Background:
Breast augmentation is one of the most common aesthetic surgical procedures. Tissue expansion followed by permanent implants is the most frequent postmastectomy breast reconstruction method. Implant contamination remains a critical problem with these procedures‚ resulting in acute infection as well as capsular contracture. To reduce the risk of implant contamination, the “no-touch technique” utilizing the Keller funnel has been adopted by many surgeons. This systematic review aims to investigate the advantages of the Keller funnel method for breast augmentation–reconstruction.
Methods:
A systematic review of PubMed, Embase, the Cochrane database, and Google Scholar was performed between 2005 and 2021. All clinical-based, retrospective and prospective studies utilizing the Keller funnel method for breast implant insertion were selected.
Results:
Six studies were identified for evaluation: five were retrospective cohorts and one was a prospective trial. No randomized controlled trials were found. Outcomes reported included lower rates of capsular contracture (RR, 0.42; P = 0.0006; 95% CI, 0.25–0.69), shorter incision lengths (35.5 ± 2.1 mm), less insertion time (mean = 6 seconds), and decreased complications, and one paper reported ultimately greater patient satisfaction with outcomes (BREAST-Q Score: 92%).
Conclusions:
This review suggests that the Keller funnel is a useful method for no-touch breast augmentation and reconstruction surgery. The Keller funnel reduces subsequent capsular contracture rate, surgical time, and incision length and allows for easier insertion. However, our findings support recommendation of a prospective randomized controlled clinical trial with larger population size and follow-up intervals.
Takeaways
Question: What is the effect of the Keller funnel on breast implant-based surgery?
Findings: A systematic review was performed demonstrating that Keller funnel use is associated with a shorter operating time and decreased implant contamination and incision length. Additionally, this method minimizes shell trauma and leads to quick healing, reducing postoperative pain.
Meaning: Keller funnels allow for breast implants to be inserted using an enhanced “no touch” method.
INTRODUCTION
Breast augmentation (BA) is the second most-performed procedure in plastic surgery with a total of 252,022 augmentations performed by board-certified plastic surgeons alone in 2020 at a total cost of $180,084,082.1,2 Tissue expansion followed by permanent implant implantation continues to be the most common approach for postmastectomy breast reconstruction. In the United States, these operations are performed on a yearly basis by more than 137,808.3 The most common complication after BA is infection (1.7% acute and 0.8% late).4–6 Subclinical infection can also occur when the implant is contaminated. The subsequent formation of biofilms from subclinical infections has been implicated as a factor in the development of capsular contracture.7,8 Significant capsular contracture may necessitate capsulectomy and revision surgery including but not limited to implant exchange.9 Overall, capsular contracture results in a reduced quality of life for augmentation patients. Therefore, the prevention of subclinical infections and resulting capsular contracture is paramount in BA.4,10,11 It has been proposed that the predominant component to implant contamination is from the bacteria of the nipple-areola complex and surrounding skin.12 Numerous methods have been articulated to prevent tissue contact with the implant. One of the most common of these methodologies is the “no-touch technique.”
The no-touch technique was initially described as retraction of the skin around the incision from contacting the implant during placement to avoid bacterial contamination.13 Currently, several techniques utilizing barriers to achieve no-touch have been published, such as double-implant loading, reversed glove sleeve, infusion containers, packaged implant cover, tissue expander-acellular dermal matrix (ADM)-self-retaining retractor, plastic sleeve, and the Keller funnel.12–17
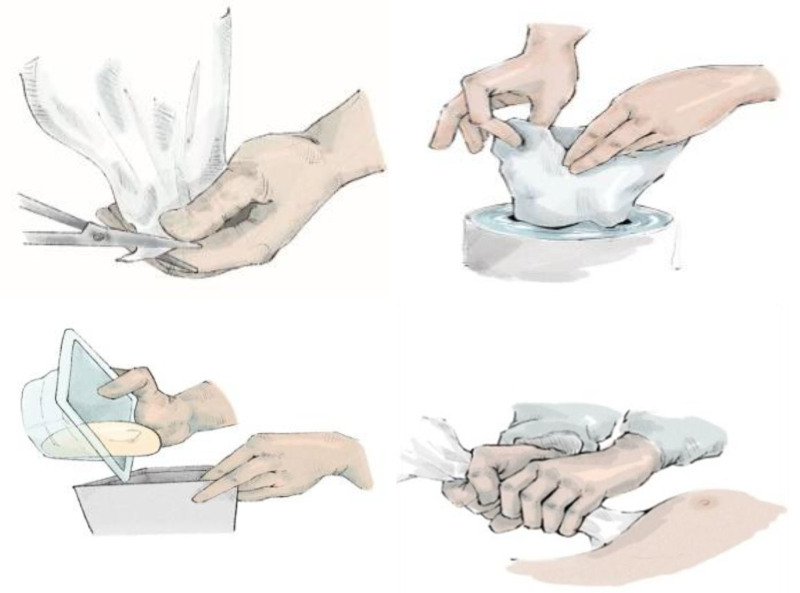
The Keller funnel is an FDA-approved class 1 prosthesis based on a nylon sleeve made of a transparent polymeric material with a hydrophilic coating that allows the implant to slide into the pocket. It contains standardized markings on the outside, which represent a specific diameter that can be cut based on implant size. After the implant is loaded into the Keller device, the funnel is inserted about 1 cm into the incision. The funnel is then squeezed from the top end providing a downward pressure to slide the implant into the breast pocket without requiring any contact with the surgeon’s gloves or the surrounding skin (Fig. 1).
