Abstract
Antimicrobial peptides, such as defensins or cathelicidins, are effector substances of the innate immune system and are thought to have antimicrobial properties that contribute to host defense. The evidence that vertebrate antimicrobial peptides contribute to innate immunity in vivo is based on their expression pattern and in vitro activity against microorganisms. The goal of this study was to investigate whether the overexpression of an antimicrobial peptide results in augmented protection against bacterial infection. C57BL/6 mice were given an adenovirus vector containing the cDNA for LL-37/hCAP-18, a human cathelicidin antimicrobial peptide. Mice treated with intratracheal LL-37/hCAP-18 vector had a lower bacterial load and a smaller inflammatory response than did untreated mice following pulmonary challenge with Pseudomonas aeruginosa PAO1. Systemic expression of LL-37/hCAP-18 after intravenous injection of recombinant adenovirus resulted in improved survival rates following intravenous injection of lipopolysaccharide with galactosamine or Escherichia coli CP9. In conclusion, the data demonstrate that expression of an antimicrobial peptide by gene transfer results in augmentation of the innate immune response, providing support for the hypothesis that vertebrate antimicrobial peptides protect against microorganisms in vivo.
The innate host defense system of vertebrates defends against infection during the initial phase of exposure. It provides a basal level of protection and interacts in various ways with the adaptive immune system (6). Antimicrobial peptides form one group of effector components of the innate host defense system which are found in myeloid cell-derived host defense cells, such as neutrophils and macrophages, as well as epithelia (18).
Several families of antimicrobial peptides that differ with respect to the presence of disulfide linkages, amino acid composition, structural conformation, and spectrum of activity have been described. In humans, a number of defensins and one cathelicidin peptide have been described (1, 2, 4, 14, 17, 19). Members of these families are thought to act as antibiotics in vivo and to interact with mediators or cellular components of the immune system (18). An assessment of the relative contribution of individual antimicrobial proteins or peptides to innate immune responses is quite challenging. Biochemical methods have been used to isolate and detect the molecules from biological samples. Functional studies have been restricted primarily to in vitro experiments with purified components and do not necessarily reflect the complexity of component interactions, such as synergism and antagonism. In fact, evidence that antimicrobial peptides actually contribute to innate immunity in vivo is largely indirect. Genetic approaches eliminating or overexpressing specific components of the host defense apparatus in model systems may help clarify these issues. For example, we previously showed that killing of Pseudomonas aeruginosa by secretions of a xenograft model of human airways is diminished if expression of hBD-1 is inhibited in situ with antisense oligonucleotides (12).
Cathelicidins are peptide antibiotics that are receiving increased attention. These peptides contain a highly conserved signal sequence and pro-region (“cathelin”) but show substantial heterogeneity in the C-terminal domain that encodes the mature peptide, which can range in size from 12 to 80 amino acids or more (23). The only human cathelicidin was isolated from human bone marrow by two groups (1, 17). The C-terminal 37-amino-acid mature peptide encoded by this gene is termed LL-37, while the unprocessed peptide is referred to as LL-37 or hCAP-18. LL-37/hCAP-18 is expressed in myeloid cells, where it resides in granules and is also found on body surfaces, such as skin and respiratory epithelia, where it is secreted by epithelial cells into the airway surface fluid (3, 9, 13). In addition to the ability of LL-37/hCAP-18 to kill microorganisms, peptides derived from it bind to lipopolysaccharide (LPS) and blunt some of its biological effects in animal models (16). This property has also been described for other host defense proteins, such as LPS-binding protein and bactericidal permeability-increasing protein, and likely minimizes the effects of bacterial infection to the host (7, 8).
The aim of this study is to analyze the impact of overexpression of a naturally occurring human antimicrobial cathelicidin peptide (LL-37/hCAP-18) in murine models of infection and sepsis.
MATERIALS AND METHODS
Mice.
Male C57BL/6 mice were obtained from Taconic and maintained in the Animal Facility of the Wistar Institute under standard care. They were used at 6 to 7 weeks of age.
Generation of recombinant adenovirus.
