Abstract
Background: Elevated blood urea nitrogen (BUN) level is associated with a higher risk of mortality in various diseases; however, the association between BUN level and in-hospital mortality in patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD) admitted to the intensive care unit (ICU) is not known. This study aimed to investigate the relationship between BUN level and in-hospital mortality in patients with AECOPD admitted to the ICU. Methods: In this retrospective cohort study, AECOPD patients were identified from the Medical Information Mart for Intensive Care (MIMIC-IV) database. Multivariate regression was used to elucidate the relationship between BUN level and in-hospital mortality, and propensity score matching (PSM) was used to adjust confounders. Receiver operating characteristics and Kaplan–Meier curves were used to evaluate the relationship between BUN level and in-hospital mortality. Results: Data from 1201 patients were analyzed. The all-cause in-hospital mortality was 13.7%. BUN levels were significantly higher in non-survivors compared to the survival group before (p < 0.001) and after (p = 0.005) PSM. Multivariate analysis indicated that elevated BUN levels were independently associated with increased risk of in-hospital mortality both before (p = 0.002) and after (p = 0.015) PSM. The optimal BUN cut-off value for in-hospital mortality in critical patients with AECOPD before (>23 mg/dL) and after (>22 mg/dL) PSM was comparable. Compared with the low BUN group, the hazard ratio (HR) of the high BUN group was 1.8987 (before PSM) and 1.7358 (after PSM). Conclusions: Higher BUN levels were significantly associated with an increased risk of in-hospital mortality in critically ill patients with AECOPD. As a widely available and rapidly measured biomarker, BUN may be useful in the risk stratification of critically ill AECOPD patients. The results need to be verified in prospective studies.
Keywords: acute exacerbation of chronic obstructive pulmonary disease, blood urea nitrogen, critical care, intensive care unit, mortality, propensity score matching
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous disease characterized by persistent respiratory symptoms and progressive airflow limitation, with episodes of exacerbations leading to substantial morbidity and mortality [1,2]. With an estimated 3.2 million deaths annually, COPD is the third leading cause of death worldwide [3]. Patients with acute exacerbations of COPD (AECOPD) often require unscheduled admission to the hospital or intensive care unit, incurring a substantial economic burden on individuals and society. The in-hospital mortality rate for AECOPD patients varies between 4% and 30% [4,5,6]. Previous studies reported various prognostic factors associated with in-hospital mortality of patients with AECOPD, including age, comorbidities, prior hospitalization due to AECOPD, exacerbation severity, and several laboratory parameters [7,8]. However, less invasive and technically easier laboratory parameters to predict in-hospital mortality, especially in the intensive care unit, represent an unmet need to minimize the negative impact of AECOPD.
Blood urea nitrogen (BUN) is a nitrogenous terminal product of protein metabolism formed in the liver and excreted by the kidneys. In addition to estimating renal function, BUN is an efficient indicator of neurohormonal activity [9]. Impaired cardiorenal function and neurohormonal activation may result in a high BUN level, which has been linked to mortality in various diseases [9,10]. Several previous studies suggested that BUN is a prognostic indicator for mortality in multiple disorders, including heart failure, aortic dissection, pancreatitis, gastrointestinal bleeding, diabetes mellitus, etc. [11,12,13,14]. Furthermore, recent studies have revealed that BUN is a significant predictor of mortality and disease severity in critically ill patients admitted with respiratory disorders such as coronavirus disease 2019 (COVID-19), hospital-acquired pneumonia, and community-acquired pneumonia (CAP) [15,16]. A recent prospective study demonstrated that the CURB-65 score is a valuable and simple tool for predicting 30- and 90-day mortality in COPD exacerbation [17]. BUN is one of the most crucial parameters in the CURB-65 score. Several factors, including hypoxia, chemoreflex, hypercapnia, and systemic inflammation, may impair cardiorenal function and neurohumoral regulation in patients with COPD exacerbations, resulting in a high BUN level [9,18].
Despite the above evidence, to our knowledge, no study has examined the association between BUN level and mortality in critically ill AECOPD patients. Therefore, we hypothesized that higher BUN levels are associated with an increased risk of in-hospital mortality in intensive care patients with AECOPD. We used the Medical Information Mart for Intensive Care—IV (MIMIC-IV) database [19] to investigate the relationship between BUN levels and in-hospital mortality in patients with AECOPD admitted to the intensive care unit (ICU).
2. Methods
2.1. Data Source
This retrospective cohort study was based on the MIMIC-IV database (version 1.0), which contains data from patients admitted to the intensive care unit of Beth Israel Deaconess Medical Center in Boston, Massachusetts, between 2008 and 2019 [19]. One author (MG) completed the training course “protecting human research participants” to access the database and was responsible for data extraction (certification number: 45355193). The establishment of the MIMIC-IV database has been approved by the institutional review boards of both Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology Affiliates. Informed consent was waived because all the patients in the database are anonymous.
2.2. Study Population and Data Extraction
We included all adult patients (≥18 years of age) diagnosed with acute exacerbation of chronic obstructive pulmonary disease (AECOPD) at hospital admission based on the International Classification of Diseases version 9 and 10 diagnosis codes (49121, J440, J441) in the MIMIC-IV database. The exclusion criteria were (i) patients with repeated ICU admissions, (ii) length of ICU stay <24 h, and (iii) more than 10% of individual data missing.