Fig. 1.
Standard protocol for implant placement using Keller funnel. The funnel is cut distally depending on desired implant size, and the funnel is lubricated. The implant is placed inside without touching the implant, the distal funnel tip is inserted into the pocket, and manual pressure is applied to guide the implant into the pocket.
Although the Keller funnel is widely used and probably the most common of the no-touch techniques, according to a survey, 69% are used in breast reconstruction surgery, and 48% are used in BA surgery, and other methods have not been widely adopted.18,19 There is a paucity of literature critically appraising its utility in mitigating capsular contracture and reoperation rates. The goal of this study is to evaluate the role of the Keller funnel as a no touch technique via systematic review.
METHOD
The systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses.20 Literature search, article screening, and data collection were conducted independently by two of the authors (S.M. and M.O.). Conflicting opinions were resolved by consensus between the two researchers, and duplicates were removed from the search process.
Search Strategy
Systematic review was conducted for all articles from January 2005 to January 2021 utilizing the keywords Keller funnel, funnel, no touch, BA, breast surgery, breast reconstruction, implant insertion, breast implant, implant capsular contracture, and postoperative complication. The search was conducted in PubMed, Embase, Google Scholar, and the Cochrane database. The bibliographies of included studies were also searched to identify additional articles.
Inclusion Criteria
Inclusion criteria included randomized controlled trials, prospective trials, retrospective studies, and studies articulating any breast implant-based surgery utilizing the Keller funnel.
Exclusion Criteria
Exclusion criteria included studies describing animal models, cadaver studies, abstracts and conference proceedings without full text, ongoing trials without complete/final data, opinion pieces, letters to the editor, or nonquantitative articles, review articles, or articles written in a language other than English.
Quality Assessment and Data Extraction
Selected articles were evaluated using the Newcastle–Ottawa Scale for methodological quality. Data extraction was performed independently by two reviewers; disagreements were resolved through consensus.
Key data were compiled, including author, date of published article, population, intervention (preoperative, perioperative, and postoperative), study type, comparator, outcomes, follow-up, limitations, type of surgical procedure, and patient number. Outcomes assessed included incision length, insertion time, immediate postoperative complications, and delayed complications including but not limited to capsular contracture. All selected articles are discussed in the text.
Data Analysis
A meta-analysis was performed on the data using RevMan 5.4.1. Risk ratio was reported with 95% confidence intervals.
RESULTS
Initially‚ 3874 articles were identified on literature review. Figure 2 shows a flow chart of studies that were included and excluded. Table 1 shows the number of reviews identified by the year of publication. Three hundred eighty-three records were screened based on titles and abstracts, and a further 3491 were removed for failing to meet the criteria. The remaining 15 full-text articles were assessed for eligibility, and of these, only five retrospective cohort studies and one prospective fulfilled the review requirements (Fig. 2). No randomized controlled trials were found. Flugstad et al21 used triple antibiotics (bacitracin, cefazolin, and gentamicin) plus betadine and silicone gel implant. No incision type was reported. Newman and Davison22 used single antibiotic (clindamycin or cefazolin) plus betadine, silicon saline implant, and periareolar incision. Montemurro et al23 used single antibiotics (clindamycin), anatomical implants, and inframammary fold (IMF) incision. Woo et al24 used double antibiotics (gentamicin and cetrazole), anatomic implant (silicone gel), and peri areolar incision. Jang and Kim25 used different implants, such as anatomic implants, smooth, cohesive gel, and round microtexture, and used IMF incision. Chen et al26 all used textured implants. There was no mention of the incision type or antibiotics.
Fig. 2.
PRISMA diagram demonstrating the process of the systematic review. Figure acquired from Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews.20
Table 1.