Recombinant adenoviruses with viral regions E1 and E3 deleted were generated as described elsewhere (5). The cDNAs coding for LL-37/hCAP-18, β-galactosidase, and alkaline phosphatase were cloned into pAd.CMV.link and used for cotransfection together with an appropriate viral backbone. After plaque purification, recombinants were grown up, purified on two sequential cesium chloride gradients, and desalted on gel filtration columns (Econo-Pac 10DG columns; Bio-Rad). Viral titers were adjusted to 5 × 1012 particles, and recombinants were stored at −80°C. All viruses contain an identical backbone and express the transgene from the 5′-flanking region of the immediate-early gene of cytomegalovirus.
Bacterial strains and LPS.
Escherichia coli CP9 is a well-characterized human sepsis isolate with documented virulence in several animal models of infection (15). Frozen bacteria were thawed and cultured overnight on Luria-Bertani (LB) agar plates. The bacteria in an arbitrarily selected single colony were cultured overnight in LB broth medium and used for the experiment. Pseudomonas aeruginosa PAO1 was grown as previously described (3). The number of CFU of the organisms was determined by quantitative cultivation on LB agar plates. LPS from wild-type Salmonella minnesota (Sigma Chemical Co.) was dissolved at 1 mg/ml in endotoxin-free H2O as a stock solution and stored at 4°C. The LPS solution was diluted appropriately in endotoxin-free phosphate-buffered saline (PBS) immediately before use in an experiment. d-(+)-Galactosamine (d-GalN) was obtained from Sigma Chemical Co.
Study protocols. (i) Lung infection model.
Mice were intratracheally given 5 × 1010 particles of vectors coding for LL-37/hCAP-18 or β-galactosidase in 50 μl of sterile PBS to analyze whether overexpression of LL-37/hCAP-18 in the respiratory system results in decreased colonization after challenge with bacterial pathogens. On day 5 of the study protocol, mice received intratracheal instillation of 5 × 106 CFU of P. aeruginosa in 50 μl of sterile PBS. The animals were euthanized after 24 h. Blood, bronchoalveolar lavage fluid (BALF), and lungs were sampled for further analysis. Transgene expression was determined in serum and BALF (see below). Levels of mouse tumor necrosis factor alpha (TNF-α) were measured in BALF by an enzyme-linked immunosorbent assay (see below). For measurement of bacterial load, the right lung was homogenized in 2 ml of sterile PBS and serial dilutions were plated onto sheep blood agar plates. After 24 h of incubation at 37°C, colonies were counted. The left lung was fixed in formalin and embedded in paraffin by standard procedures. To determine the levels of LL-37 in BALF over time, samples from mice that received 5 × 1010 particles of LL-37 vector but were not injected with bacteria were analyzed on days 1, 5, 14, and 21.
(ii) Sepsis model.
Animals were used in different study groups to investigate the effect of gene transfer of LL-37/hCAP-18. On day 1 of the protocol, mice were intravenously given 5 × 108 to 5 × 1010 particles of vector in 100 μl of sterile PBS. As a control, recombinant virus coding for β-galactosidase was used at a dose of 5 × 1010 particles. On day 5, mice were challenged by injection of LPS or bacteria. d-GalN-sensitized mice were used to determine the protective effect of expression of LL-37/hCAP-18 against the lethal effect of LPS. S. minnesota LPS (100 ng/0.2 ml) and d-GalN (18 mg/0.2 ml of saline) were sequentially injected intraperitoneally. Injections of all reagents were performed within 15 s. For bacterial challenge, fresh liquid cultures of E. coli CP9 were diluted to a final concentration of 108 CFU/ml and 100 μl of the suspension was injected intraperitoneally. These doses of LPS and bacteria killed 90 to 100% of untreated animals within 48 h. Death from infection was recorded every 24 h until day 6 after infection. All deaths occurred within 48 h of infection. On day 11, animals not treated with LPS or bacteria were sacrificed and blood and spleens were harvested for further analysis. On days 0, 2, 4, 5, 11, 21, 40, and 83 of the study, blood was withdrawn from mice not challenged with LPS or bacteria. To determine the pharmacokinetics of LL-37 in mice, 100 μg of synthetic, purified peptide (Louisiana State University Medical Center, Core Laboratories) was injected into the tail vein of mice and concentrations of peptide in serum were analyzed over time.
Detection of transgene expression.
