrahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-4′,3″-indoline]-2,2″,7(1H)-trione (4e). Yield 49 mg (24%); Rf = 0.56; white solid; mp: 217–219 °C. IR (KBr): ν 3434, 3175 (NH), 2931, 2854 (Alk), 1706, 1638, 1612 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 1.12–1.24 (m, 5H, Cy), 1.48–1.52 (m, 1H, Cy), 1.61–1.70 (m, 2H, Cy), 1.76–1.81 (m, 2H, Cy), 2.56–2.59 (m, 4H, NCH3, Cy), 2.65 (s, 3H, NCH3), 3.14 (s, 3H, 1′′-NCH3), 3.19 (d, J = 10.0 Hz, 1H, CH2), 3.42–3.50 (m, 2H, CH2), 3.72 (d, J = 10.3 Hz, 1H, CH2), 4.52 (dd, J = 5.9, 2.5 Hz, 1H, 9a-H), 4.60 (d, J = 5.8 Hz, 1H, 3a-H), 6.80 (d, J = 2.5 Hz, 1H, 9-NH), 7.02–7.06 (m, 2H, 5′′-H, 7′′-H), 7.17 (d, J = 7.2 Hz, 1H, 4′′-H), 7.36 (t, J = 7.4 Hz, 1H, 6′′-H). 13C NMR (75 MHz, DMSO-d6): δ 24.45, 24.59, 26.15, 26.57, 27.21, 28.26, 31.45, 31.89 (3NCH3, Cy), 57.77, 59.70, 59.88, 60.10, 61.09, 65.46, 66.14 (C-2′, C-3′, C-3′′, C-5′, C-3a, C-9a, Cy), 109.41 (C-7′′), 123.50, 123.86, 124.84, 130.11 (C-3a′′, C-4′′, C-5′′, C-6′′), 145.11 (C-7a′′), 148.85 (4a-C=N), 159.13 (2-C=O), 168.39 (7-C=O), 176.38 (2′′-C=O). HRMS (ESI): Calculated for C25H31N7O3S [M + H]+: 510.2282, Found: 510.2274.
3.3. General Procedure for the Preparation of Dispiro[imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine-7,3′-pyrrolidine-4′,3″-indole] 6a–e and 7a–e
Method A. Dipolarophile 5a–d (0.5 mmol), paraformaldehyde (0.060 g, 2.0 mmol) and N-alkylglycine 1a,b (2.0 mmol) were charged in a flask and acetonitrile (40 mL) was added. The resulting suspension was refluxed with stirring for 10–16 h until the red color disappeared. The solvent was then removed under reduced pressure to afford a solid product. This solid was dissolved in isopropyl alcohol (10 mL) and separated via column chromatography using isopropyl alcohol as eluent. Anti-diastereomers 6a–e have a retention factor Rf = 0.35–0.54, syn-diastereomers 7a–e have a retention factor Rf = 0.58–0.73.
Method B. To a stirred suspension of compound 3a (0.442 g, 0.1 mmol) or 4a (0.442 g, 0.1 mmol) in refluxing methanol (2 mL), 0.010 mL of 40% aqueous KOH (0.1 mmol) was added. The resulting mixture was refluxed with stirring for 1 h. After cooling, the precipitate of compounds 7a or 6a was filtered off, washed with methanol and dried.
(3aR*,4′R*,7S*,9aS*)-1,1′,1″,3-Tetramethyl-1,3a,4,9a-tetrahydro-8H-dispiro[imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine-7,3′-pyrrolidine-4′,3″-indoline]-2,2″,8(3H)-trione (6a). Method A: yield 97 mg (44%); method B: yield 41 mg (92%); Rf = 0.35; white solid; mp: 218–220 °C. IR (KBr): ν 3414 (NH), 3429, 3310 (ArH), 2967, 2941, 2853 (Alk), 1727, 1684, 1651 (C=O, C=N) cm−1. 1H NMR (500 MHz, DMSO-d6): δ 2.46 (s, 3H, NCH3), 2.47 (s, 3H, 1′-NCH3), 2.90 (s, 3H, NCH3), 3.10 (d, J = 10.2 Hz, 1H, CH2), 3.13 (s, 3H, 1′′-NCH3), 3.44–3.47 (m, 2H, CH2), 3.65 (d, J = 10.6 Hz, 1H, CH2), 4.54 (dd, J = 5.6, 2.0 Hz, 1H, 3a-H), 5.36 (d, J = 5.7 Hz, 1H, 9a-H), 6.98 (t, J = 7.5 Hz, 1H, 5′′-H), 7.03 (d, J = 7.8 Hz, 1H, 7′′-H), 7.19 (d, J = 7.5 Hz, 1H, 4′′-H), 7.33 (t, J = 7.7 Hz, 1H, 6′′-H), 7.47 (d, J = 1.9 Hz, 1H, 4-NH). 13C NMR (75 MHz, DMSO-d6): δ 26.02, 27.87, 31.62 (1,1′′,3-NCH3), 41.88 (1′-NCH3), 60.29, 61.67, 62.54, 63.77, 64.77, 66.24 (C-2′, C-3a, C-3′, C-3′′, C-5′, C-9a), 108.88 (C-7′′), 122.43, 123.52, 124.66, 129.50 (C-3a′′, C-4′′, C-5′′, C-6′′), 134.15 (5a-C=N), 144.42 (C-7a′′), 159.25 (2-C=O), 171.97 (8-C=O), 176.25 (2′′-C=O). HRMS (ESI): Calculated for C20H23N7O3S [M + H]+: 442.1655, Found: 442.1656.