Results of the Systematic Review Broken Down by Study Type, Incision Placement, Patient Demographics, Funding, Limitations, and Follow-up
| Study | Study Type | Procedure (Protocol, Population) | Insertion and Implant Type | Funding | Follow-up | Outcome | Limitations | Demographics | Incision Type |
|---|---|---|---|---|---|---|---|---|---|
| Flugstad, 201621 | Retrospective multicenter | Primary BA | KF 1, Silicone gel implants | Keller Medical, Inc. (Stuart, Florida) | 1 y | Capsular contracture rate decreased | Specific BA-related complications not reported | NR | NR |
| No Funnel: 1177 BA (2354 implants) | |||||||||
| Overall reoperation rate decreased | |||||||||
| Funnel: 1620 BA (3240 implants) | |||||||||
| Newman, 201822 | Retrospective single center | Periareolar BA | KF1, KF2, silicone, and saline implants | N/A | 12 mo to 7 y | Significantly decreased rate of capsular contracture with KF | Small sample size | NR | Periareolar |
| No funnel: 15 BA (30 implants) | |||||||||
| Funnel: 151 BA (300 implants) | |||||||||
| Montemurro, 201923 | Prospective single center | Primary BA | KF (Allergan, Dublin, Ireland) anatomical implants | N/A | 4 y (time) and 12 mo (length) |
Less implant insertion time | Small sample size for surgery time | All female Mean age: manual insertion: 34.4 and KF insertion: 35 |
Inframammary fold (IMF) |
| Surgical time: 10 KF, 10 without | |||||||||
| Shorter incision | |||||||||
| Lack of imaging for rupture surveilance | |||||||||
| Less damage to implant surface | |||||||||
| Incision length: 118 no KF, 50 KF | |||||||||
| Woo, 202024 | Retrospective single center | Nipple-sparing mastectomy of 21 cases (23 implants) |
Art funnel, anatomic-textured implants, and ADM | N/A | January 2017 and July 2019 | ADM-covered implant: easy insertion, short scar, and less pain | Small sample size | NR | Periareolar incision |
| Not compared with other incisions | |||||||||
| Useful for large implants | Short follow-up | ||||||||
| Jang, 202025 | Retrospective single center | Total mastectomy after radiotherapy, 15 patients | Art funnel | N/A | 6 and 12 mo | Short incision | Small sample size | Mean age: 44 (38–54) | Prior incision |
| Round (microtexture) implants | Less wound-related complications (postadjuvant radiotherapy) | ||||||||
| Short follow-up | |||||||||
| No control group | |||||||||
| Anatomical (macro texture) implants | |||||||||
| Chen, 201826 | Prospective trial | BA, unknown sample size | Keller funnel, textured implants | NR | NR | Easy textured implant insertion | Unknown sample size | NR | NR |
| No foreign body reaction | Unknown incision | ||||||||
| Unknown follow-up |
NR, not recorded; N/A, not applicable; KF, Keller funnel.
Capsular Contracture
Two of six studies evaluated capsular contracture. One retrospective study analyzed the results of seven different surgery centers from March 2006 to December 2012‚ and 2797 BAs were separated into two groups based on whether a funnel was utilized or not. The risk of reoperation due to capsular contracture was significantly greater when an insertion funnel was not utilized (1.49% versus 0.68%, P = 0.004). The incidence of Baker Grade III and IV capsules was reduced by 54.4% when a funnel was utilized. Moreover, after logistic regression, the odds ratio for capsular contracture necessitating reoperation when a funnel is not utilized was 2.31 (95% CI, 1.18–4.53) (P = 0.023).21
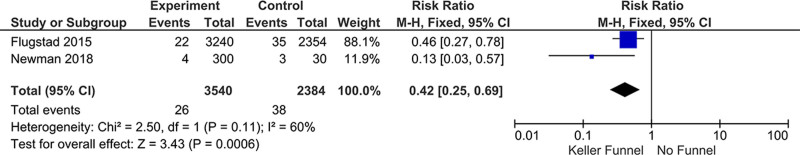
The other study that met inclusion criteria was a single-center retrospective cohort of 166 patients undergoing periareolar BA (mean follow-up = 23 months [12–94 months]). The study compared rates of Baker grades III–IV capsular contracture in implants placed without a funnel (n = 15 patients; 30 implants) with an incidence of 10%, compared with an incidence of 1.3% for patients who had implants placed with a funnel (n: 151,300 implants). This represented an 87% decrease (P = 0.0019).22 Meta-analysis of these studies resulted in a risk ratio reduction of 0.42 (95% CI, 0.25–0.69) (Fig. 3).
Fig. 3.
Results of the meta-analysis comparing studies that met criteria.
Incision Time and Incision Length
Montemurro et al23 compared implant insertion time and incision length using the Keller funnel to standard hand insertion with anatomical breast implants. Less time was required to place the implant with the Keller funnel (mean, 6 seconds; range, 3–10 seconds) than with manual insertion (mean, 16 seconds; range, 13–40 seconds; P = 0.04). However, when utilizing the Keller funnel, the mean total insertion time, measured from opening the implant’s sterile package to its final placing in the pocket, was 35 seconds (range, 13–76 seconds). This was opposed to a mean of 25 seconds (range, 13–43 seconds) when performing implantation by hand alone. Incision length was also appraised. Fifty patients had implants inserted with a Keller funnel (215–450 ml) versus 50 with manually inserted implants (165–495 ml) for an overall average base diameter of 12.2 cm. The Keller funnel group had a significantly shorter mean incision length than the manual insertion group (35.5 ± 2.1 mm against 46.2 ± 3.2 mm, <0.001).
