Background:
The fillet flap is a reliable flap for reconstruction of large deformities following oncologic resection. It provides healthy, nonradiated tissue for coverage with the secondary benefit of preserving other potential donor sites for reconstruction.
Methods:
A retrospective review of the medical records of eight patients who underwent fillet flap reconstruction from 2013 to 2021 at Mayo Clinic, Arizona, were analyzed.
Results:
Eight patients who underwent four hemipelvectomies, three forequarter amputations, and one below the knee amputation were identified. Patients’ ages ranged between 24 and 66 years. All indications for oncologic ablation were curative. Defect sizes ranged from 16 × 20 to 30 × 60 cm. Four pedicled flaps and four free fillet flaps were performed. Indication for free fillet flap was tumor invasion of local vascular structures. There was no flap loss in the pedicled group (follow-up ranged from 1 to 9 years), and one of four free fillet flaps had a successful long-term outcome (follow-up 36 months).
Conclusions:
Successful free fillet flap reconstruction in the setting of oncologic resection is a difficult task to achieve. Changes to the management of case 3F allowed for a successful transfer. Immediate elevation and anastomosis of the flap before oncologic resection, large caliber recipient vessels and isolation from the zone of injury, protection of the anastomosis, and delay in flap inset all contributed to flap survival. It is our belief that applying these general considerations in large oncologic resections with free fillet flap transfer may aid in successful flap transfer and improve its survival odds.
Takeaways
Question: What steps lead to a successful free fillet flap transfer?
Findings: Immediate elevation and anastomosis of the flap, large caliber recipient vessels, isolation from the zone of injury, protection of the anastomosis while the other teams ablate the tumor, and delay in flap inset during hemodynamic stabilization contributed to flap survival.
Meaning: Consider initiating the free fillet flap case by being the first team in the operating room that is elevating and anastomosing the flap before limb amputation in an anatomic region distant from the tumor resection.
INTRODUCTION
A fillet flap is a reliable flap that provides healthy nonradiated otherwise discarded tissue for coverage of large oncologic resections without creating a new donor site.1 Decrease in healing time may expedite adjuvant treatment therapy and prosthetic fitting and preserve other potential myocutaneous flaps for any future reconstruction.
We reviewed eight cases of reconstruction following four hemipelvectomies, one below the knee amputation, and three forequarter amputations. All eight cases resulted in large defects that required a fillet flap for coverage (Table 1). The aim of our study was to analyze the progression of the free fillet flap outcomes and identify the steps that lead to a successful reconstruction.
Table 1.
Patient Characteristics
| Case | Year of Surgery | Age, y | Sex | Diagnosis | Origin | Invasion | Prior Surgery |
|---|---|---|---|---|---|---|---|
| 1 | 2013 | 24 | M | Osteomyelitis | MVC—tibia and fibula nonunion | None | Hardware placement, multiple wound management surgeries |
| 2 | 2016 | 56 | M | High-grade pleomorphic sarcoma | Unknown | None | R shoulder mass lanced and packed in local ER 7 mo prior |
| 3 | 2021 | 43 | F | Peripheral nerve sheath tumor | Brachial plexus | None | No |
| 4 | 2021 | 26 | F | Mucinous adenocarcinoma | GI origin | None | No |
| 1F | 2014 | 54 | F | High-grade osteoblastic osteosarcoma | Right iliac bone | Right iliac vessels | LAR and ileostomy creation for stage 3 rectal cancer |
| 2F | 2019 | 66 | F | High-grade spindle cell carcinoma as well as radiation-induced sarcoma | Recurrence of a radiation-induced sarcoma | Right axilla, scapula, gleno-humeral joint, chest wall, axillary neurovascular bundle, and brachial plexus | Right axillary carcinoma resection, 2× right axillary mass resection, right axillary and anterior 2nd and 3rd rib, clavicle and pectoralis muscle resection |
| 3F | 2020 | 34 | M | High-grade chondroblastic osteosarcoma | Right iliac bone | Right common iliac, IVC | No |
| 4F | 2021 | 50 | M | Pelvic chondrosarcoma | Right hemipelvis | Mass effect on the iliac vessels, bowel, bladder, and sciatic nerve/lumbosacral plexus | No |
PATIENTS AND METHODS
The case series was deemed exempt by the institutional review board. A retrospective review of eight patient medical records was done between the years of 2013 and 2021 at the Mayo Clinic in Arizona.