(3aR*,4′R*,7S*,9aS*)-1,3-Diethyl-1′,1″-dimethyl-1,3a,4,9a-tetrahydro-8H-dispiro[imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine-7,3′-pyrrolidine-4′,3″-indoline]-2,2″,8(3H)-trione (6b). Yield 101 mg (43%); Rf = 0.38; white solid; mp: 205–207 °C. IR (KBr): ν 3287 (NH), 3070 (ArH), 2972, 2939, 2876 (Alk), 1717, 1655, 1613 (C=O, C=N) cm−1. 1H NMR (500 MHz, DMSO-d6): δ 0.89 (t, J = 7.1 Hz, 3H, CH3), 1.09 (t, J = 7.0 Hz, 3H, CH3), 2.46 (s, 3H, 1′-NCH3), 2.85–2.90 (m, 1H, NCH2), 3.07–3.14 (m, 6H, 1′′-NCH3, CH2, NCH2), 3.43–3.50 (m, 3H, CH2, NCH2), 3.63 (d, J = 10.5 Hz, 1H, CH2), 4.60 (dd, J = 5.5, 1.9 Hz, 1H, 3a-H), 5.44 (d, J = 5.5 Hz, 1H, 9a-H), 6.98–7.04 (m, 2H, 5′′-H, 7′′-H), 7.18 (d, J = 7.5 Hz, 1H, 4′′-H), 7.33 (t, J = 7.7 Hz, 1H, 6′′-H), 7.43 (d, J = 1.9 Hz, 1H, 4-NH). 13C NMR (150 MHz, DMSO-d6): δ 13.69, 13.87 (2CH3), 27.27 (1′′-NCH3), 35.83, 38.61 (1,3-NCH2), 43.07 (1′-NCH3), 61.56, 62.41, 63.01, 63.88, 64.67, 66.15 (C-2′, C-3a, C-3′, C-3′′, C-5′, C-9a), 110.05 (C-7′′), 123.80, 124.91, 125.92, 130.68 (C-3a′′, C-4′′, C-5′′, C-6′′), 135.19 (5a-C=N), 145.64 (C-7a′′), 159.11 (2-C=O), 173.22 (8-C=O), 177.52 (2′′-C=O). HRMS (ESI): Calculated for C22H27N7O3S [M + H]+: 470.1967, Found: 470.1969.
(3aR*,4′R*,7S*,9aR*)-1,1′,1″,3-Tetramethyl-2-thioxo-1,2,3,3a,4,9a-hexahydro-8H-dispiro[imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine-7,3′-pyrrolidine-4′,3″-indoline]-2″,8-dione (6c). Yield 84 mg (37%); Rf = 0.41; white solid; mp: 228–231 °C. IR (KBr): ν 3436 (NH), 3027 (ArH), 2969, 2945, 2916, 2798 (Alk), 1727, 1703, 1653, 1612 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 2.47 (s, 3H, CH3), 2.78 (s, 3H, CH3), 3.10–3.17 (m, 7H, 2CH3, CH2), 3.45–3.52 (m, 2H, CH2), 3.66 (d, J = 10.4 Hz, 1H, CH2), 4.85 (d, J = 5.9 Hz, 1H, 3a-H), 5.57 (d, J = 5.6 Hz, 1H, 9a-H), 7.02–7.08 (m, 2H, 5′′-H, 7′′-H), 7.19 (d, J = 7.2 Hz, 1H, 4′′-H), 7.33 (t, J = 7.5 Hz, 1H, 6′′-H), 7.66 (s, 1H, 4-NH). 13C NMR (75 MHz, DMSO-d6): δ 26.07 (1′′-NCH3), 31.46, 35.53 (1,3-NCH3), 41.90 (1′-NCH3), 60.19, 61.76, 62.57, 65.01 (C-2′, C-3′,C-3′′, C-5′), 66.71, 67.53 (C-3a), C-9a), 108.95 (C-7′′), 122.93, 123.55, 124.69, 129.47 (C-3a′′, C-4′′, C-5′′, C-6′′), 134.68 (5a-C=N), 144.34 (C-7a′′), 171.65 (8-C=O), 176.20 (2′′-C=O), 184.33 (2-C=S). HRMS (ESI): Calculated for C20H23N7O2S2 [M + H]+: 458.1427, Found: 458.1427.