Reconstruction
Two studies were identified discussing the role of Keller funnels in breast reconstruction. Woo et al24 assessed nipple-sparing mastectomies with periareolar incisions and immediate alloplastic breast reconstruction utilizing a funnel and ADMs, and one patient had radiotherapy. There were 21 participating patients (mean age, 45 years), with two of them receiving bilateral breast reconstruction. All procedures were completed with the use of funnels without further incision, which was purported to allow the minimum length of periareolar incisions. The BREAST-Q questionnaire, a way of evaluating patient-reported outcomes to examine the efficacy and effects of breast surgery from the patient’s perspective, was used to measure quality of life and reported the BREAST-Q score was 92%.27 Periodically, from the time a patient exited the operating room until 7 days following surgery, pain-related data were gathered. The visual analog scale was used to record pain ratings from 0 (no pain) to 10 (excruciating pain). Pain scale reduction at 7 days versus 12 hours (P = 0.035).28 However, specific data were not recorded, and this was not included in meta-analysis.
Jang and Kim25 reported the results of 15 patients who underwent delayed two-stage alloplastic reconstruction after mastectomy with neoadjuvant or adjuvant radiotherapy (eight patients had prior radiotherapy). Implants were placed in the subpectoral pocket with funnel assistance (ART funnel). The mean patient age was 44 years (range, 38–54). The entire implant procedure was performed via the prior scar incision. Mean incision length was 4.73 cm across all patients. In eight patients (53.3%), a round smooth implant was utilized, while in five patients, a round microtextured implant was utilized (33.3%). In two patients (13.3%), an anatomical implant (macrotextured) was used. Implant volumes varied from 125 to 425 mL. At the 6- and 12-month follow-ups (March 2018–January 2019), there was no evidence of Baker grade III or IV capsular contracture.
Usage of Different Implants, Lubrication
We also attempted to analyze the impact of implant type and type of lubrication. However, no studies met criteria or provided qualitative data for assessment.
DISCUSSION
Augmentation mammoplasty is the most common aesthetic surgical procedure‚ and the most common form of breast reconstruction after mastectomy is tissue expansion followed by implant implantation. Despite this, reoperation rates have been documented up to 15%29–32 and approximately 21% for implant-based reconstruction.33 Thus, strategies to mitigate immediate postoperative and long-term complications are imperative. Specifically, regarding contamination and subsequent biofilms yielding capsular contracture, prevention strategies that prevent implant contamination are critical. The introduction of the Keller funnel aimed to facilitate a simple and reliable “no touch” implant insertion while allowing for insertion of implants through a smaller incision.34,35
Capsular contracture is one of the most common complications associated with breast implants. Baker grades III and IV are considered symptomatic and may require revisional surgery, including capsulectomy and implant exchange or removal.35–37 Revision surgery often results in an inferior cosmetic outcome and a higher rate of repeat contracture recurrence, decreased patient satisfaction, and overall higher cost.38,39 Numerous etiologies have been proposed for the formation of capsular contracture including immunological response to foreign body, surgical site infection, radiation therapy, implant type, incision type, and choice of pocket.37,38,40–42 However, the prevailing theory is that overt pocket infection or subclinical infection from implant contamination is the predominant cause.43–48 Additionally, several of the theories probably include a component of predisposition to implant contamination (ie, incision placement).47,49,50 The thought is that bacterial contamination results in the formation of a biofilm. The biofilm stimulates inflammation and fibrosis, which may lead to capsular contracture and potentially anaplastic large-cell lymphoma.41,51–53 Biofilm-related implant infections are most likely caused by bacterial seeding of the implant during insertion due to contact with the skin.46,54–56 Triple antibiotic solution and povidone iodine have both successfully been used to irrigate breast implant pockets, decreasing the rate and incidence of capsular contracture.57–60 Despite appropriate skin and device preparation, positive skin cultures can still be seen.61–65 The Keller funnel was designed as a simple and reliable method of achieving the no-touch technique to avoid implant contact with skin and thus subsequent contamination. One of the first studies assessed the efficacy of the funnel in avoiding contact with the skin on cadaver models. When compared with manual implantation, the funnel demonstrated a 27-fold reduction in skin touch. Moreover, bacterial migration from the breast parenchyma to the implant shell was seen in 62.5% of digital insertions and 37.5% of Keller funnel insertions.35
This theory was supported by the findings of the systematic review and meta-analysis. In the multicenter retrospective study, the rate of capsular contracture-related reoperation was higher in the group without the funnel (1.49% versus 0.68%; P = 0.004), and presentation of Baker grade III and IV capsules was reduced by 54.4%.21 The other retrospective study included in analysis evaluated 166 patients, 15 without the funnel (30 implants) and 151 with the funnel (300 implants) for periareolar BAs. The rate of capsular contracture was significantly reduced (P = 0.0019) when the funnel was used for insertion (1.3%) compared with when no funnel was used (10%).22 Additionally, these retrospective studies demonstrated lower rates of capsular contracture compared with the literature.29–32 This was especially notable as both studies assessed periareolar incisions, which are associated with higher rates of contracture (2.4%–18.9% in the literature) compared with 1.3% in the studies noting the use of a funnel.22,23,39,66,67