RESULTS
We identified four women and four men with ages ranging between 24 and 66 years. All indications for ablation were curative. Defect sizes ranged from 16 × 20 to 30 × 60 cm. Four pedicled and four free flaps were reviewed. Indication for free fillet flaps was oncologic invasion of local vascular structures. Pedicled fillet flaps were considered a successful outcome secondary to no flap loss with follow-up of 1–9 years. Three free fillet flaps (N = 3/4, 75%) had successful intraoperative transfers with viable flaps. However, only one (N=1/4, 25%) flap had a successful long-term survival with follow-up of 36 months (Table 2).
Table 2.
Perioperative Details
| Case | Surgery | Flap Vascular Supply | Flap | Donor Vessels | Recipient Vessels | Ischemia Time | Anas-tomosis | Defect Size | Perioperative Complications |
|---|---|---|---|---|---|---|---|---|---|
| 1 | BKA | Pedicled | Distal lower leg and foot fillet flap | Anterior and posterior tibial | Pedicled | None | Pedicled | Not recorded | None |
| 1F | Hemipelvectomy | Free | Right thigh soft tissue | Femoral artery and vein | Common iliac artery and vein | 2+ hs | End to end (1 cm in diameter) |
16 × 20 cm | Massive transfusion protocol, venous thrombosis |
| 2 | Forequarter | Pedicled | Right forearm fillet flap | Brachial artery | Pedicled | None | Pedicled | 33 × 42 cm | None |
| 2F | Forequarter | Free | Right arm fillet flap | Brachial artery and veins (venae comitantes) | Lingual artery and anterior cervical vein | 2+ h | End to side (4 mm in diameter) |
17 × 25 cm | Massive transfusion protocol, coagulopathy, flap failure—arterial and venous thrombosis |
| 3 | Forequarter | Pedicled | Trapezius | Distal blood supply | Pedicled | None | Pedicled | 60 × 30 cm | None |
| 3F | Hemipelvectomy | Free | Right lower leg fillet flap | Femoral artery and vein | Subclavian artery and vein | 50 min | End to side subclavian (1 cm in diameter) |
25 × 20 cm | Massive transfusion protocol, kinking of the femoral vein in the subcutaneous tunnel |
| 4 | Hemipelvectomy | Pedicled | Lower leg filet | Femoral artery and vein | Pedicled | None | Pedicled | 52 × 21 cm | None |
| 4F | Hemipelvectomy | Free | Lower leg filet | Femoral artery and vein | AV loop on contralateral side | 50 min | End to end (4–5 mm diameter) | 16 × 20 cm | Massive transfusion protocol. Postoperative day 0—PEA, clamping the vessels supplying the flap |
FREE FILLET FLAP OPERATIVE DETAILS
In cases 1F and 2F of lower and upper extremity free fillet flap reconstruction, the extremities were partially filleted on the operating room (OR) table and amputated. Final flap elevation was completed on the back table while the ablation continued. Both cases required venous and arterial thrombectomies with re-anastomosis. The flaps were removed with final reconstruction done with free latissimus dorsi and pedicled transverse rectus abdominis myocutaneous flaps, respectively.