Navicat Premium (version 15.0) was used to extract the raw data of patients diagnosed with AECOPD from the MIMIC-IV database (version 1.0). The extracted data included demographics, vital signs, laboratory tests, comorbidities, and intervention. The following demographic data were extracted: age, gender, length of hospital stay, length of ICU stay, and hospital death sign. Monitoring parameters such as heart rate, blood pressure, respiratory rate, and oxyhemoglobin saturation (SpO2) were also collected. The extracted laboratory data include routine blood tests, glucose, creatinine, blood urea nitrogen, and bicarbonate. Comorbidities including hypertension, diabetes, congestive heart failure, coronary artery disease, chronic kidney disease, severe liver disease, obesity, malignant cancer, and cerebrovascular disease were extracted. Information on whether the patients were on mechanical ventilation was also extracted. We used the average value for the variable that was assessed multiple times on the first day of admission.
2.3. Statistical Analysis
The Kolmogorov–Smirnov test was used to determine if variables were normally distributed. If the data were normally distributed, we reported it as a mean ± standard deviation (SD) and used the independent sample t-test to compare all variables. When the data were not normally distributed, the variables were expressed as the median with interquartile range (IQR), and the Mann–Whitney test was used. Categorical variables were presented as total numbers and percentages. The baseline characteristics of the survival group and death group were evaluated by the chi-square test or Fisher’s exact test for categorical variables and the Mann–Whitney test for continuous variables. We carried out a binomial logistic regression analysis to determine the impact of BUN on in-hospital mortality in patients with AECOPD. Parameters with p < 0.1 in the univariate analysis and potential confounders determined by clinical expertise were included in multivariable logistic regression. Propensity score matching (PSM) was used to adjust for confounders between the survival and death groups. Confounders such as age, gender, hemoglobin, white blood cells, platelets, creatinine, red cell distribution width, hematocrit, bicarbonate, hypertension, diabetes, congestive heart failure, coronary artery disease, chronic kidney disease, severe liver disease, obesity, malignant cancer, cerebrovascular disease, heart rate, mean arterial pressure, the respiratory rate on the first day of admission, and mechanical ventilation were included in PSM. We used 1:1 nearest-neighbor matching without replacement within a caliper of 0.05 SD of the logit of the propensity score to create matched cohort. Logistic regression analysis was also performed on the matched cohort. Receiver operating characteristic (ROC) curves were generated to evaluate the association of BUN level with in-hospital mortality, and the area under the curve (AUC) was calculated. The optimal cut-off values of BUN associated with longer hospital mortality were determined by the Youden index. The BUN cut-off value was used to divide all patients into two groups: the high BUN group and the low BUN group. Survival analysis was performed using the Kaplan–Meier method, and the log-rank test was used to compare survival rates between high BUN and low BUN groups. Statistical analyses were performed with SPSS version 26.0 software and MedCalc version 19.6. A p-value of <0.05 was considered significant.
3. Results
3.1. Baseline Characteristics
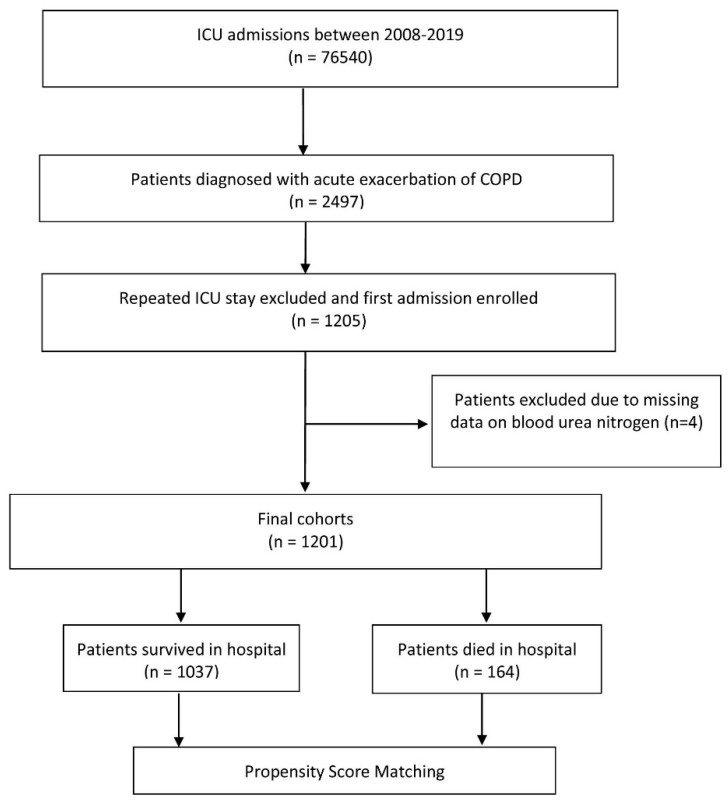
Among 76,540 records of ICU admissions in the MIMIC-IV database, we included a total of 1201 critically patients with AECOPD in the present study. The flowchart of the patient selection is presented in Figure 1. The median age of the patients was 72 years (range 41 to 97), and 50.2% were male. The overall rate of in-hospital mortality was 13.7% (164/1201). Table 1 summarizes the characteristics of the survival and death groups. No statistically significant differences were observed (all p > 0.05) between the survival and death groups regarding gender and the length of hospital stay. Patients in the death group had higher age, creatinine, red cell distribution width, and heart rate than those in the survival group (all p < 0.05) and lower mean arterial pressure, hemoglobin, platelets, hematocrit, and bicarbonate levels (all p < 0.05). Compared with the survival group, the death group had a significantly higher prevalence of prior cancer (26.8% vs. 12.7%, p = 0.001) and more often required mechanical ventilation during their hospital stay (40.2% vs. 31.5%, p = 0.031). Patients in the death group had significantly higher baseline BUN levels than those in the survival group.
Figure 1.
Flow chart of the patient selection process.
Table 1.
Demographic and clinical characteristics of the study population.