Montemurro et al23 described assessed incision and insertion variations between utilization of a funnel and cases in which the implant was inserted directly. In the insertion time cohort, mean total insertion time (from implant sterile-package opening to final positioning in the pocket) was 35 seconds (range, 13–76 seconds) with the Keller funnel and 25 seconds (range 13–43 seconds) using manual insertion (P = 0.7). The mean time needed to push the implant through the incision was 6 s (range, 3–10 seconds) with the Keller funnel and 16 seconds (range, 13–40 seconds) with manual insertion (P = 0.04). Shorter surgery time can affect general complications in plastic surgery.68 Furthermore, the study reported that there was no implant damage during insertion of different implants, from small to larger sizes, with visual inspection. If excessively short incisions are avoided, insertion funnels may decrease implant trauma. Further studies with larger sample sizes and follow-up are needed to determine if insertion funnels affect gel fracture and subsequent implant rupture. Additionally, the patient in the Keller funnel group all had shorter incisions. The authors postulated that patients would have improved recovery and healing with shorter scars. However, the single-center study, small sample size in terms of insertion time, short follow-up time for the incision-length cohort, and lack of ultrasound imaging for implant trauma during insertion are confounding factors that limit the significance of these findings.
Although traditionally used in aesthetic procedures, barrier funnels also have utility in breast reconstruction. Woo et al24 performed prepectoral implant placement via a periareolar incision. To mitigate the size of periareolar incisions in nipple-sparing mastectomies, the authors used a funnel (ART funnel) for insertion of an implant completely covered with ADM. Due to the higher rate of complications with single-state alloplastic reconstruction, including flap necrosis and implant extrusion, many surgeons prefer to delay implant-based reconstruction. Moreover, extending the periareolar incision in nipple-sparing mastectomies can disrupt perfusion and lead to skin flap and nipple-areola necrosis.69 The funnel allows for implant placement without the need for incision extension. Additionally, this method allows easy insertion of ADM-covered implants and reduces complications, pain, and scar tissue development all leading to increased patient satisfaction.70 Jang and Kim25 reported the results of 15 patients who received delayed breast reconstruction via subpectoral expander and subsequent implant placement with funnel assistance (ART funnel). The entire implant procedure was performed via the prior scar incision. Mean incision length was 4.73 cm. At the 6- and 12-month follow-ups, there was no evidence of Baker grade III or IV capsular contracture. Furthermore, no hypertrophic scars or anatomical implant malpositions were noted. Additionally, there were no wound-related complications, major necrosis, or infection during the two-stage breast reconstruction. The authors reported a smaller incision, appropriate implant position, and reduced finger manipulation during insertion without any complications. However, the small sample size, lack of long-term follow-up, and lack of control group without funnel versus the funnel-assisted group remain the main limitations of this study.
Limitations
This systematic review compiles data from six different studies reporting outcomes in BA or reconstruction using the Keller funnel for no-touch implant placement. The lack of randomized controlled trials or prospective studies is a major weakness of this review. No study compares the Keller funnel with other no-touch techniques. Despite our stringent inclusion criteria, some of the studies were retrospective single center, and one did not have a clear patient sample size, which introduces significant bias and limitations. Furthermore, the small size of the control groups, short follow-up term, and lack of comparison between incision type and area remain limitations of these studies. Touching the implants by surgeons after their placement to adjust their position can cause different results and biases, and this is not reported in studies. Additionally, different protocols such as antibiotics and combination of saline or betadine can cause bias. Although the total amount of studies included in the review is relatively small with six studies, the Keller funnel is so widely used in our field that a comprehensive and exhaustive appraisal of the literature on its use is necessary. However, further investigation should be conducted to bolster the findings in this study.
CONCLUSIONS
This systematic review presents an analysis of the use of the Keller funnel as a method for the no-touch technique in cosmetic and reconstructive breast surgery. From this analysis, it appears that the funnel does allow breast implants to be inserted using an enhanced no-touch method. Its use is associated with a shorter operating time and decreased incision length. Additionally, by using this technique, the risk of shell trauma and pain following surgery may be reduced. It may also be used with alternative access incisions, such as transaxillary or periareolar. Whether this translates to reduced capsular contracture in the medium to long term remains to be proven. To generate strong evidence for its efficacy, high-quality multicenter prospective comparative trials with long-term follow-up and outcome assessments are needed.
Footnotes
Published online 23 November 2022.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.The Aesthetic Society’s Cosmetic Surgery National Data Bank: Statistics 2020. Available at https://cdn.theaestheticsociety.org/media/statistics/aestheticplasticsurgerynationaldatabank-2020stats.pdf. Accessed February 25, 2022.
