Abstract
Opioid-induced microglia reactivity affects opioid reward and analgesic processes in ways that may contribute to the neurocognitive impairment observed in opioid addicted individuals. Opioids elicit microglia reactivity through the actions of opioid metabolites at TLR4 receptors, that are located primarily on microglia but are also present on astrocytes. Specifically, the M3G metabolite, which has no affinity for opioid receptors, exerts off-target effects on TLR4 receptors that can trigger downstream immunologic consequences. This off-target microglial reactivity, and the subsequent increase in microglial release of TNFα, IL-1β, and BDNF, have been suggested to modulate both opioid-induced reward and opioid-induced analgesia. Despite occurring independently of each other, these neuroimmune effects could converge and result in overactivation of the insula. This would produce an imbalance between the “impulsive system” and the “executive system”, such that the impulsive system’s influence over behavior becomes dominant. This state, derived from changes in microglial reactivity, could contribute to impairment in a range of neurocognitive domains that are intricately involved in addiction and lead to increases in addiction-related behaviors.
Keywords: opioids, microglia reactivity, neuroinflammation, reward, analgesia, hyperalgesia, cognitive impairment, TNFα, IL-1β, BDNF
1. Introduction
The seriousness of the opioid epidemic in the United States was highlighted by a recent report from the Center for Disease Control (CDC), which reported that 70% of all overdose deaths in the United States in 2017 was due to opioid misuse (Center for Disease Control and Prevention [CDC], 2019). That same 2018 report stated that over 10 million Americans admitted to misusing opioids while more than 2 million reported having opioid use disorder (i.e., opioid addiction) (CDC, 2019). The vast majority of previous opioid research has focused on the direct effect opioids have on neuronal function and neurotransmission through the classical opioid receptors (e.g., μ, δ, and κ opioid receptors). More recently, however, there has been increased investigation into how opioid-induced microglia reactivity can influence both opioid addiction and its neurocognitive consequences. This effect is mediated through byproducts that are released in response to microglial reactivity that includes but is not limited to BDNF as well as pro-inflammatory factors like TNFα and IL1β. This line of research has resulted in an accumulation of evidence suggesting that opioid-induced neuroinflammatory mechanisms, along with BDNF, can significantly impact both opioid reward and opioid-induced analgesia (i.e., pain relief) in ways that may also contribute to the neurocognitive dysfunction observed across various domains in those with opioid addiction (Baldacchino et al., 2012, 2017; Eidson et al., 2017; Eidson & Murphy, 2013a, 2019; Hymel et al., 2016; H. Zhang et al., 2020). With that said, it should be noted that the actions of opioid-induced microglia reactivity across opioid reward and analgesia are independent phenomena that rely on different neurobiological processes at varying levels of the CNS. Which will be shown more clearly in later sections of the review. While this paradigm shift in opioid addiction research has produced promising findings, there are still questions that need to be answered. One area that needs further investigation is determining whether opioid-induced neuroinflammatory products (TNFα, and IL-1β) increase or decrease opioid reward processes (Hymel et al., 2016; H. Zhang et al., 2020). Additionally, the detailed mechanisms by which opioid-induced microglia reactivity exerts these effects on opioid reward and analgesia have yet to be fleshed out.
This review will focus on the effect opioid-induced microglia reactivity has on 1.) opioid reward, opioid-induced analgesia, and opioid-induced hyperalgesia, 2.) how these effects are intertwined with cognitive dysfunction, and 3.) the ultimate ramifications these off-target immunologic effects may have on opioid addiction. Opioid reward is an important piece of the puzzle because opioids are known to have very powerful rewarding properties which mediate their substantial abuse potential (Fields & Margolis, 2015). Therefore, if opioid-induced neuroinflammation influences opioid reward processing, then these off-target effects could substantively and directly influence opioid addiction. Alternatively, it is also important to understand the immunologic effects of opioids on pain processing because hyperalgesia could directly promote higher use of opioids (i.e., pain is a potent negative reinforcer for opioid consumption) but also produces cognitive impairments that indirectly contribute to the development and maintenance of opioid addiction.
2. Opioid Addiction and Cognitive Dysfunction
2.1. Development of Opioid Addiction
Addiction can best be defined as “a chronic, relapsing brain disorder in which initial, voluntary drug use progresses to compulsive, uncontrollable drug-seeking and drug taking" (Moningka et al., 2019). This chronic, relapsing disorder involves a number of physiological and psychological states that include the primary effects of opioids (e.g., pain relief and reward), tolerance to the primary effects of opioids, and dependence/withdrawal. The opponent process theory provides a framework for understanding how addiction develops and it posits that there are two opposing processes involved in the development of addiction (Solomon & Corbit, 1974). The initial use of opioids triggers a positive hedonic process (e.g., pain relief and reward/pleasure) termed “a-process” which develops and decays quickly. The activation of a-process triggers the development of a negative hedonic (e.g., reduced pain relief and negative emotional state) “b-process” that reduces the effects of a-process and develops and decays more slowly than a-process. Overtime with repeated opioid use, b-process is strengthened so that it has a faster onset, greater intensity, and longer duration. This is a state termed tolerance, in which b-process “masks” the effects of a-process requiring higher doses of opioids to remove the b-process and leads to a state of opioid dependence (Koob, 2021; Solomon & Corbit, 1974). Opioid dependence can be loosely defined as a state in which the absence of opioids results in the emergence of withdrawal symptoms. There are two different types of dependence that are important in the context of opioid addiction, physical and psychological dependence. Physical dependence involves the emergence of physical withdrawal symptoms when opioid use is ceased, such as hyperalgesia (i.e., low pain threshold). While psychological dependence involves the need to continue opioid use to produce pleasure/reward and avoid the development of negative affective states associated with reward deficit (Koob, 2021).
2.2. Opioid Addiction and Cognitive Dysfunction
Although it is largely derived from observational studies, there is considerable evidence linking opioid addiction with cognitive impairment. This section will introduce these associative findings, and we will further explore potential underlying mechanisms in sections 4.3 and 5.3. Multiple meta-analyses consisting of a total of 22,999 participants have consistently shown that opioid users exhibit global neurocognitive impairment (Baldacchino et al., 2012, 2017; Wollman et al., 2019). These studies also indicate that opioid-induced neurocognitive impairment is attenuated as a function of abstinence duration, with longer abstinence resulting in improvement in the dysfunctional neurocognitive domains. However, while there is a substantial amount of research related to short-term opioid abstinence (sober for less than a year), little research has been conducted on long-term abstinence (sober for more than a year). Specifically, Baldacchino et al., 2012 conducted a quantitative review and meta-analysis that comprised 20 studies investigating the neuropsychology of chronic opioid users (767 participants) with healthy controls (15,196 participants). The results of the meta-analysis suggested that chronic opioid use was associated with deficits in multiple neuropsychological domains, with the greatest impairments observed in verbal working memory, cognitive impulsivity (risk-taking), and cognitive flexibility. These neuropsychological domains exhibited a medium effect size according to Cohen’s benchmark criteria. Baldacchino et al. 2017 conducted an additional systemic review and meta-analysis comparing the neurocognitive functioning between chronic methadone users (CM), short-term abstinence participants (SAP), and healthy controls (HCs). This analysis reports global cognitive impairment for the CM cohort relative to the HC participants, but no significant difference between CM and SAP cohorts. The interpretation of these results is limited due to methodological issues such as small sample sizes, heterogeneity, and relatively poor quality of included studies, but it is notable that the cognitive performance of SAP and CM cohorts were indistinguishable. In addition, with these behavioral findings, Wollman et al. 2017 conducted a systematic review and meta-analysis comparing gray matter volume between 293 healthy controls and 286 opioid-dependent patients. In line with the previously reported cognitive deficits, this volumetric MRI analysis revealed significantly less gray matter in several regions of the brain that are critical to cognitive and affective processing for the cohort of opioid-dependent patients. More specifically, areas with reduced gray matter volume in chronic opioid users include 1.) the fronto-cerebellar system which plays roles in impulsivity, compulsive behaviors, and affective disturbances, 2.) the fronto-insular system which plays important roles in cognition and decision making, and 3.) bilateral fronto-temporal areas of the brain that could affect emotion regulation, drug-cravings, and decision-making (Wollman et al., 2017). The reduction in gray matter reported by Wollman and colleagues is congruent with behavioral findings from Baldacchino’s meta-analyses where opioid users exhibited robust deficits in cognitive impulsivity and cognitive flexibility.
The above studies provide substantial evidence supporting the idea that opioids cause cognitive dysfunction across multiple neurocognitive domains, even if only temporarily. A recent line of research suggests that one contributing factor to this global cognitive dysfunction is opioid-induced increases in microglia reactivity. Specifically, opioid-induced microglia reactivity may lead to overactivation of the insula (via reward inhibition/induction of negative emotional states and increased pain perception) which in turn leads to an imbalance between the impulsive and executive systems (Bechara et al., 2019; Eidson & Murphy, 2019; Hutchinson et al., 2010; Koob, 2021; Van Heesch et al., 2014). There is a large body of evidence showing that opioid-induced microglia reactivity has considerable effects related to the development and maintenance of opioid reward (whether that be reward inhibition or potentiation), tolerance, dependence, and withdrawal (Eidson et al., 2017; Eidson & Murphy, 2013b, 2013a, 2019; Felger & Treadway, 2017; Frank et al., 2017; Hutchinson et al., 2007; Koob, 2020; Van Heesch et al., 2014; H. Zhang et al., 2020).
3. Opioid-Induced Microglia Reactivity
3.1. Microglia and Their Role in the Central Nervous System
Microglia function as the principal innate immune cells in the central nervous system (CNS), and are relatively numerous within the CNS with the microglial population comprising 10% of the total cells found within the CNS (Salter & Stevens, 2017). It is clear that microglia from different neuroanatomical compartments of the brain and spinal column are very different in their molecular signature, inflammatory response, proliferation, entry time and route during development (Xuan et al., 2019). A study conducted by Baskar Jesudasan and colleagues (2014) highlighted the differences in reactivity profiles between spinal and brain populations of microglia when they reported that spinal microglia exhibit a reduced inflammatory profile (e.g., reduced release of TNFα and IL-1β) after exposure to lipopolysaccharide (LPS) when compared to brain-derived microglia in neonatal mice (Baskar Jesudasan et al., 2014).In a healthy and mature CNS, microglia are in a ramified morphology and monitor the health of neurons and synapses within their local environments. They carry out this critical function by using the fine processes that extend from their cell surfaces to make brief contact with the local neurons and synapses (Kettenmann et al., 2011; Perry & Holmes, 2014).