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| Characteristics | Survival (n = 1037) |
Death (n = 164) |
p | Survival (n = 159) |
Death (n = 159) |
p Value |
| * Age, year | 71 (64–80) | 76 (70–82.5) | 0.001 | 74 (66–84) | 76 (70–82.5) | 0.258 |
| * Gender, male | 524 (50.5) | 79 (48.2) | 0.574 | 84 (52.8) | 79 (49.7) | 0.575 |
| LOS hospital, day | 8 (4.9–13.9) | 8.5 (4.0–15.8) | 0.666 | 8.8 (5.9–16.3) | 8.7 (4–15.8) | 0.227 |
| LOS ICU, day | 2.4 (1.3–4.9) | 3.9 (1.7–7.9) | 0.001 | 2.3 (1.2–4.6) | 3.9 (1.7–7.9) | 0.001 |
| Laboratory tests | ||||||
| * Hemoglobin, g/dL | 11.2 (9.8–12.7) | 10.9 (9.2–12.2) | 0.001 | 10.4 (9.4–12.5) | 10.9 (9.1–12.2) | 0.797 |
| * WBC, 109/L | 10.9 (7.9–14.6) | 12.7 (9.2–17.5) | 0.854 | 11.4 (8.1–15.6) | 12.7 (9.3–17.2) | 0.076 |
| * Platelets, 109/L | 217 (163–280) | 193 (133.2–271.7) | 0.001 | 217 (149.8–265.5) | 192 (133–276.5) | 0.187 |
| Glucose, mg/dL | 144 (119.4–180.3) | 144 (121.7–178.4) | 0.828 | 138.6 (117.5–175) | 142.3 (118.8–174) | 0.501 |
| * Creatinine, ng/dL | 1 (0.8–1.5) | 1.1 (0.8–1.8) | 0.040 | 1.1 (0.8–1.6) | 1.1 (0.8–1.8) | 0.618 |
| MCHC, g/dL | 32.2 (31.1–33.2) | 32.1 (31–33.2) | 0.377 | 31.9 (30.9–33) | 32.1 (31–33.2) | 0.456 |
| * RDW, % | 14.8 (13.8–16.1) | 15.2 (14–16.9) | 0.006 | 15.1 (14.2–17) | 15.2 (14–16.9) | 0.958 |
| * Hematocrit, % | 35 (30.5–39.5) | 33.7 (28.9–37.7) | 0.004 | 33.7 (29.1–38) | 33.4 (28.2–37.6) | 0.698 |
| BUN, mg/dL | 22.5 (16–35.5) | 30 (20.5–45) | 0.001 | 25.5 (16.5–37.5) | 30 (20.5–44.5) | 0.005 |
| * Bicarbonate, mEq/L | 26 (23–30) | 25 (21.3–30) | 0.036 | 26 (22.5–29) | 25 (21.5–30) | 0.269 |
| Comorbidities | ||||||
| * Hypertension | 423 (40.8) | 57 (34.8) | 0.146 | 57 (35.8) | 55 (34.6) | 0.907 |
| * Diabetes | 321 (31) | 48 (29.3) | 0.716 | 47 (29.6) | 46 (28.9) | 0.902 |
| * Congestive heart failure | 516 (49.8) | 93 (56.7) | 0.110 | 87 (54.7) | 90 (56.6) | 0.735 |
| * Coronary artery disease | 309 (29.8) | 57 (34.8) | 0.202 | 45 (28.5) | 55 (34.6) | 0.227 |
| * Chronic kidney disease | 247 (23.8) | 41 (25) | 0.768 | 39 (25.5) | 40 (25.2) | 0.897 |
| * Severe liver disease | 18 (1.7) | 5 (3) | 0.228 | 4 (2.5) | 5 (3.1) | 0.735 |
| * Obesity | 161 (15.5) | 24 (14.5) | 0.817 | 25 (17.5) | 24 (15.1) | 0.877 |
| * Malignant cancer | 132 (12.7) | 44 (26.8) | 0.001 | 37 (23.3) | 41 (25.8) | 0.602 |
| * Cerebrovascular disease | 82 (7.9) | 17 (10.4) | 0.286 | 15 (9.4) | 17 (10.7) | 0.709 |
| Monitoring parameters | ||||||
| * Heart rate, Bpm | 87 (77–99) | 91 (79–106) | 0.004 | 89 (80–102) | 91 (79–105) | 0.654 |
| * MAP, mmHg | 78 (71–85) | 75 (69–82) | 0.004 | 77 (71–84) | 75 (69–82) | 0.101 |
| * RR, breaths/minutes | 20 (18.1–23) | 21 (19–24) | 0.022 | 21 (19–24) | 21 (19–24) | 0.588 |
| SpO2,% | 95.4 (93.8–97) | 95 (93.5–97.3) | 0.649 | 95 (94–97) | 95 (93–97) | 0.834 |
| Intervention | ||||||
| * Mechanical ventilation | 327 (31.5) | 66 (40.2) | 0.031 | 50 (31.4) | 62 (39) | 0.159 |
Values are expressed as the median (IQR) or n (%). PSM, propensity score matching; LOS, length of stay; ICU, intensive care unit; WBC, white blood cells; MCHC, mean corpuscular hemoglobin concentration; RDW, red blood cell distribution width; BUN, blood urea nitrogen; MAP, mean arterial pressure; RR, respiratory rate. * Covariables included in the PSM.
3.2. Propensity Score Matching Analysis
Propensity score matching was used to balance the baseline characteristics, and 159 patients were allocated to each group. After PSM, most of the baseline characteristics were comparable between the death and survival groups (all p > 0.05). However, the length of ICU stay was longer in the death group than in the survival group even after the PSM (p < 0.001). Similarly, BUN levels remained significantly higher in the death group compared to the survival group (p < 0.05). The baseline characteristics are shown in Table 1.