- 2.Patel BC, Wong CS, Wright T, et al. Breast implants. 2022. Available at http://www.ncbi.nlm.nih.gov/pubmed/28723027. Accessed February 25, 2022. [PubMed]
- 3.ASPS National Clearinghouse of Plastic Surgery Procedural Statistics: Plastic Surgery Statistics Report 2020. Available at https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf. Accessed February 25, 2022.
- 4.Montemurro P, Hedén P, Behr B, et al. Controllable factors to reduce the rate of complications in primary breast augmentation: a review of the literature. Aesthetic Plast Surg. 2021;45:498–505. [DOI] [PubMed] [Google Scholar]
- 5.Wang C, Luan J, Panayi AC, et al. Complications in breast augmentation with textured versus smooth breast implants: a systematic review protocol. BMJ Open. 2018;8:e020671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prantl L, Momeni A, Brebant V, et al. Recommendations for the use of antibiotics in primary and secondary esthetic breast surgery. Plast Reconstr Surg Glob Open. 2020;8:e2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong SJ, Deva AK. Understanding the etiology and prevention of capsular contracture. Clin Plast Surg. 2015;42:427–436. [DOI] [PubMed] [Google Scholar]
- 8.Bachour Y. Capsular contracture in breast implant surgery: where are we now and where are we going? Aesthetic Plast Surg. 2021;45:1328–1337. [DOI] [PubMed] [Google Scholar]
- 9.Zingaretti N, Vittorini P, Savino V, et al. Surgical treatment of capsular contracture (CC): literature review and outcomes utilizing implants in revisionary surgery. Aesthetic Plast Surg. 2021;45:2036–2047. [DOI] [PubMed] [Google Scholar]
- 10.Adams WP, Culbertson EJ, Deva AK, et al. Macrotextured breast implants with defined steps to minimize bacterial contamination around the device: experience in 42,000 implants. Plast Reconstr Surg. 2017;140:427–431. [DOI] [PubMed] [Google Scholar]
- 11.Luvsannyam E, Patel D, Hassan Z, et al. Overview of risk factors and prevention of capsular contracture following implant-based breast reconstruction and cosmetic surgery: a systematic review. Cureus. 2020;12:e10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson HB. Early results show reduced infection rate using no-touch technique for expander/ADM breast reconstruction. Plast Reconstr Surg Glob Open. 2015;3:e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mladick RA. No-touch? Submuscular saline breast augmentation technique. Aesthetic Plast Surg. 1993;17:183–192. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg P, Rios L. Double loading of breast implants in aesthetic and reconstructive plastic surgery with the iNPLANT funnel. Aesthetic Surg J Open Forum. 2021;3:ojab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barker AS, Law J, Nicholson M, et al. The reversed glove sleeve. Plast Reconstr Surg Glob Open. 2020;8:e2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molinar V, Chopra K, Gryskiewicz J. A simple alternative: a minimal-touch technique for placing breast implants. Aesthetic Surg J Open Forum. 2020;2:ojaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller K, Preissman H. How the Keller funnel got its start. Am J Cosmet Surg. 2012;29:283–285. [Google Scholar]
- 18.Gowda AU, Chopra K, Brown EN, et al. Preventing breast implant contamination in breast reconstruction: a national survey of current practice. Ann Plast Surg. 2017;78:153–156. [DOI] [PubMed] [Google Scholar]
- 19.Heidekrueger PI, Sinno S, Hidalgo DA, et al. Current trends in breast augmentation: an international analysis. Aesthet Surg J. 2018;38:133–148. [DOI] [PubMed] [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flugstad NA, Pozner JN, Baxter RA, et al. Does implant insertion with a funnel decrease capsular contracture? A preliminary report. Aesthetic Surg J. 2016;36:550–556. [DOI] [PubMed] [Google Scholar]
- 22.Newman AN, Davison SP. Effect of Keller funnel on the rate of capsular contracture in periareolar breast augmentation. Plast Reconstr Surg Glob Open. 2018;6:e1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montemurro P, Fischer S, Schyllander S, et al. Implant insertion time and incision length in breast augmentation surgery with the Keller funnel: results from a comparative study. Aesthetic Plast Surg. 2019;43:881–889. [DOI] [PubMed] [Google Scholar]
- 24.Woo J, Seung IH, Hong SE. Funnel usefulness in direct-to-implant breast reconstruction using periareolar incision with prepectoral implant placement and complete coverage with acellular dermal matrix. J Plast Reconstr Aesthet Surg. 2020;73:2016–2024. [DOI] [PubMed] [Google Scholar]