(3aS*,3′R*,3′′S*,9aS*)-1,3-Diethyl-1′,1′′-dimethyl-2-thioxo-1,3,3a,4,9,9a-hexahydrodispiro[imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine-7,3′-pyrrolidine-4′,3′′-indole]-2′′,8(1′′H)-dione (6d). Yield 102 mg (42%); Rf = 0.44; white solid; mp: 225–227 °C. IR (KBr): ν 3327 (NH), 3069, 3055 (ArH), 2967, 2936, 2884, 2829, 2795 (Alk), 1707, 1640, 1612 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.96 (t, J = 7.0 Hz, 3H, CH3), 1.13 (t, J = 7.0 Hz, 3H, CH3), 2.46 (s, 3H, 1′-NCH3), 3.08–3.26 (m, 6H, 1′′-NCH3, NCH2, CH2), 3.42–3.51 (m, 2H, CH2), 3.61–3.66 (m, 2H, CH2, NCH2), 3.93–3.99 (m, 1H, NCH2), 4.85 (d, J = 5.6 Hz, 1H, 3a-H), 5.61 (d, J = 5.4 Hz, 1H, 9a-H), 7.02–7.10 (m, 2H, 5′′-H, 7′′-H), 7.19 (d, J = 6.9 Hz, 1H, 4′′-H), 7.33 (t, J = 6.3 Hz, 1H, 6′′-H), 7.60 (d, J = 1.9 Hz, 1H, 4-NH). 13C NMR (75 MHz, DMSO-d6): δ 11.84, 11.91 (2CH3), 26.07 (1′′-NCH3), 38.13, 40.13 (1,3-NCH2), 41.77 (1′-NCH3), 60.26, 61.99, 62.75, 63.76, 64.97, 65.33 (C-2′, C-3′, C-3′′, C-3a, C-5′, C-9a), 108.88 (C-7′′), 122.95, 123.85, 124.74, 129.41 (C-3a′′, C-4′′, C-5′′, C-6′′), 134.28 (5a-C=N), 144.32 (C-7a′′), 171.68 (8-C=O), 176.24 (2′′-C=O), 182.04 (2-C=S). HRMS (ESI): Calculated for C22H27N7O2S2 [M + H]+: 486.1733, Found: 486.1740.
(3aR*,4′R*,7S*,9aS*)-1′-Cyclohexyl-1,1″,3-trimethyl-1,3a,4,9a-tetrahydro-8H-dispiro[imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine-7,3′-pyrrolidine-4′,3″-indoline]-2,2″,8(3H)-trione (6e). Yield 117 mg (46%); Rf = 0.54; white solid; mp: 244–245 °C. IR (KBr): ν 3435, 3306 (NH), 2931, 2854 (Alk), 1711, 1651, 1611, 1577 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 1.18–1.27 (m, 5H, Cy), 1.49–1.53 (m, 1H, Cy), 1.67–1.71 (m, 2H, Cy), 1.80–1.84 (m, 2H, Cy), 2.49 (s, 3H, NCH3), 2.57–2.60 (m, 1H, Cy), 2.92 (s, 3H, NCH3), 3.15 (s, 3H, 1′′-NCH3), 3.19 (d, J = 9.9 Hz, 1H, CH2), 3.50 (d, J = 10.0 Hz, 1H, CH2), 3.59 (d, J = 10.4 Hz, 1H, CH2), 3.68 (d, J = 10.3 Hz, 1H, CH2), 4.55 (d, J = 4.4 Hz, 1H, 3a-H), 5.38 (d, J = 5.7 Hz, 1H, 9a-H), 6.97–7.05 (m, 2H, 5′′-H, 7′′-H), 7.23 (d, J = 7.5 Hz, 1H, 4′′-H), 7.34 (t, J = 7.8 Hz, 1H, 6′′-H). 7.47 (s, 1H, 4-NH). 13C NMR (125 MHz, DMSO-d6): δ 23.99, 24.11, 25.74, 26.12, 27.96, 31.05, 31.52, 31.74 (3NCH3, Cy), 57.46, 59.21, 60.60, 60.65, 61.93, 63.88, 66.38 (C-2′, C-3′, C-3′′, C-5′, C-3a, C-9a, Cy), 108.95 (C-7′′), 122.52, 123.97, 124.84, 129.58 (C-3a′′, C-4′′, C-5′′, C-6′′), 134.42 (5a-C=N), 144.56 (C-7a′′), 159.38 (2-C=O), 171.88 (8-C=O), 176.29 (2′′-C=O). HRMS (ESI): Calculated for C25H31N7O3S [M + H]+: 510.2282, Found: 510.2277.