To determine the levels of expressed LL-37/hCAP-18 in tissue culture medium, serum, or BALF, a quantitative dot blot assay was used. A 3-μl volume of sample was dotted onto nitrocellulose and detected with a polyclonal antibody against LL-37/hCAP-18 as previously described (3). Concentrations were determined by quantification of the signal intensity by using the AlphaImager 2000 analysis system (Alpha Innotec) and comparison to signals from known amounts of synthetic peptide. Immunoblots of samples were prepared with both crude material and fractions obtained from separation by reverse-phase high-pressure liquid chromatography. Serum or BALF samples were run on a 0.5- by 25-cm Dynamax-300Å C18 reverse-phase high-pressure liquid chromatography column (Rainin Instrument Co.) with a linear gradient of acetonitrile and 0.1% trifluoroacetic acid. Fractions were dried by vacuum centrifugation and resuspended in 50 μl of distilled water before being used in assays. Samples were separated in Tris-Tricine gels (10 to 20%; Bio-Rad) run under denaturing and reducing conditions prior to immunoblotting.
To analyze the expression of LL-37/hCAP-18 after gene transfer, mouse organs were harvested 5 or 6 days after injection of the recombinant vectors and used for immunohistochemistry or detection of specific transcripts by reverse transcription-PCR (RT-PCR). Poly(A)+ RNA was isolated by using Trizol (Life Technologies) and oligo(dT) column chromatography (Oligotex mRNA purification kit; Qiagen). Two primers specific to the LL-37/hCAP-18 cDNA were designed from the published sequence (Genbank accession no. Z38026) for RT-PCR as follows: GAA TTC CGG CCA TGA AGA CCC (FALL 1, nucleotides 1 to 21) and CAG AGC CCA GAA GCC TGA GC (FALL 2, nucleotides 541 to 560). The PCR products were analyzed in a 1.5% agarose gel and blotted onto a nylon membrane (Boehringer Mannheim). The Southern blots were hybridized with a [32P]dCTP random primer-labeled LL-37/hCAP-18 cDNA probe (Rediprime DNA labeling system; Amersham Life Science) and washed at high stringency. The results were recorded by autoradiography. The reverse transcriptase was omitted in the negative control, and RT-PCR with primers specific for mouse glyceraldehyde-3-phosphate dehydrogenase was used as a positive control. For immunohistochemistry of liver and lung samples, formalin-fixed and paraffin-embedded livers were sectioned at a thickness of 5 μm. The sections were dewaxed and treated with 0.3% H2O2 in PBS for 30 min. After a 30-min incubation in 5% normal goat serum at room temperature a solution of the polyclonal antibody against LL-37 (1:100 in 1% goat serum–PBS) was used for overnight incubation at 4°C. After washings, a goat anti-rabbit antibody (1:100 in 1% goat serum–PBS; Sigma Chemical Co.) was applied for 1 h at room temperature. Finally, diaminobenzidine (10 mg in 20 ml PBS–0.05% H2O2) was used as substrate. After washes in PBS and distilled water, the sections were counterstained with hematoxylin and mounted.
Detection of TNF-α levels.
To detect the levels of TNF-α in serum, BALF, and supernatants of ground organs, samples were cleared by centrifugation and assayed with a commercial enzyme-linked immunosorbent assay kit (R & D Systems). Samples were run in duplicate.
Statistical analysis.
Survival data were analyzed by the χ2 test. Differences in bacterial load and TNF-α concentrations were analyzed by Student's t test and the Mann-Whitney rank sum test, respectively. Values are displayed as mean ± standard deviation. Results were considered significant for P < 0.05 (two-tailed).
RESULTS
Pulmonary overexpression of LL-37/hCAP-18 protects against lung colonization, infection, and inflammation.
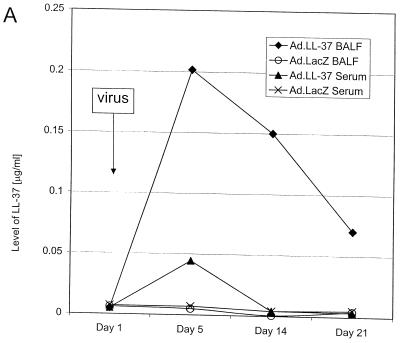
Mice were intratracheally given recombinant adenovirus vectors carrying the cDNA for LL-37/hCAP-18 or the gene for β-galactosidase as a control. Levels of peptide in blood and BALF were analyzed by a quantitative dot blot assay. Whereas application of the control vector did not result in a detectable signal over background in these biological samples, administration of the LL-37/hCAP-18-encoding vector yielded high concentrations of the mature peptide in BALF and smaller amounts in the serum (Fig. 1A).