- 25.Jang HU, Kim SY. Determining the indications for funnel-assisted implant insertion using a short incision in reconstructive breast surgery. Arch Aesthetic Plast Surg. 2020;26:57–63. [Google Scholar]
- 26.Chen SH, Yang ST, Huang WC. Lubricating the insertion funnel with autologous fat tissue for inserting breast implants. Plast Reconstr Surg Glob Open. 2018;6:e1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124:345–353. [DOI] [PubMed] [Google Scholar]
- 28.Labaste F, Ferré F, Combelles H, Rey V, et al. Validation of a visual analogue scale for the evaluation of the postoperative anxiety: A prospective observational study. Nurs Open. 2019;6:1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cunningham B. The mentor core study on silicone memorygel breast implants. Plast Reconstr Surg. 2007;120(suppl 1):19S–29S. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham B, McCue J. Safety and effectiveness of mentor’s memorygel implants at 6 years. Aesthetic Plast Surg. 2009;33:440–444. [DOI] [PubMed] [Google Scholar]
- 31.Stevens WG, Calobrace MB, Harrington J, et al. Nine-year core study data for Sientra’s FDA-approved round and shaped implants with high-strength cohesive silicone gel. Aesthetic Surg J. 2016;36:404–416. [DOI] [PubMed] [Google Scholar]
- 32.Spear SL, Murphy DK, Slicton A, et al. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg. 2007;120(supplement 1):8S–16S. [DOI] [PubMed] [Google Scholar]
- 33.Clarke-Pearson EM, Lin AM, Hertl C, et al. Revisions in implant-based breast reconstruction: how does direct-to-implant measure up? Plast Reconstr Surg. 2016;137:1690–1699. [DOI] [PubMed] [Google Scholar]
- 34.Home. Accessed February 25, 2022. https://www.kellerfunnel.com/.
- 35.Moyer HR, Ghazi B, Saunders N, et al. Contamination in smooth gel breast implant placement: testing a funnel versus digital insertion technique in a cadaver model. Aesthetic Surg J. 2012;32:194–199. [DOI] [PubMed] [Google Scholar]
- 36.Headon H, Kasem A, Mokbel K. Capsular contracture after breast augmentation: an update for clinical practice. Arch Plast Surg. 2015;42:532–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montemurro P, Agko M, Quattrini Li A, et al. Implementation of an integrated biodimensional method of breast augmentation with anatomic, highly cohesive silicone gel implants: short-term results with the first 620 consecutive cases. Aesthetic Surg J. 2017;37:782–792. [DOI] [PubMed] [Google Scholar]
- 38.Maxwell GP, Van Natta BW, Murphy DK, et al. Natrelle style 410 form-stable silicone breast implants: core study results at 6 years. Aesthetic Surg J. 2012;32:709–717. [DOI] [PubMed] [Google Scholar]
- 39.Spear SL, Murphy DK. Natrelle round silicone breast implants. Plast Reconstr Surg. 2014;133:1354–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolfram D, Rabensteiner E, Grundtman C, et al. T regulatory cells and TH17 cells in peri–silicone implant capsular fibrosis. Plast Reconstr Surg. 2012;129:327e–337e. [DOI] [PubMed] [Google Scholar]
- 41.Swanson E. Open capsulotomy. Plast Reconstr Surg Glob Open. 2016;4:e1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bachour Y, Verweij SP, Gibbs S, et al. The aetiopathogenesis of capsular contracture: a systematic review of the literature. J Plast Reconstr Aesthet Surg. 2018;71:307–317. [DOI] [PubMed] [Google Scholar]
- 43.Conte MP, Superti F, Moio M, et al. Bacterial biofilm associated with a case of capsular contracture. New Microbiol. 2018;41:238–241. [PubMed] [Google Scholar]
- 44.Ajdic D, Zoghbi Y, Gerth D, et al. The relationship of bacterial biofilms and capsular contracture in breast implants. Aesthetic Surg J. 2016;36:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rieger UM, Mesina J, Kalbermatten DF, et al. Bacterial biofilms and capsular contracture in patients with breast implants. Br J Surg. 2013;100:768–774. [DOI] [PubMed] [Google Scholar]
- 46.Pajkos A, Deva AK, Vickery K, et al. Detection of subclinical infection in significant breast implant capsules. Plast Reconstr Surg. 2003;111:1605–1611. [DOI] [PubMed] [Google Scholar]
- 47.Virden CP, Dobke MK, Paul S, et al. Subclinical infection of the silicone breast implant surface as a possible cause of capsular contracture. Aesthetic Plast Surg. 2020;44:1141–1147. [DOI] [PubMed] [Google Scholar]
- 48.Tamboto H, Vickery K, Deva AK. Subclinical (biofilm) infection causes capsular contracture in a porcine model following augmentation mammaplasty. Plast Reconstr Surg. 2010;126:835–842. [DOI] [PubMed] [Google Scholar]