Reflecting a notable shift in terminology, this manuscript will discuss microglial reactivity to acknowledge that microglial activity is far more complex than a binary state of active or quiescence. Microglial reactivity is a highly dynamic process that is characterized by morphological change from ramified to amoeboid morphology and significant alterations to gene expression and functional behaviors (Cherry et al., 2014; Davis et al., 2017). The changes in the gene expression in microglia results in the release of numerous proinflammatory cytokines and chemokines, including tumor necrosis factor-alpha (TNFα), interleukins-1β, 2, and 6 (IL-1β, IL-2, IL-6), and interferon-gamma (IFN-γ) (H. Zhang et al., 2020). These proinflammatory factors (e.g., TNFα and IL-1β) cause an inflammatory response within the CNS, but they also can have effects on synaptic transmission and plasticity. For example, TNF-α released from microglia can have effects on both GABA and glutamate receptors on nearby neurons and astrocytes and in this way TNF-α can increase synaptic transmission.
3.2. Microglia Reactivity and TLR4
The innate immune system response in the CNS, carried out by microglia, has been shown to be initiated by activation of the various types of receptors in the toll-like receptor (TLR) family, including TLR2 and TLR4 (Kawai & Akira, 2010). The most widely studied of these receptors is TLR4 which has been found to be located predominantly on the surface of microglia, and to a lesser extent astrocytes (Eidson & Murphy, 2013a; Lehnardt et al., 2003). TLR4 is a pattern recognition receptor (PRR) that is activated by binding of various pathogen-associated molecular patterns (PAMPs) such as lipids, lipoproteins, proteins, and nucleic acids that are common components on the surface membranes of a wide range of microbes (Kawai & Akira, 2010; H. Zhang et al., 2020).
The most widely used PAMP to study TLR4 activation and systemic inflammation is lipopolysaccharides (LPS), which is a component on the outer membrane of gram-negative bacteria. On the cell surface of microglia, TLR4 forms a complex with myeloid differentiation factor 2 (MD-2), which functions as the main site for LPS binding. Upon LPS, binding the TLR4-MD2 complex becomes activated and initiates a MyD88-dependent signaling cascade that increases the intracellular activity of mitogen-activated protein kinases (MAPKs). MAPKs, in turn, alter the gene expression of microglia to increase production and secretion of proinflammatory cytokines (e.g., TNFα and IL-1β), certain neurotrophic factors (e.g., BDNF), and glial activation markers that can then exert effects on synaptic plasticity and neurotransmission (Cahill et al., 2016; Kawai & Akira, 2010; Wang et al., 2012; Wollman et al., 2017; H. Zhang et al., 2020; X. Q. Zhang et al., 2012).
3.3. Mechanism of Opioid-Induced Microglia Reactivity
TLR4 is significant in the context of opioid-induced microglia reactivity because opioids bind to the same site as LPS on the TLR4-MD2 complex, which initiates the same neuroinflammatory signaling cascade. Remarkably, as a function of this mimicry, it has been empirically demonstrated that LPS activation of the microglial TLR4-MD2 complex promotes development of morphine tolerance (Eidson et al., 2017). Considering tolerance is an important stage in the development of opioid dependence (Koob, 2021; Solomon & Corbit, 1974), this finding by Eidson and colleagues (2017) suggests that the off-target immunologic effects of opioids in the CNS can indirectly increase the likelihood of opioid dependence.
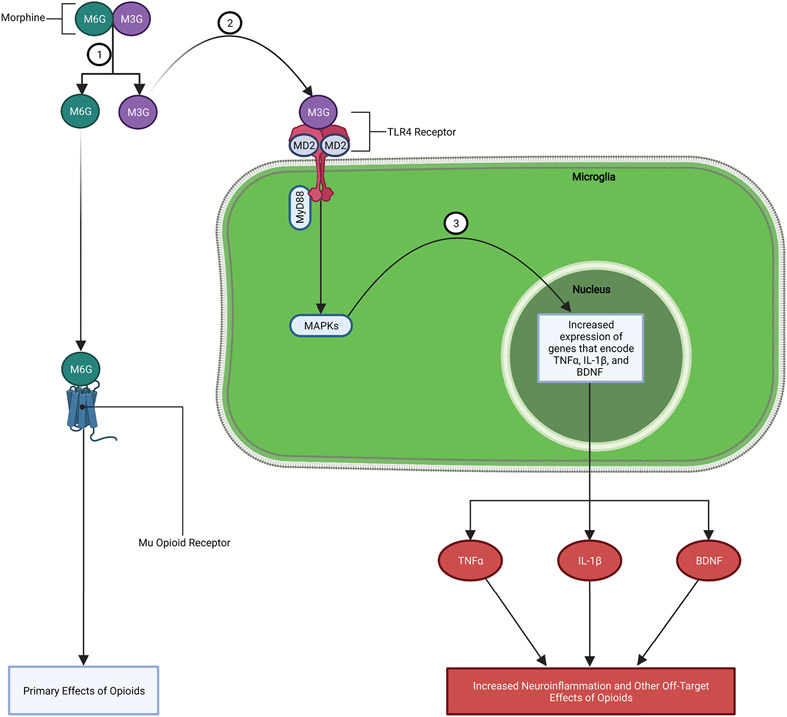
While most of the research regarding opioid binding to TLR4 has focused on morphine, it has been shown that a structurally diverse range of opioids trigger activation of TLR4 (Hutchinson, Zhang, et al., 2009; Hutchinson et al., 2010). To understand this broad action of opioids on microglial reactivity via TLR4, it is important to know that morphine and other opioids are metabolized by the body and CNS into different metabolite forms with different neuroactive effects. Morphine, for example, is metabolized into two main metabolite forms: morphine-3-gluconide (M3G) and morphine-6-gluconide (M6G). M6G has a high affinity for classical opioid receptors (especially mu-opioid receptors) and is the metabolite responsible for delivering the targeted analgesic effects. Alternatively, M3G has no affinity for opioid receptors and preferentially binds to the TLR4-MD2 complex located on microglia, which spawns a signaling cascade of off-target immunologic effects. (Lewis et al., 2010; H. Zhang et al., 2020). More specifically, M3G acts on the microglial TLR4-MD2 complex causing an increase in MAPK activity that promotes increased expression and release of proinflammatory factors (e.g., TNFα, IL-1β, etc.) and BDNF (FIGURE 1). Through this TLR4-MD2 dependent pathway opioids are able to modulate microglia reactivity and associated immunologic outcomes (e.g., increased release of TNFα, IL-1β, and BDNF) that have been suggested to play a role in various stages of opioid addiction (I.e., reward, tolerance, and dependence/withdrawal) and the cognitive dysfunctions associated with these various phases (Bland et al., 2009; Eidson & Murphy, 2013a; Frank et al., 2017; Hutchinson et al., 2007; Hutchinson, Coats, et al., 2008; Koob, 2020; H. Zhang et al., 2020). Importantly, these are off-target effects of opioid metabolites (e.g., M3G) that do not directly bind to opioid receptors.
Figure 1: Mechanism of Opioid-induced Microglia Reactivity:
1.) Morphine is broken down into two main metabolite forms: M3G and M6G. 2.) M3G binds to TLR4-MD2 complex on microglia causing an increase in MAPK signaling. 3.) Increased MAPK signaling leads to increased expression and release of proinflammatory factors (e.g., TNFα, IL-1β, etc.), chemokines, BDNF, and other substances.
While there are many different substances and proinflammatory factors released by microglia in response to opioid activation of TLR4-MD2 dependent pathways, this review will focus on the three that have appeared in the literature the most frequently and have been shown to have important effects on opioid reward and pain processing: TNFα, IL-1β, and BDNF. Below, we introduce the mechanisms for each of these three microglia-derived factors and their potential influence on opioid reward, analgesia, and tolerance. It is important to emphasize that opioid-induced reward and analgesia are fundamentally distinct processes that act independently of each other, which will be further elucidated in sections 4 and 5 that follow.