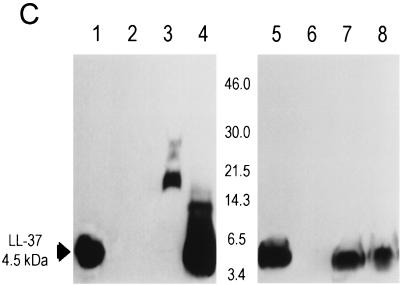
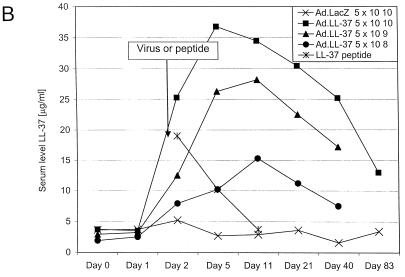
FIG. 1.
LL-37 in BALF and serum after administration of the recombinant virus or synthetic peptide. (A) Levels of LL-37 in BALF as determined by quantitative dot blot analysis. Virus was injected on day 1 of the experiment, and concentrations in BALF were determined on the following days. (B) Levels of LL-37 in serum as determined by quantitative dot blot analysis. Virus or peptide was injected on day 1 of the experiment, and concentrations in serum were determined by bleeding the animals and using the serum for qualitative dot blot analysis. (C) Western blots following denaturing polyacrylamide gel electrophoresis under reducing conditions with Tricine gels of mouse serum and BALF, using a polyclonal antibody against LL-37/hCAP-18. Lanes: 1, 20 ng of synthetic LL-37 peptide; 2, serum from a mouse that received the control vector coding for β-galactosidase; 3, serum from a mouse that received the vector coding for LL-37/hCAP-18 (crude); 4, serum from a mouse that received the vector coding for LL-37/hCAP-18 (RP-HPLC purified); 5, 20 ng of synthetic LL-37 peptide; 6, BALF from a mouse that received the vector coding for β-galactosidase (crude); 7 and 8, BALF from a mouse that received the vector coding for LL-37/hCAP-18 (crude, lane 7; HPLC purified, lane 8).
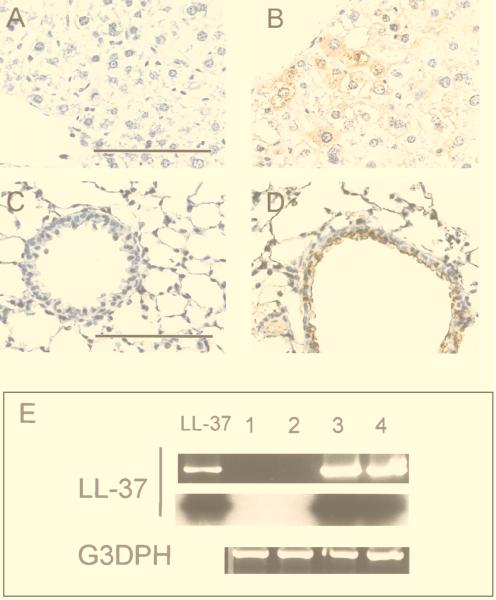
To analyze the structure of the secreted peptide, BALFs were used for immunoblotting with a polyclonal antibody to LL-37/hCAP-18. LL-37 in crude BALF migrated as a single band of approximately 4.5 kDa, which contrasts to the results in serum, in which the peptide appeared in a complex of high-molecular-mass proteins (Fig. 1C). RT-PCR and immunohistochemistry were used to detect transgene expression in lungs of mice. Immunohistochemistry showed LL-37/hCAP-18-positive cells in the large airways of mice that received the vector coding for LL-37/hCAP-18 (Fig. 2D); these cells were not seen in animals that received a control vector (Ad.AlkPhos, Fig. 2C); RT-PCR revealed the presence of recombinant-derived LL-37/hCAP-18 mRNA in the lungs of treated mice (Fig. 2E). Omission of the reverse transcriptase resulted in no PCR product (data not shown).