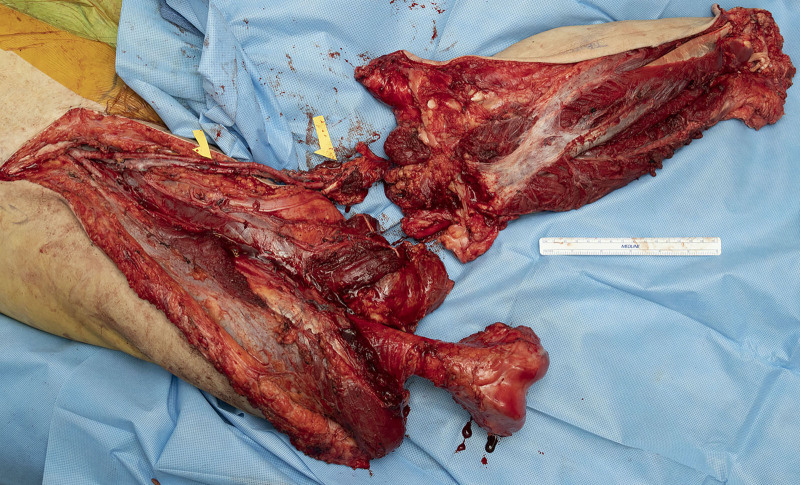
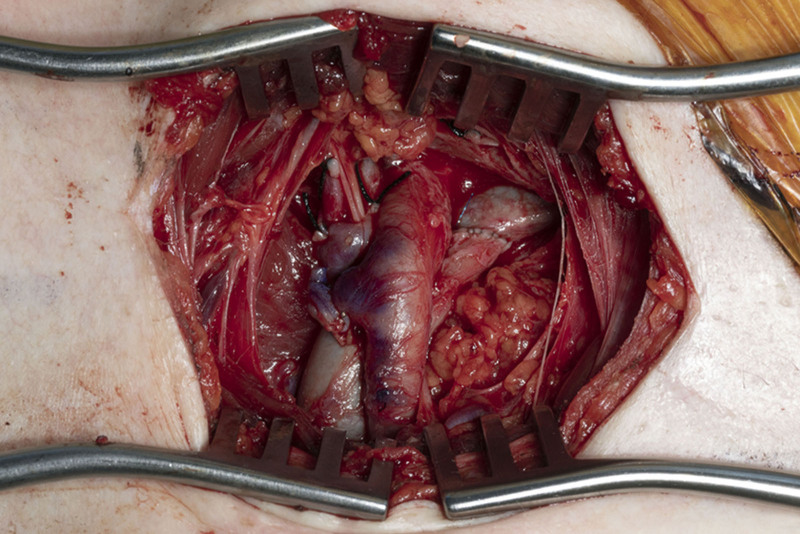
Case 3F was a lower extremity free fillet flap. The flap was elevated on the superficial femoral artery and vein (SFA/V). (See Video [online], which displays the perfusion through the SFA and SFV before removal of the flap and initiation of ischemia time.) The vessels were tunneled subcutaneously, and anastomosed to the subclavian vasculature before tumor ablation (Figs. 1 and 2). The flap was not inset while the patient underwent hemodynamic stabilization in the intensive care unit (ICU). The patient was taken back to the OR on postoperative day 0 for SFV evaluation in the subcutaneous tunnel and on the second day for flap inset.
Fig. 1.
Filleted right lower extremity below the knee connected through the patent SFA and SFV.
Fig. 2.
End to side anastomosis of SFA and SFV to subclavian artery and vein in the right upper chest.
Video 1. displays the perfusion through the SFA and SFV before removal of the flap and initiation of ischemia time.
Case 4F was a lower extremity free fillet flap which was carried out in a similar fashion to case 3F. However, during hemodynamic stabilization in the ICU, the patient experienced pulseless electrical activity (PEA). Secondary to hemodynamic instability, the decision to clamp off the vessels supplying the free fillet flap was made. The fillet flap was removed, and the final reconstruction was done with a free serratus and latissimus dorsi myocutaneous flap.
DISCUSSION
Local flap coverage options for large defect reconstructions have shown an overall 39% complication rare with 35% wound infection rates and 16% flap necrosis rates. In contrast, the free fillet flap reconstruction has shown a 15%–18% complication rate consisting of partial flap necrosis and vascular compromise.2–9 Kreutz-Rodriguez et al8 in their two case series of 10 and 12 free fillet flaps report only one flap loss and a 14% complication rate with wound healing and venous thrombosis.9
In cases with early flap elevation protocols, ischemia time is directly dependent on the final flap elevation after limb amputation and ablation of the tumor. Furthermore, with delayed final flap elevation, donor vessels may become thrombosed secondary to stasis, hypercoagulable state, and chronic inflammation. Ver Halen et al3 showed a decrease in ischemia time from 6 to 2.3 hours with routine flap harvest before limb amputation, and reported one case of flap revascularization before the ablation.