3.3. Logistic Regression Analysis
The univariable and multivariable relationships between patient characteristics and in-hospital mortality before PSM are shown in Table 2. Prior to matching, BUN was a risk factor for in-hospital mortality in ICU patients with AECOPD in univariable analyses (OR:1.018, 95% CI:1.010–1.025, p < 0.001), and this risk persisted in multivariable analyses (OR:1.014, 95% CI:1.005–1.022, p = 0.002). After PSM, BUN was still a risk factor for in-hospital mortality in patients with AECOPD in univariable analyses (OR:1.016, 95% CI:1.004–1.027, p = 0.008) and multivariable analyses (OR:1.015, 95% CI:1.003–1.027, p < 0.05). Table 3 presents relevant factors associated with in-hospital mortality in patients with AECOPD after PSM.
Table 2.
Binomial logistic regression analysis of BUN for in-hospital mortality among critically ill patients with AECOPD (before PSM).
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age | 1.039 (1.022–1.056) | 0.001 | 1.042 (1.023–1.061) | 0.001 |
| Gender (male) | 0.910 (0.574–0.910) | 0.574 | ||
| LOS hospital | 1.005 (0.987–1.023) | 0.606 | ||
| LOS ICU | 1.047 (1.021–1.073) | 0.001 | 1.038 (1.008–1.069) | 0.012 |
| Hemoglobin | 0.897 (0.829–0.970) | 0.006 | 1.078 (0.758–1.532) | 0.675 |
| WBC | 1.040 (1.017–1.063) | 0.001 | 1.029 (1.004–1.054) | 0.021 |
| Platelets | 0.998 (0.997–1.000) | 0.049 | 0.998 (0.996–1.000) | 0.033 |
| Glucose | 1.002 (0.999–1.005) | 0.206 | ||
| Creatinine | 1.087 (0.949–1.243) | 0.228 | ||
| MCHC | 0.958 (0.865–1.060) | 0.401 | ||
| RDW | 1.113 (1.042–1.188) | 0.002 | 1.076 (0.988–1.172) | 0.093 |
| Hematocrit | 0.966 (0.942–0.992) | 0.010 | 0.974 (0.872–1.089) | 0.644 |
| BUN | 1.018 (1.010–1.025) | 0.001 | 1.014 (1.005–1.022) | 0.002 |
| Bicarbonate | 0.972 (0.944–1.001) | 0.059 | 1.012 (0.979–1.046) | 0.485 |
| Hypertension (yes) | 0.773 (0.548–1.091) | 0.143 | ||
| Diabetes (yes) | 0.923 (0.643–1.325) | 0.664 | ||
| Congestive heart failure (yes) | 1.323 (0.949–1.843) | 0.099 | 1.108 (0.762–1.609) | 0.592 |
| Coronary artery disease (yes) | 1.255 (0.886–1.777) | 0.201 | ||
| Chronic kidney disease (yes) | 1.066 (0.728–1.561) | 0.742 | ||
| Severe liver disease (yes) | 1.780 (0.652–4.862) | 0.261 | ||
| Obesity (yes) | 0.933 (0.586–1.484) | 0.769 | ||
| Malignant cancer (yes) | 2.514 (1.701–3.716) | 0.001 | 2.971 (1.929–4.577) | 0.001 |
| Cerebrovascular disease (yes) | 1.347 (0.777–2.335) | 0.289 | ||
| Hear rate | 1.016 (1.005–1.027) | 0.003 | 1.018 (1.005–1.030) | 0.005 |
| Mean arterial pressure | 0.975 (0.959–0.992) | 0.015 | 0.993 (0.974–1.012) | 0.447 |
| Respiratory rate | 1.056 (1.011–1.104) | 0.001 | 1.030 (0.979–1.084) | 0.259 |
| SpO2 | 0.966 (0.900–1.036) | 0.332 | ||
| Mechanical ventilation (yes) | 1.462 (1.042–2.051) | 0.028 | 1.654 (1.093–2.505) | 0.017 |
AECOPD, acute exacerbation of chronic obstructive pulmonary disease; PSM, propensity score matching; OR, odds ratio; CI, confidence interval; LOS, length of stay; ICU, intensive care unit; WBC, white blood cells; BUN, blood urea nitrogen; MCHC, mean corpuscular hemoglobin concentration; RDW, red blood cell distribution width.
Table 3.
Binomial logistic regression analysis of BUN for in-hospital mortality among critically ill patients with AECOPD (after PSM).
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age | 1.013 (0.992–1.035) | 0.233 | ||
| Gender (male) | 0.882 (0.568–1.369) | 0.575 | ||
| LOS hospital | 0.999 (0.976–1.022) | 0.919 | ||
| LOS ICU | 1.075 (1.026–1.172) | 0.002 | 1.077 (1.027–1.130) | 0.002 |
| Hemoglobin | 0.993 (0.901–1.095) | 0.890 | ||
| WBC | 1.008 (0.980–1.036) | 0.571 | ||
| Platelets | 0.999 (0.997–1.001) | 0.575 | ||
| Glucose | 1.022 (0.998–1.005) | 0.375 | ||
| Creatinine | 1.017 (0.806–1.283) | 0.885 | ||
| MCHC | 1.048 (0.920–1.193) | 0.481 | ||
| RDW | 1.009 (0.927–1.099) | 0.830 | ||
| Hematocrit | 0.995 (0.964–1.027) | 0.747 | ||
| BUN | 1.016 (1.004–1.027) | 0.008 | 1.015 (1.003–1.027) | 0.015 |
| Bicarbonate | 0.984 (0.948–1.022) | 0.408 | ||
| Hypertension (yes) | 0.946 (0.597–1.499) | 0.814 | ||
| Diabetes (yes) | 0.970 (0.598–1.573) | 0.902 | ||
| Congestive heart failure (yes) | 1.079 (0.693–1.680) | 0.735 | ||
| Coronary artery disease (yes) | 1.340 (0.833–2.155) | 0.228 | ||
| Chronic kidney disease (yes) | 1.034 (0.622–1.720) | 0.897 | ||
| Severe liver disease (yes) | 1.258 (0.332–4.774) | 0.736 | ||
| Obesity (yes) | 0.953 (0.518–1.752) | 0.877 | ||
| Malignant cancer (yes) | 1.146 (0.687–1.911) | 0.602 | ||
| Cerebrovascular disease (yes) | 1.149 (0.553–2.390) | 0.709 | ||
| Heart rate | 1.002 (0.988–1.016) | 0.815 | ||
| Mean arterial pressure | 0.981 (0.960–1.002) | 0.081 | 0.987 (0.965–1.009) | 0.251 |
| Respiratory rate | 1.016 (0.962–1.074) | 0.566 | ||
| SpO2 | 0.967 (0.886–1.057) | 0.464 | ||
| Mechanical ventilation (yes) | 1.393 (0.878–2.212) | 0.159 | ||
AECOPD, acute exacerbation of chronic obstructive pulmonary disease; PSM, propensity score matching; OR, odds ratio; CI, confidence interval; LOS, length of stay; ICU, intensive care unit; WBC, white blood cells; BUN, blood urea nitrogen; MCHC, mean corpuscular hemoglobin concentration; RDW, red blood cell distribution width.
