ABSTRACT
Primary hepatic leiomyomas are rare tumors most commonly in immunosuppressed individuals who are coinfected with Epstein-Barr virus (EBV). From our literature review, there have been 50 published cases, of which 24 were immunocompetent individuals and only 5 were negative for EBV infection. We report a case of primary hepatic leiomyomas in an asymptomatic middle-aged woman without a history of immunosuppression or EBV infection.
INTRODUCTION
Leiomyomas are mesenchymal neoplasms originating from benign smooth muscle proliferation. Most commonly found in the genitourinary tract of women in their reproductive age, leiomyomas can be found in any organ containing smooth muscle of men and women.1,2 Primary hepatic leiomyomas (PHLs) are rare tumors with limited knowledge of natural history because of low prevalence.3,4 Most cases have been described in immunocompromised patients.5 We present a case of a healthy immunocompetent woman who was incidentally found with benign hepatic leiomyoma proven by biopsy.
CASE REPORT
A 52-year-old woman with prior acute hepatitis B infection with spontaneous clearance was referred to our hepatology office for evaluation of an incidental 5 cm lesion in the right hepatic lobe on abdominal ultrasound. The mass was heterogenous and slightly hypoechoic compared with the rest of the liver. Having recently established with a new primary care provider, the ultrasound had been obtained after having positive hepatitis B core (HBc) total antibody during routine labs. She was asymptomatic reporting no nausea, vomiting, abdominal pain, or change in appetite or weight. Family history was negative for inflammatory bowel disease, colorectal cancers, or liver cancers. Routine labs including complete blood count, metabolic panel, and liver enzymes were all within normal ranges. Hepatitis serologies were repeated with positive antibodies for hepatitis B surface antigen (HBs), HBc total, and hepatitis B e (HBe) with negative hepatitis C virus antibodies, HBs antigen, HBe antigen, and hepatitis B virus DNA polymerase chain reaction. Epstein-Barr virus (EBV) serologies were also negative. Tumor markers cancer antigen 19, cancer antigen 125, carcinoembryonic antigen, and α-fetoprotein were all negative. The patient was up to date on preventative immunizations and screening, including HIV screening. Given the patient's lack of common risk factors of chronic liver disease, evaluation for liver fibrosis was deferred at the time.
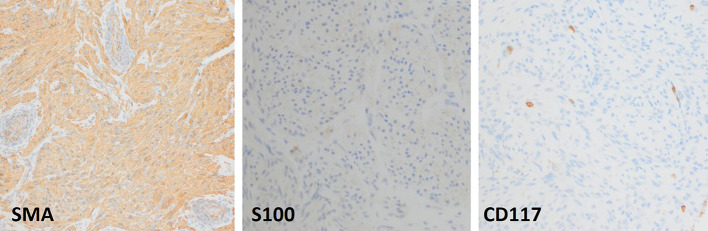
Multiphase computed tomography (CT) was obtained showing a 1.9 × 5.0 × 6.3 cm fusiform mass along with the capsule of the liver anterior to hepatic segments 8/4a with mild arterial enhancement and progressive delayed enhancement (Figure 1). Sagittal and coronal CT images showed a large hypoattenuating lesion in the right hepatic lobe (Figure 2). These CT findings were discussed with the patient, and options for further evaluation were discussed including serial imaging or biopsy. The patient chose to undergo biopsy for definitive diagnosis. Image-guided 3-core biopsy of the right lobe liver mass showed low-grade spindle cell neoplasm with myogenic differentiation (favor leiomyoma) (Figure 3). Immunohistochemistry showed the spindle cells to be positive for smooth muscle actin (SMA), negative for S100, and negative for CD117. She was diagnosed with PHL. Being asymptomatic and benign, the patient has been followed for the past three and half years with yearly CT imaging. There was an insignificant increase in size in the first year of follow-up, followed by a stable size in the subsequent years. There was no development of symptoms over the past three and half years. Given the slow growth and lack of symptoms, surgical resection was not urgent, and the medical team presented both resection and continued monitoring as options to the patient. She elected surgical removal at her 3-year follow-up, but plans for resection were ultimately canceled by the patient because of her inability to obtain leave from work during the postoperative recovery period.