FIG. 2.
Expression of LL-37/hCAP-18 in mouse liver and lungs after gene transfer. Organs were harvested 5 days after gene transfer and analyzed for transgene expression by RT-PCR and immunohistochemistry. Immunohistochemistry with polyclonal antibodies to LL-37/hCAP-18 revealed signals in hepatocytes (B) or epithelial cells of airways (D) of mice that received LL-37 vector but not in those of mice treated with Ad.AlkPhos vector (A and C). Bar, 100 μm. (E) RT-PCR was performed with LL-37/hCAP-18-specific primers and revealed the presence of transcripts only in mice after application of LL-37 vector. Amplification of glyceraldehyde-3-phosphate dehydrogenase (G3PDH) was used as a positive control. The PCR products were blotted and hybridized to an LL-37/hCAP-18-specific probe. Lane LL-37, positive control with plasmid DNA; lanes 1 and 2, PCR on RNA extracted from lungs (lane 1) or liver (lane 2) obtained from a mouse treated with lacZ vector; lanes 3 and 4, PCR on RNA extracted from lungs (lane 3) or liver (lane 4) obtained from a mouse treated with LL-37 vector.
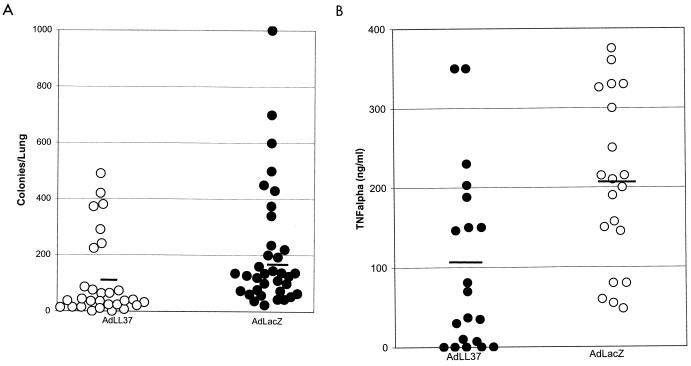
To investigate the functional consequences of LL-37/hCAP-18 overexpression in mouse airways, vector-treated animals were challenged 5 days later with a sublethal dose of P. aeruginosa PAO1. Compared to the control animals, which received the lacZ vector, mice expressing LL-37/hCAP-18 had a significantly smaller number of bacteria in their lungs (Fig. 3A) and a lower level of TNF-α in the BALF (Fig. 3B).
FIG. 3.
Effect of gene transfer of LL-37/hCAP-18 to the respiratory tracts of mice on the bacterial load and inflammatory response. Mice were injected intratracheally on day 1, challenged with bacteria on day 5, and euthanized on day 6. Individual data points are presented. The bar represents the mean. (A) The bacterial load was significantly decreased in mice that received the LL-37/hCAP-18-encoding vector (Ad.LL-37) (P < 0.005). (B) Levels of TNF-α were significantly lower in mice that received the LL-37/hCAP-18-encoding vector (P < 0.05) (40 mice per group).
Systemic overexpression of LL-37/hCAP-18 protects against septic death.
Intravenous administration of the vector coding for LL-37/hCAP-18 resulted in an acute rise in the concentration of the LL-37 peptide in serum, which peaked at 36 ± 7 μg/ml after 5 days (Fig. 1B). The level of LL-37 in serum slowly declined, but the peptide was still present 83 days after injection. The control vector did not result in a detectable change of the signal over background. Peak levels of LL-37 in serum correlated with the dose of LL-37 vector (Fig. 1B). To study the pharmacokinetics of LL-37 after intravenous administration, 100 μg of synthetic peptide was injected into mice and the levels in serum were monitored and indicated a half-life of approximately 3.4 days (Fig. 1B). Crude serum samples electrophoresed under denaturing and reducing conditions demonstrated a high-molecular-weight smear with few distinct bands (Fig. 1C). Fractionation of serum by RP-HPLC before electrophoresis resulted in a band that comigrated with synthetic LL-37 (C-terminal 37 amino acids) (Fig. 1C). The distribution of LL-37 expression in the liver was evaluated by immunohistochemistry. LL-37/hCAP-18-expressing hepatocytes could be detected diffusely throughout the hepatic lobes of mice that received the vector coding for LL-37/hCAP-18 (Fig. 2B); this was not seen in animals that received the lacZ vector (Fig. 2A). RT-PCR showed the presence of LL-37/hCAP-18 mRNA (Fig. 2E). Omitting the reverse transcriptase resulted in no PCR product (data not shown). Overexpression of LL-37/hCAP-18 did not affect antigen-specific B- or T-cell responses to the vector (data not shown).