(3aR*,4′S*,7R*,9aS*)-1,1′,1″,3-Tetramethyl-1,3a,4,9a-tetrahydro-8H-dispiro[imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine-7,3′-pyrrolidine-4′,3″-indoline]-2,2″,8(3H)-trione (7a). Method A: yield 79 mg (36%); method B: yield 35 mg (80%); Rf = 0.58; white solid; mp: 264–266 °C. IR (KBr): ν 3357 (NH), 3065, 3025 (ArH), 2966, 2934, 2894, 2851 (Alk), 1736, 1717, 1704, 1646 (C=O, C=N) cm−1. 1H NMR (500 MHz, DMSO-d6): δ 2.49 (s, 3H, NCH3), 2.50 (s, 3H, NCH3), 2.91 (s, 3H, NCH3), 3.10 (d, J = 10.2 Hz, 1H, CH2), 3.14 (s, 3H, 1′′-NCH3), 3.41 (d, J = 10.7 Hz, 1H, CH2), 3.45 (d, J = 10.3 Hz, 1H, CH2), 3.66 (d, J = 10.7 Hz, 1H, CH2), 4.59 (d, J = 5.6 Hz, 1H, 3a-H), 5.34 (d, J = 5.6 Hz, 1H, 9a-H), 7.03–7.06 (m, 2H, 5′′-H, 7′′-H), 7.16 (d, J = 7.4 Hz, 1H, 4′′-H), 7.36 (t, J = 7.7 Hz, 1H, 6′′-H), 7.45 (s, 1H, 4-NH). 13C NMR (125 MHz, DMSO-d6): δ 26.16, 27.68, 31.35 (1,1′′,3-NCH3), 42.13 (1′-NCH3), 60.44, 61.48, 63.19, 63.67, 65.24, 66.39 (C-2′, C-3a, C-3′, C-3′′, C-5′, C-9a), 109.09 (C-7′′), 122.85, 123.32, 123.93, 129.76 (C-3a′′, C-4′′, C-5′′, C-6′′), 135.13 (5a-C=N), 144.74 (C-7a′′), 158.86 (2-C=O), 172.09 (8-C=O), 176.36 (2′′-C=O). HRMS (ESI): Calculated for C20H23N7O3S [M + H]+: 442.1643, Found: 442.1656.
(3aR*,4′S*,7R*,9aS*)-1,3-Diethyl-1′,1″-dimethyl-1,3a,4,9a-tetrahydro-8H-dispiro[imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine-7,3′-pyrrolidine-4′,3″-indoline]-2,2″,8(3H)-trione (7b). Yield 96 mg (41%); Rf = 0.60; white solid; mp: 252–254 °C. IR (KBr): ν 3325 (NH), 3093, 3053 (ArH), 2988, 2974, 2966, 2936, 2899, 2865, 2837 (Alk), 1707, 1641, 1611 (C=O, C=N) cm−1. 1H NMR (500 MHz, DMSO-d6): δ 0.91 (t, J = 7.1 Hz, 3H, CH3), 1.13 (t, J = 7.1 Hz, 3H, CH3), 2.49 (s, 3H, 1′-NCH3), 2.91–2.95 (m, 1H, NCH2), 3.09–3.15 (m, 5H, 1′′-NCH3, CH2, NCH2), 3.21–3.27 (m, 1H, NCH2), 3.38 (d, J = 10.6 Hz, 1H, CH2), 3.45–3.51 (m, 2H, CH2, NCH2), 3.67 (d, J = 10.6 Hz, 1H, CH2), 4.68 (d, J = 6.6 Hz, 1H, 3a-H), 5.60 (d, J = 6.6 Hz, 1H, 9a-H), 7.04–7.07 (m, 2H, 5′′-H, 7′′-H), 7.17 (d, J = 7.5 Hz, 1H, 4′′-H), 7.35 (t, J = 7.5 Hz, 1H, 6′′-H), 7.43 (s, 1H, 4-NH). 13C NMR (75 MHz, DMSO-d6): δ 12.26, 13.61 (2CH3), 26.02 (1′′-NCH3), 34.46, 37.97 (1,3-NCH2), 41.96 (1′-NCH3), 60.30. 61.33, 62.80, 63.13, 63.33, 64.27 (C-2′, C-3a, C-3′, C-3′′, C-5′, C-9a), 108.91 (C-7′′), 122.72, 123.20, 123.85, 129.60 (C-3a′′, C-4′′, C-5′′, C-6′′), 134.71 (5a-C=N), 144.63 (C-7a′′), 157.75 (2-C=O), 171.73 (8-C=O), 176.25 (2′′-C=O). HRMS (ESI): Calculated for C22H27N7O3S [M + H]+: 470.1960, Found: 470.1969.