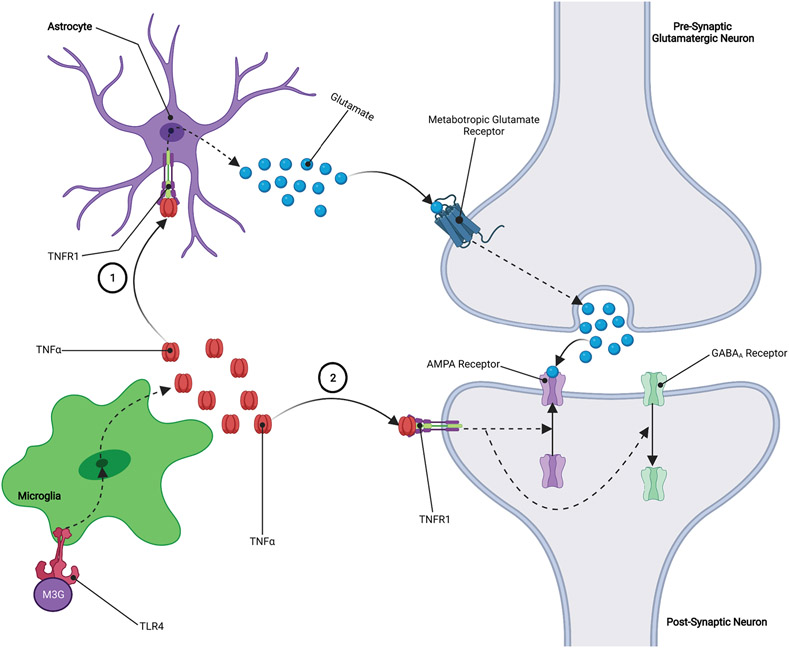
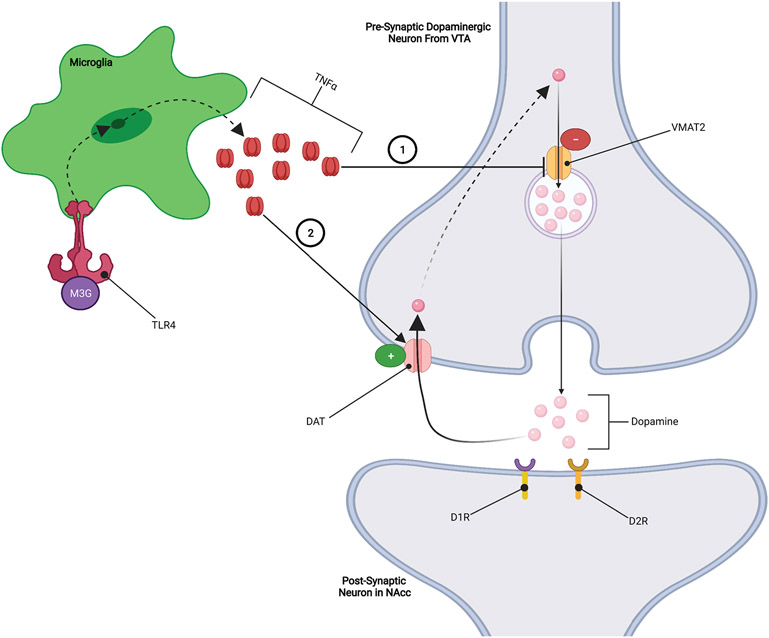
TNFα is a proinflammatory factor that has the ability to regulate neurotransmission and synaptic strength by increasing glutamate release from presynaptic neurons while also increasing the surface expression of Ca2+-permeable AMPA receptors on postsynaptic neurons, both of which increase glutamatergic tone and neurotransmission (FIGURE 2). This microglial induced action on glutamatergic tone has been implicated as a key mechanism in the opposition to the intended pain-relieving effects of opioids which results hyperalgesia (i.e., lower pain threshold) and increased opioid tolerance (Eidson et al., 2017; Eidson & Murphy, 2013a, 2019; H. Zhang et al., 2020). For example, an experimental murine model demonstrated that inhibiting TNFα in the periaqueductal gray (PAG) area prevented both opioid tolerance and the substantial reduction in pain threshold that typically accompanies it. That same study went on to demonstrate that inhibiting TNFα attenuates the opioid-induced increase in PAG TLR4 and IL-1β expression (Eidson et al., 2017). Notably, this influence of TNFα on TLR4 and IL-1β expression highlights the importance of disrupting the deleterious positive feedback loop of opioid-induced neuroinflammation. This data suggests that TNFα may play an important, and perhaps even necessary, role in the development of hyperalgesia that is characteristic of opioid tolerance and dependence. It has also been shown that opioid-induced increases in TNFα may decrease the expression and/or function of vesicular monoamine transporter 2 (VMAT2) and/or increase the expression or function of dopamine transporter (DAT) (FIGURE 3) (Felger & Treadway, 2017; Van Heesch et al., 2014; Wu et al., 2014). As we will show later, increases in DAT activity coupled with a reduction in VMAT2 activity may have significant implications for opioid reward (Felger & Treadway, 2017; Van Heesch et al., 2013; Wu et al., 2014).
Figure 2: TNFα Increases Glutamatergic Tone:
1.) TNFα binds to TNFR1 receptors located on astrocytes leading to increased release of astrocytic glutamate. Astrocytic glutamate binds to metabotropic glutamate receptors located on the presynaptic neuron, leading to increased release of glutamate into the synapse. 2.) TNFα binds to TNFR1 receptors located on the postsynaptic neuron, resulting in an upregulation of AMPA receptors and a downregulation of GABAA receptors on the surface of the postsynaptic neuron. The overall effect of these processes is an increase in glutamatergic signaling which causes an increase in neuroactivity at the synapse.
Figure 3: TNFα Inhibition of Dopaminergic Signaling:
1.) TNFα inhibits VMAT2 expression and/or function, resulting in less dopamine being packed into vesicles leading to reduced dopamine release. 2.) TNFα increases the expression and/or function of DAT dopamine transporters, which results in increased reuptake of dopamine from the synapse reducing the effects of dopamine.
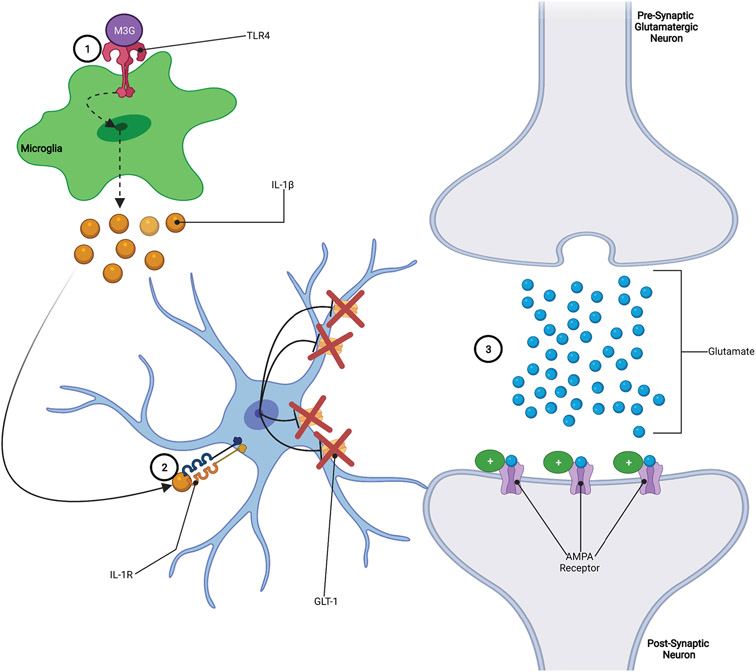
IL-1β is a proinflammatory factor that has been shown to be the primary immune regulator of long-term potentiation (LTP) in the CNS and has been suggested to play a role in reward perception and pain perception, both of which play roles in the development and maintenance of opioid addiction (Koob, 2020; Rizzo et al., 2018; H. Zhang et al., 2020). While the mechanism for how IL-1β influences LTP is unknown, a proposed mechanism involves the phosphorylation of NMDA receptor subunits that alters the conductance properties of the NMDA receptors (Viviani et al., 2003; H. Zhang et al., 2020). The fact that IL-1β affects NMDA receptors is significant because these receptors can alter the strength of synaptic connections which underlies learning and memory processes. Another possible mechanism by which IL-1β modulates neurotransmission involves IL-1β induced downregulation of the GLT-1 glutamate transporter. Downregulation of GLT-1 allows glutamate to remain in the synapse longer thereby increasing the ability of glutamate to excite neurons (FIGURE 4) (Nakagawa & Satoh, 2004; Ozawa et al., 2001; Tilleux & Hermans, 2007; Yan et al., 2014). Notably, similar to TNFα, this would achieve a similar effect of heightened glutamatergic tone. The IL-1β induced reduction in GLT-1 expression and the associated increase in glutamatergic signaling could potentially serve as a mechanism for IL-1β to modulate both opioid reward and pain processing. For example, if IL-1β exerts these effects in the VTA at synapses between presynaptic glutamatergic neurons and postsynaptic GABAergic neurons, then opioid-induced increases in IL-1β levels could result in increased GABAergic tone and inhibition of opioid reward (Frank et al., 2017; Tilleux & Hermans, 2007).
Figure 4: IL-1β Increases Glutamatergic Tone:
1.) Opioids bind to TLR4 receptors, increasing the release of IL-1β from microglia. 2.) IL-1β binds to IL-1R receptors on astrocytes, leading to the downregulation of GLT-1 glutamate transporters. 3.) Downregulation of GLT-1 on astrocytes results in less uptake of glutamate from the synapse, allowing glutamate to remain in the synapse longer and exert stronger effects on the postsynaptic neuron.
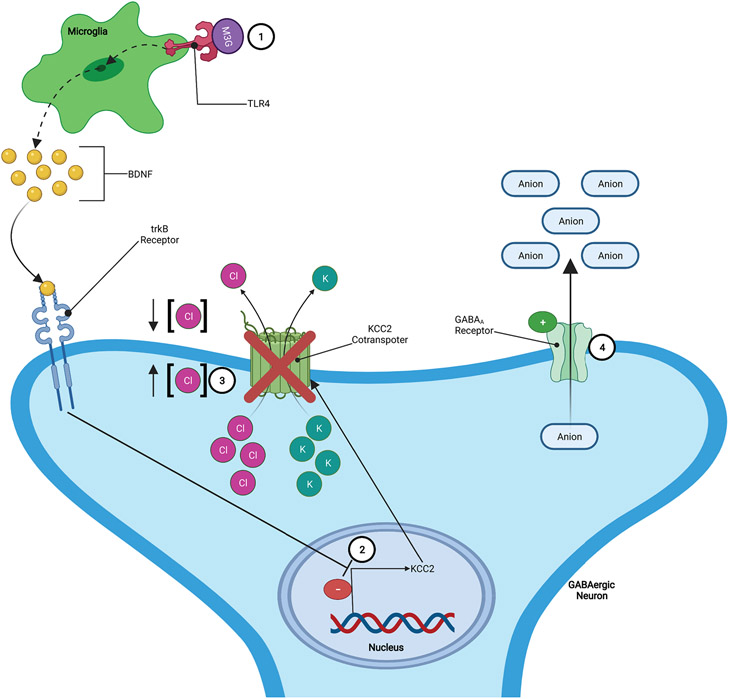
BDNF is a neurotrophic factor secreted from neurons as well as from microglia (Cahill et al., 2016). Microglial-derived BDNF has been shown to have effects on both opioid reward and opioid-induced analgesia. While the exact mechanism by which BDNF exerts its effects is not known, there is evidence suggesting that it involves BDNF induced downregulation of KCC2 potassium-chloride cotransporters located on GABAergic neurons. This downregulation of the KCC2 cotransporters results in disruption of chloride (Cl−) homeostasis by increasing the intracellular concentration of Cl−, in such conditions the activation of GABAA receptors on the GABAergic neurons result in the efflux of anions which leads to a more positive/depolarized state, increasing the excitability of the GABAergic neurons (FIGURE 5) (Cahill et al., 2016; Ferrini et al., 2013; Koo et al., 2012; Taylor et al., 2016; Vargas-Perez et al., 2009, 2014). This increase in GABAergic activity may then alter the neurotransmission in brain regions important for both pain and reward processing allowing BDNF to contribute to the development and/or maintenance of opioid addiction.