Similarly, in case 3F, the key difference leading to a successful outcome was flap elevation and immediate anastomosis to large subclavian vessels outside the zone of injury before limb amputation and tumor ablation (Figs. 1 and 2).
Vascular anastomosis to subclavian vasculature created a technically reliable anastomosis with fast flap reperfusion and protected the patency of the anastomosis while tumor ablation continued. A designated plastic surgery team member along with Vioptix near-infrared spectroscopy were also used to safeguard the flap, while the other teams continued to work.
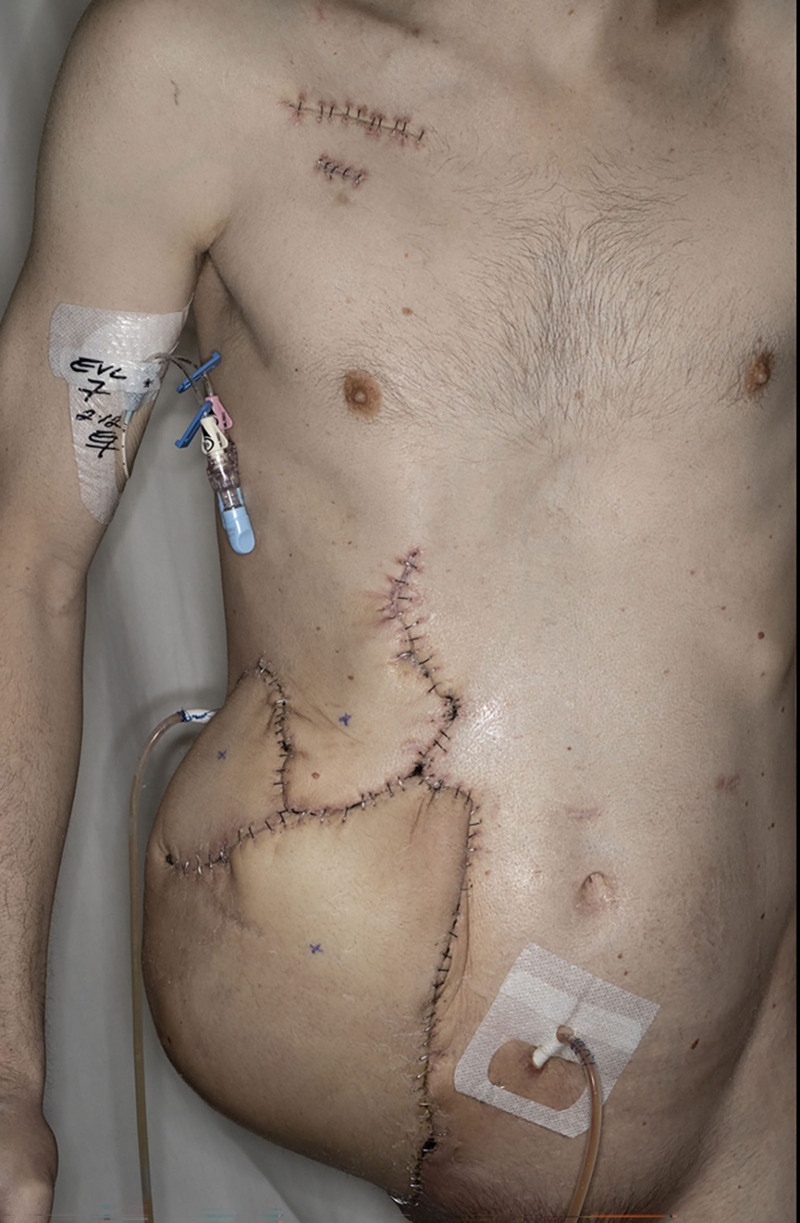
The decision to not inset the flap further optimized flap survival. The flap experienced congestion due to the kinking of the vein in the subcutaneous tunnel, which was quickly identified and immediately explored. The flap was successfully inset and the patient was able to begin his physical therapy and adjuvant chemotherapy within 2 months of his primary resection and reconstruction (Fig. 3).