3.4. Receiver Operating Characteristic Analysis
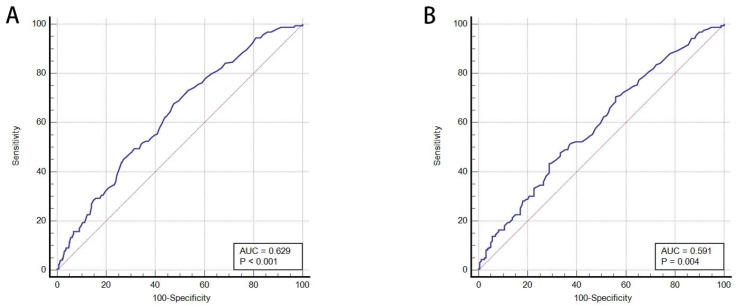
A receiver operating characteristic (ROC) curve was drawn, and the area under the curve was calculated to assess the relationship between BUN level and in-hospital mortality. Before PSM, the optimal cut-off value of BUN level for in-hospital mortality was 23 mg/dL with a sensitivity of 67.68% and specificity of 52.53%. The AUC was 0.629 (95% CI: 0.601–0.656, p < 0.001) (Figure 2). After PSM, the AUC value was 0.591 (95% CI: 0.535–0.646; p = 0.004), with sensitivity and specificity of 70.44% and 44.03%, respectively, and an optimal BUN cut-off value of 22 mg/dL (Figure 2).
Figure 2.
(A) Receiver operating characteristic (ROC) curve for BUN (before propensity score matching); (B) ROC curve for BUN (after propensity score matching). AUC, area under the curve.
3.5. Survival Analysis
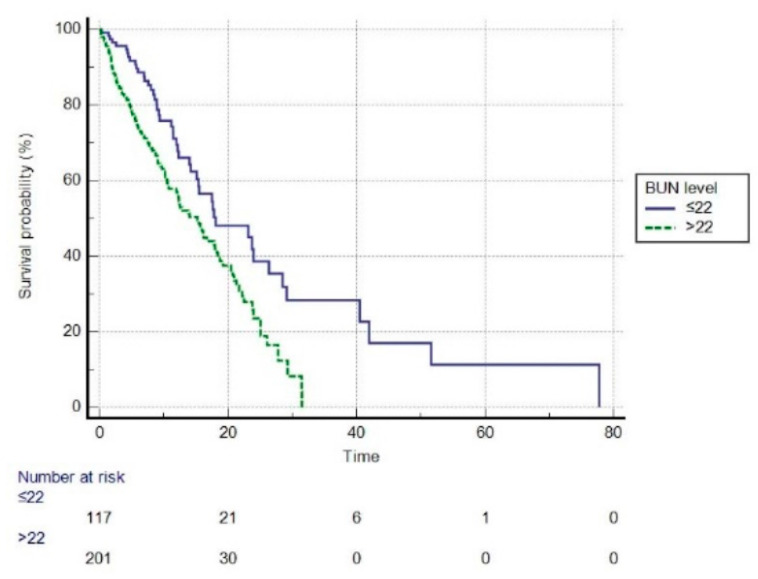
Before PSM, based on the optimal cut-off value of 23 mg/dL, patients were divided into high BUN (BUN level > 23 mg/dL) and low BUN (BUN level ≤ 23 mg/dL) groups. We performed a survival analysis using Kaplan–Meier plots in the high and low BUN groups (Figure 3). The mean survival time was 36.418 days (95% CI: 33.120–39.71) in the high BUN group vs. 49.522 days (95% CI: 40.919–58.126) in the low BUN group, and statistically significant differences were found between the two groups (log-rank test, p < 0.001). Compared to the low BUN group, the high BUN group had a hazard ratio (HR) of 1.8987 (95% CI: 1.3975–2.5796, p < 0.01). After PSM, the optimal cut-off value for BUN was 22 mg/dL (Figure 4). The high BUN group had a mean survival time of 15.183 days (95% CI: 13.550–16.816), whereas the low BUN group had a mean survival time of 27.087 days (95% CI: 19.926–34.249), and the difference was statistically significant (log-rank test, p < 0.001). Compared with the low BUN group, the HR of the high BUN group was 1.7358 (95% CI: 1.2671–2.3780, p < 0.01).
Figure 3.
The Kaplan–Meier survival curve of high and low BUN groups (before propensity score matching, log-rank p < 0.001). BUN, blood urea nitrogen (mg/dL).