Figure 5: BDNF Increases GABAergic Activity:
1.) Opioid metabolite (e.g., M3G) binding to TLR4 induces increased release of BDNF. 2.) Elevated levels of BDNF downregulates KCC2 potassium-chloride cotransporters located on GABAergic neurons. 3.) The downregulation of the KCC2 cotransporters results in disruption of chloride (Cl−) homeostasis by increasing the intracellular concentration of Cl−. 4.) In such conditions, the activation of GABAA receptors on the GABAergic neurons results in the efflux of anions which leads to a more positive/depolarized state, increasing the excitability of the GABAergic neurons.
4. Opioid-Induced Microglia Reactivity and Opioid Reward
4.1. Opioid Reward Pathway
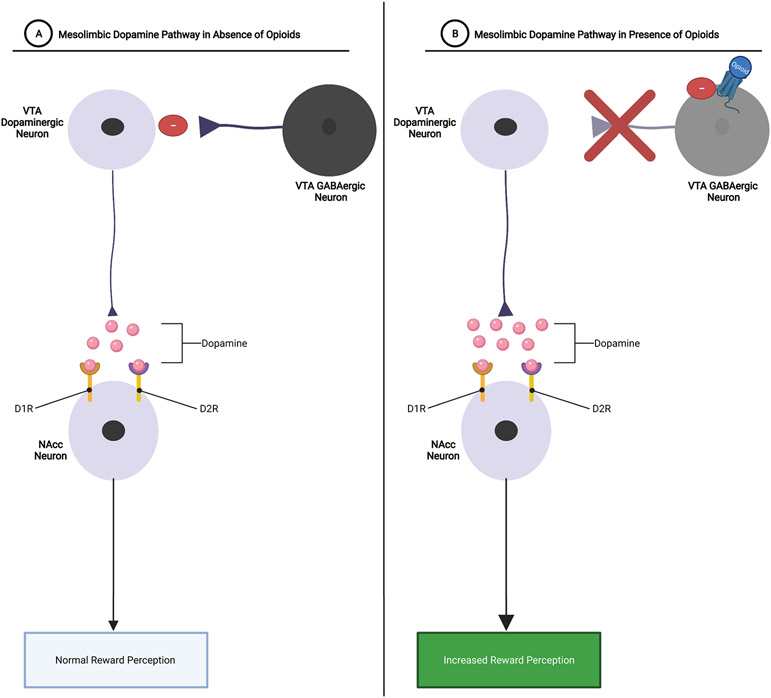
Historically, it has been the view that opioid reward is the result of increased neuronal activity in the dopaminergic mesolimbic pathway (H. Zhang et al., 2020). Reward processing via the mesolimbic pathway involves signaling between two main mesolimbic structures, the ventral tegmental area (VTA) and the nucleus accumbens (NAcc) (Volkow & Morales, 2015; H. Zhang et al., 2020). The neurons in the VTA are thought to be responsible for the encoding of reward and/or reward prediction by rapidly releasing dopamine into the NAcc. Increased dopamine release in the NAcc results in feelings of euphoria, which serves as a strong positive reinforcer for repeating the behaviors that induced the large dopamine release in the NAcc (e.g., opioid use). However, this dopamine release in the NAcc is opposed by inhibitory GABAergic neurons originating in the VTA, rostro-medial tegmental nucleus (RMTN), NAcc, and ventral pallidum (VP) (FIGURE 6A) (Bechara et al., 2019; Fields & Margolis, 2015; Volkow & Morales, 2015; H. Zhang et al., 2020). Opioids are able to exert their euphoric/rewarding effects via increasing the dopaminergic activity in the mesolimbic pathway by inhibiting the GABAergic neurons, effectively removing the “brake system” of the mesolimbic pathway, allowing for significantly more dopamine release from VTA neurons into the NAcc and thereby strengthening the positive reinforcing effects of opioids (FIGURE 6B) (Fields & Margolis, 2015; H. Zhang et al., 2020).
Figure 6: Mesolimbic Dopaminergic Signaling with and without Opioids:
A.) Mesolimbic Dopamine Pathway in Absence of Opioids: VTA dopaminergic neurons release dopamine into the NAcc. Dopamine then binds to D1R and/or D2R dopamine receptors on NAcc neurons, resulting in reward perception. GABAergic neurons function as a reward “brake system” by inhibiting dopaminergic signaling within the mesolimbic pathway, thereby mediating how rewarding a particular stimulus is.
B.) Mesolimbic Dopamine Pathway in Presence of Opioids: Opioids bind to Mu-receptors on VTA GABAergic neurons, thereby removing the reward brake system and allowing for larger amounts of dopamine to be released into the NAcc. Increased release of dopamine within the NAcc results in increased reward perception.
4.2. Opioid-Induced Microglia Reactivity Modulates Opioid Reward
When examining the effects of microglia reactivity on opioid reward processes, it is important to acknowledge that there are contradictory results related to the effects of TNFα and IL-1β on opioid reward. With some studies reporting that opioid-induced microglia reactivity increases opioid reward processes (Bland et al., 2009; Coller & Hutchinson, 2012; Frank et al., 2017; Hutchinson et al., 2007, 2008, 2009; Narita et al., 2006), while other studies have suggested that the proinflammatory products of opioid-induced microglia reactivity (e.g., TNFα and IL-1β) decreases or inhibits opioid reward (Felger & Treadway, 2017; Merali et al., 2003; Niwa et al., 2007; Van Heesch et al., 2013; Wu et al., 2014). Potential explanations for the differences in findings could be the source, dose, and time between elevated levels of immunologic factors (e.g., TNFα and IL-1β) and/or opioid administration (Hymel et al., 2016). Despite this ambiguous body of evidence, this section will further explore the potential implications of reward inhibition via these immunologic factors (e.g., TNFα and IL-1β) and BDNF.
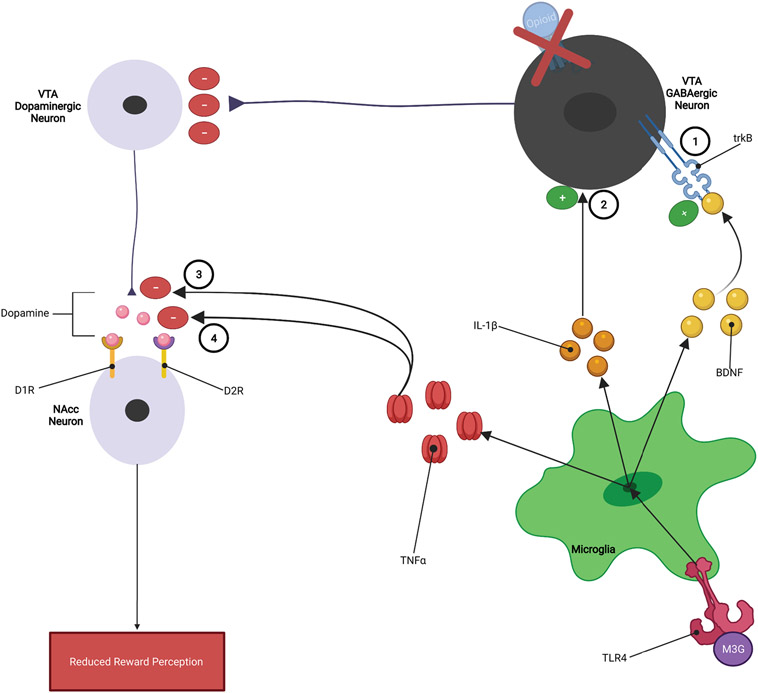
While TNFα, IL-1β, and BDNF have different mechanisms of action, they all appear to inhibit opioid reward by reducing the dopaminergic activity within the mesolimbic dopamine pathway (FIGURE 7). It has been reported that opioid-induced increases in TNFα within the mesolimbic pathway may decrease the expression and/or function of VMAT2 and/or increase the expression or function of DAT dopamine transporters (Felger & Treadway, 2017; Van Heesch et al., 2014; Wu et al., 2014). VMAT2 functions to package dopamine into vesicles to allow for the release of dopamine into synapses, while DAT functions to reuptake dopamine that has been released into the synapse. Therefore, TNFα-induced reduction in VMAT2 would decrease the amount of dopamine released into the synapse, while the increase in DAT activity would quickly remove dopamine from the synapse. Both of these processes would actively oppose opioid reward by reducing the amount of dopamine available to exert its rewarding effects thereby contributing to the development of tolerance to opioid reward (FIGURE 3) (Felger & Treadway, 2017; Van Heesch et al., 2014; Wu et al., 2014).
Figure 7: Microglia Inhibition of Opioid Reward:
1.) Elevated levels of BDNF results in increased GABAergic tone within the VTA, 2.) IL-1β may increase neurotransmission of GABAergic neurons within the mesolimbic pathway, 3.) TNFα reduces VMAT2 activity which reduces the amount of dopamine released from VTA dopaminergic neurons, and 4.) TNFα increases DAT reuptake of dopamine within the NAcc, reducing the ability of dopamine to exert rewarding effects.
While studies on the effects IL-1β have on opioid reward processes have reported contradictory results, there’s a body of evidence that suggests that IL-1β inhibits the rewarding effects of opioids (Hymel et al., 2016). An example of these contradictory findings is that Hutchinson et al., 2008 reported that the introduction of the glial activation inhibitor minocycline resulted in a decrease in morphine reward. While another study conducted by Merali et al., 2003 found that a single intraperitoneal injection of IL-1β resulted in a reduction in responding to a progressive ratio schedule for sucrose reward for up to 72 hours in rats, suggesting IL-1β may reduce the rewarding effects of a stimulus (Hutchinson, Northcutt, et al., 2008; Merali et al., 2003). A possible mechanism by which IL-1β inhibits opioid reward could involve an increase in glutamatergic activity within the VTA between a presynaptic glutamatergic neuron and a postsynaptic GABAergic neuron. This would result in an increased ability of GABAergic neurons to inhibit VTA dopaminergic neurons that project to the NAcc, resulting in a reduction in NAcc dopamine levels and opioid reward.