Figure 1.
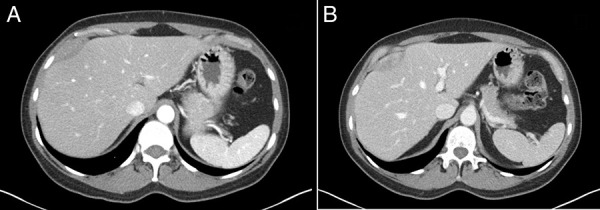
Contrast-enhanced 3-phase computed tomography demonstrating a 1.9 × 5.0 × 6.3 cm fusiform mass along the capsule of the liver anterior to hepatic segments 8/4a with mild arterial enhancement (A) and progressive delayed enhancement (B).
Figure 2.
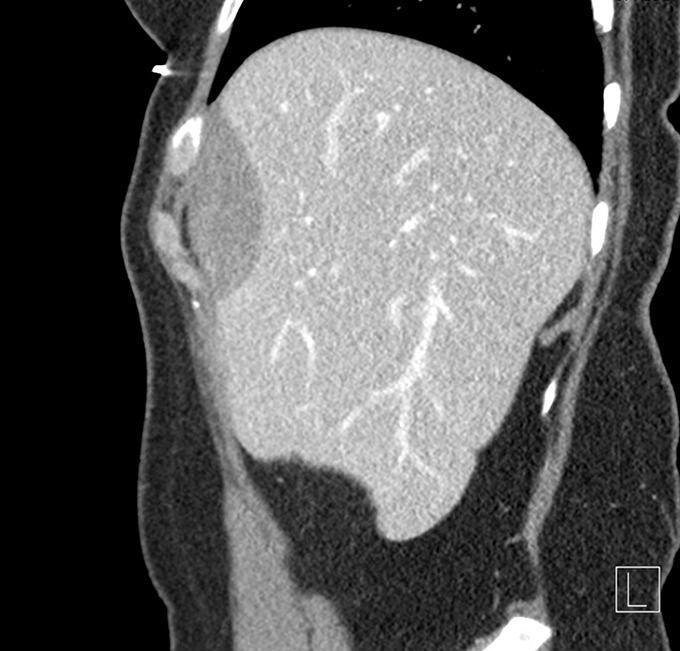
Sagittal view of contrast-enhanced computed tomography slices demonstrating fusiform mass of the anterior liver segments.
Figure 3.
Image-guided three-core biopsy of the right lobe liver mass shows low-grade spindle cell neoplasm with myogenic differentiation. Immunohistochemistry showed the spindle cells to be positive for SMA, negative for S100, and negative for CD117. Such combination of staining is diagnostic of leiomyoma. SMA, smooth muscle actin.
DISCUSSION
PHL is a rare condition with few reported cases in the literature. In most cases, patients present with symptoms related to a mass effect of the lesion, including abdominal pain and obstructive jaundice, especially in adult women.4 Occasionally such as in our case, PHL can be discovered incidentally with no symptoms reported. The neoplasm is most commonly reported in individuals who are HIV-positive or on immunosuppressive therapy after transplant, mostly in combination with EBV infection.6 Given these associations, the pathogenesis of PHL has been postulated to be a result of immunosuppression. However, our case adds to the growing evidence that EBV infection and immunocompromised states do not entirely explain the pathogenesis of this disease and argues in favor of a more multifactorial etiology. Since the first described case in 1926 by Demel et al, there have been less than 50 cases of PHL reported in the medical literature.7 Among all reported cases, only 24 cases were among immunocompetent patients.8
PHL diagnostic criteria as described by Hawkins et al require that the tumor in question originates from hepatic mesenchymal tissue and that there are no primary tumors elsewhere in the body including the urogenital organs.4 Histologic evidence of primary spindle cells, bundles of smooth muscle cells, and eosinophilic cytoplasm are ideal for accurate diagnosis. Despite these well-known criteria, diagnosis of PHL remains a challenge because of similar imaging features as other benign hepatic tumors, wide variability in laboratory testing capabilities, and difficulties defining specific tumor markers. Furthermore, it should be noted that lesions identified on contrast-enabled cross-sectional imaging are often nonspecific. As such histologic evidence is of paramount importance in making such a diagnosis. Although positive staining for SMA is the hallmark in most cases of PHL, few cases of positive staining for desmin and H-caldesmon have been observed.9 Furthermore, negative staining for Dog-1 and CD117 distinguished PHLs from the gastrointestinal stromal tumors while negative reactivity for S-100 rules out leiomyoma of the central nervous system and CD34 excludes vascular leiomyoma. Therefore, our patient fulfilled criteria for biomarkers because negative CD117 ruled out gastrointestinal stromal tumors and S100 ruled out central nervous system leiomyoma. Most importantly, positive testing for SMA was confirmatory.