Figure 4.
The Kaplan–Meier survival curve of high and low BUN groups (after propensity score matching, log-rank p < 0.001). BUN, blood urea nitrogen (mg/dL).
4. Discussion
To the best of our knowledge, this is the first study to investigate the associations between BUN and in-hospital mortality in a cohort of ICU patients with AECOPD, where potential confounding factors were mitigated through PSM. In this study of ICU patients with AECOPD, BUN levels were significantly higher in non-survivors compared to the survival group before and after PSM. Prior to PSM, multivariate analysis indicated that elevated BUN levels were independently associated with an increased risk of in-hospital mortality after adjusting for possible confounders. After PSM, high BUN levels were still associated with an increased risk of in-hospital mortality. Moreover, our findings show that BUN is an independent risk factor for patients with AECOPD. In the present study, before PSM, the optimal BUN cut-off value was 23 mg/dL. The hazard ratio (HR) for the high BUN group was 1.8987 compared to the low BUN group, indicating that the risk of in-hospital mortality in the high BUN group was 1.8987 times higher than in the low BUN group. Based on the Youden index, the optimal BUN cut-off value for in-hospital mortality in critical patients with AECOPD after PSM was 22 mg/dL, which was almost the same as before PSM. AECOPD patients with a high BUN group had an HR of 1.7358 compared with the low BUN group, implying that the high BUN group patients had a 1.7358 times higher risk of in-hospital death than the low BUN group.
Blood urea nitrogen (BUN) is not only an indicator of renal function but is also an effective marker of neurohormonal activity. BUN has been shown to be an important predictor of in-hospital mortality in various diseases [11,12,13]. In a retrospective study that included 4176 patients admitted to a German ICU, Arihan et al. [20] revealed that a high BUN level at admission was significantly associated with adverse outcomes in critically ill patients admitted to an ICU; this association existed even after correction for relevant confounders in multivariate analyses. Furthermore, previous studies [21,22] reported that the BUN/albumin ratio is an independent predictor of mortality in community-acquired and hospital-acquired pneumonia. Moreover, Küçükceran et al. [23] evaluated the association between serum BUN and albumin levels on admission in patients with COVID-19 in-hospital mortality. They demonstrated that BUN levels in the death group were considerably higher than in the survivor group, with an AUC of 0.771 for predicting in-hospital mortality. In the present study, we also found that a higher BUN level was associated with in-hospital mortality in AECOPD, with an AUC of 0.629 before PSM and 0.591 after PSM.
A recent study [24] found that high BUN levels at admission were significantly associated with hospital mortality in AECOPD patients who presented to the emergency department, and the optimal cut-off value for hospital mortality was 7.63 mmol/L. In this large, hospital-based propensity score matching study, we also found that AECOPD patients admitted to the ICU with higher baseline BUN levels (>23 mg/dL before PSM; >22 mg/dL after PSM) had a higher risk of mortality than those with lower BUN. Although the exact mechanism underlying this relationship is unclear, several mechanisms may be attributable to elevated BUN levels in AECOPD patients. First, exacerbation of COPD results in prolonged hypoxemia and hypercapnia, which activates the renin–angiotensin–aldosterone system (RAAS) and increases flow-dependent urea reabsorption in the distal tubules, resulting in increased BUN levels [9,25,26]. Secondly, cardiovascular comorbidities such as heart failure are common in COPD patients. In patients with cardiovascular disease, a complex neurohormonal mechanism is activated, which activates the renal sympathetic nervous system and the RAAS, resulting in urea reabsorption dysregulation [27]. Finally, COPD is linked to persistent blood and airway neutrophilia, as well as systemic and tissue hypoxia. Patients with COPD have higher levels of hypoxia-upregulated neutrophil-derived proteins and elevated concentrations of circulating proinflammatory mediators, which may affect the cardiorenal function and neurohumoral regulation, raising BUN levels [9,25,28].
Our results showed that the in-hospital mortality rate was 13.7% in AECOPD patients admitted to the ICU. Mohan et al. [29] also reported an in-hospital mortality rate of 13.7% in patients with AECOPD requiring admission to the ICU, which is consistent with our findings. In contrast, Warwick et al. [30] reported a high hospital mortality rate (27.2%) in patients with AECOPD admitted to the ICU. Variability in published mortality rates for AECOPD patients could be attributed to differences in patient characteristics, quality of care, prolonged ICU stay, and mechanical ventilation use. Furthermore, our findings imply that the risk of in-hospital mortality was found to be higher in critically ill patients with a BUN level >23 mg/dl. Our results suggest that BUN is a widely and rapidly available laboratory test that may allow physicians and hospital staff to identify COPD patients with increased risk of adverse outcomes requiring early intensive care.
This study has several limitations. First, this retrospective cohort study may have led to selection bias. Nevertheless, PSM and multivariate analyses were used to minimize selection bias and adjust for confounding variables. Second, although we used PSM to reduce bias, there may have been some residual and unmeasured confounders. Third, some individuals with cardiovascular comorbidities may have taken medications that influence BUN levels. However, even after adjusting for multiple comorbidities, BUN levels remained an independent predictor of in-hospital mortality. Fourth, the BUN levels could have been affected by many factors, such as diuretic use and protein intake; nevertheless, due to the study’s retrospective nature, these conditions could not be differentiated. Fifth, the AUC for BUN did not perform well in ROC analysis, which may have influenced the accuracy of the results. Finally, this is a single-center study, which limits the generalizability of our findings.
5. Conclusions
In this cohort study, elevated BUN levels were independently associated with increased in-hospital mortality in critically ill patients with AECOPD. BUN is a routine laboratory test that may be useful in the risk stratification of these patients. Future prospective multicenter studies with a larger sample size are needed to evaluate further the association between BUN level and mortality in patients with AECOPD.