As with TNFα and IL-1β, evidence also suggests that BDNF inhibits the rewarding effects of opioids. As mentioned earlier, elevated levels of BDNF disrupts chloride ion homeostasis in such a way that GABAergic activity is increased (Cahill et al., 2016; Ferrini et al., 2013; Koo et al., 2012; Taylor et al., 2016; Vargas-Perez et al., 2009, 2014). Importantly, it has been shown that opioid-dependent rats have increased levels of BDNF in the VTA (150% greater than in controls) (Vargas-Perez et al., 2009). Considering it has been shown that elevated BDNF can lead to increased GABAergic activity, this supports the possibility that BDNF contributes to the inhibition of VTA dopaminergic signaling that is associated with the reduced opioid reward that occurs in opioid dependence (Vargas-Perez et al., 2009). This line of evidence suggests that elevated levels of BDNF in VTA could be one physiological mechanism that contributes to the development of tolerance to opioids in chronic opioid users.
4.3. Cognitive Effects of Reward Deficit
The microglia-induced reward inhibition results in a state of reward deficit that manifests as a negative emotional state which could have significant effects on executive function (Cheetham et al., 2010; Koob, 2021; Morie et al., 2014). This negative emotional state consists of a cluster of symptoms characterized by the presence of anhedonia (inability to feel pleasure), malaise (a general feeling of discomfort, illness, and/or uneasiness), and dysphoria (state of generalized dissatisfaction with life) (Koob, 2020, 2021; Koob et al., 2014). The presence of this negative emotional state is thought to signal drug craving through negative reinforcement processes, in which an addicted person consumes opioids in an attempt to escape these negative feelings and feel hedonically normal. In fact, it has been suggested that the negative emotional state is one of the most influential motivating forces for the continued use of drugs in addicted individuals (Cheetham et al., 2010). For example, it has been reported that anhedonia scores are correlated with opioid craving and use in people with opioid use disorder and is strongly associated with more severe drug use (Kiluk et al., 2019; Morie et al., 2014). The presence of this negative emotional state has been shown to have effects on the executive system, that could help explain why global neurocognitive impairment is commonly reported in people with opioid addiction. It has been shown that the presence of a negative emotional state and anhedonia may increase the attentional bias toward drug related cues, which limits cognitive resources available for normal executive system functioning (e.g., verbal working memory, cognitive control, and cognitive fluency). Compounding this issue is the fact that with repeated drug use the drug-related cues are attributed motivational salience, allotting them even greater amounts of attentional resources, and thereby further depleting the cognitive resources that are available to exert control over addictive behavior and cease drug taking behaviors (Cheetham et al., 2010; Robinson et al., 2001).
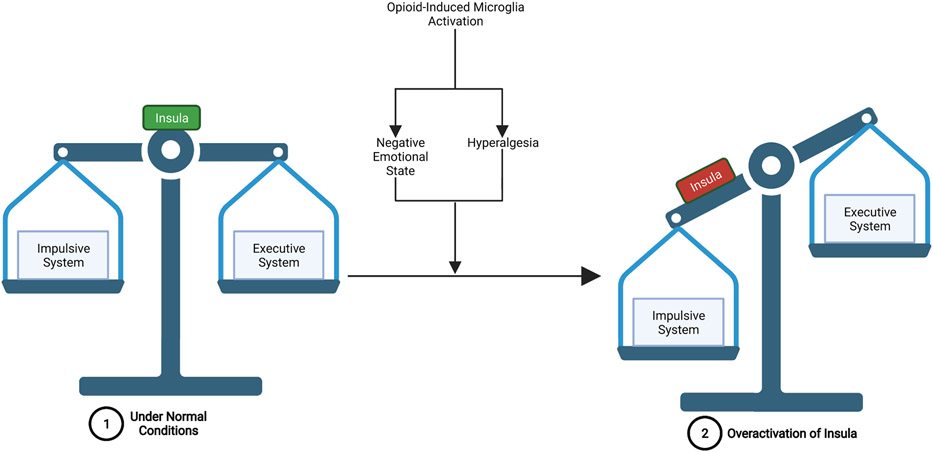
Another possible mechanism by which the negative emotional state can result in executive system dysfunction involves the over-activation of the insula. The insula is a brain structure that processes interoceptive signals from throughout the body such that visceral and autonomic information is integrated with emotion and motivation, and allows for conscious awareness of urges to take drugs (i.e., drug-cravings) (Bechara et al., 2019; Koob & Volkow, 2010; Naqvi & Bechara, 2009). Insular activity is elicited from disruptions to homeostasis such as deprivation states (e.g., physical pain and withdrawal), emotional states (e.g., anxiety, anhedonia, malaise, dysphoria, stress, etc.), and drug-cues (e.g., conditioned drug stimuli). Through its projections to the amygdala and prefrontal cortex (OFC-ACC), the insula plays a critical role in mediating the balance between the “impulsive system” (amygdala-NAcc) which mediates motivational wanting and habitual/compulsive seeking of drug reward and the “executive system” (OFC-ACC) that normally exerts control over the impulsive system (Bechara et al., 2019; Volkow & Morales, 2015). Opioid tolerance, as well as dependence/withdrawal, impairs the insula’s ability to properly maintain the balance between these two opposing systems because the interoceptive signals that increase activation of the insula, such as physical pain and negative emotional states, will increase the influence of the impulsive system over the executive system. This imbalance then results in the reduced ability of the executive system to exert self-control to resist the motivation to seek out drug-reward (FIGURE 8) (Bechara et al., 2019). This suggests that in addiction, the impulsive system becomes over-powered while the executive system simultaneously weakens, producing drug-induced psychological and neurobiological changes that make abstaining from drug use extremely difficult (Bechara et al., 2019). This may be an underlying mechanism that explains why global neurocognitive impairment, such as reduced cognitive flexibility and increased cognitive impulsivity, is commonly observed in opioid addiction (Baldacchino et al., 2012; Wollman et al., 2017).
Figure 8: Overactivation of the Insula Leads to an Imbalance Between the Impulsive and Executive Systems:
1.) Under normal conditions-the insula functions to maintain the balance between the impulsive system and the executive system, such that the executive system is able to exert control over the impulsive system when required. 2.) Opioid-induced microglia reactivity- contributes to the development of a negative emotional state and hyperalgesia, both of which lead to increased activation of the insula. This overactivation of the insula can then lead to impulsive system dominance over the executive system and an inability of the executive system to exert self-control over drug-related, impulsive behaviors.
5. Opioid-induced Microglia Reactivity and Analgesia Opposition
5.1. Pain Processing Pathway
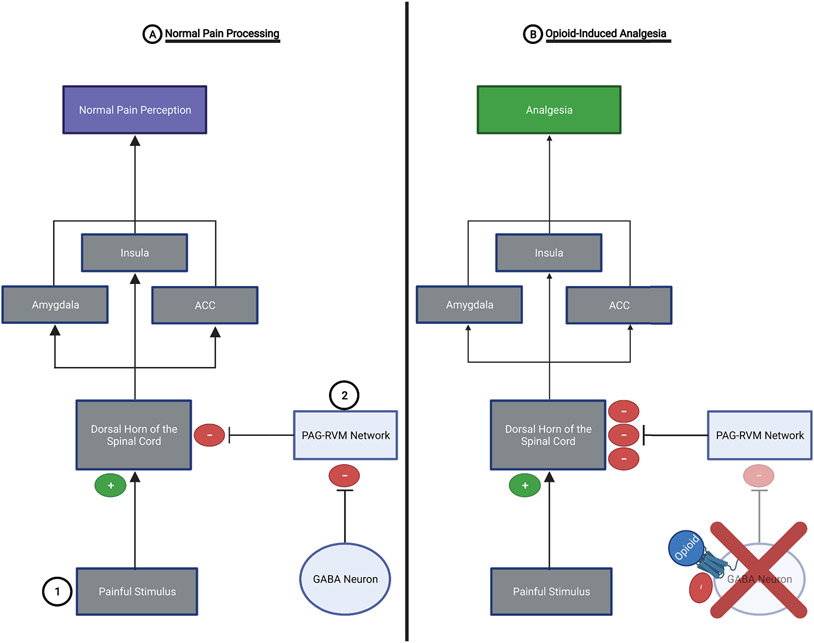
Pain signals from the periphery reach the central nervous system through the lateral spinothalamic pathway (i.e., the pain pathway). Pain signaling through this pathway starts with activation of nociceptors at the site of a painful stimulus. The pain signal is then transmitted down the axons of nociceptors to the dorsal horn of the spinal cord where they synapse with second order neurons that carry the pain signal to the contralateral thalamus. Third order neurons within the thalamus then propagate the pain signal from the thalamus to cortical areas including the insula, amygdala, and the anterior cingulate cortex (ACC) where the perception of pain occurs (Ossipov et al., 2010). A network including the periaqueductal gray (PAG) and the rostro-ventromedial medulla (RVM) exerts analgesic effects by inhibiting the strength of the pain signal at the dorsal horn of the spinal cord (FIGURE 9A). Opioids reduce perception of pain by increasing the activation of PAG neurons by inhibiting GABAergic neurons within the PAG that function to inhibit the glutamatergic PAG neurons. The disinhibited PAG neurons are then able to release larger amounts of glutamate into the RVM, which results in increased activation of RVM neurons that in turn suppress the pain signal at the level of the dorsal horn of the spinal cord (Eidson et al., 2017; Eidson & Murphy, 2013a, 2019; Ossipov et al., 2010). At the same time, opioids also inhibit GABAergic neurons within the RVM, disinhibiting the RVM neurons, and allowing for increased suppression of the pain signal at the dorsal horn of the spinal cord (FIGURE 9B). While the PAG, in general, is associated with analgesia, Tortorici et al., 1999 were able to elucidate that it was the ventrolateral PAG specifically that is crucial to the development of tolerance to the pain-relieving effects of opioids (Tortorici et al., 1999).
Figure 9: Pain Processing with and without the Presence of Opioids:
A.) Normal Pain Processing: 1.) Spinothalamic pain pathway- painful stimuli activate nociceptors which propagate a pain signal to the dorsal horn of the spinal cord. The pain signal is then sent to the contralateral thalamus and then to the pain perception areas of the brain (e.g., insula, amygdala, and ACC). 2.) PAG-RVM network- exerts analgesic effects by inhibiting the pain signal at the dorsal horn of the spinal cord. However, the PAG-RVM network is inhibited by GABAergic neurons in the PAG and RVM.