(3aR*,4′S*,7R*,9aR*)-1,1′,1″,3-Tetramethyl-2-thioxo-1,2,3,3a,4,9a-hexahydro-8H-dispiro[imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine-7,3′-pyrrolidine-4′,3″-indoline]-2″,8-dione (7c). Yield 101 mg (42%); Rf = 0.66; white solid; mp: 224–226 °C. IR (KBr): ν 3398 (NH), 3112, 3056 (ArH), 2969, 2930, 2838, 2785 (Alk), 1711, 1698, 1636, 1609 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 2.50 (s, 3H, 1′-NCH3), 2.81 (s, 3H, NCH3), 3.10–3.15 (m, 4H, CH2, NCH3), 3.22 (s, 3H, NCH3), 3.38–3.47 (m, 2H, CH2), 3.68 (d, J = 10.7 Hz, 1H, CH2), 4.98 (d, J = 6.7 Hz, 1H, 3a-H), 5.82 (d, J = 6.9 Hz, 1H, 9a-H), 7.03–7.08 (m, 2H, 5′′-H, 7′′-H), 7.17 (d, J = 7.7 Hz, 1H, 4′′-H), 7.37 (t, J = 7.7 Hz, 1H, 6′′-H), 7.66 (s, 1H, 4-NH). 13C NMR (150 MHz, DMSO-d6): δ 27.29 (1′′-NCH3), 32.17, 36.39 (1,3-NCH3), 43.21 (1′-NCH3), 61.54, 62.53, 64.38, 64.78, 68.95, 69.58 (C-2′, C-3′, C-3′′, C-3a, C-9a), 110.22 (C-7′′), 124.00, 124.34, 125.04, 130.92 (C-3a′′, C-4′′, C-5′′, C=6′′), 137.15 (5a-C=N), 145.86 (C-7a′′), 172.81 (8-C=O), 177.41 (2′′-C=O), 184.61 (2-C=S). HRMS (ESI): Calculated for C20H23N7O2S2 [M + H]+:458.1421, Found: 458.1427.
(3aR*,4′S*,7R*,9aR*)-1,3-Diethyl-1′,1″-dimethyl-2-thioxo-1,2,3,3a,4,9a-hexahydro-8H-dispiro[imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine-7,3′-pyrrolidine-4′,3″-indoline]-2″,8-dione (7d). Yield 107 mg (44%); Rf = 0.67; white solid; mp: 148–150 °C. IR (KBr): ν 3311 (NH), 3056 (ArH), 2971, 2935, 2867, 2849, 2796 (Alk), 1709, 1648, 1612 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.97 (t, J = 6.6 Hz, 3H, CH3), 1.19 (t, J = 6.9 Hz, 3H, CH3), 2.50 (s, 3H, 1′-NCH3), 3.09–3.15 (m, 4H, 1′′-NCH3, CH2), 3.19–3.29 (m, 1H, CH2), 3.37–3.61 (m, 4H, CH2, NCH2), 3.67 (d, J = 10.5 Hz, 1H, CH2), 3.97–4.04 (m, 1H, NCH2), 5.01 (d, J = 6.9 Hz, 1H, 3a-H), 5.85 (d, J = 6.9 Hz, 1H, 9a-H), 7.04–7.08 (m, 2H, 5′′-H, 7′′-H), 7.17 (d, J = 7.5 Hz, 1H, 4′′-H), 7.37 (t, J = 7.5 Hz, 1H, 6′′-H), 7.64 (d, J = 1.9 Hz, 1H, 4-NH). 13C NMR (75 MHz, DMSO-d6): δ 11.49, 13.08 (2CH3), 26.06 (1′′-NCH3), 37.80, 41.24, 41.97 (1,3-NCH2, 1′-NCH3), 60.30, 61.25, 63.21, 65.57, 66.34 (C-2′, C-3′, C-3′′, C-3a, C-5′, C-9a), 108.97 (C-7′′), 122.81, 123.08, 123.83, 129.67 (C-3a′′, C-4′′, C-5′′, C-6′′), 135.47 (5a-C=N), 144.63 (C-7a′′), 171.35 (8-C=O), 176.18 (2′′-C=O), 181.83 (2-C=S). HRMS (ESI): Calculated for C22H27N7O2S2 [M + H]+: 486.1737, Found: 486.1740.
(3aR*,4′S*,7R*,9aS*)-1′-Cyclohexyl-1,1″,3-trimethyl-1,3a,4,9a-tetrahydro-8H-dispiro[imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine-7,3′-pyrrolidine-4′,3″-indoline]-2,2″,8(3H)-trione (7e). Yield 84 mg (33%); Rf = 0.73; white solid; mp: 155–156 °C. IR (KBr): ν 3371 (NH), 2933, 2852 (Alk), 1729, 1699, 1639, 1612 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 1.19–1.27 (m, 5H, Cy), 1.50–1.54 (m, 1H, Cy), 1.67–1.71 (m, 2H, Cy), 1.79–1.83 (m, 2H, Cy), 2.52 (s, 3H, NCH3), 2.59–2.63 (m, 1H, Cy), 2.92 (s, 3H, NCH3), 3.16 (s, 3H, 1′′-NCH3), 3.20 (d, J = 10.5 Hz, 1H, CH2), 3.45–3.52 (m, 2H, CH2), 3.69 (d, J = 10.5 Hz, 1H, CH2), 3.68 (d, J = 10.3 Hz, 1H, CH2), 4.61 (d, J = 6.4 Hz, 1H, 3a-H), 5.55 (d, J = 6.4 Hz, 1H, 9a-H), 7.03–7.08 (m, 2H, 5′′-H, 7′′-H), 7.19 (d, J = 7.5 Hz, 1H, 4′′-H), 7.37 (t, J = 7.6 Hz, 1H, 6′′-H), 7.46 (s, 1H, 4-NH). 13C NMR (75 MHz, DMSO-d6): δ 23.90, 23.99, 25.65, 26.04, 27.57, 30.88, 31.24, 31.34 (3NCH3, Cy), 56.92, 59.26, 59.56, 60.26, 62.28, 65.13, 66.24 (C-2′, C-3′, C-3′′, C-5′, C-3a, C-9a, Cy), 108.91 (C-7′′), 122.68, 123.60, 123.97, 129.59 (C-3a′′, C-4′′, C-5′′, C-6′′), 135.15 (5a-C=N), 144.67 (C-7a′′), 158.76 (2-C=O), 171.81 (8-C=O), 176.13 (2′′-C=O). HRMS (ESI): Calculated for C25H31N7O3S [M + H]+: 510.2282, Found: 510.2284.