Fig. 3.
Reconstructed fillet flap over the right lower abdomen 27 days postoperatively.
Although attempts to minimize operative time with prior day spine stabilization and ureter stent placement, the tumor resection results in a long operative day with significant blood loss. All four of the free fillet flap cases required initiation of massive transfusion protocols and hemodynamic stabilization in the ICU.
Prior studies have not found transfusion affecting flap survival in hemipelvectomy patients; however, only one of 160 cases was a free fillet flap.6 Nonetheless, in the head and neck literature, postoperative complications were positively correlated with the administration of one or more units of blood products.10
Hemodynamic instability and the initiation of massive transfusion protocols are the one uniting factor in the four free fillet flaps, with only one flap having survived the insult. In case 4F hemodynamic instability that resulted in PEA was the deciding factor in removal of a viable flap. As the tumor ablation and reconstruction teams, we must identify an algorithm for hemodynamic control in order to limit intraoperative blood loss.
CONCLUSIONS
We identified parameters that allowed the flap in case 3F to have a successful transfer: immediate elevation and anastomosis of the flap, large caliber recipient vessels with isolation from the zone of injury, protection of the anastomosis while the other teams work, and delay in flap inset. However, more analysis needs to be done to minimize hemodynamic decompensation and initiation of massive transfusion protocols.
Footnotes
Published online 28 November 2022.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Sánchez-García A, Perez-Garcia A, Salmeron-Gonzalez E, et al. The spare parts concept in sarcoma surgery: a systematic review of surgical strategies. Int J Orthoplastic Surg. 2019;2: 90–98. [Google Scholar]
- 2.Samant M, Chang EI, Petrungaro J, et al. Reconstruction of massive oncologic defects following extremity amputation: a 10-year experience. Ann Plast Surg. 2012;68:467–471. [DOI] [PubMed] [Google Scholar]
- 3.Ver Halen JP, Yu P, Skoracki RJ, et al. Reconstruction of massive oncologic defects using free fillet flaps. Plast Reconstr Surg. 2010;125:913–922. [DOI] [PubMed] [Google Scholar]
- 4.Senchenkov A, Moran SL, Petty PM, et al. Soft-tissue reconstruction of external hemipelvectomy defects. Plast Reconstr Surg. 2009;124:144–155. [DOI] [PubMed] [Google Scholar]
- 5.Apffelstaedt JP, Driscoll DL, Spellman JE, et al. Complications and outcome of external hemipelvectomy in the management of pelvic tumors. Ann Surg Oncol. 1996;3:304–309. [DOI] [PubMed] [Google Scholar]
- 6.Senchenkov A, Moran SL, Petty PM, et al. Predictors of complications and outcomes of external hemipelvectomy wounds: account of 160 consecutive cases. Ann Surg Oncol. 2008;15:355–363. [DOI] [PubMed] [Google Scholar]
- 7.Kreutz-Rodrigues L, Weissler J, Moran S, et al. Reconstruction of complex hemipelvectomy defects: a 17-year single-institutional experience with lower extremity free and pedicled fillet flaps. Plast Reconstr Surg Glob Open 2019;7:108–108. [DOI] [PubMed] [Google Scholar]
- 8.Kreutz-Rodrigues L, Mohan AT, Moran SL, et al. Extremity free fillet flap for reconstruction of massive oncologic resection-Surgical technique and outcomes. J Surg Oncol. 2020;121:465–473. [DOI] [PubMed] [Google Scholar]
- 9.Küntscher MV, Erdmann D, Homann HH, et al. The concept of fillet flaps: classification, indications, and analysis of their clinical value. Plast Reconstr Surg. 2001;108:885–896. [DOI] [PubMed] [Google Scholar]
- 10.Grill FD, Wasmaier M, Mücke T, et al. Identifying perioperative volume-related risk factors in head and neck surgeries with free flap reconstructions—an investigation with focus on the influence of red blood cell concentrates and noradrenaline use. J Craniomaxillofac Surg. 2020;48:67–74. [DOI] [PubMed] [Google Scholar]