B.) Opioid-Induced Analgesia: Opioids inhibit the activity of GABAergic neurons within the PAG and RVM, thereby removing the “brake” from the PAG-RVM network. This allows this analgesic system to exert potent inhibition of the pain signal at the dorsal horn of the spinal cord. This results in a weaker pain signal reaching the brain and a substantial reduction in pain perception (i.e., analgesia).
5.2. Opioid-Induced Microglia Reactivity Opposes Opioid-Induced Analgesia
Historically, the majority of research has been focused on the neuronal mechanisms involved in the development and maintenance of increased tolerance to the targeted effects of opioid-induced analgesia. In recent years, however, there has been an increased interest in investigating the possible non-neuronal mechanisms involved in the reduced ability of opioids to relieve pain, namely microglia and their associated processes. This vein of research has led to a substantial amount of evidence showing that microglia reactivity plays a crucial role in the development of tolerance to the pain-relieving effects of opioids (Eidson & Murphy, 2019; Hutchinson, Coats, et al., 2008; Hutchinson, Lewis, et al., 2009; Koob, 2020). It has even been shown that opioid tolerance still develops in mice with knocked-out neuronal opioid receptors (i.e., Mu-opioid receptors), suggesting the classical targets of opioids (i.e., opioid receptors) are not required for the development of tolerance to opioid-induced analgesia (Eidson & Murphy, 2019; Juni et al., 2007). Additionally, a study conducted by Eidson et al., in 2013 found that central antagonism of TLR4 in rats, dose-dependently inhibited the development of tolerance to the pain-relieving effects of morphine. In that same study, it was reported that centrally administered TLR4 agonists resulted in the development of morphine tolerance even in opioid “naïve” rats (Eidson & Murphy, 2013a). This suggests that microglial involvement is required for the development of tolerance to opioid-induced analgesia. The most researched proinflammatory factor in relation to tolerance to opioid-induced analgesia has been TNFα, but evidence does suggest that IL-1β and BDNF exacerbate the anti-analgesic effects of TNFα.
Importantly, glial release of proinflammatory cytokines (TNFα, IL-1β, etc.) increases exponentially with the repeated administration of opioids. In other words, these off-target effects that oppose the analgesic effects of opioids compound over time. This pernicious cycle was elegantly demonstrated by Eidson and colleagues whereby TNFα increased the expression of IL-1β in the PAG (Eidson et al., 2017). This may indicate that glial mechanisms contribute to the potentiation of opioid tolerance and thereby dependency as the duration of opioid use/abuse increases (Eidson & Murphy, 2019; Johnston et al., 2004).
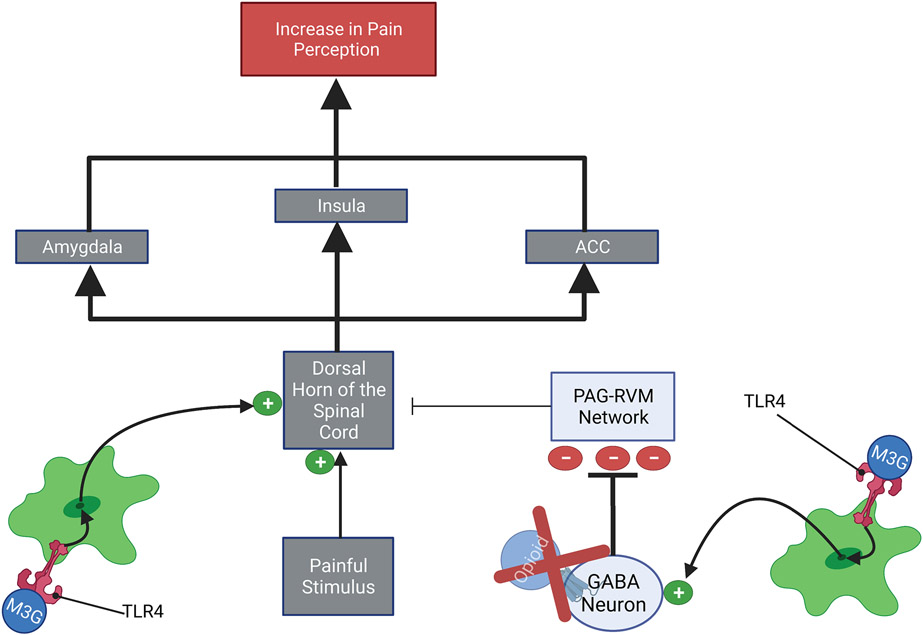
While the mechanisms by which opioid-induced microglia reactivity reduces the pain-relieving effects of opioids have not been clearly elucidated, a possible mechanism involves increased microglia activities at the vlPAG-RVM network and the dorsal horn of the spinal cord (FIGURE 10). First, at the level of the vlPAG-RVM network, microglia induce increased GABAergic inhibition of the vlPAG-RVM network. This increased inhibition of the vlPAG-RVM network would reduce the ability of this potent analgesic network to inhibit the pain signal at the level of the dorsal horn of the spinal cord. Secondly, opioid-induced microglia reactivity increases the neurotransmission of the pain signal by increasing glutamatergic signaling within the dorsal horn of the spinal cord (Eidson & Murphy, 2019). Together, these two phenomena would result in an increased nociceptive signal reaching the brain, thereby increasing the perception of pain (i.e., hyperalgesia). The importance of TNFα in opioid tolerance at the level of the spinal cord was illustrated by the fact that inhibition of TNFα signaling in the spinal cord decreased morphine-induced cytokine release and TLR4-MAPK signaling. Suggesting that TNFα plays a positive feedback role in the neuroinflammatory responses within the spinal cord that are associated with the development of opioid tolerance (Eidson & Murphy, 2019; Shen, Tsai, Shih, et al., 2011; Shen, Tsai, Tai, et al., 2011). Furthermore, immunomodulators that decrease the expression of TNFα have been shown to reduce and even reverse opioid tolerance (Eidson & Murphy, 2019).
Figure 10: Opioid-Induced Microglia Reactivity Opposes Analgesia:
1.) At the dorsal horn of the spinal cord- Microglia derived TNFα, IL-1β, and BDNF may increase the neuroactivity within the dorsal horn of the spinal cord, this leads to an amplification of the pain signal that will be propagated to the pain processing centers of the brain. 2.) At the PAG-RVM network- Microglia derived TNFα, IL-1β, and BDNF may increase GABAergic tone within the PAG-RVM network. This results in increased GABAergic inhibition of the PAG-RVM network, thereby reducing the ability of this analgesic system to inhibit the pain signal at the dorsal horn of the spinal cord. The effects of microglia reactivity at both the PAG-RVM network and the dorsal horn of the spinal cord would lead to a stronger pain signal reaching pain processing centers of the brain, thereby increasing the perception of pain (i.e., hyperalgesia).
At the level of the vlPAG-RVM network, it has been reported that morphine tolerance development is paralleled by increases in the gene expression of three substances that are associated with the proinflammatory responses of microglia (TNFα, IL-1β, TLR4) (Eidson et al., 2017; Eidson & Murphy, 2013a, 2013b, 2019; Koob, 2020). It has also been shown that development of morphine tolerance is paralleled by increases in the levels of OX-42 in the PAG, indicating that microglial activity within the PAG is significantly increased during the development of opioid tolerance. The pattern of increased OX-42 levels were such that the highest levels of OX-42 occurred in animals who were tolerant to the analgesic effects of morphine, further implicating the involvement of microglia in opioid tolerance (Eidson & Murphy, 2013b, 2019).
5.3. Cognitive Effects of Hyperalgesia
An important brain region in understanding how microglia-induced hyperalgesia could impair cognitive ability in opioid addiction is the insula. As mentioned earlier in section 4.3, the insula is a brain region that functions to maintain the balance between the “impulsive system” and the “executive system” and when the insula becomes over-activated in response to homeostatic imbalances, such as increased pain, the impulsive system becomes strengthened while the executive system weakens (FIGURE 8) (Bechara, 2005; Bechara et al., 2019; Contreras et al., 2007; Naqvi & Bechara, 2009). This paradigm results in a psychological and neurobiological state in which the executive system is unable to effectively rein in the impulsive system, which could potentially result in dysfunction in different neurocognitive domains (e.g., verbal working memory, cognitive fluency, and cognitive impulsivity) that are commonly observed in opioid addiction (Baldacchino et al., 2012; Bechara et al., 2019; Naqvi & Bechara, 2009; Wollman et al., 2017).
The fact that an increased pain signal reaching the insula could disrupt the ability of the executive system to properly function lends further support for opioid-induced microglia reactivity contributing to the neurocognitive dysfunctions that occur in opioid addiction. Specifically, opioid-induced microglia reactivity may contribute to the shift in the balance between the executive system and impulsive system by inhibiting the vlPAG-RVM network’s ability to exert analgesic effects and thereby increases the strength of the pain signal reaching the insular cortex. Therefore, the contribution of opioid-induced microglia reactivity to the development of hyperalgesia maybe one driving force behind the strengthening of the impulsive system and weakening of the executive system, where drug-cues induce such powerful drug-cravings that the executive system is simply just not strong enough to exert control over.