4. Conclusions
In the present study, the synthesis of two types of isomeric dispirocompounds based on imidazothiazolotriazine and pyrrolidineoxindole, differing in the linear and angular structure of imidazothiazolotriazine fragment, was demonstrated. This article has shown that the 1,3-dipolar cycloaddition of azomethine ylides generated in situ from paraformaldehyde and N-alkylglycine derivatives to corresponding oxindolylidene derivatives of imidazothiazolotriazine allows the formation of the desired spirooxindoles of both typs in good yields but low diastereoselectivity. Meanwhile, all individual diastereomers were isolated chromatographically. Hitherto unavailable angular dispiro[imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine-7,3′-pyrrolidine-4′,3″-indolines] can also be prepared via rearrangement of linear dispiro[imidazo[4,5-e]thiazolo- [3,2-b][1,2,4]triazine-6,3′-pyrrolidine-4′,3″-indolines] upon treatment with KOH with high yield and selectivity.
Acknowledgments
X-ray diffraction studies were performed with the financial support of the Ministry of Science and Higher Education of the Russian Federation (Contract/agreement No. 075-00697-22-00) employing the equipment of Center for molecular composition studies of INEOS RAS. High resolution mass spectra were recorded in the Department of Structural Studies of N. D. Zelinsky Institute of Organic Chemistry, Moscow.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232213820/s1.
Author Contributions
Conceptualization, A.N.I. and G.A.G.; investigation, A.N.I., V.A.K. and A.A.L.; methodology, A.N.I. and G.A.G.; resources, A.N.K. and L.L.F.; supervision, G.A.G.; visualization, A.N.I. and G.A.G.; writing—original draft, A.N.I., V.A.K. and G.A.G.; writing—review and editing, L.L.F. and G.A.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Batista V.F., Pinto D.C.G.A., Silva A.M.S. Recent in vivo advances of spirocyclic scaffolds for drug discovery. Expert Opin. Drug Discov. 2022;17:603–618. doi: 10.1080/17460441.2022.2055544. [DOI] [PubMed] [Google Scholar]
- 2.Babar K., Zahoor A.F., Ahmad S., Akhtar R. Recent synthetic strategies toward the synthesis of spirocyclic compounds comprising six-membered carbocyclic/heterocyclic ring systems. Mol. Divers. 2021;25:2487–2532. doi: 10.1007/s11030-020-10126-x. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Y., Tice C.M., Singh S.B. The use of spirocyclic scaffolds in drug discovery. Bioorg. Med. Chem. Lett. 2014;24:3673–3682. doi: 10.1016/j.bmcl.2014.06.081. [DOI] [PubMed] [Google Scholar]
- 4.Chupakhin E., Babich O., Prosekov A., Asyakina L., Krasavin M. Spirocyclic Motifs in Natural Products. Molecules. 2019;24:4165. doi: 10.3390/molecules24224165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aldeghi M., Malhotra S., Selwood D.L., Chan A.W.E. Two- and three-dimensional rings in drugs. Chem. Biol. Drug Des. 2014;83:450–461. doi: 10.1111/cbdd.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y.-T., Zhu J.-F., Liao G., Xu H.-J., Yu B. The development of biologically important spirooxindoles as new antimicrobial agents. Curr. Med. Chem. 2018;25:2233–2244. doi: 10.2174/0929867325666171129131311. [DOI] [PubMed] [Google Scholar]
- 7.Pavlovska T.L., Redkin R.G., Lipson V.V., Atamanuk D.V. Molecular diversity of spirooxindoles. Synthesis and biological activity. Mol. Divers. 2016;20:299–344. doi: 10.1007/s11030-015-9629-8. [DOI] [PubMed] [Google Scholar]
- 8.Bora D., Kaushal A., Shankaraiah N. Anticancer potential of spirocompounds in medicinal chemistry: A pentennial expedition. Eur. J. Med. Chem. 2021;215:113263. doi: 10.1016/j.ejmech.2021.113263. [DOI] [PubMed] [Google Scholar]
- 9.Yu B., Yu D.-Q., Liu H.-M. Spirooxindoles: Promising scaffolds for anticancer agents. Eur. J. Med. Chem. 2015;97:673–698. doi: 10.1016/j.ejmech.2014.06.056. [DOI] [PubMed] [Google Scholar]