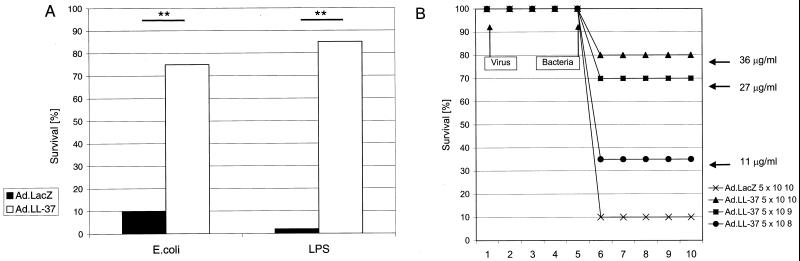
To investigate whether the high levels of mature LL-37 in serum after gene transfer have a biological effect in protecting animals from consequences of endotoxemia, d-GalN-sensitized mice were challenged with LPS or bacteria. Related experiments have shown that intravenous or intraperitoneal injection of various forms of LL-37/hCAP-18 peptides does protect animals against septic death by binding to endotoxins and inhibiting the release of TNF-α (16). Mice were injected with LPS together with galactosamine or with E. coli CP9 5 days after vector administration and monitored for mortality. The control animals treated with vector expressing β-galactosidase experienced 90 to 100% mortality within 24 h (Fig. 4A), which is equivalent to results in animals not treated with vector. Mortality was decreased to 15 to 25% in animals that received LL-37/hCAP-18 vector (Fig. 4A). A clear relationship between the dose of vector, the LL-37 concentration in serum, and improved survival was demonstrated (Fig. 4B).
FIG. 4.
Effect of the systemic overexpression of LL-37/hCAP-18 in mice on survival after intraperitoneal injection of LPS (in galactosamine-sensitized mice) or gram-negative bacteria. (A) Mice received either lacZ or LL-37 vector on day 1 and were injected with LPS plus galactosamine or E. coli CP9 on day 5. Survival of the animals that were treated with LL-37 vector (Ad.LL-37) (5 × 1010 particles) was significantly (∗∗, P < 0.05) increased compared to survival of the mice that received the same dose of lacZ control vector (Ad.lacZ) (20 mice in each group). (B) Dose-dependent survival of animals treated with different amounts of LL-37/hCAP-18-encoding virus. Whereas the control group treated with β-galactosidase-encoding vector showed high mortality after injection, the animals that received LL-37 vector showed increased survival rates that correlated with the amount of virus applied and therefore with the level of the LL-37 peptide in serum (10 mice in each group).
DISCUSSION
Antimicrobial peptides are part of the innate immune system of many species and are thought to provide protection against bacteria, fungi, and viruses, either by directly killing or binding to bacterial endotoxin and blunting the biological effects of infection (18). It has been shown previously that systemic administration of peptide derivatives of LL-37/hCAP-18 blunts the clinical consequences of septic shock in animal models. These effects are mediated by binding of the cationic peptide antibiotics to endotoxin, neutralizing LPS, and decreasing the release of TNF-α (16). Evidence that mammalian antimicrobial peptides actually contribute to innate immunity in vivo is based primarily on their expression patterns and in vitro activity against microorganisms. Functional studies have been restricted primarily to in vitro experiments with purified peptides and do not necessarily reflect the complexity of in vivo interactions, such as synergism and antagonism between individual substances. Genetic approaches eliminating or overexpressing host defense substances in model systems provide one way of analyzing the function of individual antimicrobial proteins or peptides.