- 49.Bartsich S, Ascherman JA, Whittier S, et al. The breast: a clean-contaminated surgical site. Aesthetic Surg J. 2011;31:802–806. [DOI] [PubMed] [Google Scholar]
- 50.Manav S, Ayhan MS, Deniz E, et al. Capsular contracture around silicone miniimplants following bacterial contamination: an in vivo comparative experimental study between textured and polyurethane implants. J Plast Reconstr Aesthet Surg. 2020;73:1747–1757. [DOI] [PubMed] [Google Scholar]
- 51.Mempin M, Hu H, Chowdhury D, et al. The A, B and C’s of silicone breast implants: anaplastic large cell lymphoma, biofilm and capsular contracture. Mater (Basel, Switzerland). 2018;11:231–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rastogi P, Deva AK, Prince HM. Breast implant-associated anaplastic large cell lymphoma. Curr Hematol Malig Rep. 2018;13:516–524. [DOI] [PubMed] [Google Scholar]
- 53.Poppler L, Cohen J, Dolen UC, et al. Histologic, molecular, and clinical evaluation of explanted breast prostheses, capsules, and acellular dermal matrices for bacteria. Aesthetic Surg J. 2015;35:653–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCann MT, Gilmore BF, Gorman SP. Staphylococcus epidermidis device-related infections: pathogenesis and clinical management. J Pharm Pharmacol. 2008;60:1551–1571. [DOI] [PubMed] [Google Scholar]
- 55.Walker JN, Hanson BM, Pinkner CL, et al. Insights into the microbiome of breast implants and periprosthetic tissue in breast implant-associated anaplastic large cell lymphoma. Sci Rep. 2019;9:10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thornton JW, Argenta LC, McClatchey KD, et al. Studies on the endogenous flora of the human breast. Ann Plast Surg. 1988;20:39–42. [DOI] [PubMed] [Google Scholar]
- 57.Drinane JJ, Bergman RS, Folkers BL, et al. Revisiting triple antibiotic irrigation of breast implant pockets: a placebo-controlled single practice cohort study. Plast Reconstr surgery Glob open. 2013;1:e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Awad AN, Heiman AJ, Patel A. Implants and breast pocket irrigation: outcomes of antibiotic, antiseptic, and saline irrigation. Aesthetic Surg J. 2022;42:NP102–NP111. [DOI] [PubMed] [Google Scholar]
- 59.Drinane JJ, Chowdhry T, Pham T-H, et al. Examining the role of antimicrobial irrigation and capsular contracture: a systematic review and meta-analysis. Ann Plast Surg. 2017;79:107–114. [DOI] [PubMed] [Google Scholar]
- 60.Baker NF, Hart AM, Carlson GW, et al. A systematic review of breast irrigation in implant-based breast surgery. Ann Plast Surg. 2021;86:359–364. [DOI] [PubMed] [Google Scholar]
- 61.Carvajal J, Carvajal M, Hernández G. Back to basics: could the preoperative skin antiseptic agent help prevent biofilm-related capsular contracture? Aesthetic Surg J. 2019;39:848–859. [DOI] [PubMed] [Google Scholar]
- 62.Nai GA, Medina DAL, Martelli CAT, et al. Does washing medical devices before and after use decrease bacterial contamination?: An in vitro study. Medicine (Baltim). 2021;100:e25285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saltzman MD, Nuber GW, Gryzlo SM, et al. Efficacy of surgical preparation solutions in shoulder surgery. J Bone Joint Surg Am. 2009;91:1949–1953. [DOI] [PubMed] [Google Scholar]
- 64.Swenson BR, Hedrick TL, Metzger R, et al. Effects of preoperative skin preparation on postoperative wound infection rates: a prospective study of 3 skin preparation protocols. Infect Control Hosp Epidemiol. 2009;30:964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Özkaya O, Ergan Şahin A. Can’t touch this. J Plast Reconstr Aesthet Surg. 2018;71:771–772. [DOI] [PubMed] [Google Scholar]
- 66.Jacobson JM, Gatti ME, Schaffner AD, et al. Effect of incision choice on outcomes in primary breast augmentation. Aesthetic Surg J. 2012;32:456–462. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen HH, To LT. Comparison of endoscopic transaxillary and peri-areolar approaches in breast augmentation with smooth implants. Aesthetic Plast Surg. 2021;45:2665–2675. [DOI] [PubMed] [Google Scholar]
- 68.Hardy KL, Davis KE, Constantine RS, et al. The impact of operative time on complications after plastic surgery: a multivariate regression analysis of 1753 cases. Aesthet Surg J. 2014;34:614–622. [DOI] [PubMed] [Google Scholar]
- 69.Peled AW, Foster RD, Stover AC, et al. Outcomes after total skin-sparing mastectomy and immediate reconstruction in 657 breasts. Ann Surg Oncol. 2012;19:3402–3409. [DOI] [PubMed] [Google Scholar]
- 70.Kim YJ, Kim YW, Cheon YW. Prevention of implant malposition in inframammary augmentation mammaplasty. Du C, ed. Arch Plast Surg. 2014;41:407. [DOI] [PMC free article] [PubMed] [Google Scholar]