6. Conclusion
There is clear evidence that opioid abuse and addiction is associated with deficits in a range of neurocognitive domains that play crucial roles in controlling one’s addictive behaviors (e.g., verbal working memory, cognitive flexibility, and cognitive impulsivity) (Baldacchino et al., 2012). It has also been well established that opioid-induced increases in microglia reactivity affects cognitive functions in ways that would increase the likelihood that addiction will continue. While the exact mechanism by which opioid-induced microglia reactivity achieves this remains elusive, accumulating evidence suggests that it may occur through the inhibition of opioid reward and analgesic processes by neuroinflammatory products (TNF-α and IL-1β) and BDNF (Cheetham et al., 2010; Eidson et al., 2017; Eidson & Murphy, 2013a, 2013b, 2019; H. Zhang et al., 2020). This inhibition of opioid reward and analgesia could then result in overactivation of the insula which could in turn lead to impulsive system dominance over the executive system. Thereby, increasing the likelihood that the executive system will be overwhelmed and be unable to effectively control impulsive, addictive behaviors (i.e., opioid use) (Bechara et al., 2019; Contreras et al., 2007; Naqvi & Bechara, 2009). Despite the progress that has been made in this line of research, there are still areas that require further investigation. This includes getting a better understanding of why there are contradictory findings related to the effects that TNF-α and IL-1β have on opioid reward (Bland et al., 2009; Coller & Hutchinson, 2012; Frank et al., 2017; Hutchinson et al., 2007, 2008, 2009; Narita et al., 2006), with some studies reporting these factors increase opioid rewarding effects, while other findings indicate they reduce opioid reward (Felger & Treadway, 2017; Merali et al., 2003; Niwa et al., 2007; Van Heesch et al., 2013; Wu et al., 2014). One possible explanation is that these contradictory results are due to time/dose-dependent mechanisms (Hymel et al., 2016). This heterogeneity of results provides a good avenue for further research in the future to determine if TNF-α and IL-1β increase or decrease opioid reward and to what extent these effects are time and/or dosage-dependent. Another gap in knowledge that needs to be addressed in the future is elucidating the exact, detailed mechanisms by which opioid-induced microglia reactivity exerts its effects on opioid reward, analgesia, and cognitive processes that are crucial to controlling addiction-related behaviors. While answers to these questions have remained elusive, filling in these gaps in knowledge in the future would allow for better understanding of opioid addiction and could play important roles in controlling the opioid epidemic.
Highlights.
Opioids induce microglia reactivity via TLR4 receptors
Microglial derived products (TNFα, IL-1β, and BDNF) may inhibit opioid reward and analgesia
Opioid addiction is associated with impairment in a range of neurocognitive domains
Negative emotional states and hyperalgesia could lead to cognitive impairment via insula overactivation
7. Funding
This work was supported by the National Institutes of Health R01 AG062543 (PI: Y.-H. C.).
Footnotes
Declaration of Interest
None
9. References
- Baldacchino A, Armanyous M, Balfour DJK, Humphris G, & Matthews K (2017). Neuropsychological functioning and chronic methadone use: A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews, 73, 23–38. 10.1016/j.neubiorev.2016.11.008 [DOI] [PubMed] [Google Scholar]
- Baldacchino A, Balfour DJK, Passetti F, Humphris G, & Matthews K (2012). Neuropsychological consequences of chronic opioid use: A quantitative review and meta-analysis. Neuroscience and Biobehavioral Reviews, 36(9), 2056–2068. 10.1016/j.neubiorev.2012.06.006 [DOI] [PubMed] [Google Scholar]
- Baskar Jesudasan SJ, Todd KG, & Winship IR (2014). Reduced inflammatory phenotype in microglia derived from neonatal rat spinal cord versus brain. PLoS ONE, 9(6). 10.1371/journal.pone.0099443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A (2005). Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nature Neuroscience, 8(11), 1458–1463. 10.1038/nn1584 [DOI] [PubMed] [Google Scholar]
- Bechara A, Berridge KC, Bickel WK, Morón JA, Williams SB, & Stein JS (2019). A neurobehavioral approach to addiction: Implications for the opioid epidemic and the psychology of addiction. Psychological Science in the Public Interest, 20(2), 96–127. 10.1177/1529100619860513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ST, Hutchinson MR, Maier SF, Watkins LR, & Johnson KW (2009). The glial activation inhibitor AV411 reduces morphine-induced nucleus accumbens dopamine release. Brain, Behavior, and Immunity, 23(4), 492–497. 10.1016/j.bbi.2009.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Walwyn W, Taylor AMW, Pradhan AAA, & Evans CJ (2016). Allostatic Mechanisms of Opioid Tolerance Beyond Desensitization and Downregulation. Trends in Pharmacological Sciences, 37(11), 963–976. 10.1016/j.tips.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. (2019). Annual surveillance drug-related risks and outcomes: United States, 2019. Deparment of Health and Human Services, 1–129. https://www.cdc.gov/drugoverdose/pdf/pubs/2019-cdc-drug-surveillance-report.pdf [Google Scholar]
- Cheetham A, Allen NB, Yücel M, & Lubman DI (2010). The role of affective dysregulation in drug addiction. Clinical Psychology Review, 30(6), 621–634. 10.1016/j.cpr.2010.04.005 [DOI] [PubMed] [Google Scholar]
- Cherry JD, Olschowka JA, & Banion MKO (2014). Neuroinflammation and M2 microglia : the good , the bad , and the inflamed. Journal of Neuroinflammation, 11(98), 1–15. 10.1186/1742-2094-11-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras M, Ceric F, & Torrealba F (2007). Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science, 318(5850), 655–658. 10.1126/science.1145590 [DOI] [PubMed] [Google Scholar]
- Davis BM, Salinas-navarro M, Cordeiro MF, & Moons L (2017). Characterizing microglia activation : a spatial statistics approach to maximize information extraction. Scientific Reports, 7(1576), 1–12. 10.1038/s41598-017-01747-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson LN, Inoue K, Young LJ, Tansey MG, & Murphy AZ (2017). Toll-like receptor 4 mediates morphine-induced neuroinflammation and tolerance via soluble tumor necrosis factor signaling. Neuropsychopharmacology, 42(3), 661–670. 10.1038/npp.2016.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson LN, & Murphy AZ (2013a). Blockade of toll-like receptor 4 attenuates morphine tolerance and facilitates the pain relieving properties of morphine. Journal of Neuroscience, 33(40), 15952–15963. 10.1523/JNEUROSCI.1609-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson LN, & Murphy AZ (2013b). Persistent peripheral inflammation attenuates morphine-induced periaqueductal gray glial cell activation and analgesic tolerance in the male rat. Journal of Pain, 14(4), 393–404. 10.1016/j.jpain.2012.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson LN, & Murphy AZ (2019). Inflammatory mediators of opioid tolerance: Implications for dependency and addiction. Peptides, 115, 51–58. 10.1016/j.peptides.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, & Treadway MT (2017). Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology, 42, 216–241. 10.1038/npp.2016.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrini F, Trang T, Mattioli TAM, Laffray S, Del’Guidice T, Lorenzo LE, Castonguay A, Doyon N, Zhang W, Godin AG, Mohr D, Beggs S, Vandal K, Beaulieu JM, Cahill CM, Salter MW, & De Koninck Y (2013). Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl-homeostasis. Nature Neuroscience, 16(2), 183–192. 10.1038/nn.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, & Margolis EB (2015). Understanding opioid reward. Trends in Neurosciences, 38(4), 217–225. 10.1016/j.tins.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, & Maier SF (2017). Stress- and glucocorticoid-induced priming of neuroinflammatory responses: Potential mechanisms of stress-induced vulnerability to drugs of abuse. Brain, Behavior, and Immunity, 25(Suppl 1), 1–18. 10.1016/j.bbi.2011.01.005.Stress- [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, & Watkins LR (2007). Opioid-Induced glial activation: Mechanisms of activation and implications for opioid analgesia, dependence, and reward. TheScientificWorldJournal, 7, 98–111. 10.1100/tsw.2007.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, Baker EM, Jekich BM, Wieseler JL, Somogyi AA, Martin D, Poole S, Judd CM, Maier SF, & Watkins LR (2008). Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain, Behavior, and Immunity, 22(8), 1178–1189. 10.1016/j.bbi.2008.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Rezvani N, Zhang Y, Wieseler JL, Somogyi AA, Yin H, Maier SF, Rice KC, & Watkins LR (2010). Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience, 167(3), 880–893. 10.1016/j.neuroscience.2010.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A, Vietz CM, Judd CM, Maier SF, Watkins LR, & Johnson KW (2009). Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast). Brain, Behavior, and Immunity, 23(2), 240–250. 10.1016/j.bbi.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Chao LW, Kearney JJ, Zhang Y, Berkelhammer DL, Loram LC, Rozeske RR, Bland ST, Maier SF, Gleeson TT, & Watkins LR (2008). Minocycline suppresses morphine-induced respiratory depression, suppresses morphine-induced reward, and enhances systemic morphine-induced analgesia. Brain, Behavior, and Immunity, 22(8), 1248–1256. 10.1016/j.bbi.2008.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, … Watkins LR (2009). Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain, Behavior, and Immunity, 24(1), 83–95. 10.1016/j.bbi.2009.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymel KA, Paris JJ, & McLaughlin JP (2016). Modulation of opioid analgesic reward by inflammatory agents. In Neuropathology of Drug Addictions and Substance Misuse (Vol. 3). Elsevier. 10.1016/B978-0-12-800634-4.00055-X [DOI] [Google Scholar]
- Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, & Watkins LR (2004). A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. Journal of Neuroscience, 24(33), 7353–7365. 10.1523/JNEUROSCI.1850-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni A, Klein G, Pintar JE, & Kest B (2007). Nociception increases during opioid infusion in opioid receptor triple knock-out mice. Neuroscience, 147(2), 439–444. 10.1016/j.neuroscience.2007.04.030 [DOI] [PubMed] [Google Scholar]