In the present study, we demonstrated that expression of an antimicrobial peptide after pulmonary or systemic gene transfer results in high concentrations of mature peptide in BALF and serum, respectively. Systemic overexpression of LL-37/hCAP-18 improved the survival of mice after injection of LPS or E. coli CP9, while intrapulmonary expression of recombinant peptide lowered the bacterial load and inflammatory response after pulmonary infection. LL-37/hCAP-18 was chosen because it has been implicated in the host defense system of phagocytes and mucosal surfaces and its function is reasonably well understood. LL-37/hCAP-18 resides in neutrophil granulocytes and epithelia, such as the skin and respiratory system (3, 9, 10, 13, 17). Concentrations of this peptide in plasma in humans are astonishingly high (1.18 μg/ml), indicating that this antimicrobial peptide has sources other than neutrophils and that its presence in serum might have protective functions (20). High concentrations of mature peptide could be achieved in BALF and serum of mice after in vivo gene transfer. After intrapulmonary administration of vector, expression was localized in the large airways, resulting in LL-37 concentrations in BALF of 0.2 μg/ml, which translates to 10 to 20 μg/ml in the airway surface fluid, assuming that BALF is diluted 1:100 with respect to airway surface fluid. The peptide in BALF was clearly dissociated from other proteins during denaturing electrophoresis, as evidenced by its migration pattern as single band of approximately 4.5 kDa. In serum, however, LL-37 is seen as a larger, high-molecular-mass smear. It is well known that LL-37 binds serum proteins tightly (22). After intravenous administration of the recombinant adenovirus vector, most transgene expression is usually found in the liver. In our study, expression of LL-37/hCAP-18 was detected by immunohistochemistry in hepatocytes distributed throughout hepatic lobes. Cultured primary hepatocytes synthesized mature 37-amino-acid LL-37 following transduction with the LL-37 vector (data not shown). As described for humans, LL-37 secreted into the blood of the mice is mainly bound to high-molecular-mass proteins not dissociated in denaturing gels, possibly blunting most of the killing activity of the peptide against bacteria but still allowing binding to LPS (21, 22).
The mechanism of action of LL-37 in the blood most probably involves binding to LPS and blunting its biological activities. This was demonstrated previously by injection of various forms of LL-37/hCAP-18 peptides into mice (16). In the lungs, peptide had antimicrobial activity, as shown by decreased bacterial counts in the murine model of pneumonia. Further, the inflammatory response, as measured by TNF-α concentrations, was decreased in animals that were intratracheally given the LL-37/hCAP-18-encoding vector. This is probably due to decreased bacterial load and binding of bacterial endotoxins.
In summary, the data presented in this study support the notion that expression of a mammalian antimicrobial peptide in a different species provides protection against bacterial pathogens. This was demonstrated for septic shock as well as for colonization and infection of mucosal surfaces. This supports the suggested role of LL-37/hCAP-18 as a host defense molecule in phagocytes, epithelia, and the blood.
In addition to the basic biological questions addressed in this study, the results of our experiments suggest that overexpression of antimicrobial substances by means of gene transfer or upregulation of gene expression with inducers may be a feasible way to treat infection and endotoxemia. The administration of several endogenous host defense peptides or proteins, such as LPS-binding protein and bactericidal permeability-increasing protein, has already been used in animal models and clinical trials for prevention of septic shock (11).
The data presented in this study provide proof that antimicrobial peptides protect against the consequences of bacterial infection in vivo, highlighting the role of these substances as part of the innate host defense system. The protective effect resulting from LL-37 gene transfer suggests new strategies for developing treatment of infections and favorably modifying the host response.
ACKNOWLEDGMENTS
We thank the Animal Model Group and the Cell and Morphology Core of the Institute for Human Gene Therapy.