- 10.Nasri S., Bayat M., Mirzaei F. Recent Strategies in the Synthesis of Spiroindole and Spirooxindole Scaffolds. Top. Curr. Chem. 2021;379:25. doi: 10.1007/s41061-021-00337-7. [DOI] [PubMed] [Google Scholar]
- 11.Zhu G., Wei Q., Chen H., Zhang Y., Shen W., Qu J., Wang B. Asymmetric [3 + 2] Cycloaddition of 3-Amino Oxindole-Based Azomethine Ylides and α,β-Enones with Divergent Diastereocontrol on the Spiro[pyrrolidine-oxindoles] Org. Lett. 2017;19:1862–1865. doi: 10.1021/acs.orglett.7b00625. [DOI] [PubMed] [Google Scholar]
- 12.Bhardwaj D., Singh R. A strategic approach for synthesis of benzimidazo [2,1-b]thiazolidinone appended dispirooxindole hybrids via [3 + 2]cycloaddition using fluoroethanol as solvent. Tetrahedron Lett. 2021;85:153491. doi: 10.1016/j.tetlet.2021.153491. [DOI] [Google Scholar]
- 13.Toumi A., Boudriga S., Hamden K., Daoud I., Askri M., Soldera A., Lohier J.-F., Strohmann C., Brieger L., Knorr M. Diversity-oriented synthesis of spiropyrrolo[1,2-a]isoquinoline Derivatives via Diastereoselective and Regiodivergent Three-Component 1,3-dipolar cycloaddition reactions: In Vitro and in Vivo Evaluation of the Antidiabetic Activity of Rhodanine Analogues. J. Org. Chem. 2021;86:13420–13445. doi: 10.1021/acs.joc.1c01544. [DOI] [PubMed] [Google Scholar]
- 14.Izmest’ev A.N., Streltsov A.A., Karnoukhova V.A., Kolotyrkina N.G., Strelenko Y.A., Kravchenko A.N., Gazieva G.A. 5-Indolylidene-2-iminothiazolidin-4-ones–Convenient Starting Compounds for Stereoselective Synthesis of Novel Dispirooxindole Derivatives. ChemistrySelect. 2022;7:e20210412. doi: 10.1002/slct.202104128. [DOI] [Google Scholar]
- 15.Novotortsev V.K., Kukushkin M.E., Tafeenko V.A., Skvortsov D.A., Kalinina M.A., Timoshenko R.V., Chmelyuk N.S., Vasilyeva L.A., Tarasevich B.N., Gorelkin P.V., et al. Dispirooxindoles Based on 2-Selenoxo-imidazolidin-4-ones: Synthesis, Cytotoxicity and ROS Generation Ability. Int. J. Mol. Sci. 2021;22:2613. doi: 10.3390/ijms22052613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korotaev V.Y., Barkovskii S.V., Kutyashev I.B., Ulitko M.V., Barkov A.Y., Zimnitskiy N.S., Kochnev I.A., Sosnovskikh V.Y. Two approaches toward the regio- and stereoselective synthesis of N-unsubstituted 3-aryl-4-(trifluoromethyl)-4H-spiro[chromeno[3,4-c]pyrrolidine-1,3′-oxindoles] Chem. Heterocycl. Compd. 2021;57:679–690. doi: 10.1007/s10593-021-02967-7. [DOI] [Google Scholar]
- 17.Katowah D.F., Hassaneen H.M.E., Farghaly T.A. Novel Spiro-pyrrolizidine-Oxindole and Spiropyrrolidine-Oxindoles: Green synthesis under Classical, Ultrasonic, and microwave conditions and Molecular docking simulation for antitumor and type 2 diabetes. Arab. J. Chem. 2022;25:103930. doi: 10.1016/j.arabjc.2022.103930. [DOI] [Google Scholar]
- 18.Izmest’ev A.N., Vinogradov D.B., Kolotyrkina N.G., Kravchenko A.N., Gazieva G.A. Synthesis of functionalized imidazo[4,5-e]thiazolo[3,2-b]triazines by condensation of imidazo[4,5-e]triazinethiones with DMAD or DEAD and rearrangement to imidazo[4,5-e]thiazolo[2,3-c]triazines. Beilstein J. Org. Chem. 2021;17:1141–1148. doi: 10.3762/bjoc.17.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izmest’ev A.N., Motornov V.A., Vinogradov D.B., Ioffe S.L., Kravchenko A.N., Gazieva G.A. Tandem Michael addition/elimination—Novel reactivity of pyridinium ylides in reaction with electron-deficient alkenes. Org. Chem. Front. 2022;9:4998–5004. doi: 10.1039/D2QO00911K. [DOI] [Google Scholar]
- 20.Izmest’ev A.N., Gazieva G.A., Kulikov A.S., Anikina L.V., Kolotyrkina N.G., Kravchenko A.N. Synthesis and Biological Activity of Oxindolylidene Derivatives of Imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazin-7-ones and Imidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazin-8-ones. Rus. J. Org. Chem. 2017;53:753–763. doi: 10.1134/S1070428017050177. [DOI] [Google Scholar]