Author Contributions
M.G., L.H., and T.H.: Designed the study and prepared the manuscript. M.G., L.H., A.P., T.H., X.Z., and H.D.: Collected the literature, collected the data, and prepared the manuscript. M.G., L.H., A.P., T.H., X.Z., H.D., and S.G.: Acquisition, analysis, or interpretation of data. M.G., L.H., A.P., T.H., X.Z., H.D., and S.G.: Critical revision of the manuscript. S.G.: Study supervision. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The MIMIC-IV database has received ethical approval from the Institutional Review Boards of Massachusetts Institute of Technology and BIDMC. Because the database does not contain protected health information, written informed consent for participation was not required for this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data used in this study were available from MIMIC-IV, a freely accessible critical care database (https://mimic.physionet.org/gettingstarted/access) (accessed on 28 September 2021). We completed the online course and passed the online exams to access and use the data (record ID: 45355193).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by a 2020 Municipal Subsidy for Training Senior Medical Talents (project number 0202czzx2027 (YC006)) and a China Postdoctoral Research Grant (number P0465).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Christenson S.A., Smith B.M., Bafadhel M., Putcha N. Chronic Obstructive Pulmonary Disease. Lancet. 2022;399:2227–2242. doi: 10.1016/S0140-6736(22)00470-6. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z., Locantore N., Haldar K., Ramsheh M.Y., Beech A.S., Ma W., Brown J.R., Tal-Singer R., Barer M.R., Bafadhel M., et al. Inflammatory Endotype-Associated Airway Microbiome in Chronic Obstructive Pulmonary Disease Clinical Stability and Exacerbations: A Multicohort Longitudinal Analysis. Am. J. Respir. Crit. Care Med. 2021;203:1488–1502. doi: 10.1164/rccm.202009-3448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth G.A., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A., et al. Global, Regional, and National Age-Sex-Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singanayagam A., Schembri S., Chalmers J.D. Predictors of Mortality in Hospitalized Adults with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2013;10:81–89. doi: 10.1513/AnnalsATS.201208-043OC. [DOI] [PubMed] [Google Scholar]
- 5.Bustamante-Fermosel A., De Miguel-Yanes J.M., Duffort-Falcó M., Muñoz J. Mortality-Related Factors after Hospitalization for Acute Exacerbation of Chronic Obstructive Pulmonary Disease: The Burden of Clinical Features. Am. J. Emerg. Med. 2007;25:515–522. doi: 10.1016/j.ajem.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Steer J., Gibson J., Bourke S.C. The DECAF Score: Predicting Hospital Mortality in Exacerbations of Chronic Obstructive Pulmonary Disease. Thorax. 2012;67:970–976. doi: 10.1136/thoraxjnl-2012-202103. [DOI] [PubMed] [Google Scholar]
- 7.Cao Y., Xing Z., Long H., Huang Y., Zeng P., Janssens J.-P., Guo Y. Predictors of Mortality in COPD Exacerbation Cases Presenting to the Respiratory Intensive Care Unit. Respir. Res. 2021;22:77. doi: 10.1186/s12931-021-01657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morasert T., Jantarapootirat M., Phinyo P., Patumanond J. Prognostic Indicators for In-Hospital Mortality in COPD with Acute Exacerbation in Thailand: A Retrospective Cohort Study. BMJ Open Respir. Res. 2020;7:e000488. doi: 10.1136/bmjresp-2019-000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kazory A. Emergence of Blood Urea Nitrogen as a Biomarker of Neurohormonal Activation in Heart Failure. Am. J. Cardiol. 2010;106:694–700. doi: 10.1016/j.amjcard.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 10.Testani J.M., Coca S.G., Shannon R.P., Kimmel S.E., Cappola T.P. Influence of Renal Dysfunction Phenotype on Mortality in the Setting of Cardiac Dysfunction: Analysis of Three Randomized Controlled Trials. Eur. J. Heart Fail. 2011;13:1224–1230. doi: 10.1093/eurjhf/hfr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar N.L., Claggett B.L., Cohen A.J., Nayor J., Saltzman J.R. Association between an Increase in Blood Urea Nitrogen at 24 Hours and Worse Outcomes in Acute Nonvariceal Upper GI Bleeding. Gastrointest. Endosc. 2017;86:1022–1027.e1. doi: 10.1016/j.gie.2017.03.1533. [DOI] [PubMed] [Google Scholar]
- 12.Xie Y., Bowe B., Li T., Xian H., Yan Y., Al-Aly Z. Higher Blood Urea Nitrogen Is Associated with Increased Risk of Incident Diabetes Mellitus. Kidney Int. 2018;93:741–752. doi: 10.1016/j.kint.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 13.Lan Q., Zheng L., Zhou X., Wu H., Buys N., Liu Z., Sun J., Fan H. The Value of Blood Urea Nitrogen in the Prediction of Risks of Cardiovascular Disease in an Older Population. Front. Cardiovasc. Med. 2021;8:614117. doi: 10.3389/fcvm.2021.614117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koutroumpakis E., Wu B.U., Bakker O.J., Dudekula A., Singh V.K., Besselink M.G., Yadav D., Mounzer R., van Santvoort H.C., Whitcomb D.C., et al. Admission Hematocrit and Rise in Blood Urea Nitrogen at 24 h Outperform Other Laboratory Markers in Predicting Persistent Organ Failure and Pancreatic Necrosis in Acute Pancreatitis: A Post Hoc Analysis of Three Large Prospective Databases. Am. J. Gastroenterol. 2015;110:1707–1716. doi: 10.1038/ajg.2015.370. [DOI] [PubMed] [Google Scholar]