This work was supported by the Cystic Fibrosis Foundation and the NIH (P30 DK47757, P50 DK49136, and P01 HL 49040), as well as Genovo, Inc., a biotechnology company that J.M.W. founded and in which he has equity. R.B. was a recipient of a fellowship of the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Agerberth B, Gunne H, Odeberg J, Kogner P, Boman H G, Gudmundsson G H. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci USA. 1995;92:195–199. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bals R, Wang X, Wu Z, Freeman T, Banfa V, Zasloff M, Wilson J M. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Investig. 1998;102:874–880. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bals R, Wang X, Zasloff M, Wilson J M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA. 1998;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bensch K W, Raida M, Magert H-J, Schulz-Knappe P, Forssmann W-G. hBD-1: a novel β-defensin from human plasma. FEBS Lett. 1995;368:331–335. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 5.Engelhardt J F, Yang Y, Stratford-Perricaudet L D, Allen E D, Kozarsky K, Perricaudet M, Yankaskas J R, Wilson J M. Direct gene transfer of human CFTR into human bronchial epithelia of xenografts with E1-deleted adenoviruses. Nat Genet. 1993;4:27–34. doi: 10.1038/ng0593-27. [DOI] [PubMed] [Google Scholar]
- 6.Fearon D, Locksley R. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–54. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 7.Fenton M, Golenbock D. LPS-binding proteins and receptors. J Leukoc Biol. 1998;64:25–32. doi: 10.1002/jlb.64.1.25. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher M, Kloczewiak M, Loiselle P, Ogata M, Vermeulen M, Zanzot E, Warren H. A novel peptide-IgG conjugate, CAP18(106–138)-IgI, that binds and neutralizes endotoxin and kills gram-negative bacteria. J Infect Dis. 1997;175:621–632. doi: 10.1093/infdis/175.3.621. [DOI] [PubMed] [Google Scholar]
- 9.Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, Gudmundsson G H. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 10.Frohm M, Gunne H, Bergman A-C, Agerberth B, Bergman T, Boman A, Liden S, Jornvall H, Boman H. Biochemical and antibacterial analysis of human wound and blister fluid. Eur J Biochem. 1996;237:86–92. doi: 10.1111/j.1432-1033.1996.0086n.x. [DOI] [PubMed] [Google Scholar]
- 11.Giroir B, Quint P, Barton P, Kirsch E, Kitchen L, Goldstein B, Nelson B, Wedel N, Carroll S, Scanon P. Preliminary evaluation of recombinant amino-terminal fragment of human bactericidal/permeability-increasing protein in children with severe meningicoccal sepsis. Lancet. 1997;350:1439–1442. doi: 10.1016/s0140-6736(97)06468-4. [DOI] [PubMed] [Google Scholar]
- 12.Goldman M J, Anderson G M, Stolzenberg E D, Kari U P, Zasloff M, Wilson J M. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–560. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 13.Gudmundsson G H, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem. 1996;238:325–332. doi: 10.1111/j.1432-1033.1996.0325z.x. [DOI] [PubMed] [Google Scholar]
- 14.Harder J, Bartels J, Christophers E, Schroeder J-M. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 15.Johnson J, Brown J. Defining inoculation conditions for the mouse model of ascending urinary tract infection that avoid vesicoureteral reflux yet produce bladder infection. J Infect Dis. 1996;173:746–749. doi: 10.1093/infdis/173.3.746. [DOI] [PubMed] [Google Scholar]
- 16.Kirikae T, Hirata M, Yamasu H, Kirikae F, Tamura H, Kayama F, Nakatsuka K, Yokochi T, Nakano M. Protective effects of a human 18-kilodalton cationic antimicrobial protein (CAP18)-derived peptide against murine endotoxemia. Infect Immun. 1998;66:1861–1868. doi: 10.1128/iai.66.5.1861-1868.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larrick J, Hirata M, Balint R, Lee J, Zhong J, Wright S. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehrer R, Ganz T. Antimicrobial peptides in mammalian and insect host defense. Curr Opin Immunol. 1999;11:23–27. doi: 10.1016/s0952-7915(99)80005-3. [DOI] [PubMed] [Google Scholar]
- 19.Lehrer R, Ganz T, Selsted M. Defensins: endogenous antibiotic peptides of animal cells. Cell. 1991;64:229–230. doi: 10.1016/0092-8674(91)90632-9. [DOI] [PubMed] [Google Scholar]
- 20.Sorensen O, Arnljots K, Cowland J B, Bainton D F, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–2803. [PubMed] [Google Scholar]
- 21.Turner J, Cho Y, Dinh N-N, Waring A, Lehrer R. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother. 1998;42:2206–2214. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Agerberth B, Lothgren A, Almstedt A, Johansson J. Apolipoprotein A-I binds and inhibits the human antibacterial/cytotoxic peptide LL-37. J Biol Chem. 1998;273:33115–33118. doi: 10.1074/jbc.273.50.33115. [DOI] [PubMed] [Google Scholar]
- 23.Zanetti M, Gennaro R, Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]