- Kawai T, & Akira S (2010). The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nature Immunology, 11(5), 373–384. 10.1038/ni.1863 [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, & Verkhratsky A (2011). Physiology of microglia. Physiological Reviews, 91(2), 461–553. 10.1152/physrev.00011.2010 [DOI] [PubMed] [Google Scholar]
- Kiluk BD, Yip SW, DeVito EE, Carroll KM, & Sofuoglu M (2019). Anhedonia as a key clinical feature in the maintenance and treatment of opioid use disorder. Clinical Psychological Science, 7(6), 1190–1206. 10.1177/2167702619855659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Mazei-Robison MS, Chaudhury D, Juarez B, LaPlant Q, Ferguson D, Feng J, Sun H, Scobie KN, Damez-Werno D, Crumiller M, Ohnishi YN, Ohnishi YH, Mouzon E, Dietz DM, Lobo MK, Neve RL, Russo SJ, Han MH, & Nestler EJ (2012). BDNF is a negative modulator of morphine action. Science, 338(6103), 124–128. 10.1126/science.1222265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2020). Neurobiology of Opioid Addiction: Opponent Process, Hyperkatifeia, and Negative Reinforcement. Biological Psychiatry, 87(1), 44–53. 10.1016/j.biopsych.2019.05.023 [DOI] [PubMed] [Google Scholar]
- Koob GF (2021). Drug addiction: Hyperkatifeia/Negative reinforcement as a framework for medications development. Pharmacological Reviews, 73(1), 163–201. 10.1124/pharmrev.120.000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW, & George O (2014). Addiction as a stress surfeit disorder. Neuropharmacology, 76(PART B), 370–382. 10.1016/j.neuropharm.2013.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology, 35(1), 217–238. 10.1038/npp.2009.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, & Vartanian T (2003). Activation of innate immunity in the CNS triggers neurodegeneration through a toll-like receptor 4-dependent pathway. Proceedings of the National Academy of Sciences of the United States of America, 100(14), 8514–8519. 10.1073/pnas.1432609100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SS, Hutchinson MR, Rezvani N, Loram LC, Zhang Y, Maier SF, Rice KC, & Watkins LR (2010). Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1β. Neuroscience, 165(2), 569–583. 10.1016/j.neuroscience.2009.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Brennan K, Brau P, & Anisman H (2003). Dissociating anorexia and anhedonia elicited by interleukin-1β: Antidepressant and gender effects on responding for “free chow” and “earned” sucrose intake. Psychopharmacology, 165(4), 413–418. 10.1007/s00213-002-1273-1 [DOI] [PubMed] [Google Scholar]
- Moningka H, Lichenstein S, & Yip SW (2019). Current Understanding of the Neurobiology of Opioid Use Disorder: an Overview. Current Behavioral Neuroscience Reports, 6(1), 1–11. 10.1007/s40473-019-0170-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morie KP, De Sanctis P, Garavan H, & Foxe JJ (2014). Executive dysfunction and reward dysregulation: A high-density electrical mapping study in cocaine abusers. Neuropharmacology, 85, 397–407. 10.1016/j.neuropharm.2014.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, & Satoh M (2004). Involvement of glial glutamate transporters in morphine dependence. Annals of the New York Academy of Sciences, 1025, 383–388. 10.1196/annals.1307.047 [DOI] [PubMed] [Google Scholar]
- Naqvi NH, & Bechara A (2009). The hidden island of addiction: the insula. Trends in Neurosciences, 32(1), 56–67. 10.1016/j.tins.2008.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov MH, Dussor GO, & Porreca F (2010). Review series central modulation of pain. The Journal of Clinical Investigation, 120(11), 3779–3787. 10.1172/JCI43766.reduced [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa T, Nakagawa T, Shige K, Minami M, & Satoh M (2001). Changes in the expression of glial glutamate transporters in the rat brain accompanied with morphine dependence and naloxone-precipitated withdrawal. Brain Research, 905(1–2), 254–258. 10.1016/S0006-8993(01)02536-7 [DOI] [PubMed] [Google Scholar]
- Perry VH, & Holmes C (2014). Microglial priming in neurodegenerative disease. Nature Reviews Neurology, 10, 217–224. 10.1038/nrneurol.2014.38 [DOI] [PubMed] [Google Scholar]
- Rizzo FR, Musella A, De Vito F, Fresegna D, Bullitta S, Vanni V, Guadalupi L, Stampanoni Bassi M, Buttari F, Mandolesi G, Centonze D, & Gentile A (2018). Tumor necrosis factor and interleukin-1β modulate synaptic plasticity during neuroinflammation. Neural Plasticity, 2018, 12 pages. 10.1155/2018/8430123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC, Robinson TE, & Berridge KC (2001). Incentive-sensitization and addiction. Addiction, 96(1), 103–114. 10.1080/09652140020016996 [DOI] [PubMed] [Google Scholar]
- Salter MW, & Stevens B (2017). Microglia emerge as central players in brain disease. Nature Medicine, 23(9), 1018–1027. 10.1038/nm.4397 [DOI] [PubMed] [Google Scholar]
- Shen CH, Tsai RY, Shih MS, Lin SL, Tai YH, Chien CC, & Wong CS (2011). Etanercept restores the antinociceptive effect of morphine and suppresses spinal neuroinflammation in morphine-tolerant rats. Anesthesia and Analgesia, 112(2), 454–459. 10.1213/ANE.0b013e3182025b15 [DOI] [PubMed] [Google Scholar]
- Shen CH, Tsai RY, Tai YH, Lin SL, Chien CC, & Wong CS (2011). Intrathecal etanercept partially restores morphines antinociception in morphine-tolerant rats via attenuation of the glutamatergic transmission. Anesthesia and Analgesia, 113(1), 184–190. 10.1213/ANE.0b013e318217f7eb [DOI] [PubMed] [Google Scholar]
- Solomon RL, & Corbit JD (1974). An opponent-process theory of motivation: I. Temporal dynamics of affect. Psychological Review, 81(2), 119–145. 10.1037/h0036128 [DOI] [PubMed] [Google Scholar]
- Taylor AMW, Castonguay A, Ghogha A, Vayssiere P, Pradhan AAA, Xue L, Mehrabani S, Wu J, Levitt P, Olmstead MC, De Koninck Y, Evans CJ, & Cahill CM (2016). Neuroimmune regulation of GABAergic neurons within the ventral tegmental area during withdrawal from chronic morphine. Neuropsychopharmacology, 41(4), 949–959. 10.1038/npp.2015.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilleux S, & Hermans E (2007). Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. Journal of Neuroscience Research, 85(10), 2059–2070. 10.1002/jnr [DOI] [PubMed] [Google Scholar]
- Tortorici V, Robbins CS, & Morgan MM (1999). Tolerance to the antinociceptive effect of morphine microinjections into the ventral but not lateral-dorsal periaqueductal gray of the rat. Behavioral Neuroscience, 113(4), 833–839. 10.1037/0735-7044.113.4.833 [DOI] [PubMed] [Google Scholar]
- Van Heesch F, Prins J, Konsman JP, Korte-Bouws GAH, Westphal KGC, Rybka J, Olivier B, Kraneveld AD, & Korte SM (2014). Lipopolysaccharide increases degradation of central monoamines: An in vivo microdialysis study in the nucleus accumbens and medial prefrontal cortex of mice. European Journal of Pharmacology, 725(1), 55–63. 10.1016/j.ejphar.2014.01.014 [DOI] [PubMed] [Google Scholar]
- Van Heesch F, Prins J, Korte-Bouws GAH, Westphal KGC, Lemstra S, Olivier B, Kraneveld AD, & Korte SM (2013). Systemic tumor necrosis factor-alpha decreases brain stimulation reward and increases metabolites of serotonin and dopamine in the nucleus accumbens of mice. Behavioural Brain Research, 253, 191–195. 10.1016/j.bbr.2013.07.038 [DOI] [PubMed] [Google Scholar]
- Vargas-Perez H, Bahi A, Bufalino MR, Ting-A-Kee R, Maal-Bared G, Lam J, Fahmy A, Clarke L, Blanchard JK, Larsen BR, Steffensen S, Dreyer JL, & van der Kooy D (2014). BDNF signaling in the VTA links the drug-dependent state to drug withdrawal aversions. Journal of Neuroscience, 34(23), 7899–7909. 10.1523/JNEUROSCI.3776-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Perez H, Kee RT, Walton CH, Hansen DM, Clarke L, Bufalino MR, Allison DW, Steffensen SC, & Van Der. Kooy D (2009). Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naive rats. Science, 324(5035), 1732–1734. https://doi.org/doi: 10.1126/science.1168501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, & Marinovich M (2003). Interleukin-1β enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. Journal of Neuroscience, 23(25), 8692–8700. 10.1523/jneurosci.23-25-08692.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, & Morales M (2015). The brain on drugs: From reward to addiction. Cell, 162(4), 712–725. 10.1016/j.cell.2015.07.046 [DOI] [PubMed] [Google Scholar]
- Wang X, Loram LC, Ramos K, De Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR, & Yin H (2012). Morphine activates neuroinflammation in a manner parallel to endotoxin. Proceedings of the National Academy of Sciences of the United States of America, 109(16), 6325–6330. 10.1073/pnas.1200130109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman SC, Alhassoon OM, Hall MG, Stern MJ, Connors EJ, Kimmel CL, Allen KE, Stephan RA, & Radua J (2017). Gray matter abnormalities in opioid-dependent patients: A neuroimaging meta-analysis. The American Journal of Drug and Alcohol Abuse, 43(5), 505–517. 10.1080/00952990.2016.1245312 [DOI] [PubMed] [Google Scholar]
- Wollman SC, Hauson AO, Hall MG, Connors EJ, Allen KE, Stern MJ, Stephan RA, Kimmel CL, Sarkissians S, Barlet BD, & Flora-Tostado C (2019). Neuropsychological functioning in opioid use disorder: A research synthesis and meta-analysis. The American Journal of Drug and Alcohol Abuse, 45(1), 11–25. 10.1080/00952990.2018.1517262 [DOI] [PubMed] [Google Scholar]
- Wu Y, Na X, Zang Y, Yu C, Xin W, Pang R, Zhou L, Wei X, Li Y, & Liu X (2014). Upregulation of tumor necrosis factor-alpha in nucleus accumbens attenuates morphine-induced rewarding in a neuropathic pain model. Biochemical and Biophysical Research Communications, 449(4), 502–507. 10.1016/j.bbrc.2014.05.025 [DOI] [PubMed] [Google Scholar]
- Xuan FL, Chithanathan K, Lilleväli K, Yuan X, & Tian L (2019). Differences of Microglia in the Brain and the Spinal Cord. Frontiers in Cellular Neuroscience, 13(November), 1–5. 10.3389/fncel.2019.00504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Yadav R, Gao M, & Weng HR (2014). Interleukin-1 beta enhances endocytosis of glial glutamate transporters in the spinal dorsal horn through activating protein kinase C. Glia, 62(7), 1093–1109. 10.1002/glia.22665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Largent-Milnes TM, & Vanderah TW (2020). Glial neuroimmune signaling in opioid reward. Brain Research Bulletin, 155, 102–111. 10.1016/j.brainresbull.2019.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XQ, Cui Y, Cui Y, Chen Y, Na XD, Chen FY, Wei XH, Li YY, Liu XG, & Xin WJ (2012). Activation of p38 signaling in the microglia in the nucleus accumbens contributes to the acquisition and maintenance of morphine-induced conditioned place preference. Brain, Behavior, and Immunity, 26(2), 318–325. 10.1016/j.bbi.2011.09.017 [DOI] [PubMed] [Google Scholar]