- 21.Gazieva G.A., Izmest’ev A.N., Anikina L.V., Pukhov S.A., Meshchaneva M.E., Khakimov D.V., Kolotyrkina N.G., Kravchenko A.N. The influence of substituents on the reactivity and cytotoxicity of imidazothiazolotriazinones. Mol. Divers. 2018;22:585–599. doi: 10.1007/s11030-018-9813-8. [DOI] [PubMed] [Google Scholar]
- 22.Izmest’ev A.N., Gazieva G.A., Anikina L.V., Pukhov S.A., Karnoukhova V.A., Kolotyrkina N.G., Kravchenko A.N. Synthesis and evaluation of antiproliferative activity of new hetarylmetylidene derivatives of imidazothiazolotriazinones. New J. Chem. 2021;45:12271–12285. doi: 10.1039/D1NJ02163J. [DOI] [Google Scholar]
- 23.Izmest’ev A.N., Anikina L.V., Zanin I.E., Kolotyrkina N.G., Izmalkova E.S., Kravchenko A.N., Gazieva G.A. Design, synthesis and in vitro evaluation of the hybrids of oxindolylidene and imidazothiazolotriazine as efficient antiproliferative agents. New J. Chem. 2022;46:11632–11647. doi: 10.1039/D2NJ01454H. [DOI] [Google Scholar]
- 24.Cai D., Li T., Xie Q., Yu X., Xu W., Chen Y., Jin Z., Hu C. Synthesis, Characterization, and Biological Evaluation of Novel 7-Oxo-7 H-thiazolo[3,2-b]-1,2,4-triazine-2-carboxylic Acid Derivatives. Molecules. 2020;25:1307. doi: 10.3390/molecules25061307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Shehry M.F., El-Hag F.A.A., Ewies E.F. Synthesis and Antimicrobial Study of New Fused Thiazolo[3,2-b]triazine, Triazolo [4,3-b] triazine, and 1,2,4-Triazinone Derivatives. Russ. J. Org. Chem. 2020;56:129–136. doi: 10.1134/S1070428020010200. [DOI] [Google Scholar]
- 26.Izmest’ev A.N., Gazieva G.A., Sigay N.V., Serkov S.A., Karnoukhova V.A., Kachala V.V., Shashkov A.S., Zanin I.E., Kravchenko A.N., Makhova N.N. An effective one-pot access to polynuclear dispiroheterocyclic structures comprising pyrrolidinyloxindole and imidazothiazolotriazine moieties via a 1,3-dipolar cycloaddition strategy. Beilstein J. Org. Chem. 2016;12:2240–2249. doi: 10.3762/bjoc.12.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izmest’ev A.N., Gazieva G.A., Karnoukhova V.A., Kravchenko A.N. Diastereodivergent synthesis of dispiroheterocyclic structures comprising pyrrolidinyloxindole and imidazothiazolotriazine moieties. Org. Biomol. Chem. 2020;18:6905–6911. doi: 10.1039/D0OB01628D. [DOI] [PubMed] [Google Scholar]
- 28.Gazieva G.A., Izmest’ev A.N., Nelyubina Y.V., Kolotyrkina N.G., Zanin I.E., Kravchenko A.N. Synthesis of imidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine-2,8-diones via a rearrangement of imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7-diones in the reaction with isatins. RSC Adv. 2015;5:43990–44002. doi: 10.1039/C5RA07669B. [DOI] [Google Scholar]
- 29.Gazieva G.A., Kolotyrkina N.G., Kravchenko A.N., Makhova N.N. Synthesis of novel spiro[indole-3,3′-pyrrolidin]-2(1H)-ones. Russ. Chem. Bull. 2014;63:431–434. doi: 10.1007/s11172-014-0449-2. [DOI] [Google Scholar]
- 30.Bruno I.J., Cole J.C., Kessler M., Luo J., Motherwell W.D.S., Purkis L.H., Smith B.R., Taylor R., Cooper R.I., Harris S.E., et al. Retrieval of Crystallographically-Derived Molecular Geometry Information. J. Chem. Inf. Comput. Sci. 2004;44:2133–2144. doi: 10.1021/ci049780b. [DOI] [PubMed] [Google Scholar]
- 31.Bruker, SAINT . Bruker, APEX-III. Bruker AXS Inc.; Madison, WI, USA: 2019. v8.34A, 2013. [Google Scholar]
- 32.Krause L., Herbst-Irmer R., Sheldrick G.M., Stalke D.J. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. Appl. Cryst. 2015;48:3–10. doi: 10.1107/S1600576714022985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheldrick G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015;71:3–8. doi: 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheldrick G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015;71:3–8. doi: 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spek A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015;71:9–18. doi: 10.1107/S2053229614024929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the article and in the Supplementary Materials.