- 15.Ye B., Deng H., Zhao H., Liang J., Ke L., Li W. Association between an Increase in Blood Urea Nitrogen at 24 h and Worse Outcomes in COVID-19 Pneumonia. Ren. Fail. 2021;43:347–350. doi: 10.1080/0886022X.2021.1879855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milas G.P., Issaris V., Papavasileiou V. Blood Urea Nitrogen to Albumin Ratio as a Predictive Factor for Pneumonia: A Meta-Analysis. Respir. Med. Res. 2022;81:100886. doi: 10.1016/j.resmer.2022.100886. [DOI] [PubMed] [Google Scholar]
- 17.Gayaf M., Karadeniz G., Güldaval F., Polat G., Türk M. Which One Is Superior in Predicting 30 and 90 Days Mortality after COPD Exacerbation: DECAF, CURB-65, PSI, BAP-65, PLR, NLR. Expert Rev. Respir. Med. 2021;15:845–851. doi: 10.1080/17476348.2021.1901584. [DOI] [PubMed] [Google Scholar]
- 18.Clementi A., Virzì G.M., Battaglia G.G., Ronco C. Neurohormonal, Endocrine, and Immune Dysregulation and Inflammation in Cardiorenal Syndrome. Cardiorenal Med. 2019;9:265–273. doi: 10.1159/000500715. [DOI] [PubMed] [Google Scholar]
- 19.Johnson A., Bulgarelli L., Pollard T., Horng S., Celi L.A., Mark R. MIMIC-IV (Version 1.0) PhysioNet. 2021 doi: 10.13026/S6n6-Xd98. [DOI] [Google Scholar]
- 20.Arihan O., Wernly B., Lichtenauer M., Franz M., Kabisch B., Muessig J., Masyuk M., Lauten A., Schulze P.C., Hoppe U.C., et al. Blood Urea Nitrogen (BUN) Is Independently Associated with Mortality in Critically Ill Patients Admitted to ICU. PLoS ONE. 2018;13:e0191697. doi: 10.1371/journal.pone.0191697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ugajin M., Yamaki K., Iwamura N., Yagi T., Asano T. Blood Urea Nitrogen to Serum Albumin Ratio Independently Predicts Mortality and Severity of Community-Acquired Pneumonia. Int. J. Gen. Med. 2012;5:583–589. doi: 10.2147/IJGM.S33628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng D.-Y., Zhou Y.-Q., Zou X.-L., Zhou M., Yang H.-L., Chen X.-X., Zhang T.-T. Elevated Blood Urea Nitrogen-to-Serum Albumin Ratio as a Factor That Negatively Affects the Mortality of Patients with Hospital-Acquired Pneumonia. Can. J. Infect. Dis. Med. Microbiol. 2019;2019:1547405. doi: 10.1155/2019/1547405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Küçükceran K., Ayrancı M.K., Girişgin A.S., Koçak S., Dündar Z.D. The Role of the BUN/Albumin Ratio in Predicting Mortality in COVID-19 Patients in the Emergency Department. Am. J. Emerg. Med. 2021;48:33–37. doi: 10.1016/j.ajem.2021.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L., Chen L., Zheng H., Wu S., Wang S. The Association of Blood Urea Nitrogen Levels upon Emergency Admission with Mortality in Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Chron. Respir. Dis. 2021;18:14799731211060052. doi: 10.1177/14799731211060051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lodge K.M., Vassallo A., Liu B., Long M., Tong Z., Newby P.R., Agha-Jaffar D., Paschalaki K., Green C.E., Belchamber K.B.R., et al. Hypoxia Increases the Potential for Neutrophil-Mediated Endothelial Damage in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2022;205:903–916. doi: 10.1164/rccm.202006-2467OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kent B.D., Mitchell P.D., McNicholas W.T. Hypoxemia in Patients with COPD: Cause, Effects, and Disease Progression. Int. J. Chron. Obstruct. Pulmon. Dis. 2011;6:199–208. doi: 10.2147/COPD.S10611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsue Y., van der Meer P., Damman K., Metra M., O’Connor C.M., Ponikowski P., Teerlink J.R., Cotter G., Davison B., Cleland J.G., et al. Blood Urea Nitrogen-to-Creatinine Ratio in the General Population and in Patients with Acute Heart Failure. Heart. 2017;103:407–413. doi: 10.1136/heartjnl-2016-310112. [DOI] [PubMed] [Google Scholar]
- 28.Pinto-Plata V.M., Livnat G., Girish M., Cabral H., Masdin P., Linacre P., Dew R., Kenney L., Celli B.R. Systemic Cytokines, Clinical and Physiological Changes in Patients Hospitalized for Exacerbation of COPD. Chest. 2007;131:37–43. doi: 10.1378/chest.06-0668. [DOI] [PubMed] [Google Scholar]
- 29.Mohan A., Premanand R., Reddy L.N., Rao M.H., Sharma S.K., Kamity R., Bollineni S. Clinical Presentation and Predictors of Outcome in Patients with Severe Acute Exacerbation of Chronic Obstructive Pulmonary Disease Requiring Admission to Intensive Care Unit. BMC Pulm. Med. 2006;6:27. doi: 10.1186/1471-2466-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warwick M., Fernando S.M., Aaron S.D., Rochwerg B., Tran A., Thavorn K., Mulpuru S., McIsaac D.I., Thompson L.H., Tanuseputro P., et al. Outcomes and Resource Utilization Among Patients Admitted to the Intensive Care Unit Following Acute Exacerbation of Chronic Obstructive Pulmonary Disease. J. Intensive Care Med. 2021;36:1091–1097. doi: 10.1177/0885066620944865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data used in this study were available from MIMIC-IV, a freely accessible critical care database (https://mimic.physionet.org/gettingstarted/access) (accessed on 28 September 2021). We completed the online course and passed the online exams to access and use the data (record ID: 45355193).