Although rare, PHL should be considered in differential for solid lesion of the liver. Confirmation of diagnosis is accomplished by appropriate tissue staining and immunohistochemistry for smooth muscle antibodies. Whenever a diagnosis of PHL is suspected or established (through biopsy and histology), imaging (CT, magnetic resonance imaging) should be performed to evaluate the rest of the abdomen and pelvis for other possible leiomyomas.
DISCLOSURES
Author contributions: All authors contributed to the study's conception and design. Material preparation, patient consent, and clinical care were obtained and provided by K. Qureshi. The first draft of the manuscript was written by EC Nwankwo Jr, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. EC Nwankwo Jr is the article guarantor.
Financial disclosure: None to report.
Previous presentation: This case was presented at the American College of Gastroenterology National Meeting; October 2021; Las Vegas, NV.
Informed consent was obtained for this case report.
Contributor Information
Samuel J. Burton, Email: samuel.burton@health.slu.edu.
Tim Brotherton, Email: tim.brotherton@health.slu.edu.
Danielle Carpenter, Email: danielle.carpenter@health.slu.edu.
Kamran Qureshi, Email: kamran.qureshi@health.slu.edu.
REFERENCES
- 1.Omiyale AO. Primary leiomyoma of the liver: A review of a rare tumour. HPB Surg. 2014;2014:959202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blas Laina JL, González Ruiz Y, Gonzalvo González E, Sanz Moncasi MP, Rodríguez Borobia A. Primary hepatic leiomyoma: A rare liver mass. Gastroenterol Hepatol. 2017;40(9):617–9. [DOI] [PubMed] [Google Scholar]
- 3.Vyas S, Psica A, Watkins J, Yu D, Davidson B. Primary hepatic leiomyoma: Unusual cause of an intrahepatic mass. Ann Transl Med. 2015;3(5):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo XZ, Ming CS, Chen XP, Gong NQ. Epstein-Barr virus negative primary hepatic leiomyoma: Case report and literature review. World J Gastroenterol. 2013;19(25):4094–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demel R. Ein operierter fall von leber-myom. Virchows Arch Pathol AnatPhysiol Klin Med. 1926;261(3):881–4. [Google Scholar]
- 6.Magg T, Schober T, Walz C, et al. Epstein-Barr virus + smooth muscle tumors as manifestation of primary immunodeficiency disorders. Front Immunol. 2018;9:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Djokic M, Hadzialjevic B, Luzar B, Trotovsek B. Primary hepatic leiomyoma in a healthy middle-aged woman: Literature review and case report. Front Surg. 2021;8:691674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coletta D, Parrino C, Nicosia S, et al. Primary leiomyoma of the liver in an immunocompetent patient. Intractable Rare Dis Res. 2020;9(4):251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marin D, Catalano C, Rossi M, et al. Gadobenate dimeglumine-enhanced magnetic resonance imaging of primary leiomyoma of the liver. J Magn Reson Imaging. 2008;28(3):755–8. [DOI] [PubMed] [Google Scholar]